Spontaneous Hinge-Bending Motions of Angiotensin I Converting Enzyme: Role in Activation and Inhibition
Abstract
1. Introduction
2. Results
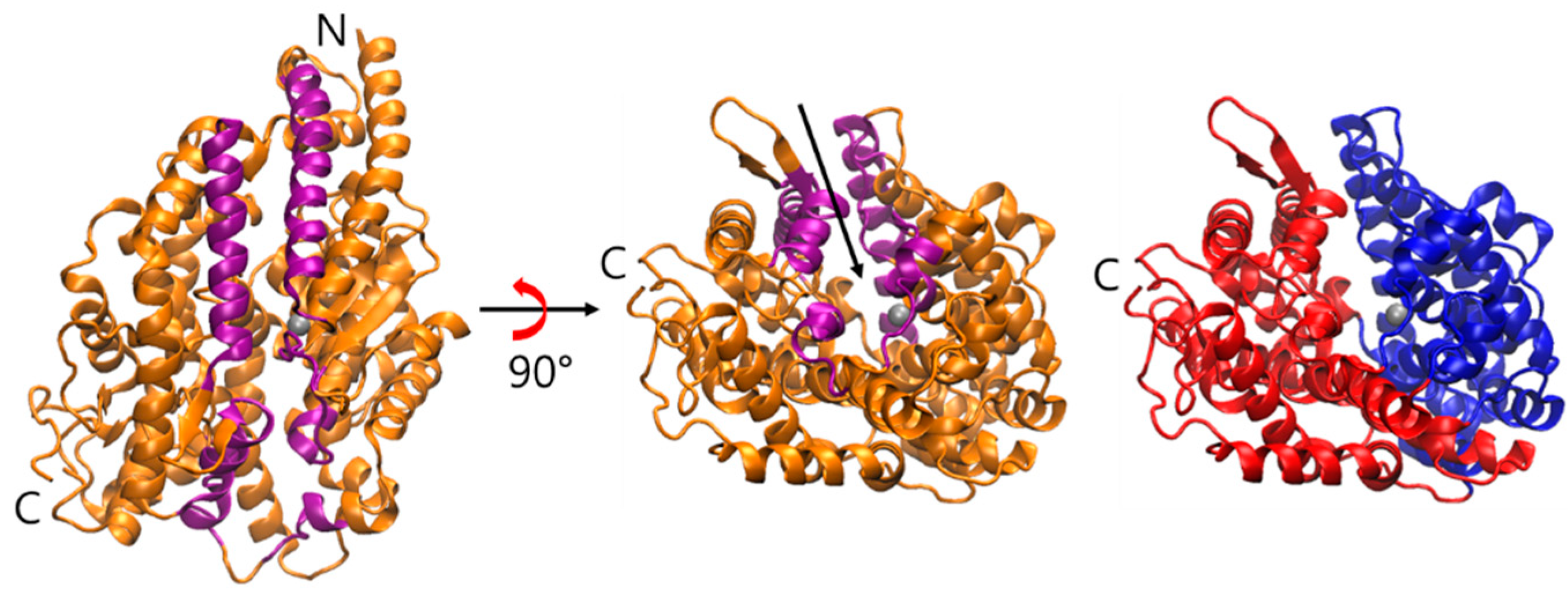
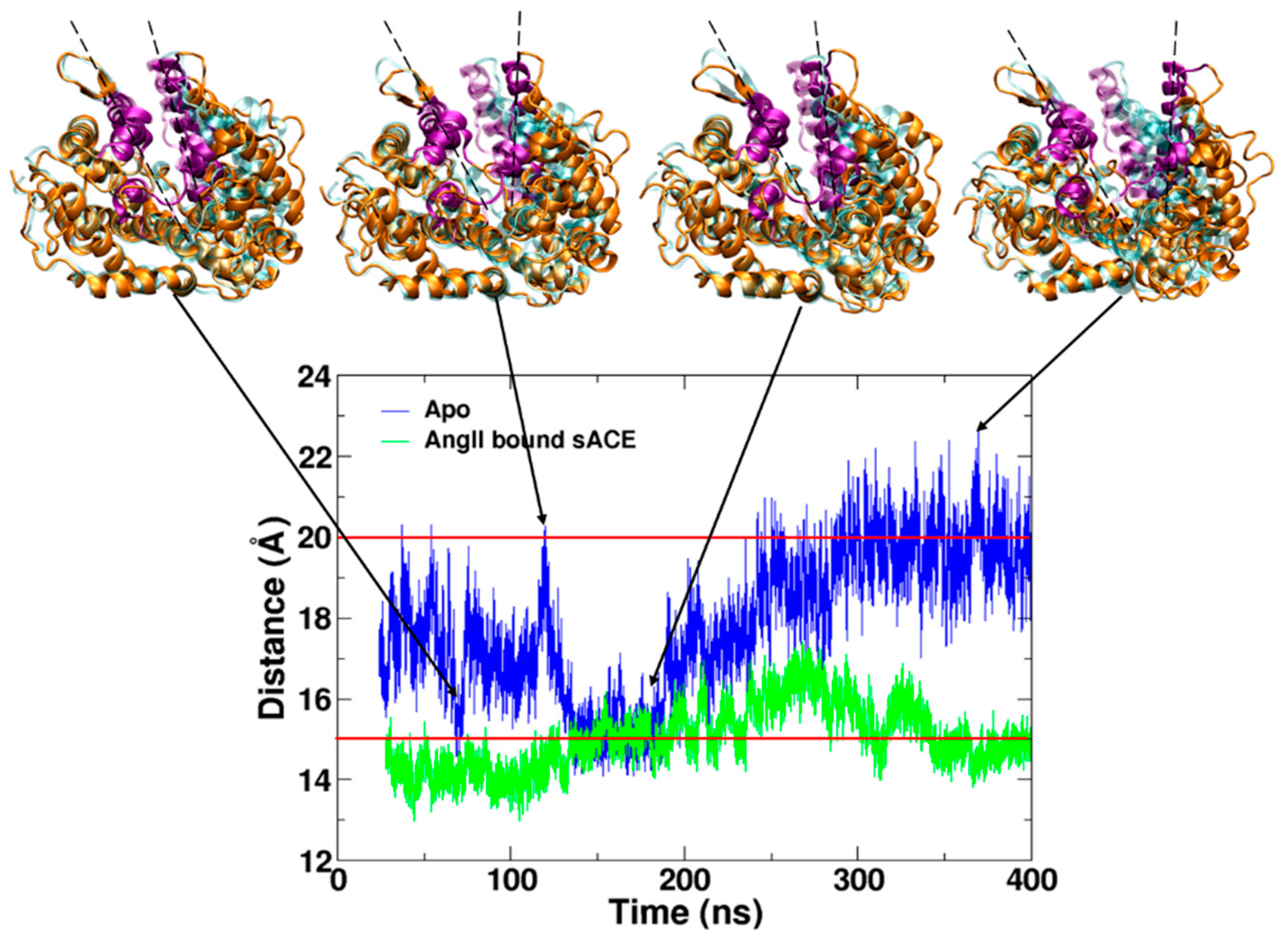
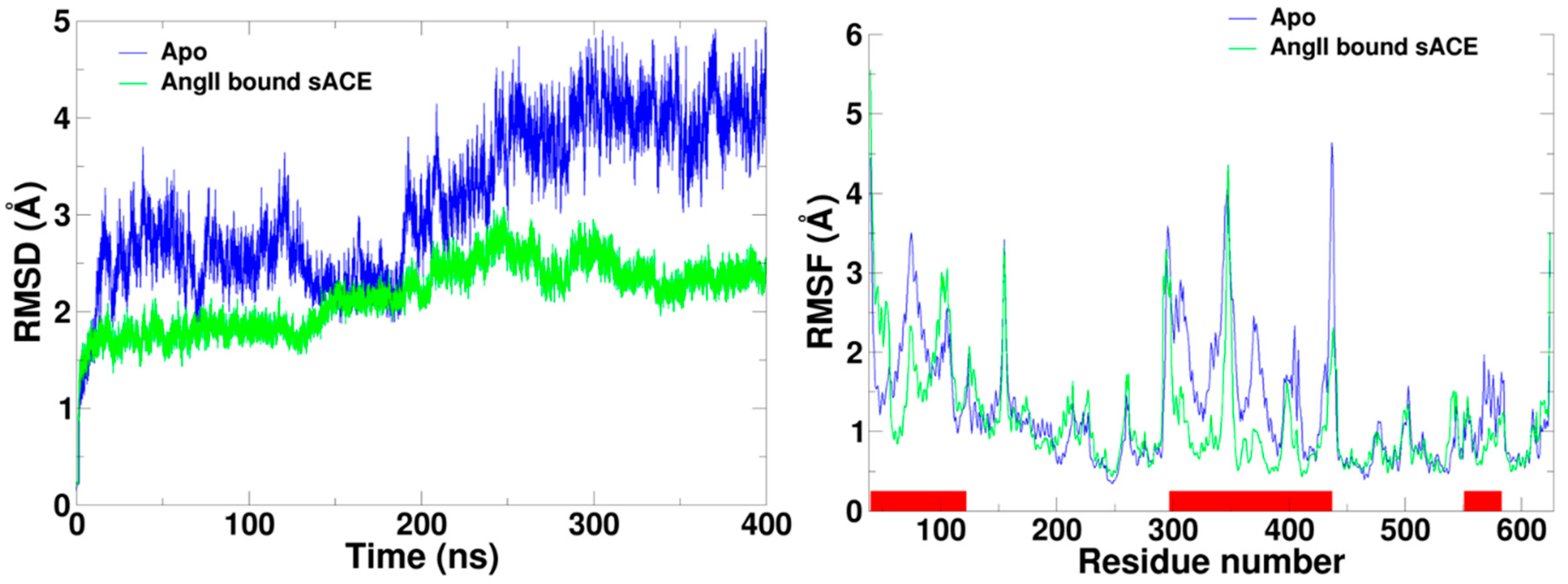
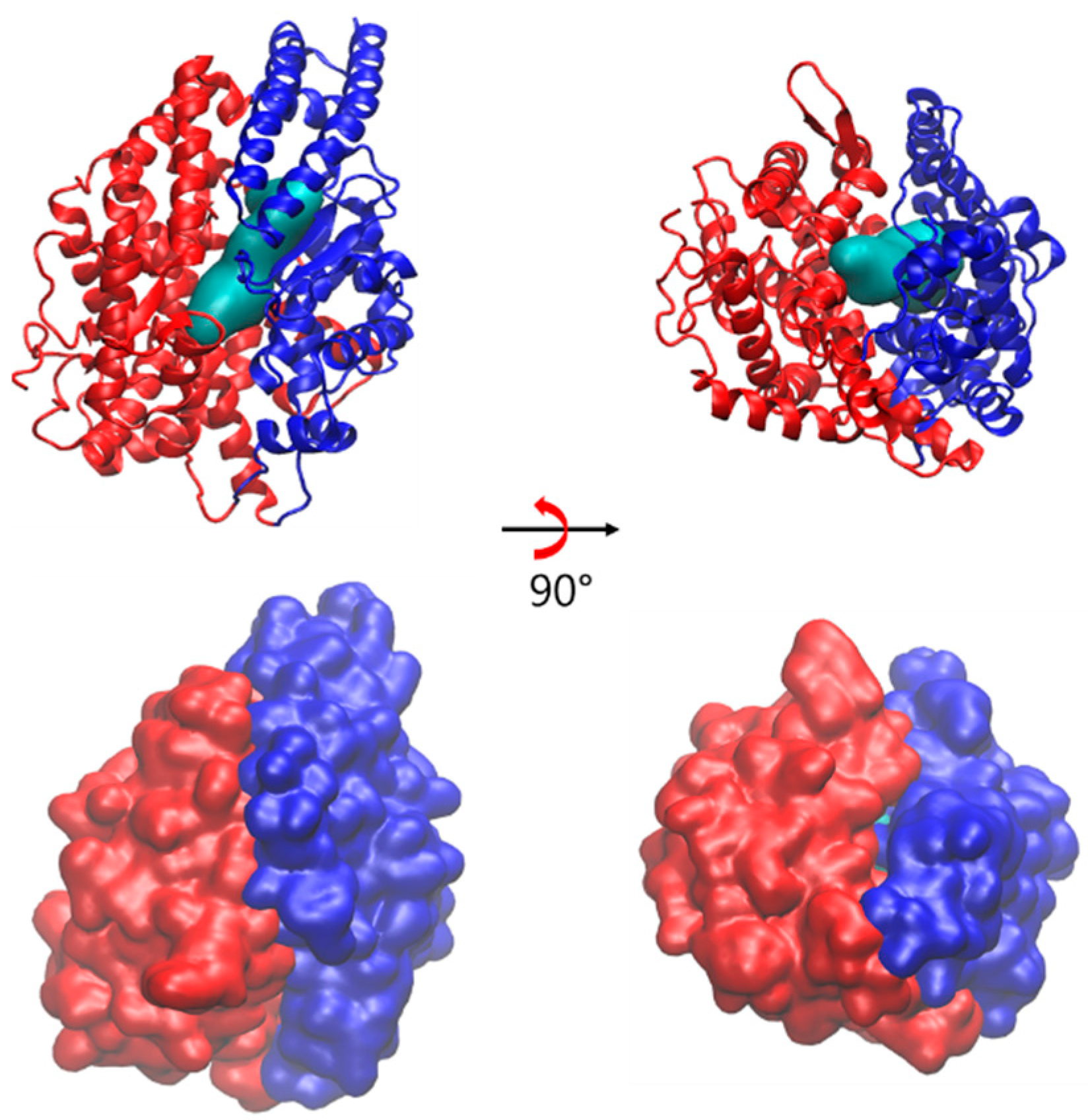
2.1. Spontaneous Conformational Changes
2.2. Flexibility of sACE
2.3. Open Conformation of ACE2
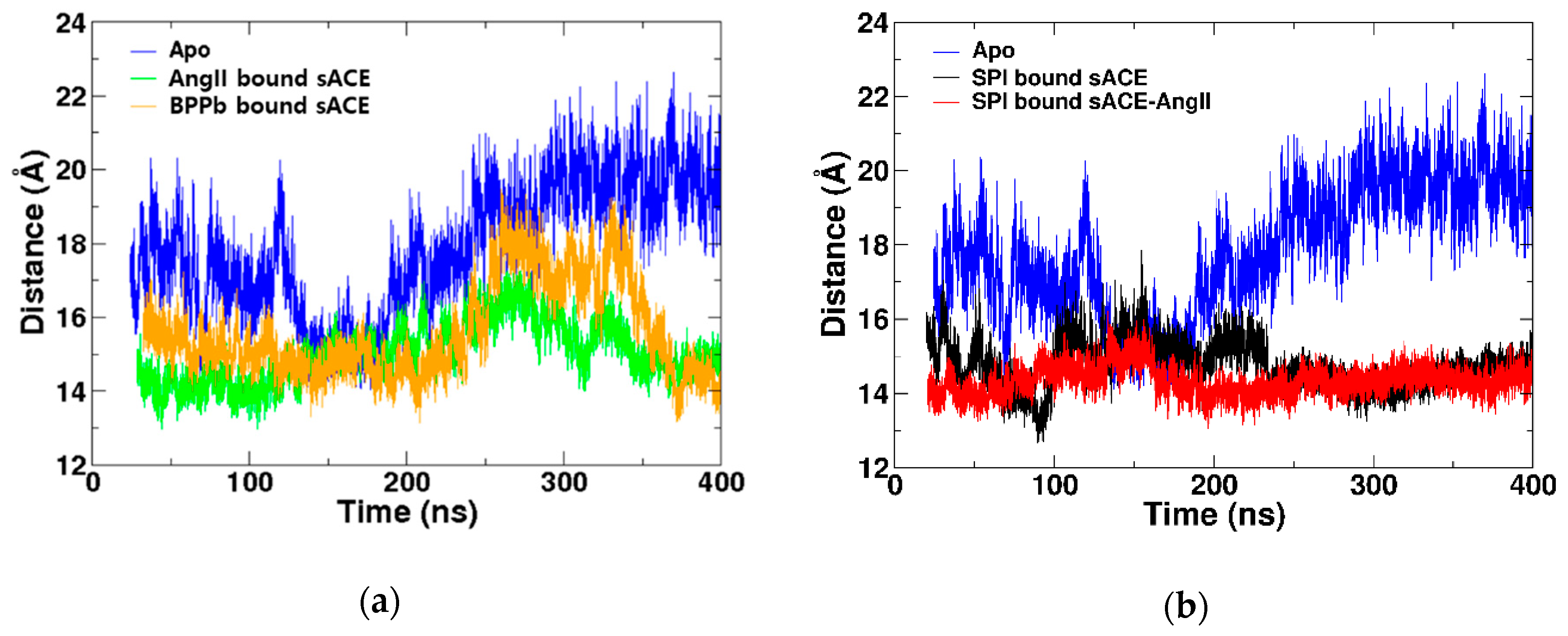
2.4. Competitive Inhibitor Binding
2.5. Mixed Non-competitive Inhibitor Binding
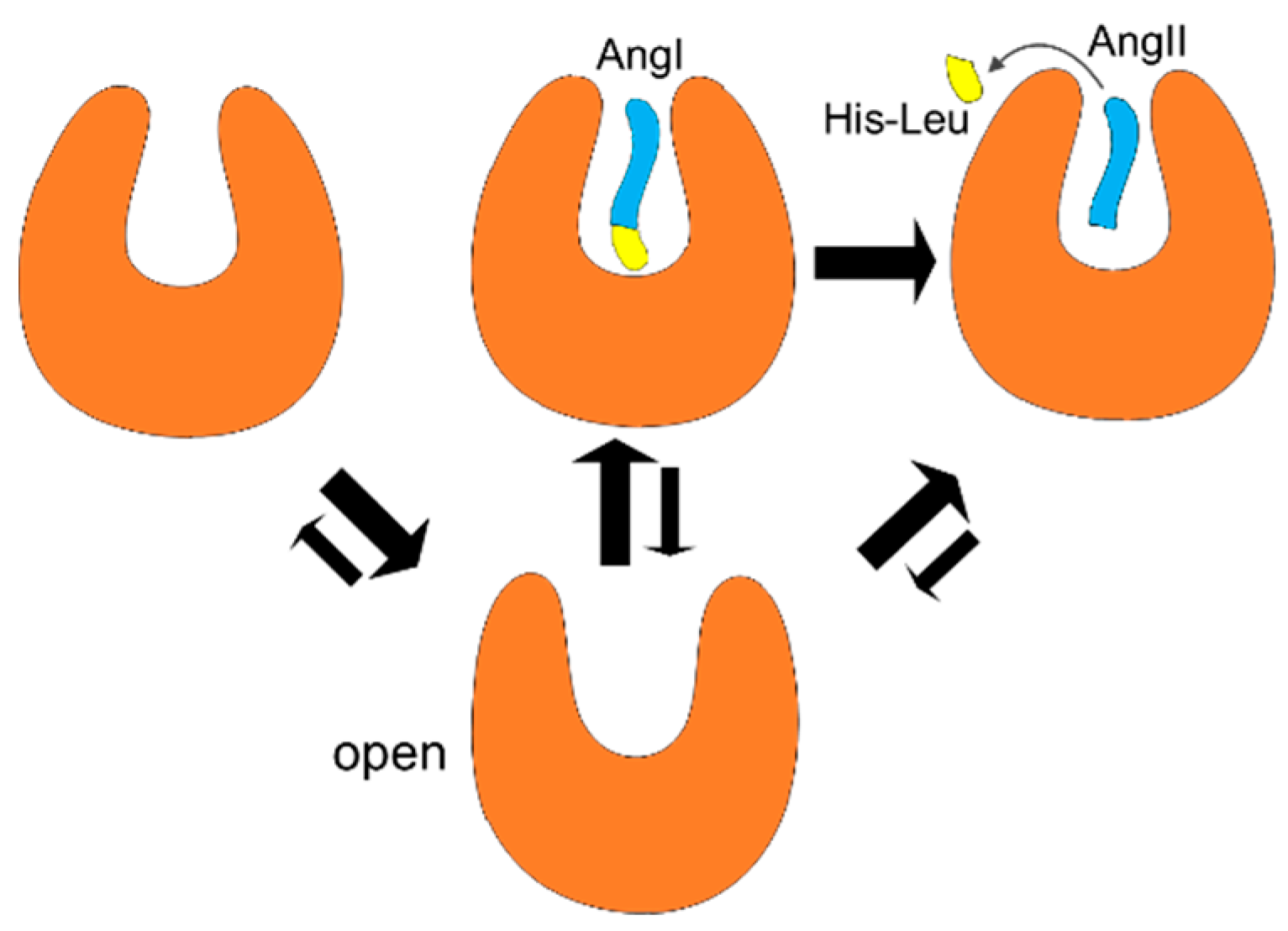
3. Discussion
4. Materials and Methods
4.1. Molecular Docking for SPI
4.2. Preparation of MD Simulations
4.3. Molcular Dynamics Simulations
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kearney, P.M.; Whelton, M.; Reynolds, K.; Muntner, P.; Whelton, P.K.; He, J. Global Burden of Hypertension: Analysis of Worldwide Data. Lancet 2005, 365, 217–223. [Google Scholar] [CrossRef]
- Inagami, T. The Renin-Angiotensin System. Essays Biochem. 1994, 28, 147–164. [Google Scholar] [PubMed]
- Rawlings, N.D.; Barrett, A.J. Evolutionary Families of Peptidases. Biochem. J. 1993, 290, 205–218. [Google Scholar] [CrossRef]
- Fernandez, J.H.; Hayashi, M.A.F.; Camargo, A.C.M.; Neshich, G. Structural Basis of the Lisinopril-Binding Specificity in N- and C-Domains of Human Somatic ACE. Biochem. Biophys. Res. Commun. 2003, 308, 219–226. [Google Scholar] [CrossRef]
- Wei, L.; Alhenc-Gelas, F.; Corvol, P.; Clauser, E. The Two Homologous Domains of Human Angiotensin I-Converting Enzyme are both Catalytically Active. J. Biol. Chem. 1991, 266, 9002–9008. [Google Scholar]
- Dell’Italia, L.J.; Meng, Q.C.; Balcells, E.; Wei, C.C.; Palmer, R.; Hageman, G.R.; Durand, J.; Hankes, G.H.; Oparil, S. Compartmentalization of Angiotensin II Generation in the Dog Heart. Evidence for Independent Mechanisms in Intravascular and Interstitial Spaces. J. Clin. Investig. 1997, 100, 253–258. [Google Scholar] [CrossRef]
- Wei, C.C.; Meng, Q.C.; Palmer, R.; Hageman, G.R.; Durand, J.; Bradley, W.E.; Farrell, D.M.; Hankes, G.H.; Oparil, S.; Dell’Italia, L.J. Evidence for Angiotensin-Converting Enzyme- and Chymase-Mediated Angiotensin II Formation in the Interstitial Fluid Space of the Dog Heart in Vivo. Circulation 1999, 99, 2583–2589. [Google Scholar] [CrossRef][Green Version]
- Erdös, N.; Deddish, N.; Marcic, N. Potentiation of Bradykinin Actions by ACE Inhibitors. Trends Endocrinol. Metab. 1999, 10, 223–229. [Google Scholar] [CrossRef]
- Turner, A.J.; Hooper, N.M. The Angiotensin-Converting Enzyme Gene Family: Genomics and Pharmacology. Trends Pharmacol. Sci. 2002, 23, 177–183. [Google Scholar] [CrossRef]
- Acharya, K.R.; Sturrock, E.D.; Riordan, J.F.; Ehlers, M.R.W. Ace Revisited: A New Target for Structure-Based Drug Design. Nat. Rev. Drug. Discov. 2003, 2, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Je, J.; Kim, S. Antihypertensive Effect of Angiotensin I Converting Enzyme-Inhibitory Peptide from Hydrolysates of Bigeye Tuna Dark Muscle, Thunnus Obesus. J. Agric. Food Chem. 2007, 55, 8398–8403. [Google Scholar] [CrossRef] [PubMed]
- Cohn, J.N.; Kowey, P.R.; Whelton, P.K.; Prisant, L.M. New Guidelines for Potassium Replacement in Clinical Practice: A Contemporary Review by the National Council on Potassium in Clinical Practice. Arch. Intern. Med. 2000, 160, 2429–2436. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, L.J.; Morris, A.A.; Chapman, S.A. Angiotensin-Converting Enzyme Inhibitor Induced Angioedema: Predictors of Mechanical Ventilation and Treatment Approaches. Intensive Care Med. 2015, 41, 2233–2234. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Wang, C.; Song, Y.; Zhu, J.; Zhang, X. Discovery of Novel Angiotensin-Converting Enzyme Inhibitory Peptides from Todarodes Pacificus and their Inhibitory Mechanism: In Silico and in Vitro Studies. Int. J. Mol. Sci. 2019, 20, 4159. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Geng, M.; Liu, C.; Wang, J.; Min, W.; Liu, J. Structural and Molecular Basis of Angiotensin-Converting Enzyme by Computational Modeling: Insights into the Mechanisms of Different Inhibitors. PLoS ONE 2019, 14, e0215609. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhang, H.; Bian, X.; Li, J.; Li, J.; Zhang, H. Insight into the Binding of ACE-Inhibitory Peptides to Angiotensin-Converting Enzyme: A Molecular Simulation. Mol. Simul. 2018, 45, 215–222. [Google Scholar] [CrossRef]
- Cotton, J.; Hayashi, M.A.F.; Cuniasse, P.; Vazeux, G.; Ianzer, D.; De Camargo Antonio, C.M.; Dive, V. Selective Inhibition of the C-Domain of Angiotensin I Converting Enzyme by Bradykinin Potentiating Peptides. Biochemistry 2002, 41, 6065–6071. [Google Scholar] [CrossRef]
- Heo, S.; Ko, S.; Kim, C.S.; Oh, G.; Ryu, B.; Qian, Z.; Kim, G.; Park, W.S.; Choi, I.; Phan, T.T.V.; et al. A Heptameric Peptide Purified from Spirulina sp. Gastrointestinal Hydrolysate Inhibits Angiotensin I-Converting Enzyme- and angiotensin II-Induced Vascular Dysfunction in Human Endothelial Cells. Int. J. Mol. Med. 2017, 39, 1072–1082. [Google Scholar] [CrossRef]
- Masuyer, G.; Schwager, S.L.; Sturrock, E.D.; Isaac, R.E.; Acharya, K.R. Molecular Recognition and Regulation of Human Angiotensin-I Converting Enzyme (ACE) Activity by Natural Inhibitory Peptides. Sci. Rep. 2012, 2, 717. [Google Scholar] [CrossRef]
- Natesh, R.; Schwager, S.L.U.; Sturrock, E.D.; Acharya, K.R. Crystal Structure of the Human Angiotensin-Converting Enzyme-Lisinopril Complex. Nature 2003, 421, 551–554. [Google Scholar] [CrossRef]
- Natesh, R.; Schwager, S.L.U.; Evans, H.R.; Sturrock, E.D.; Acharya, K.R. Structural Details on the Binding of Antihypertensive Drugs Captopril and Enalaprilat to Human Testicular Angiotensin I-Converting Enzyme. Biochemistry 2004, 43, 8718–8724. [Google Scholar] [CrossRef] [PubMed]
- Corradi, H.R.; Schwager, S.L.U.; Nchinda, A.T.; Sturrock, E.D.; Acharya, K.R. Crystal Structure of the N Domain of Human Somatic Angiotensin I-Converting Enzyme Provides a Structural Basis for Domain-Specific Inhibitor Design. J. Mol. Biol. 2006, 357, 964–974. [Google Scholar] [CrossRef] [PubMed]
- Frauenfelder, H.; Sligar, S.G.; Wolynes, P.G. The Energy Landscapes and Motions of Proteins. Science 1991, 254, 1598–1603. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Colletier, J.P.; Jiang, H.; Silman, I.; Sussman, J.L.; Weik, M. Induced-Fit or Preexisting Equilibrium Dynamics? Lessons from Protein Crystallography and MD Simulations on Acetylcholinesterase and Implications for Structure-Based Drug Design. Protein Sci. 2008, 17, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, E.R. Molecular Dynamics Simulations. Methods Mol. Biol. 2008, 443, 3–23. [Google Scholar] [CrossRef]
- Jalkute, C.B.; Barage, S.H.; Dhanavade, M.J.; Sonawane, K.D. Molecular Dynamics Simulation and Molecular Docking Studies of Angiotensin Converting Enzyme with Inhibitor Lisinopril and Amyloid Beta Peptide. Protein J. 2013, 32, 356–364. [Google Scholar] [CrossRef]
- Pac-Man. Available online: https://en.wikipedia.org/wiki/Pac-Man (accessed on 8 January 2020).
- Teague, S.J. Implications of Protein Flexibility for Drug Discovery. Nat. Rev. Drug. Discov. 2003, 2, 527–541. [Google Scholar] [CrossRef]
- Kirchdoerfer, R.N.; Wang, N.; Pallesen, J.; Wrapp, D.; Turner, H.L.; Cottrell, C.A.; Corbett, K.S.; Graham, B.S.; McLellan, J.S.; Ward, A.B. Stabilized Coronavirus Spikes are Resistant to Conformational Changes Induced by Receptor Recognition Or Proteolysis. Sci. Rep. 2018, 8, 15701. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM Structure of the 2019-nCoV Spike in the Prefusion Conformation. Science 2020. [Google Scholar] [CrossRef]
- Ehlers, M.R.; Riordan, J.F. Angiotensin-Converting Enzyme: Zinc- and Inhibitor-Binding Stoichiometries of the Somatic and Testis Isozymes. Biochemistry 1991, 30, 7118–7126. [Google Scholar] [CrossRef]
- Towler, P.; Staker, B.; Prasad, S.G.; Menon, S.; Tang, J.; Parsons, T.; Ryan, D.; Fisher, M.; Williams, D.; Dales, N.A.; et al. ACE2 X-Ray Structures Reveal a Large Hinge-Bending Motion Important for Inhibitor Binding and Catalysis. J. Biol. Chem. 2004, 279, 17996–18007. [Google Scholar] [CrossRef] [PubMed]
- Koshland, D.E. Application of a Theory of Enzyme Specificity to Protein Synthesis. Proc. Natl. Acad. Sci. USA 1958, 44, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.; Milburn, D.; Gerstein, M. Conformational Changes Associated with Protein-Protein Interactions. Curr. Opin. Struct. Biol. 2004, 14, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Watermeyer, J.M.; Sewell, B.T.; Schwager, S.L.; Natesh, R.; Corradi, H.R.; Acharya, K.R.; Sturrock, E.D. Structure of Testis ACE Glycosylation Mutants and Evidence for Conserved Domain Movement. Biochemistry (N. Y.) 2006, 45, 12654–12663. [Google Scholar] [CrossRef] [PubMed]
- Andújar-Sánchez, M.; Cámara-Artigas, A.; Jara-Pérez, V. A Calorimetric Study of the Binding of Lisinopril, Enalaprilat and Captopril to Angiotensin-Converting Enzyme. Biophys. Chem. 2004, 111, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Raveh, B.; London, N.; Schueler-Furman, O. Sub-Angstrom Modeling of Complexes between Flexible Peptides and Globular Proteins. Proteins 2010, 78, 2029–2040. [Google Scholar] [CrossRef]
- Best, R.B.; Zhu, X.; Shim, J.; Lopes, P.E.M.; Mittal, J.; Feig, M.; Mackerell, A.D. Optimization of the Additive CHARMM all-Atom Protein Force Field Targeting Improved Sampling of the Backbone Φ, Ψ and Side-Chain Χ(1) and Χ(2) Dihedral Angles. J. Chem. Theory Comput. 2012, 8, 3257–3273. [Google Scholar] [CrossRef]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMM General Force Field: A Force Field for Drug-Like Molecules Compatible with the CHARMM all-Atom Additive Biological Force Fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kalé, L.; Schulten, K. Scalable Molecular Dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef]
- Toukmaji, A.; Sagui, C.; Board, J.; Darden, T. Efficient Particle-Mesh Ewald Based Approach to Fixed and Induced Dipolar Interactions. J. Chem. Phys. 2000, 113, 10913–10927. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]

| Complex | Average Number of Hydrogen Bond |
|---|---|
| AngII bound sACE | 6.52 ± 2.15 |
| BPPb bound sACE | 6.05 ± 1.64 |
| SPI bound sACE | 5.86 ± 1.42 |
| SPI bound sACE-AngII complex | 6.73 ± 1.57 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phan, T.T.V.; Heo, S.-Y.; Jung, W.-K.; Yi, M. Spontaneous Hinge-Bending Motions of Angiotensin I Converting Enzyme: Role in Activation and Inhibition. Molecules 2020, 25, 1288. https://doi.org/10.3390/molecules25061288
Phan TTV, Heo S-Y, Jung W-K, Yi M. Spontaneous Hinge-Bending Motions of Angiotensin I Converting Enzyme: Role in Activation and Inhibition. Molecules. 2020; 25(6):1288. https://doi.org/10.3390/molecules25061288
Chicago/Turabian StylePhan, Thi Tuong Vy, Seong-Yeong Heo, Won-Kyo Jung, and Myunggi Yi. 2020. "Spontaneous Hinge-Bending Motions of Angiotensin I Converting Enzyme: Role in Activation and Inhibition" Molecules 25, no. 6: 1288. https://doi.org/10.3390/molecules25061288
APA StylePhan, T. T. V., Heo, S.-Y., Jung, W.-K., & Yi, M. (2020). Spontaneous Hinge-Bending Motions of Angiotensin I Converting Enzyme: Role in Activation and Inhibition. Molecules, 25(6), 1288. https://doi.org/10.3390/molecules25061288








