A Targeted Approach by High Resolution Mass Spectrometry to Reveal New Compounds in Raisins
Abstract
1. Introduction
2. Results and Discussion
2.1. Phenolic Profile of Commercial Raisins
2.1.1. Phenolic Acids and Derivatives
2.1.2. Flavanols (Proanthocyanidins)
2.1.3. Flavonols, Flavones, and Derivatives
2.1.4. Flavanones and Derivatives
2.1.5. Stilbenes and Derivatives
3. Materials and Methods
3.1. Standards and Reagents
3.2. Extraction of Polyphenols
3.3. LC-LTQ-Orbitrap-MS Analyses
3.4. Analysis of Total Polyphenols
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Olmo-Cunillera, A.; Escobar-Avello, D.; Pérez, A.J.; Marhuenda-Muñoz, M.; Lamuela-Raventós, R.M.; Vallverdú-Queralt, A. Is Eating Raisins Healthy? Nutrients 2019, 12, 54. [Google Scholar] [CrossRef]
- Karadeniz, F.; Durst, R.W.; Wrolstad, R.E. Polyphenolic composition of raisins. J. Agric. Food Chem. 2000, 48, 5343–5350. [Google Scholar] [CrossRef]
- Parker, T.L.; Wang, X.H.; Pazmiño, J.; Engeseth, N.J. Antioxidant capacity and phenolic content of grapes, sun-dried raisins, and golden raisins and their effect on ex vivo serum antioxidant capacity. J. Agric. Food Chem. 2007, 55, 8472–8477. [Google Scholar] [CrossRef]
- Williamson, G.; Carughi, A. Polyphenol content and health benefits of raisins. Nutr. Res. 2010, 30, 511–519. [Google Scholar] [CrossRef]
- Fabani, M.P.; Baroni, M.V.; Luna, L.; Lingua, M.S.; Monferran, M.V.; Paños, H.; Tapia, A.; Wunderlin, D.A.; Feresin, G.E. Changes in the phenolic profile of Argentinean fresh grapes during production of sun-dried raisins. J. Food Compos. Anal. 2017, 58, 23–32. [Google Scholar] [CrossRef]
- Chira, K.; Suh, J.H.; Saucier, C.; Teissèdre, P.L. Grape phenolics. Phytotherapie 2008, 6, 75–82. [Google Scholar] [CrossRef]
- Kelebek, H.; Jourdes, M.; Selli, S.; Teissedre, P.L. Comparative evaluation of the phenolic content and antioxidant capacity of sun-dried raisins. J. Sci. Food Agric. 2013, 93, 2963–2972. [Google Scholar] [CrossRef]
- Chiou, A.; Panagopoulou, E.A.; Gatzali, F.; De Marchi, S.; Karathanos, V.T. Anthocyanins content and antioxidant capacity of Corinthian currants (Vitis vinifera L., var. Apyrena). Food Chem. 2014, 146, 157–165. [Google Scholar] [CrossRef]
- Medina-Remon, A.; Estruch, R.; Tresserra-Rimbau, A.; Vallverdu-Queralt, A.; Lamuela-Raventos, R.M. The Effect of Polyphenol Consumption on Blood Pressure. Mini-Rev. Med. Chem. 2013, 13, 1137–1149. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Arranz, S.; Eder, M.; Vallverdu-Queralt, A. Dietary Polyphenols in the Prevention of Stroke. Oxid. Med. Cell. Longev. 2017, 2017, 7467962. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Fang, Y.; Zhang, A.; Chen, S.; Xu, T.; Ren, Z.; Han, G.; Liu, J.; Li, H.; Zhang, Z.; et al. Phenolic content and antioxidant capacity of Chinese raisins produced in Xinjiang Province. Food Res. Int. 2011, 44, 2830–2836. [Google Scholar] [CrossRef]
- Olivati, C.; de Oliveira Nishiyama, Y.P.; de Souza, R.T.; Janzantti, N.S.; Mauro, M.A.; Gomes, E.; Hermosín-Gutiérrez, I.; da Silva, R.; Lago-Vanzela, E.S. Effect of the pre-treatment and the drying process on the phenolic composition of raisins produced with a seedless Brazilian grape cultivar. Food Res. Int. 2019, 116, 190–199. [Google Scholar] [CrossRef]
- Panagopoulou, E.A.; Chiou, A.; Nikolidaki, E.K.; Christea, M.; Karathanos, V.T. Corinthian raisins (Vitis vinifera L., var. Apyrena) antioxidant and sugar content as affected by the drying process: a 3-year study. J. Sci. Food Agric. 2019, 99, 915–922. [Google Scholar] [CrossRef]
- Mencarelli, F.; Bellincontro, A.; Nicoletti, I.; Cirilli, M.; Muleo, R.; Corradini, D. Chemical and biochemical change of healthy phenolic fractions in winegrape by means of postharvest dehydration. J. Agric. Food Chem. 2010, 58, 7557–7564. [Google Scholar] [CrossRef]
- Zhao, B.; Hall, C.A. Composition and antioxidant activity of raisin extracts obtained from various solvents. Food Chem. 2008, 108, 511–518. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Lamuela-Raventós, R.M. Foodomics: A new tool to differentiate between organic and conventional foods. Electrophoresis 2016, 37, 1784–1794. [Google Scholar] [CrossRef]
- Delgado, A.M.; Issaoui, M.; Chammem, N. Analysis of main and healthy Phenolic compounds in foods. J. AOAC Int. 2019, 102, 1356–1364. [Google Scholar] [CrossRef]
- Kafkas, N.E.; Kosar, M.; Öz, A.T.; Mitchell, A.E. Advanced Analytical Methods for Phenolics in Fruits. J. Food Qual. 2018, 2018, 3836064. [Google Scholar] [CrossRef]
- Makarov, A.; Grinfeld, D.; Ayzikov, K. Fundamentals of Orbitrap analyzer. In Fundamentals and Applications of Fourier Transform Mass Spectrometry; Kanawati, B., Schmitt-Kopplin, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 37–61. [Google Scholar]
- Escobar-Avello, D.; Mardones, C.; Pérez, A.J.; Saéz, V.; Von Baer, D.; Vallverdú-Queralt, A. Phenolic Profile of Grape Canes: Novel Compounds Identified by LC-ESI-LTQ-Orbitrap-MS. 1–21. Molecules 2019, 24, 3763. [Google Scholar]
- Vallverdú-Queralt, A.; Meudec, E.; Eder, M.; Lamuela-Raventos, R.M.; Sommerer, N.; Cheynier, V. The Hidden Face of Wine Polyphenol Polymerization Highlighted by High-Resolution Mass Spectrometry. ChemistryOpen 2017, 6, 336–339. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Meudec, E.; Eder, M.; Lamuela-Raventos, R.M.; Sommerer, N.; Cheynier, V. Targeted filtering reduces the complexity of UHPLC-Orbitrap-HRMS data to decipher polyphenol polymerization. Food Chem. 2017, 227, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Vallverdú-Queralt, A.; Regueiro, J.; Rinaldi-Alvarenga, J.F.; Martínez-Huélamo, M.; Leal, L.N.; Lamuela-Raventós, R. Characterization of the phenolic and antioxidant profiles of selected culinary herbs and spices: caraway, turmeric, dill, marjoram and nutmeg. Food Sci. Technol. 2015, 35, 189–195. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Jáuregui, O.; Medina-Remón, A.; Andrés-Lacueva, C.; Lamuela-Raventós, R.M. Improved characterization of tomato polyphenols using liquid chromatography electrospray ionization linear ion trap quadrupole Orbitrap mass spectrometry and liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 2986–2992. [Google Scholar] [CrossRef] [PubMed]
- Fayeulle, N.; Vallverdu-Queralt, A.; Meudec, E.; Hue, C.; Boulanger, R.; Cheynier, V.; Sommerer, N. Characterization of new flavan-3-ol derivatives in fermented cocoa beans. Food Chem. 2018, 259, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Chiou, A.; Karathanos, V.T.; Mylona, A.; Salta, F.N.; Preventi, F.; Andrikopoulos, N.K. Currants (Vitis vinifera L.) content of simple phenolics and antioxidant activity. Food Chem. 2007, 102, 516–522. [Google Scholar] [CrossRef]
- Breksa, A.P.; Takeoka, G.R.; Hidalgo, M.B.; Vilches, A.; Vasse, J.; Ramming, D.W. Antioxidant activity and phenolic content of 16 raisin grape (Vitis vinifera L.) cultivars and selections. Food Chem. 2010, 121, 740–745. [Google Scholar] [CrossRef]
- Serratosa, M.P.; Lopez-Toledano, A.; Merida, J.; Medina, M. Changes in Color and Phenolic Compounds during the Raisining of Grape Cv. Pedro Ximenez. J. Agric. Food Chem. 2008, 56, 2810–2816. [Google Scholar] [CrossRef]
- Saibabu, V.; Fatima, Z.; Khan, L.A.; Hameed, S. Therapeutic Potential of Dietary Phenolic Acids. Adv. Pharmacol. Sci. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Boix, N.; Piqué, E.; Gómez-Catalan, J.; Medina-Remon, A.; Sasot, G.; Mercader-Martí, M.; Llobet, J.M.; Lamuela-Raventos, R.M. Identification of phenolic compounds in red wine extract samples and zebrafish embryos by HPLC-ESI-LTQ-Orbitrap-MS. Food Chem. 2015, 181, 146–151. [Google Scholar] [CrossRef]
- Zoubiri, L.; Bakir, S.; Barkat, M.; Carrillo, C.; Capanoglu, E. Changes in the phenolic profile, antioxidant capacity and in vitro bioaccessibility of two Algerian grape varieties, Cardinal and Dabouki (Sabel), during the production of traditional sun-dried raisins and homemade jam. J. Berry Res. 2019, 9, 1–16. [Google Scholar] [CrossRef]
- Verbaere, A.; Meudec, E.; Cheynier, V.; Sommerer, N. Straightforward method to quantify GSH, GSSG, GRP, and hydroxycinnamic acids in wines by UPLC-MRM-MS. J. Agr. Food Chem. 2015, 63, 142–149. [Google Scholar]
- Di Lecce, G.; Arranz, S.; Jáuregui, O.; Tresserra-Rimbau, A.; Quifer-Rada, P.; Lamuela-Raventós, R.M. Phenolic profiling of the skin, pulp and seeds of Albariño grapes using hybrid quadrupole time-of-flight and triple-quadrupole mass spectrometry. Food Chem. 2014, 145, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef] [PubMed]
- García, D.E.; Delgado, N.; Aranda, F.L.; Toledo, M.A.; Cabrera-Barjas, G.; Sintjago, E.M.; Escobar-Avello, D.; Paczkowski, S. Synthesis of maleilated polyflavonoids and lignin as functional bio-based building-blocks. Ind. Crops Prod. 2018, 123, 154–163. [Google Scholar] [CrossRef]
- García, D.E.; Gavino, J.; Escobar, D.; Cancino, R.A. Maleinated polyflavonoids and lignin as functional additives for three kinds of thermoplastics. Iran. Polym. J. 2017, 26, 295–304. [Google Scholar] [CrossRef]
- García, D.E.; Fuentealba, C.A.; Salazar, J.P.; Pérez, M.A.; Escobar, D.; Pizzi, A. Mild hydroxypropylation of polyflavonoids obtained under pilot-plant scale. Ind. Crops Prod. 2016, 87, 350–362. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; García-Villanova, B.; Guerra-Hernández, E.; Verardo, V. Grape seeds proanthocyanidins: An overview of in vivo bioactivity in animal models. Nutrients 2019, 11, 2435. [Google Scholar] [CrossRef]
- Jiménez-Aspee, F.; Theoduloz, C.; Soriano, M.D.; Ugalde-Arbizu, M.; Alberto, M.R.; Zampini, I.C.; Isla, M.I.; Simirigiotis, M.J.; Schmeda-Hirschmann, G. The native fruit geoffroea decorticans from arid Northern Chile: Phenolic composition, antioxidant activities and in vitro inhibition of pro-inflammatory and metabolic syndrome-associated enzymes. Molecules 2017, 22, 1–18. [Google Scholar]
- Perez-Vizcaino, F.; Duarte, J. Flavonols and cardiovascular disease. Mol. Aspects Med. 2010, 31, 478–494. [Google Scholar] [CrossRef]
- Li, Z.H.; Guo, H.; Xu, W.B.; Ge, J.; Li, X.; Alimu, M.; He, D.J. Rapid Identification of Flavonoid Constituents Directly from PTP1B Inhibitive Extract of Raspberry (Rubus idaeus L.) Leaves by HPLC-ESI-QTOF-MS-MS. J. Chromatogr. Sci. 2016, 54, 805–810. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Quispe, C.; Bórquez, J.; Areche, C.; Sepúlveda, B. Fast detection of phenolic compounds in extracts of easter pears (Pyrus communis) from the atacama desert by ultrahigh-performance liquid chromatography and mass spectrometry (UHPLC-Q/Orbitrap/MS/MS). Molecules 2016, 21, 92. [Google Scholar] [CrossRef] [PubMed]
- Vallverdú-Queralt, A.; Jáuregui, O.; Di Lecce, G.; Andrés-Lacueva, C.; Lamuela-Raventós, R.M. Screening of the polyphenol content of tomato-based products through accurate-mass spectrometry (HPLC-ESI-QTOF). Food Chem. 2011, 129, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Meyvaci, K.B.; Aksoy, U.; Eltem, R.; Altindili, A.; Akun, T.; Takin, E. Effect of yearly conditions and management practices on ochratoxin a production in sultana seedless vineyards. Food Addit. Contam. - Part A Chem. Anal. Control. Expo. Risk Assess. 2012, 29, 1157–1167. [Google Scholar] [CrossRef]
- Barreca, D.; Gattuso, G.; Bellocco, E.; Calderaro, A.; Trombetta, D.; Smeriglio, A.; Laganà, G.; Daglia, M.; Meneghini, S.; Nabavi, S.M. Flavanones: Citrus phytochemical with health-promoting properties. BioFactors 2017, 43, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Sasot, G.; Martínez-Huélamo, M.; Vallverdú-Queralt, A.; Mercader-Martí, M.; Estruch, R.; Lamuela-Raventós, R.M. Identification of phenolic metabolites in human urine after the intake of a functional food made from grape extract by a high resolution LTQ-Orbitrap-MS approach. Food Res. Int. 2017, 100, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Perestrelo, R.; Lu, Y.; Santos, S.A.O.; Silvestre, A.J.D.; Neto, C.P.; Câmara, J.S.; Rocha, S.M. Phenolic profile of Sercial and Tinta Negra Vitis vinifera L. grape skins by HPLC-DAD-ESI-MSn: Novel phenolic compounds in Vitis vinifera L. grape. Food Chem. 2012, 135, 94–104. [Google Scholar] [CrossRef]
- Ramírez-Garza, S.L.; Laveriano-Santos, E.P.; Marhuenda-Muñoz, M.; Storniolo, C.E.; Tresserra-Rimbau, A.; Vallverdú-Queralt, A.; Lamuela-Raventós, R.M. Health effects of resveratrol: Results from human intervention trials. Nutrients 2018, 10, 1892. [Google Scholar] [CrossRef]
- Xue, Y.Q.; Di, J.M.; Luo, Y.; Cheng, K.J.; Wei, X.; Shi, Z. Resveratrol oligomers for the prevention and treatment of cancers. Oxid. Med. Cell. Longev. 2014, 2014, 765832. [Google Scholar] [CrossRef]
- Riquelme, S.; Sáez, V.; Escobar, D.; Vergara, C.; Fuentealba, C.; Bustamante, L.; von Baer, D.; Jara, P.; Lamperti, L.; Mardones, C. Bench-scale extraction of stilbenoids and other phenolics from stored grape canes (Vitis vinifera): Optimization process, chemical characterization, and potential protection against oxidative damage. J. Chil. Chem. Soc. 2019, 64, 4414–4420. [Google Scholar] [CrossRef]
- Mattivi, F.; Vrhovsek, U.; Malacarne, G.; Masuero, D.; Zulini, L.; Stefanini, M.; Mose, C.; Velasco, R.; Guella, G. Profiling of resveratrol oligomers, important stress metabolites, accumulating in the leaves of hybrid Vitis vinifera (Merzling × Teroldego) genotypes infected with Plasmopara viticola. J. Agric. Food Chem. 2011, 59, 5364–5375. [Google Scholar] [CrossRef] [PubMed]
- Pugajeva, I.; Perkons, I.; Górnaś, P. Identification and determination of stilbenes by Q-TOF in grape skins, seeds, juice and stems. J. Food Compos. Anal. 2018, 74, 44–52. [Google Scholar] [CrossRef]
- Gorena, T.; Saez, V.; Mardones, C.; Vergara, C.; Winterhalter, P.; Von Baer, D. Influence of post-pruning storage on stilbenoid levels in Vitis vinifera L. canes. Food Chem. 2014, 155, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Vallverdú-Queralt, A.; Arranz, S.; Medina-Remón, A.; Casals-Ribes, I.; Lamuela-Raventós, M. Changes in phenolic content of tomato products during storage. J. Agr. Food Chem. 2011, 59, 9358–9365. [Google Scholar]
- Quifer-Rada, P.; Vallverdú-Queralt, A.; Martínez-Huélamo, M.; Chiva-Blanch, G.; Jáuregui, O.; Estruch, R.; Lamuela-Raventós, R. A comprehensive characterisation of beer polyphenols by high resolution mass spectrometry (LC-ESI-LTQ-Orbitrap-MS). Food Chem. 2015, 169, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Calani, L.; Dall’Asta, C.; Galaverna, G.; García-Viguera, C.; Bruni, R.; Crozier, A.; Del Rio, D. Rapid and comprehensive evaluation of (Poly)phenolic compounds in pomegranate (Punica granatum L.) Juice by UHPLC-MSn. Molecules 2012, 17, 14821–14840. [Google Scholar] [CrossRef]
- Las Heras, R.M.; Quifer-Rada, P.; Andrés, A.; Lamuela-Raventós, R. Polyphenolic profile of persimmon leaves by high resolution mass spectrometry (LC-ESI-LTQ-Orbitrap-MS). J. Funct. Foods 2016, 23, 370–377. [Google Scholar] [CrossRef]
- Prodanov, M.; Vacas, V.; Hernández, T.; Estrella, I.; Amador, B.; Winterhalter, P. Chemical characterisation of Malvar grape seeds (Vitis vinifera L.) by ultrafiltration and RP-HPLC-PAD-MS. J. Food Compos. Anal. 2013, 31, 284–292. [Google Scholar] [CrossRef]
- Regueiro, J.; Sánchez-González, C.; Vallverdú-Queralt, A.; Simal-Gándara, J.; Lamuela-Raventós, R.; Izquierdo-Pulido, M. Comprehensive identification of walnut polyphenols by liquid chromatography coupled to linear ion trap-Orbitrap mass spectrometry. Food Chem. 2014, 152, 340–348. [Google Scholar] [CrossRef]
- Mena, P.; Cirlini, M.; Tassotti, M.; Herrlinger, K.A.; Dall’Asta, C.; Del Rio, D. Phytochemical profiling of flavonoids, phenolic acids, terpenoids, and volatile fraction of a rosemary (Rosmarinus officinalis L.) extract. Molecules 2016, 21, 1576. [Google Scholar] [CrossRef]
Sample Availability: Samples are not available from the authors. |
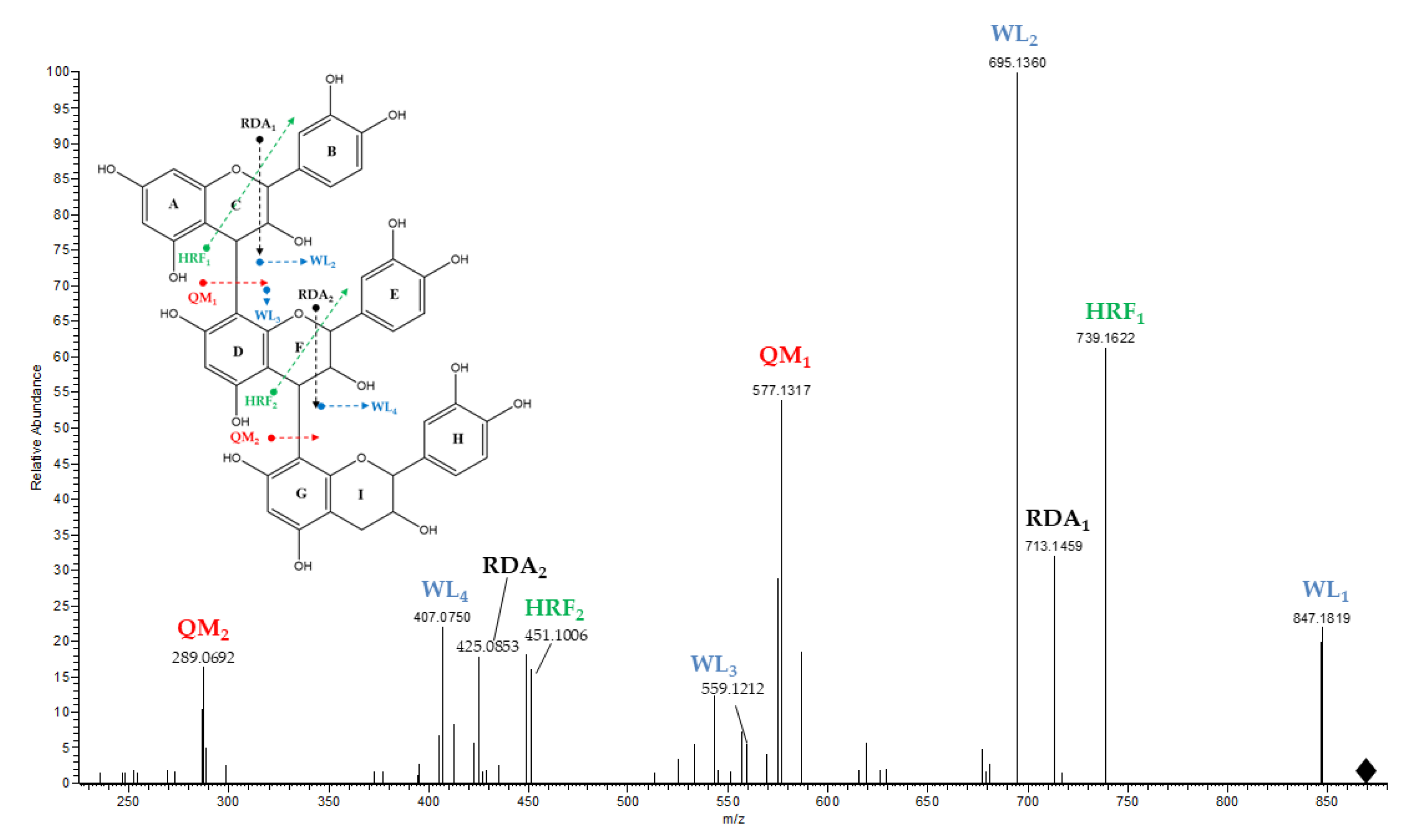
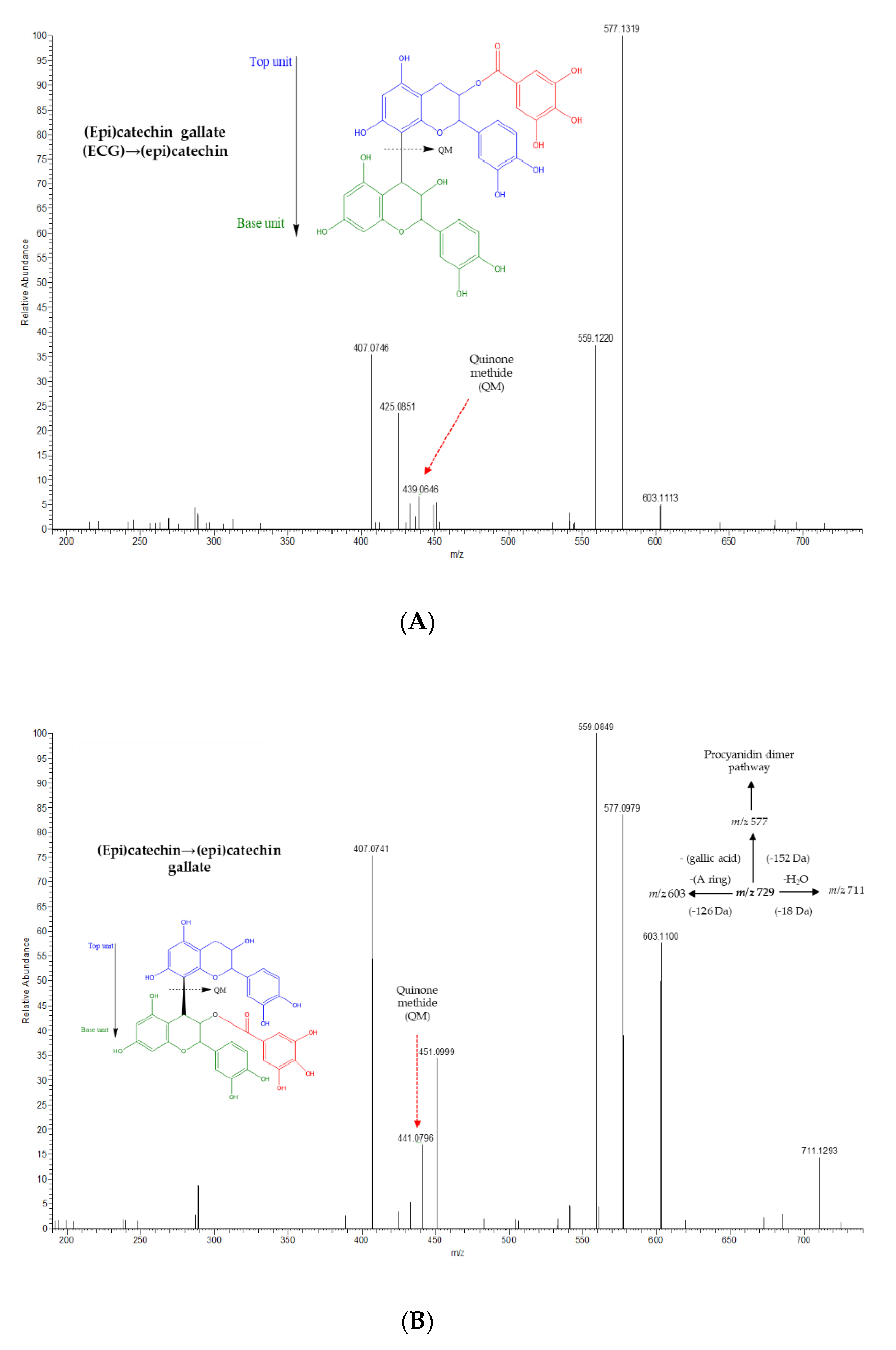
| Compounds | Raisins | Accurate Mass [M − H]− | MS/MS Ions (% Relative Abundance) | MF |
|---|---|---|---|---|
| PHENOLIC ACIDS AND DERIVATIVES | ||||
| HYDROXYBENZOIC ACIDS | ||||
| Galloyl-hexoside (1) | M,S | 331.0657 | 169.0133(100), 125.0236(5) | C13H16O10 |
| Gallic acid* | T,M,S | 169.0135 | 125.0237(100) | C7H6O5 |
| Protocatechuic acid-O-hexoside (1) | T,M,S | 315.0709 | 153.0184(100), 109.0289(10) | C13H16O9 |
| Galloyl-hexoside (2) | M,S | 331.0656 | 169.0132 (100), 125.0236(5) | C13H16O10 |
| Protocatechuic acid | T,M,S | 153.0186 | 109.0289(100) | C7H6O4 |
| Hydroxybenzoic acid hexoside | M,S | 299.0761 | 137.0238(100), 179.0342(75), 239.0551(70), 209.0449(20) | C13H16O8 |
| 2-Hydroxybenzoic acid* | T,M,S | 137.0238 | 93.0340(100) | C7H6O3 |
| Gallic acid ethyl ester (ethylgallate)* | M | 197.0446 | 169.0136(100) | C9H10O5 |
| 4-Hydroxybenzoic acid* | T,S | 137.0238 | 93.0340(100) | C7H6O3 |
| HYDROXYCINNAMIC ACIDS | ||||
| Caftaric acid (1) | M,S | 311.0394 | 149.0079(100), 179.0337(40) | C13H12O9 |
| Dihydroxy cinnamic acid (1) | T,M,S | 179.0342 | 135.0444(100) | C9H8O4 |
| Caftaric acid (2) | T,M,S | 311.0395 | 149.0084(100), 179.0343(20) | C13H12O9 |
| Coutaric acid | T,M,S | 295.0447 | 163.0391(100) | C13H12O8 |
| Coumaric acid-O-hexoside | T,M | 325.0914 | 163.0390(100) | C15H18O8 |
| Dihydroxy cinnamic acid (2)* | T,M,S | 179.0343 | 135.0444(100) | C9H8O4 |
| Fertaric acid | M,S | 325.0550 | 193.0494(100), 149.0082(10) | C14H14O9 |
| Ferulic acid-O-hexoside | T,M,S | 355.1020 | 193.0498(100) | C16H20O9 |
| FLAVONOIDS | ||||
| FLAVANOLS (PROANTHOCYANIDINS) | ||||
| B-type procyanidin dimer (1) | M | 577.1330 | 425.0853(100), 451.1008(60), 407.0750(50), 289.0699(40), 559.1217(20) | C30H26O12 |
| B-type procyanidin dimer (2) | M | 577.1329 | 425.0852(100), 451.1007(60), 407.0749(50), 289.0698(30), 559.1218(10) | C30H26O12 |
| Catechin* | M,S | 289.0707 | 245.0807(100), 205.0496(40), 179.0342(15), | C15H14O6 |
| B-type procyanidin trimer (1) | M | 865.1942 | 695.1360(100), 739.1622(60), 577.1317(55), 575.1165(30), 713.1459(30), 847.1819(20), 449.0850(20), 407.0750(20), 451.1006(15), 425.0853(15), 287.0540(15) | C45H38O18 |
| B-type procyanidin dimer (3) | M | 577.1328 | 425.0850(100), 451.1006(60), 407.0747(50), 289.0697(30), 559.1215(15) | C30H26O12 |
| B-type procyanidin dimer (4) | M | 577.1326 | 425.0853(100), 451.1009(60), 407.0750(40), 289.0699(30), 559.1221(10) | C30H26O12 |
| Epicatechin* | M,S | 289.0705 | 245.0809(100), 205.0497(40), 179.0343(20) | C15H14O6 |
| (Epi)catechin gallate→(Epi)catechin (1) | M | 729.1436 | 577.1319(100), 559.1220(40), 425.0851(20), 407.0746(35), 439.0646(5), 603.1113(5) | C37H30O16 |
| (Epi)catechin gallate→(Epi)catechin (2) | M | 729.1432 | 577.1312(100), 559.1214(40), 407.0747(30), 425.0850(20), 439.0644(5), 603.1092(5) | C37H30O16 |
| B-type procyanidin trimer (2) | M | 865.1944 | 695.1354(100), 739.1614(60). 577.1322(60), 575.1161(40), 713.1462(40), 407.0743(25), 449.0839(20), 425.0851(20), 847.1826(20), 287.0540(20), 543.0894(15), 451.1001(15), 289.0692(10) | C45H38O18 |
| (Epi)catechin→(Epi)catechin gallate (1) | M | 729.1429 | 559.0857(100), 407.0746(90), 577.0980(85), 603.1100(65), 441.0800(20), 451.1010(35), 711.1313(15) | C37H30O16 |
| (Epi)catechin→(Epi)catechin gallate (2) | M | 729.1436 | 407.0747(100), 603.1107(85), 577.1265(75), 559.0879(65), 451.1012(65), 441.0799(55), 711.1306(25), 425.0859(10) | C37H30O16 |
| B-type procyanidin dimer (5) | M | 577.1334 | 425.0849(100), 451.1003(70), 407.0744(60), 289.0699(30), 559.1204(20), 287.0540(10) | C30H26O12 |
| Epicatechin gallate* | M | 441.0811 | 289.0699(100), 331.0439(25), 169.0132(20), 271.0595(10). | C22H18O10 |
| FLAVONOLS | ||||
| Rutin (quercetin-3-rutinoside)* | S | 609.1428 | 301.0334(100), 300.0256(30) | C27H30O16 |
| Quercetin-O-hexoside (1) | M,S | 463.0864 | 301.0330(100) | C21H20O12 |
| Quercetin-3-O-glucuronide* | T,M,S | 477.0658 | 301.0334(100) | C21H18O13 |
| Quercetin-O-hexoside (2) | T,M,S | 463.0865 | 301.0337(100) | C21H20O12 |
| Kaempferol-3-O-glucoside* | M,S | 447.0915 | 284.0311(100), 285.0387(60), 327.0491(15), 255.0284(5) | C21H20O11 |
| Kaempferol-O-rutinoside | S | 593.1491 | 285.0385(100), 255.0284(5) | C27H30O15 |
| Kaempferol-O-hexoside | T,M,S | 447.0917 | 284.0311(100), 285.0388(85), 255.0282(10) | C21H20O11 |
| Isorhamnetin-O-hexoside | T,M,S | 477.1021 | 314.0410(100), 315.0489(35) | C22H22O12 |
| Quercetin* | T,M,S | 301.0341 | 178.9977(100), 151.0029(60) | C15H10O7 |
| FLAVONES | ||||
| Luteolin-O-glucuronide | S | 461.0710 | 285.0387(100), 175.0231(5) | C21H18O12 |
| FLAVANONES | ||||
| Eriodictyol-O-hexoside | M | 449.1070 | 287.0546(100), 269.0441(30), 259.0598(30) | C21H22O11 |
| Hesperidin (hesperitin-7-rutinoside)* | T,M,S | 609.1802 | 301.0699(100) | C28H34015 |
| STILBENES | ||||
| Stilbenoid tetramer (Hopeaphenol/Isohopeaphenol) | S | 905.2557 | 811.2121(100), 717.1716(35) | C56H42O12 |
| Stilbenoids dimer (viniferin) | M,S | 453.1330 | 359.0905(100), 347.0903(65) | C28H22O6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escobar-Avello, D.; Olmo-Cunillera, A.; Lozano-Castellón, J.; Marhuenda-Muñoz, M.; Vallverdú-Queralt, A. A Targeted Approach by High Resolution Mass Spectrometry to Reveal New Compounds in Raisins. Molecules 2020, 25, 1281. https://doi.org/10.3390/molecules25061281
Escobar-Avello D, Olmo-Cunillera A, Lozano-Castellón J, Marhuenda-Muñoz M, Vallverdú-Queralt A. A Targeted Approach by High Resolution Mass Spectrometry to Reveal New Compounds in Raisins. Molecules. 2020; 25(6):1281. https://doi.org/10.3390/molecules25061281
Chicago/Turabian StyleEscobar-Avello, Danilo, Alexandra Olmo-Cunillera, Julián Lozano-Castellón, María Marhuenda-Muñoz, and Anna Vallverdú-Queralt. 2020. "A Targeted Approach by High Resolution Mass Spectrometry to Reveal New Compounds in Raisins" Molecules 25, no. 6: 1281. https://doi.org/10.3390/molecules25061281
APA StyleEscobar-Avello, D., Olmo-Cunillera, A., Lozano-Castellón, J., Marhuenda-Muñoz, M., & Vallverdú-Queralt, A. (2020). A Targeted Approach by High Resolution Mass Spectrometry to Reveal New Compounds in Raisins. Molecules, 25(6), 1281. https://doi.org/10.3390/molecules25061281










