Synthesis and Antiproliferative Screening Of Novel Analogs of Regioselectively Demethylated Colchicine and Thiocolchicine
Abstract
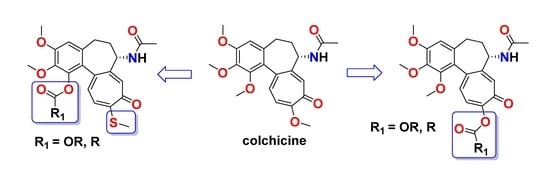
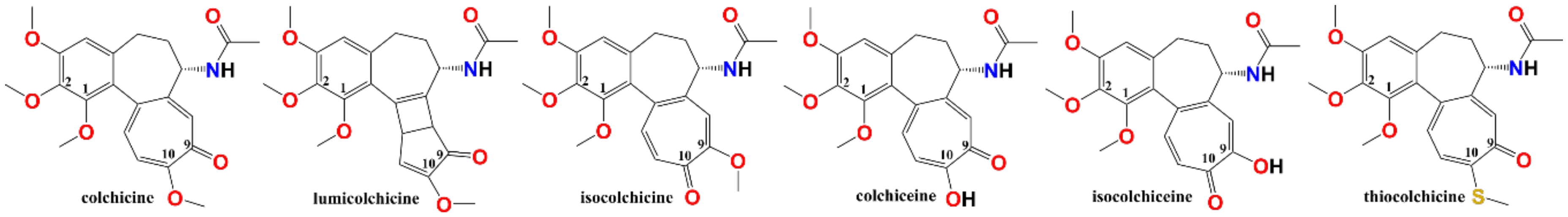
1. Introduction
2. Results and Discussion
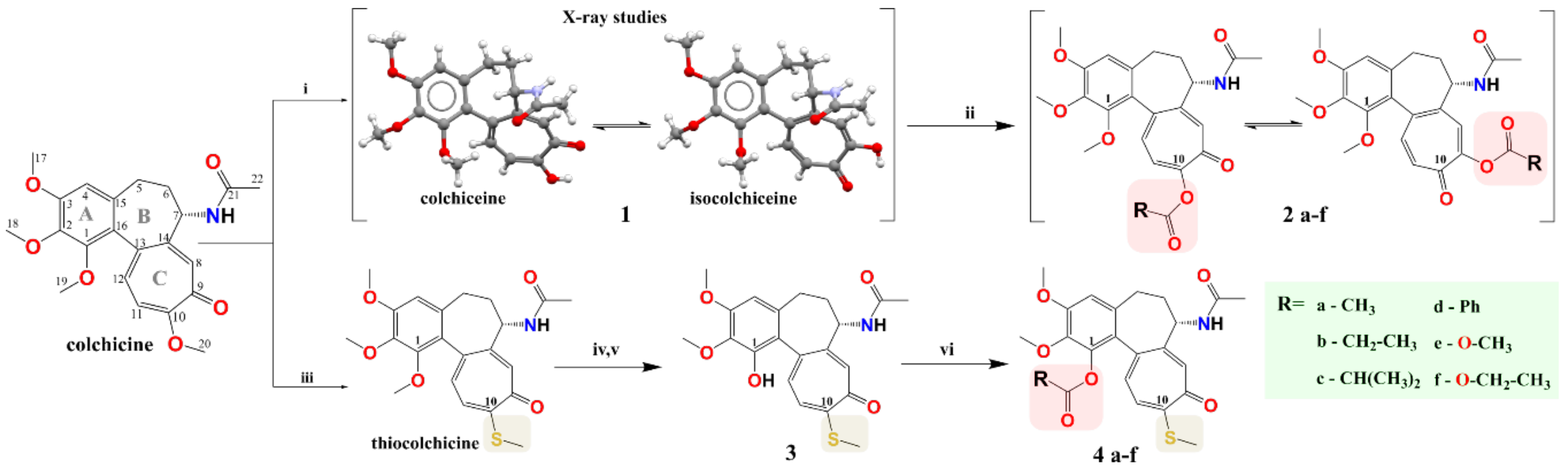
2.1. Chemistry
2.2. In Silico Calculations of the Physicochemical Properties
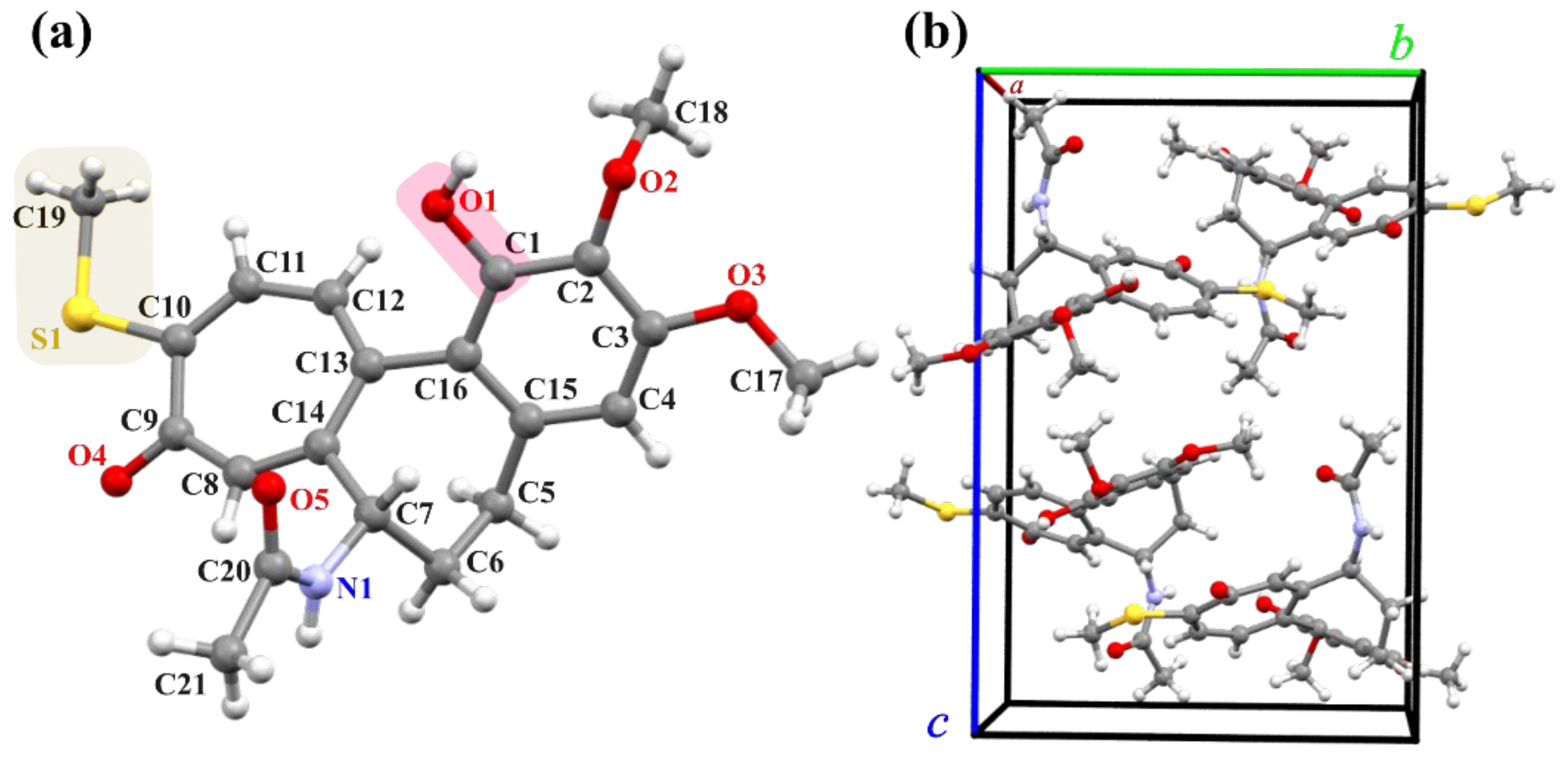
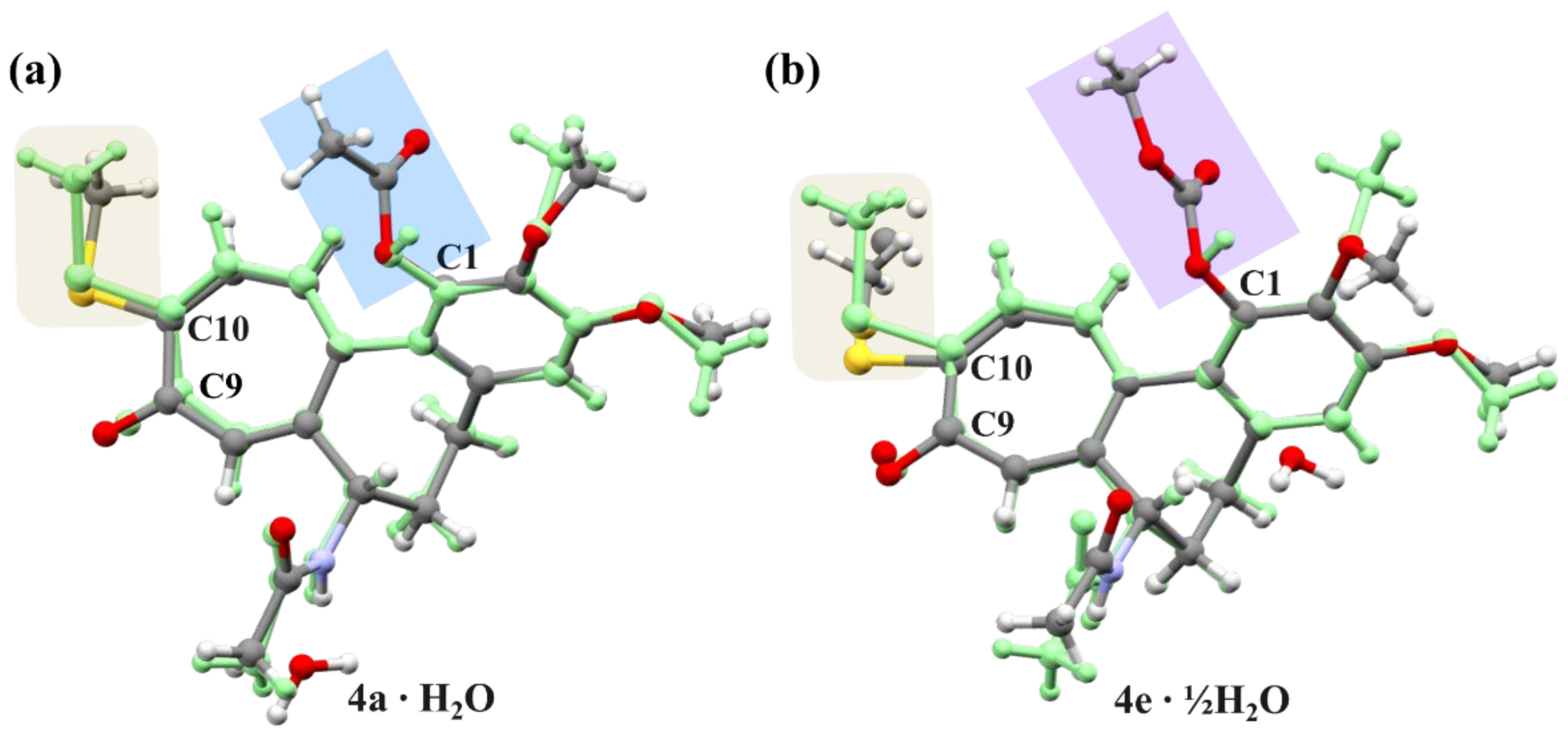
2.3. X-ray Analysis
2.4. Antiproliferative Activity
3. Materials and Methods
3.1. General
3.1.1. Synthesis and Characterization of 10-Demethylcolchicine (colchiceine, 1), Thiocolchicine and 1-Demethylthiocolchicine (3)
3.1.2. General Route for Synthesis of Compounds 2a–f and 4a–f
3.1.3. Characterization of Acetyl Ester of Colchiceine 2a
3.1.4. Characterization of Propionyl Ester of Colchiceine 2b
3.1.5. Characterization of Isobutyryl Ester of Colchiceine 2c
3.1.6. Characterization of Benzyl Ester of Colchiceine 2d
3.1.7. Characterization of Methyl Carbonate of Colchiceine 2e
3.1.8. Characterization of Ethyl Carbonate of Colchiceine 2f
3.1.9. Characterization of Acetyl Ester of 1-demethylthiocolchicine 4a
3.1.10. Characterization of Propionyl Ester of 1-demethylthiocolchicine 4b
3.1.11. Characterization of Isobutyryl Ester of 1-demethylthiocolchicine 4c
3.1.12. Characterization of Benzyl Ester of 1-demethylthiocolchicine 4d
3.1.13. Characterization of Methyl Carbonate of 1-demethylthiocolchicine 4e
3.1.14. Characterization of Ethyl Carbonate of 1-demethylthiocolchicine 4f
3.2. Antiproliferative Activity
3.3. X-ray Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Slobodnick, A.; Shah, B.; Pillinger, M.H.; Krasnokutsky, S. Colchicine: Old and New. Am. J. Med. 2015, 128, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Graham, W.; Roberts, J.B. Intravenous colchicine in the management of gouty arthritis. Ann. Rheum. Dis. 1953, 12, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Fitzgerald, J.D.; Khanna, P.P.; Bae, S.; Singh, M.K.; Neogi, T.; Pillinger, M.H.; Merill, J.; Lee, S.; Prakash, S.; et al. American college of rheumatology guidelines for management of gout. Part 1: Systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. 2012, 64, 1431–1446. [Google Scholar] [CrossRef]
- Grattagliano, I.; Bonfrate, L.; Ruggiero, V.; Scaccianoce, G.; Palasciano, G.; Portincasa, P. Novel therapeutics for the treatment of familial mediterranean fever: From colchicine to biologics. Clin. Pharmacol. Ther. 2014, 95, 89–97. [Google Scholar] [CrossRef]
- Imazio, M.; Gaita, F.; LeWinter, M. Evaluation and treatment of pericarditis: A systematic review. J. Am. Med. Assoc. 2015, 314, 1498–1506. [Google Scholar] [CrossRef]
- Vindya, N.G.; Sharma, M.; Yadav, M.; Ethiraj, K.R. Tubulins—The Target for Anticancer Therapy. Curr. Top. Med. Chem. 2015, 15, 73–82. [Google Scholar] [CrossRef]
- Seligmann, J.; Twelves, C. Tubulin: An example of targeted chemotherapy. Future Med. Chem. 2013, 5, 339–352. [Google Scholar] [CrossRef]
- Katsetos, C.D.; Draber, P. Tubulins as Therapeutic Targets in Cancer: From Bench to Bedside. Curr. Pharm. Des. 2012, 18, 2778–2792. [Google Scholar] [CrossRef]
- Cocco, G.; Chu, D.C.C.; Pandolfi, S. Colchicine in clinical medicine. A guide for internists. Eur. J. Intern. Med. 2010, 21, 503–508. [Google Scholar] [CrossRef]
- Yang, L.P.H. Oral Colchicine (Colcrys): In the treatment and prophylaxis of gout. Drugs 2010, 70, 1603–1613. [Google Scholar] [CrossRef]
- Avendaño, C.; Menéndez, J.C. Medicinal Chemistry of Anticancer Drugs; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar] [CrossRef]
- Majcher, U.; Klejborowska, G.; Kaik, M.; Maj, E.; Wietrzyk, J.; Moshari, M.; Preto, J.; Tuszynski, J.; Huczyński, A. Synthesis and Biological Evaluation of Novel Triple-Modified Colchicine Derivatives as Potent Tubulin-Targeting Anticancer Agents. Cells 2018, 7, 216. [Google Scholar] [CrossRef] [PubMed]
- Klejborowska, G.; Urbaniak, A.; Preto, J.; Maj, E.; Moshari, M.; Wietrzyk, J.; Tuszynski, J.; Chambers, T.C.; Huczyński, A. Synthesis, biological evaluation and molecular docking studies of new amides of 4-bromothiocolchicine as anticancer agents. Bioorg. Med. Chem. 2019, 23, 115–144. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa-Goto, K.; Chen, C.X.; Hamel, E.; Wu, C.C.; Bastow, K.F.; Brossi, A.; Lee, K.H. Antitumor agents. Part 236: Synthesis of water-soluble colchicine derivatives. Bioorg. Med. Chem. Lett. 2015, 15, 235–238. [Google Scholar] [CrossRef]
- Shchegravina, E.S.; Maleev, A.A.; Ignatov, S.K.; Gracheva, I.A.; Stein, A.; Schmalz, H.G.; Gavryushin, A.E.; Zubareva, A.A.; Svirshchevskaya, E.V.; Fedorov, A.Y. Synthesis and biological evaluation of novel non-racemic indole-containing allocolchicinoids. Eur. J. Med. Chem. 2017, 141, 51–60. [Google Scholar] [CrossRef]
- Yasobu, N.; Kitajima, M.; Kogure, N.; Shishido, Y.; Matsuzaki, T.; Nagaoka, M.; Takayama, H. Design, synthesis, and antitumor activity of 4-halocolchicines and their pro-drugs activated by cathepsin B. ACS Med. Chem. Lett. 2011, 2, 348–352. [Google Scholar] [CrossRef]
- Huczyński, A.; Majcher, U.; Maj, E.; Wietrzyk, J.; Janczak, J.; Moshari, M.; Tuszynski, J.A.; Bartl, F. Synthesis, antiproliferative activity and molecular docking of Colchicine derivatives. Bioorg. Chem. 2016, 64, 103–112. [Google Scholar] [CrossRef]
- Zhang, X.; Kong, Y.; Zhang, J.; Su, M.; Zhou, Y.; Zang, Y.; Li, J.; Chen, Y.; Fang, Y.; Zhang, X.; et al. Design, synthesis and biological evaluation of colchicine derivatives as novel tubulin and histone deacetylase dual inhibitors. Eur. J. Med. Chem. 2015, 95, 127–135. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Valiulin, R.A.; Pokorski, J.K.; Chang, V.; Chen, J.S. Bio-inspired synthesis and biological evaluation of a colchicine-related compound library. Bioorg. Med. Chem. Lett. 2012, 22, 3776–3780. [Google Scholar] [CrossRef]
- Chang, D.J.; Yoon, E.Y.; Lee, G.B.; Kim, S.O.; Kim, W.J.; Kim, Y.M.; Jung, J.W.; An, H.; Suh, Y.G. Design, synthesis and identification of novel colchicine-derived immunosuppressant. Bioorg. Med. Chem. Lett. 2009, 19, 4416–4420. [Google Scholar] [CrossRef]
- Marzo-Mas, A.; Barbier, P.; Breuzard, G.; Allegro, D.; Falomir, E.; Murga, J.; Carda, M.; Peyrot, V.; Marco, J.A. Interactions of long-chain homologues of colchicine with tubulin. Eur. J. Med. Chem. 2017, 126, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Goping, I.S.; Rieger, A.; Mane, J.Y.; Huzil, T.; Banerjee, A.; Luduena, R.; Hassani, B.; Winter, P.; Tuszynski, J.A. Novel Colchicine Derivatives and their Anti-cancer Activity. Curr. Top. Med. Chem. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharma, P.R.; Mondhe, D.M. Potential anticancer role of colchicine-based derivatives: An overview. Anticancer Drugs 2016, 28, 250–262. [Google Scholar] [CrossRef]
- Kozaka, T.; Nakagawa-Goto, K.; Shi, Q.; Lai, C.Y.; Hamel, E.; Bastow, K.F.; Brossi, A.; Lee, K.H. Antitumor agents 273. Design and synthesis of N-alkyl-thiocolchicinoids as potential antitumor agents. Bioorg. Med. Chem. Lett. 2010, 20, 4091–4094. [Google Scholar] [CrossRef]
- Andreu, J.M.; Timasheff, S.N. Tubulin bound to colchicine forms polymers different from microtubules. Proc. Natl. Acad. Sci. USA 1982, 79, 6753–6756. [Google Scholar] [CrossRef]
- Cortese, F.; Bhattacharyya, B.; Wolff, J. Podophyllotoxin as a probe for the colchicine binding site of tubulin. J. Biol. Chem. 1977, 252, 1134–1140. [Google Scholar]
- Chen, J.; Liu, T.; Dong, X.; Hu, Y. Recent Development and SAR Analysis of Colchicine Binding Site Inhibitors. Mini Rev. Med. Chem. 2009, 9, 1174–1190. [Google Scholar] [CrossRef]
- Hastie, S.B.; Williams, R.C.; Puett, D.; Macdonald, T.L. The binding of isocolchicine to tubulin. Mechanisms of ligand association with tubulin. J. Biol. Chem. 1989, 264, 6682–6688. [Google Scholar]
- Gohar, M.A.; Makkawi, M. The Antibacterial Action of Colchicine and Colchiceine. J. Pharm. Pharmacol. 1951, 3, 415–419. [Google Scholar] [CrossRef]
- Boyland, E.; Mawson, E.H. The conversion of colchicine into colchiceine. Biochem. J. 1938, 32, 1204–1206. [Google Scholar] [CrossRef]
- Klein, A.E.; Davis, P.J. Determination of Colchicine and Colchiceine in Microbial Cultures by High-Performance Liquid Chromatography. Anal. Chem. 1980, 52, 2432–2435. [Google Scholar] [CrossRef]
- Elguero, J.; Muller, R.N.; Blade-Font, A.; Faure, R.; Vincent, E.J. Carbon-13 Magnetic Resonance Spectroscopy. A Study Of Colchicine And Related Compounds. Bull. Soc. Chim. Belges 1980, 89, 193–204. [Google Scholar] [CrossRef]
- Mackay, M.F.; Morrison, J.D.; Gulbis, J.M. Crystal Structure of Triclinic Colchiceine Hemihydrate. Aust. J. Phys. 1985, 38, 413. [Google Scholar] [CrossRef]
- Kurek, J.; Barczynski, P. Colchiceine complexes with lithium, sodium and potassium salts-spectroscopic studies. Croat. Chem. Acta 2016, 89, 297–308. [Google Scholar] [CrossRef]
- Shi, Q.; Verdier-Pinard, P.; Brossi, A.; Hamel, E.; Lee, K.H. Antitumor Agents-CLXXV. Anti-tubulin action of (+)-thiocolchicine prepared by partial synthesis. Bioorg. Med. Chem. 1997, 5, 2277–2282. [Google Scholar] [CrossRef]
- Prajapati, P.B.; Bodiwala, K.B.; Marolia, B.P.; Bhingradiya, N.; Shah, S. Oxidative Degradation Kinetic Study of Thiocolchicoside using Stability Indicating High Performance Thin Layer Chromatographic Method. Pharm. Methods 2014, 5, 69–78. [Google Scholar] [CrossRef]
- Banerjee, A.; Kasmala, L.T.; Hamel, E.; Sun, L.; Lee, K.H. Interaction of novel thiocolchicine analogs with the tubulin isoforms from bovine brain. Biochem. Biophys. Res. Commun. 1999, 254, 334–337. [Google Scholar] [CrossRef]
- Kerekes, P.; Sharma, P.N.; Brossi, A.; Chignell, C.F.; Quinn, F.R. Synthesis and Biological Effects of Novel Thiocolchicines. 3. Evaluation of N-Acyldeacetylthiocolchicines, N-(Alkoxycarbonyl)deacetylthiocolchicines, and O-Ethyldemethylthiocolchicines. New Synthesis of Thiodemecolcine and Antileukemic Effects of 2-Demeth. J. Med. Chem. 1985, 28, 1204–1208. [Google Scholar] [CrossRef]
- Alkadi, H.; Khubeiz, M.J. Colchicine: A Review About Chemical Structure and Clinical Using. Infect. Disord. Drug Targets 2017, 17. [Google Scholar] [CrossRef]
- Shi, Q.; Chen, K.; Morris-Natschke, S.L.; Lee, K.H. Recent progress in the development of tubulin inhibitors as antimitotic antitumor agents. Curr. Pharm. Des. 1998, 4, 219–248. [Google Scholar]
- Dumont, R.; Brossi, A.; Chignell, C.F.; Quinn, F.R.; Suffness, M. A Novel Synthesis of Colchicide and Analogues from Thiocolchicine and Congeners: Reevaluation of Colchicide as a Potential Antitumor Agent. J. Med. Chem. 1987, 30, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Voitovich, Y.V.; Shegravina, E.S.; Sitnikov, N.S.; Faerman, V.I.; Fokin, V.V.; Schmalz, H.G.; Combes, S.; Allegro, D.; Barbier, P.; Beletskaya, I.P.; et al. Synthesis and biological evaluation of furanoallocolchicinoids. J. Med. Chem. 2015, 58, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Blade-Font, A. New chemistry of colchicine and related compounds IV. Selective demethylation of colchicine with Lewis acids and tge structure of Zeisel’s dimethylcolchicinic acid. Afinidad 1979, 36, 329. [Google Scholar]
- Molinspiration Property Calculation Service. Available online: http://www.molinspiration.com (accessed on 15 January 2020).
- Lipinski, C. Lead-and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 4, 337–341. [Google Scholar] [CrossRef]
- Lipinski, C.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Khankari, R.K.; Grant, D.J.W. Pharmaceutical hydrates. Thermochim. Acta 1995, 248, 61–79. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1, 2a–f, 3 and 4a–f are available from the authors. |
| Compound | MW | clogP | tPSA | n(O,N) | N(OH,NH) | Rotb | MV |
|---|---|---|---|---|---|---|---|
| 1 | 385.42 | 0.83 | 94.10 | 7 | 2 | 4 | 346.63 |
| 2a | 427.45 | 0.60 | 100.18 | 8 | 1 | 6 | 383.14 |
| 2b | 441.48 | 1.27 | 100.18 | 8 | 1 | 7 | 399.94 |
| 2c | 455.51 | 1.51 | 100.18 | 8 | 1 | 7 | 416.53 |
| 2d | 489.52 | 2.92 | 100.18 | 8 | 1 | 7 | 437.99 |
| 2e | 443.45 | 0.95 | 109.41 | 9 | 1 | 7 | 392.12 |
| 2f | 457.48 | 1.33 | 109.41 | 9 | 1 | 8 | 408.93 |
| 3 | 401.48 | 1.89 | 84.86 | 6 | 2 | 4 | 355.77 |
| 4a | 443.52 | 1.66 | 90.94 | 7 | 1 | 6 | 392.28 |
| 4b | 457.55 | 2.33 | 90.94 | 7 | 1 | 7 | 409.08 |
| 4c | 471.57 | 2.57 | 90.94 | 7 | 1 | 7 | 425.67 |
| 4d | 505.59 | 3.98 | 90.94 | 7 | 1 | 7 | 447.13 |
| 4e | 459.52 | 2.02 | 100.18 | 8 | 1 | 7 | 401.27 |
| 4f | 473.55 | 2.39 | 100.18 | 8 | 1 | 8 | 418.07 |
| colchicine | 399.44 | 1.10 | 83.11 | 7 | 1 | 5 | 364.15 |
| Label | 3 | 4a·H2O | 4e·½H2O | |
|---|---|---|---|---|
| Formula | C21H22NO5S | C23H25NO6S · H2O | C23H25NO7S· ½H2O | |
| CCDC number | 1966196 | 1966194 | 1966195 | |
| Crystal system | orthorhombic | monoclinic | monoclinic | |
| Space group | P212121 | P21 | P21 | |
| Unit cell dimensions | a (Å) | 9.1005(17) | 10.600(2) | 10.8835(10) |
| b (Å) | 11.866(2) | 6.9635(11) | 9.2158(6) | |
| c (Å) | 17.881(4) | 16.712(3) | 12.0632(12) | |
| β (°) | 90 | 107.07(2) | 104.613(9) | |
| Volume (Å3) | 1930.8(7) | 1179.2(4) | 1170.80(18) | |
| Z/Z’ | 4/1 | 2/1 | 2/1 | |
| Dx (g/cm3) | 1.378 | 1.300 | 1.329 | |
| Compound | A549 | SI | MCF-7 | SI | LoVo | SI | BALB/3T3 |
|---|---|---|---|---|---|---|---|
| IC50 (μM) | IC50 (μM) | IC50 (μM) | IC50 (μM) | ||||
| 1 | 12.99 ± 1.79 | 0.79 | 11.23 ± 2.52 | 0.91 | 6.00 ± 1.88 | 1.71 | 10.25 ± 0.96 |
| 2a | 12.47 ± 2.77 | 0.68 | 10.03 ± 2.18 | 0.85 | 6.18 ± 1.48 | 1.37 | 8.50 ± 0.50 |
| 2b | 9.97 ± 0.70 | 0.75 | 7.56 ± 2.84 | 0.99 | 2.39 ± 1.20 | 3.13 | 7.47 ± 0.50 |
| 2c | 20.39 ± 11.30 | 0.41 | 11.95 ± 1.54 | 0.70 | 6.47 ± 0.35 | 1.30 | 8.42 ± 1.14 |
| 2d | 8.75 ± 0.33 | 1.02 | 8.21 ± 3.35 | 1.09 | 4.87 ± 2.17 | 1.83 | 8.92 ± 3.93 |
| 2e | 0.98 ± 0.16 | 0.84 | 0.98 ± 0.33 | 0.84 | 0.48 ± 0.32 | 1.70 | 0.82 ± 0.11 |
| 2f | 9.67 ± 0.84 | 0.86 | 9.07 ± 1.90 | 0.92 | 6.41 ± 1.57 | 1.30 | 8.36 ± 0.17 |
| 3 | 0.82 ± 0.02 | 0.74 | 0.12 ± 0.05 | 4.92 | 0.11 ± 0.03 | 5.38 | 0.61 ± 0.14 |
| 4a | 0.48 ± 0.15 | 1.36 | 0.10 ± 0.02 | 6.85 | 0.11 ± 0.02 | 5.92 | 0.65 ± 0.12 |
| 4b | 0.97 ± 0.04 | 0.77 | 0.96 ± 0.13 | 0.78 | 0.50 ± 0.19 | 1.53 | 0.75 ± 0.10 |
| 4c | 0.89 ± 0.07 | 0.80 | 0.86 ± 0.10 | 0.83 | 0.56 ± 0.06 | 1.27 | 0.71 ± 0.03 |
| 4d | 0.60 ± 0.31 | 0.75 | 0.78 ± 0.22 | 0.58 | 0.10 ± 0.08 | 4.71 | 0.45 ± 0.23 |
| 4e | 0.90 ± 0.09 | 0.87 | 1.02 ± 0.10 | 0.77 | 0.43 ± 0.19 | 1.84 | 0.78 ± 0.06 |
| 4f | 0.96 ± 0.13 | 1.08 | 0.95 ± 0.20 | 1.09 | 0.79 ± 0.16 | 1.31 | 1.03 ± 0.20 |
| colchicine | 0.07 ± 0.01 | 0.63 | 0.01 ± 0.01 | 4.30 | 0.01 ± 0.01 | 5.38 | 0.04 ± 0.01 |
| cisplatin | 3.60 ± 0.25 | 0.81 | 3.05 ± 0.67 | 0.95 | 3.80 ± 0.28 | 0.77 | 2.91 ± 1.83 |
| doxorubicin | 0.16 ± 0.03 | 0.25 | 0.15 ± 0.05 | 0.26 | 0.08 ± 0.03 | 0.49 | 0.04 ± 0.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czerwonka, D.; Sobczak, S.; Maj, E.; Wietrzyk, J.; Katrusiak, A.; Huczyński, A. Synthesis and Antiproliferative Screening Of Novel Analogs of Regioselectively Demethylated Colchicine and Thiocolchicine. Molecules 2020, 25, 1180. https://doi.org/10.3390/molecules25051180
Czerwonka D, Sobczak S, Maj E, Wietrzyk J, Katrusiak A, Huczyński A. Synthesis and Antiproliferative Screening Of Novel Analogs of Regioselectively Demethylated Colchicine and Thiocolchicine. Molecules. 2020; 25(5):1180. https://doi.org/10.3390/molecules25051180
Chicago/Turabian StyleCzerwonka, Dominika, Szymon Sobczak, Ewa Maj, Joanna Wietrzyk, Andrzej Katrusiak, and Adam Huczyński. 2020. "Synthesis and Antiproliferative Screening Of Novel Analogs of Regioselectively Demethylated Colchicine and Thiocolchicine" Molecules 25, no. 5: 1180. https://doi.org/10.3390/molecules25051180
APA StyleCzerwonka, D., Sobczak, S., Maj, E., Wietrzyk, J., Katrusiak, A., & Huczyński, A. (2020). Synthesis and Antiproliferative Screening Of Novel Analogs of Regioselectively Demethylated Colchicine and Thiocolchicine. Molecules, 25(5), 1180. https://doi.org/10.3390/molecules25051180