Ultrasonic-Assisted Extraction, Structural Characterization, Chain Conformation, and Biological Activities of a Pectic-Polysaccharide from Okra (Abelmoschus esculentus)
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Characteristics
2.1.1. Chemical Compositions of OPP-D
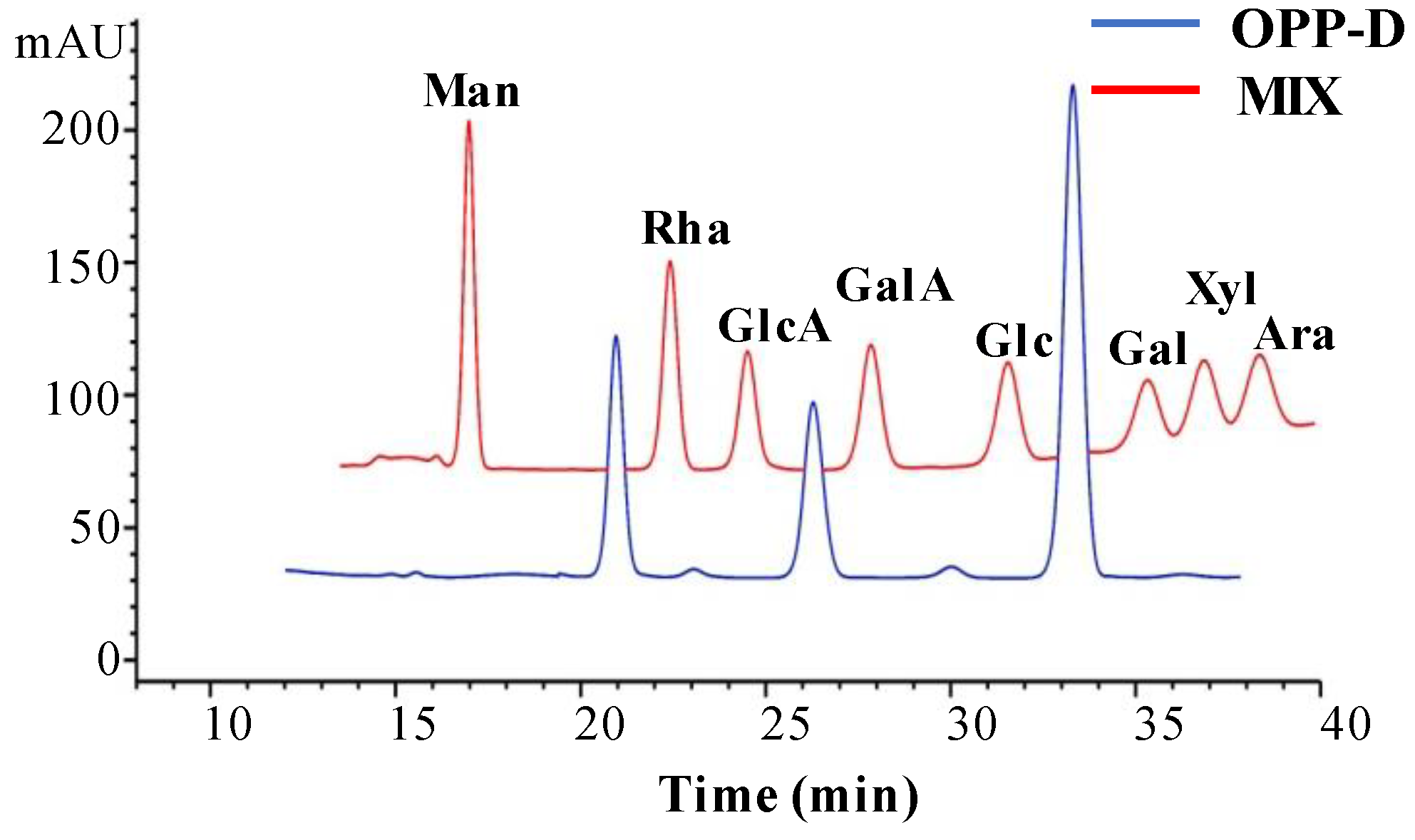
2.1.2. Constituent Monosaccharides of OPP-D
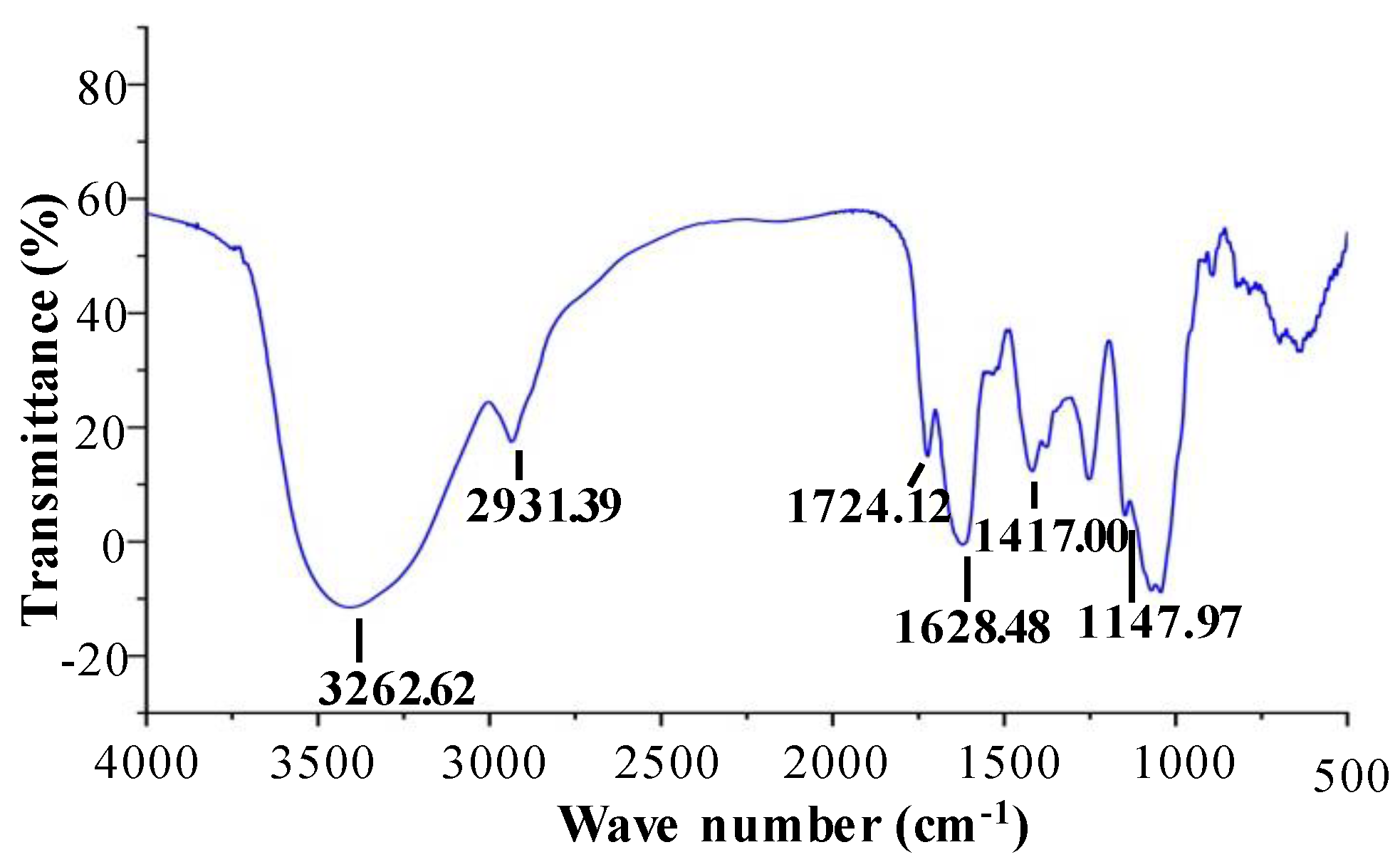
2.1.3. FT–IR Spectra and Esterification Degree of OPP-D
2.1.4. Structure Prediction of OPP-D by NMR Analysis
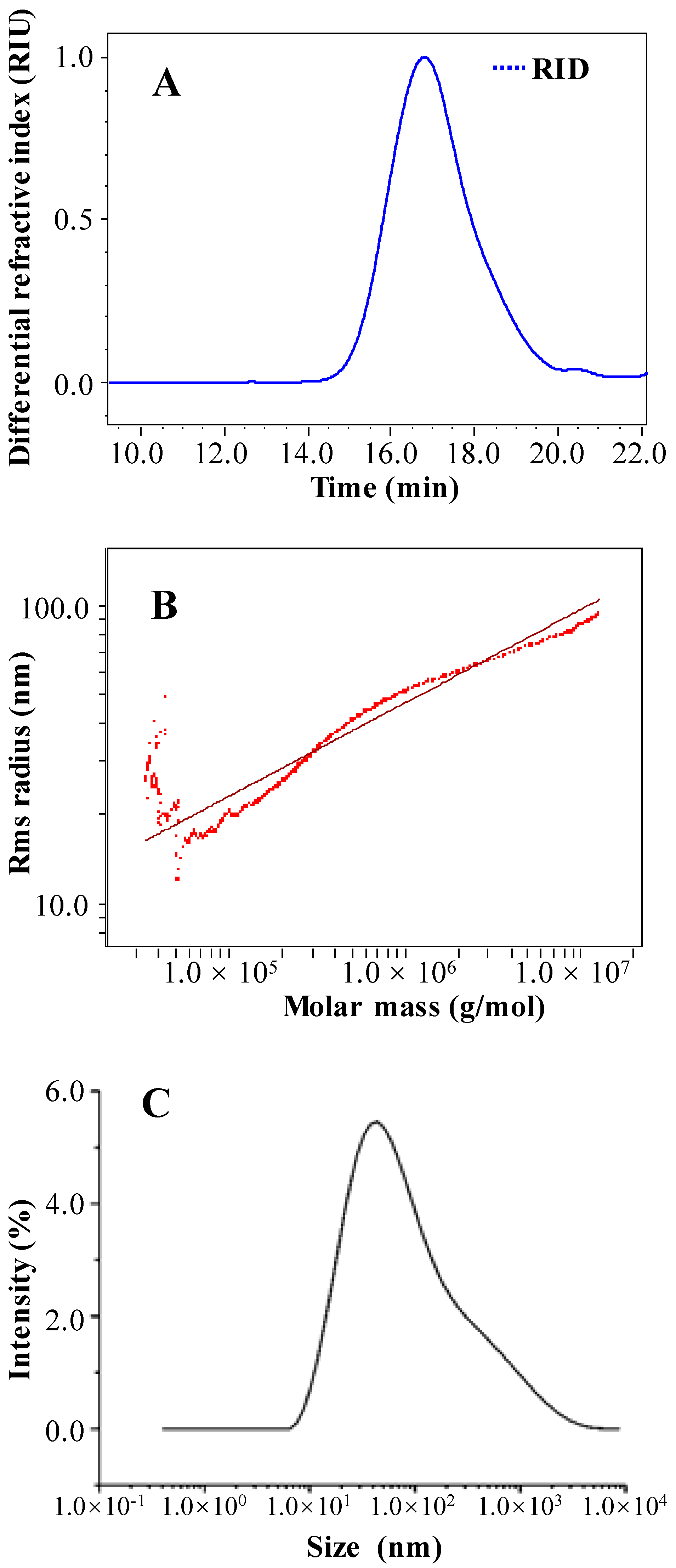
2.1.5. Molecular Weight and Chain Conformation of OPP-D
2.1.6. Apparent Viscosity of OPP-D
2.2. Antioxidant Activities of OPP-D
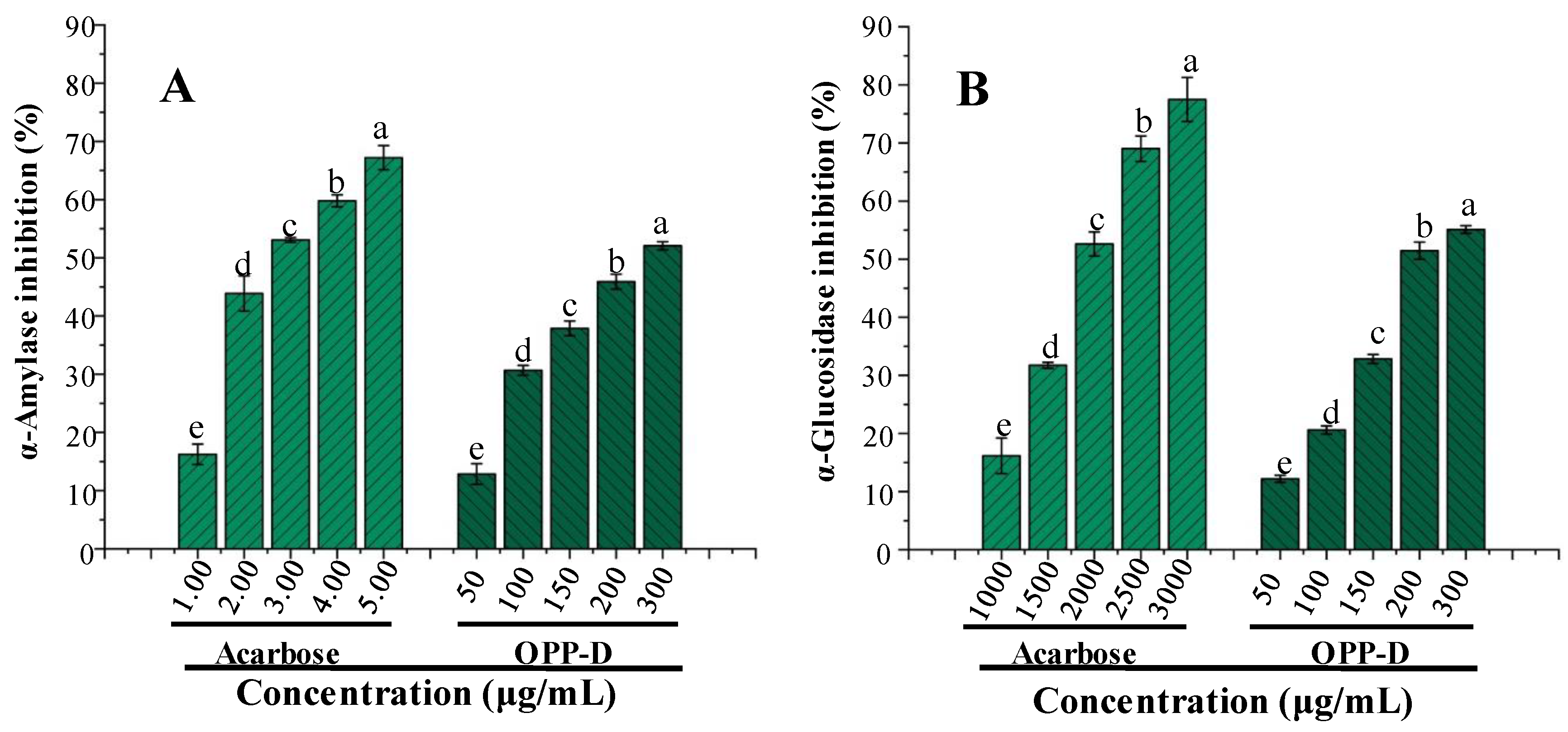
2.3. In Vitro α-amylase and α-glucosidase Inhibitory Activities of OPP-D
2.4. In Vitro Binding Properties of OPP-D
2.5. In Vitro Prebiotic Activities of OPP-D
3. Materials and Methods
3.1. Material, Chemicals, and Lactobacillus Strains
3.2. Extraction and Preparation of OPPs
3.3. Isolation and Purification of OPPs
3.4. Structural Characterization of OPP-D
3.4.1. Chemical Composition Analysis
3.4.2. Determination of Constituent Monosaccharides
3.4.3. Fourier Transform Infrared Spectroscopy Analysis
3.4.4. NMR Analysis
3.4.5. Determination of Molecular Weight and Particle Size
3.4.6. Determination of Apparent Viscosity
3.5. Evaluation of Bioactivities of OPP-D
3.5.1. Determination of In Vitro Antioxidant Activities
3.5.2. Determination of In Vitro α-amylase and α-glucosidase Inhibitory Activities
3.5.3. Determination of In Vitro Binding Properties
3.5.4. Determination of In Vitro Prebiotic Activity
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Peng, C.H.; Chyau, C.C.; Wang, C.J.; Lin, H.T.; Huang, C.N.; Ker, Y.B. Abelmoschus esculentus fractions potently inhibited the pathogenic targets associated with diabetic renal epithelial to mesenchymal transition. Food Funct. 2016, 7, 728–740. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Zhong, Y.; Li, M.; Chang, Q.; Liao, Y.; Liu, X.; Pan, R. Antioxidant and anti-fatigue constituents of okra. Nutrients 2015, 7, 8846–8858. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Liu, C.; Li, D.; Zhang, Z.; Liu, C.; Wang, D.; Niu, L.; Zhang, M. Evaluation of freeze drying combined with microwave vacuum drying for functional okra snacks: Antioxidant properties, sensory quality, and energy consumption. LWT-Food Sci. Technol. 2017, 82, 216–226. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Wen, X.; Chen, X.; He, Z.; Ni, Y. Optimization of ultrasound-assisted extraction of okra (Abelmoschus esculentus (L.) Moench) polysaccharides based on response surface methodology and antioxidant activity. Int. J. Biol. Macromol. 2018, 114, 1056–1063. [Google Scholar] [CrossRef]
- Zhang, T.; Xiang, J.; Zheng, G.; Yan, R.; Min, X. Preliminary characterization and anti-hyperglycemic activity of a pectic polysaccharide from okra (Abelmoschus esculentus (L.) Moench). J. Funct. Foods 2018, 41, 19–24. [Google Scholar] [CrossRef]
- Islam, M.T. Phytochemical information and pharmacological activities of okra (Abelmoschus esculentus): A literature-based review. Phytother. Res. 2019, 33, 72–80. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.G.; Sun, H.J.; Wei, Z.J. Pectin from Abelmoschus esculentus: Optimization of extraction and rheological properties. Int. J. Biol. Macromol. 2014, 70, 498–505. [Google Scholar] [CrossRef]
- Kpodo, F.M.; Agbenorhevi, J.K.; Alba, K.; Bingham, R.J.; Oduro, I.N.; Morris, G.A.; Kontogiorgos, V. Pectin isolation and characterization from six okra genotypes. Food Hydrocoll. 2017, 72, 323–330. [Google Scholar] [CrossRef]
- Nie, X.R.; Li, H.Y.; Du, G.; Lin, S.; Hu, R.; Li, H.Y.; Zhao, L.; Zhang, Q.; Chen, H.; Wu, D.T.; et al. Structural characteristics, rheological properties, and biological activities of polysaccharides from different cultivars of okra (Abelmoschus esculentus) collected in China. Int. J. Biol. Macromol. 2019, 139, 459–467. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Wu, Q.; John, A.; Jiang, Y.; Yang, J.; Liu, H.; Yang, B. Structure characterisation of polysaccharides in vegetable “okra” and evaluation of hypoglycemic activity. Food Chem. 2018, 242, 211–216. [Google Scholar] [CrossRef]
- Yuan, Q.; Lin, S.; Fu, Y.; Nie, X.R.; Liu, W.; Su, Y.; Han, Q.H.; Zhao, L.; Zhang, Q.; Lin, D.R.; et al. Effects of extraction methods on the physicochemical characteristics and biological activities of polysaccharides from okra (Abelmoschus esculentus). Int. J. Biol. Macromol. 2019, 127, 178–186. [Google Scholar] [CrossRef]
- Tao, Y.; Sun, D.-W. Enhancement of food processes by ultrasound: A review. Crit. Rev. Food. Sci. Nutr. 2014, 55, 570–594. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Wan, Y.; Xu, J.Y.; Wu, G.H.; Li, L.; Yao, X.H. Ultrasound extraction of polysaccharides from mulberry leaves and their effect on enhancing antioxidant activity. Carbohydr. Polym. 2016, 137, 473–479. [Google Scholar] [CrossRef]
- Cheung, Y.C.; Yin, J.; Wu, J.Y. Effect of polysaccharide chain conformation on ultrasonic degradation of curdlan in alkaline solution. Carbohydr. Polym. 2018, 195, 298–302. [Google Scholar] [CrossRef]
- Duan, M.; Shang, H.; Chen, S.; Li, R.; Wu, H. Physicochemical properties and activities of comfrey polysaccharides extracted by different techniques. Int. J. Biol. Macromol. 2018, 115, 876–882. [Google Scholar] [CrossRef]
- Guo, H.; Lin, S.; Lu, M.; Gong, J.D.B.; Wang, L.; Zhang, Q.; Lin, D.R.; Qin, W.; Wu, D.T. Characterization, in vitro binding properties, and inhibitory activity on pancreatic lipase of beta-glucans from different Qingke (Tibetan hulless barley) cultivars. Int. J. Biol. Macromol. 2018, 120 (Pt B), 2517–2522. [Google Scholar] [CrossRef]
- Zhi, Z.; Chen, J.; Li, S.; Wang, W.; Huang, R.; Liu, D.; Ding, T.; Linhardt, R.J.; Chen, S.; Ye, X. Fast preparation of RG-I enriched ultra-low molecular weight pectin by an ultrasound accelerated Fenton process. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Thole, C.; Brandt, S.; Ahmed, N.; Hensel, A. Acetylated rhamnogalacturonans from immature fruits of Abelmoschus esculentus inhibit the adhesion of helicobacter pylori to human gastric cells by interaction with outer membrane proteins. Molecules 2015, 20, 16770–16787. [Google Scholar] [CrossRef]
- Guo, H.; Yuan, Q.; Fu, Y.; Liu, W.; Su, Y.H.; Liu, H.; Wu, C.Y.; Zhao, L.; Zhang, Q.; Lin, D.R.; et al. Extraction optimization and effects of extraction methods on the chemical structures and antioxidant activities of polysaccharides from snow chrysanthemum (Coreopsis tinctoria). Polymers 2019, 11, 215. [Google Scholar] [CrossRef]
- Fu, Y.; Yuan, Q.; Lin, S.; Liu, W.; Du, G.; Zhao, L.; Zhang, Q.; Lin, D.R.; Liu, Y.T.; Qin, W.; et al. Physicochemical characteristics and biological activities of polysaccharides from the leaves of different loquat (Eriobotrya japonica) cultivars. Int. J. Biol. Macromol. 2019, 135, 274–281. [Google Scholar] [CrossRef]
- Han, Q.H.; Liu, W.; Li, H.Y.; He, J.L.; Guo, H.; Lin, S.; Zhao, L.; Chen, H.; Liu, Y.W.; Wu, D.T.; et al. Extraction optimization, physicochemical characteristics, and antioxidant activities of polysaccharides from Kiwifruit (Actinidia chinensis Planch.). Molecules 2019, 24, 461. [Google Scholar] [CrossRef] [PubMed]
- Alba, K.; Laws, A.P.; Kontogiorgos, V. Isolation and characterization of acetylated LM-pectins extracted from okra pods. Food Hydrocoll. 2015, 43, 726–735. [Google Scholar] [CrossRef]
- Sengkhamparn, N.; Verhoef, R.; Schols, H.A.; Sajjaanantakul, T.; Voragen, A.G.J. Characterisation of cell wall polysaccharides from okra (Abelmoschus esculentus (L.) Moench). Carbohydr. Res. 2009, 344, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, L.-M. Chemical structural and chain conformational characterization of some bioactive polysaccharides isolated from natural sources. Carbohydr. Polym. 2009, 76, 349–361. [Google Scholar] [CrossRef]
- Liu, W.; Liu, Y.; Zhu, R.; Yu, J.; Lu, W.; Pan, C.; Yao, W.; Gao, X. Structure characterization, chemical and enzymatic degradation, and chain conformation of an acidic polysaccharide from Lycium barbarum L. Carbohydr. Polym. 2016, 147, 114–124. [Google Scholar] [CrossRef]
- Wu, D.T.; Lam, S.C.; Cheong, K.L.; Wei, F.; Lin, P.C.; Long, Z.R.; Lv, X.J.; Zhao, J.; Ma, S.C.; Li, S.P. Simultaneous determination of molecular weights and contents of water-soluble polysaccharides and their fractions from Lycium barbarum collected in China. J. Pharm. Biomed. Anal. 2016, 129, 210–218. [Google Scholar] [CrossRef]
- Li, L.; Liao, B.Y.; Thakur, K.; Zhang, J.G.; Wei, Z.J. The rheological behavior of polysaccharides sequential extracted from Polygonatum cyrtonema Hua. Int. J. Biol. Macromol. 2018, 109, 761–771. [Google Scholar] [CrossRef]
- Benchabane, A.; Bekkour, K. Rheological properties of carboxymethyl cellulose (CMC) solutions. Colloid Polym. Sci. 2008, 286, 1173–1180. [Google Scholar] [CrossRef]
- Jin, Q.; Cai, Z.; Li, X.; Yadav, M.P.; Zhang, H. Comparative viscoelasticity studies: Corn fiber gum versus commercial polysaccharide emulsifiers in bulk and at air/liquid interfaces. Food Hydrocoll. 2017, 64, 85–98. [Google Scholar] [CrossRef]
- Zhong, K.; Zhang, Q.; Tong, L.; Liu, L.; Zhou, X.; Zhou, S. Molecular weight degradation and rheological properties of schizophyllan under ultrasonic treatment. Ultrason. Sonochem. 2015, 23, 75–80. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, H.; Wang, Z. Ultrasonic degradation of polysaccharides from Auricularia auricula and the antioxidant activity of their degradation products. LWT-Food Sci. Technol. 2019, 113, 108266. [Google Scholar] [CrossRef]
- Yan, J.K.; Wu, L.X.; Qiao, Z.R.; Cai, W.D.; Ma, H. Effect of different drying methods on the product quality and bioactive polysaccharides of bitter gourd (Momordica charantia L.) slices. Food Chem. 2019, 271, 588–596. [Google Scholar] [CrossRef]
- Podsedek, A.; Majewska, I.; Redzynia, M.; Sosnowska, D.; Koziolkiewicz, M. In vitro inhibitory effect on digestive enzymes and antioxidant potential of commonly consumed fruits. J. Agric. Food Chem. 2014, 62, 4610–4617. [Google Scholar] [CrossRef]
- Ren, Y.Y.; Zhu, Z.Y.; Sun, H.Q.; Chen, L.J. Structural characterization and inhibition on α-glucosidase activity of acidic polysaccharide from Annona squamosa. Carbohydr. Polym. 2017, 174, 1–12. [Google Scholar] [CrossRef]
- Liu, H.; He, P.; He, L.; Li, Q.; Cheng, J.; Wang, Y.; Yang, G.; Yang, B. Structure characterization and hypoglycemic activity of an arabinogalactan from Phyllostachys heterocycla bamboo shoot shell. Carbohydr. Polym. 2018, 201, 189–200. [Google Scholar] [CrossRef]
- Yang, L.; Fu, S.; Zhu, X.; Zhang, L.; Yang, Y.; Yang, X.; Liu, H. Hyperbranched Acidic Polysaccharide from Green Tea. Biomacromolecules 2010, 11, 3395–3405. [Google Scholar] [CrossRef]
- Huang, F.; Liu, H.; Zhang, R.; Dong, L.; Liu, L.; Ma, Y.; Jia, X.; Wang, G.; Zhang, M. Physicochemical properties and prebiotic activities of polysaccharides from longan pulp based on different extraction techniques. Carbohydr. Polym. 2019, 206, 344–351. [Google Scholar] [CrossRef]
- Chen, G.; Chen, X.; Yang, B.; Yu, Q.; Wei, X.; Ding, Y.; Kan, J. New insight into bamboo shoot (Chimonobambusa quadrangularis) polysaccharides: Impact of extraction processes on its prebiotic activity. Food Hydrocoll. 2019, 95, 367–377. [Google Scholar] [CrossRef]
- Mueller, M.; Cavarkapa, A.; Unger, F.M.; Viernstein, H.; Praznik, W. Prebiotic potential of neutral oligo- and polysaccharides from seed mucilage of Hyptis suaveolens. Food Chem. 2017, 221, 508–514. [Google Scholar] [CrossRef]
- Khodaei, N.; Fernandez, B.; Fliss, I.; Karboune, S. Digestibility and prebiotic properties of potato rhamnogalacturonan I polysaccharide and its galactose-rich oligosaccharides/oligomers. Carbohydr. Polym. 2016, 136, 1074–1084. [Google Scholar] [CrossRef]
- Wang, X.; Huang, M.; Yang, F.; Sun, H.; Zhou, X.; Guo, Y.; Wang, X.; Zhang, M. Rapeseed polysaccharides as prebiotics on growth and acidifying activity of probiotics in vitro. Carbohydr. Polym. 2015, 125, 232–240. [Google Scholar] [CrossRef]
Sample Availability: Samples of the raw material of okra and the purified okra polysaccharides are available from the authors. |



| Bacteria | Carbon Source | OD600 | Total SCFAs (mM) |
|---|---|---|---|
| L. acidophilus CICC 6089 | Control | 0.23 ± 0.01 d | 8.47 ± 0.98 d |
| 1% OPP-D | 0.28 ± 0.02 cd | 16.17 ± 1.53 d | |
| 2% OPP-D | 0.31 ± 0.01 cd | 28.46 ± 2.27 c | |
| 3% OPP-D | 0.35 ± 0.01 c | 31.32 ± 2.78 c | |
| 1% Inulin | 0.47 ± 0.03 b | 30.34 ± 1.92 c | |
| 2% Inulin | 0.63 ± 0.01 a | 41.99 ± 3.25 b | |
| 3% Inulin | 0.65 ± 0.06 a | 51.56 ± 3.76 a | |
| L. rhamnosus CICC 6133 | Control | 0.29 ± 0.01 c | 3.51 ± 0.45 e |
| 1% OPP-D | 0.33 ± 0.02 b | 10.28 ± 0.87 de | |
| 2% OPP-D | 0.36 ± 0.01 b | 13.24 ± 2.16 cde | |
| 3% OPP-D | 0.40 ± 0.01 a | 20.81 ± 1.85 bc | |
| 1% Inulin | 0.35 ± 0.01 b | 16.94 ± 2.65 cd | |
| 2% Inulin | 0.40 ± 0.00 a | 28.40 ± 2.79 b | |
| 3% Inulin | 0.43 ± 0.00 a | 67.04 ± 6.04 a | |
| L. rhamnosus CICC 6151 | Control | 0.24 ± 0.02 e | 6.36 ± 1.17 c |
| 1% OPP-D | 0.32 ± 0.01 d | 54.38 ± 4.38 b | |
| 2% OPP-D | 0.33 ± 0.01 cd | 77.64 ± 6.13 a | |
| 3% OPP-D | 0.40 ± 0.02 bc | 90.98 ± 10.52 a | |
| 1% Inulin | 0.47 ± 0.01 b | 54.14 ± 3.56 b | |
| 2% Inulin | 0.55 ± 0.01 a | 89.67 ± 6.76 a | |
| 3% Inulin | 0.62 ± 0.05 a | 100.89 ± 8.57 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, X.-R.; Fu, Y.; Wu, D.-T.; Huang, T.-T.; Jiang, Q.; Zhao, L.; Zhang, Q.; Lin, D.-R.; Chen, H.; Qin, W. Ultrasonic-Assisted Extraction, Structural Characterization, Chain Conformation, and Biological Activities of a Pectic-Polysaccharide from Okra (Abelmoschus esculentus). Molecules 2020, 25, 1155. https://doi.org/10.3390/molecules25051155
Nie X-R, Fu Y, Wu D-T, Huang T-T, Jiang Q, Zhao L, Zhang Q, Lin D-R, Chen H, Qin W. Ultrasonic-Assisted Extraction, Structural Characterization, Chain Conformation, and Biological Activities of a Pectic-Polysaccharide from Okra (Abelmoschus esculentus). Molecules. 2020; 25(5):1155. https://doi.org/10.3390/molecules25051155
Chicago/Turabian StyleNie, Xi-Rui, Yuan Fu, Ding-Tao Wu, Ting-Ting Huang, Qin Jiang, Li Zhao, Qing Zhang, De-Rong Lin, Hong Chen, and Wen Qin. 2020. "Ultrasonic-Assisted Extraction, Structural Characterization, Chain Conformation, and Biological Activities of a Pectic-Polysaccharide from Okra (Abelmoschus esculentus)" Molecules 25, no. 5: 1155. https://doi.org/10.3390/molecules25051155
APA StyleNie, X.-R., Fu, Y., Wu, D.-T., Huang, T.-T., Jiang, Q., Zhao, L., Zhang, Q., Lin, D.-R., Chen, H., & Qin, W. (2020). Ultrasonic-Assisted Extraction, Structural Characterization, Chain Conformation, and Biological Activities of a Pectic-Polysaccharide from Okra (Abelmoschus esculentus). Molecules, 25(5), 1155. https://doi.org/10.3390/molecules25051155








