Semi-Synthesis of C-Ring Cyclopropyl Analogues of Fraxinellone and Their Insecticidal Activity Against Mythimna separata Walker
Abstract
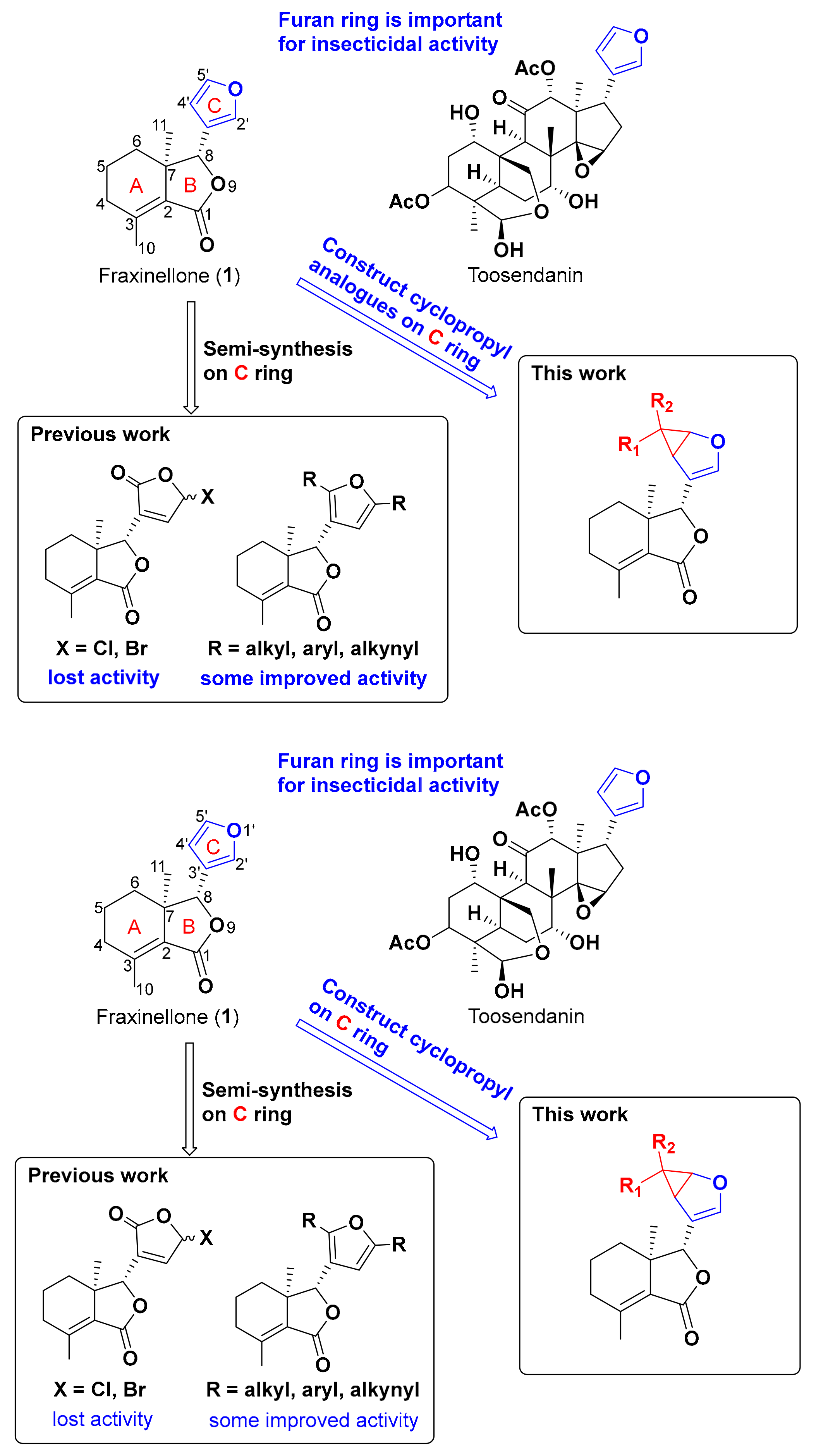
1. Introduction
2. Results and Discussion
2.1. Semi-Synthesis
2.2. Insecticidal Activity
3. Materials and Methods
3.1. General
3.2. Synthesis of α-Diazocarbonyl Esters (2a–2c)
3.3. Synthesis of C-Ring Cyclopropyl Analogues (3a–3c) of Fraxinellone
3.4. X-Ray Experimental of 3a
3.5. The Insecticidal Activity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Machida, S.; Mukai, S.; Kono, R.; Funato, M.; Saito, H.; Uchiyama, T. Synthesis and comparative structure-activity study of carbohydrate-based phenolic compounds as alpha-glucosidase inhibitors and antioxidants. Molecules 2019, 24, 4340. [Google Scholar] [CrossRef] [PubMed]
- Richers, J.; Pothig, A.; Herdtweck, E.; Sippel, C.; Hausch, F.; Tiefenbacher, K. Synthesis and neurotrophic activity studies of illicium sesquiterpene natural product analogues. Chem.-Eur. J. 2017, 23, 3178–3183. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.-X.; Song, X.-M.; Wang, L.-C.; Lv, H.-N.; Chen, J.-F.; Liu, D.; Fu, G.; Zhao, M.-B.; Jiang, Y.; Zeng, K.-W.; et al. Highly selective inhibition of IMPDH2 provides the basis of antineuroinflammation therapy. Proc. Natl. Acad. Sci. 2017, 114, E5986–E5994. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, C.L.; Dayan, F.E.; Duke, S.O. Natural products as sources for new pesticides. J. Nat. Prod. 2012, 75, 1231–1242. [Google Scholar] [CrossRef]
- Bolzani Vda, S.; Davies-Coleman, M.; Newman, D.J.; Singh, S.B.; Gordon, M.; Cragg, D.; Phil, D. Sc. (h.c.): A man for all natural products. J. Nat. Prod. 2012, 75, 309–310. [Google Scholar] [CrossRef]
- Xu, H. Natural products as leads for new drugs and pesticides discovery. Mini Rev. Org. Chem. 2012, 9, 125. [Google Scholar] [CrossRef]
- Fuse, S.; Matsumura, K.; Johmoto, K.; Uekusa, H.; Tanaka, H.; Hirose, T.; Sunazuka, T.; Omura, S.; Takahashi, T. The design, synthesis, and evaluation of 1,5,7-trisubstituted-3-pyridyl-xanthones for use as insecticides starting from pyripyropene A. Chem.-Eur. J. 2016, 22, 18450–18455. [Google Scholar] [CrossRef]
- Copping, L.G.; Duke, S.O. Natural products that have been used commercially as crop protection agents. Pest. Manag. Sci. 2007, 63, 524–554. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Liu, Z.L.; Ho, S.H.; Goh, S.H. Effect of fraxinellone on growth and digestive physiology of Asian corn borer, Ostrinia furnacalis Guenee. Pestic. Biochem. Physiol. 2008, 91, 122–127. [Google Scholar] [CrossRef]
- Liu, Z.L.; Ho, S.H.; Goh, S.H. Modes of action of fraxinellone against the tobacco budworm, Heliothis virescens. Insect Sci. 2009, 16, 147–155. [Google Scholar] [CrossRef]
- Zhao, W.; Wolfender, J.L.; Hostettmann, K.; Xu, R.; Qin, G. Antifungal alkaloids and limonoid derivatives from Dictamnus dasycarpus. Phytochemistry 1998, 47, 7–11. [Google Scholar] [CrossRef]
- Riley, A.P.; Day, V.W.; Navarro, H.A.; Prisinzano, T.E. Palladium-catalyzed transformations of salvinorin A, a neoclerodane diterpene from Salvia divinorum. Org. Lett. 2013, 15, 5936–5939. [Google Scholar] [CrossRef] [PubMed]
- Harding, W.W.; Schmidt, M.; Tidgewell, K.; Kannan, P.; Holden, K.G.; Dersch, C.M.; Rothman, R.B.; Prisinzano, T.E. Synthetic studies of neoclerodane diterpenes from Salvia divinorum: Selective modification of the furan ring. Bioorg. Med. Chem. Lett. 2006, 16, 3170–3174. [Google Scholar] [CrossRef] [PubMed]
- Riley, A.P.; Groer, C.E.; Young, D.; Ewald, A.W.; Kivell, B.M.; Prisinzano, T.E. Synthesis and kappa-opioid receptor activity of furan-substituted salvinorin A analogues. J. Med. Chem. 2014, 57, 10464–10475. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Isman, M.B.; Chiu, S.F. Antifeedant and growth-inhibitory effects of the limonoid toosendanin and melia-toosendan extracts on the variegated cutworm, Peridroma-Saucia (Lep, Noctuidae). J. Appl. Entomol. 1995, 119, 367–370. [Google Scholar] [CrossRef]
- Shi, Y.L.; Wang, W.P.; Liao, C.Y.; Chiu, S.F. Effect of toosendanin on the sensory inputs of chemoreceptors of the armyworm larvae Mythimna-Separata. Acta Entomol. Sin. 1986, 29, 233–238. [Google Scholar]
- Guo, Y.; Qu, H.; Zhi, X.Y.; Yu, X.; Yang, C.; Xu, H. Semisynthesis and insecticidal activity of some fraxinellone derivatives modified in the B ring. J. Agric. Food Chem. 2013, 61, 11937–11944. [Google Scholar] [CrossRef]
- Dong, Q.M.; Dong, S.; Shen, C.; Cao, Q.H.; Song, M.Y.; He, Q.R.; Wang, X.L.; Yang, X.J.; Tang, J.J.; Gao, J.M. Furan-site bromination and transformations of fraxinellone as insecticidal agents against Mythimna separata Walker. Sci. Rep. 2018, 8, 8372. [Google Scholar] [CrossRef]
- Xiang, P.; Cao, Q.H.; Dong, Q.M.; Yang, X.J.; Tang, J.J.; Bai, H. Furan-site transformations of obacunone as potent insecticidal agents. Heliyon 2018, 4, e01064. [Google Scholar] [CrossRef]
- Wang, D.M.; Zhang, C.C.; Zhang, Q.; Shafiq, N.; Pescitelli, G.; Li, D.W.; Gao, J.M. Wightianines A-E, Dihydro-beta-agarofuran sesquiterpenes from Parnassia wightiana, and their antifungal and insecticidal activities. J. Agric. Food Chem. 2014, 62, 6669–6676. [Google Scholar] [CrossRef] [PubMed]
- Fan, N.J.; Wei, S.P.; Gao, J.M.; Tang, J.J. Potential insecticidal activity of steroidal C-17 pyrazolinyl derivatives. J. Braz. Chem. Soc. 2015, 26, 389–392. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, Q.; Gao, Y.Q.; Tang, J.J.; Zhang, A.L.; Gao, J.M. Secondary metabolites from the endophytic Botryosphaeria dothidea of Melia azedarach and their antifungal, antibacterial, antioxidant, and cytotoxic activities. J. Agric. Food Chem. 2014, 62, 3584–3590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Shen, J.; Zhou, Y.; Wei, Z.P.; Gao, J.M. Insecticidal constituents from Buddlej aalbiflora Hemsl. Nat. Prod. Res. 2017, 31, 1446–1449. [Google Scholar] [CrossRef]
- Wenkert, E.; Khatuya, H. The effect of substituents of alpha-alkyl sidechains on furan-diazoester interactions. Tetrahedron Lett. 1999, 40, 5439–5442. [Google Scholar] [CrossRef]
- Wenkert, E.; Khatuya, H.; Klein, P.S. Reactions of ethyl diazoacetate with beta-methylfurans. Tetrahedron Lett. 1999, 40, 5171–5174. [Google Scholar] [CrossRef]
- Lindsay, V.N.G.; Lin, W.; Charette, A.B. Experimental evidence for the all-up reactive conformation of chiral rhodium(II) carboxylate catalysts: Enantioselective synthesis of cis-cyclopropane alpha-amino acids. J. Am. Chem. Soc. 2009, 131, 16383–16385. [Google Scholar] [CrossRef]
- Liao, K.B.; Pickel, T.C.; Oyarskikh, V.B.; Acsa, J.B.; Usaev, D.G.M.; Davies, H.M.L. Site-selective and stereoselective functionalization of non-activated tertiary C-H bonds. Nature 2017, 551, 609–613. [Google Scholar] [CrossRef]
- Lehner, V.; Davies, H.M.L.; Reiser, O. Rh(II)-catalyzed cyclopropanation of furans and its application to the total synthesis of natural product derivatives. Org. Lett. 2017, 19, 4722–4725. [Google Scholar] [CrossRef]
- Xu, H.; He, X.Q. Natural products-based insecticidal agents 6. Design, semisynthesis, and insecticidal activity of novel monomethyl phthalate derivatives of podophyllotoxin against Mythimna separata Walker in vivo. Bioorg. Med. Chem. Lett. 2010, 20, 4503–4506. [Google Scholar] [CrossRef]
- Guo, Y.; Yan, Y.Y.; Yu, X.; Wang, Y.; Zhi, X.Y.; Hu, Y.; Xu, H. Synthesis and insecticidal activity of some novel fraxinellone-based esters. J. Agric. Food Chem. 2012, 60, 7016–7021. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yan, Y.Y.; Yang, C.; Yu, X.; Zhi, X.Y.; Xu, H. Regioselective synthesis of fraxinellone-based hydrazone derivatives as insecticidal agents. Bioorg. Med. Chem. Lett. 2012, 22, 5384–5387. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Palatinus, L.; Chapuis, G. SUPERFLIP-a computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Crystallogr. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2007, 64, 112–122. [Google Scholar] [CrossRef]
- Tang, J.J.; Zhang, F.Y.; Wang, D.M.; Tian, J.M.; Dong, S.; Gao, J.M. Semisynthesis and antifeedant activity of new derivatives of a dihydro-beta-agarofuran from Parnassia wightiana. Int. J. Mol. Sci. 2013, 14, 19484–19493. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


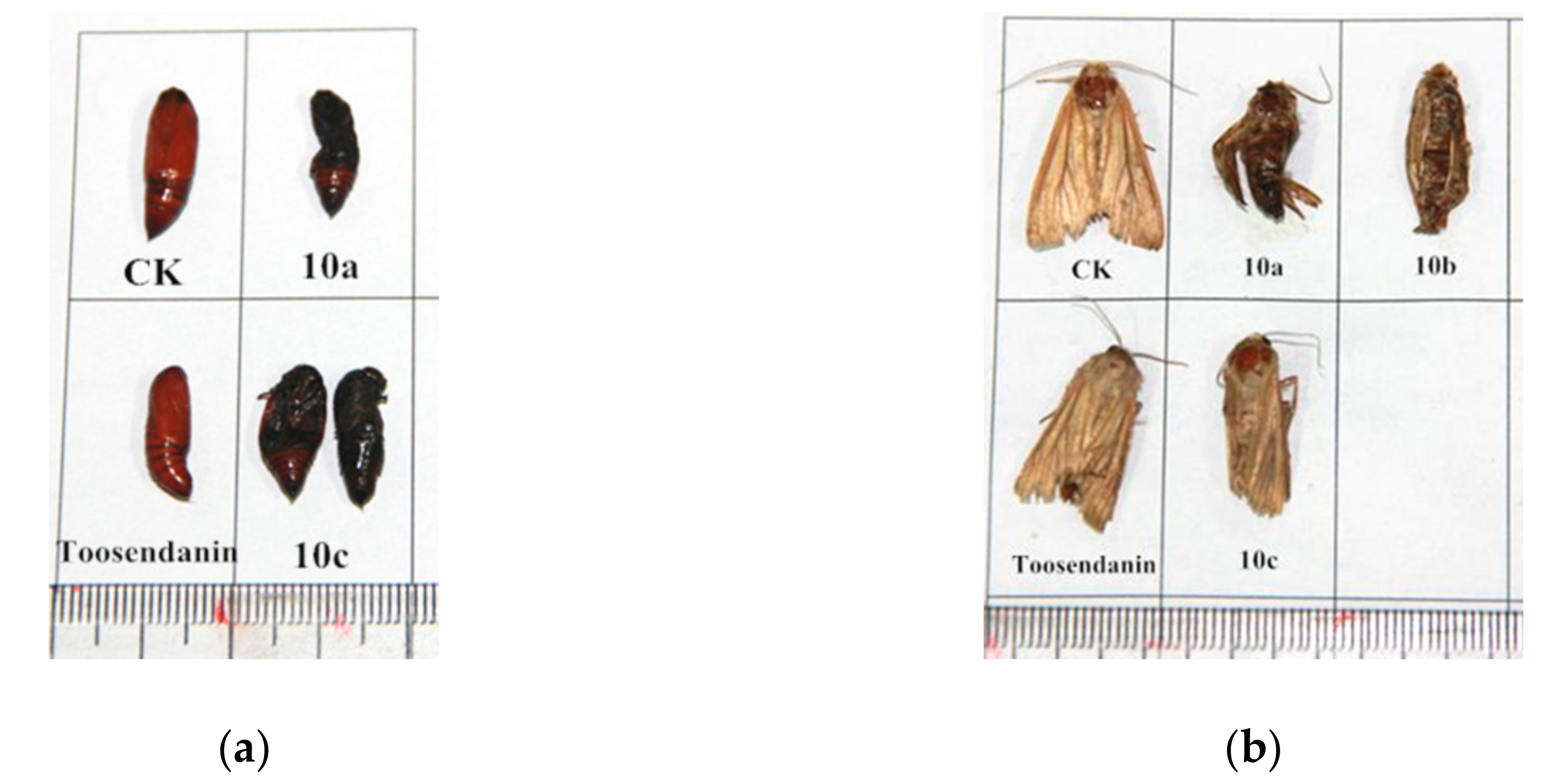
| Compound | Corrected Mortality Rate [%]1 | ||
|---|---|---|---|
| 10 days | 20 days | 35 days | |
| 1 | 0.0 | 11.3 | 17.4 |
| 3a | 8.3 | 12.5 | 29.2 |
| 3b | 4.2 | 8.3 | 33.3 |
| 3c | 4.2 | 12.5 | 37.5 |
| toosendanin | 29.2 | 29.2 | 33.3 |
| blank control | 0.0 | 0.0 | 0.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.-J.; Dong, Q.-M.; Wang, M.-R.; Tang, J.-J. Semi-Synthesis of C-Ring Cyclopropyl Analogues of Fraxinellone and Their Insecticidal Activity Against Mythimna separata Walker. Molecules 2020, 25, 1109. https://doi.org/10.3390/molecules25051109
Yang X-J, Dong Q-M, Wang M-R, Tang J-J. Semi-Synthesis of C-Ring Cyclopropyl Analogues of Fraxinellone and Their Insecticidal Activity Against Mythimna separata Walker. Molecules. 2020; 25(5):1109. https://doi.org/10.3390/molecules25051109
Chicago/Turabian StyleYang, Xiao-Jun, Qing-Miao Dong, Min-Ran Wang, and Jiang-Jiang Tang. 2020. "Semi-Synthesis of C-Ring Cyclopropyl Analogues of Fraxinellone and Their Insecticidal Activity Against Mythimna separata Walker" Molecules 25, no. 5: 1109. https://doi.org/10.3390/molecules25051109
APA StyleYang, X.-J., Dong, Q.-M., Wang, M.-R., & Tang, J.-J. (2020). Semi-Synthesis of C-Ring Cyclopropyl Analogues of Fraxinellone and Their Insecticidal Activity Against Mythimna separata Walker. Molecules, 25(5), 1109. https://doi.org/10.3390/molecules25051109







