Study on Absorption Mechanism and Tissue Distribution of Fucoidan
Abstract
1. Introduction
2. Results
2.1. Fluorescence Labeling of Fucoidan
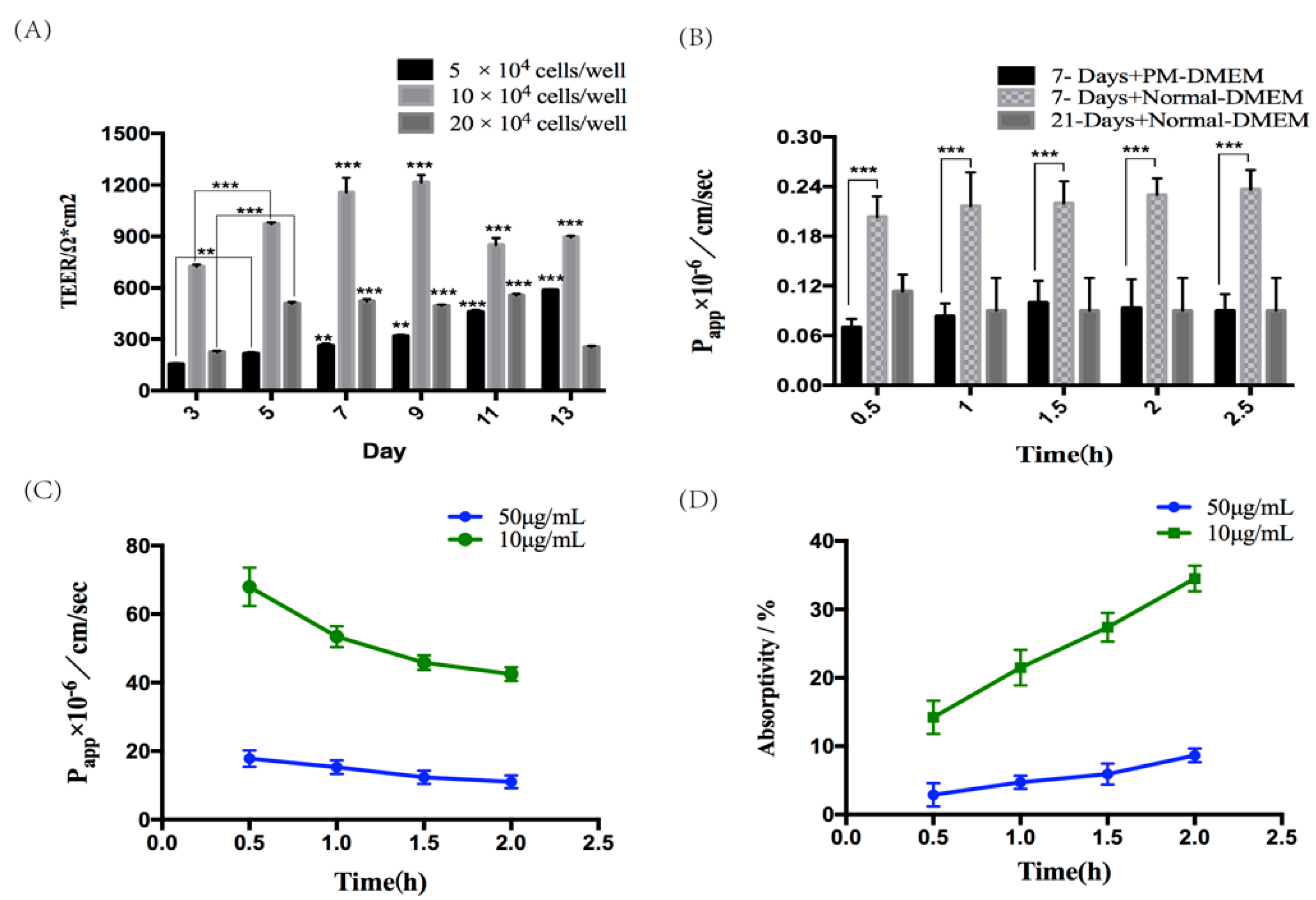
2.2. Establishment and Assessment of Seven-Day Absorption Model of Caco-2 Cells
2.3. Verification of the Absorption and Transport Function of Caco-2 Monolayer Cell Model
2.4. The Mechanism of Fucoidan Absorption and Transport
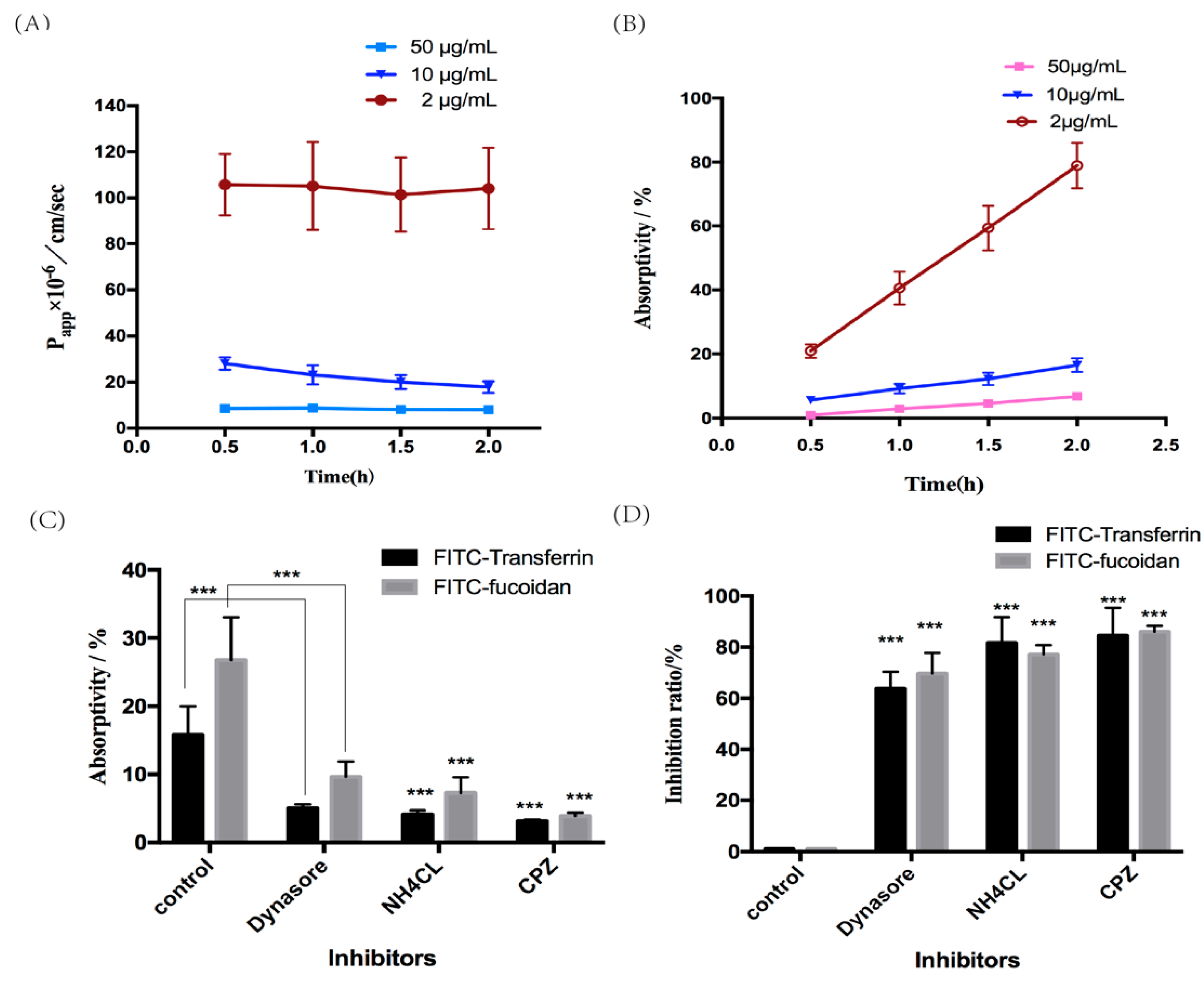
2.4.1. Absorption and Transport of Fucoidan
2.4.2. Effect of Inhibition of Clathrin-Mediated Endocytosis on the Absorption and Transport of Fucoidan
2.5. Tissue Distribution of Fucoidan in Mice
2.5.1. Toxicity of Fucoidan in Mice
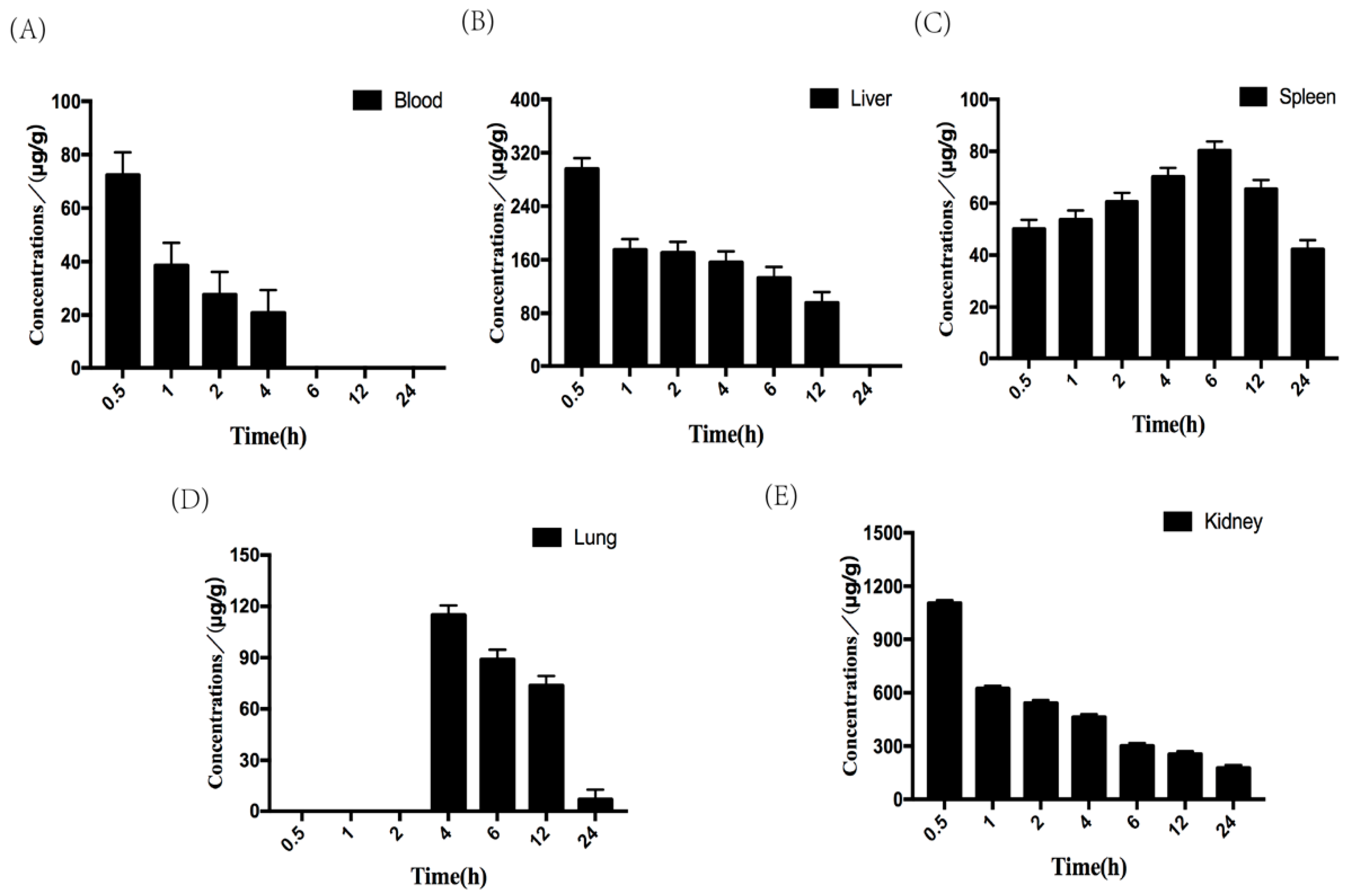
2.5.2. Tissue Distribution of Fucoidan
3. Materials and Methods
3.1. Materials
3.2. Fluorescent Labeling of Fucoidan
3.3. Effect of FITC Labeling on the Content of Fucose and Sulfate
3.4. MTT Assay for Cell Viability
3.5. Caco-2 Cell Culture and Establishment of Caco-2 Monolayer Cell Model
3.6. Verification of the Absorption and Transport Function of Caco-2 Monolayer Cell Model
3.7. The Mechanism of Fucoidan Absorption and Transport
3.8. Tissue distribution of Fucoidan in Mice
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cao, H.T.T.; Mikkelsen, M.D.; Lezyk, M.J.; Bui, L.M.; Tran, V.T.T.; Silchenko, A.S.; Kusaykin, M.I.; Pharm, T.D.; Truong, B.H.; Holck, J.; et al. Novel Enzyme Actions for Sulphated Galactofucan Depolymerisation and a New Engineering Strategy for Molecular Stabilisation of Fucoidan Degrading Enzymes. Mar. Drugs 2018, 16, E422. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Q.; Zhang, Z.; Zhang, H.; Niu, X. Structural studies on a novel fucogalactan sulfate extracted from the brown seaweed Laminaria japonica. Int. J. Biol. Macromol. 2010, 47, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Anastyuk, S.D.; Imbs, T.I.; Shevchenko, N.M.; Dmitrenok, N.M.; Zvyagintseva, T.N. ESIMS analysis of fucoidan preparations from Costaria costata, extracted from alga at different life-stages. Carbohydr. Polym. 2012, 90, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Usoltseva, R.V.; Shevchenko, N.M.; Malyarenko, O.S.; Ishina, I.A.; Ivannikova, S.I.; Ermakova, S.P. Structure and anticancer activity of native and modified polysaccharides from brown alga Dictyota dichotoma. Carbohydr. Polym. 2018, 180, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Ko, C.I.; Jee, Y.; Jeong, Y.; Kim, M.; Kim, J.S.; Jeon, Y.J. Anti-inflammatory effect of fucoidan extracted from Ecklonia cava in zebrafish model. Carbohydr. Polym. 2013, 92, 84–89. [Google Scholar]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. Interuniversitario Nazionale per la Bio-Oncologia, A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef]
- Chen, M.C.; Hsu, W.L.; Hwang, P.A.; Chou, T.C. Low Molecular Weight Fucoidan Inhibits Tumor Angiogenesis through Downregulation of HIF-1/VEGF Signaling under Hypoxia. Mar. Drugs 2015, 13, 4436–4451. [Google Scholar] [CrossRef]
- Wei, H.; Gao, Z.; Zheng, L.; Zhang, C.; Liu, Z.; Yang, Y.; Teng, H.; Hou, L.; Yin, Y.; Zou, X. Protective Effects of Fucoidan on Abeta25-35 and d-Gal-Induced Neurotoxicity in PC12 Cells and d-Gal-Induced Cognitive Dysfunction in Mice. Mar. Drugs 2017, 15, E77. [Google Scholar] [CrossRef]
- Wijesinghe, W.A.; Jeon, Y.J. Exploiting biological activities of brown seaweed Ecklonia cava for potential industrial applications: A review. Int. J. Food Sci. Nutr. 2012, 63, 225–235. [Google Scholar] [CrossRef]
- Kim, S.K.; Pangestuti, R. Biological activities and potential health benefits of fucoxanthin derived from marine brown algae. Adv. Food Nutr. Res. 2011, 64, 11–28. [Google Scholar]
- Mathew, L.; Burney, M.; Gaikwad, A.; Nyshadham, P.; Nugent, E.K.; Gonzalez, A.; Smith, J.A. Preclinical Evaluation of Safety of Fucoidan Extracts From Undaria pinnatifida and Fucus vesiculosus for Use in Cancer Treatment. Integr. Cancer Ther. 2017, 16, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Sae-Lao, T.; Luplertlop, N.; Janvilisri, T.; Tohtong, R.; Bates, D.O.; Wongprasert, K. Sulfated galactans from the red seaweed Gracilaria fisheri exerts anti-migration effect on cholangiocarcinoma cells. Phytomedicine 2017, 36, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Chu, F.; Xu, L.; Liang, H.; Song, S.; Ji, A. Use of fluorescein isothiocyanate isomer I to study the mechanism of intestinal absorption of fucoidan sulfate in vivo and in vitro. Biopharm. Drug Dispos. 2018, 39, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xie, M.; Wan, P.; Chen, D.; Ye, H.; Chen, L.; Zeng, X.; Liu, Z. Digestion under saliva, simulated gastric and small intestinal conditions and fermentation in vitro by human intestinal microbiota of polysaccharides from Fuzhuan brick tea. Food Chem. 2018, 244, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, H.; Shen, Y.; Zhao, X.; Wang, X.; Wang, J.; Fan, K.; Zhan, X. Characterization of a novel polysaccharide from Ganoderma lucidum and its absorption mechanism in Caco-2 cells and mice model. Int. J. Biol. Macromol. 2018, 118, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Cheng, F.; Pan, X.; Zhou, T.; Liu, X.; Zheng, Z.; Luo, L.; Zhang, Y. Investigation of the transport and absorption of Angelica sinensis polysaccharide through gastrointestinal tract both in vitro and in vivo. Drug Deliv. 2017, 24, 1360–1371. [Google Scholar] [CrossRef]
- Azhdarinia, A.; Ghosh, P.; Ghosh, S.; Wilganowski, N.; Sevick-Muraca, E.M. Dual-labeling strategies for nuclear and fluorescence molecular imaging: A review and analysis. Mol. Imaging Biol. 2012, 14, 261–276. [Google Scholar] [CrossRef]
- Ticha, M.; Kocourek, J. Fluorescein-labeled O-glycosyloxyalkenyl-aminoalkenyl-acrylamide copolymers in lectin-saccharide binding studies. Carbohydr. Res. 1991, 213, 339–343. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, F.; Hu, J.; Zhang, L.; Xue, C.; Zhang, Z.; Li, B. Antithrombotic activity of oral administered low molecular weight fucoidan from Laminaria Japonica. Thromb. Res. 2016, 144, 46–52. [Google Scholar] [CrossRef]
- Lin, X.; Wang, Z.; Sun, G.; Shen, L.; Xu, D.; Feng, Y. A sensitive and specific HPGPC-FD method for the study of pharmacokinetics and tissue distribution of Radix Ophiopogonis polysaccharide in rats. Biomed. Chromatogr. 2010, 24, 820–825. [Google Scholar] [CrossRef]
- Yu, M.; Wang, Y.; Ma, F.; Yu, W.; Jiang, T.; Lv, Z. Pharmacokinetics, Tissue Distribution and Excretion Study of Fluoresceinlabeled PS916 in Rats. Curr. Pharm. Biotechnol. 2017, 18, 391–399. [Google Scholar]
- Sevin, E.; Dehouck, L.; Costa, A.F.A.; Cecchelli, R.; Dehouck, M.P.; Lundquist, S.; Culot, M. Accelerated Caco-2 cell permeability model for drug discovery. J. Pharmacol. Toxicol. Methods 2013, 68, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wu, J.; Cheng, F.; Huang, X.; Zeng, F.; Zhang, Y. Acidic Polysaccharide from Angelica sinensis Reverses Anemia of Chronic Disease Involving the Suppression of Inflammatory Hepcidin and NF-kappaB Activation. Oxid. Med. Cell Longev. 2017, 2017, 7601592. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhu, B.; Ai, C.; Lu, J.; Wu, S.; Liu, Y.; Wang, L.; Yang, J.; Song, S.; Liu, X. Development and application of a HPLC-MS/MS method for quantitation of fucosylated chondroitin sulfate and fucoidan in sea cucumbers. Carbohydr. Res. 2018, 466, 11–17. [Google Scholar] [CrossRef]
- Ale, M.T.; Maruyama, H.; Tamauchi, H.; Mikkelsen, J.D.; Meyer, A.S. Fucoidan from Sargassum sp. and Fucus vesiculosus reduces cell viability of lung carcinoma and melanoma cells in vitro and activates natural killer cells in mice in vivo. Int. J. Biol. Macromol. 2011, 49, 331–336. [Google Scholar] [CrossRef]
- Oliveira, C.; Granja, S.; Neves, N.M.; Reis, R.L.; Baltazar, F.; Silva, T.H.; Martins, A. Fucoidan from Fucus vesiculosus inhibits new blood vessel formation and breast tumor growth in vivo. Carbohydr. Polym. 2019, 223, 115034. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wu, T.C.; Hsieh, S.L.; Tsai, Y.H.; Yeh, C.W.; Huang, C.Y. Antioxidant activity and growth inhibition of human colon cancer cells by crude and purified fucoidan preparations extracted from Sargassum cristaefolium. J. Food Drug Anal. 2015, 23, 766–777. [Google Scholar] [CrossRef]
- Lim, S.J.; Aida, W.M.W.; Schiehser, S.; Rosenau, T.; Bohmdorfer, S. Structural elucidation of fucoidan from Cladosiphon okamuranus (Okinawa mozuku). Food Chem. 2019, 272, 222–226. [Google Scholar] [CrossRef]
- Martirosyan, A.; Grintzalis, K.; Polet, M.; Laloux, L.; Schneider, Y.J. Tuning the inflammatory response to silver nanoparticles via quercetin in Caco-2 (co-)cultures as model of the human intestinal mucosa. Toxicol. Lett. 2016, 253, 36–45. [Google Scholar] [CrossRef]
- Lin, A.; Su, X.; She, D.; Qiu, K.; He, Q.; Liu, Y. LC-MS/MS determination and comparative pharmacokinetics of strychnine, brucine and their metabolites in rat plasma after intragastric administration of each monomer and the total alkaloids from Semen Strychni. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1008, 65–73. [Google Scholar] [CrossRef]
- Kim, O.K.; Nam, D.E.; Lee, M.; Kwon, H.O.; Park, J.; You, Y.; Kim, S.I.; Lee, J.; Jun, W. The Effects of Costaria costata Extracts on Atopic Dermatitis in an In Vitro Model. J. Med. Food 2016, 19, 945–951. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the fucoidan are available from the authors. |

| Fucoidan | Content% | |
|---|---|---|
| Before Labeled | After Labeled | |
| Fucose content | 42.86 ± 0.24 | 45.11 ± 0.88 |
| Sulfate content | 25.37 ± 0.25 | 25.33 ± 0.34 |
| Tissue | Standard Curve | R2 |
|---|---|---|
| Blood | Y = 1028.62 X + 48217.63 | 0.999 |
| Heart | Y = 1595.96 X + 153165.43 | 0.994 |
| Liver | Y = 3217.36 X + 164583.03 | 0.998 |
| Spleen | Y = 2902.54 X + 26586.43 | 0.998 |
| Pulmonary | Y = 3194.69 X + 35547.02 | 0.995 |
| Renal | Y = 1791.22 X + 215822.65 | 0.99 |
| Brain | Y = 2427.88 X + 48044.49 | 0.998 |
| Parameter | Unit | Tissue | ||||
|---|---|---|---|---|---|---|
| Blood | Liver | Spleen | Lung | Kidney | ||
| Kel | 1/h | 0.25 ± 0.10 | 0.07 ± 0.01 | 0.003 ± 0.02 | 0.18 ± 0.05 | 0.03 ± 0.04 |
| T1/2 | h | 2.77 ± 0.82 | 10.67 ± 3.73 | 209.22 ± 6.94 | 3.79 ± 0.97 | 22.27 ± 1.75 |
| Tmax | h | 0.5 | 0.5 ± 0.29 | 6 ± 1.15 | 4 ± 1 | 0.5 |
| Cmax | μg/g | 66.37 ± 25.56 | 284.27 ± 211.88 | 77.79 ± 30.05 | 110.92 ± 81.897 | 1092.31 ± 297.66 |
| C0 | μg/g | 135.28 ± 59.91 | 495.25 ± 159.14 | 47.61 ± 17.28 | 188.58 ± 48.32 | 1949.94 ± 1448.45 |
| AUC 0-t | μg/g×h | 138.71 ± 20.64 | 1653.86 ± 567.04 | 1597.28 ± 394.38 | 1694.21 ± 580.70 | 7520.11 ± 2110.44 |
| AUC 0-∞ | μg/g×h | 198.11 ± 41.10 | 2947.506 ± 992.52 | 22886.97 ± 1301.13 | 1709.85 ± 588.22 | 12834.30 ± 5247.13 |
| MRT 0-∞ | h | 3.23 ± 1.30 | 14.66 ± 5.94 | 303.96 ± 13.44 | 6.88 ± 0.37 | 28.14 ± 12.324 |
| CL | (mg)/(μg/g)/h | 0.25 ± 0.04 | 0.02 ± 0.001 | 0.002 ± 0.01 | 0.03 ± 0.004 | 0.004 ± 0.003 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, X.; Zhang, E.; Hu, B.; Liang, H.; Song, S.; Ji, A. Study on Absorption Mechanism and Tissue Distribution of Fucoidan. Molecules 2020, 25, 1087. https://doi.org/10.3390/molecules25051087
Bai X, Zhang E, Hu B, Liang H, Song S, Ji A. Study on Absorption Mechanism and Tissue Distribution of Fucoidan. Molecules. 2020; 25(5):1087. https://doi.org/10.3390/molecules25051087
Chicago/Turabian StyleBai, Xu, E Zhang, Bo Hu, Hao Liang, Shuliang Song, and Aiguo Ji. 2020. "Study on Absorption Mechanism and Tissue Distribution of Fucoidan" Molecules 25, no. 5: 1087. https://doi.org/10.3390/molecules25051087
APA StyleBai, X., Zhang, E., Hu, B., Liang, H., Song, S., & Ji, A. (2020). Study on Absorption Mechanism and Tissue Distribution of Fucoidan. Molecules, 25(5), 1087. https://doi.org/10.3390/molecules25051087




