Design of Deep Eutectic Systems: A Simple Approach for Preselecting Eutectic Mixture Constituents
Abstract
1. Introduction
2. Results and Discussion
2.1. Melting Properties of Pure Components
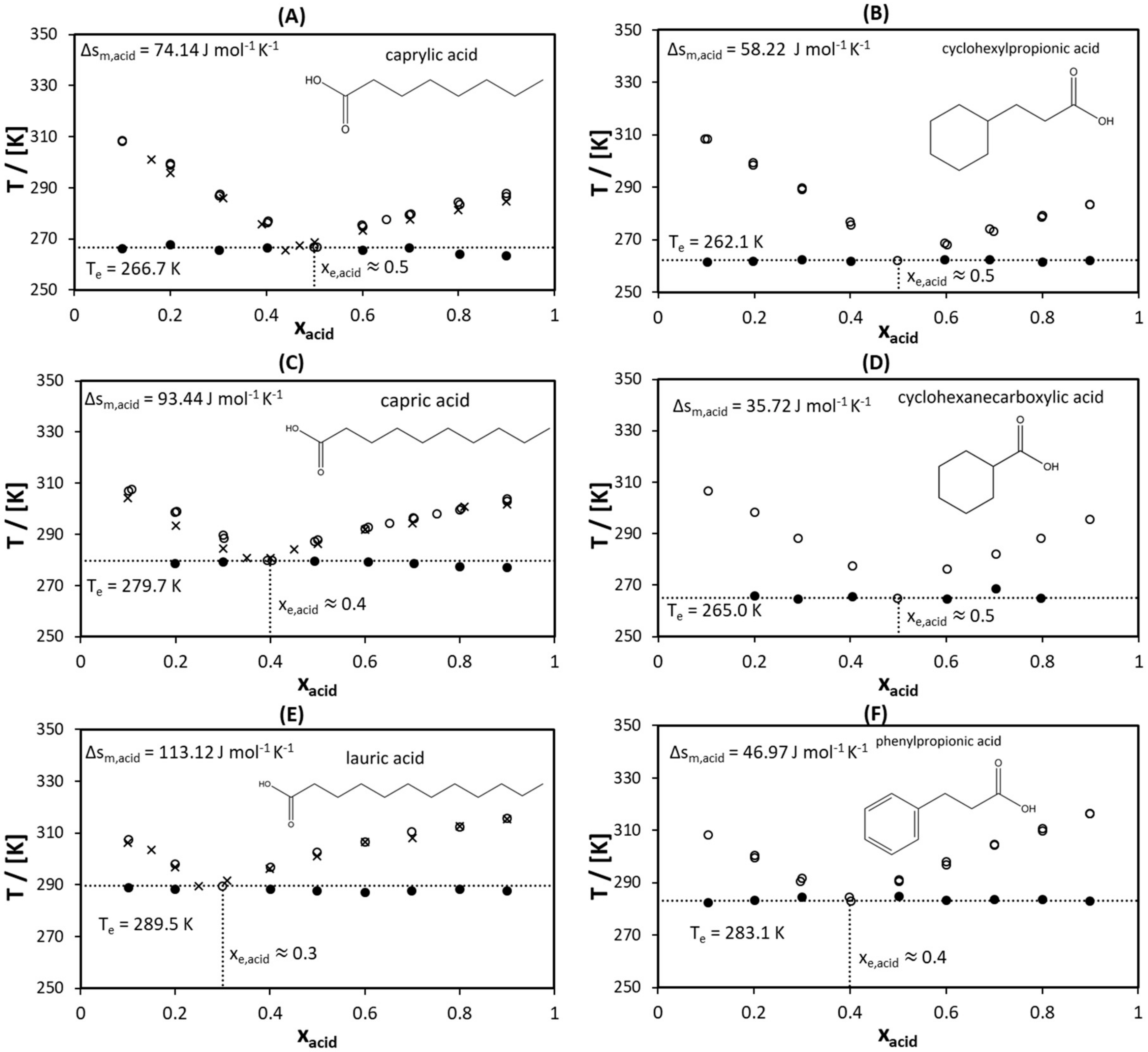
2.2. Solid–Liquid Equilibria
3. Materials and Methods
3.1. Prediction of Melting Entropy
3.2. Eutectic Mixture Constituents
- (i)
- Caprylic acid and 3-cyclohexylpropionic acid with a melting temperature of approximately 15 °C,
- (ii)
- Capric acid and cyclohexanecarboxylic acid with a melting temperature of approximately 30 °C,
- (iii)
- Lauric acid and 3-phenylpropionic acid with a melting temperature of approximately 45 °C.
3.3. Eutectic Mixture Preparation
3.4. Differential Scanning Calorimetry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gamsjäger, H.; Lorimer J., W.; Scharlin, P.; Shaw David, G. Glossary of terms related to solubility (IUPAC Recommendations 2008). Pure Appl. Chem. 2008, 80, 233. [Google Scholar]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Cullis, P.M.; Gibson, M.J.; Harris, R.C.; Raven, E. Extraction of glycerol from biodiesel into a eutectic based ionic liquid. Green Chem. 2007, 9, 868–872. [Google Scholar] [CrossRef]
- Abbott, A.P.; Harris, R.C.; Ryder, K.S.; D’Agostino, C.; Gladden, L.F.; Mantle, M.D. Glycerol eutectics as sustainable solvent systems. Green Chem. 2011, 13, 82–90. [Google Scholar] [CrossRef]
- Bezold, F.; Minceva, M. A water-free solvent system containing an L-menthol-based deep eutectic solvent for centrifugal partition chromatography applications. J. Chromatogr. A 2019, 1587, 166–171. [Google Scholar] [CrossRef]
- Gouveia, A.S.L.; Oliveira, F.S.; Kurnia, K.A.; Marrucho, I.M. Deep Eutectic Solvents as Azeotrope Breakers: Liquid–Liquid Extraction and COSMO-RS Prediction. ACS Sustain. Chem. Eng. 2016. [Google Scholar] [CrossRef]
- Roehrer, S.; Bezold, F.; Garcia, E.M.; Minceva, M. Deep eutectic solvents in countercurrent and centrifugal partition chromatography. J. Chromatogr. A 2016, 1434, 102–110. [Google Scholar] [CrossRef]
- Alonso, D.A.; Baeza, A.; Chinchilla, R.; Guillena, G.; Pastor, I.M.; Ramón, D.J. Deep Eutectic Solvents: The Organic Reaction Medium of the Century. Eur. J. Org. Chem. 2016, 2016, 612–632. [Google Scholar] [CrossRef]
- Hayyan, A.; Ali Hashim, M.; Mjalli, F.S.; Hayyan, M.; AlNashef, I.M. A novel phosphonium-based deep eutectic catalyst for biodiesel production from industrial low grade crude palm oil. Chem. Eng. Sci. 2013, 92. [Google Scholar] [CrossRef]
- Hayyan, A.; Hashim, M.A.; Hayyan, M.; Mjalli, F.S.; AlNashef, I.M. A new processing route for cleaner production of biodiesel fuel using a choline chloride based deep eutectic solvent. J. Clean. Prod. 2014, 65. [Google Scholar] [CrossRef]
- Khandelwal, S.; Tailor, Y.K.; Kumar, M. Deep eutectic solvents (DESs) as eco-friendly and sustainable solvent/catalyst systems in organic transformations. J. Mol. Liq. 2016, 215, 345–386. [Google Scholar] [CrossRef]
- Wagle, D.V.; Zhao, H.; Baker, G.A. Deep eutectic solvents: Sustainable media for nanoscale and functional materials. Acc. Chem. Res. 2014, 47. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Hao, J.-W.; Mo, L.-P.; Zhang, Z.-H. Recent advances in the application of deep eutectic solvents as sustainable media as well as catalysts in organic reactions. RSC Advances 2015, 5, 48675–48704. [Google Scholar] [CrossRef]
- Abbott, A.P.; Griffith, J.; Nandhra, S.; O’Connor, C.; Postlethwaite, S.; Ryder, K.S.; Smith, E.L. Sustained electroless deposition of metallic silver from a choline chloride-based ionic liquid. Surf. Coat. Technol. 2008, 202, 2033–2039. [Google Scholar] [CrossRef]
- Boisset, A.; Menne, S.; Jacquemin, J.; Balducci, A.; Anouti, M. Deep eutectic solvents based on N-methylacetamide and a lithium salt as suitable electrolytes for lithium-ion batteries. PCCP 2013, 15, 20054–20063. [Google Scholar] [CrossRef]
- Chen, Z.; McLean, B.; Ludwig, M.; Stefanovic, R.; Warr, G.G.; Webber, G.B.; Page, A.J.; Atkin, R. Nanostructure of Deep Eutectic Solvents at Graphite Electrode Interfaces as a Function of Potential. J. Phys. Chem. C 2016, 120, 2225–2233. [Google Scholar] [CrossRef]
- Gómez, E.; Cojocaru, P.; Magagnin, L.; Valles, E. Electrodeposition of Co, Sm and SmCo from a Deep Eutectic Solvent. J. Electroanal. Chem. 2011, 658, 18–24. [Google Scholar] [CrossRef]
- Zaidi, W.; Boisset, A.; Jacquemin, J.; Timperman, L.; Anouti, M. Deep Eutectic Solvents Based on N-Methylacetamide and a Lithium Salt as Electrolytes at Elevated Temperature for Activated Carbon-Based Supercapacitors. J. Phys. Chem. C 2014, 118, 4033–4042. [Google Scholar] [CrossRef]
- Pätzold, M.; Siebenhaller, S.; Kara, S.; Liese, A.; Syldatk, C.; Holtmann, D. Deep Eutectic Solvents as Efficient Solvents in Biocatalysis. Trends Biotechnol. 2019, 37, 943–959. [Google Scholar] [CrossRef]
- Oh, Y.; Park, S.; Yoo, E.; Jo, S.; Hong, J.; Kim, H.J.; Kim, K.J.; Oh, K.K.; Lee, S.H. Dihydrogen-bonding deep eutectic solvents as reaction media for lipase-catalyzed transesterification. Biochem. Eng. J. 2019, 142, 34–40. [Google Scholar] [CrossRef]
- Jablonský, M.; Škulcová, A.; Šima, J. Use of Deep Eutectic Solvents in Polymer Chemistry–A Review. Molecules 2019, 24, 3978. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Matias, A.A.; Paiva, A.; Duarte, A.R.C. Polymer Science and Engineering Using Deep Eutectic Solvents. Polymers 2019, 11, 912. [Google Scholar] [CrossRef] [PubMed]
- Gómez, A.V.; Biswas, A.; Tadini, C.C.; Furtado, R.F.; Alves, C.R.; Cheng, H.N. Use of natural deep eutectic solvents for polymerization and polymer reactions. J. Braz. Chem. Soc. 2019, 30, 717–726. [Google Scholar] [CrossRef]
- Abbott, A.P.; Ahmed, E.I.; Prasad, K.; Qader, I.B.; Ryder, K.S. Liquid pharmaceuticals formulation by eutectic formation. Fluid Phase Equilib. 2017, 448, 2–8. [Google Scholar] [CrossRef]
- Aroso, I.M.; Craveiro, R.; Rocha, Â.; Dionísio, M.; Barreiros, S.; Reis, R.L.; Paiva, A.; Duarte, A.R.C. Design of controlled release systems for THEDES—Therapeutic deep eutectic solvents, using supercritical fluid technology. Int. J. Pharm. 2015, 492, 73–79. [Google Scholar] [CrossRef]
- Aroso, I.M.; Silva, J.C.; Mano, F.; Ferreira, A.S.; Dionísio, M.; Sá-Nogueira, I.; Barreiros, S.; Reis, R.L.; Paiva, A.; Duarte, A.R.C. Dissolution enhancement of active pharmaceutical ingredients by therapeutic deep eutectic systems. Eur. J. Pharm. Sci. 2016, 98, 57–66. [Google Scholar] [CrossRef]
- Jeong, K.M.; Ko, J.; Zhao, J.; Jin, Y.; Yoo, D.E.; Han, S.Y.; Lee, J. Multi-functioning deep eutectic solvents as extraction and storage media for bioactive natural products that are readily applicable to cosmetic products. J. Clean. Prod. 2017, 151, 87–95. [Google Scholar] [CrossRef]
- Van Osch, D.J.G.P.; Dietz, C.H.J.T.; van Spronsen, J.; Kroon, M.C.; Gallucci, F.; van Sint Annaland, M.; Tuinier, R. A Search for Natural Hydrophobic Deep Eutectic Solvents Based on Natural Components. ACS Sustain. Chem. Eng. 2019, 7, 2933–2942. [Google Scholar] [CrossRef]
- Francisco, M.; van den Bruinhorst, A.; Kroon, M.C. New natural and renewable low transition temperature mixtures (LTTMs): Screening as solvents for lignocellulosic biomass processing. Green Chem. 2012, 14, 2153–2157. [Google Scholar] [CrossRef]
- Van den Bruinhorst, A.; Kollau, L.J.B.M.; Kroon, M.C.; Meuldijk, J.; Tuinier, R.; Esteves, A.C.C. A centrifuge method to determine the solid–liquid phase behavior of eutectic mixtures. J. Chem. Phys. 2018, 149, 224505. [Google Scholar] [CrossRef]
- Silva, L.P.; Fernandez, L.; Conceição, J.H.F.; Martins, M.A.R.; Sosa, A.; Ortega, J.; Pinho, S.P.; Coutinho, J.A.P. Design and Characterization of Sugar-Based Deep Eutectic Solvents Using Conductor-like Screening Model for Real Solvents. ACS Sustain. Chem. Eng. 2018. [Google Scholar] [CrossRef]
- Rodriguez Rodriguez, N.; van den Bruinhorst, A.; Kollau, L.J.B.M.; Kroon, M.C.; Binnemans, K. Degradation of Deep-Eutectic Solvents Based on Choline Chloride and Carboxylic Acids. ACS Sustain. Chem. Eng. 2019, 7, 11521–11528. [Google Scholar] [CrossRef]
- Abranches, D.O.; Larriba, M.; Silva, L.P.; Melle-Franco, M.; Palomar, J.F.; Pinho, S.P.; Coutinho, J.A.P. Using COSMO-RS to design choline chloride pharmaceutical eutectic solvents. Fluid Phase Equilib. 2019, 497, 71–78. [Google Scholar] [CrossRef]
- Wolbert, F.; Brandenbusch, C.; Sadowski, G. Selecting Excipients Forming Therapeutic Deep Eutectic Systems-A Mechanistic Approach. Mol. Pharm. 2019, 16, 3091–3099. [Google Scholar] [CrossRef] [PubMed]
- Kollau, L.J.B.M.; Vis, M.; van den Bruinhorst, A.; Esteves, A.C.C.; Tuinier, R. Quantification of the liquid window of deep eutectic solvents. Chem. Commun. 2018, 54, 13351–13354. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.A.R.; Pinho, S.P.; Coutinho, J.A.P. Insights into the Nature of Eutectic and Deep Eutectic Mixtures. J. Solut. Chem. 2018. [Google Scholar] [CrossRef]
- Alhadid, A.; Mokrushina, L.; Minceva, M. Modeling of Solid-Liquid Equilibria in Deep Eutectic Solvents: A Parameter Study. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Williams, D.H.; O’Brien, D.P.; Bardsley, B. Enthalpy/Entropy Compensation as a Competition between Dynamics and Bonding: The Relevance to Melting of Crystals and Biological Aggregates. J. Am. Chem. Soc. 2001, 123, 737–738. [Google Scholar] [CrossRef]
- Jain, A.; Yalkowsky, S.H. Estimation of Melting Points of Organic Compounds-II. J. Pharm. Sci. 2006, 95, 2562–2618. [Google Scholar] [CrossRef]
- Yalkowsky, S.H. Carnelley’s Rule and the Prediction of Melting Point. J. Pharm. Sci. 2014, 103, 2629–2634. [Google Scholar] [CrossRef] [PubMed]
- Dearden, J.C. The QSAR prediction of melting point, a property of environmental relevance. Sci. Total Environ. 1991, 109, 59–68. [Google Scholar] [CrossRef]
- Brown, R.J.C.; Brown, R.F.C. Melting Point and Molecular Symmetry. J. Chem. Educ. 2000, 77, 724. [Google Scholar] [CrossRef]
- Jain, A.; Yang, G.; Yalkowsky, S.H. Estimation of Total Entropy of Melting of Organic Compounds. Ind. Eng. Chem. Res. 2004, 43, 4376–4379. [Google Scholar] [CrossRef]
- Preiss, U.P.; Beichel, W.; Erle, A.M.; Paulechka, Y.U.; Krossing, I. Is universal, simple melting point prediction possible? Chemphyschem 2011, 12, 2959–2972. [Google Scholar] [CrossRef] [PubMed]
- Bondi, A. Physical Properties of Molecular Crystals, Liquids, and Glasses; John Wiley and Sons: New York, NY, USA, 1968. [Google Scholar]
- Crespo, E.A.; Silva, L.P.; Martins, M.A.R.; Bülow, M.; Ferreira, O.; Sadowski, G.; Held, C.; Pinho, S.P.; Coutinho, J.A.P. The Role of Polyfunctionality in the Formation of [Ch]Cl-Carboxylic Acid-Based Deep Eutectic Solvents. Ind. Eng. Chem. Res. 2018, 57, 11195–11209. [Google Scholar] [CrossRef]
- Crespo, E.A.; Silva, L.P.; Martins, M.A.R.; Fernandez, L.; Ortega, J.; Ferreira, O.; Sadowski, G.; Held, C.; Pinho, S.P.; Coutinho, J.A.P. Characterization and Modeling of the Liquid Phase of Deep Eutectic Solvents Based on Fatty Acids/Alcohols and Choline Chloride. Ind. Eng. Chem. Res. 2017, 56, 12192–12202. [Google Scholar] [CrossRef]
- Martins, M.A.R.; Crespo, E.A.; Pontes, P.V.A.; Silva, L.P.; Bülow, M.; Maximo, G.J.; Batista, E.A.C.; Held, C.; Pinho, S.P.; Coutinho, J.A.P. Tunable Hydrophobic Eutectic Solvents Based on Terpenes and Monocarboxylic Acids. ACS Sustain. Chem. Eng. 2018, 6, 8836–8846. [Google Scholar] [CrossRef]
- Domańska, U.; Morawski, P.; Piekarska, M. Solubility of perfumery and fragrance raw materials based on cyclohexane in 1-octanol under ambient and high pressures up to 900MPa. J. Chem. Thermodyn. 2008, 40, 710–717. [Google Scholar] [CrossRef]
- Pontes, P.V.A.; Crespo, E.A.; Martins, M.A.R.; Silva, L.P.; Neves, C.M.S.S.; Maximo, G.J.; Hubinger, M.D.; Batista, E.A.C.; Pinho, S.P.; Coutinho, J.A.P.; et al. Measurement and PC-SAFT modeling of solid-liquid equilibrium of deep eutectic solvents of quaternary ammonium chlorides and carboxylic acids. Fluid Phase Equilib. 2017, 448, 69–80. [Google Scholar] [CrossRef]
- Monte, M.J.S.; Hillesheim, D.M. Thermodynamic study on the sublimation of 3-phenylpropionic acid and of three methoxy-substituted 3-phenylpropionic acids. J. Chem. Thermodyn. 2001, 33, 837–847. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are no longer available from the authors. |

| Compound | σ | SP3 | SP2 | Ring | τ | Φ | Δsm (J mol–1 K–1) |
|---|---|---|---|---|---|---|---|
| 3-cyclohexylpropionic acid | 1 | 2 | 1 | 1 | 2 | 5.93 | 64.80 |
| caprylic acid | 1 | 6 | 1 | 0 | 5.5 | 133.58 | 90.69 |
| cyclohexanecarboxylic acid | 1 | 0 | 1 | 1 | 0 | 1 | 50 |
| capric acid | 1 | 8 | 1 | 0 | 7.5 | 792.03 | 105.49 |
| 3-phenylpropionic acid | 1 | 2 | 1 | 1 | 2 | 5.93 | 64.80 |
| lauric acid | 1 | 10 | 1 | 0 | 9.5 | 4696.13 | 120.29 |
| Compound | Tm (K) | Δhm (kJ mol–1) | ||
|---|---|---|---|---|
| This Work * | Lit. | This Work * | Lit. | |
| l-menthol | 314.6 ± 0.1 | 315.68 [49] | 13.74 ± 0.5 | 12.89 [49] |
| 3-cyclohexylpropionic acid | 291.3 ± 0.1 | – | 16.96 ± 0.5 | – |
| caprylic acid | 288.0 ± 0.7 | 288.20 [49] | 21.43 ± 0.3 | 19.80 [49] |
| cyclohexanecarboxylic acid | 299.4 ± 1.1 | 301.9 [50] | 10.69 ± 0.2 | 9.20 [50] |
| capric acid | 303.9 ± 0.1 | 304.75 [51] | 28.39 ± 0.7 | 27.50 [51] |
| 3-phenylpropionic acid | 321.6 ± 0.1 | 321.2 [52] | 15.11 ± 0.1 | 15.68 [52] |
| lauric acid | 316.6 ± 0.1 | 317.48 [51] | 35.81 ± 0.4 | 37.83 [51] |
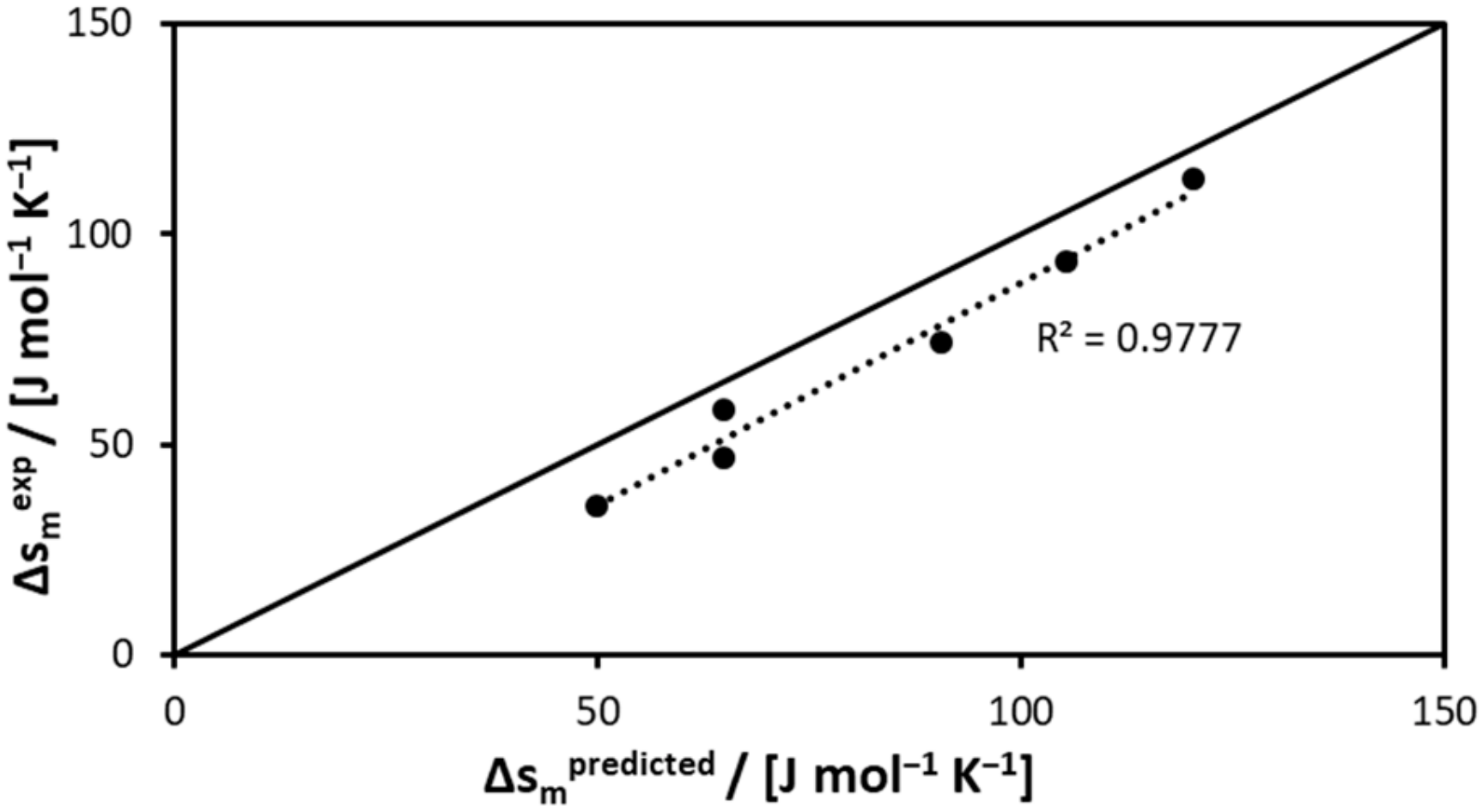
| Compound | Δsm predicted | Δsm experimental |
|---|---|---|
| 3-cyclohexylpropionic acid | 64.80 | 58.22 |
| caprylic acid | 90.69 | 74.41 |
| cyclohexanecarboxylic acid | 50 | 35.72 |
| capric acid | 105.49 | 93.44 |
| 3-phenylpropionic acid | 64.80 | 46.97 |
| lauric acid | 120.29 | 113.12 |
| Name | CAS Number | Supplier | Purity * |
|---|---|---|---|
| l-menthol | 2216–51–5 | Sigma Aldrich Chemie GmbH | ≥ 99 % |
| 3-cyclohexylpropionic acid | 701–97–3 | ThermoFisher (Kandel) GmbH | > 98 % |
| caprylic acid | 124–07–2 | Merck KGaA | 99 % |
| cyclohexanecarboxylic acid | 98–89–5 | ThermoFisher (Kandel) GmbH | 98 % |
| capric acid | 334–48–5 | Alfa Aesar GmbH | 99 % |
| 3-phenylpropionic acid | 501–52–0 | Alfa Aesar GmbH | 99 % |
| lauric acid | 143–07–7 | Merck KGaA | 99 % |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhadid, A.; Mokrushina, L.; Minceva, M. Design of Deep Eutectic Systems: A Simple Approach for Preselecting Eutectic Mixture Constituents. Molecules 2020, 25, 1077. https://doi.org/10.3390/molecules25051077
Alhadid A, Mokrushina L, Minceva M. Design of Deep Eutectic Systems: A Simple Approach for Preselecting Eutectic Mixture Constituents. Molecules. 2020; 25(5):1077. https://doi.org/10.3390/molecules25051077
Chicago/Turabian StyleAlhadid, Ahmad, Liudmila Mokrushina, and Mirjana Minceva. 2020. "Design of Deep Eutectic Systems: A Simple Approach for Preselecting Eutectic Mixture Constituents" Molecules 25, no. 5: 1077. https://doi.org/10.3390/molecules25051077
APA StyleAlhadid, A., Mokrushina, L., & Minceva, M. (2020). Design of Deep Eutectic Systems: A Simple Approach for Preselecting Eutectic Mixture Constituents. Molecules, 25(5), 1077. https://doi.org/10.3390/molecules25051077






