On the Formation and Stability of Chitosan/Hyaluronan-Based Complex Coacervates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Coacervates
2.3. Physical Characterization of Coacervates
2.3.1. Dynamic Light Scattering (DLS) Analyses
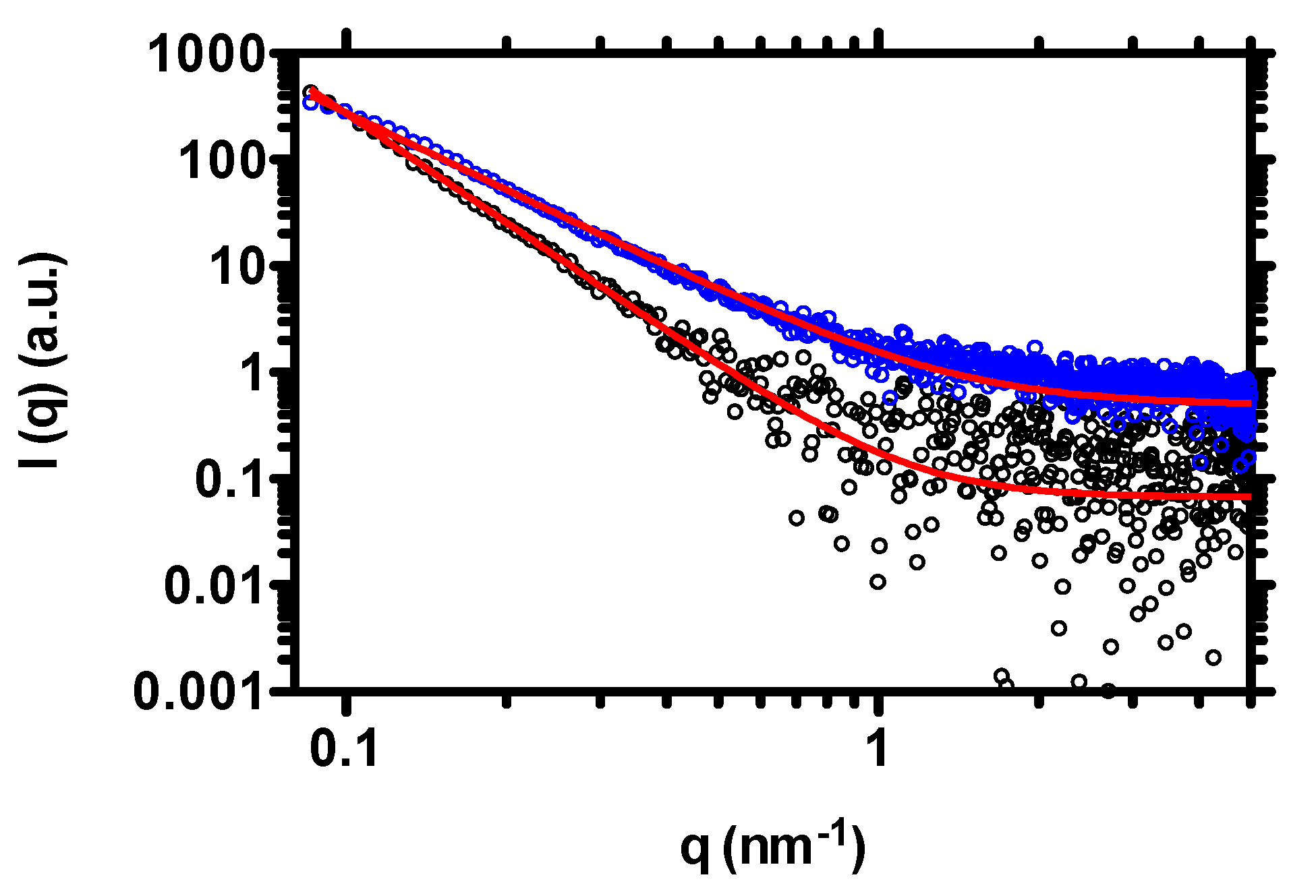
2.3.2. Small Angle X-Ray Scattering (SAXS) Measurements
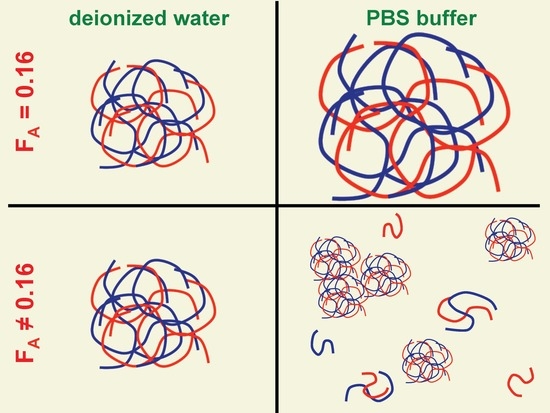
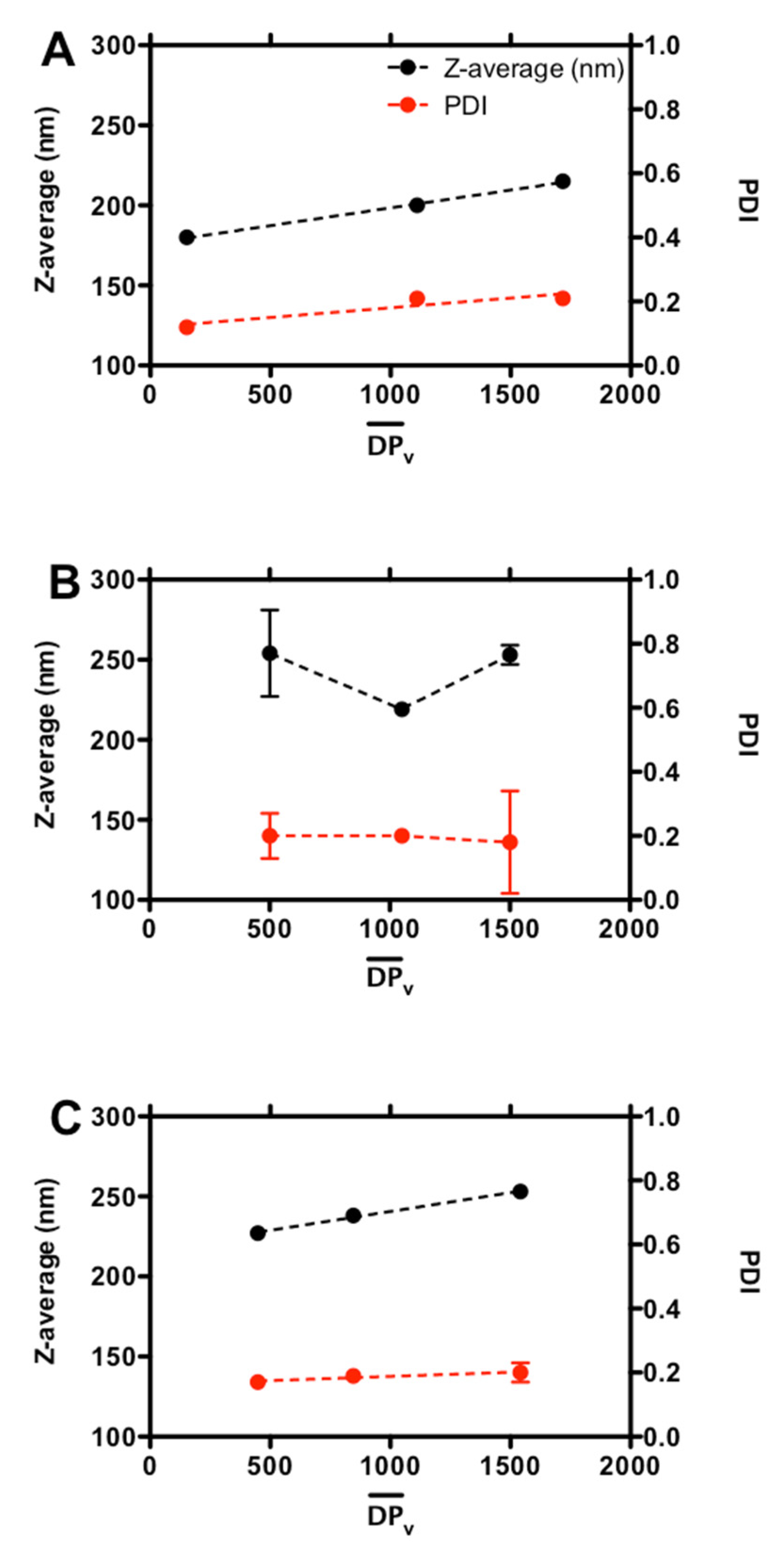
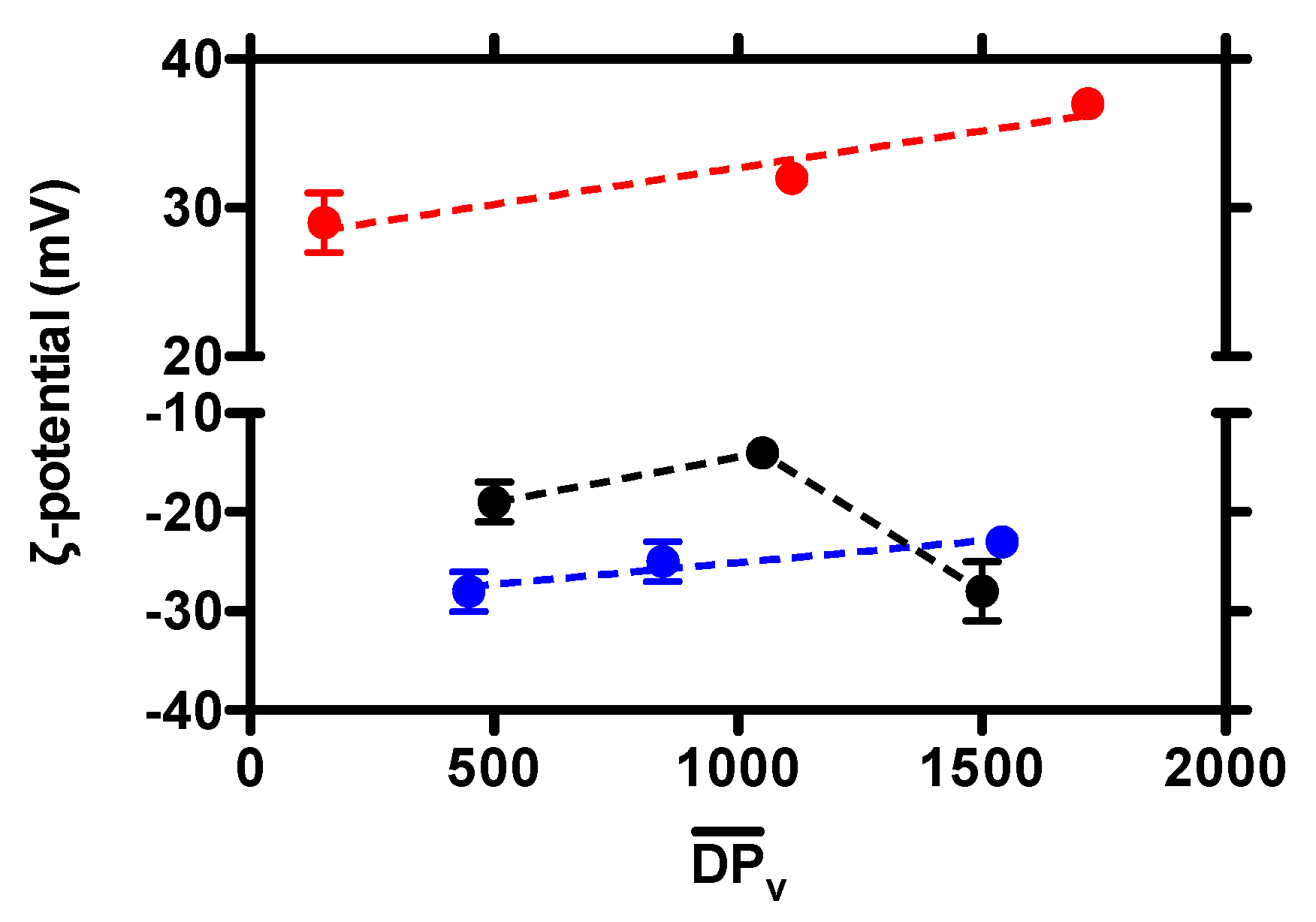
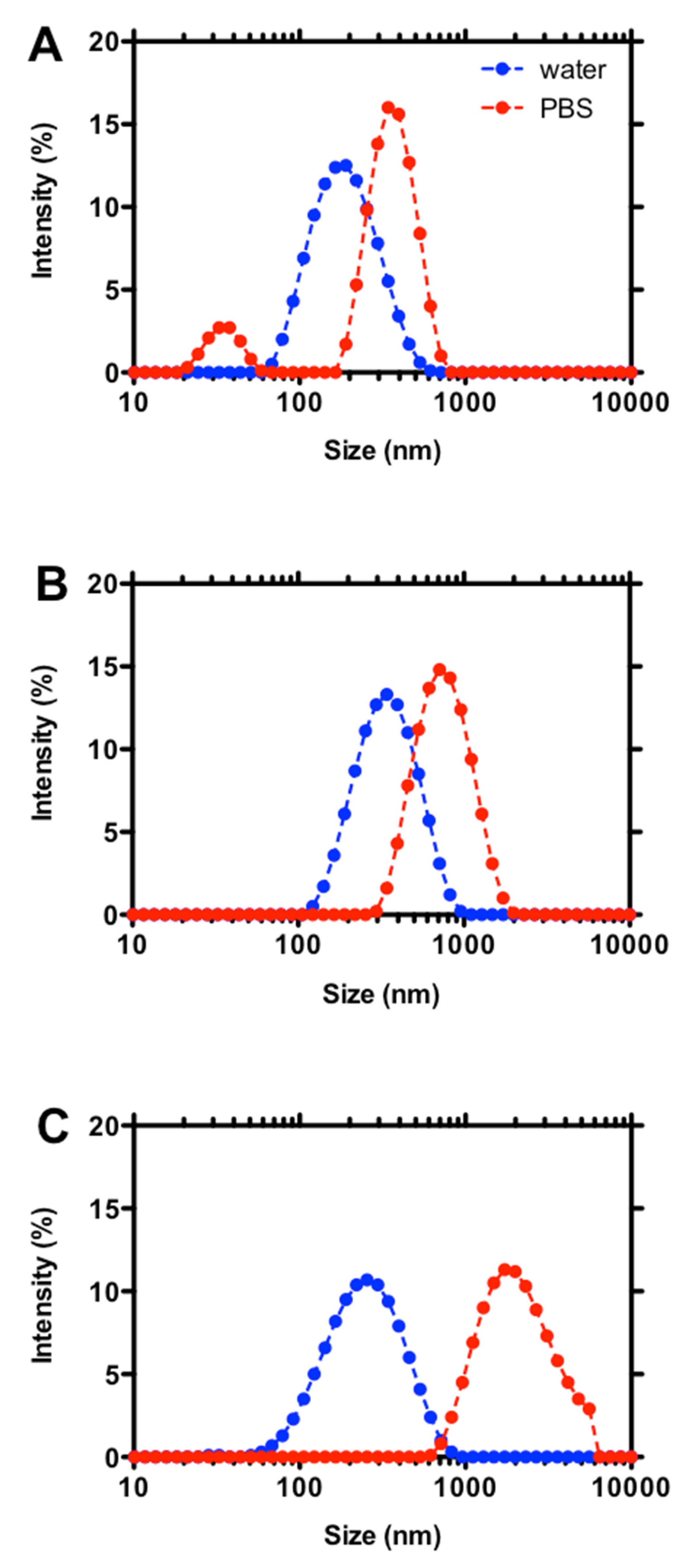
3. Results and Discussion
3.1. Effect of Chitosan Variables on Coacervates Formation and Physical Properties
3.2. The Stability of Coacervates as a Function of Chitosan Variables
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sacco, P.; Furlani, F.; de Marzo, G.; Marsich, E.; Paoletti, S.; Donati, I. Concepts for developing physical gels of chitosan and of chitosan derivatives. Gels 2018, 4, 67. [Google Scholar] [CrossRef]
- Vårum, K.M.; Smidsrød, O. Structure-property relationship in chitosans. In Polysaccharides: Structural Diversity and Functional Versatility; Dumitriu, S., Ed.; CRC Press: Boca Raton, FL, USA, 2004; pp. 625–642. [Google Scholar]
- Racine, L.; Texier, I.; Auzély-Velty, R. Chitosan-based hydrogels: Recent design concepts to tailor properties and functions. Polym. Int. 2017, 66, 981–998. [Google Scholar] [CrossRef]
- Sacco, P.; Cok, M.; Asaro, F.; Paoletti, S.; Donati, I. The role played by the molecular weight and acetylation degree in modulating the stiffness and elasticity of chitosan gels. Carbohydr. Polym. 2018, 196, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Remuñán-López, C.; Vila-Jato, J.L.; Alonso, M.J. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Furlani, F.; Sacco, P.; Marsich, E.; Donati, I.; Paoletti, S. Highly monodisperse colloidal coacervates based on a bioactive lactose-modified chitosan: From synthesis to characterization. Carbohydr. Polym. 2017, 174, 360–368. [Google Scholar] [CrossRef]
- Sacco, P.; Brun, F.; Donati, I.; Porrelli, D.; Paoletti, S.; Turco, G. On the correlation between the microscopic structure and properties of phosphate-cross-linked chitosan gels. ACS Appl. Mater. Interfaces 2018, 10, 10761–10770. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef]
- Liu, Q.; Sacco, P.; Marsich, E.; Furlani, F.; Arib, C.; Djaker, N.; De La Chapelle, L.M.; Donati, I.; Spadavecchia, J. Lactose-modified chitosan gold(III)-pegylated complex-bioconjugates: From synthesis to interaction with targeted Galectin-1 protein. Bioconjug. Chem. 2018, 29, 3352–3361. [Google Scholar] [CrossRef]
- Cok, M.; Sacco, P.; Porrelli, D.; Travan, A.; Borgogna, M.; Marsich, E.; Paoletti, S.; Donati, I. Mimicking mechanical response of natural tissues. Strain hardening induced by transient reticulation in lactose-modified chitosan (chitlac). Int. J. Biol. Macromol. 2018, 106, 656–660. [Google Scholar] [CrossRef]
- Kizilay, E.; Kayitmazer, A.B.; Dubin, P.L. Complexation and coacervation of polyelectrolytes with oppositely charged colloids. Adv. Colloid Interface Sci. 2011, 167, 24–37. [Google Scholar] [CrossRef]
- Umerska, A.; Paluch, K.J.; Inkielewicz-Stepniak, I.; Santos-Martinez, M.J.; Corrigan, O.I.; Medina, C.; Tajber, L. Exploring the assembly process and properties of novel crosslinker-free hyaluronate-based polyelectrolyte complex nanocarriers. Int. J. Pharm. 2012, 436, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Zambito, Y.; Felice, F.; Fabiano, A.; Di Stefano, R.; Di Colo, G. Mucoadhesive nanoparticles made of thiolated quaternary chitosan crosslinked with hyaluronan. Carbohydr. Polym. 2013, 92, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Cao, M.; Zhang, J.; Hu, K.; Yin, Z.; Zhou, Z.; Xiao, X.; Yang, Y.; Sheng, W.; Wu, Y.; et al. Hyaluronic acid-chitosan nanoparticles for co-delivery of MiR-34a and doxorubicin in therapy against triple negative breast cancer. Biomaterials 2014, 35, 4333–4344. [Google Scholar] [CrossRef] [PubMed]
- Almalik, A.; Day, P.J.; Tirelli, N. HA-coated chitosan nanoparticles for CD44-mediated nucleic acid delivery. Macromol. Biosci. 2013, 13, 1671–1680. [Google Scholar] [CrossRef]
- de la Fuente, M.; Seijo, B.; Alonso, M.J. Novel hyaluronan-based nanocarriers for transmucosal delivery of macromolecules. Macromol. Biosci. 2008, 8, 441–450. [Google Scholar] [CrossRef]
- de la Fuente, M.; Seijo, B.; Alonso, M.J. Novel hyaluronic acid-chitosan nanoparticles for ocular gene therapy. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2016–2024. [Google Scholar] [CrossRef]
- de la Fuente, M.; Seijo, B.; Alonso, M.J. Design of novel polysaccharidic nanostructures for gene delivery. Nanotechnology 2008, 19. [Google Scholar] [CrossRef]
- Lallana, E.; Rios De La Rosa, J.M.; Tirella, A.; Pelliccia, M.; Gennari, A.; Stratford, I.J.; Puri, S.; Ashford, M.; Tirelli, N. Chitosan/hyaluronic acid nanoparticles: Rational design revisited for RNA delivery. Mol. Pharm. 2017, 14, 2422–2436. [Google Scholar] [CrossRef]
- Sacco, P.; Decleva, E.; Tentor, F.; Menegazzi, R.; Borgogna, M.; Paoletti, S.; Kristiansen, K.A.; Vårum, K.M.; Marsich, E. Butyrate-loaded chitosan/hyaluronan nanoparticles: A suitable tool for sustained inhibition of ROS release by activated neutrophils. Macromol. Biosci. 2017, 17. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int. J. Biol. Macromol. 2014, 64, 353–367. [Google Scholar] [CrossRef]
- Oyarzun-Ampuero, F.A.; Brea, J.; Loza, M.I.; Torres, D.; Alonso, M.J. Chitosan-hyaluronic acid nanoparticles loaded with heparin for the treatment of asthma. Int. J. Pharm. 2009, 381, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Vecchies, F.; Sacco, P.; Decleva, E.; Menegazzi, R.; Porrelli, D.; Donati, I.; Turco, G.; Paoletti, S.; Marsich, E. Complex coacervates between a lactose-modified chitosan and hyaluronic acid as radical-scavenging drug carriers. Biomacromolecules 2018, 19, 3936–3944. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Delair, T. Stabilization of chitosan/hyaluronan colloidal polyelectrolyte complexes in physiological conditions. Carbohydr. Polym. 2015, 119, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cai, Y.; Lapitsky, Y. Factors affecting the stability of chitosan/tripolyphosphate micro- and nanogels: Resolving the opposing findings. J. Mater. Chem. B 2015, 3, 5957–5970. [Google Scholar] [CrossRef]
- Goycoolea, F.M.; Brunel, F.; Gueddari, N.E.; El Coggiola, A.; Lollo, G.; Moerschbacher, B.M.; Remunan-Lopez, C.; Delair, T.; Domard, A.; Alonso, M.J. Physical properties and stability of soft gelled chitosan-based nanoparticles. Macromol. Biosci. 2016, 1–10. [Google Scholar] [CrossRef]
- Furlani, F.; Sacco, P.; Decleva, E.; Menegazzi, R.; Donati, I.; Paoletti, S.; Marsich, E. Chitosan acetylation degree influences the physical properties of polysaccharide nanoparticles: Implication for the innate immune cells response. ACS Appl. Mater. Interfaces 2019, 11, 9794–9803. [Google Scholar] [CrossRef]
- Berth, G.; Dautzenberg, H. The degree of acetylation of chitosans and its effect on the chain conformation in aqueous solution. Carbohydr. Polym. 2002, 47, 39–51. [Google Scholar] [CrossRef]
- Vårum, K.M.; Anthonsen, M.W.; Grasdalen, H.; Smidsrød, O. Determination of the degree of N-acetylation and the distribution of N-acetyl groups in partially N-deacetylated chitins (chitosans) by high-field n.m.r. spectroscopy. Carbohydr. Res. 1991, 211, 17–23. [Google Scholar] [CrossRef]
- Parajó, Y.; D’Angelo, I.; Welle, A.; Garcia-Fuentes, M.; Alonso, M.J. Hyaluronic acid/Chitosan nanoparticles as delivery vehicles for VEGF and PDGF-BB. Drug Deliv. 2010, 17, 596–604. [Google Scholar] [CrossRef]
- Amenitsch, H.; Rappolt, M.; Kriechbaum, M.; Mio, H.; Laggner, P.; Bernstorff, S. First performance assessment of the small-angle X-ray scattering beamline at ELETTRA. J. Synchrotron Radiat. 1998, 5, 506–508. [Google Scholar] [CrossRef]
- Hammersley, A.P.; Svensson, S.O.; Hanfland, M.; Fitch, A.N.; Hausermann, D. Two-dimensional detector software: From real detector to idealised image or two-theta scan. High Press. Res. 1996, 14, 235–248. [Google Scholar] [CrossRef]
- Costalat, M.; Alcouffe, P.; David, L.; Delair, T. Controlling the complexation of polysaccharides into multi-functional colloidal assemblies for nanomedicine. J. Colloid Interface Sci. 2014, 430, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Lalevée, G.; Sudre, G.; Montembault, A.; Meadows, J.; Malaise, S.; Crépet, A.; David, L.; Delair, T. Polyelectrolyte complexes via desalting mixtures of hyaluronic acid and chitosan—Physicochemical study and structural analysis. Carbohydr. Polym. 2016, 154, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Tallian, C.; Herrero-Rollett, A.; Stadler, K.; Vielnascher, R.; Wieland, K.; Weihs, A.M.; Pellis, A.; Teuschl, A.H.; Lendl, B.; Amenitsch, H.; et al. Structural insights into pH-responsive drug release of self-assembling human serum albumin-silk fibroin nanocapsules. Eur. J. Pharm. Biopharm. 2018, 133, 176–187. [Google Scholar] [CrossRef]
- Beaucage, G. Small-angle scattering from polymeric mass fractals of arbitrary mass-fractal dimension. J. Appl. Crystallogr. 1996, 29, 134–146. [Google Scholar] [CrossRef]
- Ben Messaoud, G.; Promeneur, L.; Brennich, M.; Roelants Sophie, L.K.W.; Le Griel, P.; Baccile, N. Complex coacervation of natural sophorolipid bolaamphiphile micelles with cationic polyelectrolytes. Green Chem. 2018, 20, 3371–3385. [Google Scholar] [CrossRef]
| FA | ||||
|---|---|---|---|---|
| 0.63 | 300 | 90,000 | 201 | 448 |
| 550 | 170,000 | 846 | ||
| 950 | 310,000 | 1542 | ||
| 0.46 | 340 | 100,000 | 200 | 500 |
| 650 | 210,000 | 1050 | ||
| 920 | 300,000 | 1500 | ||
| 0.16 | 110 | 30,000 | 198 | 152 |
| 681 | 220,000 | 1111 | ||
| 1026 | 340,000 | 1717 |
| FA | Hydrodynamic Diameter (nm) | PDI | -Potential (mV) | Notes | |
|---|---|---|---|---|---|
| 0.16 | 30,000 | 180 ± 1 | 0.12 ± 0.01 | 29 ± 2 | no aggregation |
| 220,000 | 200 ± 4 | 0.21 ± 0.01 | 32 ± 1 | no aggregation | |
| 340,000 | 215 ± 3 | 0.21 ± 0.01 | 37 ± 1 | no aggregation | |
| 0.46 | 100,000 | 254 ± 27 | 0.20 ± 0.07 | −19 ± 2 | no aggregation |
| 210,000 | 219 ± 4 | 0.20 ± 0.01 | −14 ± 1 | limited aggregation | |
| 300,000 | 253 ± 6 | 0.18 ± 0.16 | −28 ± 3 | no aggregation | |
| 0.63 | 90,000 | 227 ± 1 | 0.17 ± 0.02 | −28 ± 2 | no aggregation |
| 170,000 | 238 ± 1 | 0.19 ± 0.02 | −25 ± 3 | no aggregation | |
| 310,000 | 253 ± 3 | 0.20 ± 0.03 | −23 ± 1 | limited aggregation |
| FA | Hydrodynamic Diameter (nm) | PDI | Notes | |
|---|---|---|---|---|
| 0.16 | 30,000 | [270 ± 42] (*) | [0.40 ± 0.03] (*) | unstable |
| 220,000 | 773 ± 21 | 0.08 ± 0.07 | stable | |
| 340,000 | [1307 ± 461] (*) | [0.35 ± 0.36] (*) | unstable | |
| 0.46 | 100,000 | [194 ± 38] (*) | [0.49 ± 0.23] (*) | unstable |
| 210,000 | [982 ± 743] (*) | [0.76 ± 0.28] (*) | unstable | |
| 300,000 | [213 ± 49] (*) | [0.32 ± 0.06] (*) | unstable | |
| 0.63 | 90,000 | [1060 ± 823] (*) | [0.82 ± 0.20] (*) | unstable |
| 170,000 | [1037 ± 21] (*) | [0.38 ± 0.04] (*) | unstable | |
| 310,000 | [103 ± 24] (*) | [0.66 ± 0.02] (*) | unstable |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furlani, F.; Parisse, P.; Sacco, P. On the Formation and Stability of Chitosan/Hyaluronan-Based Complex Coacervates. Molecules 2020, 25, 1071. https://doi.org/10.3390/molecules25051071
Furlani F, Parisse P, Sacco P. On the Formation and Stability of Chitosan/Hyaluronan-Based Complex Coacervates. Molecules. 2020; 25(5):1071. https://doi.org/10.3390/molecules25051071
Chicago/Turabian StyleFurlani, Franco, Pietro Parisse, and Pasquale Sacco. 2020. "On the Formation and Stability of Chitosan/Hyaluronan-Based Complex Coacervates" Molecules 25, no. 5: 1071. https://doi.org/10.3390/molecules25051071
APA StyleFurlani, F., Parisse, P., & Sacco, P. (2020). On the Formation and Stability of Chitosan/Hyaluronan-Based Complex Coacervates. Molecules, 25(5), 1071. https://doi.org/10.3390/molecules25051071