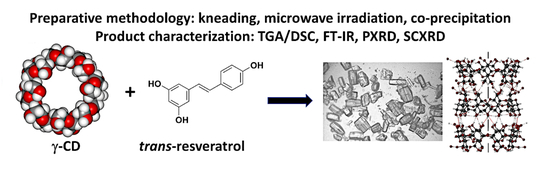
Inclusion of the Phytoalexin trans-Resveratrol in Native Cyclodextrins: A Thermal, Spectroscopic, and X-Ray Structural Study
Abstract
1. Introduction
2. Results
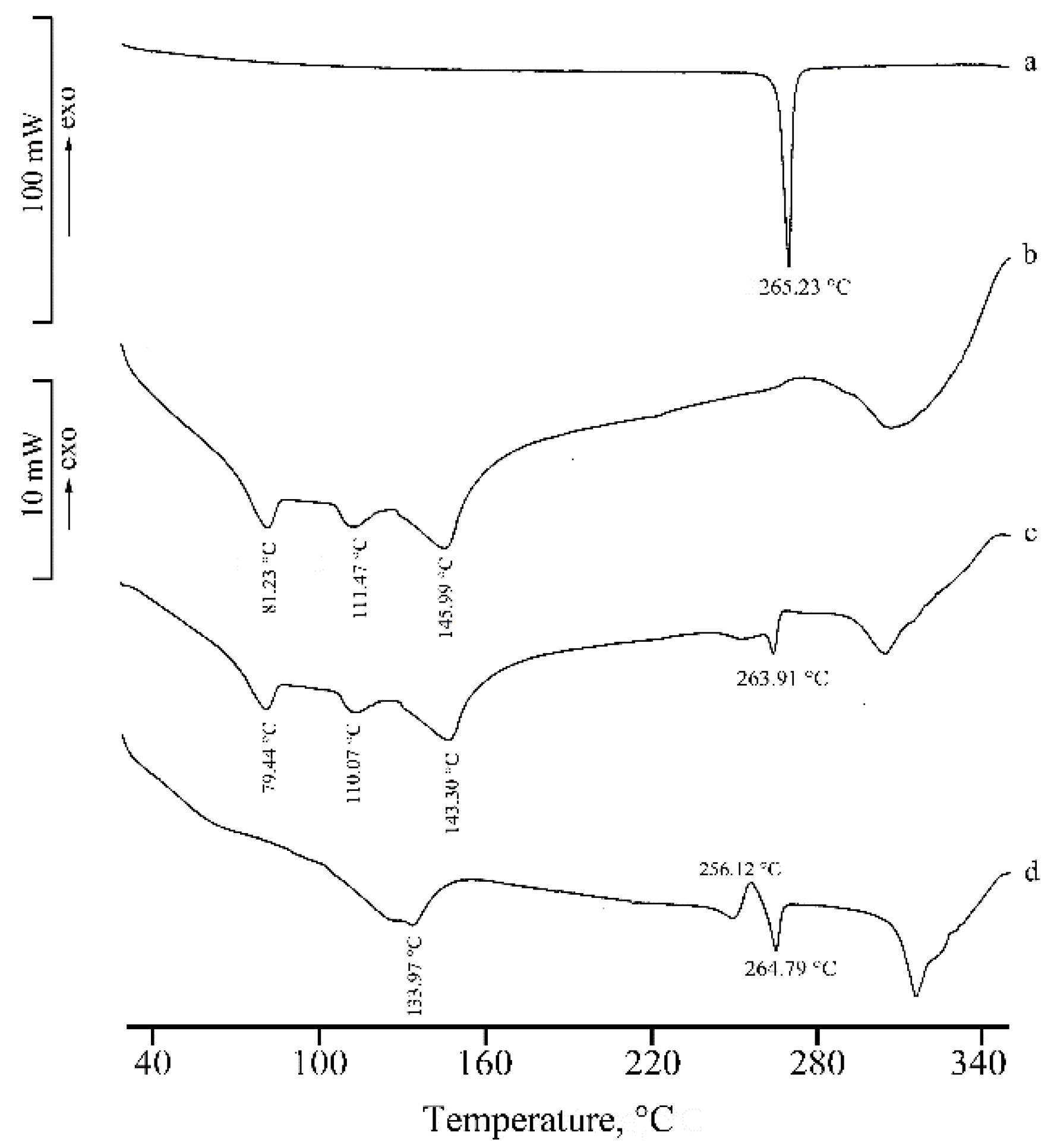
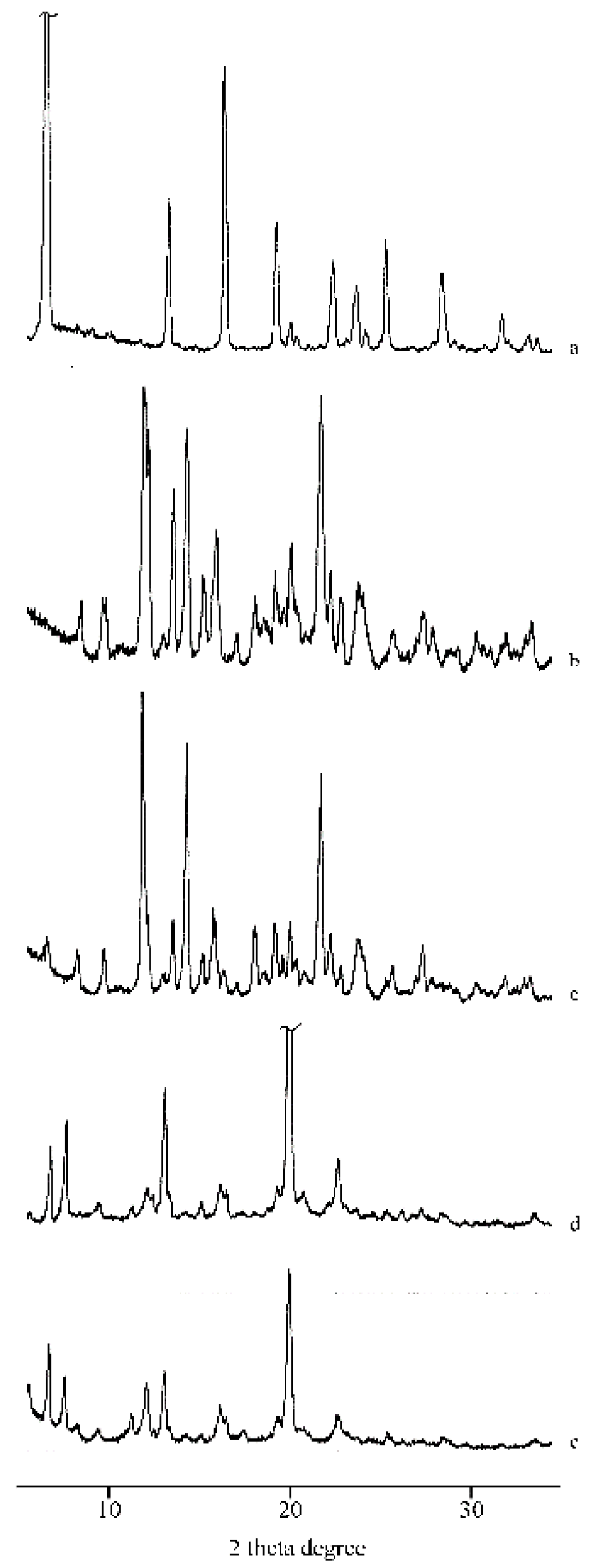
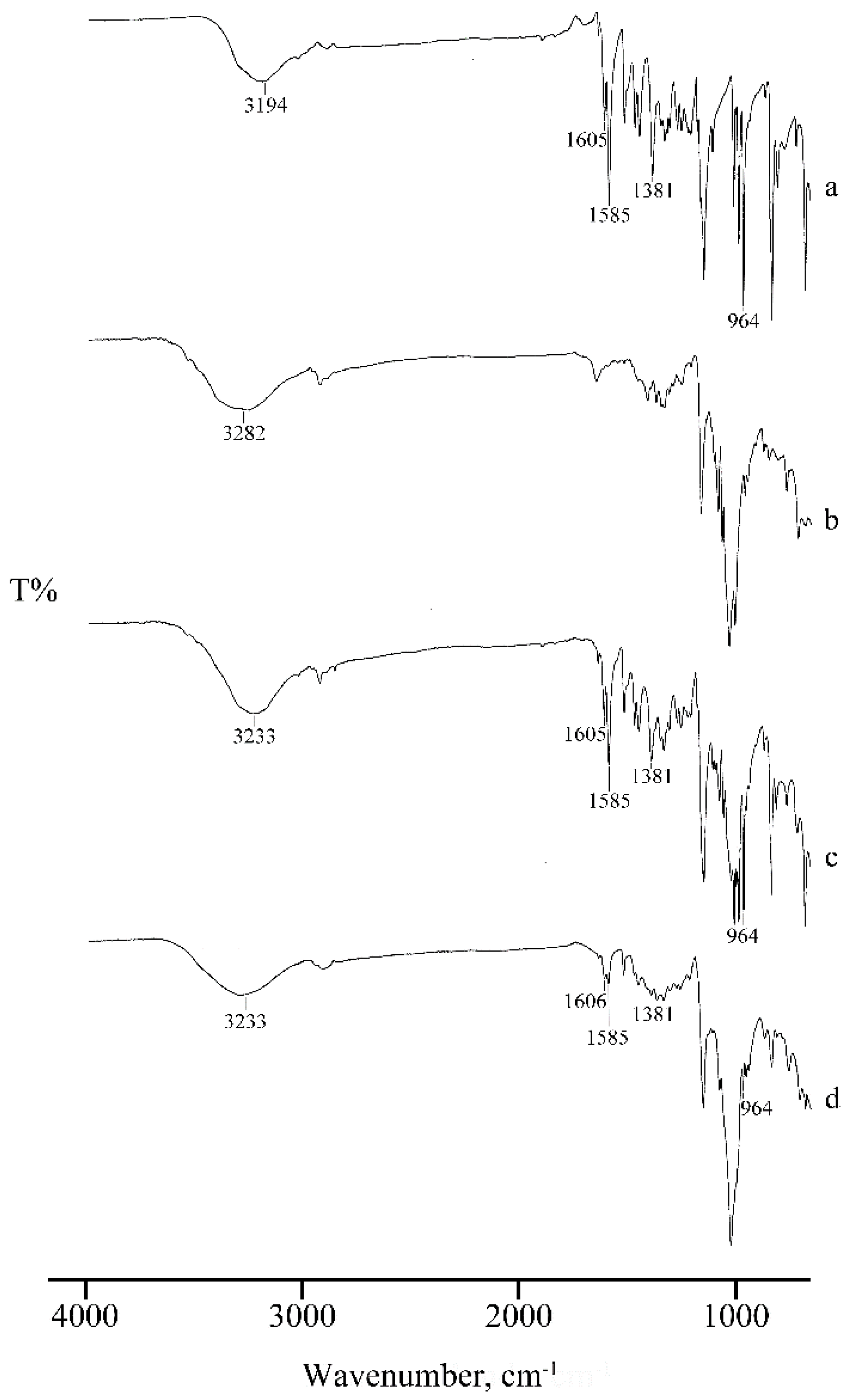
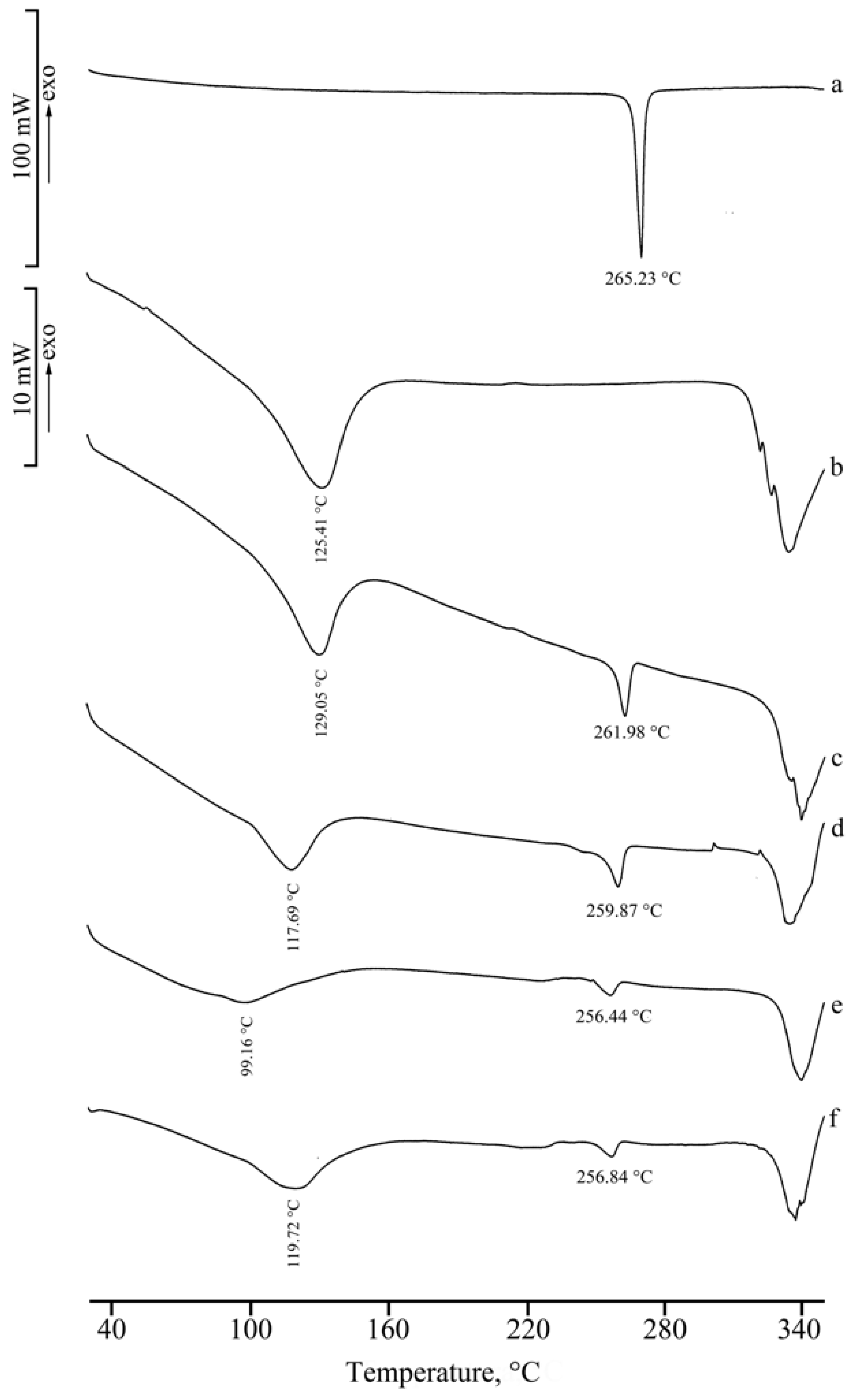
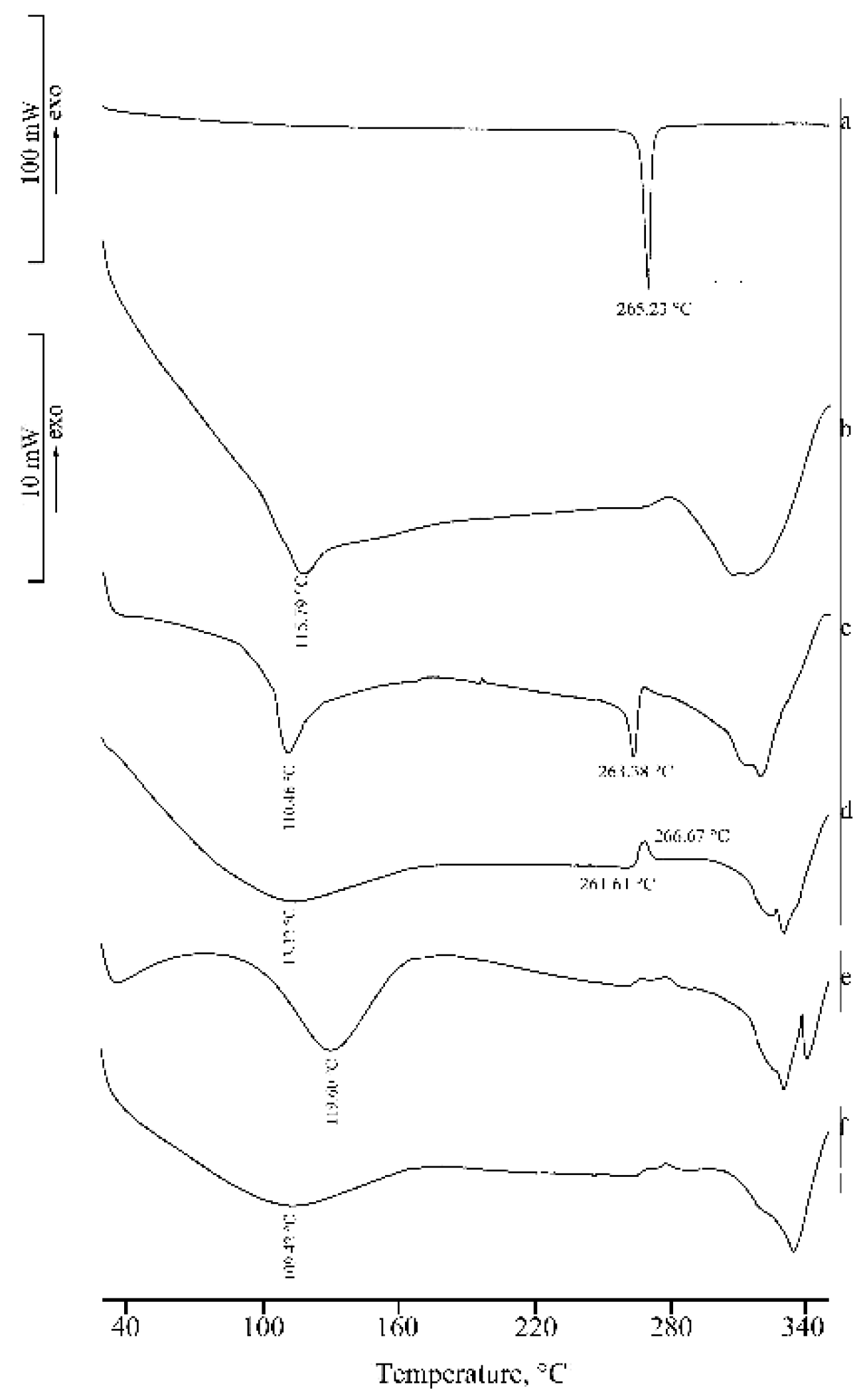
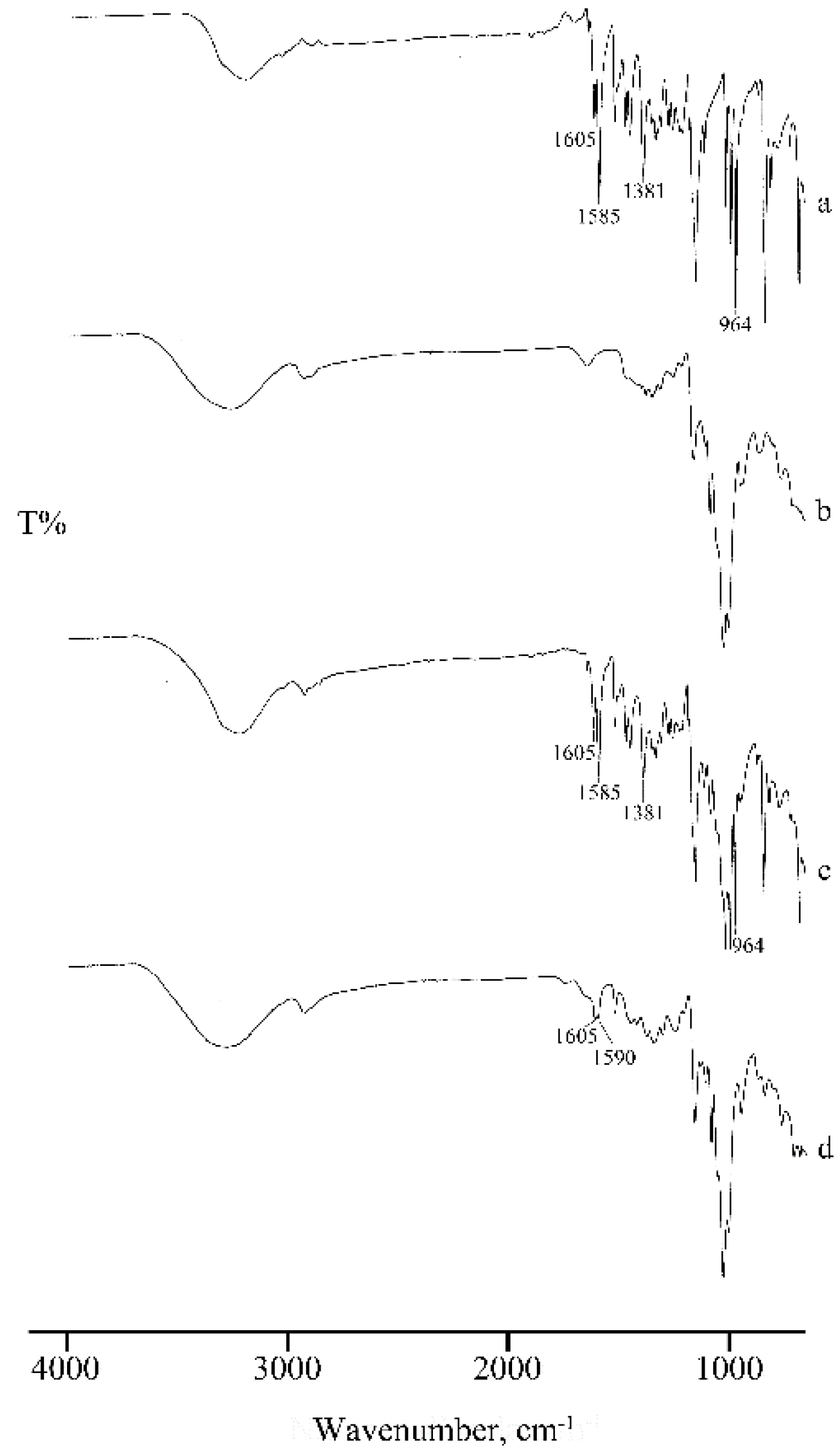
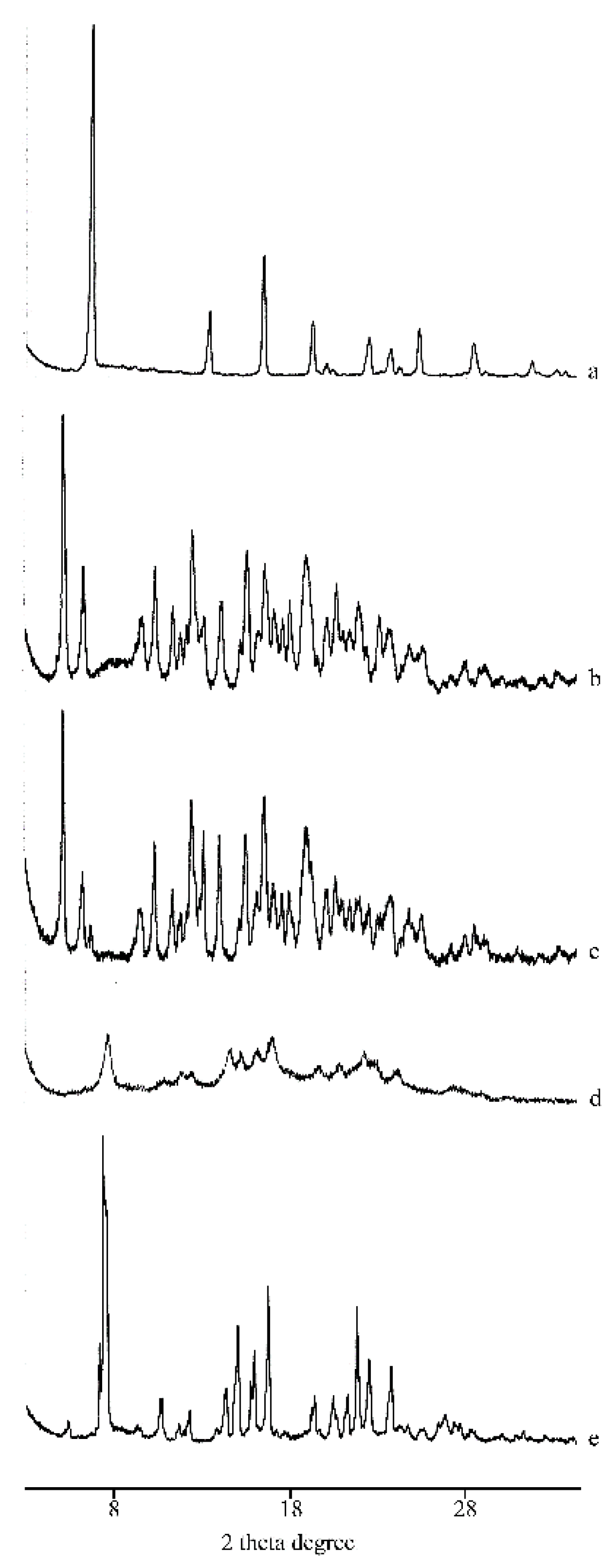
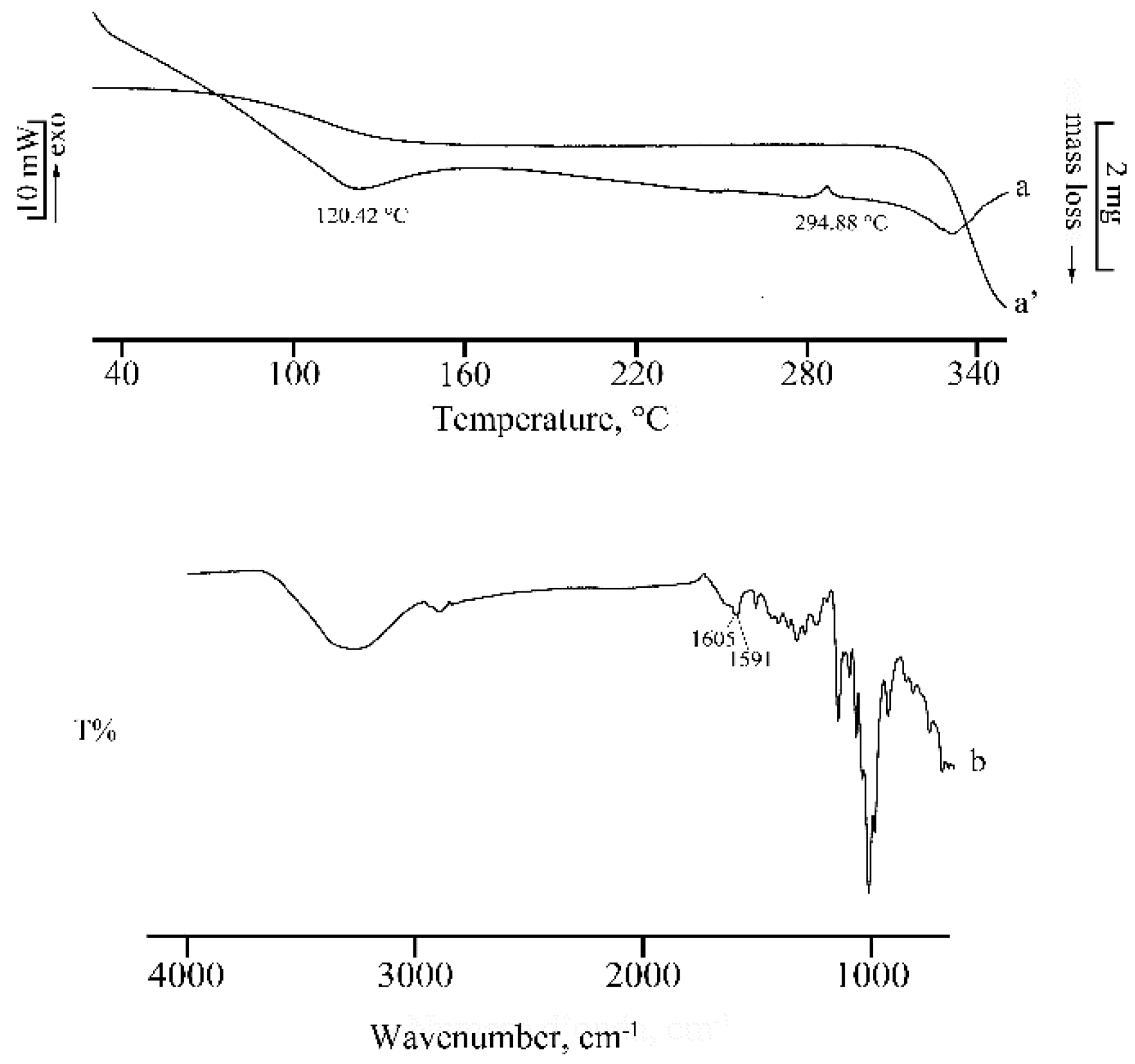
2.1. Thermal, PXRD, and FT-IR Characterization of Prepared Solid Phases
2.1.1. RSV + α-CD
2.1.2. RSV + β-CD
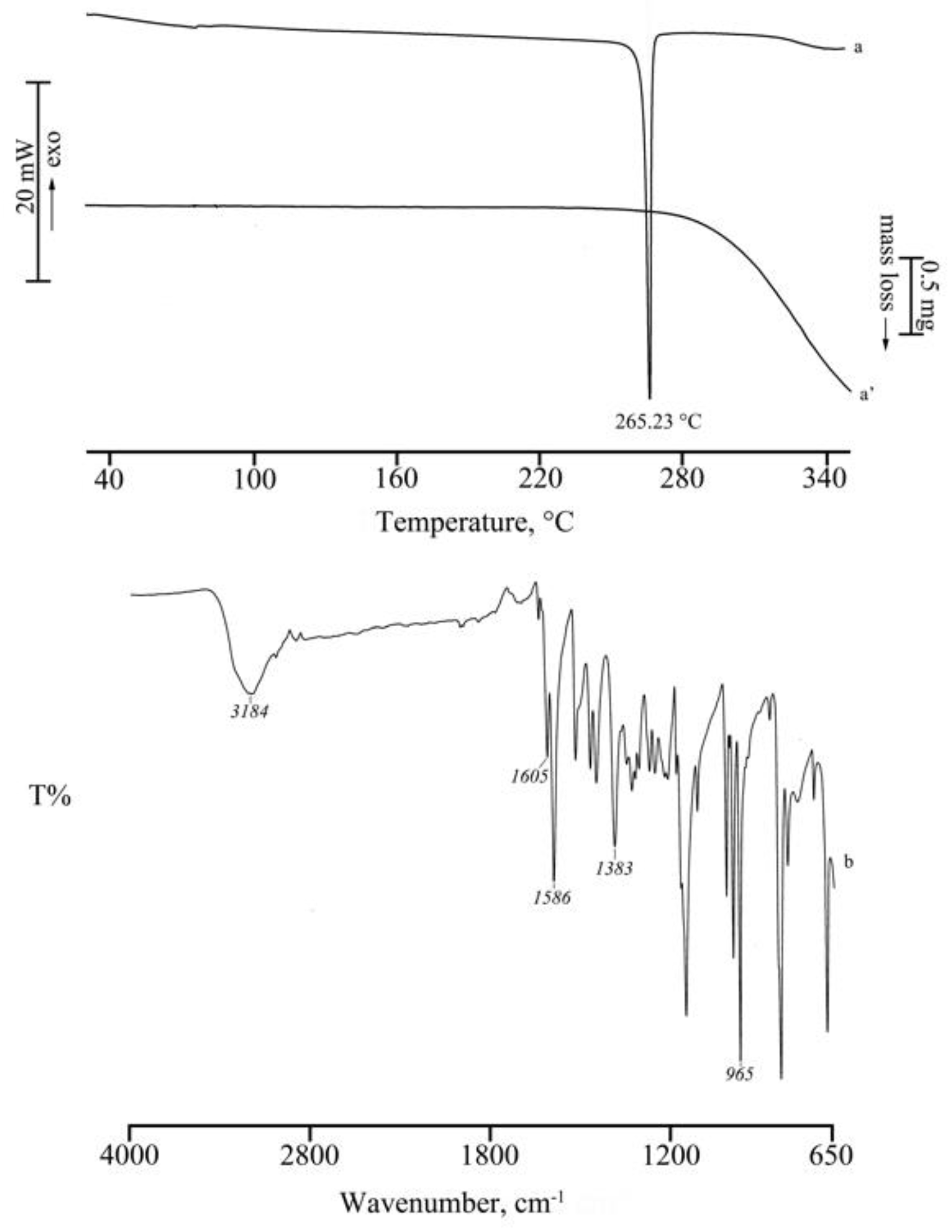
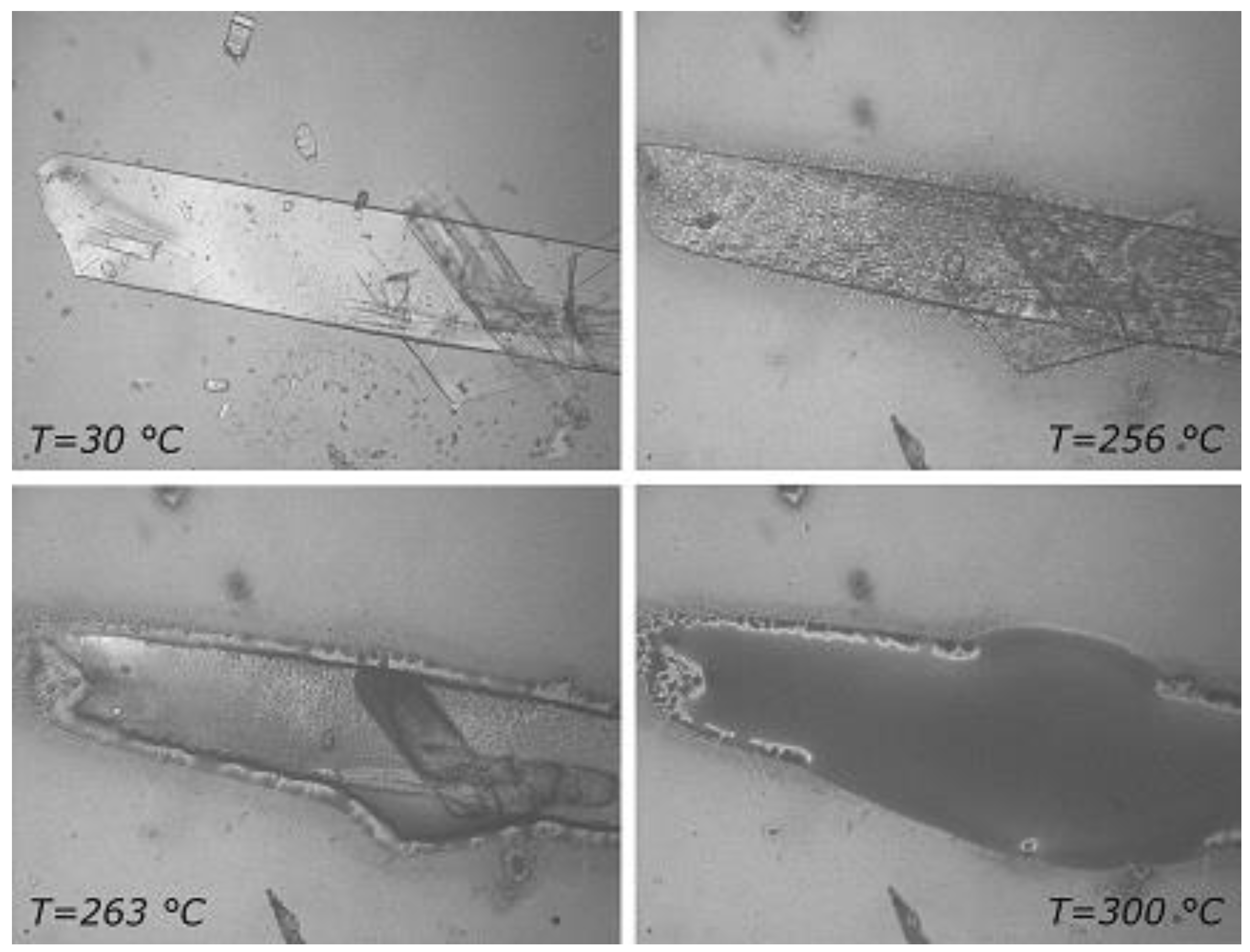
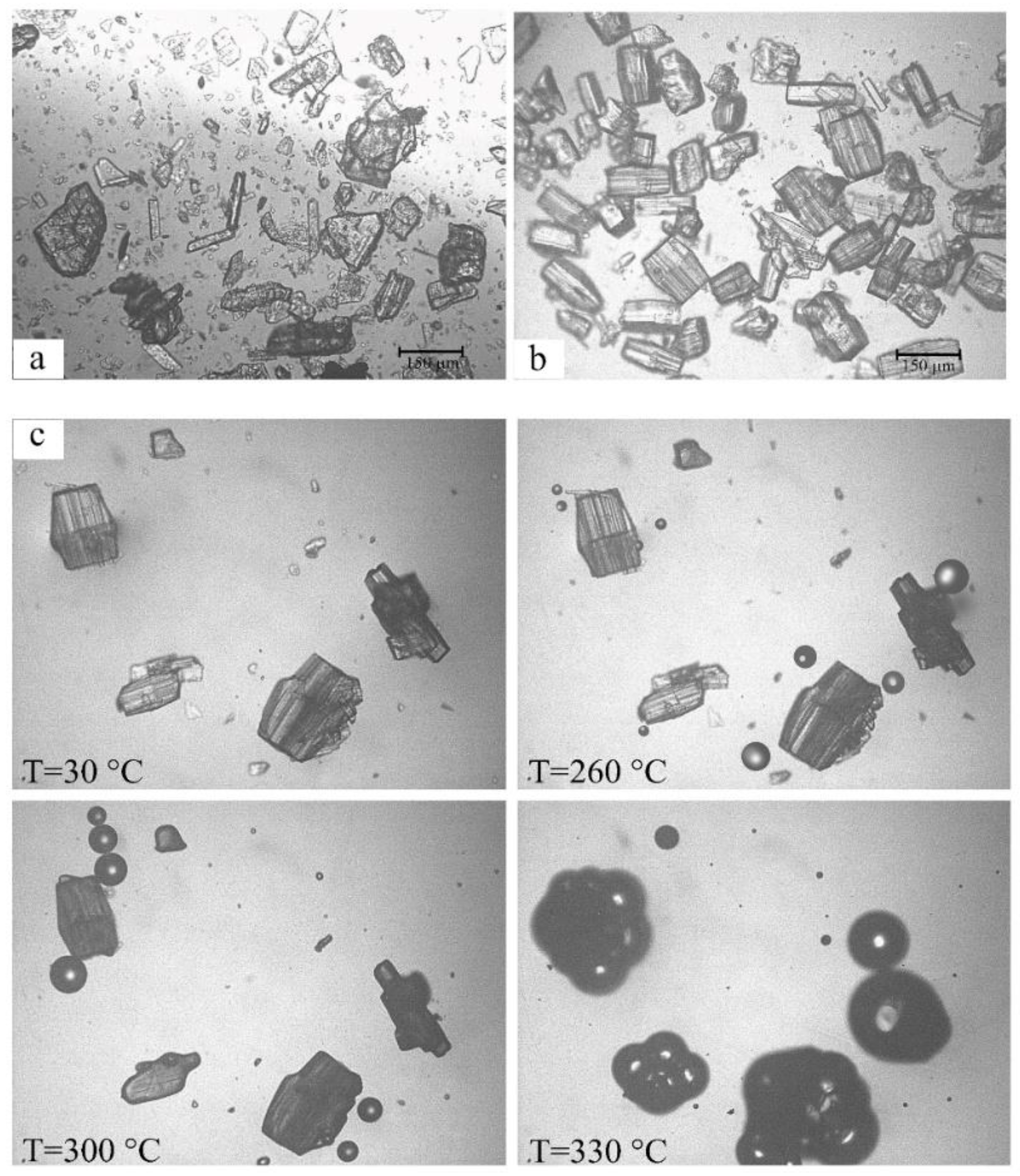
2.1.3. RSV + γ -CD
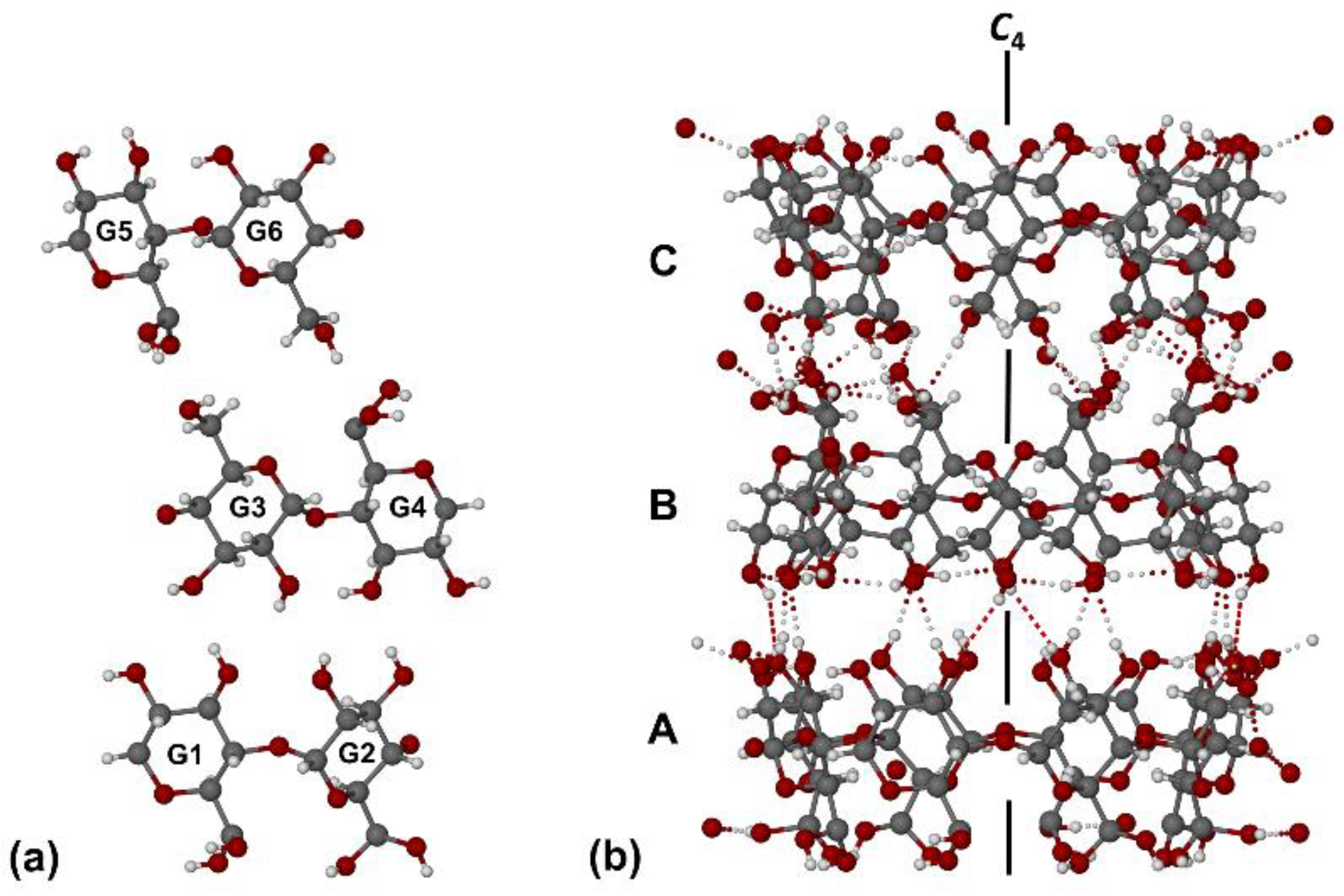
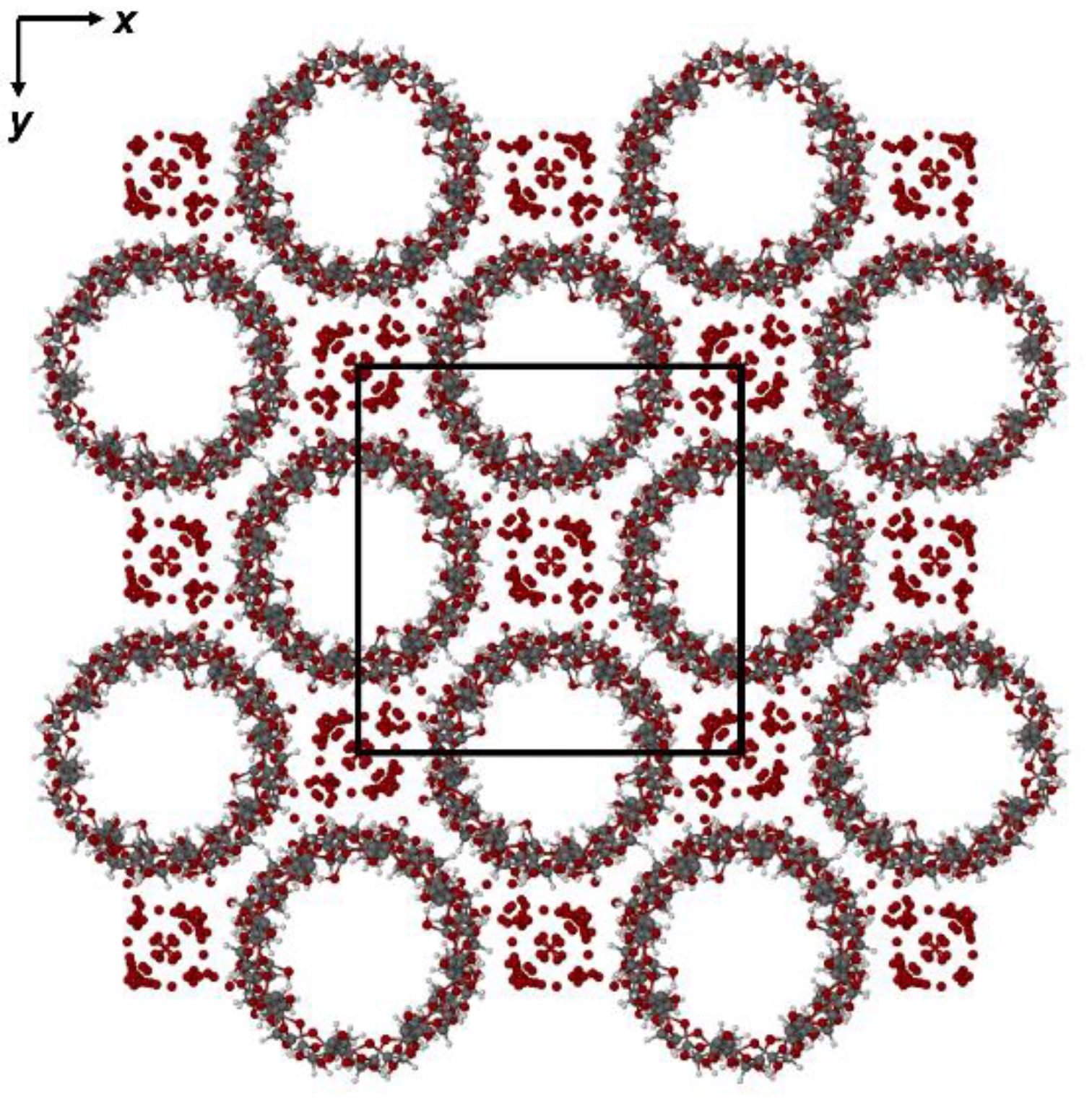
2.2. X-Ray Structural Analysis of the γ-CD·RSV Complex
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Recrystallization of RSV from Various Solvents
4.3. Preparation of the Binary Systems
4.4. Thermal and Spectroscopic Analytical Methods
4.5. X-Ray Diffraction Methods
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frémont, L. Biological effects of resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar] [CrossRef]
- Pirola, L.; Fröjdö, S. Resveratrol: One molecule, many targets. Life 2008, 6, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Nakata, R.; Takahashi, S.; Inoue, H. Recent advances in the study on resveratrol. Biol. Pharm. Bull. 2012, 35, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an anti-cancer agent: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1428–1447. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zhu, W.; Feng, W.; Lee, S.S.; Leung, A.W.; Shen, J.; Gao, L.; Xu, C. A review of resveratrol as a potent chemoprotective and synergistic agent in cancer chemotherapy. Front. Pharmacol. 2019, 9, 1534. [Google Scholar] [CrossRef] [PubMed]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef]
- Newman, A. Specialized solid form screening techniques. Org. Process Res. Dev. 2013, 17, 457–471. [Google Scholar] [CrossRef]
- Aaltonen, J.; Allesø, M.; Mirza, S.; Koradia, V.; Gordon, K.C.; Rantanen, J. Solid form screening—A review. Eur. J. Pharm. Biopharm. 2009, 71, 23–37. [Google Scholar] [CrossRef]
- Sorrenti, M.; Catenacci, L.; Cruickshank, D.L.; Caira, M.R. Lisinopril dihydrate: Single-crystal X-ray structure and physicochemical characterization of derived solid forms. J. Pharm. Sci. 2013, 102, 3596–3603. [Google Scholar] [CrossRef]
- Sorrenti, M.; Catenacci, L.; Bruni, G.; Luppi, B.; Bigucci, F.; Bettinetti, G. Solid-state characterization of tacrine hydrochloride. J. Pharm. Biomed. Anal. 2012, 63, 53–61. [Google Scholar] [CrossRef]
- Figueiras, T.S.; Neves-Petersen, M.T.; Petersen, S.B. Activation energy of light induced isomerization of resveratrol. J. Fluoresc. 2011, 21, 1897–1906. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Lucas-Abellan, C.; Fortea, I.; Lopez-Nicolas, J.M.; Nunez-Delicado, E. Cyclodextrins as resveratrol carrier system. Food Chem. 2007, 104, 39–44. [Google Scholar] [CrossRef]
- Das, S.; Lin, H.-S.; Ho, P.C.; Ng, K.-Y. The impact of aqueous solubility and dose on the pharmacokinetic profiles of resveratrol. Pharm. Res. 2008, 25, 2593–2600. [Google Scholar] [CrossRef]
- Pinho, E.; Grootveld, M.; Soares, G.; Henriques, M. Cyclodextrins as encapsulation agents for plant bioactive compounds. Carbohydr. Polym. 2014, 101, 21–135. [Google Scholar] [CrossRef]
- Loftsson, T.; Duchêne, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef]
- Rasheed, A.; Ashok Kumar, C.K.; Sravanthi, V.V.N.S.S. Cyclodextrins as drug carrier molecule: A review. Sci. Pharm. 2008, 76, 567–598. [Google Scholar] [CrossRef]
- Oberholzer, T.; Perchyonok, V.T. Cyclodextrins as oral drug carrier molecular devices: Origins, reasons and in-vitro model applications. Curr. Org. Chem. 2012, 16, 2365–2378. [Google Scholar]
- Jacob, S.; Nair, A.B. Cyclodextrin complexes: Perspective from drug delivery and formulation. Drug Dev. Res. 2018, 79, 201–217. [Google Scholar] [CrossRef]
- Ali, N.; Kaur, A.; Harikumar, S.L. Cyclodextrins: An excipient tool in drug delivery. Int. J. Res. Pharm. 2012, 3, 44–50. [Google Scholar]
- Laza-Knoerr, A.L.; Gref, R.; Couvreur, P. Cyclodextrins for drug delivery. J. Drug Target. 2010, 18, 645–656. [Google Scholar] [CrossRef]
- Zhong, L.; Bo, C.; Yeli, H.; Youhong, Z.; Guolin, Z. Complexation of resveratrol with cyclodextrins: Solubility and antioxidant activity. Food Chem. 2009, 113, 17–20. [Google Scholar]
- Bertacche, V.; Lorenzi, N.; Nava, D.; Pini, E.; Sinico, C. Host–guest interaction study of resveratrol with natural and modified cyclodextrins. J. Incl. Phenom. Macro. 2006, 55, 279–287. [Google Scholar] [CrossRef]
- Trollope, L.; Cruickshank, D.L.; Noonan, T.; Bourne, S.A.; Sorrenti, M.; Catenacci, L.; Caira, M.R. Inclusion of trans-resveratrol in methylated cyclodextrins: Synthesis and solid-state structures. Beilstein J. Org. Chem. 2014, 10, 3136–3151. [Google Scholar] [CrossRef]
- Caruso, F.; Tanski, J.; Villegas-Estrada, A.; Rossi, M. Structural basis for antioxidant activity of trans-resveratrol: Ab initio calculations and crystal and molecular structure. J. Agric. Food Chem. 2004, 52, 7279–7285. [Google Scholar] [CrossRef]
- Chadha, R.; Dureja, J.; Karan, M. Tempering trans-resveratrol to a crystal level: A contemporary approach toward polymorphic pursuits and morphological insights. Cryst. Growth Des. 2016, 16, 605–616. [Google Scholar] [CrossRef]
- Zarychta, B.; Gianopoulos, C.G.; Pinkerton, A.A. Revised structure of trans-resveratrol: Implications for its proposed mechanism. Biorg. Med. Chem. Lett. 2016, 26, 1416–1418. [Google Scholar] [CrossRef]
- Fronczek, F.R. CSD Communication (Private Communication); Cambridge Structural Database: Cambridge, UK, 2018; CCDC 1834256. [Google Scholar]
- Fronczek, F.R. CSD Communication (Private Communication); Cambridge Structural Database: Cambridge, UK, 2018; CCDC 1834257. [Google Scholar]
- Caira, M.R. On the isostructurality of cyclodextrin inclusion complexes and its practical utility. Rev. Roum. Chim. 2001, 46, 371–386. [Google Scholar]
- Kamitori, S.; Hirotsu, K.; Higuchi, T. Crystal and molecular structure of the γ-cyclodextrin–12-crown-4 1:1 inclusion complex. J. Chem. Soc., Chem.Commun. 1986, 9, 690–691. [Google Scholar] [CrossRef]
- Yvon, K.; Jeitschko, W.; Parthe, E. Lazy pulverix, a computer program, for calculating X-ray and neutron diffraction powder patterns. J. Appl. Crystallogr. 1997, 10, 73–74. [Google Scholar] [CrossRef]
- Spek, A.L. Platon squeeze: A tool for the calculation of the disordered solvent contribution to the structure factors. Acta Cryst. 2015, C71, 9–18. [Google Scholar]
- Spek, A.L. Structure validation in chemical crystallography. Acta Cryst. 2009, D65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Martin Del Valle, E.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Program MestreNova; Version 5.1.1-092; Mestrelab Research S.L.: Santiago de Compostela, Spain, 2007.
- Otwinowski, Z.; Minor, W. Processing of X-Ray Diffraction Data Collected in Oscillation Mode. In Methods in Enzymology; Sweet, R.M., Ed.; Academic Press: New York, NY, USA, 1997; Volume 276, pp. 307–326. [Google Scholar]
- Sheldrick, G.M. A short history of shelx. Acta Crystallogr. Sect. A: Found. Crystallogr. 2008, 4, 112–122. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: No sample materials are available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catenacci, L.; Sorrenti, M.; Bonferoni, M.C.; Hunt, L.; Caira, M.R. Inclusion of the Phytoalexin trans-Resveratrol in Native Cyclodextrins: A Thermal, Spectroscopic, and X-Ray Structural Study. Molecules 2020, 25, 998. https://doi.org/10.3390/molecules25040998
Catenacci L, Sorrenti M, Bonferoni MC, Hunt L, Caira MR. Inclusion of the Phytoalexin trans-Resveratrol in Native Cyclodextrins: A Thermal, Spectroscopic, and X-Ray Structural Study. Molecules. 2020; 25(4):998. https://doi.org/10.3390/molecules25040998
Chicago/Turabian StyleCatenacci, Laura, Milena Sorrenti, Maria Cristina Bonferoni, Lee Hunt, and Mino R. Caira. 2020. "Inclusion of the Phytoalexin trans-Resveratrol in Native Cyclodextrins: A Thermal, Spectroscopic, and X-Ray Structural Study" Molecules 25, no. 4: 998. https://doi.org/10.3390/molecules25040998
APA StyleCatenacci, L., Sorrenti, M., Bonferoni, M. C., Hunt, L., & Caira, M. R. (2020). Inclusion of the Phytoalexin trans-Resveratrol in Native Cyclodextrins: A Thermal, Spectroscopic, and X-Ray Structural Study. Molecules, 25(4), 998. https://doi.org/10.3390/molecules25040998