Extraction Methods Affect the Structure of Goji (Lycium barbarum) Polysaccharides
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties and Monosaccharide Composition Analysis
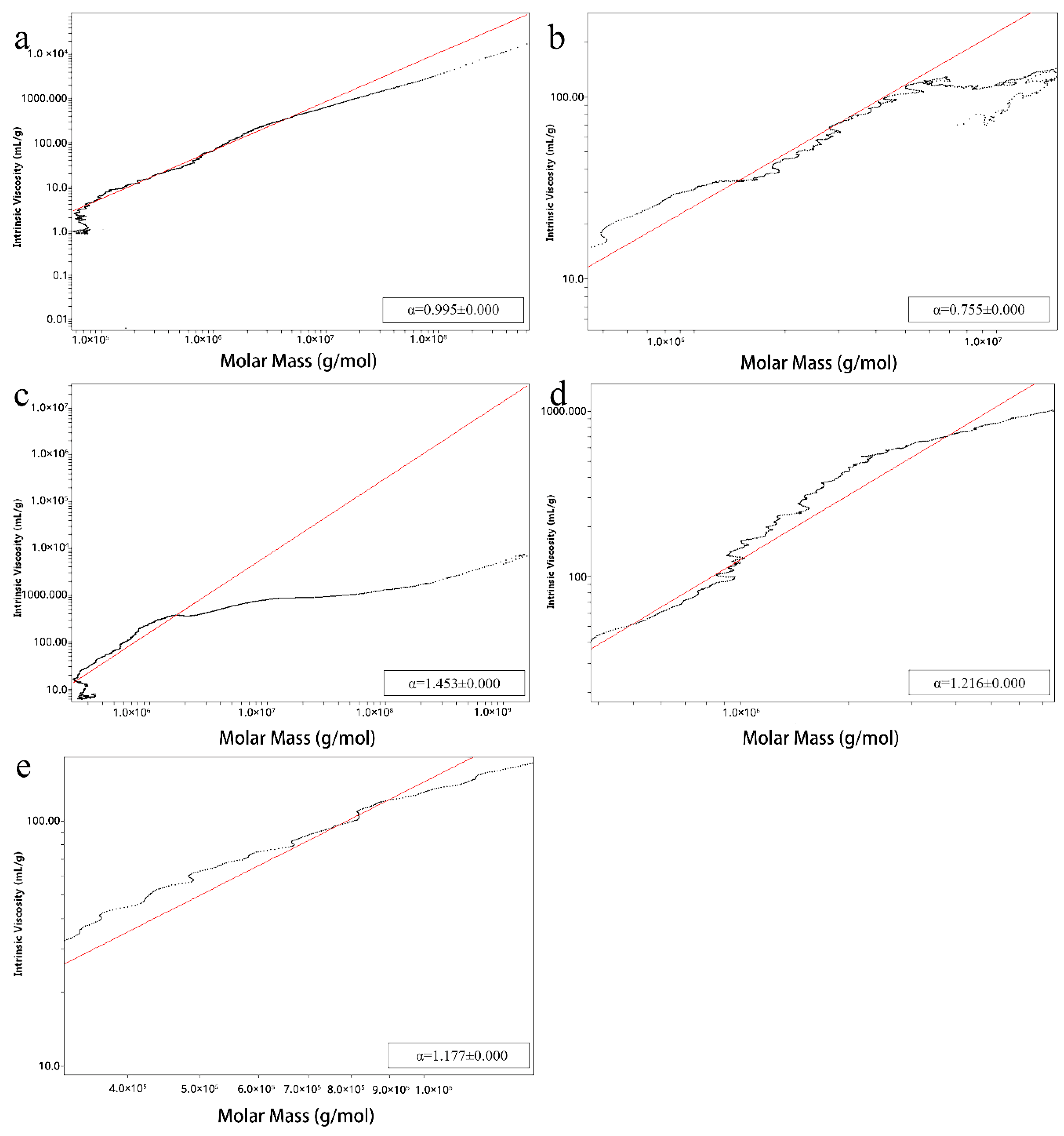
2.2. Homogeneity, Molecular Weight and Conformation of Lbps
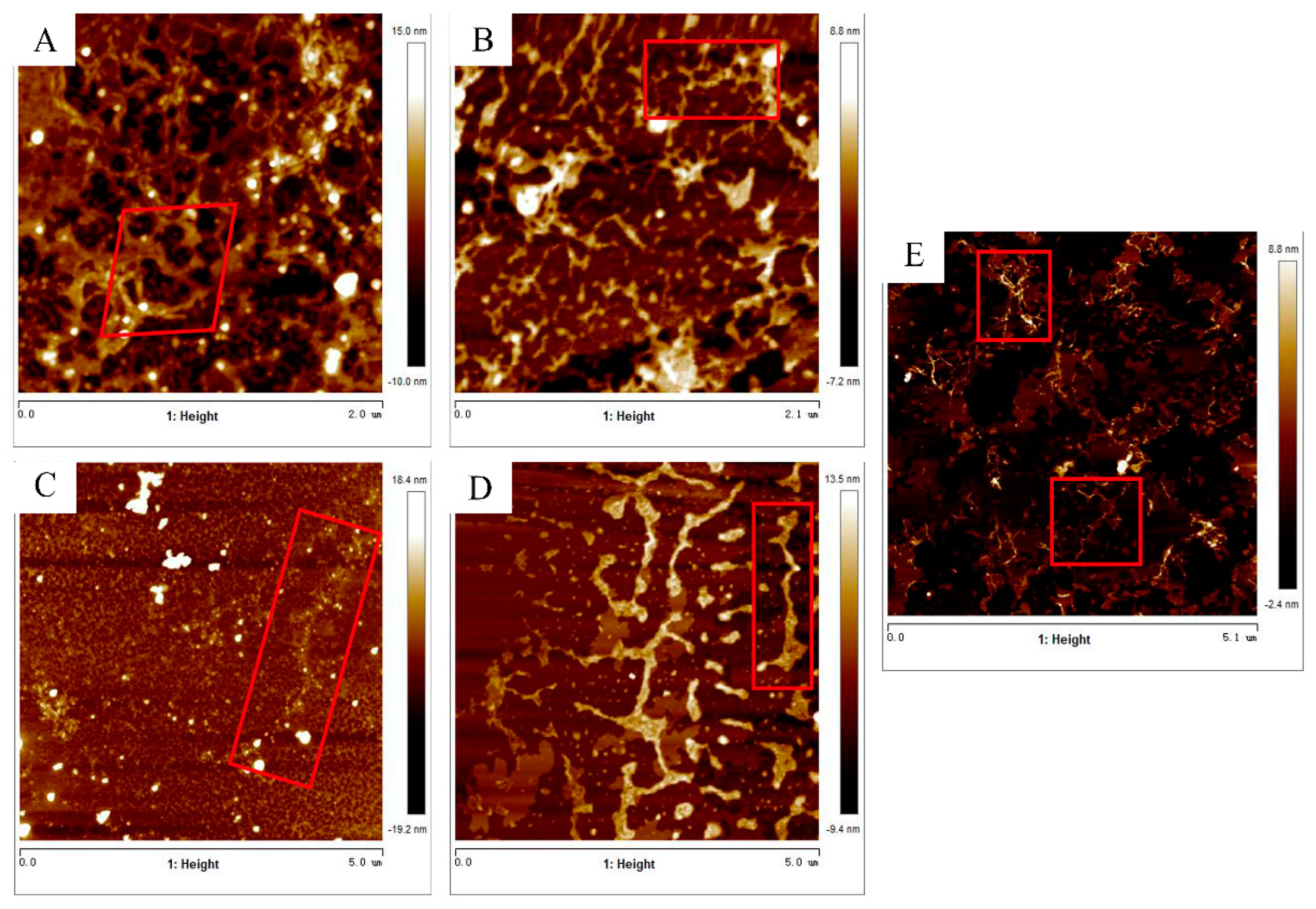
2.3. Nanostructure Analysis
2.4. FT-IR Analysis
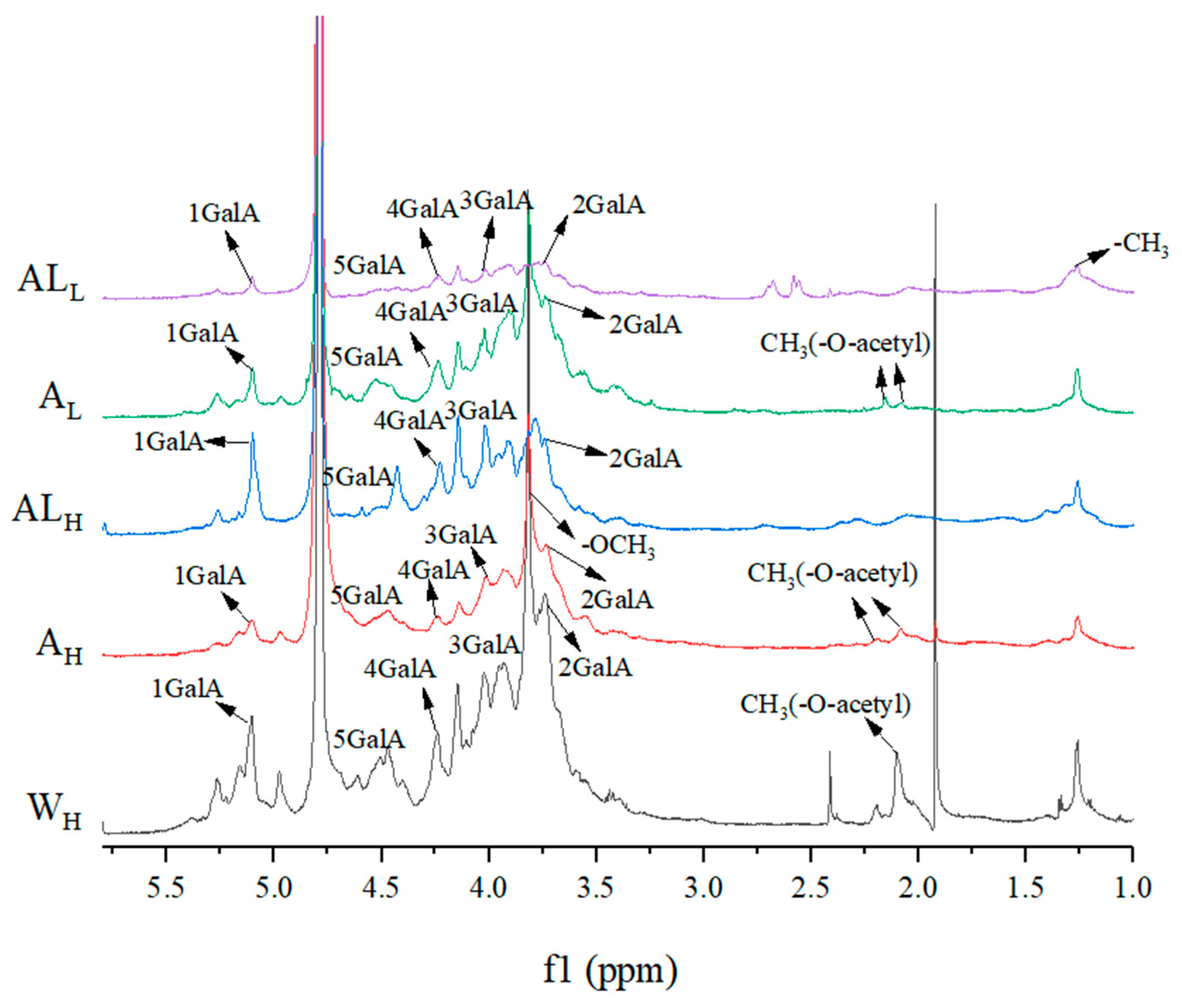
2.5. NMR Analysis
3. Materials and Methods
3.1. Materials
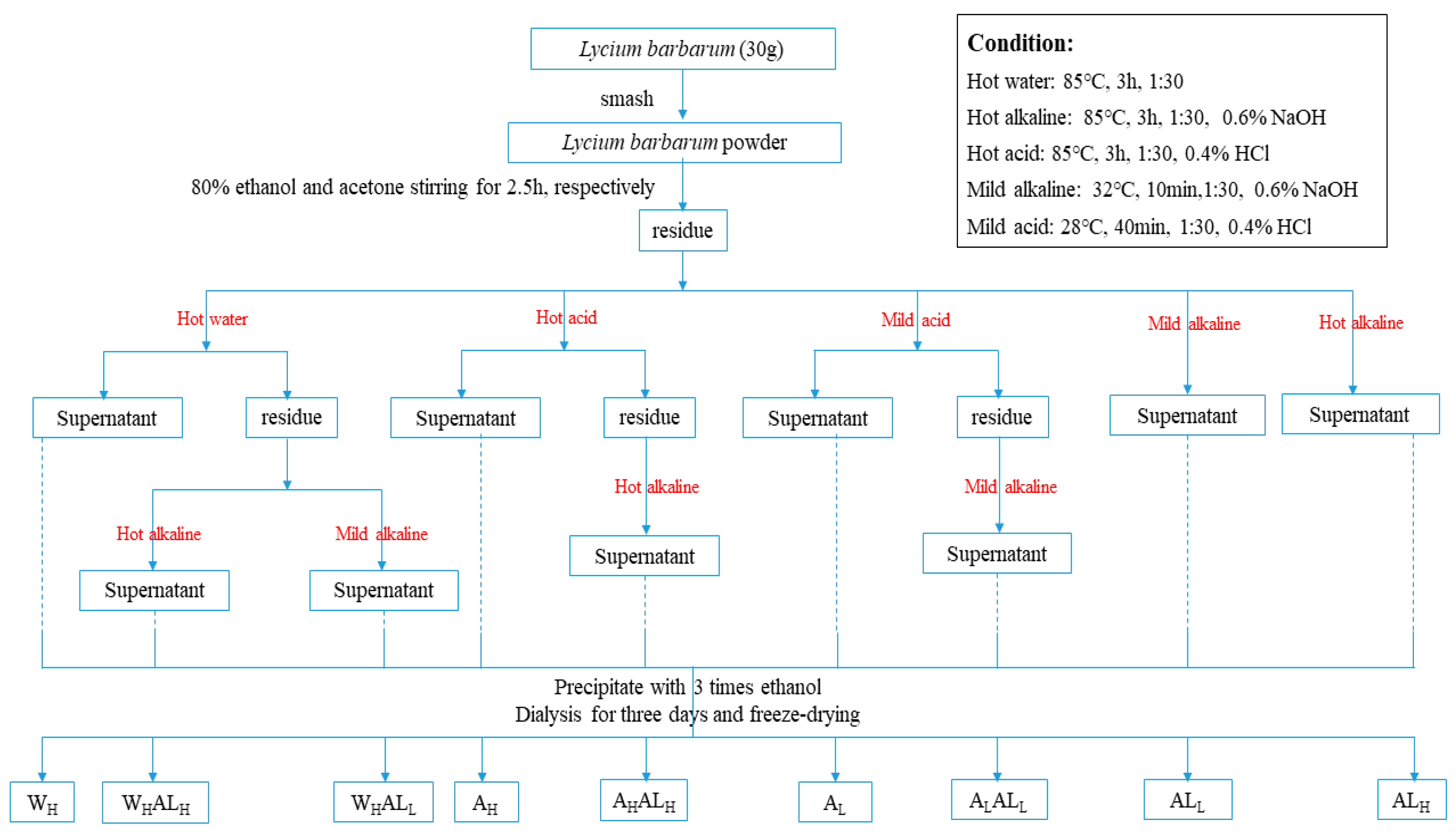
3.2. Extraction of LBPs
3.3. Determination of Total Sugar, Protein Content and Amino Acid Composition
3.4. Analysis of Monosaccharide Composition by HPLC
3.5. Determination of Molecular Weight and Conformation
3.6. Atomic Force Microscopy (AFM)
3.7. FT-IR Spectra
3.8. Nuclear Magnetic Resonance Spectroscopy (NMR)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef]
- Wu, D.M.; Zheng, J.Q.; Mao, G.Z.; Hu, W.W.; Ye, X.Q.; Linhardt, R.J.; Chen, S.G. Rethinking the impact of RG-I mainly from fruits and vegetables on dietary health. Crit. Rev. Food Sci. 2019, 2, 1–23. [Google Scholar] [CrossRef]
- Mao, G.Z.; Wu, D.M.; Wei, C.Y.; Tao, W.Y.; Ye, X.Q.; Linhardt, R.J.; Orfila, C.; Chen, S.G. Reconsidering conventional and innovative methods for pectin extraction from fruit and vegetable waste: Targeting rhamnogalacturonan I. Trends Food Sci. Tech. 2019, 94, 65–78. [Google Scholar] [CrossRef]
- Tan, L.; Eberhard, S.; Pattathil, S.; Warder, C.; Glushka, J.; Yuan, C.; Hao, Z.; Zhu, X.; Avci, U.; Miller, J.S.; et al. An Arabidopsis cell wall proteoglycan consists of pectin and arabinoxylan covalently linked to an arabinogalactan protein. Plant. Cell 2013, 25, 270–287. [Google Scholar] [CrossRef]
- Broxterman, S.E.; Schols, H.A. Interactions between pectin and cellulose in primary plant cell walls. Carbohydr. Polym. 2018, 192, 263–272. [Google Scholar] [CrossRef] [PubMed]
- May, C.D. Industrial pectins: Sources, production and applications. Carbohydr. Polym. 1990, 12, 79–99. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, M.-Y.; Nie, S.; Li, C.; Wang, Y. Purification, composition analysis and antioxidant activity of a polysaccharide from the fruiting bodies of Ganoderma atrum. Food Chem. 2008, 107, 231–241. [Google Scholar] [CrossRef]
- Lavi, I.; Friesem, D.; Geresh, S.; Hadar, Y.; Schwartz, B. An aqueous polysaccharide extract from the edible mushroom Pleurotus ostreatus induces anti-proliferative and pro-apoptotic effects on HT-29 colon cancer cells. Cancer Lett. 2006, 244, 61–70. [Google Scholar] [CrossRef]
- Li, S.P.; Zhao, K.J.; Ji, Z.N.; Song, Z.H.; Dong, T.T.X.; Lo, C.K.; Cheung, J.K.H.; Zhu, S.Q.; Tsim, K.W.K. A polysaccharide isolated from Cordyceps sinensis, a traditional Chinese medicine, protects PC12 cells against hydrogen peroxide-induced injury. Life Sci. 2003, 73, 2503–2513. [Google Scholar] [CrossRef]
- Khodaei, N.; Karboune, S. Optimization of enzymatic production of prebiotic galacto/galacto(arabino)-oligosaccharides and oligomers from potato rhamnogalacturonan I. Carbohydr. Polym. 2018, 181, 1153–1159. [Google Scholar] [CrossRef]
- Zykwinska, A.; Rondeau-Mouro, C.; Garnier, C.; Thibault, J.-F.; Ralet, M.C. Alkaline extractability of pectic arabinan and galactan and their mobility in sugar beet and potato cell walls. Carbohydr. Polym. 2006, 65, 510–520. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.; Li, J.; Yan, L.; Li, S.; Ye, X.; Liu, D.; Ding, T.; Linhardt, R.J.; Orfila, C.; et al. Extraction and characterization of RG-I enriched pectic polysaccharides from mandarin citrus peel. Food Hydrocolloid 2018, 79, 579–586. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Zheng, G. Effect of stand age on soil microbial community structure in wolfberry (Lycium barbarum L.) fields. Acta Ecol. Sin. 2017, 37, 10–17. [Google Scholar] [CrossRef]
- Cao, S.; Du, J.; Hei, Q. Lycium barbarum polysaccharide protects against neurotoxicity via the Nrf2-HO-1 pathway. Exp. Med. 2017, 14, 4919–4927. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Bao, S.; Du, Y.; Jiang, Z.; Wuliji, A.O.; Ren, X.; Zhang, C.; Chu, H.; Kong, L.; Ma, H. Antioxidant effects of Lycium barbarum polysaccharides on photoreceptor degeneration in the light-exposed mouse retina. Biomed. Pharmacother. 2018, 103, 829–837. [Google Scholar] [CrossRef]
- Shi, G.J.; Zheng, J.; Wu, J.; Qiao, H.Q.; Chang, Q.; Niu, Y.; Sun, T.; Li, Y.X.; Yu, J.Q. Beneficial effects of Lycium barbarum polysaccharide on spermatogenesis by improving antioxidant activity and inhibiting apoptosis in streptozotocin-induced diabetic male mice. Food Funct. 2017, 8, 1215–1226. [Google Scholar] [CrossRef]
- Zhang, X.R.; Zhou, W.X.; Zhang, Y.X.; Qi, C.H.; Yan, H.; Wang, Z.F.; Wang, B. Macrophages, rather than T and B cells are principal immunostimulatory target cells of Lycium barbarum L. polysaccharide LBPF4-OL. J. Ethnopharmacol. 2011, 136, 465–472. [Google Scholar] [CrossRef]
- Miao, Y.; Xiao, B.; Jiang, Z.; Guo, Y.; Mao, F.; Zhao, J.; Huang, X.; Guo, J. Growth inhibition and cell-cycle arrest of human gastric cancer cells by Lycium barbarum polysaccharide. Med. Oncol. 2010, 27, 785–790. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Q.Y.; Jiang, Q.Y.; Kang, X.M.; Zhao, L. Polysaccharides derived from Lycium barbarum suppress IGF-1-induced angiogenesis via PI3K/HIF-1α/VEGF signalling pathways in MCF-7 cells. Food Chem. 2012, 131, 1479–1484. [Google Scholar] [CrossRef]
- Ho, Y.S.; Yu, M.S.; Yang, X.F.; So, K.F.; Yuen, W.H.; Chang, R.C.C. Neuroprotective effects of polysaccharides from wolfberry, the fruits of Lycium barbarum, against homocysteine-induced toxicity in rat cortical neurons. J. Alzheimer’s Dis. 2010, 19, 813–827. [Google Scholar] [CrossRef]
- Teng, P.; Li, Y.; Cheng, W.; Zhou, L.; Shen, Y.; Wang, Y. Neuroprotective effects of Lycium barbarum polysaccharides in lipopolysaccharide-induced BV2 microglial cells. Mol. Med. Rep. 2013, 7, 1977–1981. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Chen, X.; Dang, T.; Deng, Y.; Zou, Z.; Liu, Q.; Gong, G.; Song, S.; Ma, F.-L.; Huang, L.; et al. Lycium barbarum polysaccharides extend the mean lifespan of Drosophila melanogaster. Food Funct. 2019, 10, 4231–4241. [Google Scholar] [CrossRef] [PubMed]
- Masci, A.; Carradori, S.; Casadei, M.A.; Paolicelli, P.; Petralito, S.; Ragno, R.; Cesa, S. Lycium barbarum polysaccharides: Extraction, purification, structural characterisation and evidence about hypoglycaemic and hypolipidaemic effects. A review. Food Chem. 2018, 254, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.F.; Zhao, C.; Chen, X.; Chan, S.W.; Wu, J.Y. Chemical properties and bioactivities of Goji ( Lycium barbarum ) polysaccharides extracted by different methods. J. Funct. Food 2015, 17, 903–909. [Google Scholar] [CrossRef]
- Muatasim, R.; Ma, H.; Yang, X. Effect of multimode ultrasound assisted extraction on the yield of crude polysaccharides from Lycium barbarum (Goji). Food Sci. Tech. 2018, 38, 160–166. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, S.; Liu, Y.; Wu, S.; Ran, J. Optimization of enzyme-assisted extraction of the Lycium barbarum polysaccharides using response surface methodology. Carbohydr. Polym. 2011, 86, 1089–1092. [Google Scholar] [CrossRef]
- Zhou, L.; Huang, L.; Yue, H.; Ding, K. Structure analysis of a heteropolysaccharide from fruits of Lycium barbarum L. and anti-angiogenic activity of its sulfated derivative. Int. J. Biol. Macromol. 2018, 108, 47–55. [Google Scholar] [CrossRef]
- Chang, C.Y.; Tsai, Y.R.; Chang, W.H. Models for the interactions between pectin molecules and other cell-wall constituents in vegetable tissues. Food Chem. 1993, 48, 145–157. [Google Scholar] [CrossRef]
- Methacanon, P.; Krongsin, J.; Gamonpilas, C. Pomelo (Citrus maxima) pectin: Effects of extraction parameters and its properties. Food Hydrocolloid 2014, 35, 383–391. [Google Scholar] [CrossRef]
- Oosterveld, A.; Beldman, G.; Schols, H.A.; Voragen, A.G.J. Characterization of arabinose and ferulic acid rich pectic polysaccharides and hemicelluloses from sugar beet pulp. Carbohydr. Res. 2000, 328, 185–197. [Google Scholar] [CrossRef]
- Kumar, A.; Chauhan, G.S. Extraction and characterization of pectin from apple pomace and its evaluation as lipase (steapsin) inhibitor. Carbohydr. Polym. 2010, 82, 454–459. [Google Scholar] [CrossRef]
- Bonnin, E.; Brunel, M.; Gouy, Y.; Lesage-Meessen, L.; Asther, M.; Thibault, J.F. Aspergillus niger I-1472 and Pycnoporus cinnabarinus MUCL39533, selected for the biotransformation of ferulic acid to vanillin, are also able to produce cell wall polysaccharide-degrading enzymes and feruloyl esterases. Enzym. Microb Tech. 2001, 28, 70–80. [Google Scholar] [CrossRef]
- Levigne, S.; Ralet, M.C.; Thibault, J.F. Characterisation of pectins extracted from fresh sugar beet under different conditions using an experimental design. Carbohydr. Polym. 2002, 49, 145–153. [Google Scholar] [CrossRef]
- Alba, K.; Kontogiorgos, V. Pectin at the oil-water interface: Relationship of molecular composition and structure to functionality. Food Hydrocolloid 2016, S0268005X16303149. [Google Scholar] [CrossRef]
- Bucheli, P.; Gao, Q.; Redgwell, R.; Vidal, K.; Wang, J.; Zhang, W. Biomolecular and Clinical Aspects of Chinese Wolfberry. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I., Wachtel, G.S., Eds.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Liu, W.; Liu, Y.; Zhu, R.; Yu, J.; Lu, W.; Pan, C.; Yao, W.; Gao, X. Structure characterization, chemical and enzymatic degradation, and chain conformation of an acidic polysaccharide from Lycium barbarum L. Carbohydr. Polym. 2016, 147, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Nakauma, M.; Funami, T.; Noda, S.; Ishihara, S.; Al-Assaf, S.; Nishinari, K.; Phillips, G.O. Comparison of sugar beet pectin, soybean soluble polysaccharide, and gum arabic as food emulsifiers. 1. Effect of concentration, pH, and salts on the emulsifying properties. Food Hydrocolloid 2008, 22, 1254–1267. [Google Scholar] [CrossRef]
- Zhao, R.; Qiu, B.; Li, Q.; Zhang, T.; Zhao, H.; Chen, Z.; Cai, Y.; Ruan, H.; Ge, W.; Zheng, X. LBP-4a improves insulin resistance via translocation and activation of GLUT4 in OLETF rats. Food Funct. 2014, 5, 811–820. [Google Scholar] [CrossRef]
- Redgwell, R.J.; Curti, D.; Wang, J.; Dobruchowska, J.M.; Gerwig, G.J.; Kamerling, J.P.; Bucheli, P. Cell wall polysaccharides of Chinese Wolfberry (Lycium barbarum): Part 1. Characterisation of soluble and insoluble polymer fractions. Carbohydr. Polym. 2011, 84, 1344–1349. [Google Scholar] [CrossRef]
- Burchard, W. Solution properties of branched macromolecules. In Advances in Polymer Science; Roovers, J., Ed.; Springer: Berlin, Germany, 1999. [Google Scholar]
- Chatjigakis, A.K.; Pappas, C.; Proxenia, N.; Kalantzi, O.; Rodis, P.; Polissiou, M. FT-IR spectroscopic determination of the degree of esterification of cell wall pectins from stored peaches and correlation to textural changes. Carbohydr. Polym. 1998, 37, 395–408. [Google Scholar] [CrossRef]
- Ravanat, G.; Rinaudo, M. Investigation on oligo - and polygalacturonic acids by potentiometry and circular dichroism. Biopolymers 1980, 19, 2209–2222. [Google Scholar] [CrossRef]
- Manrique, G.D.; Lajolo, F.M. FT-IR spectroscopy as a tool for measuring degree of methyl esterification in pectins isolated from ripening papaya fruit. Postharvest Biol. Technol. 2002, 25, 99–107. [Google Scholar] [CrossRef]
- Yapo, B.M.; Lerouge, P.; Thibault, J.-F.; Ralet, M.-C. Pectins from citrus peel cell walls contain homogalacturonans homogenous with respect to molar mass, rhamnogalacturonan I and rhamnogalacturonan II. Carbohydr. Polym. 2007, 69, 426–435. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, Y.B.; Jiang, Y.; Prasad, K.N.; Yang, J.; Qu, H.; Wang, Y.; Jia, Y.; Mo, H.; Yang, B. Structure identification of a polysaccharide purified from Lycium barbarium fruit. Int J. Biol. Macromol. 2015, 82, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Zhang, S.H.; Liu, Q.; Xu, H.B. A polysaccharide-protein complex from Lycium barbarum upregulates cytokine expression in human peripheral blood mononuclear cells. Eur. J. Pharm. 2003, 471, 217–222. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Chem 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Strydom, D.J. Chromatographic separation of 1-phenyl-3-methyl-5-pyrazolone-derivatized neutral, acidic and basic aldoses. J. Chromatogr. A 1994, 678, 17–23. [Google Scholar] [CrossRef]
- Arakawa, T.; Wen, J. Size-exclusion chromatography with on-line light scattering. In Current Protocols in Protein Science; John, E., Ed.; Wiely Board: Thousand Oaks, CA, USA, 2001. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |
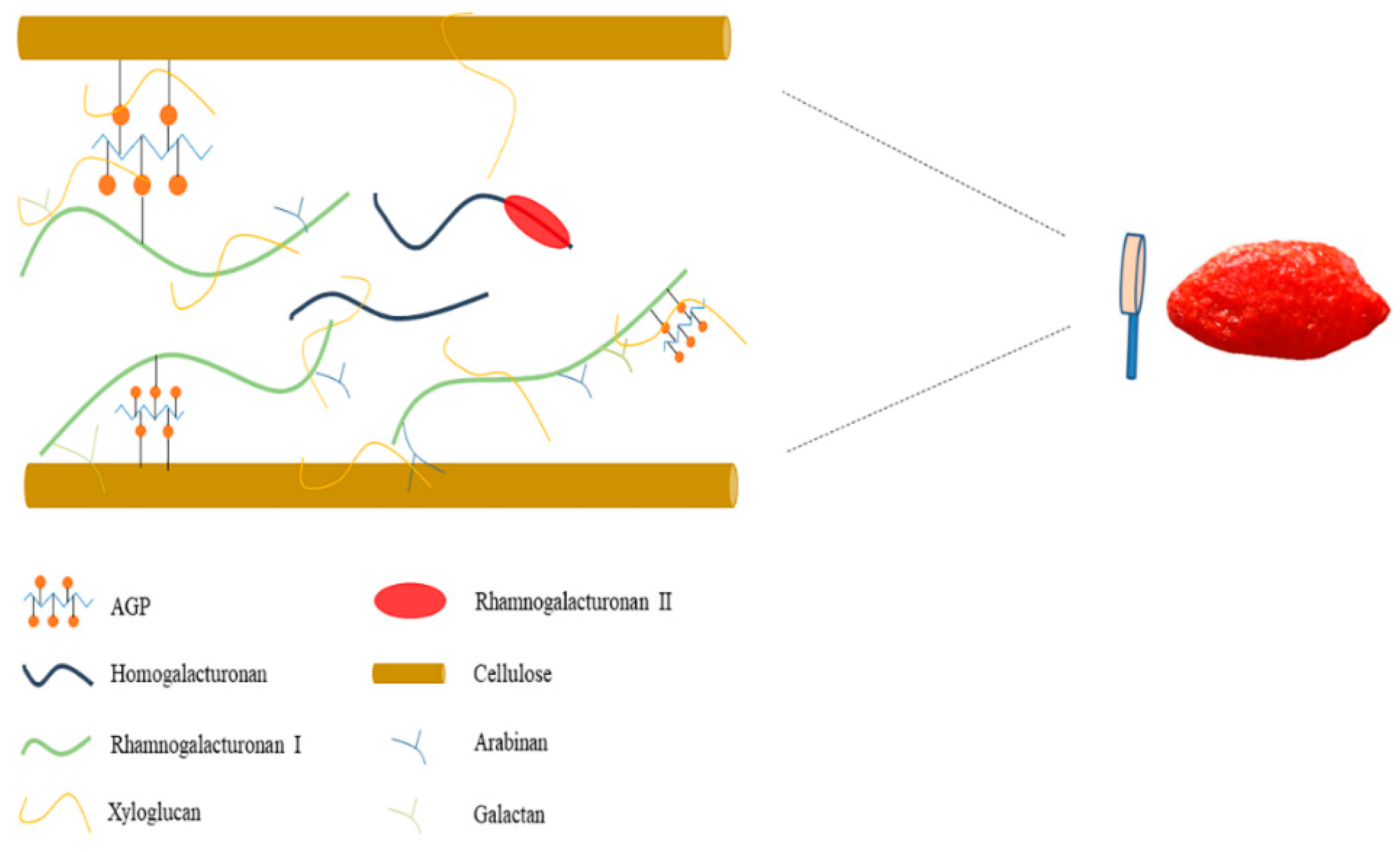

| Sample | Yield | Mol | Rha/GalA | (Ara + Gal)/Rha | Total Sugar (%) | Total Protein (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Man | Rib | Rha | GlcA | GalA | Glc | Gal | Ara | Fuc | ||||||
| WH | 1.79 ± 0.16 | 2.79 ± 0.09 | 0.57 ± 0.16 | 4.17 ± 0.29 | 1.64 ± 0.10 | 19.75 ± 0.05 | 4.59 ± 0.04 | 20.74 ± 0.23 | 39.13 ± 0.06 | 6.62 ± 0.03 | 0.211 | 14.36 | 56.68 ± 5.14 | 7.46 ± 1.13 |
| AH | 2.50 ± 0.07 | 3.78 ± 0.08 | 1.00 ± 0.54 | 9.88 ± 0.04 | 3.84 ± 1.66 | 23.67 ± 0.70 | 3.44 ± 0.12 | 24.11 ± 0.04 | 24.31 ± 0.03 | 5.37 ± 0.06 | 0.417 | 4.9 | 46.70 ± 6.51 | 15.76 ± 1.36 |
| ALH | 4.18 ± 0.05 | 7.39 ± 0.05 | 0.70 ± 0.13 | 8.21 ± 0.12 | 2.60 ± 0.16 | 6.34 ± 0.08 | 4.14 ± 0.04 | 25.23 ± 0.12 | 29.17 ± 0.04 | 16.22 ± 0.03 | 1.295 | 6.63 | 53.34 ± 0.05 | 33.48 ± 2.37 |
| AL | 1.80 ± 0.09 | 2.24 ± 0.01 | ND | 7.47 ± 0.10 | 1.84 ± 0.02 | 28.24 ± 0.05 | 9.06 ± 0.01 | 20.54 ± 0.03 | 22.94 ± 0.06 | 7.68 ± 0.04 | 0.264 | 5.82 | 40.71 ± 0.03 | 6.48 ± 1.08 |
| ALL | 2.46 ± 0.11 | 4.11 ± 0.17 | ND | 7.66 ± 0.03 | 1.61 ± 0.19 | 14.53 ± 0.13 | 6.18 ± 0.03 | 22.23 ± 0.08 | 37.26 ± 0.09 | 6.43 ± 0.28 | 0.527 | 7.77 | 58.11 ± 6.99 | 35.30 ± 1.76 |
| WHALH | 2.04 ± 0.10 | ND | ND | 16.27 ± 0.33 | ND | 13.30 ± 0.20 | 2.65 ± 0.13 | 25.65 ± 0.33 | 37.15 ± 0.02 | 4.98 ± 0.06 | 1.223 | 3.86 | 46.04 ± 4.63 | 37.11 ± 2.13 |
| WHALL | 0.89 ± 0.07 | 2.67 ± 0.03 | ND | 11.99 ± 0.24 | 0.88 ± 0.11 | 22.08 ± 0.06 | 4.19 ± 0.08 | 12.81 ± 0.03 | 43.89 ± 0.04 | 1.52 ± 0.13 | 0.543 | 4.73 | 38.61 ± 1.23 | 29.68 ± 1.39 |
| AHALH | 1.14 ± 0.07 | 6.98 ± 1.88 | 1.02 ± 0.67 | 13.9 ± 0.09 | 4.01 ± 0.02 | 25.46 ± 0.12 | 3.38 ± 0.07 | 22.25 ± 0.13 | 17.73 ± 0.03 | 5.27 ± 0.02 | 0.546 | 2.88 | 56.44 ± 6.04 | 24.55 ± 1.18 |
| ALALL | 0.92 ± 0.03 | 2.06 ± 0.13 | ND | 11.04 ± 0.10 | 1.23 ± 0.26 | 31.06 ± 0.17 | 5.33 ± 0.19 | 13.44 ± 0.13 | 31.84 ± 0.19 | 4.00 ± 0.32 | 0.355 | 4.1 | 55.49 ± 4.26 | 28.73 ± 1.26 |
| Mw a (kDa) | Mn b (kDa) | Mw/Mn | Rz c (nm) | |||||
|---|---|---|---|---|---|---|---|---|
| Sample | Fraction I | Fraction II | Fraction I | Fraction II | Fraction I | Fraction II | Fraction I | Fraction II |
| WH | 49.44 ± 0.590% | 3.078 ± 3.549% | 29.51 ± 0.785% | 2.432 ± 1.906% | 1.675 ± 0.982% | 1.266 ± 2.456% | 62.0 ± 0.7% | 68.8 ± 1.5% |
| AH | 199.2 ± 0.914% | 3.954 ± 4.782% | 150.4 ± 1.104% | 3.355 ± 1.986% | 1.145 ± 1.457% | 1.178 ± 2.668% | 62.4 ± 1.3% | 71.5 ± 2.1% |
| ALH | 223.1 ± 1.803% | 26.32 ± 3.206% | 130.9 ± 1.393% | 15.84 ± 2.424% | 1.704 ± 1.825% | 1.662 ± 3.301% | 52.0 ± 1.2% | 57.6 ± 1.9% |
| AL | 2334 ± 2.481% | 717.4 ± 3.313% | 1690 ± 3.738% | 679.6 ± 3.269% | 1.381 ± 4.486% | 1.056 ± 4.654% | 59.3 ± 3.0% | 68.2 ± 2.4% |
| ALL | 7162 ± 0.686% | 1582 ± 2.061% | 2539 ± 1.072% | 1495 ± 2.082% | 2.821 ± 1.273% | 1.058 ± 2.930% | 56.6 ± 0.6% | 64.8 ± 1.3% |
| WHALH | 207.1 ± 1.182% | 18.27 ± 2.720% | 112.6 ± 2.529% | 13.82 ± 2.544% | 1.839 ± 1.653% | 1.322 ± 2.567% | 53.1 ± 2.1% | 66.1 ± 1.7% |
| WHALL | 6371 ± 0.684% | 1239 ± 1.779% | 2289 ± 0.506% | 1037 ± 0.919% | 2.783 ± 0.771% | 1.195 ± 1.240% | 55.1 ± 0.7% | 64.4 ± 0.7% |
| AHALH | 194.6 ± 1.487% | 29.68 ± 2.960% | 105.7 ± 2.714% | 18.01 ± 2.513% | 1.841 ± 1.507% | 1.648 ± 2.519% | 47.4 ± 2.1% | 58.3 ± 3.0% |
| ALALL | 2294 ± 3.384% | 1035 ± 3.589% | 1062 ± 2.529% | 952.1 ± 3.283% | 2.160 ± 2.854% | 1.087 ± 3.281% | 53.4 ± 1.5% | 69.6 ± 2.3% |
| Samples | Conclusion |
|---|---|
| WH | The free heteropolysaccharide in Goji cell wall could be extracted by hot water |
| AH | High-temperature-acid reserved more HG regions and degraded the side chains |
| AL | Low-temperature-acid reserved not only more HG regions, but also RG-I and side chains |
| ALH | HG regions was destroyed in severe alkaline condition |
| ALL | More extensively branched RG-I domains |
| WHALH | The polysaccharides released by hot alkali were difficult to be extracted by hot water, and the yield was still high |
| WHALL | Hot water extracted free heteropolysaccharide, then more RG-I, relatively, were extracted by mild alkali |
| AHALH | The polysaccharides released by hot alkali were difficult to be extracted by hot acid, and the yield was still high |
| ALALL | The RG-I extracted by mild alkali were difficult to be extracted by mild acid |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Rahman, A.; Li, J.; Wei, C.; Chen, J.; Linhardt, R.J.; Ye, X.; Chen, S. Extraction Methods Affect the Structure of Goji (Lycium barbarum) Polysaccharides. Molecules 2020, 25, 936. https://doi.org/10.3390/molecules25040936
Zhou S, Rahman A, Li J, Wei C, Chen J, Linhardt RJ, Ye X, Chen S. Extraction Methods Affect the Structure of Goji (Lycium barbarum) Polysaccharides. Molecules. 2020; 25(4):936. https://doi.org/10.3390/molecules25040936
Chicago/Turabian StyleZhou, Shengyi, Atikur Rahman, Junhui Li, Chaoyang Wei, Jianle Chen, Robert J. Linhardt, Xingqian Ye, and Shiguo Chen. 2020. "Extraction Methods Affect the Structure of Goji (Lycium barbarum) Polysaccharides" Molecules 25, no. 4: 936. https://doi.org/10.3390/molecules25040936
APA StyleZhou, S., Rahman, A., Li, J., Wei, C., Chen, J., Linhardt, R. J., Ye, X., & Chen, S. (2020). Extraction Methods Affect the Structure of Goji (Lycium barbarum) Polysaccharides. Molecules, 25(4), 936. https://doi.org/10.3390/molecules25040936






