A Green Extraction Process for Polyphenols from Elderberry (Sambucus nigra) Flowers Using Deep Eutectic Solvent and Ultrasound-Assisted Pretreatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material and Handling
2.3. Synthesis of the Deep Eutectic Solvent (DES)
2.4. Batch Extraction Process
2.5. Ultrasound-Assisted Pretreatment
2.6. Experimental Design and Deployment of Response Surface Methodology
2.7. Total Polyphenol Determination
2.8. Total Flavonoid Determination
2.9. Determination of the Antiradical Activity (AAR)
2.10. Determination of the Reducing Power (PR)
2.11. Liquid Chromatography–Diode Array–Mass Spectrometry (LC-DAD-MS)
2.12. High-Performance Liquid Chromatography–Diode Array (HPLC-DAD)
2.13. Statistical Analysis
3. Results and Discussion
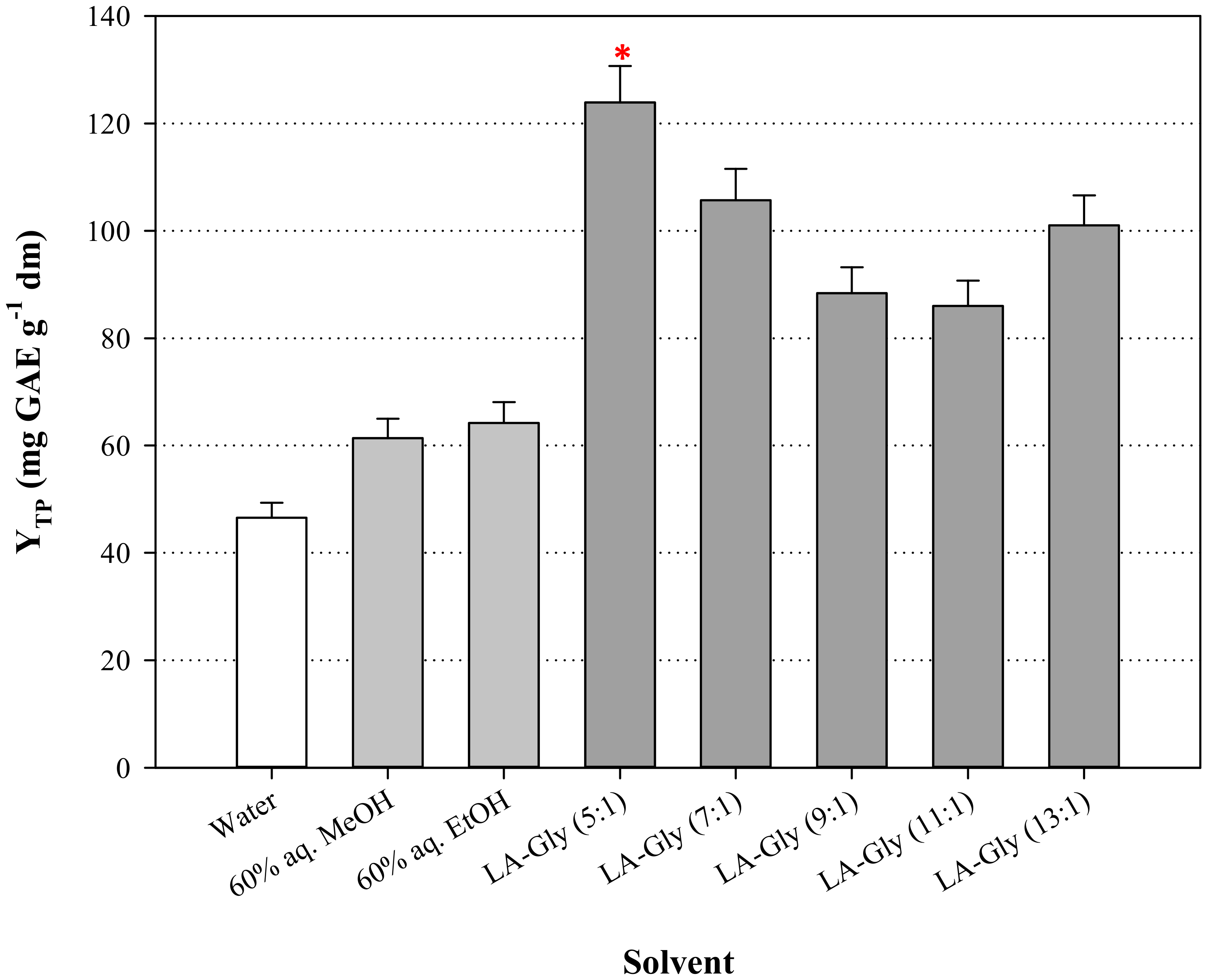
3.1. DES Synthesis and the Effect of HBD:HBA Molar Ratio ()
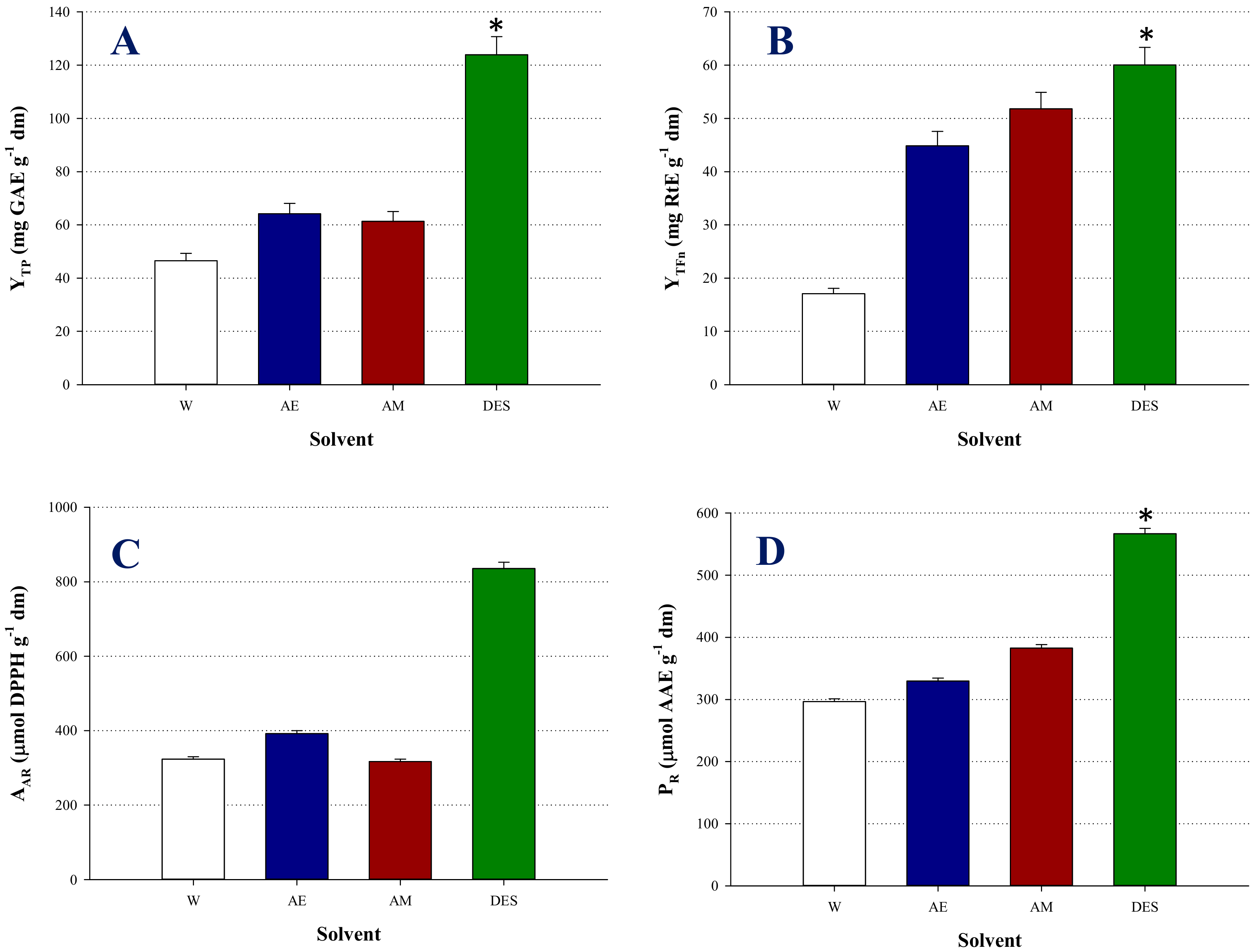
3.2. Optimization of Extraction Performance
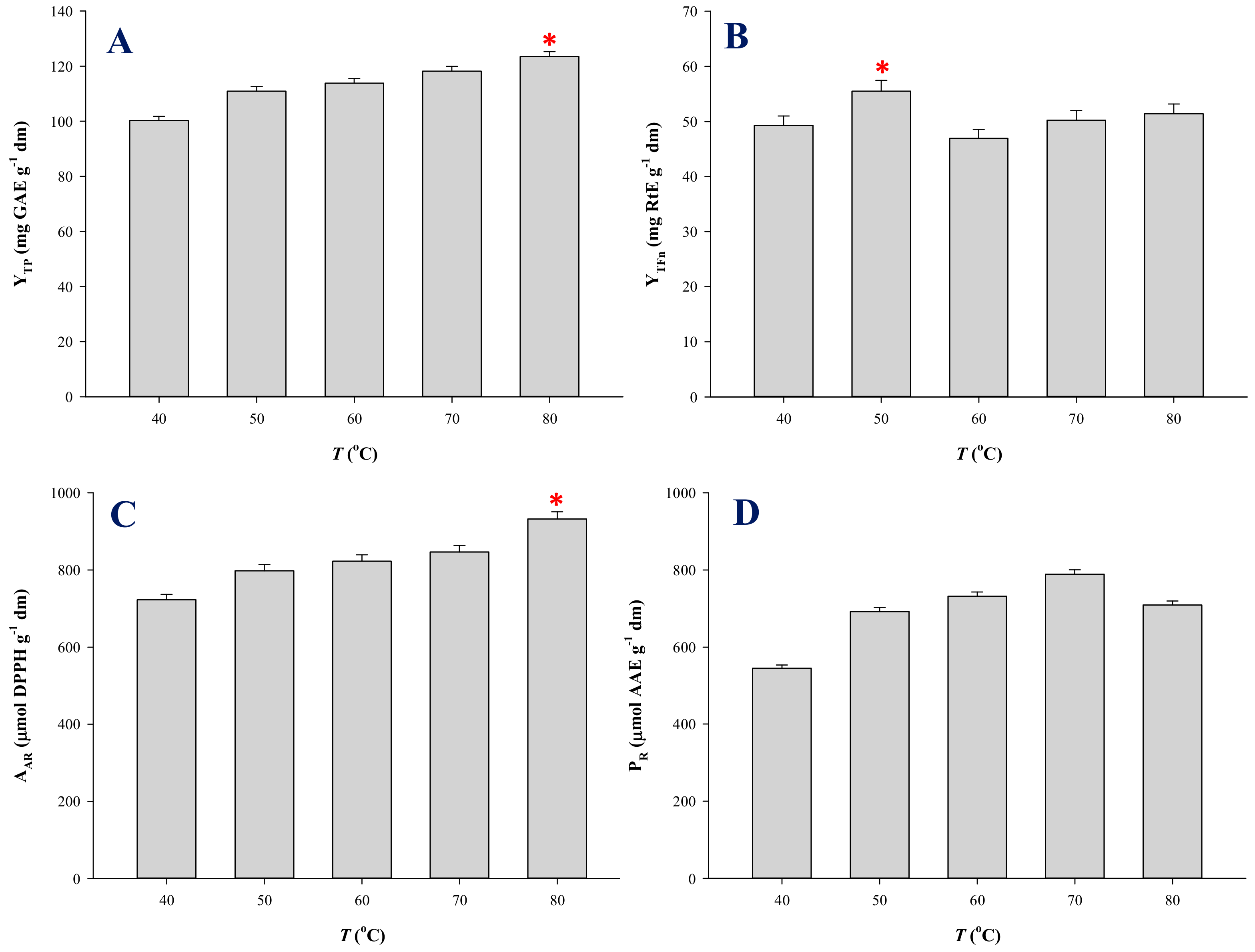
3.3. Temperature Effects
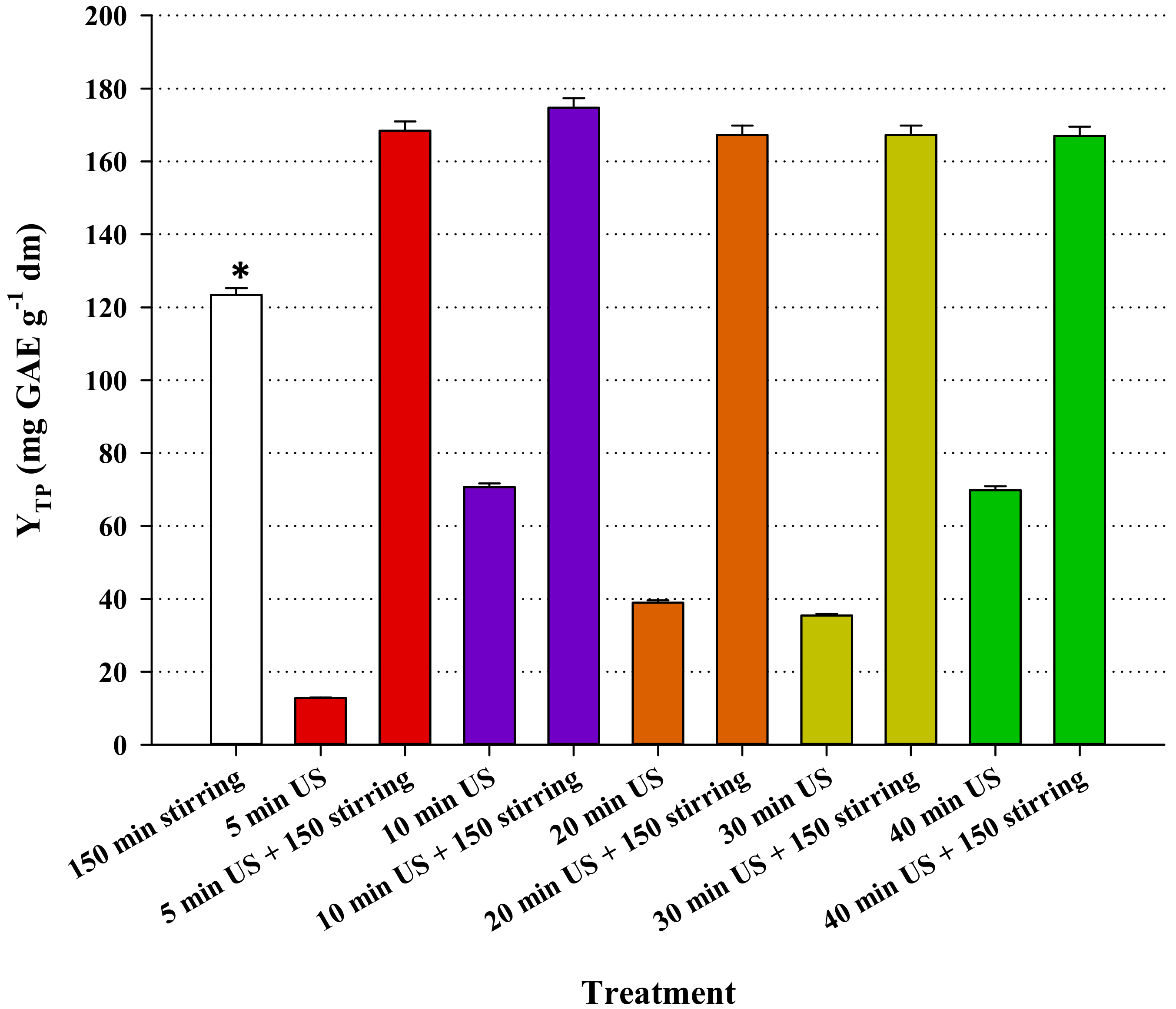
3.4. Effect of Ultrasound-Assisted Pretreatment
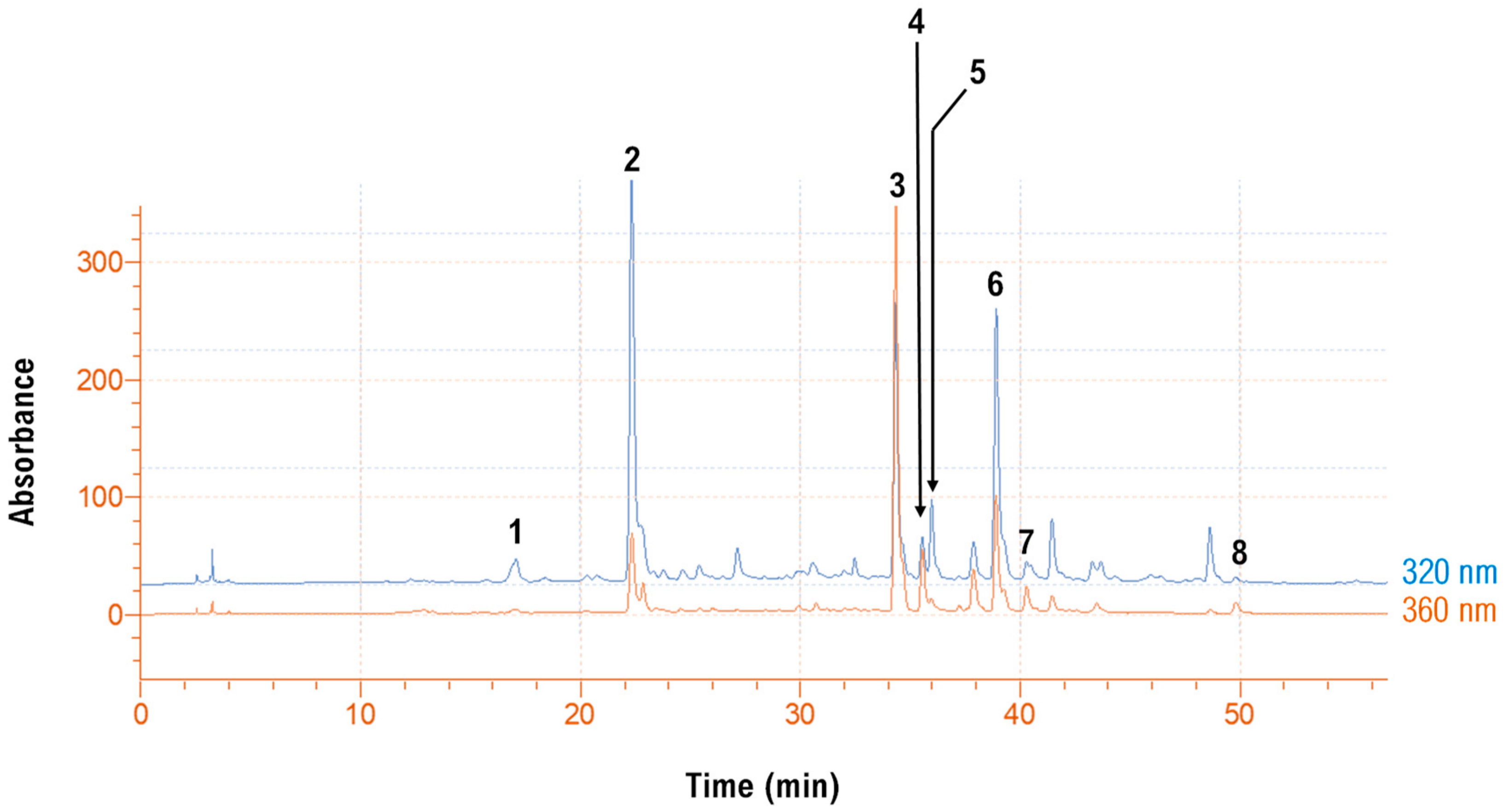
3.5. Polyphenolic Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AAR | antiradical activity (μmol DPPH g−1) |
| PR | reducing power (μmol AAE g−1) |
| RL/S | liquid-to-solid ratio (mL g−1) |
| t | time (min) |
| T | temperature (°C) |
| YTFn | yield in total flavonoids (mg RtE g−1) |
| YTP | yield in total polyphenols (mg GAE g−1) |
| AAE | ascorbic acid equivalents |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl radical |
| GAE | gallic acid equivalents |
| HBA | hydrogen bond acceptor |
| HBD | hydrogen bond donor |
| NADES | Natural deep eutectic solvents |
| TPTZ | 2,4,6-tripyridyl-s-triazine |
References
- Fernandes, L.; Casal, S.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E. Edible flowers: A review of the nutritional, antioxidant, antimicrobial properties and effects on human health. J. Food Compos. Anal. 2017, 60, 38–50. [Google Scholar] [CrossRef]
- Lu, B.; Li, M.; Yin, R. Phytochemical content, health benefits, and toxicology of common edible flowers: A review (2000–2015). Crit. Rev. Food Sci Nutr. 2016, 56, S130–S148. [Google Scholar] [CrossRef] [PubMed]
- Mlcek, J.; Rop, O. Fresh edible flowers of ornamental plants–A new source of nutraceutical foods. Trends Food Sci. Technol. 2011, 22, 561–569. [Google Scholar] [CrossRef]
- Sidor, A.; Gramza-Michałowska, A. Advanced research on the antioxidant and health benefit of elderberry (Sambucus nigra) in food–A review. J. Funct. Foods 2015, 18, 941–958. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Pugliese, A.; Bonesi, M.; Tenuta, M.C.; Menichini, F.; Xiao, J.; Tundis, R. Edible flowers: A rich source of phytochemicals with antioxidant and hypoglycemic properties. J. Agric. Food Chem. 2015, 64, 2467–2474. [Google Scholar] [CrossRef]
- Chemat, F.; Abert Vian, M.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Fabiano Tixier, A.-S. Review of alternative solvents for green extraction of food and natural products: Panorama, principles, applications and prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef]
- Cunha, S.C.; Fernandes, J.O. Extraction techniques with deep eutectic solvents. TrAC Trends Anal. Chem. 2018, 105, 225–239. [Google Scholar] [CrossRef]
- Vanda, H.; Dai, Y.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Green solvents from ionic liquids and deep eutectic solvents to natural deep eutectic solvents. Compt. Rendus Chim. 2018, 21, 628–638. [Google Scholar] [CrossRef]
- Skarpalezos, D.; Detsi, A. Deep eutectic solvents as extraction media for valuable flavonoids from natural sources. Appl. Sci. 2019, 9, 4169. [Google Scholar] [CrossRef]
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sánchez-Contreras, A.; Pacheco, N. Ultrasound assisted extraction for the recovery of phenolic compounds from vegetable sources. Agronomy 2017, 7, 47. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. TrAC Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Nayak, A.; Bhushan, B.; Rosales, A.; Turienzo, L.R.; Cortina, J. Valorisation potential of Cabernet grape pomace for the recovery of polyphenols: Process intensification, optimisation and study of kinetics. Food Bioprod. Proc. 2018, 109, 74–85. [Google Scholar] [CrossRef]
- Lakka, A.; Karageorgou, I.; Kaltsa, O.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D. Polyphenol extraction from Humulus lupulus (hop) using a neoteric glycerol/L-alanine deep eutectic solvent: Optimisation, kinetics and the effect of ultrasound-assisted pretreatment. AgriEngineering 2019, 1, 30. [Google Scholar] [CrossRef]
- Lakka, A.; Grigorakis, S.; Kaltsa, O.; Karageorgou, I.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D.P. The effect of ultrasonication pretreatment on the production of polyphenol-enriched extracts from Moringa oleifera L. (drumstick tree) using a novel bio-based deep eutectic solvent. Appl. Sci. 2020, 10, 220. [Google Scholar] [CrossRef]
- Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Kaltsa, O.; Bozinou, E.; Lalas, S.; Makris, D.P. Saffron processing wastes as a bioresource of high-value added compounds: Development of a green extraction process for polyphenol recovery using a natural deep eutectic solvent. Antioxidants 2019, 8, 586. [Google Scholar] [CrossRef]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A reproducible, rapid and inexpensive Folin–Ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Shehata, E.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Extraction optimisation using water/glycerol for the efficient recovery of polyphenolic antioxidants from two Artemisia species. Separ. Purif. Technol. 2015, 149, 462–469. [Google Scholar] [CrossRef]
- Manousaki, A.; Jancheva, M.; Grigorakis, S.; Makris, D. Extraction of antioxidant phenolics from agri-food waste biomass using a newly designed glycerol-based natural low-transition temperature mixture: A comparison with conventional eco-friendly solvents. Recycling 2016, 1, 194. [Google Scholar] [CrossRef]
- Philippi, K.; Tsamandouras, N.; Grigorakis, S.; Makris, D.P. Ultrasound-assisted green extraction of eggplant peel (Solanum melongena) polyphenols using aqueous mixtures of glycerol and ethanol: Optimisation and kinetics. Environ. Proc. 2016, 3, 369–386. [Google Scholar] [CrossRef]
- Karakashov, B.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Optimisation of polyphenol extraction from Hypericum perforatum (St. John’s Wort) using aqueous glycerol and response surface methodology. J. Appl. Res. Med. Arom. Plants 2015, 2, 1–8. [Google Scholar] [CrossRef]
- Bakirtzi, C.; Triantafyllidou, K.; Makris, D.P. Novel lactic acid-based natural deep eutectic solvents: Efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from common native Greek medicinal plants. J. Appl. Res. Med. Arom. Plants 2016, 3, 120–127. [Google Scholar] [CrossRef]
- Kottaras, P.; Koulianos, M.; Makris, D. Low-Transition temperature mixtures (LTTMs) made of bioorganic molecules: Enhanced extraction of antioxidant phenolics from industrial cereal solid wastes. Recycling 2017, 2, 3. [Google Scholar] [CrossRef]
- Karageorgou, I.; Grigorakis, S.; Lalas, S.; Makris, D.P. Enhanced extraction of antioxidant polyphenols from Moringa oleifera Lam. leaves using a biomolecule-based low-transition temperature mixture. Eur. Food Res. Technol. 2017, 243, 1839–1848. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Grigorakis, S.; Lalas, S.; Makris, D.P. Highly efficient extraction of antioxidant polyphenols from Olea europaea leaves using an eco-friendly glycerol/glycine deep eutectic solvent. Waste Biomass Valor. 2018, 9, 1985–1992. [Google Scholar] [CrossRef]
- Espino, M.; de los Ángeles Fernández, M.; Gomez, F.J.; Silva, M.F. Natural designer solvents for greening analytical chemistry. TrAC Trends Anal. Chem. 2016, 76, 126–136. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Shewale, S.; Rathod, V.K. Extraction of total phenolic content from Azadirachta indica or (neem) leaves: Kinetics study. Prep. Biochem. Biotechnol. 2018, 48, 312–320. [Google Scholar] [CrossRef]
- Jancheva, M.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Optimised extraction of antioxidant polyphenols from Satureja thymbra using newly designed glycerol-based natural low-transition temperature mixtures (LTTMs). J. Appl. Res. Med. Aromat. Plants 2017, 6, 31–40. [Google Scholar] [CrossRef]
- Stefou, I.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Development of sodium propionate-based deep eutectic solvents for polyphenol extraction from onion solid wastes. Clean Technol. Environ. Policy 2019, 21, 1563–1574. [Google Scholar] [CrossRef]
- Chakroun, D.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Enhanced-performance extraction of olive (Olea europaea) leaf polyphenols using L-lactic acid/ammonium acetate deep eutectic solvent combined with β-cyclodextrin: Screening, optimisation, temperature effects and stability. Biomass Convers. Bioref. 2019, 1–12. [Google Scholar] [CrossRef]
- Vetal, M.D.; Lade, V.G.; Rathod, V.K. Extraction of ursolic acid from Ocimum sanctum leaves: Kinetics and modeling. Food Bioprod. Process. 2012, 90, 793–798. [Google Scholar] [CrossRef]
- Khiari, Z.; Makris, D.P.; Kefalas, P. An investigation on the recovery of antioxidant phenolics from onion solid wastes employing water/ethanol-based solvent systems. Food Bioprocess Technol. 2009, 2, 337. [Google Scholar] [CrossRef]
- Katsampa, P.; Valsamedou, E.; Grigorakis, S.; Makris, D.P. A green ultrasound-assisted extraction process for the recovery of antioxidant polyphenols and pigments from onion solid wastes using Box–Behnken experimental design and kinetics. Ind. Crop. Prod. 2015, 77, 535–543. [Google Scholar] [CrossRef]
- Shang, X.; Dou, Y.; Zhang, Y.; Tan, J.-N.; Liu, X.; Zhang, Z. Tailor-made natural deep eutectic solvents for green extraction of isoflavones from chickpea (Cicer arietinum L.) sprouts. Ind. Crop. Prod. 2019, 140, 111724. [Google Scholar] [CrossRef]
- Patsea, M.; Stefou, I.; Grigorakis, S.; Makris, D.P. Screening of natural sodium acetate-based low-transition temperature mixtures (LTTMs) for enhanced extraction of antioxidants and pigments from red vinification solid wastes. Environ. Process. 2017, 4, 123–135. [Google Scholar] [CrossRef]
- Ali, M.C.; Chen, J.; Zhang, H.; Li, Z.; Zhao, L.; Qiu, H. Effective extraction of flavonoids from Lycium barbarum L. fruits by deep eutectic solvents-based ultrasound-assisted extraction. Talanta 2019, 203, 16–22. [Google Scholar] [CrossRef]
- Lavilla, I.; Bendicho, C. Fundamentals of ultrasound-assisted extraction. In Water Extraction of Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2017; pp. 291–316. [Google Scholar]
- Michail, A.; Sigala, P.; Grigorakis, S.; Makris, D.P. Kinetics of ultrasound-assisted polyphenol extraction from spent filter coffee using aqueous glycerol. Chem. Eng. Com. 2016, 203, 407–413. [Google Scholar] [CrossRef]
- Clifford, M.N.; Marks, S.; Knight, S.; Kuhnert, N. Characterization by LC-MS n of four new classes of p-coumaric acid-containing diacyl chlorogenic acids in green coffee beans. J. Agric. Food Chem. 2006, 54, 4095–4101. [Google Scholar] [CrossRef]
- Li-Li, D.; Li, D.; Long, G.; Le-Le, L.; Zhang, Y.-D.; Ping, L. An UHPLC-MS/MS method for simultaneous determination of quercetin 3-O-rutinoside, kaempferol 3-O-rutinoside, isorhamnetin 3-O-rutinoside, bilobalide and ligustrazine in rat plasma, and its application to pharmacokinetic study of Xingxiong injection. Chinese J. Nat. Med. 2017, 15, 710–720. [Google Scholar]
- Christensen, L.P.; Kaack, K.; Fretté, X.C. Selection of elderberry (Sambucus nigra L.) genotypes best suited for the preparation of elderflower extracts rich in flavonoids and phenolic acids. Eur. Food Res. Technol. 2008, 227, 293–305. [Google Scholar] [CrossRef]
- Kaack, K.; Christensen, L.P. Phenolic acids and flavonoids in tea processed from flowers of black elder (Sambucus nigra L.) stored in different packing materials. Eur. J. Hortic. Sci. 2010, 75, 214. [Google Scholar]
- Esin Çelik, S.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E.; Apak, R. Identification and antioxidant capacity determination of phenolics and their glycosides in elderflower by on-line HPLC–CUPRAC method. Phytochem. Anal. 2014, 25, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, A.L.; Wianowska, D.; Baraniak, B. The antioxidant properties of alcoholic extracts from Sambucus nigra L. (antioxidant properties of extracts). LWT 2006, 39, 308–315. [Google Scholar]
- Milena, V.; Tatjana, M.; Gökhan, Z.; Ivana, B.; Aleksandra, C.; Mohammad, M.F.; Marija, R. Advantages of contemporary extraction techniques for the extraction of bioactive constituents from black elderberry (Sambucus nigra L.) flowers. Ind. Crop. Prod. 2019, 136, 93–101. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
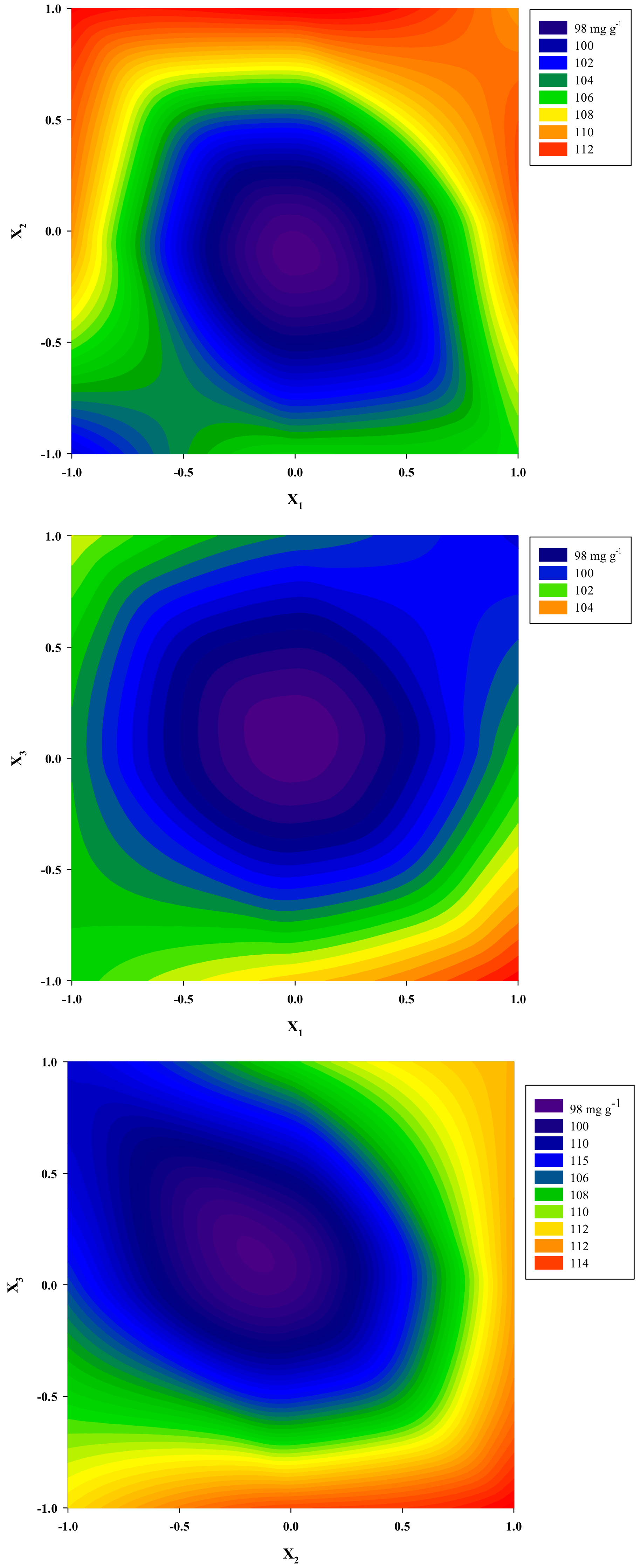
| Independent Variables | Code Units | Coded Variable Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| CDES (%, w/v) | X1 | 55 | 70 | 85 |
| RL/S (mL g−1) | X2 | 20 | 40 | 60 |
| SS (rpm) | X3 | 200 | 500 | 800 |
| Term | Standard Error | t Ratio | Probability > t | Sum of Squares | F Ratio |
|---|---|---|---|---|---|
| Intercept | 1.666545 | 57.83 | < 0001 * | 31.08661 | 3.7309 |
| CDES | 1.020546 | 1.93 | 0.1113 | 124.7410 | 14.971 |
| RL/S | 1.020546 | 3.87 | 0.0118 * | 185.6664 | 22.283 |
| SS | 1.020546 | −4.72 | 0.0052 * | 17.22250 | 2.0670 |
| CDES RL/S | 1.443270 | −1.44 | 0.2100 | 10.33622 | 1.2405 |
| CDES SS | 1.443270 | −1.11 | 0.3160 | 4.182020 | 0.5019 |
| RL/S SS | 1.443270 | 0.71 | 0.5103 | 74.05096 | 8.8874 |
| CDESCDES | 1.502204 | 2.98 | 0.0308 * | 155.9200 | 18.713 |
| RL/S RL/S | 1.502204 | 4.33 | 0.0075 * | 147.9857 | 17.761 |
| SS SS | 1.502204 | 4.21 | 0.0084 * | 31.08661 | 3.7309 |
| Lack-of-fit | 0.0817 | 39.3593 | 11.402 |
| Design Point | Independent Variables | Response (YTP, mg GAE g−1 dw) | |||
|---|---|---|---|---|---|
| X1 (CDES, % w/v) | X2 (RL/S, mL g−1) | X3 (SS, rpm) | Measured | Predicted | |
| 1 | −1 (55) | −1 (20) | 0 (500) | 100.43 | 100.87 |
| 2 | −1 (55) | 1 (60) | 0 (500) | 112.48 | 112.92 |
| 3 | 1 (85) | −1 (20) | 0 (500) | 106.39 | 105.95 |
| 4 | 1 (85) | 1 (60) | 0 (500) | 110.14 | 109.70 |
| 5 | 0 (70) | −1 (20) | −1 (200) | 109.16 | 109.60 |
| 6 | 0 (70) | −1 (20) | 1 (800) | 101.37 | 100.93 |
| 7 | 0 (70) | 1 (60) | −1 (200) | 115.01 | 115.45 |
| 8 | 0 (70) | 1 (60) | 1 (800) | 111.31 | 110.87 |
| 9 | −1 (55) | 0 (40) | −1 (200) | 109.31 | 108.43 |
| 10 | 1 (85) | 0 (40) | −1 (200) | 118.60 | 118.60 |
| 11 | −1 (55) | 0 (40) | 1 (800) | 99.00 | 102.01 |
| 12 | 1 (85) | 0 (40) | 1 (800) | 101.86 | 102.74 |
| 13 | 0 (70) | 0 (40) | 0 (500) | 97.56 | 96.38 |
| 14 | 0 (70) | 0 (40) | 0 (500) | 95.46 | 96.38 |
| 15 | 0 (70) | 0 (40) | 0 (500) | 96.13 | 96.38 |
| No | Rt (min) | UV-Vis (λmax) | [M + H]+ (m/z) | Other Ions (m/z) | Tentative Identity |
|---|---|---|---|---|---|
| 1 | 17.22 | 328 | 355 | 163 | Neochlorogenic acid |
| 2 | 22.4 | 328 | 355 | 163 | Chlorogenic acid |
| 3 | 34.32 | 255, 352 | 611 | 303 | Quercetin 3-O-rutinoside (rutin) |
| 4 | 35.50 | 254, 352 | 465 | 303 | Quercetin 3-O-glucoside (isoquercitrin) |
| 5 | 35.97 | 318 | 517 | 355, 163 | Di-caffeoylquinic acid |
| 6 | 38.90 | 318 | 485 | 147 | p-Coumaroylquinic acid derivative |
| 7 | 40.28 | 351 | 625 | 647[M + Na]+, 317 | Isorhamnetin 3-O-rutinoside (narcissin) |
| 8 | 49.4 | 259, 369 | 303 | - | Quercetin |
| Polyphenol | Content (mg g−1 dm) ± SD |
|---|---|
| Phenolic acids | |
| Neochlorogenic acid | 1.11 ± 0.01 |
| Chlorogenic acid | 10.76 ± 0.45 |
| Di-caffeoylquinic acid | 2.55 ± 0.04 |
| di-p-Coumaroylquinic acid derivative | 13.06 ± 0.89 |
| Total | 27.48 |
| Flavonols | |
| Quercetin 3-O-rutinoside (rutin) | 17.36 ± 1.10 |
| Quercetin 3-O-glucoside (isoquercitrin) | 2.06 ± 0.05 |
| Isorhamnetin 3-O-rutinoside (narcissin) | 0.96 ± 0.00 |
| Quercetin | 0.53 ± 0.00 |
| Total | 20.91 |
| Sum | 48.39 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaltsa, O.; Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D.P. A Green Extraction Process for Polyphenols from Elderberry (Sambucus nigra) Flowers Using Deep Eutectic Solvent and Ultrasound-Assisted Pretreatment. Molecules 2020, 25, 921. https://doi.org/10.3390/molecules25040921
Kaltsa O, Lakka A, Grigorakis S, Karageorgou I, Batra G, Bozinou E, Lalas S, Makris DP. A Green Extraction Process for Polyphenols from Elderberry (Sambucus nigra) Flowers Using Deep Eutectic Solvent and Ultrasound-Assisted Pretreatment. Molecules. 2020; 25(4):921. https://doi.org/10.3390/molecules25040921
Chicago/Turabian StyleKaltsa, Olga, Achillia Lakka, Spyros Grigorakis, Ioanna Karageorgou, Georgia Batra, Eleni Bozinou, Stavros Lalas, and Dimitris P. Makris. 2020. "A Green Extraction Process for Polyphenols from Elderberry (Sambucus nigra) Flowers Using Deep Eutectic Solvent and Ultrasound-Assisted Pretreatment" Molecules 25, no. 4: 921. https://doi.org/10.3390/molecules25040921
APA StyleKaltsa, O., Lakka, A., Grigorakis, S., Karageorgou, I., Batra, G., Bozinou, E., Lalas, S., & Makris, D. P. (2020). A Green Extraction Process for Polyphenols from Elderberry (Sambucus nigra) Flowers Using Deep Eutectic Solvent and Ultrasound-Assisted Pretreatment. Molecules, 25(4), 921. https://doi.org/10.3390/molecules25040921









