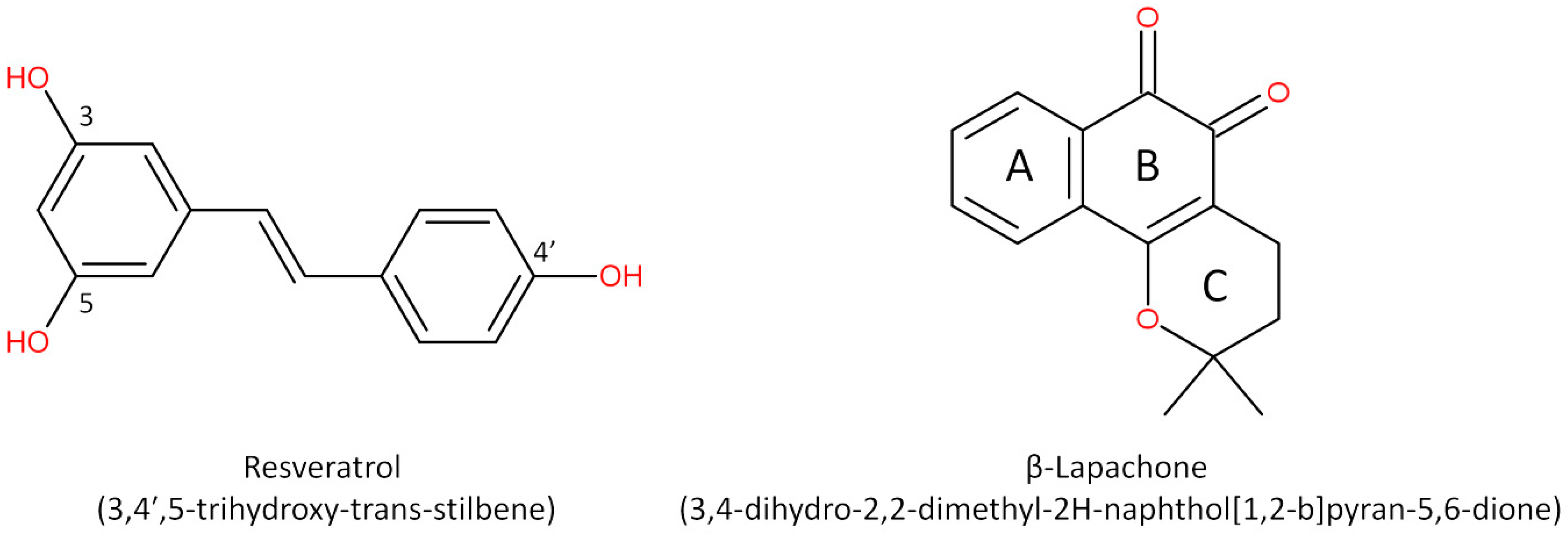
Anticancer Potential of Resveratrol, β-Lapachone and Their Analogues
Abstract
1. Introduction
2. Resveratrol and Stilbene-Based Compounds
2.1. Methoxylated Resveratrol Derivatives
2.1.1. Pterostilbene
2.1.2. Trimethoxystilbene
2.1.3. Tetramethoxystilbene
2.1.4. Pentamethoxystilbene
2.2. Hydroxylated Resveratrol Derivatives
2.2.1. Dihydroxystilbene
2.2.2. Tetrahydroxystilbene
2.2.3. Hexahydroxystilbene
3. β-Lapachone and Its Derivatives: The South American Promise for Cancer
3.1. Anticancer Effects
3.2. Mechanisms of Action
3.2.1. ROS and NQO1
3.2.2. Topoisomerase Inhibition
3.2.3. p53
3.2.4. Other Cellular or Molecular Pathways
3.3. Strategies to Overcome β-Lapachone Bioavailability and Toxicity Issues: Drug Delivery and Derivatives Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- The International Agency for Research on Cancer (IARC). Latest Global CANCER Data: Cancer Burden Rises to 18.1 Million New Cases and 9.6 Million Cancer Deaths in 2018; IARC: Lyon, France, 2018. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Roupe, K.; Remsberg, C.; Yanez, J.; Davies, N. Pharmacometrics of Stilbenes: Seguing Towards the Clinic. Curr. Clin. Pharmacol. 2008, 1, 81–101. [Google Scholar] [CrossRef] [PubMed]
- Siemann, E.H.; Creasy, L.L. Concentration of the phytoalexin resveratrol in wine. Am. J. Enol. Vitic. 1992, 43, 49–52. [Google Scholar]
- Signorelli, P.; Ghidoni, R. Resveratrol as an anticancer nutrient: Molecular basis, open questions and promises. J. Nutr. Biochem. 2005, 16, 449–466. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.; Maltese, F.; Choi, Y.H.; Verpoorte, R. Metabolic constituents of grapevine and grape-derived products. Phytochem. Rev. 2010, 9, 357–378. [Google Scholar] [CrossRef]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.W.; Fong, H.H.S.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef]
- Pezzuto, J.M. Resveratrol as an inhibitor of carcinogenesis. Pharm. Biol. 2008, 46, 443–573. [Google Scholar] [CrossRef]
- Da Costa, D.C.F.; Fialho, E.; Silva, J.L. Cancer chemoprevention by resveratrol: The P53 tumor suppressor protein as a promising molecular target. Molecules 2017, 22, 1014. [Google Scholar] [CrossRef]
- Kundu, J.K.; Surh, Y.J. Cancer chemopreventive and therapeutic potential of resveratrol: Mechanistic perspectives. Cancer Lett. 2008, 269, 243–261. [Google Scholar] [CrossRef] [PubMed]
- Varoni, E.M.; Lo Faro, A.F.; Sharifi-Rad, J.; Iriti, M. Anticancer Molecular Mechanisms of Resveratrol. Front. Nutr. 2016, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- De la Lastra, C.A.; Villegas, I. Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and clinical implications. Biochem. Soc. Trans. 2007, 35, 1156–1160. [Google Scholar] [CrossRef]
- Martins, L.A.M.; Coelho, B.P.; Behr, G.; Pettenuzzo, L.F.; Souza, I.C.C.; Moreira, J.C.F.; Borojevic, R.; Gottfried, C.; Guma, F.C.R. Resveratrol Induces Pro-oxidant Effects and Time-Dependent Resistance to Cytotoxicity in Activated Hepatic Stellate Cells. Cell Biochem. Biophys. 2014, 68, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.L.; Vieira, T.C.R.G.; Gomes, M.P.B.; Ano Bom, A.P.; Lima, L.M.T.R.; Freitas, M.S.; Ishimaru, D.; Cordeiro, Y.; Foguel, D. Ligand binding and hydration in protein misfolding: Insights from studies of prion and p53 tumor suppressor proteins. Acc. Chem. Res. 2010, 43, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.L.; Cino, E.A.; Soares, I.N.; Ferreira, V.F.; De Oliveira, G. Targeting the Prion-like Aggregation of Mutant p53 to Combat Cancer. Acc. Chem. Res. 2018, 51, 181–190. [Google Scholar] [CrossRef]
- Ano Bom, A.P.D.; Rangel, L.P.; Costa, D.C.F.; De Oliveira, G.A.P.; Sanches, D.; Braga, C.A.; Gava, L.M.; Ramos, C.H.I.; Cepeda, A.O.T.; Stumbo, A.C.; et al. Mutant p53 aggregates into prion-like amyloid oligomers and fibrils: Implications for cancer. J. Biol. Chem. 2012, 287, 28152–28162. [Google Scholar] [CrossRef]
- Silva, J.L.; Gallo, C.V.D.M.; Costa, D.C.F.; Rangel, L.P. Prion-like aggregation of mutant p53 in cancer. Trends Biochem. Sci. 2014, 39, 260–267. [Google Scholar] [CrossRef]
- Xu, J.; Reumers, J.; Couceiro, J.R.; De Smet, F.; Gallardo, R.; Rudyak, S.; Cornelis, A.; Rozenski, J.; Zwolinska, A.; Marine, J.C.; et al. Gain of function of mutant p53 by coaggregation with multiple tumor suppressors. Nat. Chem. Biol. 2011, 7, 285–295. [Google Scholar] [CrossRef]
- Ferraz Da Costa, D.C.; Campos, N.P.C.; Santos, R.A.; Guedes-Da-Silva, F.H.; Martins-Dinis, M.M.D.C.; Zanphorlin, L.; Ramos, C.; Rangel, L.P.; Silva, J.L. Resveratrol prevents p53 aggregation in vitro and in breast cancer cells. Oncotarget 2018, 9, 29112–29122. [Google Scholar]
- Ferraz da Costa, D.C.; Casanova, F.A.; Quarti, J.; Malheiros, M.S.; Sanches, D.; dos Santos, P.S.; Fialho, E.; Silva, J.L. Transient Transfection of a Wild-Type p53 Gene Triggers Resveratrol-Induced Apoptosis in Cancer Cells. PLoS ONE 2012, 7, e48746. [Google Scholar] [CrossRef] [PubMed]
- Chimento, A.; De Amicis, F.; Sirianni, R.; Sinicropi, M.S.; Puoci, F.; Casaburi, I.; Saturnino, C.; Pezzi, V. Progress to improve oral bioavailability and beneficial effects of resveratrol. Int. J. Mol. Sci. 2019, 20, 1381. [Google Scholar] [CrossRef] [PubMed]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, W.; Zhou, Z.; Deng, S.; Ma, X.; Ma, X.; Li, C.; Shu, X. Therapeutic versatility of resveratrol derivatives. Nutrients 2017, 9, 1188. [Google Scholar] [CrossRef] [PubMed]
- McCormack, D.; McFadden, D. Pterostilbene and cancer: Current review. J. Surg. Res. 2012, 173, e53–e61. [Google Scholar] [CrossRef]
- Kosuru, R.; Rai, U.; Prakash, S.; Singh, A.; Singh, S. Promising therapeutic potential of pterostilbene and its mechanistic insight based on preclinical evidence. Eur. J. Pharmacol. 2016, 789, 229–243. [Google Scholar] [CrossRef]
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother. Pharmacol. 2011, 68, 593–601. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, X.; Xu, L.; Liu, D.; Di, S.; Li, W.; Zhang, J.; Zhang, H.; Li, X.; Han, J.; et al. Pterostilbene: Mechanisms of its action as oncostatic agent in cell models and in vivo studies. Pharmacol. Res. 2019, 145, 104265. [Google Scholar] [CrossRef]
- Suh, N.; Paul, S.; Hao, X.; Simi, B.; Xiao, H.; Rimando, A.M.; Reddy, B.S. Pterostilbene, an active constituent of blueberries, suppresses aberrant crypt foci formation in the azoxymethane-induced colon carcinogenesis model in rats. Clin. Cancer Res. 2007, 13, 350–355. [Google Scholar] [CrossRef]
- Chiou, Y.S.; Tsai, M.L.; Wang, Y.J.; Cheng, A.C.; Lai, W.M.; Badmaev, V.; Ho, C.T.; Pan, M.H. Pterostilbene inhibits colorectal aberrant crypt foci (ACF) and colon carcinogenesis via suppression of multiple signal transduction pathways in azoxymethane-treated mice. J. Agric. Food Chem. 2010, 58, 8833–8841. [Google Scholar] [CrossRef]
- Wawszczyk, J.; Kapral, M.; Hollek, A.; Węglarz, L. In vitro evaluation of antiproliferative and cytotoxic properties of pterostilbene against human colon cancer cells. Acta Pol. Pharmacol. 2014, 71, 1051–1055. [Google Scholar]
- Pan, M.H.; Chang, Y.H.; Badmaev, V.; Nagabhushanam, K.; Ho, C.T. Pterostilbene induces apoptosis and cell cycle arrest in human gastric carcinoma cells. J. Agric. Food Chem. 2007, 55, 7777–7785. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Chiou, Y.S.; Chen, W.J.; Wang, J.M.; Badmaev, V.; Ho, C.T. Pterostilbene inhibited tumor invasion via suppressing multiple signal transduction pathways in human hepatocellular carcinoma cells. Carcinogenesis 2009, 30, 1234–1242. [Google Scholar] [CrossRef]
- Huang, C.S.; Ho, C.T.; Tu, S.H.; Pan, M.H.; Chuang, C.H.; Chang, H.W.; Chang, C.H.; Wu, C.H.; Ho, Y.S. Long-term ethanol exposure-induced hepatocellular carcinoma cell migration and invasion through lysyl oxidase activation are attenuated by combined treatment with pterostilbene and curcumin analogues. J. Agric. Food Chem. 2013, 61, 4326–4335. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Hong, B.H.; Ho, C.T.; Yen, G.C. Targeting cancer stem cells in breast cancer: Potential anticancer properties of 6-shogaol and pterostilbene. J. Agric. Food Chem. 2015, 63, 2432–2441. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, K.; Mukherjee, S.; Vanmanen, J.; Banerjee, P.; Fata, J.E. Dietary polyphenols, resveratrol and pterostilbene exhibit antitumor activity on an HPV E6-positive cervical cancer model: An in vitro and in vivo analysis. Front. Oncol. 2019, 9, 352. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, G.; Pourgheysari, B.; Shirzad, H.; Sourani, Z. Pterostilbene increases Fas expression in T-lymphoblastic leukemia cell lines. Res. Pharmacol. Sci. 2019, 14, 55. [Google Scholar]
- Tsai, J.H.; Hsu, L.S.; Lin, C.L.; Hong, H.M.; Pan, M.H.; Way, T.D.; Chen, W.J. 3,5,4′-Trimethoxystilbene, a natural methoxylated analog of resveratrol, inhibits breast cancer cell invasiveness by downregulation of PI3K/Akt and Wnt/β-catenin signaling cascades and reversal of epithelial-mesenchymal transition. Toxicol. Appl. Pharmacol. 2013, 272, 746–756. [Google Scholar] [CrossRef]
- Weng, C.J.; Yang, Y.T.; Ho, C.T.; Yen, G.C. Mechanisms of apoptotic effects induced by resveratrol, dibenzoylmethane, and their analogues on human lung carcinoma cells. J. Agric. Food Chem. 2009, 57, 5235–5243. [Google Scholar] [CrossRef]
- Yang, Y.T.; Weng, C.J.; Ho, C.T.; Yen, G.C. Resveratrol analog-3,5,4′-trimethoxy-trans-stilbene inhibits invasion of human lung adenocarcinoma cells by suppressing the MAPK pathway and decreasing matrix metalloproteinase-2 expression. Mol. Nutr. Food Res. 2009, 53, 407–416. [Google Scholar] [CrossRef]
- Zielińska-Przyjemska, M.; Kaczmarek, M.; Krajka-Kuźniak, V.; Łuczak, M.; Baer-Dubowska, W. The effect of resveratrol, its naturally occurring derivatives and tannic acid on the induction of cell cycle arrest and apoptosis in rat C6 and human T98G glioma cell lines. Toxicol. In Vitro 2017, 43, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Guo, X.; Chen, H.; Lin, T.; Xu, Y.; Chen, Q.; Liu, J.; Zeng, J.; Zhang, X.K.; Yao, X. A resveratrol analog, phoyunbene B, induces G2/M cell cycle arrest and apoptosis in HepG2 liver cancer cells. Bioorg. Med. Chem. Lett. 2012, 22, 2114–2118. [Google Scholar] [CrossRef] [PubMed]
- Androutsopoulos, V.P.; Fragiadaki, I.; Spandidos, D.A.; Tosca, A. The resveratrol analogue, 3,4,5,4′-trans-tetramethoxystilbene, inhibits the growth of A375 melanoma cells through multiple anticancer modes of action. Int. J. Oncol. 2016, 49, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Gosslau, A.; Pabbaraja, S.; Knapp, S.; Chen, K.Y. Trans- and cis-stilbene polyphenols induced rapid perinuclear mitochondrial clustering and p53-independent apoptosis in cancer cells but not normal cells. Eur. J. Pharmacol. 2008, 587, 25–34. [Google Scholar] [CrossRef]
- Piotrowska, H.; Myszkowski, K.; Abraszek, J.; Kwiatkowska-Borowczyk, E.; Amarowicz, R.; Murias, M.; Wierzchowski, M.; Jodynis-Liebert, J. DMU-212 inhibits tumor growth in xenograft model of human ovarian cancer. Biomed. Pharmacol. 2014, 68, 397–400. [Google Scholar] [CrossRef]
- Piotrowska-Kempisty, H.; Rucinski, M.; Borys, S.; Kucinska, M.; Kaczmarek, M.; Zawierucha, P.; Wierzchowski, M.; Lazewski, D.; Murias, M.; Jodynis-Liebert, J. 3′-hydroxy-3,4,5,4′-tetramethoxystilbene, the metabolite of resveratrol analogue DMU-212, inhibits ovarian cancer cell growth in vitro and in a mice xenograft model. Sci. Rep. 2016, 6, 32627. [Google Scholar] [CrossRef]
- Hong, M.; Park, N.; Chun, Y.J. Role of annexin A5 on mitochondria-dependent apoptosis induced by tetramethoxystilbene in human breast cancer cells. Biomol. Ther. 2014, 22, 519–524. [Google Scholar] [CrossRef]
- Xu, H. (Z)-3,4,3′,5′-Tetramethoxystilbene, a natural product, induces apoptosis and reduces viability of paclitaxel-And cisplatin-resistant osteosarcoma cells. J. Cancer Res. Ther. 2016, 12, 1261. [Google Scholar] [CrossRef]
- Pan, M.H.; Lin, C.L.I.; Tsal, J.H.; Ho, C.T.; Chen, W.J. 3,5,3′,4′,5′ -Pentamethoxystilbene (MR-5), a synthetically methoxylated analogue of resveratrol, inhibits growth and induces G1 cell cycle arrest of human breast carcinoma MCF-7 Cells. J. Agric. Food Chem. 2010, 58, 226–234. [Google Scholar] [CrossRef]
- Li, H.; Wu, W.K.K.; Zheng, Z.; Che, C.T.; Yu, L.; Li, Z.J.; Wu, Y.C.; Cheng, K.W.; Yu, J.; Cho, C.H.; et al. 2,3′,4,4′,5′-Pentamethoxy-trans-stilbene, a resveratrol derivative, is a potent inducer of apoptosis in colon cancer cells via targeting microtubules. Biochem. Pharmacol. 2009, 78, 1224–1232. [Google Scholar] [CrossRef]
- Li, H.; Wu, W.K.K.; Li, Z.J.; Chan, K.M.; Wong, C.C.M.; Ye, C.G.; Yu, L.; Sung, J.J.Y.; Cho, C.H.; Wang, M. 2,3′,4,4′,5′-Pentamethoxy-trans-stilbene, a resveratrol derivative, inhibits colitis-associated colorectal carcinogenesis in mice. Br. J. Pharmacol. 2010, 160, 1352–1361. [Google Scholar] [CrossRef]
- Fan, G.J.; Liu, X.D.; Qian, Y.P.; Shang, Y.J.; Li, X.Z.; Dai, F.; Fang, J.G.; Jin, X.L.; Zhou, B. 4,4′-Dihydroxy-trans-stilbene, a resveratrol analogue, exhibited enhanced antioxidant activity and cytotoxicity. Bioorg. Med. Chem. 2009, 17, 2360–2365. [Google Scholar] [CrossRef]
- Savio, M.; Coppa, T.; Bianchi, L.; Vannini, V.; Maga, G.; Forti, L.; Cazzalini, O.; Lazzè, M.C.; Perucca, P.; Prosperi, E.; et al. The resveratrol analogue 4,4′-dihydroxy-trans-stilbene inhibits cell proliferation with higher efficiency but different mechanism from resveratrol. Int. J. Biochem. Cell Biol. 2009, 41, 2493–2502. [Google Scholar] [CrossRef]
- Kimura, Y.; Sumiyoshi, M.; Baba, K. Antitumor and antimetastatic activity of synthetic hydroxystilbenes through inhibition of lymphangiogenesis and M2 macrophage differentiation of tumor-associated macrophages. Anticancer Res. 2016, 36, 137–148. [Google Scholar]
- Saha, B.; Patro, B.S.; Koli, M.; Pai, G.; Ray, J.; Bandyopadhyay, S.K.; Chattopadhyay, S. trans-4,4′-Dihydroxystilbene (DHS) inhibits human neuroblastoma tumor growth and induces mitochondrial and lysosomal damages in neuroblastoma cell lines. Oncotarget 2017, 8, 73905. [Google Scholar] [CrossRef]
- Saha, B.; Pai, G.B.; Subramanian, M.; Gupta, P.; Tyagi, M.; Patro, B.S.; Chattopadhyay, S. Resveratrol analogue, trans-4,4′-dihydroxystilbene (DHS), inhibits melanoma tumor growth and suppresses its metastatic colonization in lungs. Biomed. Pharmacol. 2018, 107, 1104–1114. [Google Scholar] [CrossRef]
- Chen, C.W.; Li, Y.; Hu, S.; Zhou, W.; Meng, Y.; Li, Z.; Zhang, Y.; Sun, J.; Bo, Z.; DePamphilis, M.L.; et al. DHS (trans−4,4′-dihydroxystilbene) suppresses DNA replication and tumor growth by inhibiting RRM2 (ribonucleotide reductase regulatory subunit M2). Oncogene 2019, 38, 2364–2379. [Google Scholar] [CrossRef]
- Larrosa, M.; Tomás-Barberán, F.A.; Espín, J.C. Grape polyphenol resveratrol and the related molecule 4-hydroxystilbene induce growth inhibition, apoptosis, S-phase arrest, and upregulation of cyclins A, E, and B1 in human SK-Mel-28 melanoma cells. J. Agric. Food Chem. 2003, 51, 4576–4584. [Google Scholar] [CrossRef]
- Larrosa, M.; Tomás-Barberán, F.A.; Espín, J.C. The grape and wine polyphenol piceatannol is a potent inducer of apoptosis in human SK-Mel-28 melanoma cells. Eur. J. Nutr. 2004, 43, 275–284. [Google Scholar] [CrossRef]
- Barton, B.E.; Karras, J.G.; Murphy, T.F.; Barton, A.; Huang, H.F.S. Signal transducer and activator of transcription 3 (STAT3) activation in prostate cancer: Direct STAT3 inhibition induces apoptosis in prostate cancer lines. Mol. Cancer Ther. 2004, 3, 11–20. [Google Scholar]
- Wolter, F.; Clausnitzer, A.; Akoglu, B.; Stein, J. Piceatannol, a Natural Analog of Resveratrol, Inhibits Progression through the S Phase of the Cell Cycle in Colorectal Cancer Cell Lines. J. Nutr. 2002, 132, 298–302. [Google Scholar] [CrossRef]
- Kuo, P.L.; Hsu, Y.L. The grape and wine constituent piceatannol inhibits proliferation of human bladder cancer cells via blocking cell cycle progression and inducing Fas/membrane bound Fas ligand-mediated apoptotic pathway. Mol. Nutr. Food Res. 2008, 52, 408–418. [Google Scholar] [CrossRef]
- Liu, W.H.; Chang, L. Sen Piceatannol induces Fas and FasL up-regulation in human leukemia U937 cells via Ca2+/p38α MAPK-mediated activation of c-Jun and ATF-2 pathways. Int. J. Biochem. Cell Biol. 2010, 42, 1498–1506. [Google Scholar] [CrossRef]
- Füllbeck, M.; Huang, X.; Dumdey, R.; Frommel, C.; Dubiel, W.; Preissner, R. Novel curcumin- and emodin-related compounds identified by in silico 2D/3D conformer screening induce apoptosis in tumor cells. BMC Cancer 2005, 5, 97. [Google Scholar] [CrossRef] [PubMed]
- CL, L.; SL, H.; Leung, W.; JH, J.; GC, H.; CT, L.; CC, W. 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside suppresses human colorectal cancer cell metastasis through inhibiting NF-κB activation. Int. J. Oncol. 2016, 49, 629–638. [Google Scholar]
- Lin, C.L.; Jeng, J.H.; Wu, C.C.; Hsieh, S.L.; Huang, G.C.; Leung, W.; Lee, C.T.; Chen, C.Y.; Lee, C.H. Chemopreventive Potential of 2,3,5,4′-Tetrahydroxystilbene-2-O-β-D-glucoside on the Formation of Aberrant Crypt Foci in Azoxymethane-Induced Colorectal Cancer in Rats. Biomed Res. Int. 2017, 2017, 3634915. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, Y.; Shen, H.; Pan, H.; Xu, L.; Yuan, L.; Ding, Z. The synergistic effect of 2,3,5,4′-Tetrahydroxystilbene-2-O-β-D-glucoside combined with Aadriamycin on MCF-7 breast cancer cells. Drug Des. Dev. Ther. 2018, 12, 4083–4094. [Google Scholar] [CrossRef]
- Szekeres, T.; Saiko, P.; Fritzer-Szekeres, M.; Djavan, B.; Jäger, W. Chemopreventive effects of resveratrol and resveratrol derivatives. Ann. N. Y. Acad. Sci. 2011, 1215, 89–95. [Google Scholar] [CrossRef]
- Murias, M.; Luczak, M.W.; Niepsuj, A.; Krajka-Kuzniak, V.; Zielinska-Przyjemska, M.; Jagodzinski, P.P.; Jäger, W.; Szekeres, T.; Jodynis-Liebert, J. Cytotoxic activity of 3,3′,4,4′,5,5′-hexahydroxystilbene against breast cancer cells is mediated by induction of p53 and downregulation of mitochondrial superoxide dismutase. Toxicol. In Vitro 2008, 22, 1361–1370. [Google Scholar] [CrossRef]
- Saiko, P.; Pemberger, M.; Horvath, Z.; Savinc, I.; Grusch, M.; Handler, N.; Erker, T.; Jaeger, W.; Fritzer-Szekeres, M.; Szekeres, T. Novel resveratrol analogs induce apoptosis and cause cell cycle arrest in HT29 human colon cancer cells: Inhibition of ribonucleotide reductase activity. Oncol. Rep. 2008, 19, 1621–1626. [Google Scholar]
- Kucinska, M.; Piotrowska, H.; Luczak, M.W.; Mikula-Pietrasik, J.; Ksiazek, K.; Wozniak, M.; Wierzchowski, M.; Dudka, J.; Jäger, W.; Murias, M. Effects of hydroxylated resveratrol analogs on oxidative stress and cancer cells death in human acute T cell leukemia cell line: Prooxidative potential of hydroxylated resveratrol analogs. Chem. Biol. Interact. 2014, 209, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Paulitschke, V.; Schicher, N.; Szekeres, T.; Jäger, W.; Elbling, L.; Riemer, A.B.; Scheiner, O.; Trimurtulu, G.; Venkateswarlu, S.; Mikula, M.; et al. 3,3′,4,4′,5,5′-hexahydroxystilbene impairs melanoma progression in a metastatic mouse model. J. Investig. Dermatol. 2010, 130, 1668–1679. [Google Scholar] [CrossRef] [PubMed]
- Mikuła-Pietrasik, J.; Sosińska, P.; Wierzchowski, M.; Piwocka, K.; Ksiazek, K. Synthetic resveratrol analogue, 3,3′,4,4′,5,5′-hexahydroxy-trans-stilbene, accelerates senescence in peritoneal mesothelium and promotes senescence-dependent growth of gastrointestinal cancers. Int. J. Mol. Sci. 2013, 14, 22483–22498. [Google Scholar] [CrossRef]
- Kotha, A.; Sekharam, M.; Cilenti, L.; Siddiquee, K.; Khaled, A.; Zervos, A.S.; Carter, B.; Turkson, J.; Jove, R. Resveratrol inhibits Src and Stat3 signaling and induces the apoptosis of malignant cells containing activated Stat3 protein. Mol. Cancer Ther. 2006, 5, 621–629. [Google Scholar] [CrossRef]
- Fouad, M.A.; Agha, A.M.; Merzabani, M.A.; Shouman, S.A. Resveratrol inhibits proliferation, angiogenesis and induces apoptosis in colon cancer cells: Calorie restriction is the force to the cytotoxicity. Hum. Exp. Toxicol. 2013, 32, 1067–1080. [Google Scholar] [CrossRef]
- Li, P.; Yang, S.; Dou, M.; Chen, Y.; Zhang, J.; Zhao, X. Synergic effects of artemisinin and resveratrol in cancer cells. J. Cancer Res. Clin. Oncol. 2014, 140, 2065–2075. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, B.; Cailing, E.; Liu, J.; Zhang, Q.; Liu, J.; Chen, N.; Chen, R.; Zhu, R. Resveratrol inhibits the proliferation of human melanoma cells by inducing G1/S cell cycle arrest and apoptosis. Mol. Med. Rep. 2015, 11, 400–404. [Google Scholar] [CrossRef]
- Shin, H.J.; Han, J.M.; Choi, Y.S.; Jung, H.J. Pterostilbene suppresses both cancer cells and cancer stem-like cells in cervical cancer with superior bioavailability to resveratrol. Molecules 2020, 25, 228. [Google Scholar] [CrossRef]
- Wen, W.; Lowe, G.; Roberts, C.M.; Finlay, J.; Han, E.S.; Glackin, C.A.; Dellinger, T.H. Pterostilbene, a natural phenolic compound, synergizes the antineoplastic effects of megestrol acetate in endometrial cancer. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Cheng, T.C.; Lai, C.S.; Chung, M.C.; Kalyanam, N.; Majeed, M.; Ho, C.T.; Ho, Y.S.; Pan, M.H. Potent anti-cancer effect of 39-hydroxypterostilbene in human colon xenograft tumors. PLoS ONE 2014, 9, e111814. [Google Scholar] [CrossRef]
- Schneider, Y.; Chabert, P.; Stutzmann, J.; Coelho, D.; Fougerousse, A.; Gossé, F.; Launay, J.F.; Brouillard, R.; Raul, F. Resveratrol analog (Z)-3,5,4′-trimethoxystilbene is a potent anti-mitotic drug inhibiting tubulin polymerization. Int. J. Cancer 2003, 107, 189–196. [Google Scholar] [CrossRef]
- Zaki, M.A.; Balachandran, P.; Khan, S.; Wang, M.; Mohammed, R.; Hetta, M.H.; Pasco, D.S.; Muhammad, I. Cytotoxicity and modulation of cancer-related signaling by (Z)- and (E)-3,4,3′,5′-tetramethoxystilbene isolated from Eugenia rigida. J. Nat. Prod. 2013, 76, 679–684. [Google Scholar] [CrossRef]
- Xu, M.; Wang, C.; Zhu, M.; Wang, X.; Zhang, L.; Zhao, J. 2, 3, 5, 4‑tetrahydroxy diphenylethylene‑2‑O‑glucoside inhibits the adhesion and invasion of A549 human lung cancer cells. Mol. Med. Rep. 2017, 16, 8900–8906. [Google Scholar]
- Bolton, J.L.; Trush, M.A.; Penning, T.M.; Dryhurst, G.; Monks, T.J. Role of quinones in toxicology. Chem. Res. Toxicol. 2000, 13, 135–160. [Google Scholar] [CrossRef]
- El-Najjar, N.; Gali-Muhtasib, H.; Ketola, R.A.; Vuorela, P.; Urtti, A.; Vuorela, H. The chemical and biological activities of quinones: Overview and implications in analytical detection. Phytochem. Rev. 2011, 10, 353. [Google Scholar] [CrossRef]
- Tseng, C.H.; Cheng, C.M.; Tzeng, C.C.; Peng, S.I.; Yang, C.L.; Chen, Y.L. Synthesis and anti-inflammatory evaluations of β-lapachone derivatives. Bioorg. Med. Chem. 2013, 21, 523–531. [Google Scholar] [CrossRef]
- Bolton, J.L.; Dunlap, T. Formation and biological targets of quinones: Cytotoxic versus cytoprotective effects. Chem. Res. Toxicol. 2017, 30, 13–37. [Google Scholar] [CrossRef]
- Cardoso, M.F.C.; Rodrigues, P.C.; Oliveira, M.E.I.M.; Gama, I.L.; Da Silva, I.M.C.B.; Santos, I.O.; Rocha, D.R.; Pinho, R.T.; Ferreira, V.F.; De Souza, M.C.B.V.; et al. Synthesis and evaluation of the cytotoxic activity of 1,2-furanonaphthoquinones tethered to 1,2,3-1H-triazoles in myeloid and lymphoid leukemia cell lines. Eur. J. Med. Chem. 2014, 84, 708–717. [Google Scholar] [CrossRef]
- Thomson, R.H. Distribution and Biogenesis. In Naturally Occurring Quinones; Elsevier: Amsterdam, The Netherlands, 1971. [Google Scholar]
- Rao, K.V.; Mcbride, T.J.; Oleson, J.J. Recognition and Evaluation of Lapachol as an Antitumor Agent. Cancer Res. 1968, 28, 1952–1954. [Google Scholar]
- Hussain, H.; Green, I.R. Lapachol and lapachone analogs: A journey of two decades of patent research (1997–2016). Expert Opin. Ther. Pat. 2017, 27, 1111–1121. [Google Scholar] [CrossRef]
- Salas, C.; Tapia, R.A.; Ciudad, K.; Armstrong, V.; Orellana, M.; Kemmerling, U.; Ferreira, J.; Maya, J.D.; Morello, A. Trypanosoma cruzi: Activities of lapachol and α- and β-lapachone derivatives against epimastigote and trypomastigote forms. Bioorg. Med. Chem. 2008, 16, 668–674. [Google Scholar] [CrossRef]
- Boveris, A.; Stoppani, A.O.M.; Docampo, R.; Cruz, F.S. Superoxide anion production and trypanocidal action of naphthoquinones on Trypanosoma cruzi. Comp. Biochem. Physiol. Part C Comp. 1978, 61, 327–329. [Google Scholar] [CrossRef]
- Silvers, M.A.; Deja, S.; Singh, N.; Egnatchik, R.A.; Sudderth, J.; Luo, X.; Beg, M.S.; Burgess, S.C.; DeBerardinis, R.J.; Boothman, D.A.; et al. The NQO1 bioactivatable drug, β-lapachone, alters the redox state of NQO1 pancreatic cancer cells, causing perturbation in central carbon metabolism. J. Biol. Chem. 2017, 292, 18203–18216. [Google Scholar] [CrossRef]
- Wuerzberger, S.M.; Pink, J.J.; Planchon, S.M.; Byers, K.L.; Bornmann, W.G.; Boothman, D.A. Induction of apoptosis in MCF-7: WS8 breast cancer cells by β-Lapachone. Cancer Res. 1998, 58, 1876–1885. [Google Scholar] [PubMed]
- Park, E.J.; Min, K.J.; Lee, T.J.; Yoo, Y.H.; Kim, Y.S.; Kwon, T.K. β-Lapachone induces programmed necrosis through the RIP1-PARP-AIF-dependent pathway in human hepatocellular carcinoma SK-Hep1 cells. Cell Death Dis. 2014, 5, e1230. [Google Scholar] [CrossRef] [PubMed]
- Gerber, D.E.; Beg, M.S.; Fattah, F.; Frankel, A.E.; Fatunde, O.; Arriaga, Y.; Dowell, J.E.; Bisen, A.; Leff, R.D.; Meek, C.C.; et al. Phase 1 study of ARQ 761, a β-lapachone analogue that promotes NQO1-mediated programmed cancer cell necrosis. Br. J. Cancer 2018, 119, 928. [Google Scholar] [CrossRef]
- Don, M.J.; Chang, Y.H.; Chen, K.K.; Ho, L.K.; Chau, Y.P. Induction of CDK inhibitors (p21WAF1 and p27Kip1) and BAK in the β-lapachone-induced apoptosis of human prostate cancer cells. Mol. Pharmacol. 2001, 59, 784–794. [Google Scholar] [CrossRef]
- Liu, T.J.; Lin, S.Y.; Chau, Y.P. Inhibition of poly(ADP-ribose) polymerase activation attenuates β-lapachone-induced necrotic cell death in human osteosarcoma cells. Toxicol. Appl. Pharmacol. 2002, 182, 116–125. [Google Scholar] [CrossRef]
- Yu, H.Y.; Kim, S.O.; Jin, C.Y.; Kim, G.Y.; Kim, W.J.; Yoo, Y.H.; Choi, Y.H. β-lapachone-induced apoptosis of human gastric carcinoma AGS cells is caspase-dependent and regulated by the PI3K/Akt pathway. Biomol. Ther. 2014, 22, 184. [Google Scholar] [CrossRef]
- Kee, J.Y.; Han, Y.H.; Kim, D.S.; Mun, J.G.; Park, S.H.; So, H.S.; Park, S.J.; Park, R.; Um, J.Y.; Hong, S.H. β-Lapachone suppresses the lung metastasis of melanoma via the MAPK signaling pathway. PLoS ONE 2017, 12, e0176937. [Google Scholar] [CrossRef]
- Woo, H.J.; Park, K.Y.; Rhu, C.H.; Lee, W.H.; Choi, B.T.; Kim, G.Y.; Park, Y.M.; Choi, Y.H. β-lapachone, a quinone isolated from Tabebuia avellanedae, induces apoptosis in HepG2 hepatoma cell line through induction of Bax and activation of caspase. J. Med. Food 2006, 9, 161–168. [Google Scholar] [CrossRef]
- Bey, E.A.; Bentle, M.S.; Reinicke, K.E.; Dong, Y.; Yang, C.R.; Girard, L.; Minna, J.D.; Bornmann, W.G.; Gao, J.; Boothman, D.A. An NQO1- and PARP-1-mediated cell death pathway induced in non-small-cell lung cancer cells by β-lapachone. Proc. Natl. Acad. Sci. USA 2007, 104, 11832–11837. [Google Scholar] [CrossRef]
- Huang, X.; Dong, Y.; Bey, E.A.; Kilgore, J.A.; Bair, J.S.; Li, L.S.; Patel, M.; Parkinson, E.I.; Wang, Y.; Williams, N.S.; et al. An NQO1 substrate with potent antitumor activity that selectively kills by PARP1-induced programmed necrosis. Cancer Res. 2012, 72, 3038–3047. [Google Scholar] [CrossRef] [PubMed]
- Bey, E.A.; Reinicke, K.E.; Srougi, M.C.; Varnes, M.; Anderson, V.E.; Pink, J.J.; Li, L.S.; Patel, M.; Cao, L.; Moore, Z.; et al. Catalase abrogates β-lapachone-induced PARP1 hyperactivation-directed programmed necrosis in NQO1-positive breast cancers. Mol. Cancer Ther. 2013, 12, 2110–2120. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Pardee, A.B. β-lapachone induces cell cycle arrest and apoptosis in human colon cancer cells. Mol. Med. 1999, 5, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Liu, T.; Ho, L.; Don, M.; Chau, Y. Beta-lapachone induced cell death in human hepatoma (HepA2) cells. Histol. Histopathol. 1998, 13, 89–97. [Google Scholar] [PubMed]
- Wu, Y.; Wang, X.; Chang, S.; Lu, W.; Liu, M.; Pang, X. β-lapachone induces NAD(P)H:quinone oxidoreductase-1- and oxidative stress-dependent heat shock protein 90 cleavage and inhibits tumor growth and angiogenesiss. J. Pharmacol. Exp. Ther. 2016, 357, 466–475. [Google Scholar] [CrossRef]
- Silva, E.O.; de Carvalho, T.C.; Parshikov, I.A.; dos Santos, R.A.; Emery, F.S.; Furtado, N.A.J.C. Cytotoxicity of lapachol metabolites produced by probiotics. Lett. Appl. Microbiol. 2014, 59, 108–114. [Google Scholar] [CrossRef]
- Salustiano, E.J.S.; Netto, C.D.; Fernandes, R.F.; Da Silva, A.J.M.; Bacelar, T.S.; Castro, C.P.; Buarque, C.D.; Maia, R.C.; Rumjanek, V.M.; Costa, P.R.R. Comparison of the cytotoxic effect of lapachol, α-lapachone and pentacyclic 1,4-naphthoquinones on human leukemic cells. Investig. New Drugs 2010, 28, 139–144. [Google Scholar] [CrossRef]
- Li, X.; Bian, J.; Wang, N.; Qian, X.; Gu, J.; Mu, T.; Fan, J.; Yang, X.; Li, S.; Yang, T.; et al. Novel naphtho[2,1-d]oxazole-4,5-diones as NQO1 substrates with improved aqueous solubility: Design, synthesis, and in vivo antitumor evaluation. Bioorg. Med. Chem. 2016, 24, 1006–1013. [Google Scholar] [CrossRef]
- Dias, R.B.; de Araújo, T.B.S.; de Freitas, R.D.; Rodrigues, A.C.B.D.C.; Sousa, L.P.; Sales, C.B.S.; de Valverde, L.F.; Soares, M.B.P.; dos Reis, M.G.; Coletta, R.D.; et al. β-Lapachone and its iodine derivatives cause cell cycle arrest at G2/M phase and reactive oxygen species-mediated apoptosis in human oral squamous cell carcinoma cells. Free Radic. Biol. Med. 2018, 126, 87–100. [Google Scholar]
- Ross, D.; Siegel, D. Functions of NQO1 in cellular protection and CoQ10 metabolism and its potential role as a redox sensitive molecular switch. Front. Physiol. 2017, 8, 595. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.; Yan, C.; Ross, D. NAD(P)H:quinone oxidoreductase 1 (NQO1) in the sensitivity and resistance to antitumor quinones. Biochem. Pharmacol. 2012, 83, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, G.; Moore, Z.R.; Luo, X.; Ilcheva, M.; Ali, A.; Padanad, M.; Zhou, Y.; Xie, Y.; Burma, S.; Scaglioni, P.P.; et al. Targeting glutamine metabolism sensitizes pancreatic cancer to PARP-driven metabolic catastrophe induced by ß-lapachone. Cancer Metab. 2015, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Tagliarino, C.; Pink, J.J.; Reinicke, K.E.; Simmers, S.M.; Wuerzberger-Davis, S.M.; Boothman, D.A. μ-calpain activation in β-lapachone-mediated apoptosis. Cancer Biol. Ther. 2003, 2, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Li, L.S.; Bey, E.A.; Dong, Y.; Meng, J.; Patra, B.; Yan, J.; Xie, X.J.; Brekken, R.A.; Barnett, C.C.; Bornmann, W.G.; et al. Modulating endogenous NQO1 levels identifies key regulatory mechanisms of action of β-lapachone for pancreatic cancer therapy. Clin. Cancer Res. 2011, 17, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Buranrat, B.; Chau-In, S.; Prawan, A.; Puapairoj, A.; Zeekpudsa, P.; Kukongviriyapan, V. NQO1 expression correlates with Cholangiocarcinoma prognosis. Asian Pac. J. Cancer Prev. 2012, 13, 131–136. [Google Scholar]
- Siegel, D.; Franklin, W.A.; Ross, D. Immunohistochemical detection of NAD(P)H:Quinone oxidoreductase in human lung and lung tumors. Clin. Cancer Res. 1998, 4, 2065–2070. [Google Scholar]
- Awadallah, N.S.; Dehn, D.; Shah, R.J.; Russell Nash, S.; Chen, Y.K.; Ross, D.; Bentz, J.S.; Shroyer, K.R. NQO1 expression in pancreatic cancer and its potential use as a biomarker. Appl. Immunohistochem. Mol. Morphol. 2008, 16, 24–31. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Wu, Q.; Cui, X.; Lin, Z.; Liu, S.; Chen, L. Clinical implications of high NQO1 expression in breast cancers. J. Exp. Clin. Cancer Res. 2014, 33, 14. [Google Scholar] [CrossRef]
- Ma, Y.; Kong, J.; Yan, G.; Ren, X.; Jin, D.; Jin, T.; Lin, L.; Lin, Z. NQO1 overexpression is associated with poor prognosis in squamous cell carcinoma of the uterine cervix. BMC Cancer 2014, 14, 414. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, X.; Xu, M.; Piao, J.; Zhang, Y.; Lin, Z.; Chen, L. β-Lapachone suppresses tumour progression by inhibiting epithelial-to-mesenchymal transition in NQO1-positive breast cancers. Sci. Rep. 2017, 7, 2681. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.; Ross, D. Immunodetection of NAD(P)H:quinone oxidoreductase 1 (NQO1) in human tissues. Free Radic. Biol. Med. 2000, 29, 246–253. [Google Scholar] [CrossRef]
- Strassburg, A.; Strassburg, C.P.; Manns, M.P.; Tukey, R.H. Differential gene expression of NAD(P)H:Quinone oxidoreductase and NRH:Quinone oxidoreductase in human hepatocellular and biliary tissue. Mol. Pharmacol. 2002, 61, 320–325. [Google Scholar] [CrossRef]
- Schor, N.A.; Morris, H.P. The activity of the D T diaphorase in experimental hepatomas. Cancer Biochem. Biophys. 1977, 2, 5–9. [Google Scholar]
- Cresteil, T.; Jaiswal, A.K. High levels of expression of the NAD(P)H:Quinone oxidoreductase (NQO1) gene in tumor cells compared to normal cells of the same origin. Biochem. Pharmacol. 1991, 42, 1021–1027. [Google Scholar] [CrossRef]
- Hori, T.; Kondo, T.; Lee, H.; Song, C.W.; Park, H.J. Hyperthermia enhances the effect of β-lapachone to cause γh2AX formations and cell death in human osteosarcoma cells. Int. J. Hyperth. 2011, 27, 53–62. [Google Scholar] [CrossRef]
- Dong, G.Z.; Youn, H.; Park, M.T.; Oh, E.T.; Park, K.H.; Song, C.W.; Kyung Choi, E.; Park, H.J. Heat shock increases expression of NAD(P)H:quinone oxidoreductase (NQO1), mediator of β-lapachone cytotoxicity, by increasing NQO1 gene activity and via Hsp70-mediated stabilisation of NQO1 protein. Int. J. Hyperth. 2009, 25, 477–487. [Google Scholar] [CrossRef]
- Song, C.W.; Chae, J.J.; Choi, E.K.; Hwang, T.S.; Kim, C.; Lim, B.U.L.; Park, H.J. Anti-cancer effect of bio-reductive drug β-lapachon is enhanced by activating NQO1 with heat shock. Int. J. Hyperth. 2008, 24, 161–169. [Google Scholar] [CrossRef]
- Park, H.J.; Choi, E.K.; Choi, J.; Ahn, K.J.; Kim, E.J.; Ji, I.M.; Kook, Y.H.; Ahn, S.D.; Williams, B.; Griffin, R.; et al. Heat-induced up-regulation of NAD(P)H:quinone oxidoreductase potentiates anticancer effects of β-lapachone. Clin. Cancer Res. 2005, 11, 8866–8871. [Google Scholar] [CrossRef]
- Boothman, D.A.; Trask, D.K.; Pardee, A.B. Inhibition of Potentially Lethal DNA Damage Repair in Human Tumor Cells by β-Lapachone, an Activator of Topoisomerase I. Cancer Res. 1989, 49, 605–612. [Google Scholar]
- Jones, J.C.; Stevnsner, T.; Mattern, M.R.; Bohr, V.A. Effect of specific enzyme inhibitors on replication, total genome DNA repair and on gene-specific DNA repair after UV irradiation in CHO cells. Mutat. Res. Repair 1991, 255, 155–162. [Google Scholar] [CrossRef]
- Katz, E.J.; Vick, J.S.; Kling, K.M.; Andrews, P.A.; Howell, S.B. Effect of topoisomerase modulators on cisplatin cytotoxicity in human ovarian carcinoma cells. Eur. J. Cancer Clin. Oncol. 1990, 26, 724–727. [Google Scholar] [CrossRef]
- Li, C.J.; Averboukh, L.; Pardee, A.B. β-Lapachone, a novel DNA topoisomerase I inhibitor with a mode of action different from camptothecin. J. Biol. Chem. 1993, 268, 22463–22468. [Google Scholar]
- Li, C.J.; Wang, C.; Pardee, A.B. Induction of Apoptosis by β-Lapachone in Human Prostate Cancer Cells. Cancer Res. 1995, 55, 3712–3715. [Google Scholar] [PubMed]
- Furuya, Y.; Ohta, S.; Ito, H. Apoptosis of androgen-independent mammary and prostate cell lines induced by topoisomerase inhibitors: Common pathway of gene regulation. Anticancer Res. 1997, 17, 2089–2093. [Google Scholar]
- Planchon, S.M.; Wuerzberger, S.; Boothman, D.A.; Church, D.R.; Wilding, G.; Frydman, B.; Witiak, D.T.; Hutson, P. β-Lapachone-mediated Apoptosis in Human Promyelocytic Leukemia (HL-60) and Human Prostate Cancer Cells: A p53-independent Response. Cancer Res. 1995, 55, 3706–3711. [Google Scholar]
- Li, Y.; Sun, X.; LaMont, J.T.; Pardee, A.B.; Li, C.J. Selective killing of cancer cells by β-lapachone: Direct checkpoint activation as a strategy against cancer. Proc. Natl. Acad. Sci. USA 2003, 100, 2674–2678. [Google Scholar] [CrossRef]
- Choi, Y.H.; Ho, S.K.; Yoo, M.A. Suppression of human prostate cancer cell growth by β-lapachone via down-regulation of pRB phosphorylation and induction of Cdk inhibitor P21 WAF1/CIP1. J. Biochem. Mol. Biol. 2003, 36, 223–229. [Google Scholar] [CrossRef]
- Pink, J.J.; Wuerzberger-Davis, S.; Tagliarino, C.; Planchon, S.M.; Yang, X.H.; Froelich, C.J.; Boothman, D.A. Activation of a cysteine protease in MCF-7 and T47D breast cancer cells during β-lapachone-mediated apoptosis. Exp. Cell Res. 2000, 255, 144–145. [Google Scholar] [CrossRef]
- Ough, M.; Lewis, A.; Bey, E.A.; Gao, J.; Ritchie, J.M.; Bornmann, W.; Boothman, D.A.; Oberley, L.W.; Cullen, J.J. Efficacy of β-lapachone in pancreatic cancer treatment: Exploiting the novel, therapeutic target NQO1. Cancer Biol. Ther. 2005, 4, 102–109. [Google Scholar] [CrossRef]
- Kim, I.; Kim, H.; Ro, J.; Jo, K.; Karki, S.; Khadka, P.; Yun, G.; Lee, J. Preclinical pharmacokinetic evaluation of β-lapachone: Characteristics of oral bioavailability and first-pass metabolism in rats. Biomol. Ther. 2015, 23, 296. [Google Scholar] [CrossRef] [PubMed]
- Mangas-Sanjuan, V.; Gutiérrez-Nieto, J.; Echezarreta-López, M.; González-Álvarez, I.; González-Álvarez, M.; Casabó, V.G.; Bermejo, M.; Landin, M. Intestinal Permeability of β-Lapachone and Its Cyclodextrin Complexes and Physical Mixtures. Eur. J. Drug Metab. Pharmacokinet. 2016, 41, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Bey, E.A.; Khemtong, C.; Yang, S.G.; Setti-Guthi, J.; Chen, H.; Kessinger, C.W.; Carnevale, K.A.; Bornmann, W.G.; Boothman, D.A.; et al. β-lapachone micellar nanotherapeutics for non-small cell lung cancer therapy. Cancer Res. 2010, 70, 3896–3904. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Nicoletti, C.; Ferreira, P.; Futuro, D.; da Silva, F. Strategies for Increasing the Solubility and Bioavailability of Anticancer Compounds: β-Lapachone and Other Naphthoquinones. Curr. Pharm. Des. 2016, 22, 5899–5914. [Google Scholar] [CrossRef]
- Szejtli, J. Cyclodextrin Technology; Springer: Berlin, Germany, 2013; ISBN 9788578110796. [Google Scholar]
- Dahan, A.; Lennernäs, H.; Amidon, G.L. The fraction dose absorbed, in humans, and high jejunal human permeability relationship. Mol. Pharm. 2012, 9, 1847–1851. [Google Scholar] [CrossRef]
- Seoane, S.; Díaz-Rodríguez, P.; Sendon-Lago, J.; Gallego, R.; Pérez-Fernández, R.; Landin, M. Administration of the optimized β-Lapachone-poloxamer-cyclodextrin ternary system induces apoptosis, DNA damage and reduces tumor growth in a human breast adenocarcinoma xenograft mouse model. Eur. J. Pharm. Biopharm. 2013, 84, 497–504. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Jang, S.B.; Kim, D.; Kim, S.Y.; Park, C.; Jeong, J.H.; Kuh, H.J.; Lee, J. Impact of micellar vehicles on in situ intestinal absorption properties of β-lapachone in rats. Korean J. Physiol. Pharmacol. 2013, 17, 9–13. [Google Scholar] [CrossRef]
- Dai, L.; Li, X.; Duan, X.; Li, M.; Niu, P.; Xu, H.; Cai, K.; Yang, H. A pH/ROS Cascade-Responsive Charge-Reversal Nanosystem with Self-Amplified Drug Release for Synergistic Oxidation-Chemotherapy. Adv. Sci. 2019, 6, 1801807. [Google Scholar] [CrossRef]
- Li, X.; Jia, X.; Niu, H. Nanostructured lipid carriers co-delivering lapachone and doxorubicin for overcoming multidrug resistance in breast cancer therapy. Int. J. Nanomed. 2018, 13, 4107–4119. [Google Scholar] [CrossRef]
- Yin, W.; Ke, W.; Chen, W.; Xi, L.; Zhou, Q.; Mukerabigwi, J.F.; Ge, Z. Integrated block copolymer prodrug nanoparticles for combination of tumor oxidative stress amplification and ROS-responsive drug release. Biomaterials 2019, 195, 63–74. [Google Scholar] [CrossRef]
- Da Silva Júnior, E.N.; Jardim, G.A.M.; Jacob, C.; Dhawa, U.; Ackermann, L.; de Castro, S.L. Synthesis of quinones with highlighted biological applications: A critical update on the strategies towards bioactive compounds with emphasis on lapachones. Eur. J. Med. Chem. 2019. [Google Scholar] [CrossRef]
- Di Rosso, M.E.; Barreiro Arcos, M.L.; Elingold, I.; Sterle, H.; Baptista Ferreira, S.; Ferreira, V.F.; Galleano, M.; Cremaschi, G.; Dubin, M. Novel o-naphthoquinones induce apoptosis of EL-4 T lymphoma cells through the increase of reactive oxygen species. Toxicol. In Vitro 2013, 27, 2094–2104. [Google Scholar] [CrossRef]
- Araújo, A.J.; de Souza, A.A.; da Silva Júnior, E.N.; Marinho-Filho, J.D.B.; de Moura, M.A.B.F.; Rocha, D.D.; Vasconcellos, M.C.; Costa, C.O.; Pessoa, C.; de Moraes, M.O.; et al. Growth inhibitory effects of 3′-nitro-3-phenylamino nor-beta-lapachone against HL-60: A redox-dependent mechanism. Toxicol. In Vitro 2012, 26, 585–594. [Google Scholar] [CrossRef]
- Da Rocha, D.R.; De Souza, A.C.G.; Resende, J.A.L.C.; Santos, W.C.; Dos Santos, E.A.; Pessoa, C.; De Moraes, M.O.; Costa-Lotufo, L.V.; Montenegro, R.C.; Ferreira, V.F. Synthesis of new 9-hydroxy-α- and 7-hydroxy-β-pyran naphthoquinones and cytotoxicity against cancer cell lines. Org. Biomol. Chem. 2011, 9, 4315–4322. [Google Scholar] [CrossRef]
- Bortolot, C.S.; da S.M. Forezi, L.; Marra, R.K.F.; Reis, M.I.P.; Sá, B.V.F.; Filho, R.I.; Ghasemishahrestani, Z.; Sola-Penna, M.; Zancan, P.; Ferreira, V.F.; et al. Design, Synthesis and Biological Evaluation of 1H-1,2,3-Triazole-Linked-1H-Dibenzo[b,h]xanthenes as Inductors of ROS-Mediated Apoptosis in the Breast Cancer Cell Line MCF-7. Med. Chem. 2018, 15, 119–129. [Google Scholar]
- Da Cruz, E.H.G.; Silvers, M.A.; Jardim, G.A.M.; Resende, J.M.; Cavalcanti, B.C.; Bomfim, I.S.; Pessoa, C.; De Simone, C.A.; Botteselle, G.V.; Braga, A.L.; et al. Synthesis and antitumor activity of selenium-containing quinone-based triazoles possessing two redox centres, and their mechanistic insights. Eur. J. Med. Chem. 2016, 122, 1–16. [Google Scholar] [CrossRef]
- Ma, X.; Huang, X.; Moore, Z.; Huang, G.; Kilgore, J.A.; Wang, Y.; Hammer, S.; Williams, N.S.; Boothman, D.A.; Gao, J. Esterase-activatable β-lapachone prodrug micelles for NQO1-targeted lung cancer therapy. J. Control. Release 2015, 200, 201–211. [Google Scholar] [CrossRef]
- Lamberti, M.J.; Morales Vasconsuelo, A.B.; Chiaramello, M.; Ferreira, V.F.; Macedo Oliveira, M.; Baptista Ferreira, S.; Rivarola, V.A.; Rumie Vittar, N.B. NQO1 induction mediated by photodynamic therapy synergizes with β-Lapachone-halogenated derivative against melanoma. Biomed. Pharmacother. 2018, 108, 1553–1564. [Google Scholar] [CrossRef]
- Motea, E.A.; Huang, X.; Singh, N.; Kilgore, J.A.; Williams, N.S.; Xie, X.J.; Gerber, D.E.; Beg, M.S.; Bey, E.A.; Boothman, D.A. NQO1-dependent, tumor-selective radiosensitization of non-small cell lung cancers. Clin. Cancer Res. 2019, 25, 2601–2609. [Google Scholar] [CrossRef]
- Li, L.S.; Reddy, S.; Lin, Z.H.; Liu, S.; Park, H.; Chun, S.G.; Bornmann, W.G.; Thibodeaux, J.; Yan, J.; Chakrabarti, G.; et al. NQO1-Mediated tumor-selective lethality and radiosensitization for head and neck cancer. Mol. Cancer Ther. 2016, 15, 1757–1767. [Google Scholar] [CrossRef] [PubMed]

| Compound | 2D Structure | IC50 | Study Model | References |
|---|---|---|---|---|
| Resveratrol and Resveratrol Methoxylated Derivatives | ||||
| Resveratrol |  | 15–145 µM | Breast (MCF-7, MDA-MB-231, MDA-MB-468, MDA-MB-453), lung (A549, H460), pancreatic (Colo-357, Panc-1), prostate (LNCap, DU145) and colon (HCT116, Caco2) cancer cells; cervix carcinoma (HeLa); hepatocarcinoma (HepG2); melanoma (A357, SK-MEL-31); glioma (C6, T98G) | [22,42,75,76,77,78] |
| Pterostilbene |  | 4.1–108 µM | Acute lymphoblastic leukemia (Molt-4), acute T cell leukemia (Jurkat), breast (MDA-MB-231), colon (COLO 205, HCT-116, HT-29) and endometrial (HEC-1A, ECC-1) cancer cells; melanoma (A357); hepatocarcinoma (HepG2); cervix carcinoma (HeLa, SiHa); epidermoid carcinoma (CaSki); | [27,38,79,80,81] |
| Trimethoxystilbene |  | 0.08–80.3 µM | Colon (Caco2, SW480), head and neck (KB), lymphoma (TK6) and breast (MCF-7) cancer cells; glioma (C6, T98G) | [39,42,82] |
| Tetramethoxystilbene |  | 4-60–µM | Leukemia (HL-60) and breast (BT-459) cancer cells, melanoma (SK-MEL); cervix carcinoma (HeLa) | [83] |
| Pentamethoxystilbene |  | 29.2–37.8 µM | Breast (MCF-7) and colon (Colon26) cancer cells | [50,52] |
| Hydroxylated Resveratrol Derivatives | ||||
| Dihydroxystilbene |  | 2.3–6.5 µM | Leukemia (HL-60), colon (HCT-116) and breast (MDA-MB-231) cancer cells; osteosarcoma (U2OS) | [57] |
| Tetrahydroxystilbene |  | 58.4–620.6 µM | Acute T cell leukemia (Jurkat), breast (MCF-7), lung (H1299, A549) and prostate (LNC) cancer cells; | [68,72,84] |
| Hexahydroxystilbene |  | 6.25–127.8 µM | Breast (T47D, ZR-75-1, MDA-MB-231), colon (HT-29), leukemia (HL-60) cancer cells; melanoma (M24met) | [69,70,71,73] |
| Compound | 2D Structure | IC50 | Study Model | References |
|---|---|---|---|---|
| Lapachol | 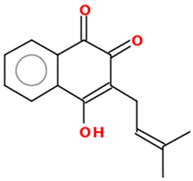 | 16.04–72.3 µM | Human chronic myelogenous leukemia (K562, Lucena), Burkitt’s lymphoma (Daudi), Breast cancer (MCF-7, SK-BR3) | [110,111] |
| ß-lapachone |  | 0.03–70.13 µM | Lung cancer cells (A549 cell line); Tongue squamous cell carcinoma (HSC-3, SCC4, SCC9, SCC15, SCC25), hepatocellular carcinoma (HEPG2), HL-60, K562, Gastric adenocarcinoma (AGP-01, ACP-02, ACP-03), colon adenocarcinoma (HT-29, HCT-116). | [112,113] |
| α-lapachone |  | 38–69 µM | K562, Lucena, Daudi, MCF-7 | [111] |
| 3-iodo-ß-lapachone |  | 0.02–5.61 µM | Tongue squamous cell carcinoma (HSC-3, SCC4, SCC9, SCC15, SCC25), hepatocellular carcinoma (HEPG2), HL-60, K562, Gastric adenocarcinoma (AGP-01, ACP-02, ACP-03), colon adenocarcinoma (HT-29, HCT-116). | [113] |
| 3-I-α-lapachone |  | 0.77–14.65 µM | ||
| naphtho[2,1-d]oxazole-4,5-diones |  | 4.6–20 µM * | Lung cancer cells (A549 cell line) | [112] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraz da Costa, D.C.; Pereira Rangel, L.; Martins-Dinis, M.M.D.d.C.; Ferretti, G.D.d.S.; Ferreira, V.F.; Silva, J.L. Anticancer Potential of Resveratrol, β-Lapachone and Their Analogues. Molecules 2020, 25, 893. https://doi.org/10.3390/molecules25040893
Ferraz da Costa DC, Pereira Rangel L, Martins-Dinis MMDdC, Ferretti GDdS, Ferreira VF, Silva JL. Anticancer Potential of Resveratrol, β-Lapachone and Their Analogues. Molecules. 2020; 25(4):893. https://doi.org/10.3390/molecules25040893
Chicago/Turabian StyleFerraz da Costa, Danielly C., Luciana Pereira Rangel, Mafalda Maria Duarte da Cunha Martins-Dinis, Giulia Diniz da Silva Ferretti, Vitor F. Ferreira, and Jerson L. Silva. 2020. "Anticancer Potential of Resveratrol, β-Lapachone and Their Analogues" Molecules 25, no. 4: 893. https://doi.org/10.3390/molecules25040893
APA StyleFerraz da Costa, D. C., Pereira Rangel, L., Martins-Dinis, M. M. D. d. C., Ferretti, G. D. d. S., Ferreira, V. F., & Silva, J. L. (2020). Anticancer Potential of Resveratrol, β-Lapachone and Their Analogues. Molecules, 25(4), 893. https://doi.org/10.3390/molecules25040893






