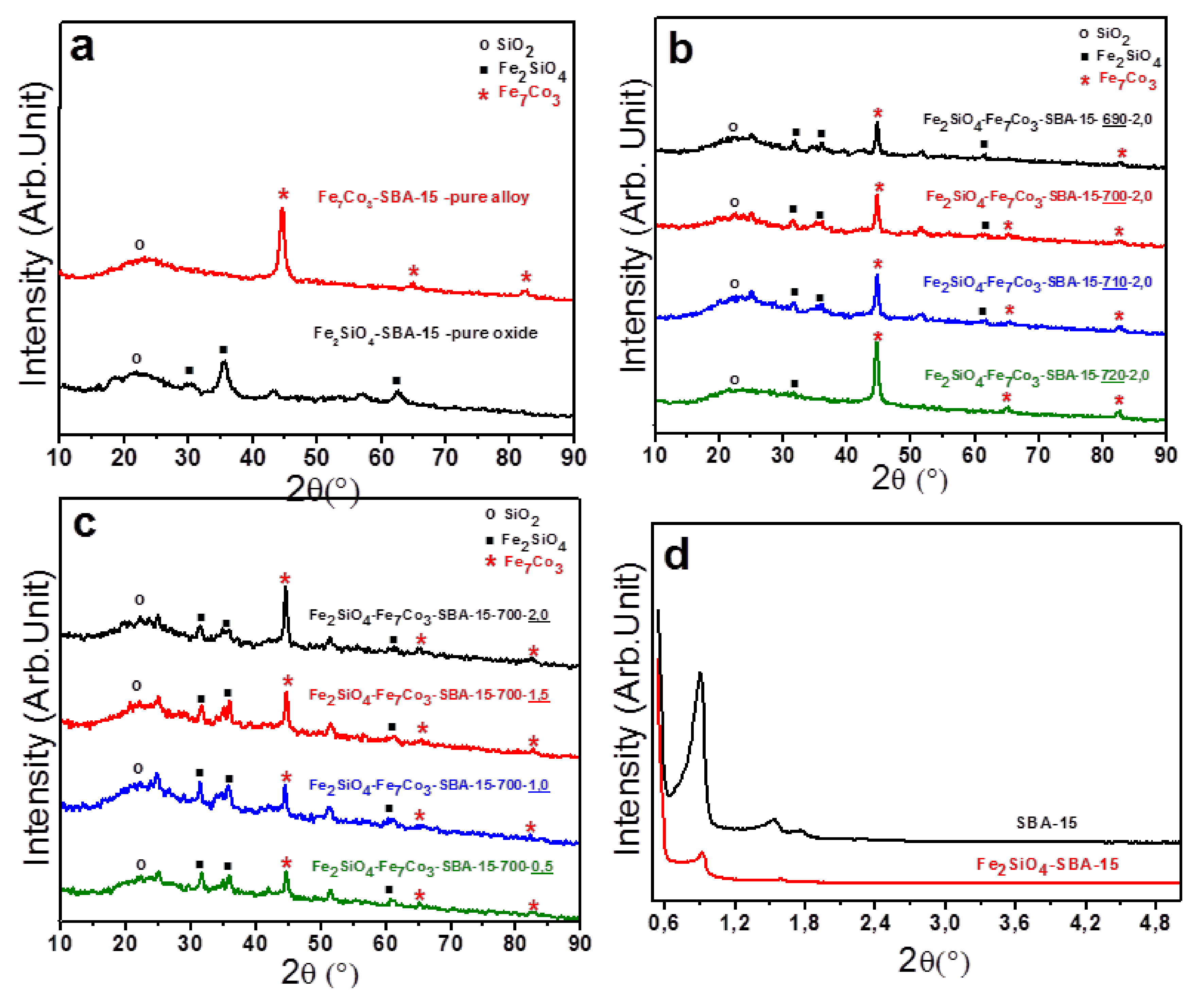
2.1. Structural Properties (X-ray Diffraction)
Based on the X-ray diffraction results, one can observe the influence of the reduction temperature and hydrogen content on the formation of the nanocomposite, since the intensities of the diffraction peaks varied depending on the temperature and the amount of H2 used in total flow rate.
Initially, in
Figure 1a, the characteristic diffractograms are presented for the samples containing oxide and pure alloy dispersed in the mesoporous SBA-15. It was also possible to observe, for both materials, the presence of a broad peak with 2θ values around 22°, characteristic of amorphous silica (SiO
2) from the SBA-15 matrix. For pure oxide supported on SBA-15, well-defined peaks with 2θ values of approximately 30, 35, 62°, respectively, associated to iron silicate (Fe
2SiO
4), fayalite (JCPDS 00-071-1678), were visualized. In addition, for the pure oxide sample, cobalt ferrite formation (JCPDS 00-022-1086) was also observed. On the other hand, for the composite containing pure alloy supported on SBA-15, it was possible to identify three characteristic peaks concerning the Fe
7Co
3 phase (JCPDS 00-048-1816) with 2θ values in approximately 44.7, 65, and 82.5°, respectively. Thus, it confirms the total reduction of the oxide to form the alloy at 900 °C using pure H
2. This is an expected result, since in this temperature range, iron and cobalt oxides are completely reduced [
38,
39]. The result of the Fe
7Co
3-SBA-15 solid indicates that the use of pure H
2 was not adequate, since the use of high reducing gas content resulted in complete oxide reduction. Thus, it is necessary to use a mixture of H
2 and N
2 to reduce oxide only partially and lead to the formation of the dispersed oxide-alloy mixture supported in mesoporous silica.
The first study showing the effect of the reduction temperature on the formation of the oxide-alloy mixture, keeping the amount of hydrogen constant. The obtained results are presented in
Figure 1b. This figure shows the diffractograms of samples starting from pure oxide (Fe
2SiO
4-SBA) submitted to different reduction temperature conditions with values ranging from 690 to 720 °C, maintaining the percentage reducing gas of 2% H
2 and 98% N
2 in the total flow rate of 25 mL/min.
One can observe that the increase in the reduction temperature leads to an increase in the peak intensity referring to Fe7Co3 alloy, which means that if the hydrogen amount is kept constant, the increase in the reduction temperature favors the reduction of oxide to alloy. Thus, it is possible to note that at 690 °C the sample Fe2SiO4- Fe7Co3-SBA-15-690-2.0 has a higher amount of oxide compared to other composites, while at 720 °C, the solid has a higher amount of alloy and only a small fraction of oxide.
In addition, the second study shows the effect of hydrogen concentration on the formation of composite, maintaining the reduction temperature at 700 °C constant, see
Figure 1c. The diffractograms show the results concerning the four different H
2 percentages. The effect of H
2 content was less significant in composite formation compared to the influence of reduction temperature. However, it can be noted that the increase in the amount of H
2 in the total flow rate leads to an increase in the amount of alloy in relation to oxide, confirming the role of hydrogen for reduction of oxide to alloy. Thus, an adequate control of the hydrogen amount in relation to nitrogen in the flow rate is fundamental to obtain the appropriate mixture of the Fe
2SiO
4 and Fe
7Co
3 phases dispersed in the silica matrix, taking into account that an excess of H
2 will lead to a complete reduction of oxide.
Although all diffractograms qualitatively show two phases concerning Fe
7Co
3 alloy and Fe
2SiO
4-based oxide, the relative amount between phases changed depending on the reaction temperature and the amount of hydrogen applied, as can be seen in the peaks intensities. The structure refinement based on the Rietveld method was performed in order to quantify the observed phases and then calculate the crystallite size using the Scherrer equation. It was not possible to obtain qualitative data for the Fe
2SiO
4-SBA-15 sample due to the broad and low intensity peak for the Fe
2SiO
4-based oxide sample, and the diffractograms were quantified only for the oxide and alloy mixture. The results are presented in
Table 1. The experimental and theoretical diffractograms obtained from the refinements are detailed in the
supplementary materials (Figures S1 and S2).
It can be observed that the sample containing pure oxide was mainly composed by iron silicate and a small amount of cobalt ferrite, while the pure alloy corresponds to 100% of the Fe
7Co
3 phase, characterizing that the oxide was completely reduced to alloy. On the other hand, in
Table 1, the refinement confirms that the increase in the reduction temperature and the increase in H
2 content in total flow rate favor the reduction of oxide to alloy. The percentage of Fe
7Co
3 phase increased comparing the highest with the lowest reduction temperature and the highest with the lowest H
2 content. This indicate that control of synthesis conditions is essential to obtain the desired percentage of alloy and oxide.
Furthermore, it was possible to observe that the crystallite size obtained from the diffractograms in
Figure 1 did not vary significantly with the reduction temperature and with the amount of H
2. This indicates that the increase from 690 to 720 °C was not significant to provide an evident increase in crystallite size due to particle sintering. The crystallites size values are between 14 and 20 nm for the different synthesized composites based on the data described in
Table 1, confirming that the synthesis method used leads to the formation of small crystallites.
Previous papers describe the possible reactions that justify the formation of fayalite from iron oxides and silicon. A series of gas-solid reactions in the presence of H
2 may be occurring, which leads to a series of redox reactions such as 2Fe
3+ + 2Fe
0 → 3Fe
2+ and 2α-Fe
3+ + 2Fe + 3SiO
2 → 3α-Fe
2SiO
4 [
40,
41].
Low-angle XRD was performed in order to characterize the mesoporous structure. The results are presented in
Figure 1d. The low angle diffractograms of the SBA-15 before and after impregnation of metals show three characteristic reflections with 2θ values of 0.9, 1.5, and 1.7° for planes (100), (110), and (200), respectively, which characterize a perfect hexagonal ordering of a typically mesoporous SBA-15. However, for the SBA-15 support after impregnation of iron and cobalt-based metals, there was a decrease in intensity and slight displacements in the characteristic reflections of the SBA-15 phase, which is due to a small disorder of the mesoporous structure. This may be related to the presence and dispersion of oxide in mesoporous silica, which will be confirmed below by N
2 adsorption and desorption isotherms as well as by images obtained from transmission electron microscopy analysis.
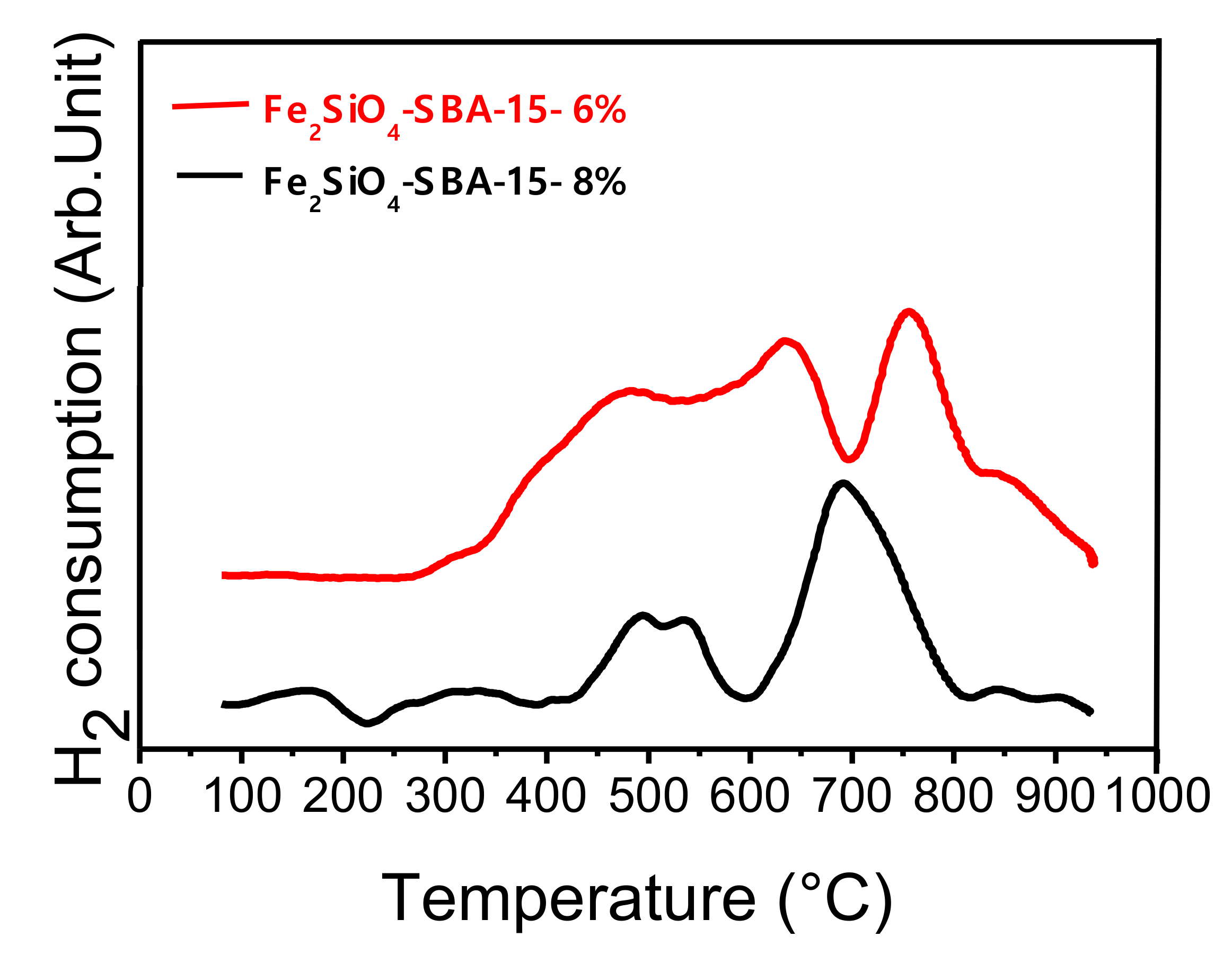
2.2. Redox Properties of Pure Oxide (TPR-H2 Analysis)
For the temperature-programmed reduction (TPR) analysis, the pure oxide sample (Fe
2SiO
4-SBA-15) was used with two different percentages of hydrogen in the reducing mixture. The analysis was performed to evaluate the reduction temperature of oxide to alloy and the effect of H
2 content. The percentages containing 6 and 8% of H
2 in N
2 were used in the total flow rate of 25 mL/min. The TPR profiles are presented in
Figure 2. According to the literature, solids containing iron and cobalt dispersed in silica begin to reduce at temperature between 250 and 300 °C. It usually has two or three peaks related to the reduction of iron and cobalt oxides concerning the phases of cobalt ferrite and fayalite dispersed in the silica matrix as observed in the diffractions of
Figure 1 [
41,
42,
43,
44,
45]. The TPR curves show two distinct reduction stages, the first between 350 and 650 °C and the second between 650 and 850 °C, approximately. These peaks were related to the cobalt oxide (Co
2+→ Co
+ →Co
0) and iron oxide reduction (Fe
3+→ Fe
2+→ Fe
0) present in the Fe
2SiO
4 and CoFe
2O
4 phases identified in the Fe
2SiO
4-SBA-15 sample before reduction (
Figure 1a).
The first reduction range can be attributed to the first stage of iron oxide (III) reduction as well as the reduction of Co2+, both present in the iron and cobalt oxides structure. The second peak can be related to the second stage of iron reduction, Fe2+ to Fe0, leading to Fe7Co3 alloy formation, visualized in diffractograms which iron and cobalt are completely reduced.
The interaction of iron and cobalt oxides in the structure of the silica matrix leads to a change in the structure of iron oxide phases, justifying the formation of the Fe
2SiO
4 phase [
39]. The second range referring to the reduction of Fe
2+ to Fe
0 may be related to the reduction of iron silicate. It is worth to highlight that the iron silicate and cobalt ferrite phases, observed in the XRD results before reduction, are very resistant structures to be reduced and, therefore, the complete reduction of these phases may not have been completely observed in the temperature range studied [
41,
45]. It is important to mention that metal oxides particles located internally in the pores are more difficult to reduce compared to particles positioned on the outer surface [
46,
47]. Thus, the reduction range at higher temperatures may also be related to particles of Fe and Co oxides located within the pores of the SBA-15.
TPR profiles can be correlated with diffractograms, since the effect of the reduction temperature on the percentage of alloy in relation to oxide was observed,
Figure 1b and
Table 1. It is possible to observe the second reduction range related to the partial reduction of Fe
2+ and Co
2+ to Fe
0 and Co
0 at the temperature range chosen between 690 and 720 °C, where the oxide and alloy mixture was observed in the diffractograms, leading to the formation of a fraction of the Fe
7Co
3 alloy. The increase in the alloy content in relation to oxide observed in the XRD results can also be seen in the TPR profiles, once that at 720 °C the consumption of H
2 is higher compared to the temperature of 690 °C, indicating a higher reduction rate with the increase in temperature.
The second effect studied concerning the influence of the H2 percentage on the ability to reduce oxide to alloy can also be seen in TPR results. In the XRD data, it has already been shown that the increase in hydrogen content intensifies the reduction of oxide to alloy. This phenomenon can be seen clearly in TPR profiles, considering that when higher H2 content was used the reduction occur at lower temperatures, confirming the agreement between the two techniques. For example, the TPR performed with 6% of H2 the total reduction occurred at approximately 780 °C, while using 8% of hydrogen the complete reduction occurred at lower temperatures around 700 °C.
2.3. Chemical State Surface of Iron, Cobalt and Silicon (XPS Analysis)
The samples Fe
2SiO
4-SBA-15, Fe
2SiO
4-Fe
7Co
3-SBA-15-700-0.5, and Fe
2SiO
4-Fe
7Co
3-SBA-15-700-1.0 were selected for XPS analysis to evaluate the chemical state surface of the composite observed in the XRD results and compare them with the two pure phases. The results are present in
Figure S5. The wide-scan XPS spectrum with binding energies from 0 to 800 eV for the Fe
2SiO
4-SBA-15 solid showed four characteristic peaks with binding energy values of 103.4, 532, 712, and 781 eV related to Si2p, O1s, Fe2p, and Co2p, respectively. The binding energy at 532 eV refers to the typical O1s of SiO4
−2 present in the Fe
2SiO
4 phase and the Si2p is related to silicon oxide from the SBA-15 matrix. In addition, the spectra for Fe
2SiO
4-Fe
7Co
3-SBA-15-700-0.5 and Fe
2SiO
4-Fe
7Co
3-SBA-15-700-1.0 solids showed peaks with binding energies of approximately 103.4, 532, 710.1, and 781.1 eV attributed to Si2p, O1s, Fe2p, and Co2p, respectively. The Co
2+, Fe
3+/Fe
2+ and Fe
0 species are related to peaks at 781.1, 712 eV and 710.1, respectively, as presented in
Figure S5. The percentage of elements from the full XPS spectrum are shown in
Table S1, indicating the quantity of each element on the surface. The observed binding energy values corroborate with other previously published papers [
48,
49,
50]. The XPS spectra are in accordance with the diffractograms, see
Figure 1, considering that binding energy of Fe
2+ and Fe
0 from the Fe
7Co
3 and Fe
2SiO
4 phases, as already indicated in the XRD results.
The XPS results indicate that the impregnation method was successfully performed, since the structure of Fe2SiO4/SBA-15 solid and the composite Fe2SiO4-Fe7Co3-SBA-15 are present on the solid surface, which is a fundamental aspect for application in the adsorption of organic molecules.
2.4. Chemical Environment of the Iron (Mössbauer Spectroscopy)
The spectrum at 300 K for the Fe
2SiO
4-SBA-15 solid is fit with two components, a sextet due to a slow relaxing Fe moments in the Fe oxide phase and a doublet due to a fast relaxation Fe moments present in Fe oxide superparamagnetic nanoparticles (SPM),
Figure S4a. A large fraction of the Fe-oxide phase is in the superparamagnetic regime. The B
hf obtained for the slow relaxing Fe moments is smaller than the thermally blocked bulk-CoFe
2O
4 particles [
51], indicating that the cobalt and iron spin moments are experiencing a slow relaxation and, therefore, diminishing the hyperfine magnetic field.
The Mössbauer spectrum for sample Fe
7Co
3-SBA-15 has two components, a magnetic and a paramagnetic,
Figure S4b. The magnetic component is ascribed to the Fe
7Co
3 alloy, it has a hyperfine magnetic field (B
hf) and isomer shift (IS) typical for the metal alloy. In order to find the sextet that best fit the spectrum, it was performed a fitting using several sextets with a distribution of B
hf and a distribution of IS. The distribution shows a peak at
Bhf = 35.7 T at an IS of 0.032 mm/s. These results indicate that those parameters are adequate to fit the component ascribed to the alloy. The paramagnetic component (SPM) has a small Mössbauer spectral contribution and IS similar to the Fe
7Co
3 phase, this component is due to a very small metal nanoparticles in the superparamagnetic regime.
Mössbauer results for the solid Fe
2SiO
4-Fe
7Co
3-SBA-15-700-2.0 is presented in
Figure S4c. The spectrum at 300 K is deconvoluted using three components, two paramagnetic and one magnetic subspectra. The component that has the largest relative area is the sextet and is ascribed to the Fe
7Co
3 alloy. It shows the nuclear transition between excited and ground states of magnetically split nuclear moment levels. Each peak is regarded to the Zeeman Effect and has an energy of
E = −Δm. B
hf where Δm is one of the allowed transition between the nuclear levels from the excited (
I = ±3/2) and the ground (
I = ±1/2) states, and B
hf is the hyperfine magnetic field. The magnetic field is provided by a non zero density of states at the Fermi level due to electrons in Fe and Co atoms. The
Bhf = 35.8 T of the present sample is very close to the B
hf of Fe
7Co
3 [
52]. Usually, the Mössbauer spectrum of pure Fe
7Co
3 alloy is fit using several sextets with B
hf ranging from 31.4 T to 36.9 T, and the sextets with the largest (>40%) absorption areas are the sextets with the largest B
hf. Herein, besides the sextet there are two paramagnetic components. These components are doublets related to Fe
2SiO
4 and Fe
3+-silicate phases. The bulk Fe
2SiO
4 has a Fe
2+ charge state and is antiferromagnetic with a Néel transition at 64.5 K [
53], thus, at 300 K this phase is in the paramagnetic regime. The Fe
2+ occupies two non-equivalent octahedral sites in equal proportions. From the literature, a bulk sample has an IS ranging from 0.99–1.02 mm/s and QS ranging from 2.91–3.02 mm/s, while nanostructured samples have similar IS values and reduced QS values [
54]. In the present results (
Table S2), it was considered a representative single doublet to fit the Fe
2SiO
4 phase, where the large IS and QS are typical of Fe
2+. On the other hand, the subspectrum for the Fe
3+-silicate is paramagnetic at 300 K and its spectrum has hyperfine parameters similar to microwaved silica coated maghemite sample [
55]. Thus, Mössbauer results are in agreement with the obtained diffractograms for the nanocomposites, since in both analyses, the presence of Fe
7Co
3 and Fe
2SiO
4 phases were observed.
2.5. N2 Adsorption and Desorption Isotherms (Textural Properties)
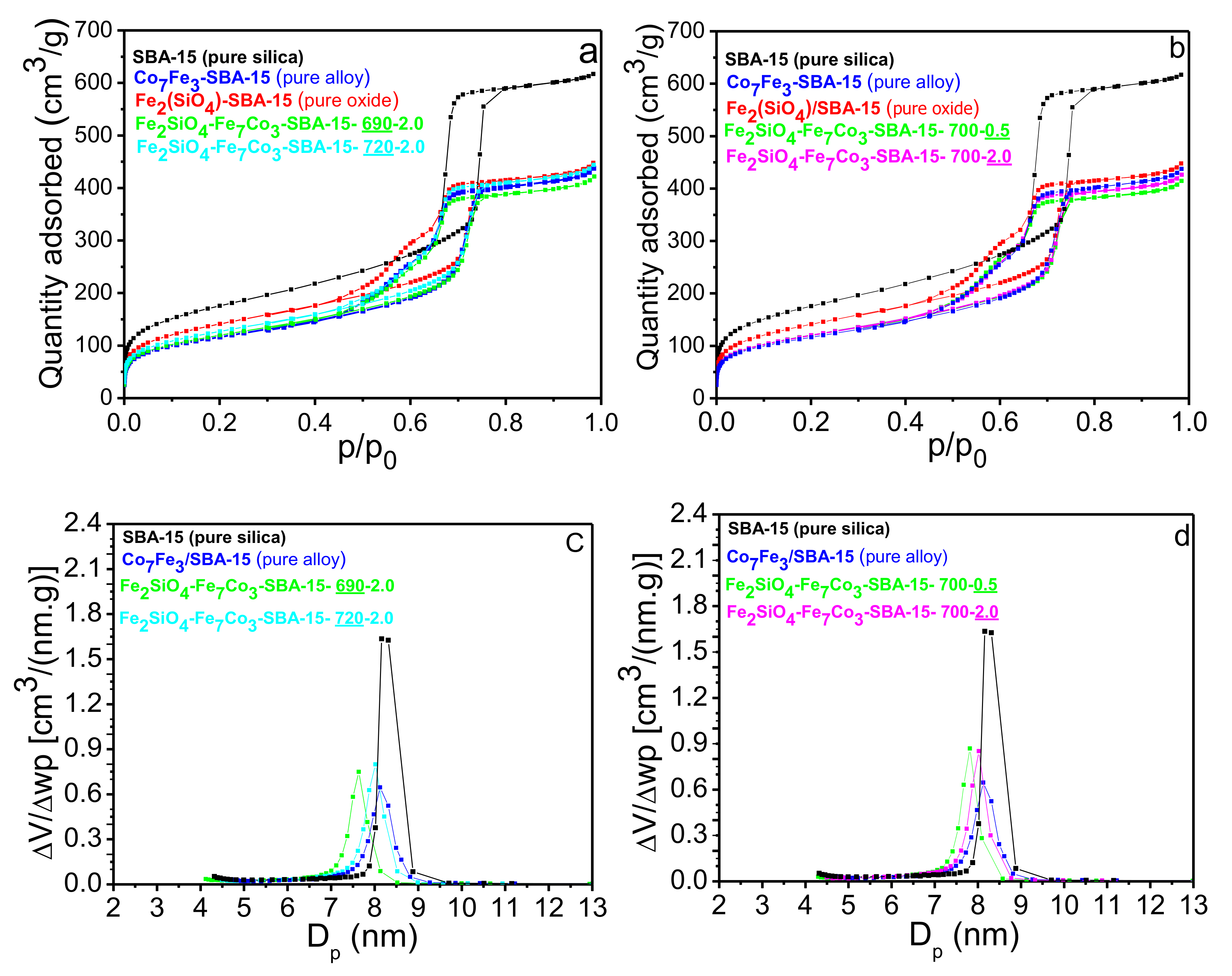
Figure 3a displays the N
2 adsorption and desorption isotherms of the reduced materials at 690 and 720 °C (two extremes of temperature) keeping the H
2 content constant as well as for oxide, alloy, and pure SBA-15. It was observed that both materials containing pure silica and impregnated silica present regular mesoporosity and type IV isotherms, typical of mesoporous materials, with type H1 hysteresis for pure silica (SBA-15) and type H2 (b) for other impregnated materials. H1 hysteresis are characteristics of cylindrical mesoporous materials and uniformly distributed typical of silica (SBA-15, MCM-41, KIT-6). The H2 (b) hysteresis type are considered an intermediate of the anterior hysteresis, characteristic of materials with complex porous structure and is associated with partially blocked pores and present an interconnected pores networks with different sizes and shapes. Therefore, this blocking effects may be associated with partial pore filling by the Fe
7Co
3 and/or Fe
2SiO
4 phases due to the impregnation methodology used.
No significant change in textural properties before and after impregnation of iron and cobalt oxides as well as after reduction in the different temperatures were observed, indicating that the different stages of oxidation and reduction in the different temperatures did not affect the isotherm type formed. On the other hand, the hysteresis type and amount of N
2 adsorbed changed significantly by comparing pure silica with the other composites that were impregnated with Fe and Co. The textural properties extracted from the N
2 physisorption isotherms are described in
Table 2. Before impregnation of Fe
2SiO
4 oxide, the pure SBA-15 material has a specific surface area of 640 m
2/g. However, after impregnation, the specific surface area values, as well as the total pore volume decreased as can be observed in
Table 2. According to the literature [
56], the oxide crystallites size and their reduction depend heavily on the pore diameter of the silica. Thus, the partial introduction of oxides in mesoporous silica promote a decrease in the specific surface area and total pore volume. For example, specific area values decreased from 640 to 510 m
2/g and total pore volume from 0.94 to 0.69 cm
3/g comparing pure silica with Fe
2SiO
4-SBA-15 solid. This confirms that the changes in the specific surface area were not too drastic and that the good textural characteristics of the silica matrix were maintained in the nanocomposite, considering that it is a fundamental aspect to provide high dispersion of the Fe
7Co
3 and Fe
2SiO
4 phases in the mesoporous matrix. After reduction at different temperatures, there is also a slight decrease in the surface area and pore volume. However, comparing the textural properties of the different temperatures used, it is perceived that temperature variation did not significantly affect the values of surface area, pore volume, and pore diameter.
The change in the hysteresis type of the isotherms due to the variation in the pore structure for the SBA-15 is in accordance with the low angle diffractions (
Figure 1d), which showed a slight change in peaks after the impregnation stage. Despite the alteration of textural properties after impregnation, the materials reduced in the different temperatures showed isotherms with very similar profile, indicating that the pore structure was not significantly affected with the increase in the reduction temperature. These results are in accordance with the crystallites size calculated from the diffractograms of
Figure 1b, since the crystallites size,
Table 1, was also not significantly affected with the reduction temperature variation.
In addition, the effect of H
2 content used for nanocomposite synthesis on the textural properties of different solids was also studied. The results of N
2 adsorption-desorption isotherms for the amounts of 0.5% and 2.0% of H
2 studied (two extremes of H
2 content) are present in
Figure 3b. It can be observed that the results of the textural properties obtained with the variation of H
2 flow rate were very similar to the study of temperature variation (
Figure 3a). The samples presented the same type of isotherm and hysteresis as well as the specific surface area values, volume and pore diameter were practically the same. These results suggest that metals inserted by impregnation are mainly on the catalyst surface, as presented in the XPS results (
Figure S2), and a small fraction of pores is partially blocked by oxide and oxide-alloy mixture (Fe
2SiO
4 and the Fe
2SiO
4-Fe
7Co
3 mixture). Despite the small change in textural properties after impregnation, the mesoporous structure of the SBA-15 support was practically maintained, corroborating with the low angle diffractions (
Figure 1d). The variation in the percentage of H
2 also did not significantly affect the crystallite size,
Table 1, corroborating with the texture properties values presented in
Table 2.
Figure 3c,d present the pore diameter distribution curves for pure SBA-15, Fe
2SiO
4-SBA-15, and Fe
2SiO
4-Fe
7Co
3-SBA-15 with their respective variations in reduction temperature and H
2 flow rate. A homogeneous distribution was observed in the mesopores range for the different samples. It can be observed that impregnated materials have an average pore size smaller than pure SBA-15 due to partial pore blocking by oxide and alloy as previously described.
2.6. Morphological Properties (SEM-FEG and TEM)
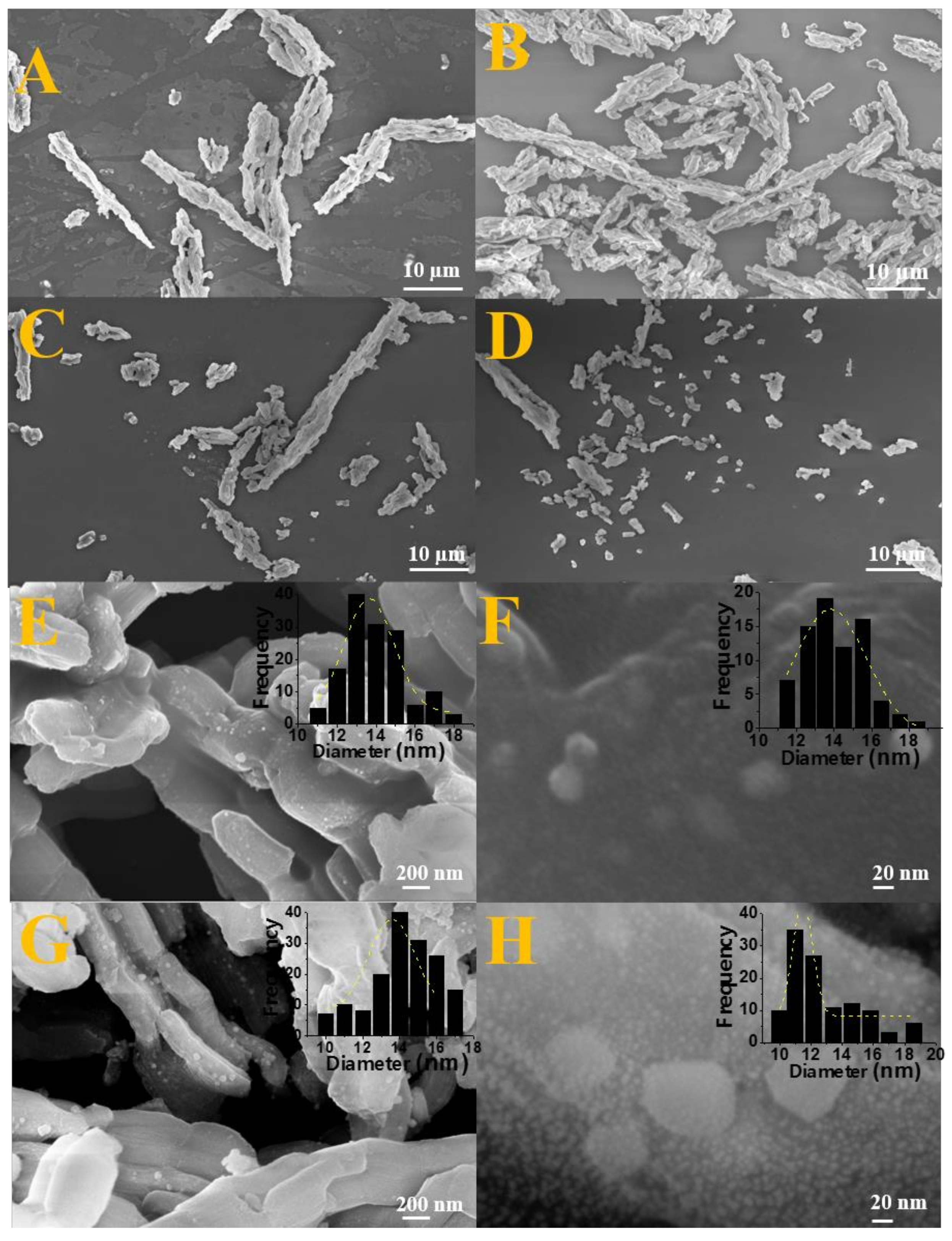
High resolution scanning electron microscopy analyses were performed to obtain information related to the morphology of synthesized nanocomposites as well as confirm the maintenance of silica matrix morphology before and after impregnation of oxide and/or alloy as well as to observe the particle size distribution after impregnation and reduction through the construction of histograms.
In
Figure 4A, associated to pure silica before impregnation, it is possible to observe a highly ordered matrix with uniform hexagonal rod-like morphology and longitudinally arranged characteristic of the SBA-15 silica [
47,
56]. It is possible to notice that the typical morphology of SBA-15 is maintained in solids after impregnation and calcination for oxide formation as well as after the reduction stage for nanocomposite formation, since the rods were preserved comparing the images of
Figure 4A–D. These results corroborate with the low angle diffractograms (
Figure 1d) that showed the maintenance of the pore structure after impregnation and they are also in accordance with the N
2 adsorption-desorption isotherms that indicated the maintenance of the typical mesoporosity for the SBA-15 after the Fe and Co insertion (
Figure 3).
Furthermore, in order to complement the FEG-SEM analyses, particle size distribution was obtained for Fe
2SiO
4-Fe
7Co
3-SBA-15-700-0.5 and Fe
2SiO
4-Fe
7Co
3-SBA-15-700-2.0 solids from the images present in
Figure 4E–H. In addition, an elementary analysis was carried out by EDS and the information is presented in
Table S3, confirming the presence of Fe, Co, Si, and O. It was observed that the sample reduced using 2.0% of H
2 has particle sizes ranging from 11 to 18 nm with an average size of 14 nm. However, the nanocomposite prepared with 0.5% of hydrogen in the reducing mixture showed particle sizes ranging from 10 to 19 nm with an average value of 16 nm according to histograms inserted in
Figure 4. The particle size values observed by SEM were in accordance with the crystallites size calculated from the diffractograms using the Scherrer equation (
Table 1) and similarly the variation of the H
2 percentage used in the synthesis did not significantly affect the particles size, considering that both samples presented average particle size very close. This result shows that there is not aggregation of nanoparticles.
Transmission electron microscopy (TEM) was performed for pure SBA-15, Fe
2SiO
4-Fe
7Co
3-SBA-15-700-0.5, and Fe
2SiO
4-Fe
7Co
3-SBA-15-700-2.0 solids in order to confirm the mesoporous structure of these solids, to visualize the partial confinement of the nanocomposite and determine the particle size distribution. The different images obtained are presented in
Figure 5. One can observe that the ordered arrangement of pores consists of long mesochannels, which once again agree with low angle XRD diffractograms (
Figure 1d).
Figure 5B shows a 2D two-dimensional hexagonal arrangement of uniformly sized cylindrical mesoporous channels characteristic of the SBA-15 [
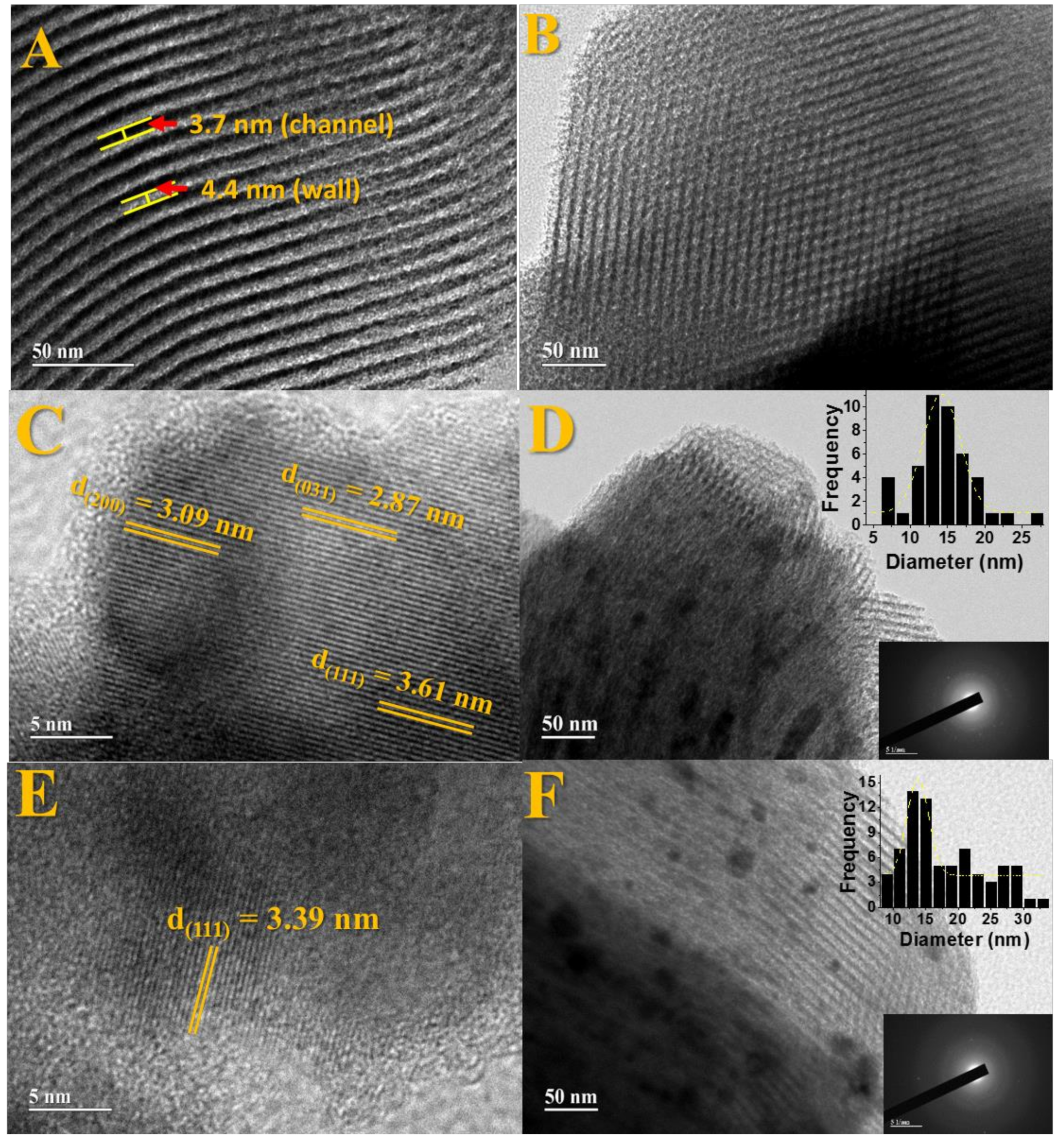
57]. For pure SBA-15,
Figure 5A, the wall thickness and the channel distance (pores) were calculated, obtaining an interpore distance of 3.7 nm and wall thickness of 4.4 nm. From the low angle diffractograms and the values obtained in the N
2 isotherms, it was possible to determine the wall thickness for the SBA-15 and compare with the value obtained by TEM. The values obtained are described in
Table S4. There is an agreement in the data obtained by the two techniques.
The images presented in
Figure 5C–F show well-defined lattice fringes related to mesoporous SBA-15 partially filled by the Fe
7Co
3 and Fe
2SiO
4 phases, which are also in accordance with low-angle XRD diffractograms (
Figure 1d) and N
2 isotherms (
Figure 3).
Figure 5C,D refers to the Fe
2SiO
4-Fe
7Co
3-SBA-15-700-2.0 solid, and
Figure 5E,F are related to the Fe
2SiO
4-Fe
7Co
3-SBA-15-700-0.5 material. Image 5C shows the interplanar distances of 2.87 nm, 3.09 nm, and 3.61 nm, respectively, for the planes (031), (200), and (111) referring to the solid Fe
2SiO
4-Fe
7Co
3-SBA-15-700-2.0, which is in accordance with the pattern shown in the diffractograms of
Figure 1.
Figure 6E also displays a distance of 3.39 nm, referring to the plane (111) for Fe
2SiO
4-Fe
7Co
3-SBA-15-700-0.5 and also corroborates with the JCPDS 00-071-1678 card.
Moreover,
Figure 5D,F present a histogram with particle size distribution. An average particle size around 17.8 nm was observed for the Fe
2SiO
4-Fe
7Co
3-SBA-15-700-2.0 composite and 14.4 nm for the Fe
2SiO
4-Fe
7Co
3-SBA-15-700-0.50 sample. The particle sizes values determined by TEM corroborate with the values obtained by the SEM images and also the crystallite size extracted from diffractograms using the Scherrer equation. The images inserted at the bottom of
Figure 5D,F, referring to selected area electron diffraction, confirm the polycrystalline nature of the nanoparticles related to the Fe
7Co
3 and Fe
2SiO
4 phases observed in XRD and Mössbauer results.
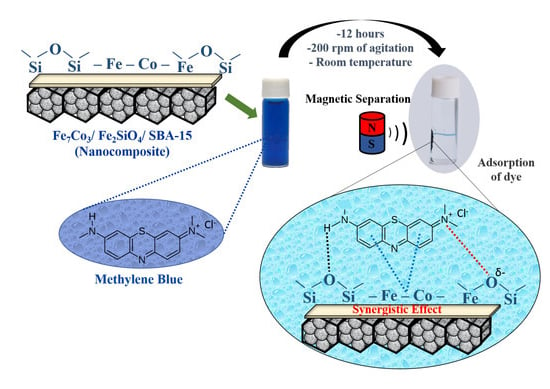
2.7. Adsorption Tests
Initially, in order to investigate the adsorption capacity of magnetic nanocomposite using three types of dyes, the Fe
2SiO
4-Fe
7Co
3-SBA-15-700-2.0 sample was selected. The results are presented in
Figure S5. Thus, it was possible to observe the performance of the material Fe
2SiO
4-Fe
7Co
3-SBA-15-700-2.0 for the adsorption of three dyes, with an adsorption capacity of 7 mg/g for methyl orange, 27 mg/g for rhodamine B, and 49 mg/g for methylene blue. Therefore, as methylene blue presented the best adsorption results for the selected solid, all samples described in
Table S5 were tested to evaluate which material has the highest adsorption capacity among the synthesized solids. In addition, a study was also conducted for the pure SBA-15, Fe
2SiO
4-SBA-15, and Fe
7Co
3-SBA-15 in order to investigate the adsorption capacity of the Fe
7Co
3 and Fe
2SiO
4 phases separately and confirm the positive effect of oxide and alloy mixture on the dye adsorption efficiency. The obtained results are presented in
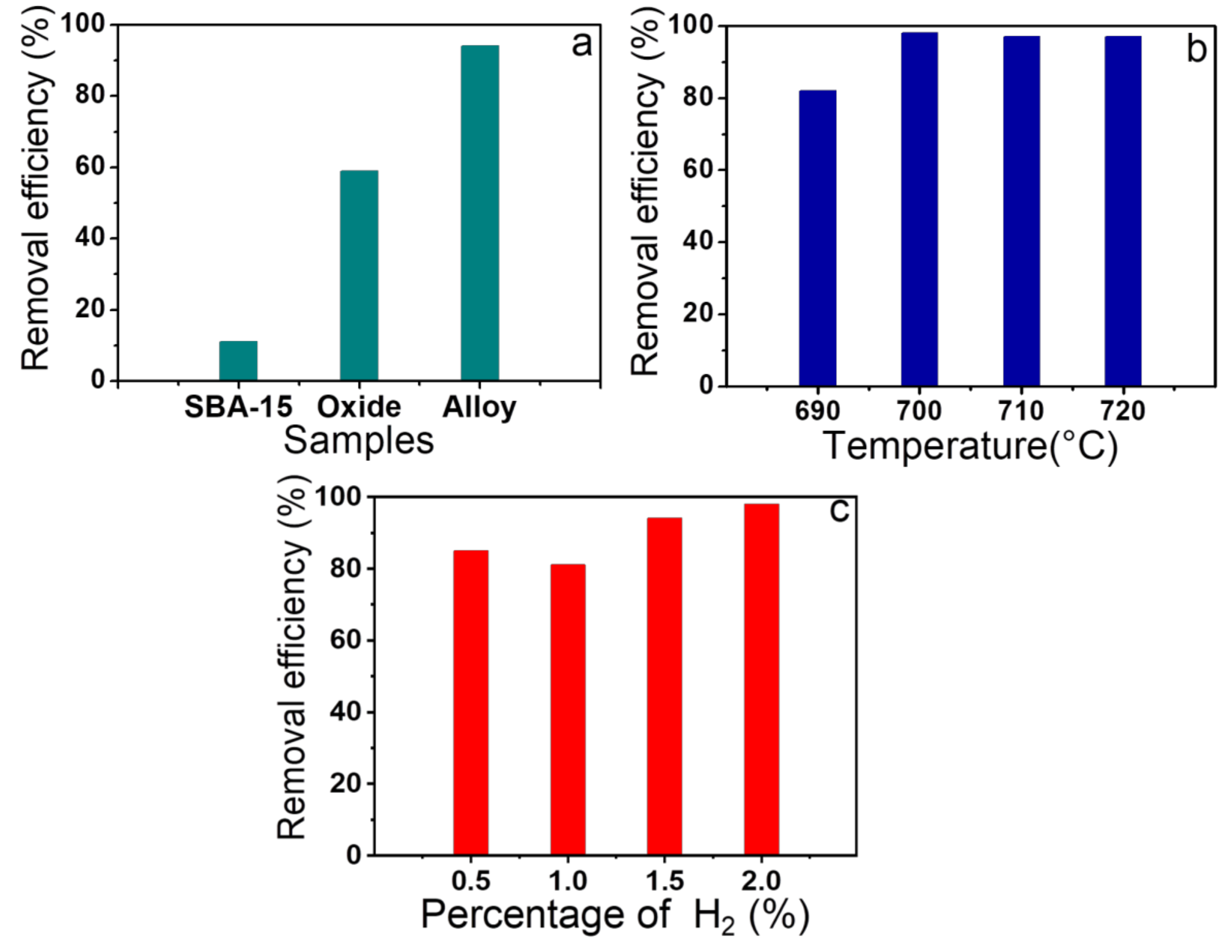
Figure 6. In
Figure 6a, one can observe that the pure SBA-15 silica matrix has low adsorption capacity compared to other solids after oxide impregnation and alloy formation, confirming that the presence of Fe and Co is fundamental to obtain better results for dye removal.
The adsorption capacities of magnetic nanocomposites for samples varying the reduction temperature used in the synthesis increases significantly with the temperature increase from 690 °C (41 mg/g) to 700 °C (49 mg/g) and remains virtually constant, with the temperatures between 710 and 720 °C (48 mg/g),
Figure 6b. These results indicate that the presence of a greater amount of alloy in relation to oxide is necessary to obtain better adsorption results, whereas the Fe
2SiO
4-Fe
7Co
3-SBA-15-690-0.5 sample has lower amount of alloy compared to the Fe
2SiO
4-Fe
7Co
3-SBA-15-700-2.0 material, justifying its best performance. However, the reduction temperature above 700 °C, despite increasing the alloy amount in relation to oxide, does not affect the adsorption capacity, considering that the percentage degradation was quite similar for the solids reduced at temperatures of 700, 710, and 720 °C, indicating that from a certain amount of the alloy in relation to oxide the adsorption performance is not significantly affected.
In addition, it was also studied the influence of the amount of hydrogen used in the synthesis on adsorption performance,
Figure 6c. Similar to the effect of the reduction temperature, adsorption capacity increases with the increase in the amount of alloy in relation to oxide, considering that the different solids presented the following results in ascending order Fe
2SiO
4-Fe
7Co
3-SBA-15-700-1.0 (40 mg/g), Fe
2SiO
4-Fe
7Co
3-SBA-15-700-0.5 (43 mg/g), Fe
2SiO
4-Fe
7Co
3-SBA-15-700-1.5 (47 mg/g), and Fe
2SiO
4-Fe
7Co
3-SBA-15-700-2.0 (49 mg/g). Thus, the higher adsorption capacity of the sample Fe
2SiO
4-Fe
7Co
3-SBA-15-700-2.0 (oxide and alloy mixture) compared to Fe
7Co
3-SBA-15 (isolated pure alloy) and Fe
2SiO
4-SBA-15 (isolated pure oxide) samples confirm the best nanocomposite properties containing the Fe
7Co
3 and Fe
2SiO
4 phase mixture in relation to the same isolated phases. Furthermore, it was observed that control of synthesis conditions such as reduction temperature and H
2 content also affect adsorption capacity, considering that the alloy amounts in relation to oxide in the mixture affect the physicochemical properties of nanocomposites as shown in previous characterizations and consequently affect the adsorption performance of methylene blue. These results suggest that there is a synergy between the Fe
7Co
3 and Fe
2SiO
4 phases that positively affects dye adsorption. Previous studies have reported a synergy between alloy and oxide phases that positively affect the interaction of organic molecules [
58].
A reusability study was carried out for the material Fe
2SiO
4-Fe
7Co
3-SBA-15-700-2.0,
Figure 7. The result related to adsorption capacity for the first test was 46 mg/g, for the second was 40 mg/g, the third was 37 mg/g, the fourth was 30 mg/g and, finally, for the fifth was 22 mg/g, indicating that there was a slight reduction in the adsorption capacity between recycle tests due to due to partial pore obstruction by methylene blue, however, the material was resistant since it has a little difference of 18 mg/g between the first and fifth reuse. It is important to highlight that this material is easily attracted by a magnet due to its magnetic properties, providing a simple way to separate the magnetic solid from the suspension.
Figure 8 (insert) shows the solution before and after adsorption, illustrating the attraction of the nanocomposite by the application of an external magnetic field, which facilitates the recycle of this material.
2.8. Magnetic Properties (VSM, Mzfc, and Mfc)
The
Figure 8a shows the measurements magnetization zero field cooling (Mzfc) and magnetization field cooling (Mfc) for nanocomposite Fe
2SiO
4-SBA-15. The Mzfc has a peak at 286 K and the Mfc show a large increase when the temperature decreases from 300 K to 6 K. The peak in the Mzfc may be related to a blocking temperature or to a spin glass transition. The M-H at 10 K shows a typical hysteresis loop characteristic of a hard magnetic material,
Figure 8b. The maximum magnetic field of ± 40 kOe is not able to saturate magnetically the sample, i.e., there is a fraction of magnetic moments that are not aligned in the direction of the applied field. Thus, the ascending and descending M-H curves join at the field of ± 40 kOe. The coercivity field (Hc) and the remanent magnetization (Mr) are of 11.3 kOe and 7.5 emu/g, respectively. The magnetization at the field of 40 kOe is of
M = 12.9 emu/g, and the ratio of the remanent magnetization and the magnetization at 40 kOe is of
Mr/M = 0.58. The high coercivity field and high M/Mr values are typical of hard magnetic materials. In fact, for CoFe
2O
4 the value of Mr/Ms of 0.5 is typical of a system with uniaxial magnetocrystalline anisotropy and Mr/Ms > 0.5 is typical for a system with a cubic magnetocrystalline anisotropy (Ms is the saturation magnetization), therefore, the present sample seems to have a cubic magnetocrystalline anisotropy.
The
Figure 8c shows the measurements Mzfc and Mfc for the solid Fe
7Co
3-SBA-15. The Mzfc does not show a peak and the Mfc data shows a quite small increase when the temperature decreases from 300 K to 6 K. The absence of a peak in the Mzfc curve indicates that the sample is thermally blocked below 300 K, i.e., the sample has a blocking temperature above 300 K.
Figure 8d shows the M-H loops at 10 K and 300 K. The hystereses have a small coercivity field. Thus, they show typical behavior of soft magnetic materials, i.e., the sample has a low magnetocrystalline anisotropy. The hysteresis at 10 K and 300 K show coercivity fields of 390 Oe and 181 Oe, respectively. The Ms at 10 K and 300 K are of 31.2 and 29.9 emu/g, respectively. The Mr at 10 K and 300 K are of 12.2 and 8.3 emu/g, respectively, thus, their Mr/Ms values will be 0.39 and 0.28, respectively. In both curves the magnetization saturate quickly, beginning this process at a magnetic field of 10 kOe. These characteristics are typical of soft magnetic solids.
Taking into account that the Fe-Co metal nominal mass fraction is of 20 wt%, the Fe-Co-based phase corresponds to a 20 wt%. Therefore, the saturation magnetization for the Co
3Fe
7 phase will be 31.2/0.2 emu/g = 156 emu/g, this value is close to the expected value of 223 emu/g obtained for Co
3Fe
7 particles with a size of 180 nm [
59]. The difference can be ascribed to surface effects due to the smaller particles size, i. e. the superficial spins are usually disordered and have a different magnetic behavior when compared with the spins at the core. The total surface area (per gram of sample) of very small particles is enhanced and the magnetic behavior of these nanoparticles is strongly affected by the superficial spins, leading to a smaller saturation magnetization.
For sample Fe
2SiO
4-Fe
7Co
3-SBA-15-700-2.0, the Mzfc does not show a peak in the range from 5 K to 300 K, indicating that the sample is in the thermal blocked regime,
Figure 8e. The blocking temperature for this sample is above 300 K. The Mfc shows a typical behavior for blocked particles with low magnetocrystalline anisotropy. During the cooling under an applied magnetic field the spins are frozen in a particular direction depending on the strength of the applied magnetic field. The increase of Mzfc will be small with decrease in temperature if the sample has a low magnetocrystalline anisotropy.
Figure 8f shows the M-H loops recorded at 10 K and 300 K. Both hysteresis show low coercivities fields of 940 Oe and 520 Oe, respectively. The magnetizations at 40 kOe were of 20.6 emu/g and 18.4 emu/g for the measurements recorded at 10 K and 300 K, respectively.
We have recorded an M-H measurement at 10 K after cooling the samples from 300 K under a magnetic field of 3 kOe, these measurements did not show a hysteresis shift due to an exchange bias effect. This result indicates that there is not a measurable magnetic coupling between the metal and the oxide phases. This coupling may occur at the interface between both phases and will shift the hysteresis to the left (right) when the coupling is ferromagnetic (antiferromagnetic). However, a chemical coupling may be present at the interface between the oxide and alloy and it will provide synergy between phases, which justifies the better adsorption capacity of the mixed phases compared to the same isolated phases (
Figure 7).
Table 3 shows the adsorption capacity of methylene blue from different adsorbents studied in the literature and compared with the present study [
60,
61,
62,
63,
64,
65]. It is possible to notice that the solid Fe
2SiO
4-Co
7Fe
3-SBA-15-700-2.0 which presented the best adsorption capacity has promising results compared to other solids containing Fe.
2.9. Mechanistic Proposal
Adsorption of environmental contaminants such as methylene blue on oxide-based surfaces and iron-containing metal alloys can occur through physical and chemical interactions between adsorbent and adsorbate. In physical adsorption, most adsorbed species occur in a very short time. However, a chemical bond between adsorbate and adsorbent requires longer contact time to reach equilibrium. Between the two stages, the adsorption rate is almost constant, since it has a large number of surface sites and vacancies available for adsorption during the initial stage, considering that all sites are free before adsorption. However, after certain interval of time, the remaining sites, present on the surface, are more difficult to be occupied, since most of the sites have been filled by the adsorbate [
66]. Specifically, for methylene blue as adsorbate, due to its aromatic ring and N-H stretch in its structure, the main interactions studied are electrostatic, acid-base, ionic exchange, coordination, π-π interaction, and hydrogen bonds [
66,
67,
68,
69,
70]. However, the interaction type is related to the intrinsic characteristics of each adsorbent [
66].
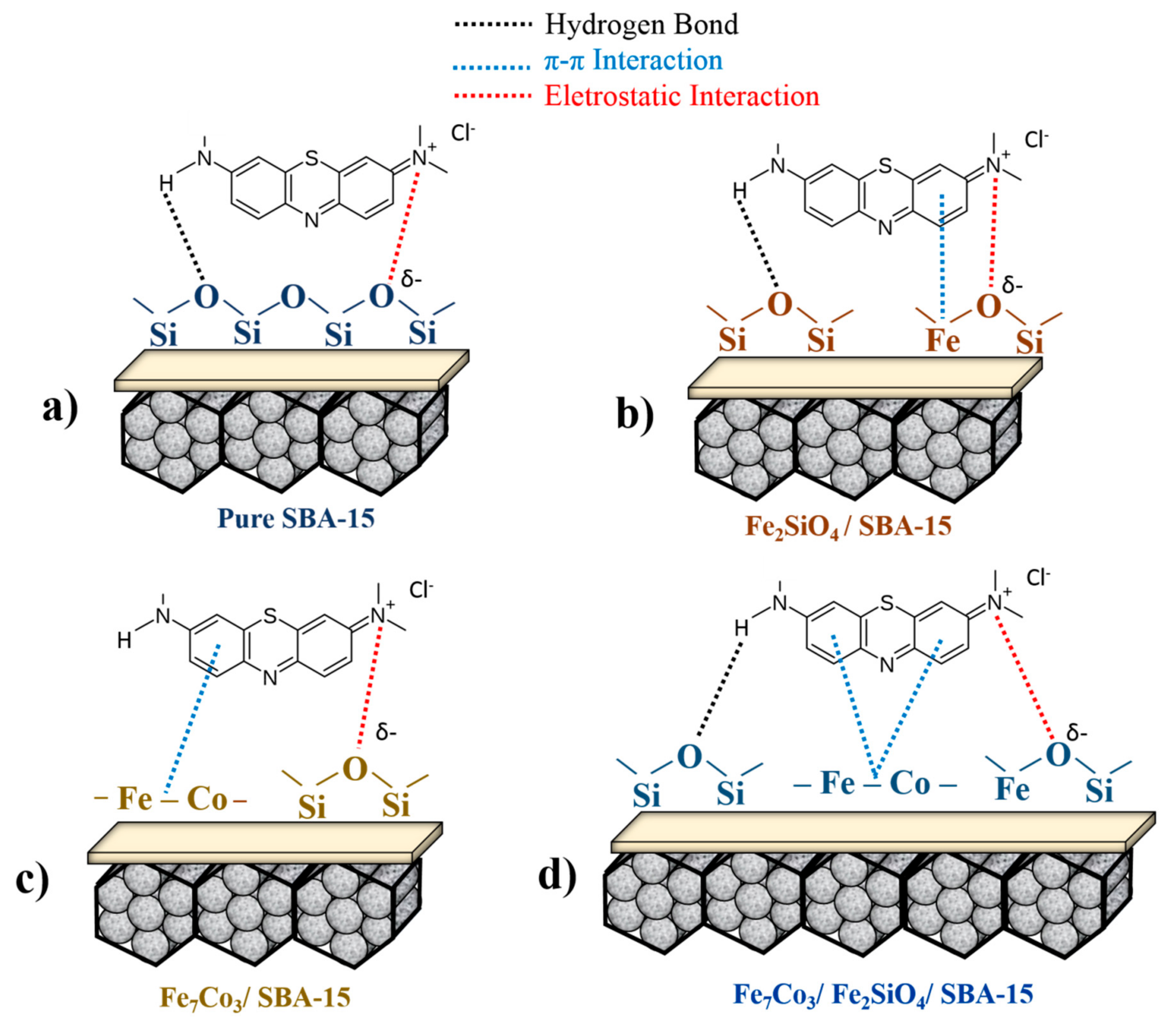
Scheme 1 presents a proposal referring to the mechanism for methylene blue adsorption in the materials synthesized in the present study, indicating the main interactions between adsorbent and adsorbate. In
Scheme 1a, the test with pure SBA-15 demonstrated low adsorption capacity (
Figure 6a), however, it is possible to predict interactions of the lattice oxygen present in the silica based matrix with N present in the methylene blue structure. In addition, it is also possible to have interactions of nitrogen bonded with H in the dye structure with the lattice oxygens present with amorphous silica through stronger hydrogen binding interactions.
In the case of
Scheme 1b, the presence of Fe
2SiO
4 oxide dispersed on SBA-15 provides a significant gain in methylene blue adsorption, considering that the number of possibilities for interactions is greater. In this case, the structure has more lattice oxygen sites present in the structure of silica and iron-cobalt oxides. Moreover, the presence of Fe causes π-π interactions with aromatic rings from the dye, justifying the higher adsorption capacity for the Fe
2SiO
4-SBA-15 sample when compared to pure SBA-15 (
Figure 6a).
Scheme 1c displays the material containing the pure Fe
7Co
3 alloy supported on SBA-15. The result was more significant when compared to the two schemes previously described. In the case of pure alloy dispersed in silica matrix, the presence of metallic Fe and Co intensifies π-π interactions with the aromatic rings of dye structure compared to Fe
2+/Fe
3+ from oxide, justifying the higher adsorption capacity of the Fe
7Co
3 -SBA-15 solid compared to the sample Fe
2SiO
4-SBA-15.
Finally,
Scheme 1d presents the composite containing the mixture of alloy and oxide-based phases supported on SBA-15. In this case, it is possible to propose the existence of three types of interactions (electrostatic, π-π, and hydrogen bonding). Fe and Co based metals present in oxide and alloy structure have the adsorption ability through π-π interaction with aromatic rings of dye. Lattice oxygens present in the structure of silica and fayalite can also adsorb dye molecules through electrostatic interaction or hydrogen binding. The higher adsorption capacity due to the presence of the mixture of phases, Fe
7Co
3 and Fe
2SiO
4, when compared to the same isolated phases, may be related to the synergistic effect between the fayalite and alloy phases. Electron transfer from π-π interactions is favored due to the synergistic effects of Fe
0 and Fe
2+, justifying the higher adsorption capacity for the Fe
2SiO
4-Fe
7Co
3-SBA-15-700-2.0 sample compared to Fe
7Co
3-SBA-15 and Fe
2SiO
4-SBA-15 solids. It is important to highlight that previous studies have already mentioned the positive effect of the Fe
0-Fe
2+-Fe
3+ mixture due to the synergistic effects on dyes adsorption, corroborating with the results obtained in the present study [
58,
70].