Identification and Characterization of Nematicidal Volatile Organic Compounds from Deep-Sea Virgibacillus dokdonensis MCCC 1A00493
Abstract
:1. Introduction
2. Results
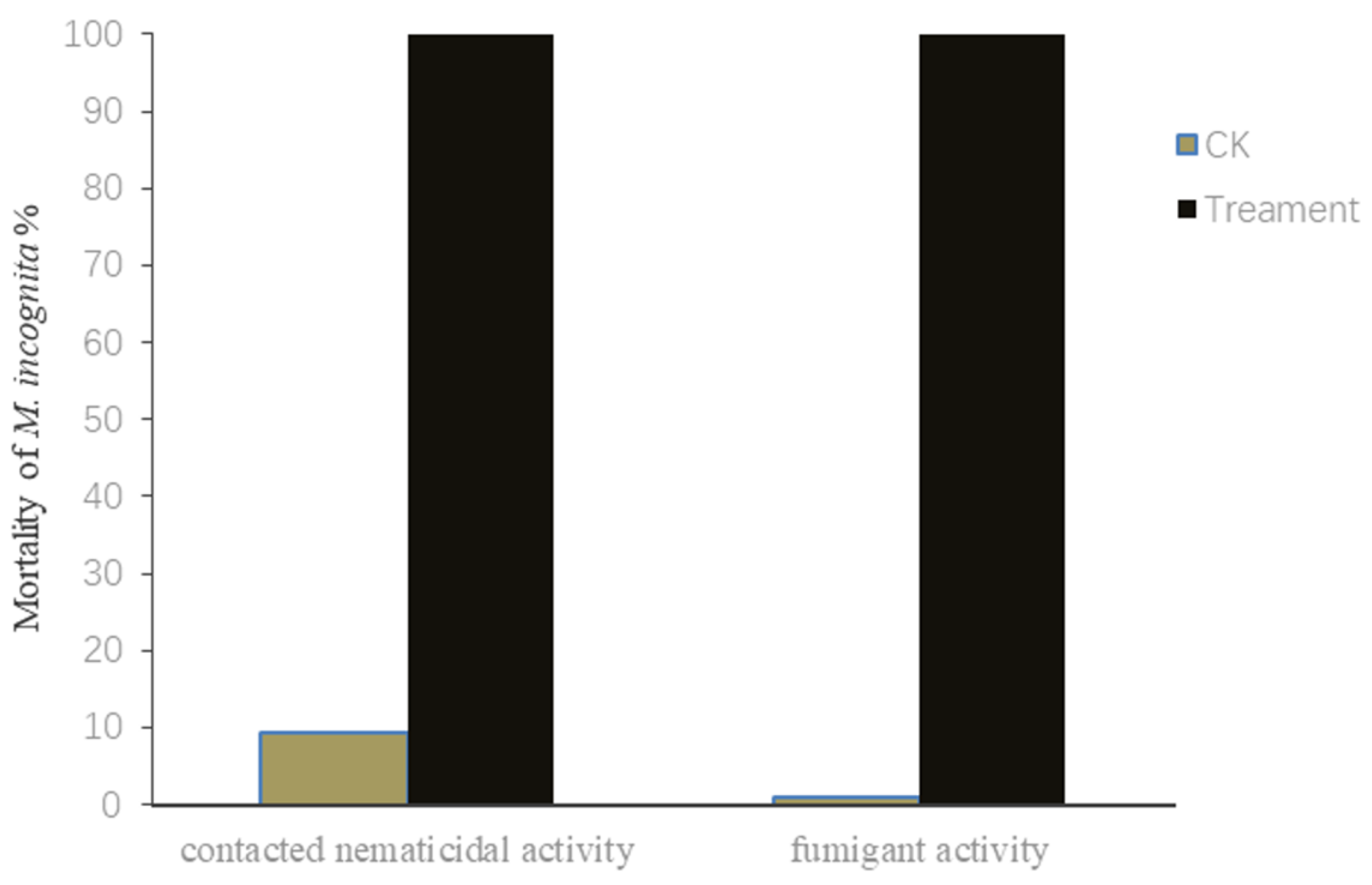
2.1. Contact and Fumigant Nematicidal Activities of V. dokdonensis’s Fermentation Supernatant against M. incognita J2s
2.2. Identification of the VOCs of V. dokdonensis
2.3. Nematicidal Activity of VOCs against M. incognita J2s
2.4. Fumigant Activity of VOCs against Juveniles and Eggs
2.5. Chemotaxis of M. incognita toward Ethylbenzene, Dimethyl disulfide, Acetaldehyde, and 2-Butanone
3. Discussion
4. Materials and Methods
4.1. Bacterial Material
4.2. Chemicals
4.3. Nematode Population
4.4. GC-MS Analysis
4.5. Nematicidal Activity Bioassays of the Fermentation Supernatant and VOCs of MCCC 1A00493
/(1 − mortality percentage in control)] × 100%.
4.6. Fumigant Activity of the Fermentation Supernatant and VOCs of MCCC 1A00493 against M. incognita Juveniles
4.7. Fumigant Activity of VOCs to Inhibit Egg Hatching
4.8. Chemotaxis Test
4.9. Data Analysis and Atatistics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, J.; Zou, C.; Xu, J.; Ji, X.; Niu, X.; Yang, J.; Huang, X.; Zhang, K.Q. Molecular mechanisms of nematode-nematophagous microbe interactions: Basis for biological control of plant-parasitic nematodes. Annu. Rev. Phytopathol. 2015, 53, 67–95. [Google Scholar] [CrossRef]
- Jones, J.T.; Aegeman, A.; Danchin, E.G.; Gaur, H.S.; Helder, J.; Jones, M.G.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Meyer, S.L.F.; Chitwood, D.J.; Chauhan, K.R.; Dong, D.; Zhang, T.T.; Li, J.; Liu, W.C. New nematotoxic indoloditerpenoid produced by Gymnoascus reessii za-130. J. Agric. Food Chem. 2017, 65, 3127–3132. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.N. Plant Parasitic Nematodes in Subtropical and Tropical Agriculture, 2nd ed.; Luc, M., Sikora, R.A., Bridge, J., Eds.; CABI Publishing: Wallingford, UK, 2005; p. 896. [Google Scholar]
- Janssen, T.; Karssen, G.; Verhaeven, M.; Coyne, D.; Bert, W. Mitochondrial coding genome analysis of tropical root-knot nematodes (Meloidogyne) supports haplotype based diagnostics and reveals evidence of recent reticulate evolution. Sci. Rep. 2016, 6, 22591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajihassani, A.; Rutter, W.; Schwarz, T.; Woldemeskel, M.; Ali, M.E.; Hamidi, N. Characterization of resistance to major tropical root-knot nematodes (Meloidogyne spp.) in Solanum sisymbriifolium. Phytopathology 2019. [Google Scholar] [CrossRef] [PubMed]
- Karssen, G.; Wesemael, M.; Moens, M. Root-knot nematodes. In Plant Nematology, 2nd ed.; Perry, R.N., Moens, M., Starr, J.L., Eds.; CABI Publishing: Wallingford, UK, 2013; pp. 74–108. [Google Scholar]
- Duncan, L.W.; Moens, M. Migratory endoparasitic nematodes. In Plant Nematology, 2nd ed.; Perry, R.N., Moens, M., Eds.; CABI Publishing: Wallingford, UK, 2006; pp. 123–152. [Google Scholar]
- Shahbaz, M.U.; Mukhtar, T.; Irfanulhaque, M.; Begum, N. Biochemical and serological characterization of ralstonia solanacearum associated with chilli seeds from pakistan. Int. J. Agric. Biol. 2015, 17, 31–40. [Google Scholar]
- Verdejo-Lucas, S.; Talavera, M. Root-knot nematodes on zucchini (Cucurbita pepo subsp. pepo): Pathogenicity and management. Crop Prot. 2019, 126, 104943. [Google Scholar]
- Al-Hazmi, A.S.; Dawabah, A.A.; Al-Nadhari, S.N.; Al-Yahya, F.A. Comparative efficacy of different approaches to managing Meloidogyne incognita on green bean. Saudi J. Bio. Sci. 2017, 24, 149–154. [Google Scholar] [CrossRef] [Green Version]
- Collange, B.; Navarrete, M.; Peyre, G.; Mateille, T.; Tchamitchian, M. Root-knot nematode (Meloidogyne) management in vegetable crop production: The challenge of an agronomic system analysis. Crop Prot. 2011, 30, 1251–1262. [Google Scholar] [CrossRef] [Green Version]
- Ntalli, N.G.; Caboni, P. Botanical nematicides in the mediterranean basin. Phytochem. Rev. 2012, 11, 351–359. [Google Scholar] [CrossRef]
- Wolfgang, A.; Taffner, J.; Guimarães, R.A.; Coyne, D.; Berg, G. Novel strategies for soil-borne diseases: Exploiting the microbiome and volatile-based mechanisms toward controlling Meloidogyne-based disease complexes. Front. Microbiol. 2019, 10, 1296. [Google Scholar] [CrossRef] [PubMed]
- Desaeger, J.A.; Watson, T.T. Evaluation of new chemical and biological nematicides for managing Meloidogyne javanica in tomato production and associated double-crops in Florida. Pest Manag. Sci. 2019, 75, 3363–3370. [Google Scholar] [CrossRef] [PubMed]
- Yagi, K.; Williams, J.; Wang, N.Y.; Cicerone, R.J. Agricultural soil fumigation as a source of atmospheric methyl bromide. Proc Natl. Acad. Sci. USA 1993, 90, 8420–8423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whorton, M.D.; Foliart, D.E. Mutagenicity, carcinogenicity and reproductive effects of dibromochloropropane (DBCP). Mutat. Res. 1983, 123, 13–30. [Google Scholar] [CrossRef]
- Zhang, X.K.; Guan, P.T.; Wang, Y.L.; Li, Q.; Zhang, S.X.; Zhang, Z.Y.; Bezemer, T.M.; Liang, W.J. Community composition, diversity and metabolic footprints of soil nematodes in differently-aged temperate forests. Soil Biol. Biochem. 2015, 80, 118–126. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Li, Y.L.; Lai, H.X.; Guo, Q.; Xue, Q.H. Effects of two strains of Streptomyces on root-zone microbes and nematodes for biocontrol of root-knot nematode disease in tomato. Appl. Soil Ecol. 2017, 112, 34–41. [Google Scholar] [CrossRef]
- Zhai, Y.L.; Shao, Z.Z.; Cai, M.M.; Zheng, L.Y.; Li, G.Y.; Yu, Z.N.; Zhang, J.B. Cyclo(l-Pro-l-Leu) of Pseudomonas putida MCCC 1A00316 isolated from Antarctic soil: Identification and characterization of activity against Meloidogyne incognita. Molecules 2019, 24, 768. [Google Scholar] [CrossRef] [Green Version]
- Arthurs, S.; Dara, S.K. Microbial biopesticides for invertebrate pests and their markets in the United States. J. Invertebr. Pathol. 2019, 165, 13–21. [Google Scholar] [CrossRef]
- Gu, Y.Q.; Mo, M.H.; Zhou, J.P.; Zou, C.S.; Zhang, K.Q. Evaluation and identification of potential organic nematicidal volatiles from soil bacteria. Soil Biol. Biochem. 2007, 39, 2567–2575. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Rahman, A.; Dung, N.T.; Huh, M.K.; Kang, S.C. In vitro inhibition of food spoilage and foodborne pathogenic bacteria by essential oil and leaf extracts of Magnolia liliflora Desr. J. Food Sci. 2008, 73, 314–320. [Google Scholar] [CrossRef]
- Ojaghian, M.R.; Jiang, H.; Xie, G.L.; Cui, Z.Q.; Zhang, Z.; Li, B. In vitro biofumigation of brassica tissues against potato stem rot caused by Sclerotinia sclerotiorum. Plant Pathol. 2012, 28, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Cheng, W.L.; Yang, J.Y.; Nie, Q.Y.; Huang, D.; Yu, C.; Zheng, L.Y.; Cai, M.M.; Thomashow, L.S.; Weller, D.M.; Yu, Z.N.; et al. Volatile organic compounds from Paenibacillus polymyxa KM2501-1 control Meloidogyne incognita by multiple strategies. Sci. Rep. 2017, 7, 16213. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.L.; Shao, Z.Z.; Cai, M.M.; Zheng, L.Y.; Li, G.Y.; Huang, D.; Cheng, W.L.; Thomasshow, L.S.; Weller, D.M.; Yu, Z.N.; et al. Multiple modes of nematode control by volatiles of Pseudomonas putida 1A00316 from Antarctic soil against Meloidogyne incognita. Front. Microbiol. 2018, 9, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessler, A.; Halitschke, R.; Diezel, C.; Baldwin, I.T. Priming of plant defense responses in nature by airborne signaling between Artemisia tridentata and Nicotiana attenuata. Oecologia 2006, 148, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Wiese, J.; Imhoff, J.F. Marine bacteria and fungi as promising source for new antibiotics. Drug Dev. Res. 2019, 80, 24–27. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Shao, Z.Z.; Yu, Y.; Cai, M.M.; Zheng, L.Y.; Li, G.Y.; Yu, Z.N.; Yi, X.F.; Zhang, J.B.; Hao, F.H. Identification, characteristics and mechanism of 1-Deoxy-N-acetylglucosamine from deep-sea Virgibacillus dokdonensis MCCC 1A00493. Mar. Drugs 2018, 16, 52. [Google Scholar] [CrossRef] [Green Version]
- Barros, A.F.; Campos, V.P.; Silva, J.C.P.; Pedroso, M.P.; Medeiros, F.H.V.; Pozza, E.A.; Reale, A.L. Nematicidal activity of volatile organic compounds emitted by Brassica juncea, Azadirachta indica, Canavalia ensiformis, Mucuna pruriens, and Cajanus cajan, against Meloidogyne incognita. Appl. Soil Ecol. 2014, 80, 34–43. [Google Scholar] [CrossRef]
- Aissani, N.; Urgeghe, P.P.; Oplos, C.; Saba, M.; Tocco, G.; Petretto, G.L.; Eloh, K.; Menkissogu-Spiroudi, U.; Ntalli, N.; Caboni, P. Nematicidal activity of the volatilome of Eruca sativa on Meloidogyne incognita. J. Agric. Food Chem. 2015, 63, 6120–6125. [Google Scholar] [CrossRef]
- Popova, A.A.; Koksharova, O.A.; Lipasova, V.A.; Zaitseva, J.V.; Katkova-Zhukotskaya, O.A.; Eremina, S.I.; Mironov, A.S.; Chernin, L.S.; Khmel, I.A. Inhibitory and toxic effects of volatiles emitted by strains of Pseudomonas and Serratia on growth and survival of selected microorganisms, Caenorhabditis elegans, and Drosophila melanogaster. Biomed Res. Int. 2014, 125704. [Google Scholar]
- Xu, Y.Y.; Lu, H.; Wang, X.; Zhang, K.Q.; Li, G.H. Effect of volatile organic compounds from bacteria on nematodes. Chem. Biodivers. 2015, 12, 1415–1421. [Google Scholar] [CrossRef]
- Yu, J.; Du, G.; Li, R.; Li, L.; Li, Z.; Zhou, C.J.; Chen, C.C.; Guo, D.S. Nematicidal activities of bacterial volatiles and components from two marine bacteria, Pseudoalteromonas marinastrain H-42 and Vibrio atlanticus strain S-16, against the pine wood nematode, Bursaphelenchus xylophilus. Nematology 2015, 17, 1011–1025. [Google Scholar] [CrossRef]
- Parsons, P.A.; Spence, G.E. Acetaldehyde: A low-concentration resource and larval attractant in 3 Drosophila, species. Experientia 1981, 37, 576–577. [Google Scholar] [CrossRef]
- Aharoni, Y.; Stewart, J.K.; Guadagni, D.G.; Mon, T.R. Thrips mortality and strawberry quality after vacuum fumigation with acetaldehyde or ethyl formate. J. AM. Soc. Hortic. Sci. 1980, 105, 926–929. [Google Scholar]
- Stewart, J.K.; Aharoni, Y.; Hartsell, P.L.; Young, D.K. Acetaldehyde fumigation at reduced pressures to control the green peach aphid on wrapped and packed head lettuce. J. Econ. Entomol. 1980, 73, 149–152. [Google Scholar] [CrossRef]
- Curto, G.; Dongiovanni, C.; Sasanelli, N.; Santori, A.; Myrta, A. Efficacy of dimethyl disulfide (DMDS) in the control of the root-knot nematode Meloidogyne incognita and the cyst nematode Heterodera carotae on carrot in field condition in Italy. Acta Hortic. 2014, 1044, 405–410. [Google Scholar] [CrossRef]
- Fritsch, J.; Fouillet, T.; Charles, P.; Fargier-Puech, P.; Ramponi- Bur, C.; Descamps, S. French experiences with dimethyl disulfide (DMDS) as a nematicide in vegetable crops. Acta Hortic. 2014, 1044, 427–434. [Google Scholar] [CrossRef]
- Faruk, M.I.; Rahman, M.L.; Mustafa, M.M.H.; Coosemans, I.J. Dimethyl disulfide-a potential biopesticide against root-knot nematode of tomato (Lycopersicon esculentum L.). Bangladesh J. Agr. Res. 2012, 36. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Xu, C.K.; Ma, L.; Zhang, K.Q.; Duan, C.Q.; Mo, M.H. Characterisation of volatiles produced from Bacillus megaterium, YFM3.25 and their nematicidal activity against Meloidogyne incognita. Eur. J. Plant Pathol. 2010, 126, 417–422. [Google Scholar] [CrossRef]
- Mao, L.; Jiang, H.; Zhang, L.; Zhang, Y.; Sial, M.U.; Yu, H.; Cao, A. Assessment of the potential of a reduced dose of dimethyl disulfide plus metham sodium on soilborne pests and cucumber growth. Sci. Rep. 2019, 9, 19806. [Google Scholar] [CrossRef]
- Fialho, M.B.; Bessi, R.; Inomoto, M.M.; Pascholati, S.F. Nematicidal effect of volatile organic compounds (VOCs) on the plant-parasitic nematode Meloidogyne javanica. Summa phytopathol. 2012, 38, 152–154. [Google Scholar] [CrossRef] [Green Version]
- Liarzi, O.; Bucki, P.; Miyara, S.B.; Ezra, D. Bioactive Volatiles from an endophytic Daldinia cf. concentrica isolate affect the viability of the plant parasitic nematode Meloidogyne javanica. PLoS ONE 2016, 11, e0168437. [Google Scholar]
- Jager, L.S.; Perfetti, G.A.; Diachenko, G.W. Comparison of headspace-SPME-GC-MS and LC-MS for the detection and quantification of coumarin, vanillin, and ethyl vanillin in vanilla extract products. Food Chem. 2008, 107, 1701–1709. [Google Scholar] [CrossRef]
- Diaz, A.; Vazquez, L.; Ventura, F.; Galceran, M.T. Estimation of measurement uncertainty for the determination of nonylphenol in water using solid-phase extraction and solid-phase microextraction procedures. Anal. Chim. Acta 2004, 506, 71–80. [Google Scholar] [CrossRef]
- Ntalli, N.G.; Ferrari, F.; Giannakou, I.; Menkissoglu-Spiroudi, U. Synergistic and antagonistic interactions of terpenes against Meloidogyne incognita and the nematicidal activity of essential oils from seven plants indigenous to Greece. Pest Manag. Sci. 2011, 67, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Ntalli, N.G.; Manconi, F.; Leonti, M.; Maxia, A.; Caboni, P. Aliphatic ketones from Ruta chalepensis (Rutaceae) induce paralysis on root knot nematodes. J. Agric. Food Chem. 2011, 59, 7098–7103. [Google Scholar] [CrossRef] [PubMed]
- Tajima, T.; Watanabe, N.; Kogawa, Y.; Takiguchi, N.; Kato, J.; Ikeda, T.; Kuroda, A.; Ohtake, H. Chemotaxis of the nematode Caenorhabditis elegans toward cycloheximide and quinine hydrochloride. J. Biosci. Bioeng. 2001, 91, 322–324. [Google Scholar] [CrossRef]
- Parida, L.; Neogi, S.; Padmanabhan, V. Effect of temperature pre-exposure on the locomotion and chemotaxis of C. elegans. PLoS ONE 2014, 9, e111342. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.M.; Zhi, D.J.; Ren, H.; Wang, D.; Wang, X.; Zhang, Z.X.; Fei, D.Q.; Zhu, H.M.; Li, H.Y. Shengmai formula ameliorates pathological characteristics in AD C. elegans. Cell. Mol. Neurobiol. 2016, 36, 1291–1302. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
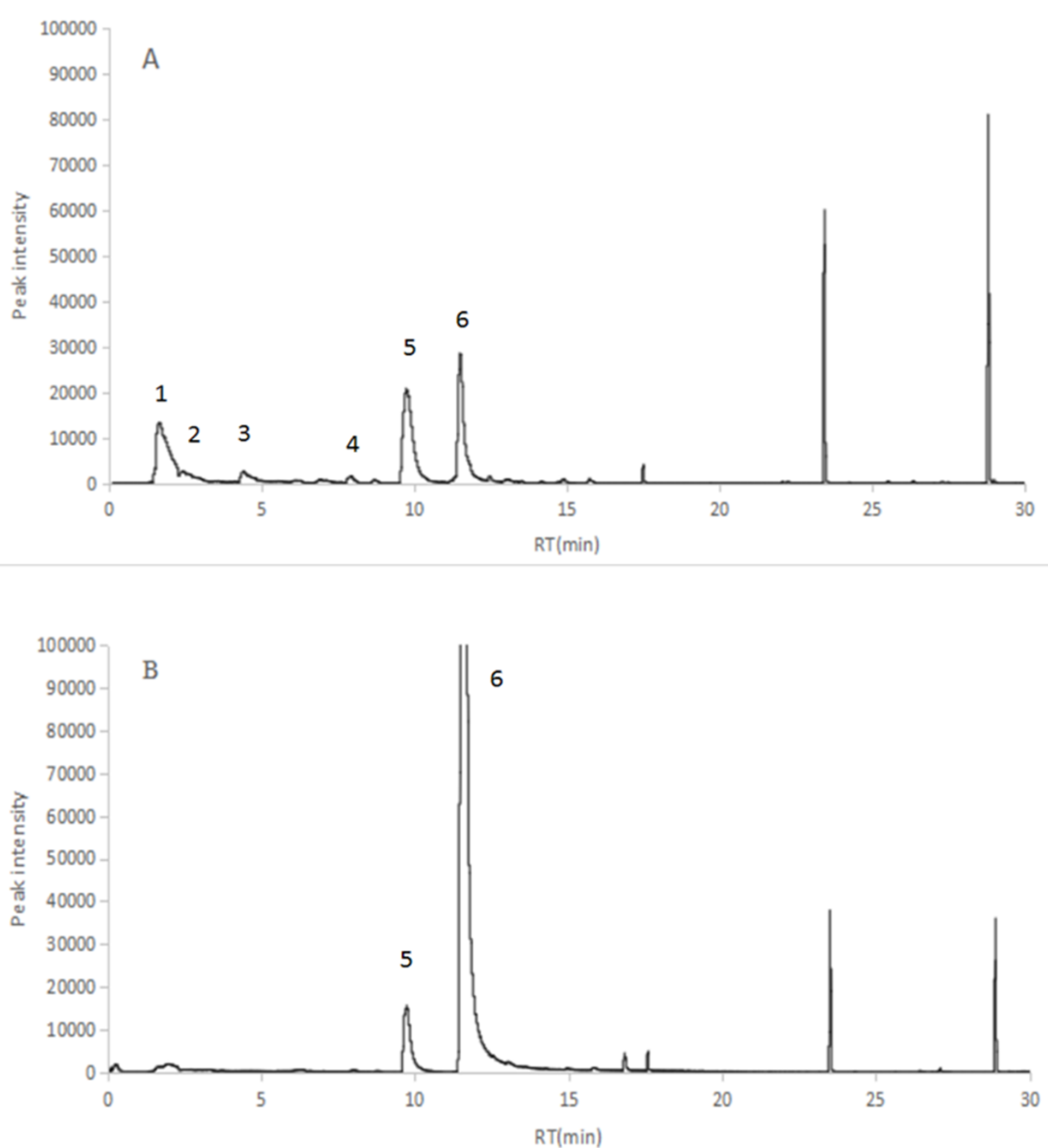


| PK | RT | Area Pct | Library/ID | CAS |
|---|---|---|---|---|
| 1 | 1.6566 | 17.7158 | Acetaldehyde | 000075-07-0 |
| 2 | 2.4153 | 3.1635 | 2-Butanone | 000078-93-3 |
| 3 | 4.4139 | 2.288 | Dimethyl disulfide | 000624-92-0 |
| 4 | 7.9255 | 0.977 | Ethylbenzene | 000100-41-4 |
| 5 | 9.7593 | 21.4172 | 2,5-Dimethyl pyrazine | 000123-32-0 |
| 6 | 11.5195 | 19.0497 | Benzaldehyde | 000100-52-7 |
| Compound | EC50 (mg/L) |
|---|---|
| 24 h | |
| Acetaldehyde | <3 |
| Dimethyl disulfide | 139.1 |
| 2-Butanone | >1000 |
| Ethylbenzene | >1000 |
| Mortality (%) ± SD | ||
|---|---|---|
| 6 h | 24 h | |
| 10 mg/mL acetaldehyde | 100 | 100 |
| 1 mg/mL acetaldehyde | 70.0 ± 12.0 | 97.9 ±2.4 |
| 10 mg/mL 2-butanone | 0 | 1.4 ± 1.9 |
| 10 mg/mL dimethyl disulfide | 0 | 7.9 ± 1.6 |
| 10 mg/mL ethylbenzene | 0 | 1.3 ± 1.6 |
| Hatched Worms Per Egg Mass ± SD | |||
|---|---|---|---|
| 1 day | 2 days | 3 days | |
| 1 mg/mL acetaldehyde | 17.3 ± 7.0 b | 37.4 ± 13.3 ab | 77.1 ± 6.0 a |
| 10 mg/mL acetaldehyde | 3.7 ± 2.3 b | 3.8 ± 2.3 c | 3.8 ± 2.3 b |
| 1A00493 culture | 18.7 ± 8.8 b | 18.9 ± 8.7 bc | 19.6 ± 8.4 b |
| control | 43.9 ± 21.0 a | 62.4 ± 23.7 a | 77.6 ± 25.7 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, D.; Yu, C.; Shao, Z.; Cai, M.; Li, G.; Zheng, L.; Yu, Z.; Zhang, J. Identification and Characterization of Nematicidal Volatile Organic Compounds from Deep-Sea Virgibacillus dokdonensis MCCC 1A00493. Molecules 2020, 25, 744. https://doi.org/10.3390/molecules25030744
Huang D, Yu C, Shao Z, Cai M, Li G, Zheng L, Yu Z, Zhang J. Identification and Characterization of Nematicidal Volatile Organic Compounds from Deep-Sea Virgibacillus dokdonensis MCCC 1A00493. Molecules. 2020; 25(3):744. https://doi.org/10.3390/molecules25030744
Chicago/Turabian StyleHuang, Dian, Chen Yu, Zongze Shao, Minmin Cai, Guangyu Li, Longyu Zheng, Ziniu Yu, and Jibin Zhang. 2020. "Identification and Characterization of Nematicidal Volatile Organic Compounds from Deep-Sea Virgibacillus dokdonensis MCCC 1A00493" Molecules 25, no. 3: 744. https://doi.org/10.3390/molecules25030744
APA StyleHuang, D., Yu, C., Shao, Z., Cai, M., Li, G., Zheng, L., Yu, Z., & Zhang, J. (2020). Identification and Characterization of Nematicidal Volatile Organic Compounds from Deep-Sea Virgibacillus dokdonensis MCCC 1A00493. Molecules, 25(3), 744. https://doi.org/10.3390/molecules25030744








