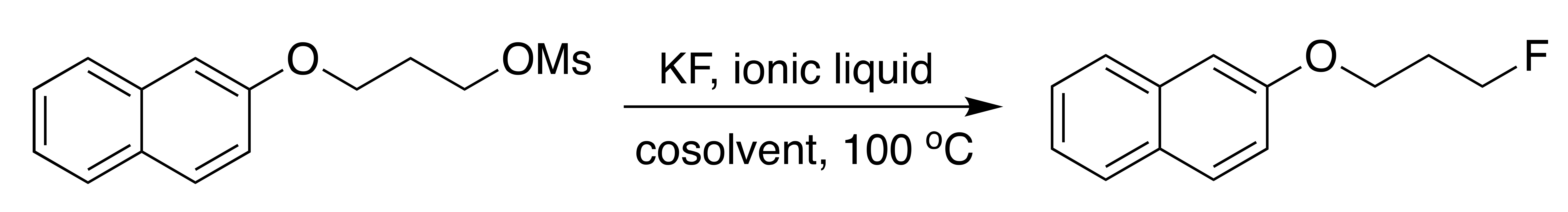Abstract
We review recent works for nucleophilic fluorination of organic compounds in which the Coulombic interactions between ionic species and/or hydrogen bonding affect the outcome of the reaction. SN2 fluorination of aliphatic compounds promoted by ionic liquids is first discussed, focusing on the mechanistic features for reaction using alkali metal fluorides. The influence of the interplay of ionic liquid cation, anion, nucleophile and counter-cation is treated in detail. The role of ionic liquid as bifunctional (both electrophilic and nucleophilic) activator is envisaged. We also review the SNAr fluorination of diaryliodonium salts from the same perspective. Nucleophilic fluorination of guanidine-containing of diaryliodonium salts, which are capable of forming hydrogen bonds with the nucleophile, is exemplified as an excellent case where ionic interactions and hydrogen bonding significantly affect the efficiency of reaction. The origin of experimental observation for the strong dependence of fluorination yields on the positions of -Boc protection is understood in terms of the location of the nucleophile with respect to the reaction center, being either close to far from it. Recent advances in the synthesis of [18F]F-dopa are also cited in relation to SNAr fluorination of diaryliodonium salts. Discussions are made with a focus on tailor-making promoters and solvent engineering based on ionic interactions and hydrogen bonding.
1. Introduction
Nucleophilic fluorination [1,2,3,4] using various sources of F fluoride exhibits several advantages over the electrophilic [5,6] counterpart, especially for introducing the isotopic F-fluorine onto organic compounds: First, it does not need to use the carrier added [18F]F2 gas that are very cumbersome to handle. This feature is especially important when the radioisotopic fluorine-18 [18F] is to be incorporated into an organic substance for clinical applications to positron emission tomography (PET) [7,8,9]. Second, [18F]F-labeled molecules can generally be produced in higher radiochemical yield and higher activity than the electrophilic substitution using carrier added [18F]F2 sources by several orders of magnitude. Third, the reaction yields from nucleophilic fluorination may improve tremendously (up to > 90%) in reasonable reaction time (<2 h) by employing a variety of promoter/catalyst with a minimum amount of by-products. Recent use of alkali metal fluoride in ionic liquids (ILs) have clearly opened this possibility by designing and applying various ‘task-specific’ ILs for nucleophilic fluorination. This novel capability of ILs as promoter/catalyst is really the results of the fact that ILs comprise the ionic species (cation and anion), but the detailed mechanism has been seldom understood. Here we review recent advances in the catalysis/promotion of SN2 fluorination by ILs, focusing on the mechanistic features of the process. We show that the rates and yields of nucleophilic fluorination may be improved by monitoring and controlling the Coulombic forces and weak interactions (hydrogen bonding, π-interactions, etc.) between IL cation, anion and substrates (especially, the leaving group).
These interactions also determine the efficiency of SNAr fluorination of diaryliodonium salts that are gaining much importance as a useful path to incorporating 18F and 19F to aromatic compounds. Since diaryliodonium salts consist of diaryliodonium cation and the counter-anion such as Br−, OTf− etc., Coulombic interactions with the alkali metal counter-cation and the nucleophile F− would be critical to determine the efficiency of fluorination. When the substrate contains functional groups such as hydroxyl (-OH), amino (-NH2, -NHR) that are amenable to hydrogen bonding, the situation becomes too complicated to scrutinize by intuition only. In the second part of this brief review, we show that nucleophilic fluorinations of diaryliodonium salts using alkali metal fluoride are indeed significantly affected by these electrostatic interactions. In some cases, the position of the nucleophile F− relative to those of the electropositive C atom and the leaving group may be determined by these ionic interactions and hydrogen bonding. Either the nucleophile F− is positioned close to the electropositive C atom for efficient fluorination, or F− may be located far from the center of reaction as the results of intricate interplay of the electrostatic interactions. In the latter case, of course, fluorination will not proceed at all. In order to scrutinize the configuration of the reacting system (diaryliodonium cation, counter-anion, leaving group, metal cation and F−) in pre-reaction complex and transition state to analyze the experimentally observed efficiency of reaction, quantum chemical calculations are indispensable, and we discuss the relevant recent works.
2. SN2 Fluorination in Ionic Liquids
Besides being considered excellent solvent for chemical reactions because of its many useful physicochemical properties such as very low vapor pressure, non-combustibility, high thermal stability, low viscosity, easy recovery and high ionic conductivity, ionic liquids (ILs) [10,11,12,13,14,15,16,17,18,19] have found further significant role as catalysts/promoters [20,21] in many chemical transformations such as SN2 [22,23,24,25,26,27,28], Diels-Alder [29], aldol condensation [30], Heck [31,32,33], and Michael addition [34] reactions. The acceleration of reaction rates of organic reactions by IL occur by the nature of the substance comprising cation and anion. However, the detailed mechanism seldom seems to be fully elucidated. Song and coworkers [26] gave a detailed review for experimental observations of increasing catalytic activity of metal triflates such as Sc(OTf)3 in ILs, attributing the phenomenon to formation of superacidic Lewis acidic catalysts by anion exchange. They systematically studied the “anion effect” for reactions such as Diels-Alder, and Friedel-Crafts Alkylation (Scheme 1). Therefore, we skip the discussion on this topic, and only review the catalytic activity of ILs in organic solvents or in solvent-free environment.
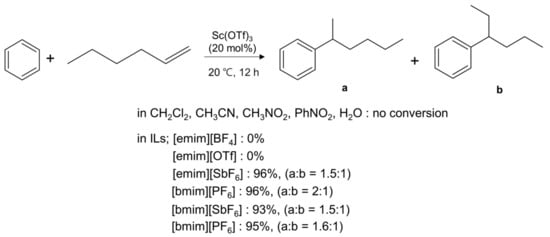
Scheme 1.
Sc(OTf)3-catalyzed Friedel-Crafts alkylation of benzene with 1-hexene in various solvents.
The first demonstration (Table 1) of promotion of nucleophilic fluorination in ionic liquids reaction media was made by Kim, Song and Chi [23,35]. They reported that KF is the source of F− in imidazolium based ILs [bmim][X] resulted in excellent yields (>90%) in reasonable reaction time (<2 h) with a minimal amount of by-products. Use of cosolvent such as acetonitrile did not affect or improve the reactivity of fluorination. They also showed that fluorination in [bmim][X] did not require anhydrous condition, demonstrating the extreme flexibility of ionic liquids for reaction conditions. However, less than stoichiometric amounts of IL (0.5 equiv) as catalyst/promoter showed rather poor performance for the nucleophilic fluorination.

Table 1.
Fluorinations using KF in various ionic liquids and cosolvents a.
The mechanism of these very interesting observations was elucidated by Lee and co-workers [24] in quantum chemical analysis. It was found that the ionic liquid anion plays a key role as a Lewis base by binding to the counter-cation K+ or Cs+, thereby reducing its retarding Coulombic influence on the nucleophile F−. That is, the counter-cation becomes a ‘ghost-like’ agent by the action of the negative charge of IL anion. The intricate interplay (Coulombic forces) of the counter-cation, the nucleophile, IL cation and anion helps to form a pre-reaction complex and transition state that is optimal for SN2 fluorination, prohibiting the formation of by-products (Figure 1). The role of IL anion in this mechanism corresponds to that of electronegative O atoms in oligoethylene glycols [36] or in bulky alcohols [37,38,39] (t-butanol and amyl alcohol) that were proved to act as catalyst/promoter in nucleophilic fluorination, but were probably better because of the explicit negative charge of IL anions.
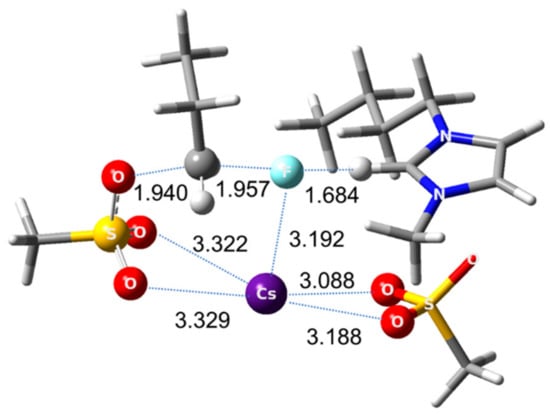
Figure 1.
Transition state for SN2 fluorination using CsF in [bmim][OMs]. MPW1K/6-311G ** method for C, H, O, N, F, S atoms, Hay-Wadt VDZ (n + 1) basis set with relevant effective core potential for alkali metal elements Cs, using Gaussian 03 set of programs.
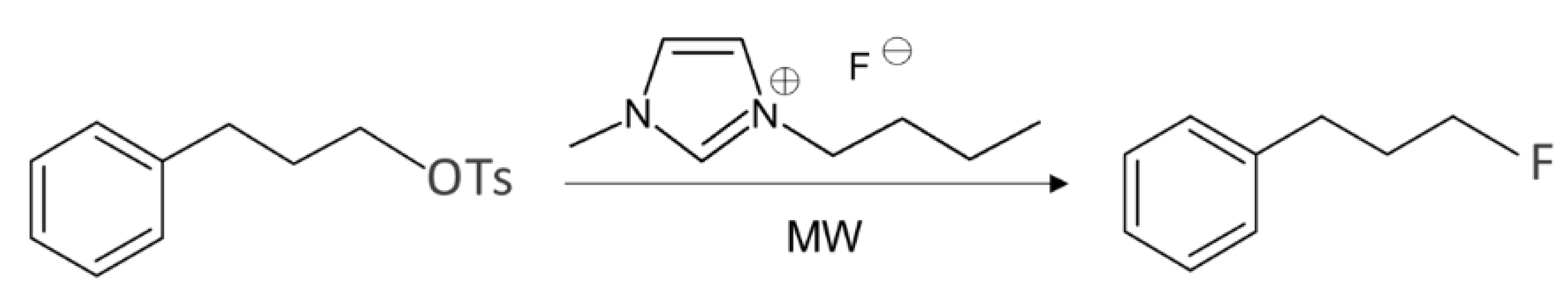
Magnier and coworkers [40] carried out a somewhat different approach to using ILs for nucleophilic fluorination. Unlike the experiments listed in Table 1, in which the source of the fluorinating agent is metal fluoride, they employed IL [bmim][F] directly, thereby in solvent-free environment. The IL [bmim][F] was prepared by the exchange of [bmim][Cl] with KF. Another interesting observation was that the reaction yields increased from 49 to 84 and 95% as the equivalent of IL increased from 1:1 to 1:2 and 1:3 (Table 2). The underlying mechanism [41] of Magnier and coworkers observations is depicted in Figure 2. When 1 eq. of IL is employed, the calculated pre-dissociation complex is shown in Figure 2a. Here, the IL cation bmim+ bridges the nucleophile F− and the leaving group -OTs, helping the formation of compact pre-reaction complex with optimal configuration for SN2 fluorination. For 2 eq. of [bmim][F] used (Figure 2b), the role of F− is two- fold: One of the two F− acts as a regular nucleophile, whereas the other one is off the electropositive carbon and the leaving group, whose action is on the two IL bmim+ cations to reduce their strong Coulombic forces on the nucleophile.

Table 2.
Fluorination of 3-phenylpropyl p-toluenesulfonate with [bmim][F].
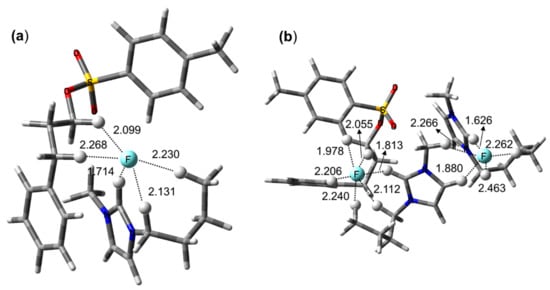
Figure 2.
Pre-reaction complexes of SN2 fluorination in [bmim][F]. (a) equivalent of IL = 1 (b) equivalent of IL = 2. M06-2X/6-311G ** method for C, H, O, N, S, F atoms, using Gaussian 09 set of programs.
It seems that Magnier and coworkers’ experiments were the first implicit demonstration of the contact ion-pair SN2 mechanism proposed by Lee and co-workers [42], in that the separation of IL cation and anion in liquid phase [bmim][F] would be extremely difficult in reaction medium because of the strong electrostatic force between them. The calculated pre-reaction complexes (whose coordinates are given in Supplementary Materials) shown in Figure 2 also support this suggestion that IL counter-cation and anion/nucleophile are in close contact. This role of IL for promoting the process of nucleophilic fluorination may also be described as an electrophile – nucleophile dual activator proposed by Lu and co-workers [43].
As a “task-specific” promoter/catalyst for organic transformations to be designed, the structure of side-chain [44] of IL cation and anion would be an important determinant of the efficiency of ILs. Modification of ILs by oligoethylene glycol chain is a good example, in which the synergistic effects of the two moieties improve the overall efficiency of the promoter/catalyst (Figure 3) [24].
Figure 3.
Promotion of SN2 fluorination by oligoethylene glycol substituted imidazolium salts.
Another example is the design and use of pyrene-tagged IL (Figure 4) [45]. As presented in Figure 4, the pyrene moiety acts as an additional Lewis base besides the IL anion in this mechanism, further strengthening the catalytic efficiency of IL. The mechanism (Figure 5) (pre-reaction complex → transition state → post-reaction complex) of the reaction is depicted in Figure 5: Pyrene - cation π interactions facilitate the reaction by giving π electrons to the counter-cation, further alleviating the Coulombic influence of the cation on the nucleophile [24].
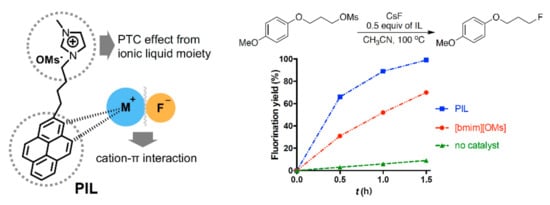
Figure 4.
SN2 fluorination in pyrene-tagged IL.
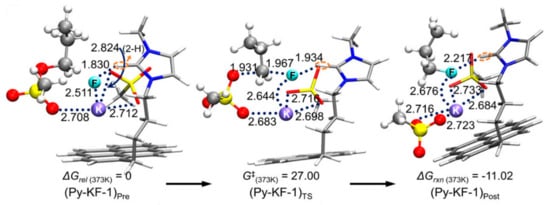
Figure 5.
Mechanism of SN2 fluorination catalyzed by pyrene-tagged IL. M06-2X/6-311G ** method for C, H, O, N, S atoms, LANL2DZ24 basis set with relevant effective core potential for alkali metal elements (Cs, K). Gaussian 09 set of programs.
3. SNAr Fluorination of Diaryliodonium Salts
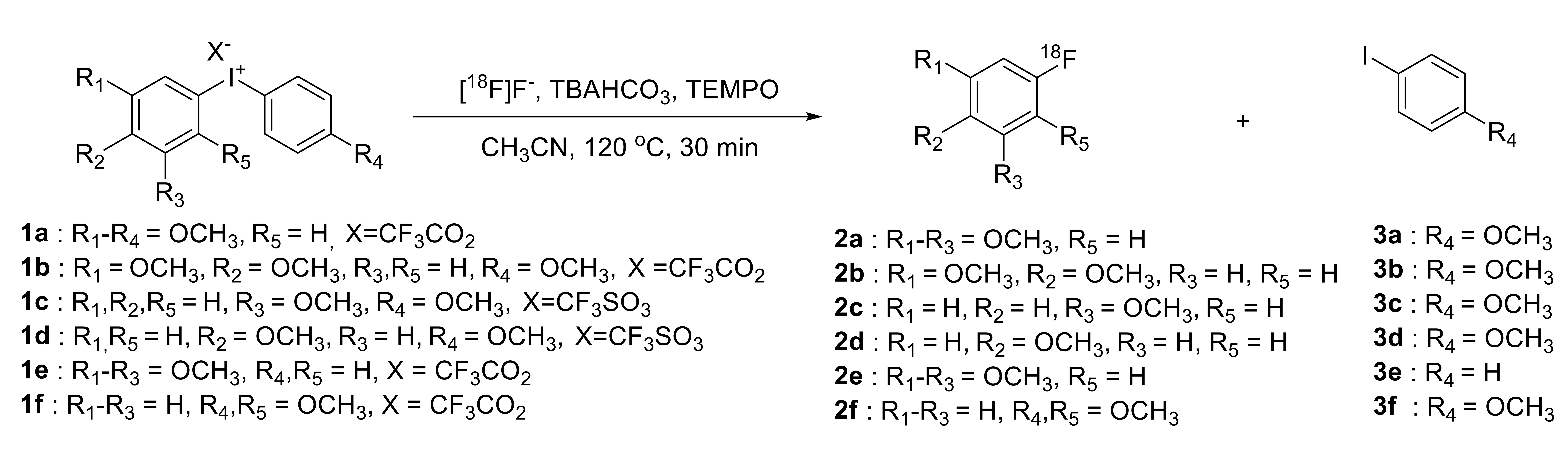
As in the case of SN2 fluorination, electrophilic SNAr fluorination such as fluorodestannylation [46] using [18F]F2 suffers similar kinds of drawbacks. A conventional approach to incorporating F to aromatic compounds was to try nucleophilic fluorination of electron deficient aromatics. Use of diaryliodonium salts have now proved very useful for the preparation of [18F]fluoroaromatics electron-rich rings at any desired ring position. This methodology is particularly important in relation to the synthesis of many useful [18F] radiophamaceutical compounds to be clinically detected by non-evasive PET technique. The seminal work by Pike and coworkers [47] was published in 1998, and since then this approach has found wide applicability [48,49,50,51,52,53]. This feature of diaryliodonium salts seems to originate from the ionic nature of the compounds. There exists an excellent review by Gouverneur and coworkers, [8] so we will not try to be comprehensive in this concise review. In some studies, metals such as copper [54,55,56] were employed to carry out SNAr fluorination of diaryliodonium salts, but we will also leave this topic to other reviews. We will only cite three typical studies: Coenen and coworkers’ [49] metal-free SNAr fluorination of diaryliodonium salts containing the 2-thienyl group (Scheme 2) is a good example of regioselective no-carrier-added [49,57,58] radiofluorination. The influence of the substitution pattern, of counteranions, and of different reaction conditions were studied carefully. Seid et al. studied one-pot synthesis of unsymmetrical aryl(2,4,6-trimethoxyphenyl)iodonium salts [57]. Chun and coworkers’ [58] chemoselective radiosynthesis of [18F]fluoroarenes using an aryl(2,4,6-trimethoxyphenyl)iodonium tosylate for 18F-incorporation on electron-rich aryl rings (Scheme 3) is a most recent study.
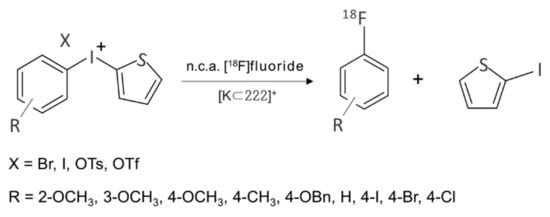
Scheme 2.
[18F]Fluorination of SNAr fluorination of diaryliodonium salts containing the 2-thienyl group.
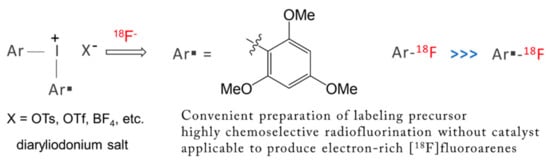
Scheme 3.
Chemoselective radiosynthesis of [18F]fluoroarenes using an aryl(2,4,6-trimethoxyphenyl)iodonium tosylate as a precursor for 18F-incorporation on electron-rich aryl rings.
SNAr fluorination of diaryliodonium salts may even give products that are contrary to what can be expected by conventional inductive effects: [18F] labeling may occur at the ring with larger electron density with RCY up to 68% in 30 min. (Table 3) [59].

Table 3.
[18F]Fluorination of various diaryliodonium salts. Fluorination may occur at the ring with more electron-donating substituents, and subsequently with larger electron density, in contrast to what is predicted by substituent inductive effects.
When aromatic fluorination is carried out for diaryliodonium salts with alkali metal fluoride, complicated ionic interactions would arise among the diaryliodonium cation, counter-anion, metal cation and the nuclephile F−, and they would certainly affect the outcomes of the reaction significantly. Presence of ring substituents and/or the leaving group prone to forming hydrogen bonding with ionic species would cause further complications. These interactions may make the elucidation of the mechanism by intuition a formidable task. However, they may constitute a very instructive approach to synthesizing [18F]fluoroaromatics that are difficult to achieve by conventional methods.
One such strategy results from the question: Could we monitor and control the position of F− relative to the SNAr center (that is, the electropositive C atom at which the SNAr occur) by harnessing these interactions? Among the numerous studies of SNAr fluorination, diaryliodonium salts containing side chains that are amenable to form hydrogen bonds seem to be the best example to address this question.
One such example is the aromatic 18F-labeling of guanidine-containing radiopharmaceutical [60,61,62,63,64,65,66,67,68,69,70] that was synthesized by nucleophilic fluorination of diaryliodonium salts. Jang and coworkers [71] found that the positions of -Boc protection profoundly affected the efficiency of 18F-labeling: The fully protected N,N’,N¨,N¨-tetrakis-Boc guanidine group (1b in Scheme 4) exhibited remarkably enhanced reactivity (yield = 39% in 5 min) and improved selectivity in contrast to N,N’-bis-Boc protected guanidine (1a, yield ≈ 0) in the absence of hydrogen bonding with fluoride ion (Scheme 4). What is the origin of these very intriguing observations? The observed difference in reactivity of (1a) and (1b) may be understood by scrutinizing the structures of corresponding pre-reaction complexes depicted in Figure 6: It was proposed by quantum chemical analysis that strong Coulombic interactions among the ionic species and hydrogen bonding between the anion and the amino group –NHR resulted in very different position of the nucleophile relative to the electropositive C atom. As shown in Figure 6, the nucleophile F− in (pre-8a) forms a hydrogen bond with the amino group in guanidine, and this interaction moves the nucleophile far away from the iodonium site. Thus, the SNAr reaction may never proceed from this complex. On the other hand, in (pre-8b), the absence of the hydrogen bonding with the fully protected (N,N’,N¨,N¨-tetrakis-Boc) amine group allows F− to be near the iodonium site, and therefore from this favorable configuration the nuclephilic attack of F− onto the electropositive C atom may easily ensue. This example seems to give a good answer to the question posed above: By carefully monitoring the ionic interactions and hydrogen bonding, it is possible to locate the nucleophile at a position that is favorable for SNAr reactions.
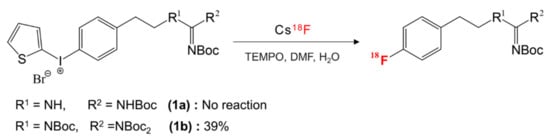
Scheme 4.
Dependence of 18F-labeling of guanidine-containing radiopharmaceutical on the positions of -Boc protection.
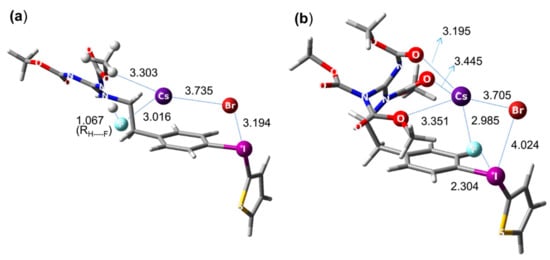
Figure 6.
Pre-reaction complexes for reactions listed in Scheme 2. In (a) the absence of hydrogen bonding positions F− favorable for fluorination, whereas in (b) hydrogen bonding with -NHR moves F− far off the reaction center. M06-2X/6-311G ** method for C, H, O, N, F, S atoms, LANL2DZ basis set with relevant effective core potential for alkali metal elements (I, Br, Cs), using Gaussian 09 set of programs.
Another 18F-labeled radiopharmaceutical that is under intense investigation recently by SNAr fluorination is [18F]F-dopa. There have been considerable amount of efforts [72,73,74,75,76] to prepare this radiotracer over the years. Neumaier and coworkers work based on Ritter and co-workers’ [74] scheme (Scheme 5) of nickel-based fluoride-derived electrophilic radiofluorination reagent is a good example. Numerous attempts to synthesize [18F]F-dopa by SNAr scheme have also experienced severe problems of poor yields along with many undesirable by-products. Besides these problems, the number of steps in the synthesis of this elusive radiotracer is of profound importance in order to be used for clinical purposes. Wirth and co-workers’ recent work [72] seems to deserve attention because of the simplicity of the synthetic scheme (Scheme 6) (SNAr fluorination of diaryl iodonium salt based on DiMagno and co-workers procedure [49]) despite the low yields (0–5%) of both “cold” (19F) and “hot” ([18F]) fluorination. The origin of the observed poor yields was also studied by quantum chemical calculations [75]: Focusing on the counter-anion Br− in diaryliodonium salt, it was proposed that SNAr bromination (Model I in Scheme 7) would be the predominant process rather than fluorination, resulting in very small amount of fluorinated product. This suggests that ionic interactions with the counter-anion (Br− in Wirth’s experiments) strongly affect the reaction yield, and using a counter-cation such as OTf− with lower nucleophilicity seems to be preferable. In ‘hot’ fluorination for synthesizing [18F]F-dopa, further complication would result from the use of base such as K2CO3 employed to extract 18F−. The alkali metal cation K+ may certainly be unfavorable due to its interactions with the nucleophile 18F−, and using Cs+ instead may be better. More recent experimental work by Maisonial-Besset et al. [76] for [18F] labelling of L-dopa (Scheme 8) without the use of base, cryptand or metal catalyst would also be of interest, because their methods gave improved RCY (27–38%) for [18F] labeling. It would be an excellent system to be investigated by quantum chemical methods. It seems that careful choice of the counter-anion (to the diaryiodonium) and counter-cation (to the nucleophile) and the base may lead to robust synthetic route to [18F]F-dopa with excellent yield and in short reaction time sufficiently satisfactory for clinical usage.
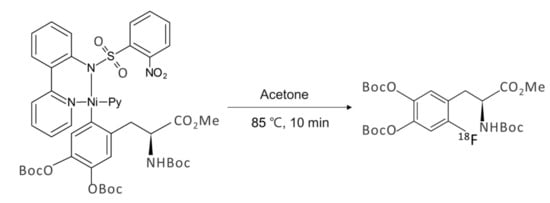
Scheme 5.
Nickel-mediated preparation of [18F]fluoroarenes.
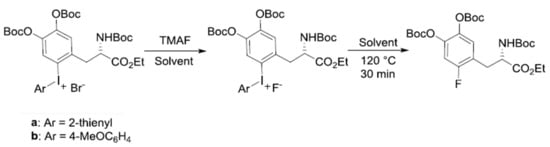
Scheme 6.
Cold fluorination of tri-Boc protected iodonium salts with tetramethylammonium fluoride (TMAF).
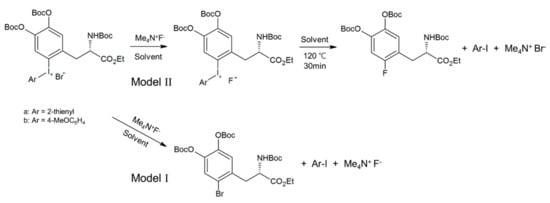
Scheme 7.
Proposed processes for Wirth and co-workers. Model I process (SNAr bromination) is predicted to be dominant over Model II process (SNAr fluorination).
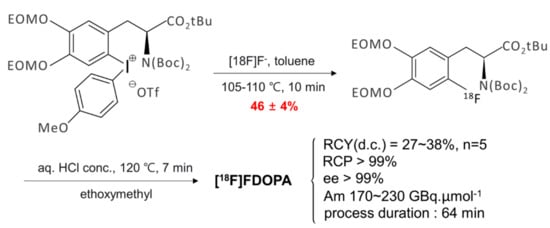
Scheme 8.
Preparation of [18F]F-dopa from tert-butyl ester precursor.
4. Conclusions
Nucleophilic fluorination, and especially [18F]Fluorination, is usually very difficult due to unfavorable solvent effects on the small-sized F fluoride when the reaction is attempted in organic solvent. Careful choice of efficient promoter/catalyst is usually required for this process. Use of alkali metal fluoride in Lewis base solvent/promoter such as bulky alcohols and oligoethylene glycols proved to be such a breakthrough for efficient nucleophilic fluorination. Employing ILs for that purpose is another example, in which the IL anion acts as Lewis base due to its ionic nature. We discussed that systematic design of the structure of ILs allowed very efficient SN2 fluorination with a minimum amount of by-products in reasonable reaction time. The harnessing of ionic interactions and hydrogen bonding also proved critical in controlling the reaction yield and rates. For SNAr fluorination of diaryliodonium salts, we presented a number of examples where quantum chemical analysis allowed the elucidation of underlying mechanism and design of experiments. We presented the synthesis of [18F]F-dopa by SNAr fluorination of diaryliodonium salts as a very recent example. We believe that our present review would help to advance further developments concerning the tailor-making of promoters/catalysts and solvent engineering based on ionic interactions and hydrogen bonding in the field of organic synthesis, medicinal chemistry, and environmental chemistry.
Supplementary Materials
The Supplementary Materials are available online.
Author Contributions
S.L., H.C. and Y.-H.O. carried out quantum chemical calculations and wrote the paper, D.W.K. and C.P. performed the experiments and wrote the paper. All authors have read and approved the manuscript.
Funding
This work was funded by Korea Research Foundation (NRF-2019R1F1A1057609), the Ministry of Science and ICT (MSIT), the MOTIE (Ministry of Trade, Industry & Energy) and KSRC (Korea Semiconductor Research Consortium) support program (grant code: 10080450), Korea.
Conflicts of Interest
The authors declare no conflict of interest
References
- Vaillancourt, F.H.; Yeh, E.; Vosburg, D.A.; Garneau-Tsodikova, S.; Walsh, C.T. Nature’s inventory of halogenation catalysts: Oxidative strategies predominate. Chem. Rev. 2006, 106, 3364–3378. [Google Scholar] [CrossRef] [PubMed]
- Champagne, P.A.; Desroches, J.; Hamel, J.D.; Vandamme, M.; Paquin, J.F. Monofluorination of Organic Compounds: 10 Years of Innovation. Chem. Rev. 2015, 115, 9073–9174. [Google Scholar] [CrossRef] [PubMed]
- Mascaretti, O.A. Modern methods for the monofluorination of aliphatic organic compounds. Aldrichim. Acta 1993, 26, 47–58. [Google Scholar]
- Lee, J.W.; Oliveira, M.T.; Jang, H.B.; Lee, S.; Chi, D.Y.; Kim, D.W.; Song, C.E. Hydrogen-bond promoted nucleophilic fluorination: Concept, mechanism and applications in positron emission tomography. Chem. Soc. Rev. 2016, 45, 4638–4650. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Neumann, C.N.; Ritter, T. Introduction of fluorine and fluorine-containing functional groups. Angew. Chem. Int. Ed. 2013, 52, 8214–8264. [Google Scholar] [CrossRef]
- Yang, X.; Wu, T.; Phipps, R.J.; Toste, F.D. Advances in catalytic enantioselective fluorination, mono-, di-, and trifluoromethylation, and trifluoromethylthiolation reactions. Chem. Rev. 2015, 115, 826–870. [Google Scholar] [CrossRef]
- Zhu, L.; Ploessl, K.; Kung, H.F. Expanding the scope of fluorine tags for PET imaging. Science 2013, 342, 429–430. [Google Scholar] [CrossRef]
- Preshlock, S.; Tredwell, M.; Gouverneur, V. 18F-Labeling of Arenes and Heteroarenes for Applications in Positron Emission Tomography. Chem. Rev. 2016, 116, 719–766. [Google Scholar] [CrossRef]
- Phelps, M.E. Positron emission tomography provides molecular imaging of biological processes. Proc. Natl. Acad. Sci. USA 2000, 97, 9226–9233. [Google Scholar] [CrossRef]
- Sheldon, R. Paul Olivier. Catalytic reactions in ionic liquids. Chem. Commun. 2001, 2399–2407. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Keim, W. Ionic Liquids—New “Solutions” for Transition Metal Catalysis. Angew. Chem. 2000, 39, 3772–3789. [Google Scholar] [CrossRef]
- Welton, T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev. 1999, 99, 2071–2083. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; De Souza, R.F.; Suarez, P.A.Z. Ionic liquid (molten salt) phase organometallic catalysis. Chem. Rev. 2002, 102, 3667–3692. [Google Scholar] [CrossRef]
- Dupont, J.; Suarez, P.A.Z. Physico-chemical processes in imidazolium ionic liquids. Phys. Chem. Chem. Phys. 2006, 8, 2441–2452. [Google Scholar] [CrossRef]
- Binnemans, K. Ionic liquid crystals. Chem. Rev. 2005, 105, 4148–4204. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Tak, H.C. Ionic-liquid-supported synthesis: A novel liquid-phase strategy for organic synthesis. Acc. Chem. Res. 2006, 39, 897–908. [Google Scholar] [CrossRef]
- Lei, Z.; Chen, B.; Li, C.; Liu, H. Predictive molecular thermodynamic models for liquid solvents, solid salts, polymers, and ionic liquids. Chem. Rev. 2008, 108, 1419–1455. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-temperature ionic liquids: Solvents for synthesis and catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef]
- Song, C.E. Methodologies in Asymmetric Catalysis. ACS Symp. Ser. 2004, 880. [Google Scholar]
- Vekariya, R.L. A review of ionic liquids: Applications towards catalytic organic transformations. J. Mol. Liq. 2017, 227, 44. [Google Scholar] [CrossRef]
- Xia, S.-M.; Chen, K.-H.; Fu, H.-C.; He, L.-N. Ionic Liquids Catalysis for Carbon Dioxide Conversion With Nucleophiles. Front. Chem. 2018, 6, 462. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.H.; Jang, H.B.; Im, S.; Song, M.J.; Kim, S.Y.; Park, S.W.; Chi, D.Y.; Song, C.E.; Lee, S. SN2 Fluorination reactions in ionic liquids: A mechanistic study towards solvent engineering. Org. Biomol. Chem. 2011, 9, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Song, C.E.; Chi, D.Y. New method of fluorination using potassium fluoride in ionic liquid: Significantly enhanced reactivity of fluoride and improved selectivity. J. Am. Chem. Soc. 2002, 124, 10278–10279. [Google Scholar] [CrossRef] [PubMed]
- Min, B.K.; Lee, S.S.; Kang, S.M.; Kim, J.; Kim, D.W.; Lee, S. Mechanism of Nucleophilic Fluorination Facilitated by a Pyrene-tagged Ionic Liquids: Synergistic Effects of Pyrene–Metal Cation π-Interactions. Bull. Korean Chem. Soc. 2018, 39, 1047–1053. [Google Scholar] [CrossRef]
- Lee, S.; Kim, D.W. Sustainable Catalysis in Ionic liquids; Lozano, P., Ed.; CRC Press: New York, NY, USA, 2018. [Google Scholar]
- Lee, J.W.; Shin, J.Y.; Chun, Y.S.; Jang, H.B.; Song, C.E.; Lee, S.G. Toward understanding the origin of positive effects of ionic liquids on catalysis: Formation of more reactive catalysts and stabilization of reactive intermediates and transition states in ionic liquids. Acc. Chem. Res. 2010, 43, 985–994. [Google Scholar] [CrossRef]
- Newington, I.; Perez-Arlandis, J.M.; Welton, T. Ionic liquids as designer solvents for nucleophilic aromatic substitutions. Org. Lett. 2007, 9, 5247–5250. [Google Scholar] [CrossRef]
- Jadhav, V.H.; Jang, S.H.; Jeong, H.J.; Lim, S.T.; Sohn, M.H.; Kim, J.Y.; Lee, S.; Lee, J.W.; Song, C.E.; Kim, D.W. Oligoethylene glycols as highly efficient mutifunctional promoters for nucleophilic-substitution reactions. Chem. A Eur. J. 2012, 18, 3918–3924. [Google Scholar] [CrossRef]
- Fischer, T.; Sethi, A.; Welton, T.; Woolf, J. Diels-Alder Reactions in Room-Temperature Ionic Liquids. Tetrahedron Lett. 1999, 2–5. [Google Scholar] [CrossRef]
- Gauchot, V.; Schmitzer, A.R. Asymmetric aldol reaction catalyzed by the anion of an ionic liquid. J. Org. Chem. 2012, 77, 4917–4923. [Google Scholar] [CrossRef]
- Xu, L.; Chen, W.; Xiao, J. Heck reaction in ionic liquids and the in situ identification of N-heterocyclic carbene complexes of palladium. Organometallics 2000, 19, 1123–1127. [Google Scholar] [CrossRef]
- Xu, L.; Chen, W.; Ross, J.; Xiao, J. Palladium-catalyzed regioselective arylation of an electron-rich olefin by aryl halides in ionic liquids. Org. Lett. 2001, 3, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Böhm, V.P.W.; Herrmann, W.A. Nonaqueous ionic liquids: Superior reaction media for the catalytic Heck-Vinylation of chloroarenes. Chem. A Eur. J. 2000, 6, 1017–1025. [Google Scholar] [CrossRef]
- Ranu, B.C.; Banerjee, S. Ionic liquid as catalyst and reaction medium. The dramatic influence of a task-specific ionic liquid, [bmIm]OH, in Michael addition of active methylene compounds to conjugated ketones, carboxylic esters, and nitriles. Org. Lett. 2005, 7, 3049–3052. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Song, C.E.; Chi, D.Y. Significantly enhanced reactivities of the nucleophilic substitution reactions in ionic liquid. J. Org. Chem. 2003, 68, 4281–4285. [Google Scholar] [CrossRef]
- Lee, J.W.; Yan, H.; Jang, H.B.; Kim, H.K.; Park, S.W.; Lee, S.; Chi, D.Y.; Song, C.E. Bis-terminal hydroxy polyethers as all-purpose, multifunctional organic promoters: A mechanistic investigation and applications. Angew. Chem. Int. Ed. 2009, 48, 7683–7686. [Google Scholar] [CrossRef]
- Kim, D.W.; Ahn, D.-S.; Oh, Y.-H.; Lee, S.; Kil, H.S.; Oh, S.J.; Lee, S.J.; Kim, J.S.; Ryu, J.S.; Moon, D.H.; et al. A new class of S<inf>N</inf>2 reactions catalyzed by protic solvents: Facile fluorination for isotopic labeling of diagnostic molecules. J. Am. Chem. Soc. 2006, 128. [Google Scholar] [CrossRef]
- Kim, D.W.; Jeong, H.J.; Lim, S.T.; Sohn, M.-H.; Katzenellenbogen, J.A.; Chi, D.Y. Facile nucleophilic fluorination reactions using tert-alcohols as a reaction medium: Significantly enhanced reactivity of alkali metal fluorides and improved selectivity. J. Org. Chem. 2008, 73, 957–962. [Google Scholar] [CrossRef]
- Shinde, S.S.; Khonde, N.S.; Kumar, P. Tri-tert-Butanolamine as an Organic Promoter in Nucleophilic Fluorination. ChemistrySelect 2017, 2, 118–122. [Google Scholar] [CrossRef]
- Bouvet, S.; Pégot, B.; Marrot, J.; Magnier, E. Solvent free nucleophilic introduction of fluorine with [bmim][F]. Tetrahedron Lett. 2014, 55, 826–829. [Google Scholar] [CrossRef]
- Choi, H.; Lee, S. Mechanistic study of SN2 reactions in [Bmim]F. (Unpublished).
- Han, H.J.; Lee, S.-S.; Kang, S.M.; Kim, Y.; Park, C.; Yoo, S.; Kim, C.H.; Oh, Y.-H.; Lee, S.; Kim., D.W. Effects of Structural Modifications of Bis-tert-Alcohol-Functionalized Crwon-Calix [4]arenes as Nucleophilic Fluorination Promotors and Relations with Computational Predictions. Eur. J. Org. Chem. 2020, 2020, 728–735, Accepted Manuscript. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, G.-p.; Cai, C. Facile aromatic nucleophilic substitution(SNAr) reactions in ionic liquids: An electrophile-nucleophile dual activation by [Omim]Br for the reaction. Green Chem. 2016, 18, 5580. [Google Scholar]
- Jadhav, V.H.; Kim, J.Y.; Chi, D.Y.; Lee, S.; Kim, D.W. Organocatalysis of nucleophilic substitution reactions by the combined effects of two promoters fused in a molecule: Oligoethylene glycol substituted imidazolium salts. Tetrahedron 2014, 70, 533–542. [Google Scholar] [CrossRef]
- Taher, A.; Lee, K.C.; Han, H.J.; Kim, D.W. Pyrene-Tagged Ionic Liquids: Separable Organic Catalysts for SN2 Fluorination. Org. Lett. 2017, 19, 3342–3345. [Google Scholar] [CrossRef] [PubMed]
- Pretze, M.; Wängler, C.; Wängler, B. 6-[18F]fluoro-L-DOPA: A well-established neurotracer with expanding application spectrum and strongly improved radiosyntheses. Biomed. Res. Int. 2014, 2014, 674063. [Google Scholar] [CrossRef]
- Shah, A.; Pike, V.W.; Widdowson, D.A. The synthesis of [18F]fluoroarenes from the reaction of cyclotron-produced [18F]fluoride ion with diaryliodonium salts. J. Chem. Soc. Perkin Trans. 1998, 1, 2043–2046. [Google Scholar] [CrossRef]
- Pike, V.W.; Aigbirhio, F.I. Reactions of cyclotron-produced [18F]fluoride with diaryliodonium salts—A novel single-step route to no-carrier-added [18F]fluoroarenes. J. Chem. Soc. Chem. Commun. 1995, 2215–2216. [Google Scholar] [CrossRef]
- Ross, T.L.; Ermert, J.; Hocke, C.; Coenen, H.H. Nucleophilic 18F-fluorination of heteroaromatic iodonium salts with no-carrier-added [18F]fluoride. J. Am. Chem. Soc. 2007, 129, 8018–8025. [Google Scholar] [CrossRef]
- Tredwell, M.; Gouverneur, V. 18F labeling of arenes. Angew. Chem. Int. Ed. 2012, 51, 11426–11437. [Google Scholar] [CrossRef]
- Wang, B.; Qin, L.; Neumann, K.D.; Uppaluri, S.; Cerny, R.L.; DiMagno, S.G. Improved arene fluorination methodology for I(III) salts. Org. Lett. 2010, 12, 3352–3355. [Google Scholar] [CrossRef][Green Version]
- Yuan, Z.; Cheng, R.; Chen, P.; Liu, G.; Liang, S.H. Efficient Pathway for the Preparation of Aryl(isoquinoline)iodonium(III) Salts and Synthesis of Radiofluorinated Isoquinolines. Angew. Chem. Int. Ed. 2016, 55, 11882–11886. [Google Scholar] [CrossRef] [PubMed]
- Zlatopolskiy, B.D.; Zischler, J.; Krapf, P.; Zarrad, F.; Urusova, E.A.; Kordys, E.; Endepols, H.; Neumaier, B. Copper-mediated aromatic radiofluorination revisited: Efficient production of PET tracers on a preparative scale. Chem. A Eur. J. 2015, 21, 5972–5979. [Google Scholar] [CrossRef] [PubMed]
- Ichiishi, N.; Canty, A.J.; Yates, B.F.; Sanford, M.S. Cu-catalyzed fluorination of diaryliodonium salts with KF. Org. Lett. 2013, 15, 5134–5137. [Google Scholar] [CrossRef] [PubMed]
- Ichiishi, N.; Brooks, A.F.; Topczewski, J.J.; Rodnick, M.E.; Sanford, M.S.; Scott, P.J.H. Copper-catalyzed [18F]fluorination of (Mesityl)(aryl)iodonium salts. Org. Lett. 2014, 16, 3224–3227. [Google Scholar] [CrossRef] [PubMed]
- Ichiishi, N.; Canty, A.J.; Yates, B.F.; Sanford, M.S. Mechanistic investigations of Cu-catalyzed fluorination of diaryliodonium salts: Elaborating the CuI/CuIII manifold in copper catalysis. Organometallics 2014, 33, 5525–5534. [Google Scholar] [CrossRef] [PubMed]
- Seidl, T.L.; Sundalam, S.K.; McCullough, B.; Stuart, D.R. Unsymmetrical Aryl(2,4,6-trimethoxyphenyl)iodonium Salts: One-Pot Synthesis, Scope, Stability, and Synthetic Studies. J. Org. Chem. 2016, 81, 1998–2009. [Google Scholar] [CrossRef]
- Kwon, Y.D.; Son, J.; Chun, J.H. Chemoselective Radiosyntheses of Electron-Rich [18F]Fluoroarenes from Aryl(2,4,6-trimethoxyphenyl)iodonium Tosylates. J. Org. Chem. 2019, 84, 3678–3686. [Google Scholar] [CrossRef]
- Jang, K.S. Nucleophilic Aromatic [18F]Fluorination of Electron Rich Aromatic Systems via Diaryliodonium Salts. Ph.D Thesis, Inha University, Incheon, Korea, 2011. [Google Scholar]
- Berry, C.R.; Garg, P.K.; Zalutsky, M.R.; Coleman, R.E.; DeGrado, T.R. Uptake and retention kinetics of para-fluorine-18-fluorobenzylguanidine in isolated rat heart. J. Nucl. Med. 1996, 37, 2011–2016. [Google Scholar]
- Garg, P.K.; Garg, S.; Zalutsky, M.R. Synthesis and preliminary evaluation of para- and meta-[18F]fluorobenzylguanidine. Nucl. Med. Biol. 1994, 21, 97–103. [Google Scholar] [CrossRef]
- Jung, Y.W.; Jang, K.S.; Gu, G.; Koeppe, R.A.; Sherman, P.S.; Quesada, C.A.; Raffel, D.M. Fluoro-Hydroxyphenethylguanidines: Efficient Synthesis and Comparison of Two Structural Isomers as Radiotracers of Cardiac Sympathetic Innervation. ACS Chem. Neurosci. 2017, 8, 1530–1542. [Google Scholar] [CrossRef]
- Higuchi, T.; Yousefi, B.H.; Kaiser, F.; Gärtner, F.; Rischpler, C.; Reder, S.; Yu, M.; Robinson, S.; Schwaiger, M.; Nekolla, S.G. Assessment of the 18F-Labeled PET tracer LMI1195 for imaging norepinephrine handling in rat hearts. J. Nucl. Med. 2013, 54, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.C.; Paik, J.Y.; Chi, D.Y.; Lee, K.H.; Choe, Y.S. Potential and Practical Adrenomedullary PET Radiopharmaceuticals as an Alternative to m-Iodobenzylguanidine: M-(ω[18F]Fluoroalkyl)benzylguanidines. Bioconjugate Chem. 2004, 15, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Raffel, D.; Loc, C.; Mardon, K.; Mazi, B.; Syrota, A. Bromobenzylguanidine in Isolated Working Rat Heart A-l. Nucl. Med. Biol. 1998, 25, 1–16. [Google Scholar] [CrossRef]
- Vaidyanathan, G.; Affleck, D.J.; Zalutsky, M.R. (4-[18F]Fluoro-3-iodobenzyl)guanidine, a Potential MIBG Analogue for Positron Emission Tomography. J. Med. Chem. 1994, 37, 3655–3662. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, C.; Affleck, D.J.; Zalutsky, M.R. Validation of 4-[fluorine-18]fluoro-3-iodobenzylguanidine as a positron- emitting analog of MIBG. J. Nucl. Med. 1995, 36, 644–650. [Google Scholar] [PubMed]
- Vaidyanathan, G.; McDougald, D.; Koumarianou, E.; Choi, J.; Hens, M.; Zalutsky, M.R. Synthesis and evaluation of 4-[18F]fluoropropoxy-3-iodobenzylguanidine ([18F]FPOIBG): A novel 18F-labeled analogue of MIBG. Nucl. Med. Biol. 2015, 42, 673–684. [Google Scholar] [CrossRef]
- Yu, M.; Bozek, J.; Lamoy, M.; Guaraldi, M.; Silva, P.; Kagan, M.; Yalamanchili, P.; Onthank, D.; Mistry, M.; Lazewatsky, J.; et al. Evaluation of LMI1195, a novel 18F-labeled cardiac neuronal PET imaging agent, in cells and animal models. Circ. Cardiovasc. Imaging 2011, 4, 435–443. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, R.; Pillarsetty, N.; Thorek, D.L.; Vaidyanathan, G.; Serganova, I.; Blasberg, R.G.; Lewis, J.S. Synthesis and evaluation of 18F-labeled benzylguanidine analogs for targeting the human norepinephrine transporter. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 322–332. [Google Scholar] [CrossRef][Green Version]
- Jang, K.S.; Lee, S.S.; Oh, Y.H.; Lee, S.H.; Kim, S.E.; Kim, D.W.; Lee, B.C.; Lee, S.; Raffel, D.M. Control of reactivity and selectivity of guanidinyliodonium salts toward 18F-Labeling by monitoring of protecting groups: Experiment and theory. J. Fluor. Chem. 2019, 227, 109387. [Google Scholar] [CrossRef]
- Edwards, R.; Westwell, A.D.; Daniels, S.; Wirth, T. Convenient synthesis of diaryliodonium salts for the production of [18F]F-DOPA. Eur. J. Org. Chem. 2015, 2015, 625–630. [Google Scholar] [CrossRef]
- Zlatopolskiy, B.D.; Zischler, J.; Urusova, E.A.; Endepols, H.; Kordys, E.; Frauendorf, H.; Mottaghy, F.M.; Neumaier, B. A Practical One-Pot Synthesis of Positron Emission Tomography (PET) Tracers via Nickel-Mediated Radiofluorination. ChemistryOpen 2015, 4, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Hooker, J.M.; Ritter, T. Nickel-mediated oxidative fluorination for PET with aqueous [18F] fluoride. J. Am. Chem. Soc. 2012, 134, 17456–17458. [Google Scholar] [CrossRef]
- Oh, Y.-H.; Choi, H.; Lee, S.-S.; Lee, S. Toward the Robust Synthesis of [18F]F-DOPA: Quantum Chemical Analysis of SNAr Cold Fluorination of Diaryl Iodonium Salt by 19F. Bull. Korean Chem. Soc. 2020, (in press). [CrossRef]
- Maisonial-Besset, A.; Serre, A.; Ouadi, A.; Schmitt, S.; Canitrot, D.; Léal, F.; Miot-Noirault, E.; Brasse, D.; Marchand, P.; Chezal, J.M. Base/Cryptand/Metal-Free Automated Nucleophilic Radiofluorination of [18F]FDOPA from Iodonium Salts: Importance of Hydrogen Carbonate Counterion. Eur. J. Org. Chem. 2018, 2018, 7058–7065. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).