Assessment of Pesticide Residue Content in Polish Agricultural Soils
Abstract
1. Introduction
2. Results and Discussion
2.1. Soil Physicochemical Properties
2.2. OCP Soil Concentrations
2.3. NCP Soil Concentrations
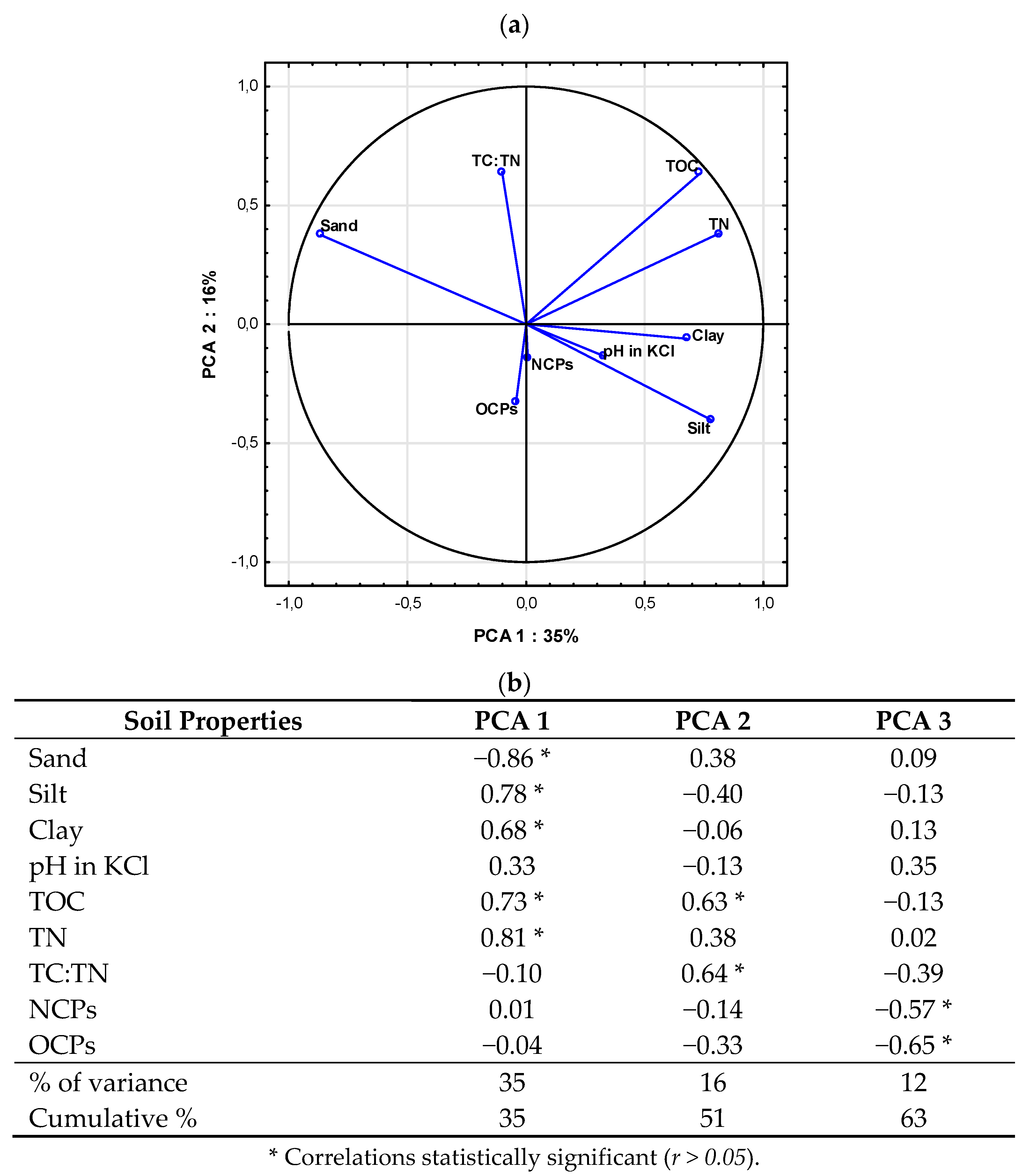
2.4. Relationship between Soil Properties and Pesticide Residue Concentrations
3. Materials and Methods
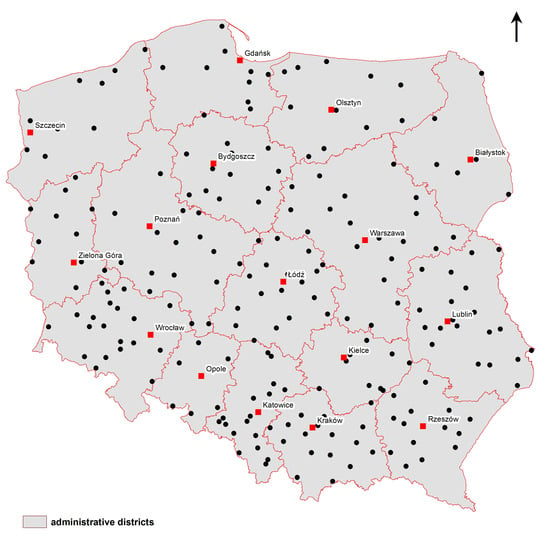
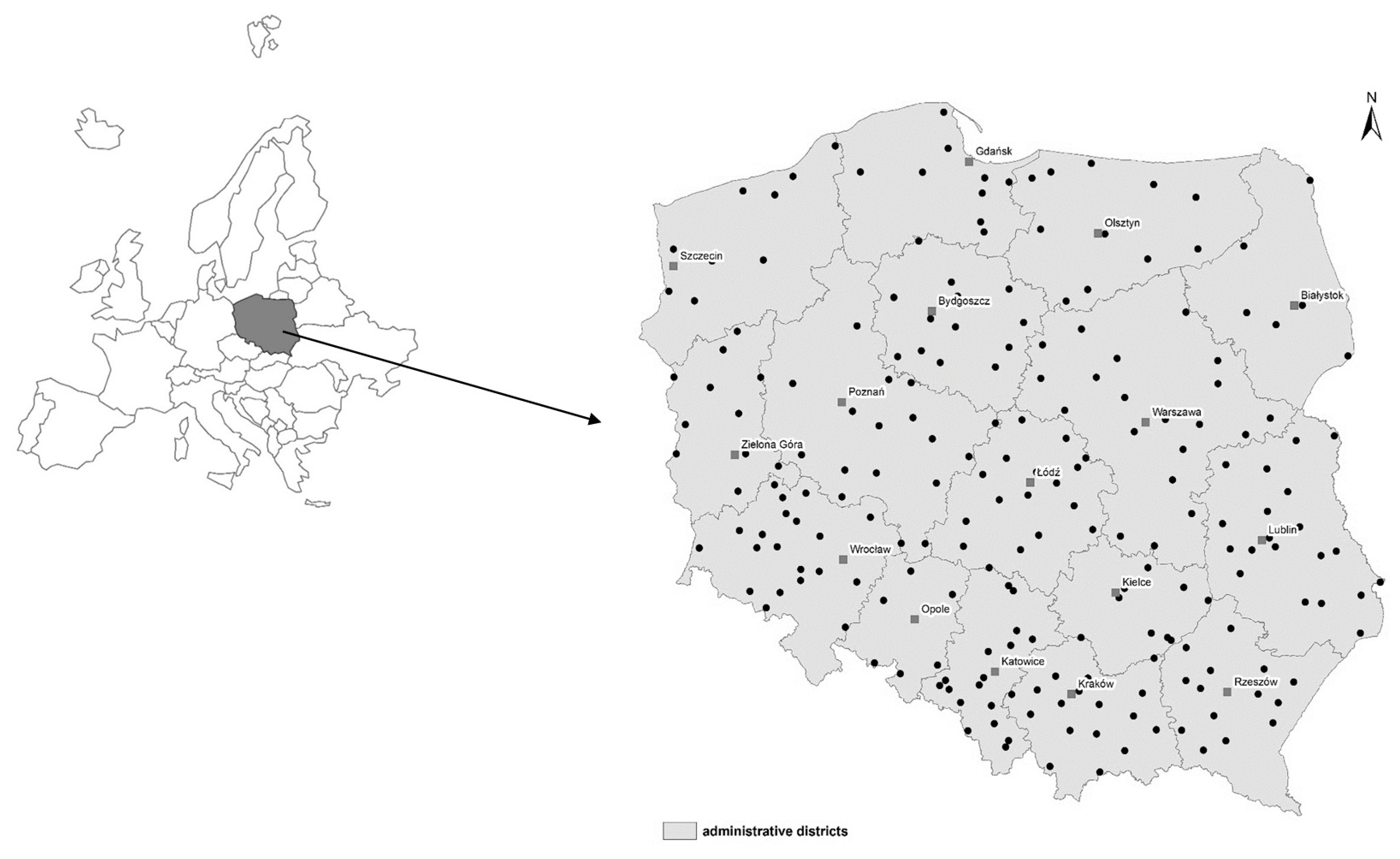
3.1. Soil Sampling
3.2. Basic Soil Property Determination
3.3. OCP Concentration Determination
3.4. NCP Concentration Determination
3.5. Statistics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mrówczyńska-Kamińska, A. The Importance of Agriculture in the Polish National Economy: Macroeconomic and Regional Analysis. Problems of World Agriculture. Sci. J. Warsaw Univ. Life Sci. 2008, 5, 96–107. (In Polish) [Google Scholar]
- Silva, V.; Mol, H.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissena, V. Pesticide Residues in European Agricultural Soils—A hidden Reality Unfolded. Sci. Total. Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef]
- Hvězdová, M.; Kosubová, P.; Košíková, M.; Scherr, K.; Šimek, Z.; Brodský, L.; Šudoma, M.; Škulcová, L.; Sáňka, M.; Svobodová, M. Currently and Recently Used Pesticides in Central European Arable Soils. Sci. Total. Environ. 2018, 613–614, 361–370. [Google Scholar]
- Jorfi, F.; Atashi, Z.; Akhbarizadeh, R.; Khorasgani, Z.; Ahmadi, M. Distribution and Health Risk Assessment of Organochlorine Pesticides in Agricultural Soils of the Aghili Plain, Southwest Iran. Environ. Earth Sci. 2019, 78, 603. [Google Scholar] [CrossRef]
- FAO. ITPS Global Assessment of the Impact of Plant Protection Products on Soil Functions and Soil Ecosystems; FAO: Rome, Italy, 2017; p. 40. [Google Scholar]
- Stolte, J.; Tesfai, M.; Øygarden, L.; Kværnø, S.; Keizer, J.; Verheijen, F.; Panagos, P.; Ballabio, C.; Hesse, R. Soil Threats in Europe: Status, Methods, Drivers and Effects on Ecosystem Services; EUR 27607 EN; Publications Office of the European Union: Brussels, Belgium, 2016. [CrossRef]
- Lechenet, M.; Dessaint, F.; Py, G.; Makowski, D.; Munier-Jolain, N. Reducing Pesticide Use While Preserving Crop Productivity and Profitability on Arable Farms. Nat. Plants 2017, 3, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dirbaba, N.B.; Li, S.; Wu, H.; Yan, X.; Wang, J. Organochlorine Pesticides, Polybrominated Diphenyl Ethers and Polychlorinated Biphenyls in Surficial Sediments of the Awash River Basin, Ethiopia. PLoS ONE 2018, 13. [Google Scholar] [CrossRef]
- Cycoń, M.; Piotrowska-Seget, Z. Transformations of Pesticides in Soil Environment—A review. Pestycydy 2006, 3–4, 45–56. [Google Scholar]
- Ehlers, G.; Loibner, A. Linking Organic Pollutant (bio)availability with Geosorbent Properties and Biomimetic Methodology: A Review of Geosorbent Characterization and (bio)availability Prediction. Environ. Pollut. 2006, 141, 494–512. [Google Scholar] [CrossRef]
- Nieder, R.; Benbi, D.K.; Reichl, Z.X. Soil-Borne Particles and Their Impact on Environment and Human Health. Soil Components and Human Health. Springer Neth. 2018, 99–177. [Google Scholar] [CrossRef]
- Yang, H.; Wu, X.; Zhou, L.; Yang, Z. Effect of Dissolved Organic Matter on Chlorotoluron sorption and desorption in soils. Pedosphere 2005, 15, 432–439. [Google Scholar]
- Pu, X.; Cutright, T. Sorption–Desorption Behavior of PCP on Soil Organic Matter and Clay Minerals. Chemosphere 2006, 64, 972–983. [Google Scholar] [CrossRef] [PubMed]
- Laha, S.; Tansel, B.; Ussawarujikulchai, A. Surfactant–Soil Interactions during Surfactantamended Remediation of Contaminated Soils by Hydrophobic Organic Compounds: A review. J. Environ. Manag. 2009, 90, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Babut, M.; Arts, G.H.; Caracciolo, A.B.; Carluer, N.; Domange, N.; Friberg, N. Pesticide risk assessment and management in a globally changing world—report from a European interdisciplinary workshop. Environ. Sci. Pollut. Res. Int. 2013, 20, 8298–8312. [Google Scholar] [CrossRef] [PubMed]
- COM 231 Final Communication from the Commission to the Council and the European Parliament, the European Economic and Social Committee and the Committee of the Regions: Towards a thematic strategy for soil protection; COM(2006)231 final Brussels, Belgium 2006.
- Carlon, C. Derivation Methods of Soil Screening Values in Europe. A Review and Evaluation of National Procedures towards Harmonization; European Commission, Joint Research Centre: Ispra, Italy, 2007; Volume 320, pp. 26–74. [Google Scholar]
- Statistical Yearbook of Agriculture 2018 (in Polish). Available online: https://stat.gov.pl/obszary-tematyczne/roczniki-statystyczne/roczniki-statystyczne/rocznik-statystyczny-rolnictwa-2018,6,12.html (accessed on 28 January 2020).
- JoL 2013 item 1686. Regulation of Minister of Agriculture and Rural Development from 13th December, 2013 on Certifying of Equipment’s Technical Efficiency. Available online: http://prawo.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20130001686 (accessed on 28 January 2020).
- JoL 2013 item 1742. Regulation of Minister of Agriculture and Rural Development from 18th December, 2013 on Requirements Regulating Equipment’s Technical Efficiency. Available online: http://prawo.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20130001742 (accessed on 28 January 2020).
- JoL 2013 item 554. Regulation of Minister of Agriculture and Rural Development from 8th May, 2013 on pesticides’ trainings. Available online: http://prawo.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20130000554 (accessed on 28 January 2020).
- JoL 2013 item 625. Regulation of Minister of Agriculture and Rural Development from 22nd May, 2013 on ways of pesticides’ use and storage. Available online: http://prawo.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20130000625 (accessed on 28 January 2020).
- JoL 2014 item 516. Regulation of Minister of Agriculture and Rural Development from 31st March, 2014 on terms of use of pesticides. Available online: http://prawo.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20140000516 (accessed on 28 January 2020).
- JoL 2016 item 1395. Regulation of Minister of Environmental Protection from 1th September, 2016 on assessment of soil surface pollution. Available online: http://prawo.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20160001395 (accessed on 28 January 2020).
- Commission Directive (EU) 2019/782 of 15 May 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32019L0782&from=EN (accessed on 28 January 2020).
- Report on the Implementation of Directive 2009/128/EC on the Sustainable Use of Pesticides (2017/2284(INI)) 2019. Available online: http://www.europarl.europa.eu/doceo/document/A-8-2019-0045_EN.html (accessed on 28 January 2020).
- Maliszewska-Kordybach, B.; Smreczak, B.; Klimkowicz-Pawlas, A. Evaluation of the Status of Contamination of Arable Soils in Poland with DDT and HCH Residues; National and Regional Scales. Pol. J. Environ. Stud. 2014, 23, 139–148. [Google Scholar]
- Bojakowska, I.; Gliwicz, T. Chlorinated Pesticides and Polychlorinated biphenyls in river sediments of Poland. Polish Geol. Rev. 2005, 53, 649–655. (In Polish) [Google Scholar]
- Łozowicka, B.; Kaczyński, P.; Wolejko, E.; Piekutin, J.; Sagitov, A.; Tolwubayev, K.; Isenova, G.; Abzeitova, E. Evaluation of Organochlorine Pesticide Residues in Soil and Plants from East Europe and Central Asia. Desalin. Water. Treat. 2016, 57, 1–12. [Google Scholar] [CrossRef]
- Tan, K.H. Humic Matter in Soil and Environment. Principles and Controversies, Boca Raton, FL 33487-2742; CRC Press: Taylor and Francis Group: Boca Raton, FL, USA, 2014; pp. 79–104. [Google Scholar]
- Holoubek, I.; Dušek, L.; Sáňka, M.; Hofman, J.; Čupr, P.; Jarkovskỳ, J.; Zbíral, J.; Klánová, J. Soil Burdens of Persistent Organic Pollutants—Their Levels, Fate and Risk. Part I. Variation of Concentration Ranges According to Different Soil Uses and Locations. Environ. Pollut. 2009, 157, 3207. [Google Scholar] [CrossRef]
- Manz, M.; Wenzel, K.D.; Dietze, U.; Schüürman, G. Persistent Organic Pollutants in Agricultural Soils of Germany. Sci. Total Environ. 2001, 277, 187. [Google Scholar] [CrossRef]
- Cabrerizo, A.; Dachs, J.; Jones, K.C.; Barcel, D. Soil-Air Exchange Controls on Background Atmospheric Concentrations of Organochlorine Pesticides. Atmos. Chem. Phys. 2011, 11, 12799–12811. [Google Scholar] [CrossRef]
- Meijer, S.N.; Halsall, C.J.; Harner, T.; Peters, A.J.; Ockenden, W.A.; Johnston, A.E.; Jones, K.C. Organochlorine Pesticide Residues in Archived UK Soil. Environ. Sci. Technol. 2001, 35, 10. [Google Scholar] [CrossRef] [PubMed]
- Maliszewska-Kordybach, B.; Smreczak, B.; Klimkowicz-Pawlas, A. The Levels and Composition of Persistent Organic Pollutants in Alluvial Agriculture Soils Affected by Flooding. Environ. Monit. Assess. 2013, 185, 9935–9948. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhou, H.; Pan, G.; Wang, J.; Chen, B. Factors Influencing the Persistence of Organochlorine Pesticides in Surface Soil from the Region Around the Hongze Lake, China. Sci. Total Environ. 2013, 443, 7–13. [Google Scholar] [CrossRef]
- Rajendran, R.B.; Imagawa, T.; Tao, H.; Ramesh, R. Distribution of PCBs, HCHs and DDTs and their Ecotoxicological Impacs in Bay of Bengal, India. Environ. Int. 2005, 31, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Junhong, B.; Guangliang, Z.; Xin, W.; Jia, J.; Baoshan, C.; Xinhui, L. Depth-Distribution, Possible Sources, and Toxic Risk Assessment of Organochlorine Pesticides (OCP s) in Different River Sediment Cores Affected by Urbanization and Reclamation in a Chinese Delta. Environ. Pollut. 2017, 230, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Messing, P.G.; Farenhorst, A.; Waite, D.T.; McQueen, D.A.R.; Sproull, J.F.; Humphries, D.A.; Thompson, L.L. Predicting Wetland Contamination from Atmospheric Deposition Measurements of Pesticides in the Canadian Prairie Pothole Region. Atmos. Environ. 2011, 45, 7227–7234. [Google Scholar] [CrossRef]
- Singh, B.; Farenhorst, A.; Gaultier, J.; Pennock, D.; Degenhardt, D.; McQueen, R. Soil Characteristics and Herbicide Sorption Coefficients in 140 Soil Profiles of two Irregular Undulating to Hummocky Terrains of Western Canada. Geoderma 2014, 232, 107–116. [Google Scholar] [CrossRef]
- Stipičević, S.; Galzina, N.; Udiković-Kolić, N.; Jurina, T.; Mendaš, G.; Dvoršćak, M.; Petrić, I.; Barić, K.; Drevenkar, V. Distribution of Terbuthylazine and Atrazine Residues in Crop-Cultivated soil: The Effect of Herbicide Application Rate on Herbicide Persistence. Geoderma 2015, 259–260, 300–309. [Google Scholar] [CrossRef]
- Chung, N.; Alexander, M. Effect of Soil Properties on Bioavailability and Extractability of Phenanthrene and Atrazine Sequestered in Soil. Chemosphere 2002, 48, 109–115. [Google Scholar] [CrossRef]
- Jablonowski, N.D.; Köppchen, S.; Hofmann, D.; Schäffer, A.; Burauel, P. Persistence of 14C-labeled Atrazine and its Residues in a Field Lysimeter Soil after 22 years. Environ. Pollut. 2009, 157, 2126–2131. [Google Scholar] [CrossRef]
- Martinsa, E.; Melob, V.; Bohonea, J.; Abatea, G. Sorption and Desorption of Atrazine on Soils: The Effect of Different Soil Fractions. Geoderma 2018, 322, 131–139. [Google Scholar] [CrossRef]
- Toccalino, P.L.; Gilliom, R.J.; Lindsey, B.D.; Rupert, M.G. Pesticides in Groundwater of the United States: Decadal-Scale Changes, 1993–2011. Groundwater 2014, 52, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Vonberg, D.; Vanderborght, J.; Cremer, N.; Pütz, T.; Herbst, M.; Vereecken, H. 20 years of Long-Term Atrazine Monitoring in a Shallow Aquifer in Western Germany. Water Res. 2014, 50, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Montagner, C.C.; Vidal, C.; Acayaba, R.D.; Jardim, W.F.; Jardim, I.C.S.F.; Umbuzeiro, G. Trace Analysis of Pesticides and An Assessment of their Occurrence in Surface and Drinking Waters from the State of São Paulo (Brazil). Anal. Methods 2014, 6, 6668–6677. [Google Scholar] [CrossRef]
- Yue, L.; Ge, C.J.; Feng, D.; Yu, G.; Deng, H.; Fu, B. Adsorption–Desorption Behavior of Atrazine on Agricultural Soils in China. J. Environ. Sci. 2017, 57, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Siampiringue, M.; Chahboune, R.; Wong-Wah-Chung, P.; Sarakha, M. Carbaryl Photochemical Degradation on Soil Model Surfaces. Soil Syst. 2019, 3, 17. [Google Scholar] [CrossRef]
- Demirbas, A. Spectrophotometric Determination of Carbaryl Pesticide and its Product in Soil and Strawberry Samples. Sci. Total Environ. 1998, 220, 235–241. [Google Scholar] [CrossRef]
- Martin, J.D.; Crawford, C.G.; Larson, S.J. Pesticides in Streams—Preliminary Results from Cycle I of the National Water Quality Assessment Program (NAWQA); United States Geological Survey: Reston, VA, USA, 2003; pp. 1992–2001.
- Walters, J.; Goh, K.S.; Li, L.; Feng, H.; Hernandez, J.; White, J.J. Environmental Monitoring of Carbaryl Applied in Urban Areas to Control the Glassy-Winged Sharpshooter in California. Environ. Monit. Assess. 2003, 82, 265–280. [Google Scholar] [CrossRef]
- Kinyunzu, J.M. Residues Concentrations of Carbaryl Pesticide in Soil and Tomatoes from Hippo, Kingfisher and Harnekop Green House Farms in Thika and Naivasha, Kenya. Ph.D. Thesis, Degree-Granting University, Nairobi, Kenya, 2015. [Google Scholar]
- Singh, R.P.; Singh, S.; Srivastava, G. Adsorption Thermodynamics of Carbaryl onto Four Texturally Different Indian Soils. Adsorp. Sci. Technol. 2011, 29, 277–288. [Google Scholar] [CrossRef]
- De Oliveira, M.F.; Johnston, C.T.; Premachandra, G.S.; Teppen, B.J.; Li, H.; Laird, D.A.; Zhu, D.; Boyd, S.A. Spectroscopic Study of Carbaryl Sorption on Smectite from Aqueous Suspension. Environ. Sci. Technol. 2005, 39, 9123–9129. [Google Scholar] [CrossRef]
- Otieno, P.; Lalah, O.; Virani, M.; Jondiko, I.; Werner Schramm, K. Soil and Water Contamination with Carbofuran Residues in Agricultural Farmlands in Kenya Following the Application of the Technical Formulation Furadan. J. Environ. Sci. Health. Part B 2010, 45, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Teerakun, M.; Reungsang, A.; Virojanakud, W. Phytoremediation of Carbofuran in Soil. Environ. Hazard. Manag. 2004, 26, 172–176. [Google Scholar]
- Lalah, J.O.; Kaigwara, P.N.; Getenga, Z.; Mghenyi, J.M.; Wandiga, S.O. The Major Environmental Factors that Influence Rapid Disappearance of Pesticides from Tropical Soils in Kenya. Toxicol. Environ. Chem. 2001, 81, 161–197. [Google Scholar] [CrossRef]
- Vyas, B.; Kumar Singh, A.; Singh Cameotra, S. Sorption Behaviour of Maneb in the Agriculture Soils and its Correlation with Soil Properties. Int. J. Eng. Sci. 2015, 1, 1–8. [Google Scholar]
- Grimalt, S.; Dehouck, P. Review of Analytical Methods for the Determination of Pesticideresidues in Grapes. J. Chromatogr. A 2016, 1433, 1–23. [Google Scholar] [CrossRef]
- Bhushan, C.; Bhardwaj, A.; Misra, S. State of Pesticide Regulations in India; Centre for Science and Environment: New Delhi, India, 2013. [Google Scholar]
- Gan, J.; Koskinen, W.C.; Becker, R.L.; Buhler, D.D. Effect of Concentration on Persistence of Alochlor in soil. J. Environ. Qual. 1995, 24, 1162–1169. [Google Scholar] [CrossRef]
- Gevao, B.; Semple, K.; Jones, K. Bound Pesticide Residues in Soils: A Review. Environ. Pollut. 2000, 108, 3–14. [Google Scholar] [CrossRef]
- Siebielec, G.; Smreczak, B.; Klimkowicz-Pawlas, A.; Kowalik, M.; Kaczyński, R.; Koza, P.; Ukalska-Jaruga, A.; Łysiak, M.; Wójtowicz, U.; Poręba, L.; et al. Report for the III Stage of the Monitoring of the Chemical Properties of Arable Soils in Poland in Years 2015–2017. Chief Inspectorate of Environmental Protection, IUNG-PIB, 2017. 2017. Available online: http://www.gios.gov.pl/images/dokumenty/pms/monitoring_jakosci_gleb/Raport_MChG_etap3.pdf (accessed on 28 January 2020).
Sample Availability: Samples of the OCP and NCP compounds are available from the authors. |
| Soil Properties | Min | Max. | Me | CoV | LQ | UQ |
|---|---|---|---|---|---|---|
| Clay (%) | 0.0 | 47.0 | 3.0 | 112 | 2.0 | 6.0 |
| Silt (%) | 2.0 | 83.0 | 28.0 | 62 | 19.0 | 56.0 |
| Sand (%) | 11 | 97 | 66 | 43 | 35 | 78 |
| pH in KCl | 3.1 | 7.4 | 5.0 | 21 | 4.2 | 5.9 |
| TOC (g kg−1) | 3.6 | 38.4 | 9.8 | 45 | 8.3 | 3.1 |
| TN (g kg−1) | 0.4 | 3.6 | 1.1 | 42 | 0.1 | 0.1 |
| TC/TN | 4.03 | 15.6 | 9.4 | 18 | 8.2 | 10.1 |
| Min | Max | Aver | Me | LQ | UQ | SD | CoV | Kurtosis | Skewness | No. and % of Samples with Detected Pesticide Compound | Acceptable Limits (JoL 2016 Item 1395) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organochlorine Pesticides (OCPs) | ||||||||||||
| pp’DDT | 0.12 | 202.68 | 18.23 | 8.54 | 2.47 | 22.50 | 26.91 | 148 | 16 | 4 | 216 (100%) | - |
| pp’DDE | <0.1 | 79.87 | 4.06 | 1.53 | 0.56 | 4.06 | 8.65 | 213 | 41 | 3 | 211 (98%) | - |
| pp’DDD | <0.1 | 267.52 | 23.60 | 9.58 | 4.25 | 25.33 | 39.56 | 168 | 18 | 6 | 211 (98%) | - |
| ∑ DDT | 0.61 | 484.64 | 44.60 | 24.73 | 8.78 | 53.47 | 64.35 | 144 | 17 | 4 | 216 (100%) | 120 |
| α-HCH | <0.1 | 192.64 | 96.96 | 96.96 | 49.12 | 144.80 | 135.32 | 140 | −3 | 1 | 4 (2%) | 25 |
| β-HCH | <0.1 | 1008.57 | 339.55 | 8.57 | 5.04 | 508.57 | 579.40 | 171 | −1 | 2 | 6 (3%) | 10 |
| γ-HCH | <0.1 | 7.27 | 3.04 | 1.53 | 1.17 | 4.79 | 2.78 | 92 | −1 | 1 | 6 (3%) | 10 |
| ∑ HCH | 0.98 | 1008.57 | 152.78 | 3.70 | 1.22 | 54.38 | 352.31 | 231 | 7 | 6 | 8 (4%) | - |
| ∑ OCPs | 4.03 | 1037.59 | 61.70 | 34.63 | 15.86 | 66.46 | 97.62 | 158 | 49 | 3 | 216 (100%) | - |
| Nonchlorinated Pesticides (NCPs) | ||||||||||||
| Atrazine | <0.01 | 15.85 | 0.63 | 0.38 | 0.28 | 0.56 | 1.27 | 201 | 122 | 10 | 173 (80%) | 50 |
| Carbaryl | <0.01 | 28.07 | 2.11 | 0.77 | 0.38 | 1.38 | 4.59 | 3 | 24 | 5 | 45 (20%) | 200 |
| Carbofuran | <0.01 | 0.54 | 0.45 | 0.46 | 0.40 | 0.51 | 0.07 | 17 | −3 | 0 | 4 (2%) | 200 |
| Maneb | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | 0 (0%) | 200 |
| ∑ NCPs | <0.01 | 43.92 | 1.17 | 0.46 | 0.30 | 1.02 | 3.58 | 308 | 118 | 10 | 176 (82%) | - |
| OCPs | NCPs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| pp’DDT | pp’DDE | pp’DDD | α-HCH | β-HCH | γ-HCH | Atrazine | Carbaryl | Carbofuran | Maneb | |
| Molecule structure | 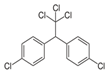 | 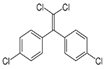 | 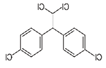 |  | 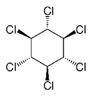 | 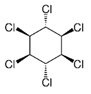 | 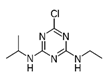 | 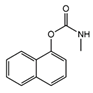 | 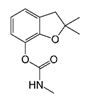 | 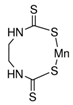 |
| Pesticide type | Insecticide | Insecticide | Insecticide | Insecticide | Insecticide | Insecticide | Herbicide | Insecticide | Insecticide | Fungicide |
| Molecular weight (g mol−1) | 354.49 | 318.02 | 320.04 | 290.82 | 290.82 | 290.82 | 215.68 | 201.22 | 221.26 | 265.30 |
| Water solublility (mg l−1) | 0.006 | 0.12 | 0.09 | 2.0 | 2.41 | 8.52 | 35 | 9.1 | 322 | 178 |
| log Ko/w | 6.91 | 6.51 | 6.02 | 3.82 | 3.57 | 3.50 | 2.7 | 2.36 | 1.8 | −0.45 |
| Vapor pressure (mPa) | 0.025 | - | 0.18 | 5.99 | 0.029 | 4.40 | 0.039 | 0.042 | 0.08 | 0.014 |
| Soil degradation, DT 50 (days) | 6200 | 5000 | 1000 | 175 | 10 | 980 | 75 | 16 | 29 | 7 |
| Bioconcentration factor (l kg−1) | 3173 | 1800 | - | 20 | 527 | 1300 | 4.3 | 44 | 12 | Low risk |
| Threshold of toxicological concern (Cramer class) | High (class III) | High (class III) | High (class III) | High (class III) | High (class III) | High (class III) | High (class III) | High (class III) | High (class III) | High (class III) |
| Parameter | GC-µECD | GC-MS/MS |
|---|---|---|
| Injection mode | Splitless | Hot-splitless; MMI injection mode |
| Injection volume | 2 μL | 2 μL |
| Inlet temperature | 225 °C | 280 °C |
| Carrier gas | He constant flow 2.00 mL/min | He, constant flow 1.00 mL min−1 (column 2 = 1.20 mL min−1) |
| Detector temperatutre | 325 °C | - |
| Makeup gas | N2, constant flow 40 mL min−1 | - |
| Oven program | 50 °C for 1 min, 30 °C/min to 180 °C, 180 °C, for 1 min, 3 °C/min to 205 °C, 205 °C for 4 min, 20 °C/min to 290 °C, 290 °C for 7 min | 70 °C for 2 min 25 °C/min to 150 °C for 0 min; 3 °C/min to 200 °C for 0 min; 8 °C/min to 280 °C for 10 min hold time |
| MS transfer line temperature | - | 280 °C |
| Backflush settings | - | 5 min during post-run/310 °C |
| Aux EPC pressure | - | ~50 psi |
| Inlet pressure | - | ~2 psi |
| Column pressure | - | ~3 psi |
| Electron energy | - | 70 eV |
| MS1 and MS2 resolution | - | Wide |
| Collision cell | - | 1.5 mL min−1 N2 and 2.25 mL min−1 He |
| Source temperature | - | 300 °C |
| Quad temperatures | - | 150 °C |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ukalska-Jaruga, A.; Smreczak, B.; Siebielec, G. Assessment of Pesticide Residue Content in Polish Agricultural Soils. Molecules 2020, 25, 587. https://doi.org/10.3390/molecules25030587
Ukalska-Jaruga A, Smreczak B, Siebielec G. Assessment of Pesticide Residue Content in Polish Agricultural Soils. Molecules. 2020; 25(3):587. https://doi.org/10.3390/molecules25030587
Chicago/Turabian StyleUkalska-Jaruga, Aleksandra, Bożena Smreczak, and Grzegorz Siebielec. 2020. "Assessment of Pesticide Residue Content in Polish Agricultural Soils" Molecules 25, no. 3: 587. https://doi.org/10.3390/molecules25030587
APA StyleUkalska-Jaruga, A., Smreczak, B., & Siebielec, G. (2020). Assessment of Pesticide Residue Content in Polish Agricultural Soils. Molecules, 25(3), 587. https://doi.org/10.3390/molecules25030587