The Effect of Protein-Rich Extract from Bombyx Batryticatus against Glutamate-Damaged PC12 Cells Via Regulating γ-Aminobutyric Acid Signaling Pathway
Abstract
1. Introduction
2. Results
2.1. Optimization of Extraction Conditions
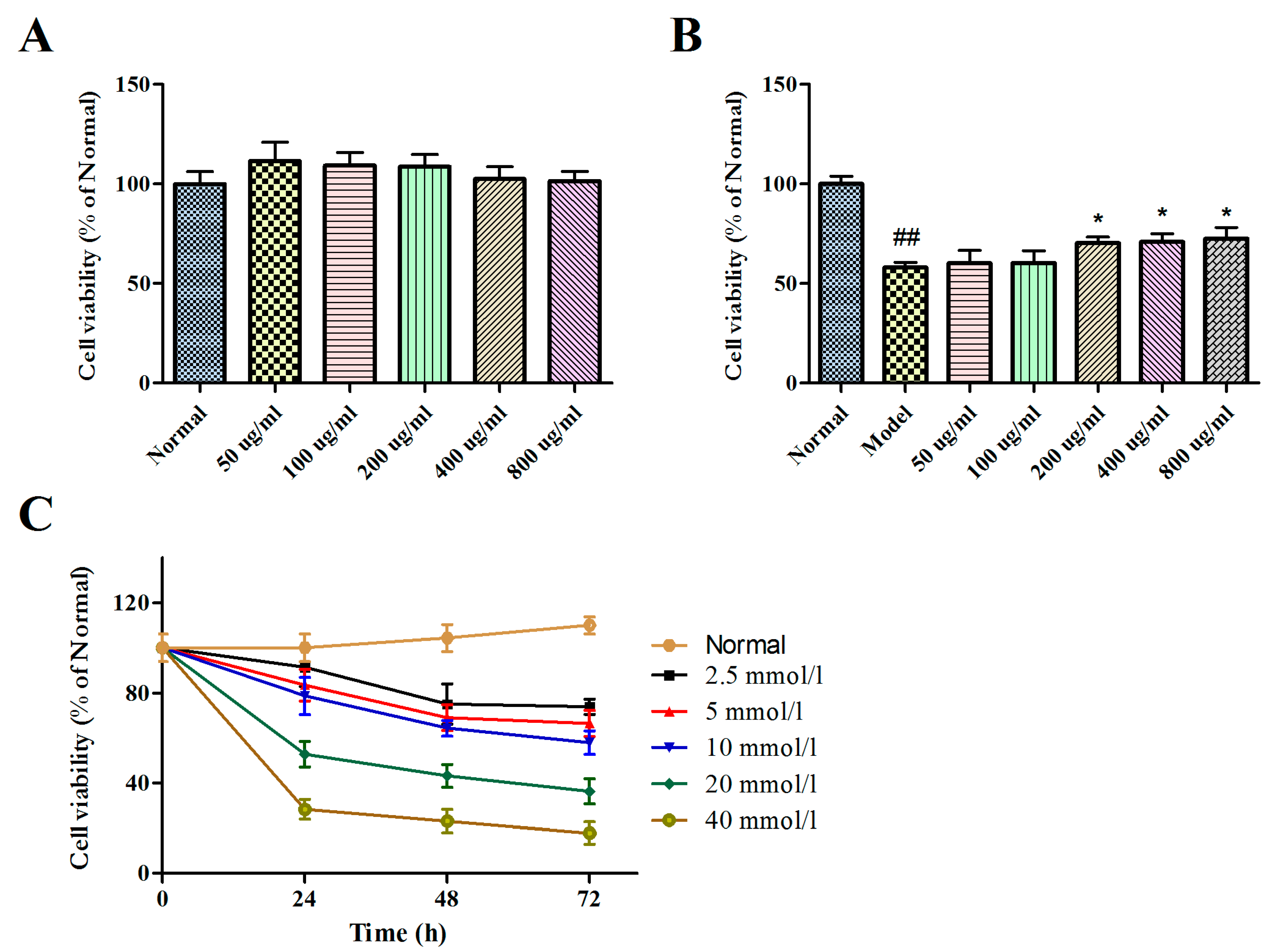
2.2. Effects of BBPs and Glu on Cell Viability of PC12 Cells
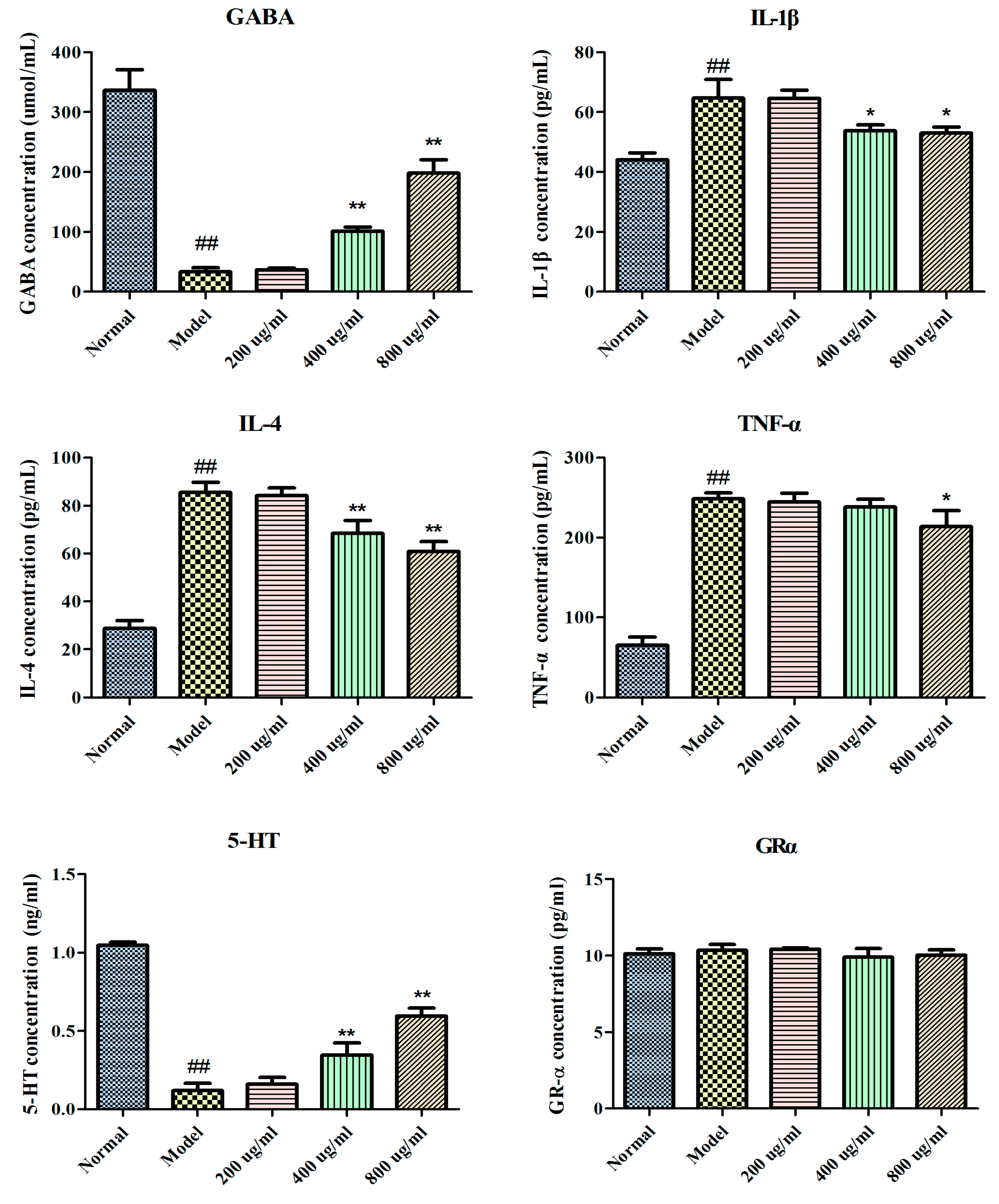
2.3. Effects of BBPs on GABA, IL-1β, IL-4, 5-HT, TNF-α and GRα in NGF-Induced PC12 Cells Injured by Glu
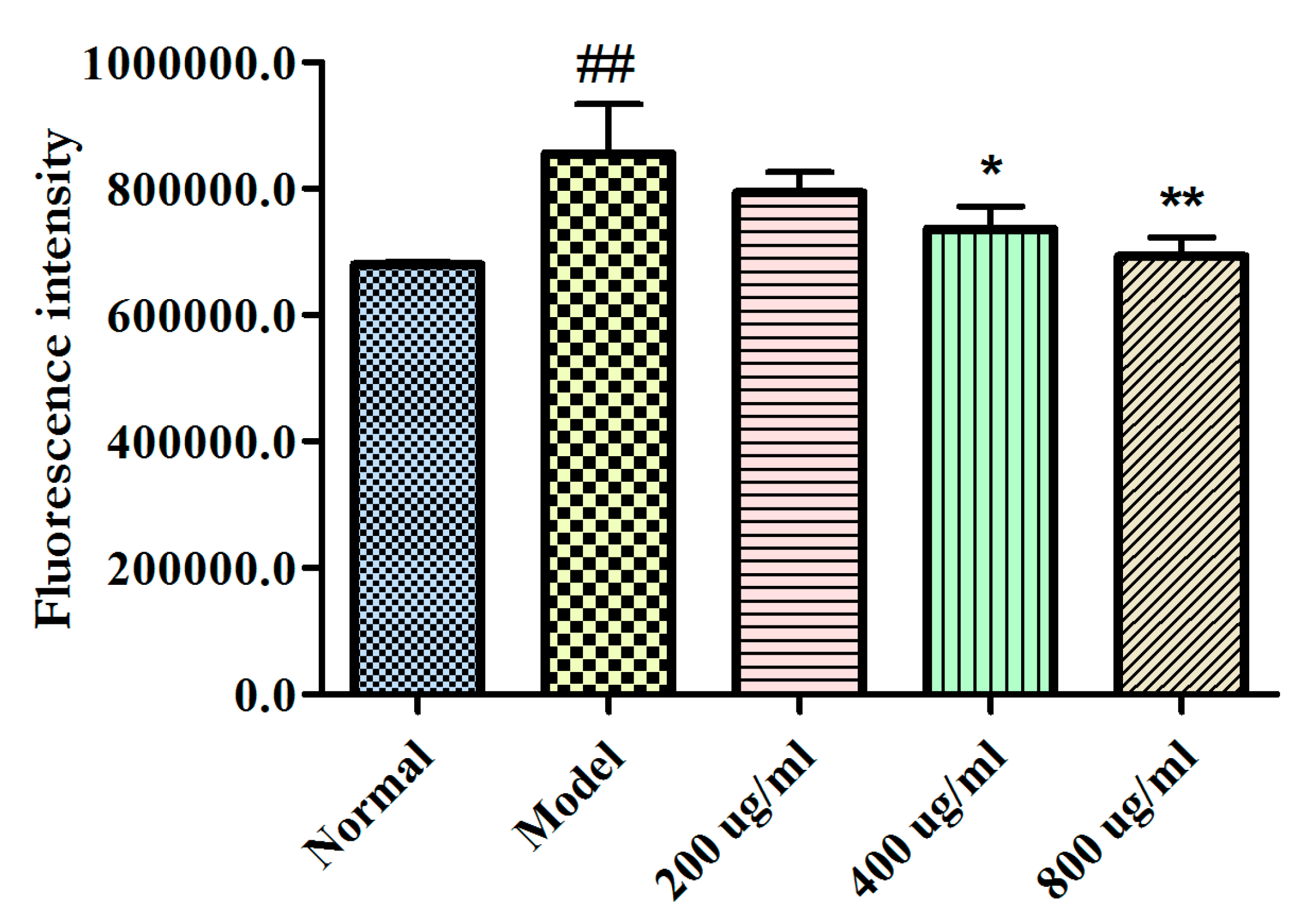
2.4. Effects of BBPs on Ca2+ Levels of NGF-Induced PC12 Cells Injured by Glu
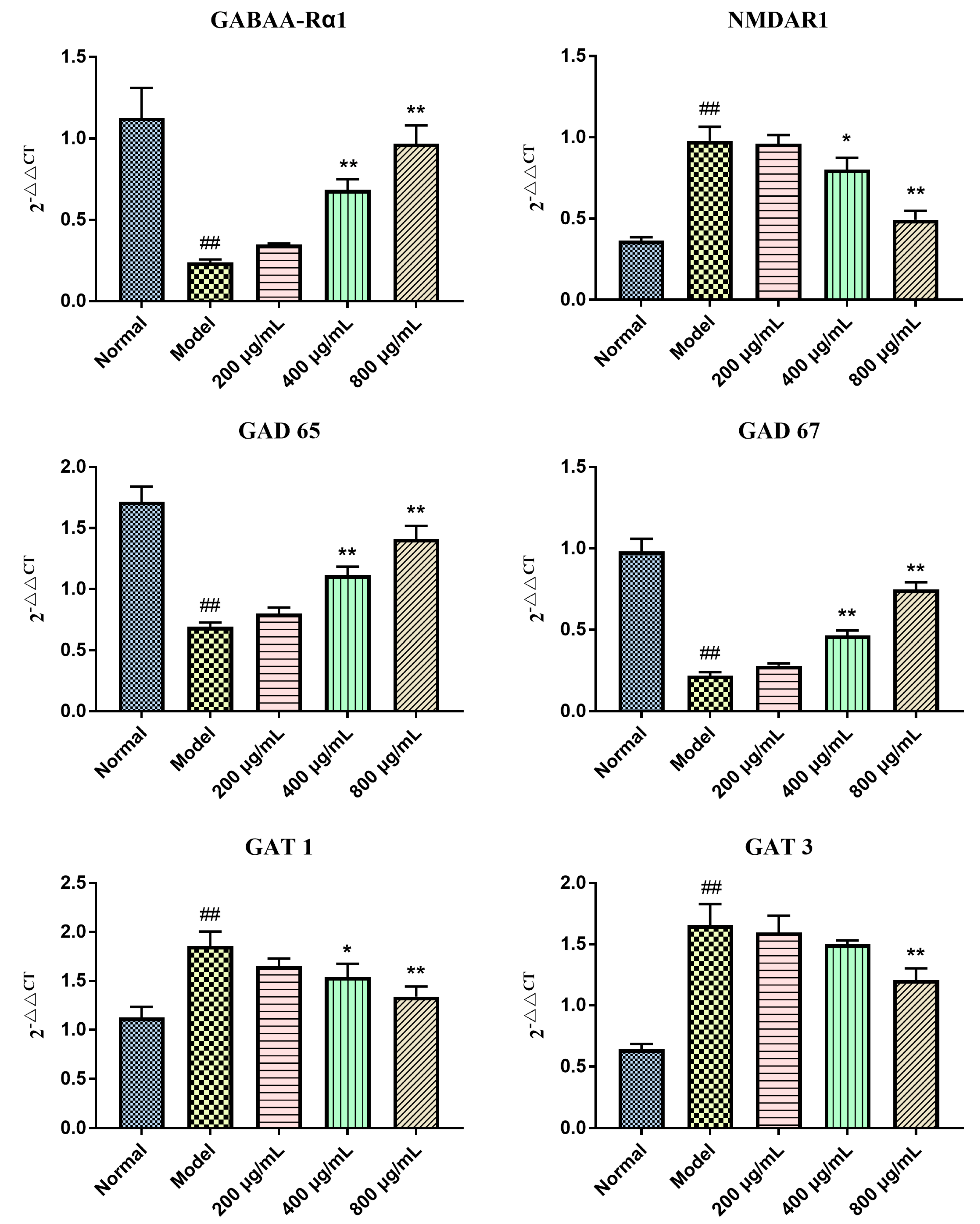
2.5. Effects of BBPs on mRNA Expressions of GABAA-Rα1, NMDAR1, GAD 65, GAT 1, GAT 3 and GAD 67 in NGF-Induced PC12 Cells Injured by Glu
2.6. Effects of BBPs on Protein Expressions of GABAA-Rα1, NMDAR1, GAD 65, GAT 1, GAT 3 and GAD 67 in NGF-Induced PC12 Cells Injured by Glu
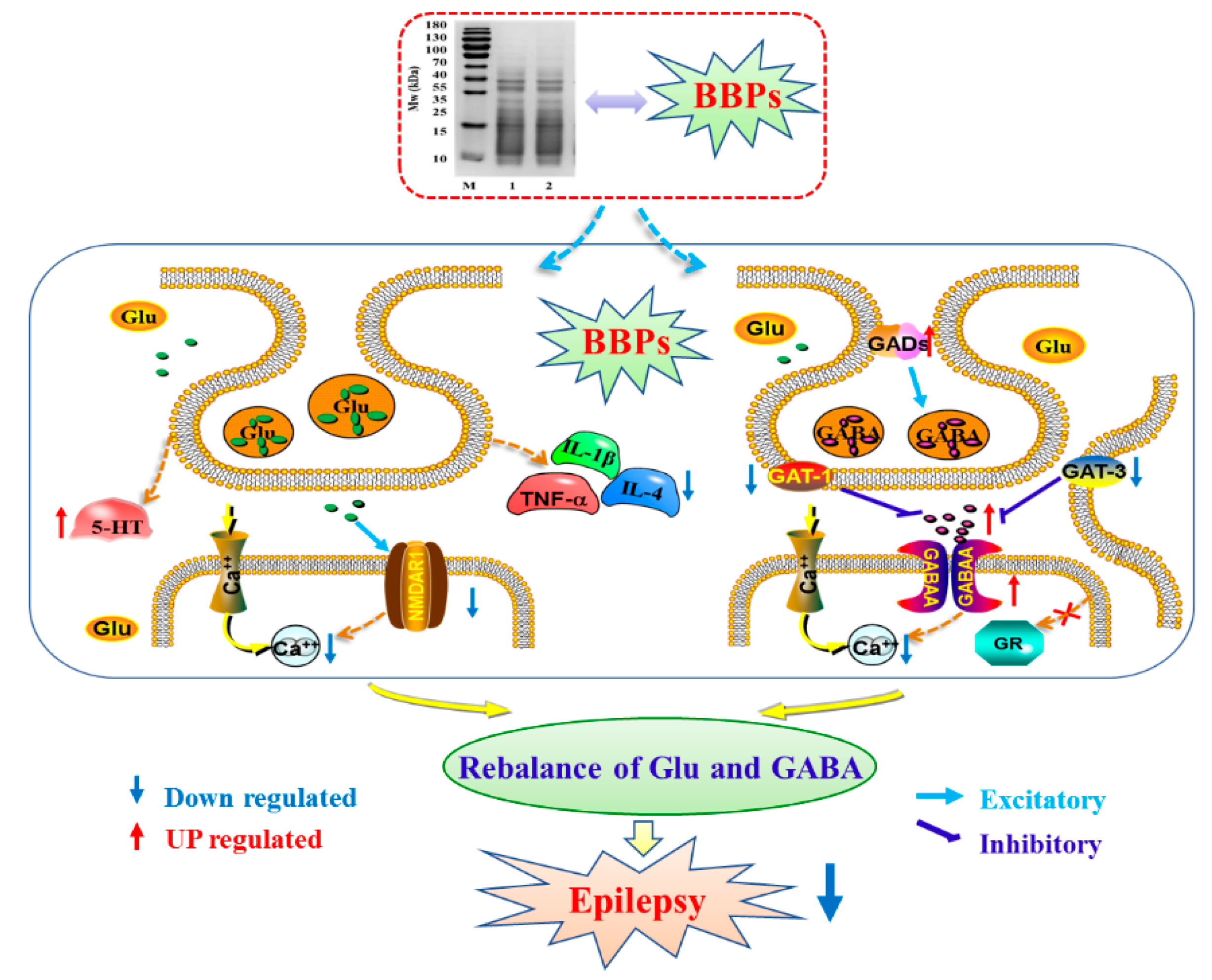
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Extraction of BBPs by Ultrasonic-Assisted Extraction (UAE)
4.3. Cell Culture
4.4. Cell Viability Assay
4.5. Determination of GABA, IL-1β, IL-4, TNF-α and GRα Content in PC12 Cells
4.6. Measurement of Cytosolic Free Calcium (Ca2+) Release
4.7. Quantitative Real-Time Polymerase Chain Reaction (RT-PCR) Assay
4.8. Western Blot Assay
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gooneratne, I.K.; Green, A.L.; Dugan, P.; Sen, A.; Franzini, A.; Aziz, T.; Cheeran, B. Comparing neurostimulation technologies in refractory focal-onset epilepsy. J. Neurol. Neurosurg. Psychiatry 2016, 87, 1174–1182. [Google Scholar] [CrossRef]
- Weber, Y.G.; Biskup, S.; Helbig, K.L.; Von Spiczak, S.; Lerche, H. The role of genetic testing in epilepsy diagnosis and management. Expert Rev. Mol. Diagn. 2017, 17, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Mula, M. Investigational new drugs for focal epilepsy. Expert Opin. Investig. Drugs 2015, 25, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Manford, M. Recent advances in epilepsy. J. Neurol. 2017, 264, 1811–1824. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yongyan, W.U.; Song, D.; Yan, Z.; Liu, T. Compounds isolated and purified from chloroform active part of bombyx batryticatus and their anticonvulsive activities. Chin. J. Pharm. 2014, 45, 431–433. [Google Scholar]
- Karpova, M.N.; Kuznetzova, L.V.; Klishina, N.Y. Gaba and its Receptors in Pathogenesis of Epilepsy. Uspekhi fiziologicheskikh nauk 2015, 46, 46–59. [Google Scholar]
- Qiu, W.J.; Hu, X.W. Research progress on pathogenesis and treatment of epilepsy. Chin. J. Clin. Phys. 2014, 8, 101–105. [Google Scholar]
- Obata, K. Synaptic inhibition and γ-aminobutyric acid in the mammalian central nervous system. Proc. Jpn. Acad. 2013, 89, 139–156. [Google Scholar] [CrossRef]
- Pinal, C.S.; Tobin, A.J. Uniqueness and redundancy in GABA production. Perspect. Dev. Neurobiol. 1998, 5, 109–118. [Google Scholar]
- Soghomonian, J.-J.; Martin, D.L. Two isoforms of glutamate decarboxylase: Why? Trends Pharmacol. Sci. 1998, 19, 500–505. [Google Scholar] [CrossRef]
- Stork, O.; Ji, F.-Y.; Kaneko, K.; Stork, S.; Yoshinobu, Y.; Moriya, T.; Shibata, S.; Obata, K. Postnatal development of a GABA deficit and disturbance of neural functions in mice lacking GAD65. Brain Res. 2000, 865, 45–58. [Google Scholar] [CrossRef]
- Dalby, N.O. Inhibition of γ-aminobutyric acid uptake: Anatomy, physiology and effects against epileptic seizures. Eur. J. Pharmacol. 2003, 479, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Jing, G.; Zhu, M.D. Advances in the pathogenesis of epilepsy. J. Shenyang Med. Coll. 2012, 14, 111–113. [Google Scholar]
- Ravizza, T.; Vezzani, A. Status epilepticus induces time-dependent neuronal and astrocytic expression of interleukin-1 receptor type I in the rat limbic system. Neuroscience 2006, 137, 301–308. [Google Scholar] [CrossRef]
- Dey, A.; Kang, X.; Qiu, J.; Du, Y.; Jiang, J. Anti-Inflammatory Small Molecules to Treat Seizures and Epilepsy: From Bench to Bedside. Trends Pharmacol. Sci. 2016, 37, 463–484. [Google Scholar] [CrossRef]
- Sonar, S.; Lal, G. Role of Tumor Necrosis Factor Superfamily in Neuroinflammation and Autoimmunity. Front. Immunol. 2015, 6, 364. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Liu, N.; Zheng, P.; Ma, L.; Guo, F.; Sun, T.; Zhou, R.; Yu, J. The Anticonvulsant Effects of Baldrinal on Pilocarpine-Induced convulsion in Adult Male Mice. Molecules 2019, 24, 1617. [Google Scholar] [CrossRef]
- Shandra, A.A.; Godlevsky, L.S.; Vastyanov, R.S.; Oleinik, A.A.; Konovalenko, V.L.; Rapoport, E.N.; Korobka, N.N. The role of TNF-alpha in amygdala kindled rats. Neurosci. Res. 2002, 42, 147–153. [Google Scholar] [CrossRef]
- Li, T.; Zhai, X.; Jiang, J.; Song, X.; Han, W.; Ma, J.; Xie, L.; Cheng, L.; Chen, H.; Jiang, L. Intraperitoneal injection of IL-4/IFN-γ modulates the proportions of microglial phenotypes and improves epilepsy outcomes in a pilocarpine model of acquired epilepsy. Brain Res. 2017, 1657, 120–129. [Google Scholar] [CrossRef]
- Pijnenburg-Kleizen, K.J.; Engels, M.; Mooij, C.F.; Griffin, A.; Krone, N.; Span, P.N.; Van Herwaarden, A.E.; Sweep, F.C.G.J.; Der Grinten, H.L.C.-V. Adrenal Steroid Metabolites Accumulating in Congenital Adrenal Hyperplasia Lead to Transactivation of the Glucocorticoid Receptor. Endocrinology 2015, 156, 3504–3510. [Google Scholar] [CrossRef]
- Yu, C.-W.; Chang, P.-T.; Hsin, L.-W.; Chern, J.-W. Quinazolin-4-one Derivatives as Selective Histone Deacetylase-6 Inhibitors for the Treatment of Alzheimer’s Disease. J. Med. Chem. 2013, 56, 6775–6791. [Google Scholar] [CrossRef] [PubMed]
- Nishina, A.; Kimura, H.; Tsukagoshi, H.; Kozawa, K.; Koketsu, M.; Ninomiya, M.; Sato, D.; Obara, Y.; Furukawa, S. Neurite outgrowth of PC12 cells by 4’-O-β-D-glucopyranosyl-3’,4-dimethoxychalcone from Brassica rapa L. ‘hidabeni’ was enhanced by pretreatment with p38MAPK inhibitor. Neurochem. Res. 2013, 38, 2397–2407. [Google Scholar] [CrossRef] [PubMed]
- Terada, K.; Matsushima, Y.; Matsunaga, K.; Takata, J.; Karube, Y.; Ishige, A. The Kampo medicine Yokukansan (YKS) enhances nerve growth factor (NGF)-induced neurite outgrowth in PC12 cells. Bosn. J. Basic Med. Sci. 2018, 18, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Yan, N.; Huang, Y.; Cao, G.; Deng, J.; Deng, Y. Effects of nerve growth factor on nerve regeneration after corneal nerve damage. Int. J. Clin. Exp. Med. 2014, 7, 4584–4589. [Google Scholar]
- Zeng, X.; Hu, K.; Chen, L.; Zhou, L.; Luo, W.; Li, C.; Zong, W.; Chen, S.; Gao, Q.; Zeng, G.; et al. The effects of ginsenoside compound k against epilepsy by enhancing the gamma-aminobutyric acid signaling pathway. Front. Pharmacol. 2018, 9, 1020. [Google Scholar] [CrossRef]
- Kim, M.H.; Lee, H.J.; Lee, S.-R.; Lee, H.-S.; Huh, J.-W.; Bae, Y.C.; Lee, D.-S. Peroxiredoxin 5 Inhibits Glutamate-Induced Neuronal Cell Death through the Regulation of Calcineurin-Dependent Mitochondrial Dynamics in HT22 Cells. Mol. Cell. Biol. 2019, 39. [Google Scholar] [CrossRef]
- Soukupová, M.; Binaschi, A.; Falcicchia, C.; Palma, E.; Roncon, P.; Zucchini, S.; Simonato, M. Increased extracellular levels of glutamate in the hippocampus of chronically epileptic rats. Neuroscience 2015, 301, 246–253. [Google Scholar] [CrossRef]
- Albrecht, J.; Zielińska, M. Mechanisms of Excessive Extracellular Glutamate Accumulation in Temporal Lobe Epilepsy. Neurochem. Res. 2017, 42, 1724–1734. [Google Scholar] [CrossRef]
- Yu, S.P.; Strasser, U.; Tian, M.; Choi, D.W.J.S. Nmda receptor-mediated k+ efflux and neuronal apoptosis. Science 1999, 284, 336–339. [Google Scholar] [CrossRef]
- Zhang, J.; An, S.; Hu, W.; Teng, M.; Wang, X.; Qu, Y.; Liu, Y.; Yuan, Y.; Wang, D. The Neuroprotective Properties of Hericium erinaceus in Glutamate-Damaged Differentiated PC12 Cells and an Alzheimer’s Disease Mouse Model. Int. J. Mol. Sci. 2016, 17, 1810. [Google Scholar] [CrossRef]
- Huang, H.; Peng, X.; Peng, Y. Modern research progress of bombyx mori. J. Hunan. Univ. Tradit. Chin. Med. 2003, 23, 62–64. [Google Scholar]
- Wu, J.-Y.; Sheikho, A.; Ma, H.; Li, T.-C.; Zhao, Y.-Q.; Zhang, Y.-L.; Wang, D. Molecular mechanisms of Bombyx batryticatus ethanol extract inducing gastric cancer SGC-7901 cells apoptosis. Cytotechnology 2017, 69, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Jia, T.Z.; Cao, Q.C.; Tian, F.; Ying, W.T. A Crude 1-DNJ Extract from Home Made Bombyx Batryticatus Inhibits Diabetic Cardiomyopathy-Associated Fibrosis in db/db Mice and Reduces Protein N-Glycosylation Levels. Int. J. Mol. Sci. 2018, 19, 1699. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Wang, G.J.; Wang, J.; Chu, Y.L. Advances in studies on constituents and pharmacological actions of bombyx mori. China. Seri. Cul. 2004, 4, 87–89. [Google Scholar]
- Hu, M.; Yu, Z.; Wang, J.; Fan, W.; Liu, Y.; Li, J.; Xiao, H.; Li, Y.; Peng, W.; Wu, C. Traditional Uses, Origins, Chemistry and Pharmacology of Bombyx batryticatus: A Review. Molecules 2017, 22, 1779. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Liu, Y.; He, L.; Yuan, X.; Peng, W.; Wu, C. Antiepileptic Effects of Protein-Rich Extract from Bombyx batryticatus on Mice and Its Protective Effects against H2O2-Induced Oxidative Damage in PC12 Cells via Regulating PI3K/Akt Signaling Pathways. Oxid. Med. Cell. Longev. 2019, 2019, 7897584. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.-S.; An, H.-G.; Moon, S.-K.; Lee, Y.-C.; Kim, H.-M.; Ko, J.-H.; Kim, C.-H. Bombycis corpus extract (BCE) protects hippocampal neurons against excitatory amino acid-induced neurotoxicity. Immunopharmacol. Immunotoxicol. 2003, 25, 191–201. [Google Scholar] [CrossRef]
- Bai, Y.; Zhao, Q.; He, M.; Ye, X.; Zhang, X. Extensive characterization and differential analysis of endogenous peptides from Bombyx batryticatus using mass spectrometric approach. J. Pharm. Biomed. Anal. 2019, 163, 78–87. [Google Scholar] [CrossRef]
- Cheng, S.-M.; Huang, J.; Wang, H.-Y.; Li, G.-Y.; Lin, R.-C.; Wang, J.-H. Two new compounds from Bombyx batryticatus. J. Asian Nat. Prod. Res. 2014, 16, 825–829. [Google Scholar] [CrossRef]
- Shindyapina, A.V.; Komarova, T.V.; Sheshukova, E.V.; Ershova, N.M.; Tashlitsky, V.N.; Kurkin, A.V.; Yusupov, I.R.; Mkrtchyan, G.V.; Shagidulin, M.Y.; Dorokhov, Y.L. The Antioxidant Cofactor Alpha-Lipoic Acid May Control Endogenous Formaldehyde Metabolism in Mammals. Front. Neurosci. 2017, 11, 651. [Google Scholar] [CrossRef]
- Caruso, G.; Fresta, C.G.; Martinez-Becerra, F.; Antonio, L.; Johnson, R.T.; De Campos, R.P.S.; Siegel, J.M.; Wijesinghe, M.B.; Lazzarino, G.; Lunte, S.M. Carnosine modulates nitric oxide in stimulated murine RAW 264.7 macrophages. Mol. Cell. Biochem. 2017, 431, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Magarkar, A.; Jurkiewicz, P.; Allolio, C.; Hof, M.; Jungwirth, P. Increased Binding of Calcium Ions at Positively Curved Phospholipid Membranes. J. Phys. Chem. Lett. 2017, 8, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Krisanova, N.; Pozdnyakova, N.; Pastukhov, A.; Dudarenko, M.; Maksymchuk, O.; Parkhomets, P.; Sivko, R.; Borisova, T. Vitamin D3 deficiency in puberty rats causes presynaptic malfunctioning through alterations in exocytotic release and uptake of glutamate/GABA and expression of EAAC-1/GAT-3 transporters. Food Chem. Toxicol. 2019, 123, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Ericsson, C.; Nistér, M. Protein extraction from solid tissue. Methods. Mol. Biol. 2011, 675, 307–312. [Google Scholar]
- Chen, Y.; Li, C.; Zhu, J.; Xie, W.; Hu, X.; Song, L.; Zi, J.; Yu, R. Purification and characterization of an antibacterial and anti-inflammatory polypeptide from Arca subcrenata. Int. J. Biol. Macromol. 2017, 96, 177–184. [Google Scholar] [CrossRef]
- Lin, K.H.; Li, C.Y.; Hsu, Y.M.; Tsai, F.J.; Tang, C.H.; Yang, J.-S.; Wang, Z.H.; Yin, M.C. Oridonin, A natural diterpenoid, protected NGF-differentiated PC12 cells against MPP+- and kainic acid-induced injury. Food Chem. Toxicol. 2019, 133, 110765. [Google Scholar] [CrossRef]
- Overbeeke, R.; Yildirim, M.; Reutelingsperger, C.P.; Haanen, C.; Vermes, I. Sequential occurrence of mitochondrial and plasma membrane alterations, fluctuations in cellular Ca2+ and pH during initial and later phases of cell death. Apoptosis 1999, 4, 455–460. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Source | Sum of Squares | df | Mean Square | F Value | p-Value (Prob > F) |
|---|---|---|---|---|---|
| Model | 2.59 | 9 | 0.29 | 151.46 | <0.0001 |
| A-A | 0.039 | 1 | 0.039 | 20.59 | 0.0027 |
| B-B | 0.070 | 1 | 0.070 | 36.94 | 0.0005 |
| C-C | 0.58 | 1 | 0.58 | 303.54 | <0.0001 |
| AB | 0.14 | 1 | 0.14 | 75.86 | <0.0001 |
| AC | 4 × 10−4 | 1 | 4 × 10−4 | 0.21 | 0.6606 |
| BC | 4.225 × 10−3 | 1 | 4.225 × 10−3 | 2.22 | 0.1799 |
| A2 | 0.64 | 1 | 0.64 | 334.28 | <0.0001 |
| B2 | 0.35 | 1 | 0.35 | 181.24 | <0.0001 |
| C2 | 0.60 | 1 | 0.60 | 313.13 | <0.0001 |
| Residual | 0.013 | 7 | 1.904 × 10−3 | ||
| Lack of Fit | 6.325 × 10−3 | 3 | 2.108 × 10−3 | 1.20 | 0.4153 |
| Pure Error | 7 × 10−3 | 4 | 1.750 × 10−3 | -- | -- |
| Cor Total | 2.61 | 16 | -- | -- | |
| Standard deviation | 0.044 | R2 | 0.9949 | -- | -- |
| C.V.% | 2.19 | Adj R2 | 0.9883 | -- | -- |
| Adeq Precision | 32.686 | Pred R2 | 0.9570 | -- | -- |
| Run | Extracting Time (A) (h) | Material Liquid Than (B) (mL/g) | Ultrasonic Power (C) (W) | Extraction Yield (%) |
|---|---|---|---|---|
| 1 | 0.75 | 3.00 | 220 | 1.64 |
| 2 | 1.25 | 3.00 | 220 | 2.18 |
| 3 | 0.75 | 5.00 | 220 | 1.83 |
| 4 | 1.25 | 5.00 | 220 | 1.61 |
| 5 | 0.75 | 4.00 | 180 | 1.38 |
| 6 | 1.25 | 4.00 | 180 | 1.48 |
| 7 | 0.75 | 4.00 | 260 | 1.95 |
| 8 | 1.25 | 4.00 | 260 | 2.09 |
| 9 | 1.00 | 3.00 | 180 | 1.71 |
| 10 | 1.00 | 5.00 | 180 | 1.46 |
| 11 | 1.00 | 3.00 | 260 | 2.13 |
| 12 | 1.00 | 5.00 | 260 | 2.01 |
| 13 | 1.00 | 4.00 | 220 | 2.48 |
| 14 | 1.00 | 4.00 | 220 | 2.44 |
| 15 | 1.00 | 4.00 | 220 | 2.51 |
| 16 | 1.00 | 4.00 | 220 | 2.47 |
| 17 | 1.00 | 4.00 | 220 | 2.55 |
| Gene Name | Forward Primer | Reverse Primer | Product Length |
|---|---|---|---|
| β-actin | GAAGATCAAGATCATTGCTCC | TACTCCTGCTTGCTGATCCA | 111 bp |
| GABAA-Rα1 | CGCTCAGTGGTTGTGGCAGAAGATGG | GTCACGGTCAGAACGGTCGTCACTCC | 272 bp |
| GAD 67 | GTGCTGCTCCAGTGTTCTGCCATCC | AATCCCACAGTGCCCTTTGCTTTCCA | 203 bp |
| GAD 65 | CAAGTGGAAGCTGAACGGTGTGGAGA | TCTGACCAGGAGAGCCGAACATTGC | 100 bp |
| NMDAR1 | GGCACACAGGAGCGGGTAAACAACAG | AAGCGGTCCAGCAGGTACAGCATCA | 296 bp |
| GAT 1 | TTGGCTGGCGGGCGTGTTTCTCTTCA | TGCGGCTGCTCAGGACCATTCTCA | 246 bp |
| GAT 3 | GGTGCTGGCTCATGGCTCTGTCCT | AAGTGCGTCTCCTTCTCTGTGATGGC | 238 bp |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, L.-Y.; Hu, M.-B.; Li, R.-L.; Zhao, R.; Fan, L.-H.; Wang, L.; Peng, W.; Liu, Y.-J.; Wu, C.-J. The Effect of Protein-Rich Extract from Bombyx Batryticatus against Glutamate-Damaged PC12 Cells Via Regulating γ-Aminobutyric Acid Signaling Pathway. Molecules 2020, 25, 553. https://doi.org/10.3390/molecules25030553
He L-Y, Hu M-B, Li R-L, Zhao R, Fan L-H, Wang L, Peng W, Liu Y-J, Wu C-J. The Effect of Protein-Rich Extract from Bombyx Batryticatus against Glutamate-Damaged PC12 Cells Via Regulating γ-Aminobutyric Acid Signaling Pathway. Molecules. 2020; 25(3):553. https://doi.org/10.3390/molecules25030553
Chicago/Turabian StyleHe, Li-Ying, Mei-Bian Hu, Ruo-Lan Li, Rong Zhao, Lin-Hong Fan, Li Wang, Wei Peng, Yu-Jie Liu, and Chun-Jie Wu. 2020. "The Effect of Protein-Rich Extract from Bombyx Batryticatus against Glutamate-Damaged PC12 Cells Via Regulating γ-Aminobutyric Acid Signaling Pathway" Molecules 25, no. 3: 553. https://doi.org/10.3390/molecules25030553
APA StyleHe, L.-Y., Hu, M.-B., Li, R.-L., Zhao, R., Fan, L.-H., Wang, L., Peng, W., Liu, Y.-J., & Wu, C.-J. (2020). The Effect of Protein-Rich Extract from Bombyx Batryticatus against Glutamate-Damaged PC12 Cells Via Regulating γ-Aminobutyric Acid Signaling Pathway. Molecules, 25(3), 553. https://doi.org/10.3390/molecules25030553





