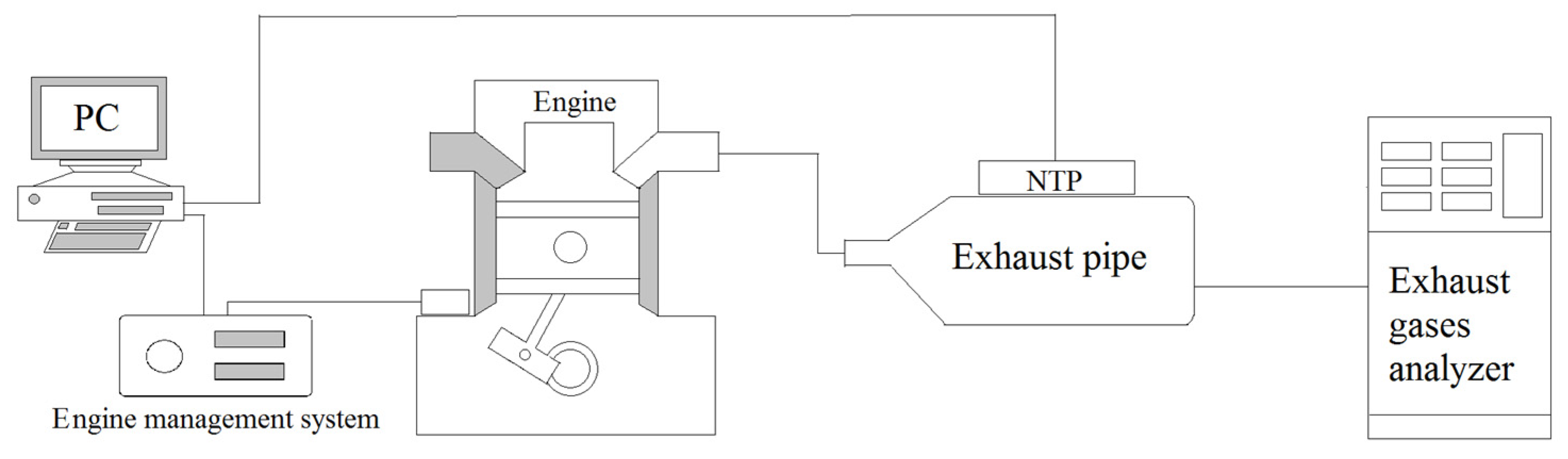
Figure 1.
Schematic of this experiment and setup location.
Figure 1.
Schematic of this experiment and setup location.
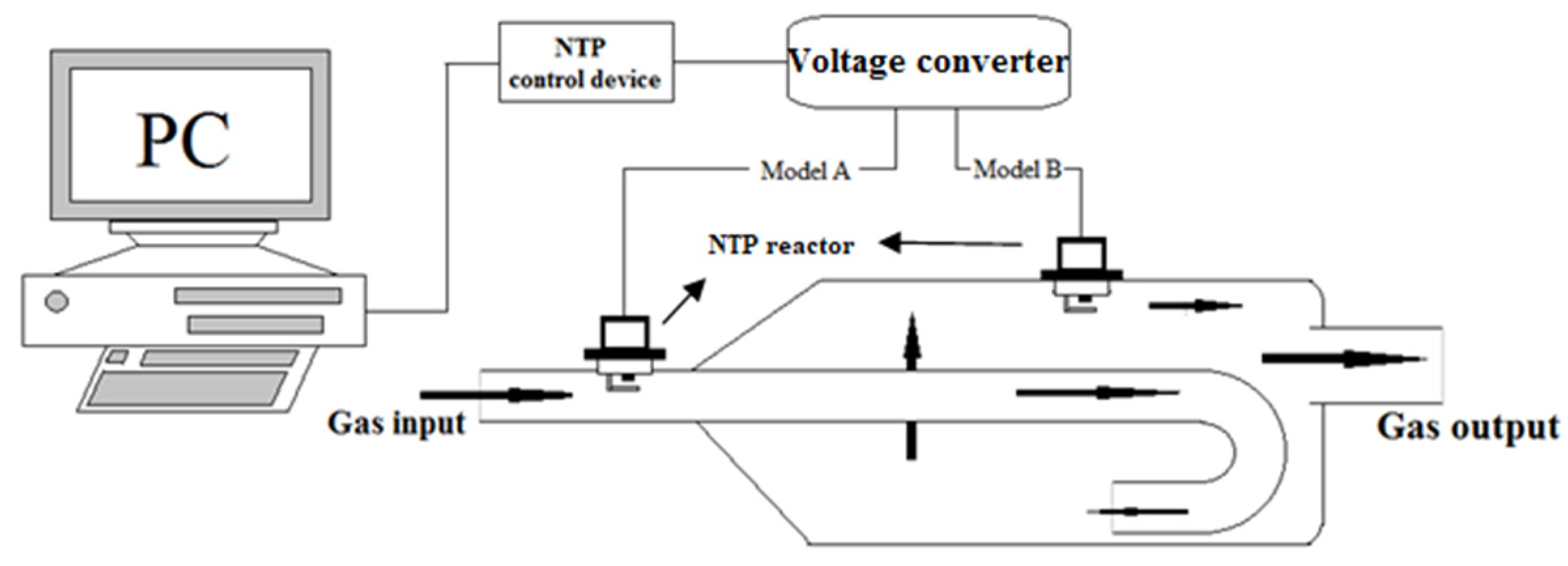
Figure 2.
The setup of non-thermal plasma (NTP) system.
Figure 2.
The setup of non-thermal plasma (NTP) system.
Figure 3.
Photographic view of the device set up on (a) model A and (b) model B of the experimental motorcycle.
Figure 3.
Photographic view of the device set up on (a) model A and (b) model B of the experimental motorcycle.
Figure 4.
The procedure of experimental.
Figure 4.
The procedure of experimental.
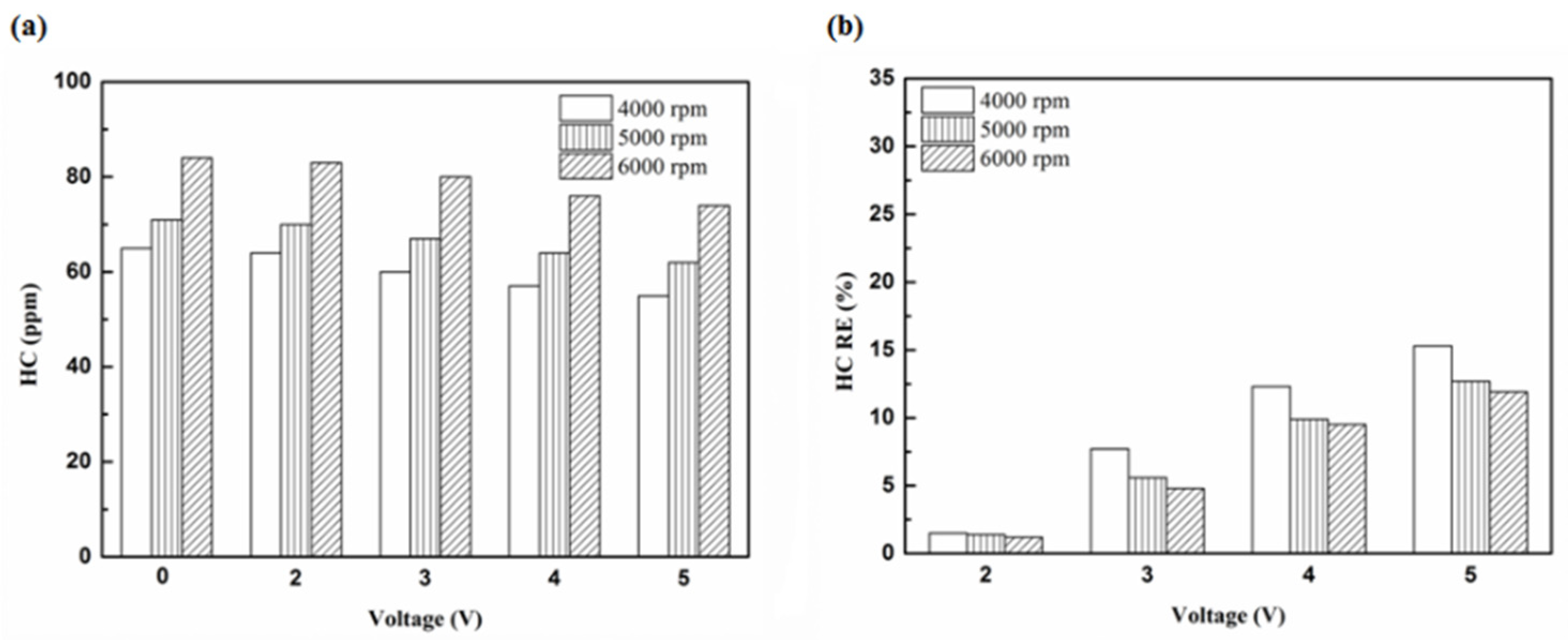
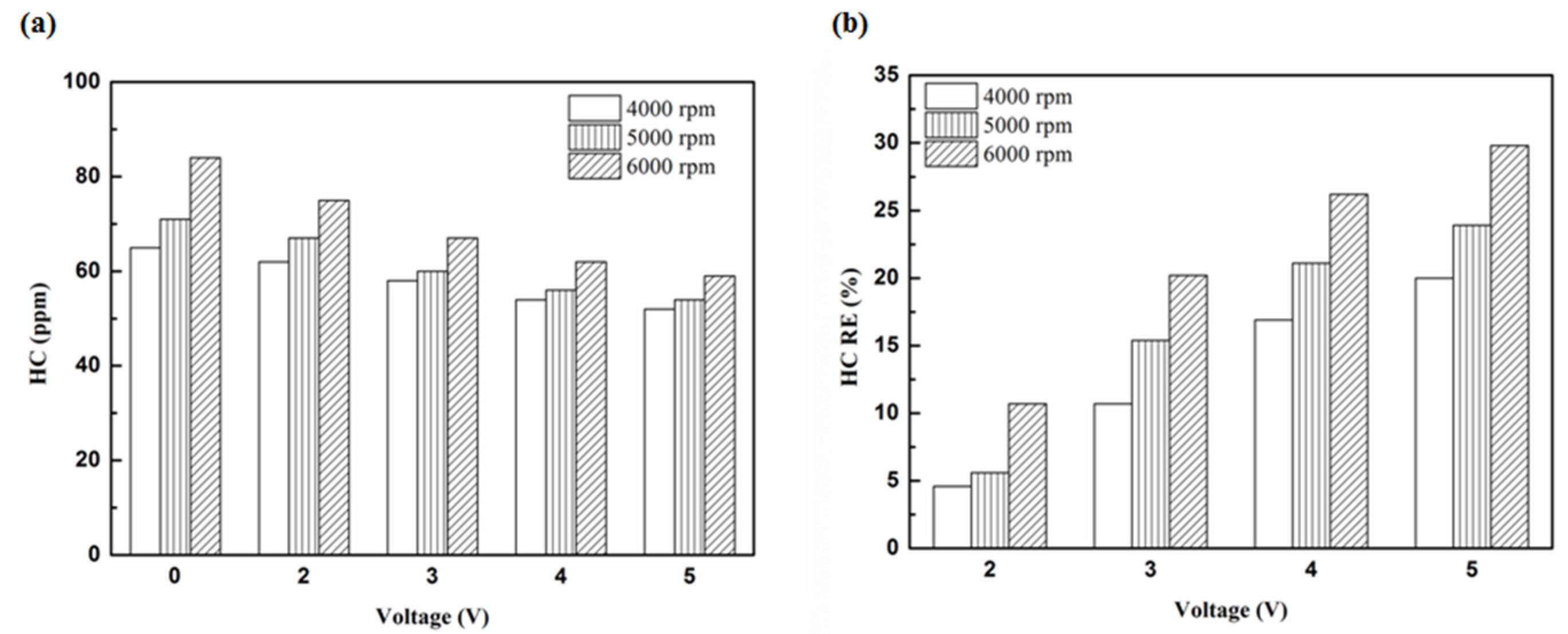
Figure 5.
The HC (a) emission values and (b) removal efficiency (RE) using platinum in the front of the exhaust pipe.
Figure 5.
The HC (a) emission values and (b) removal efficiency (RE) using platinum in the front of the exhaust pipe.
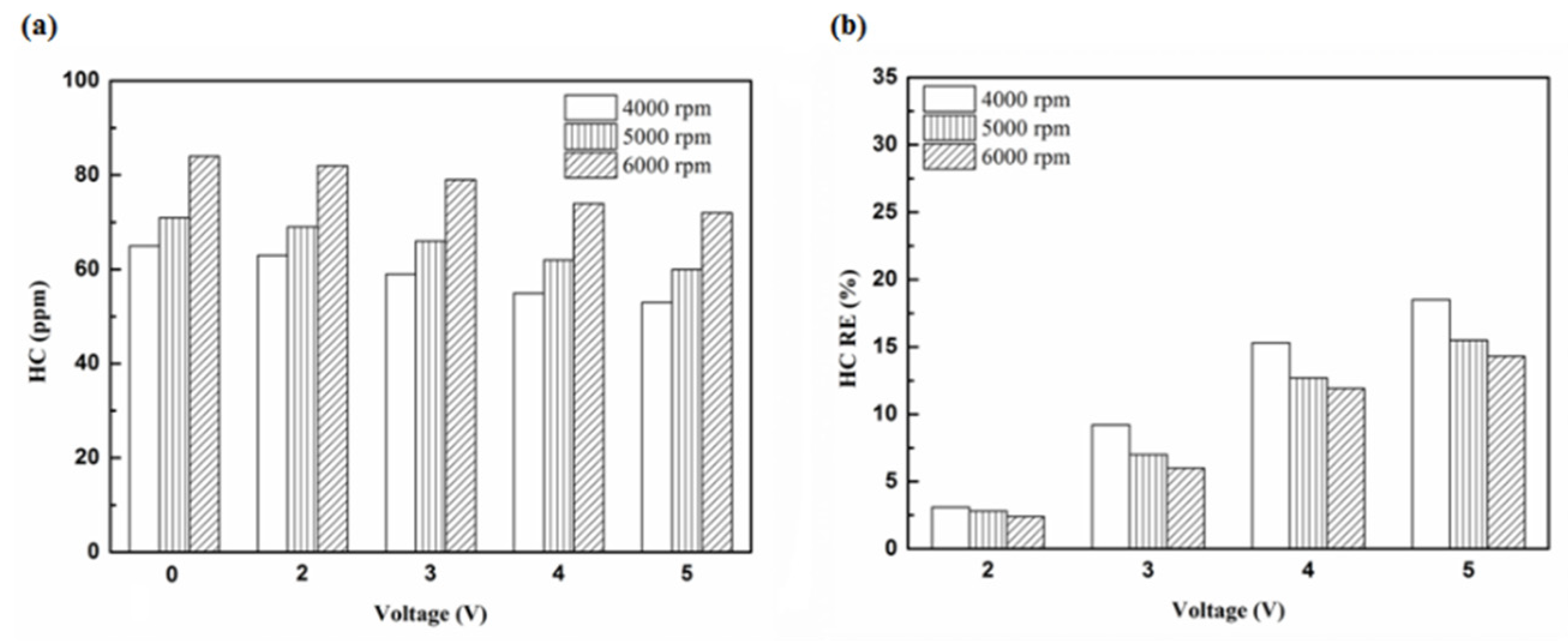
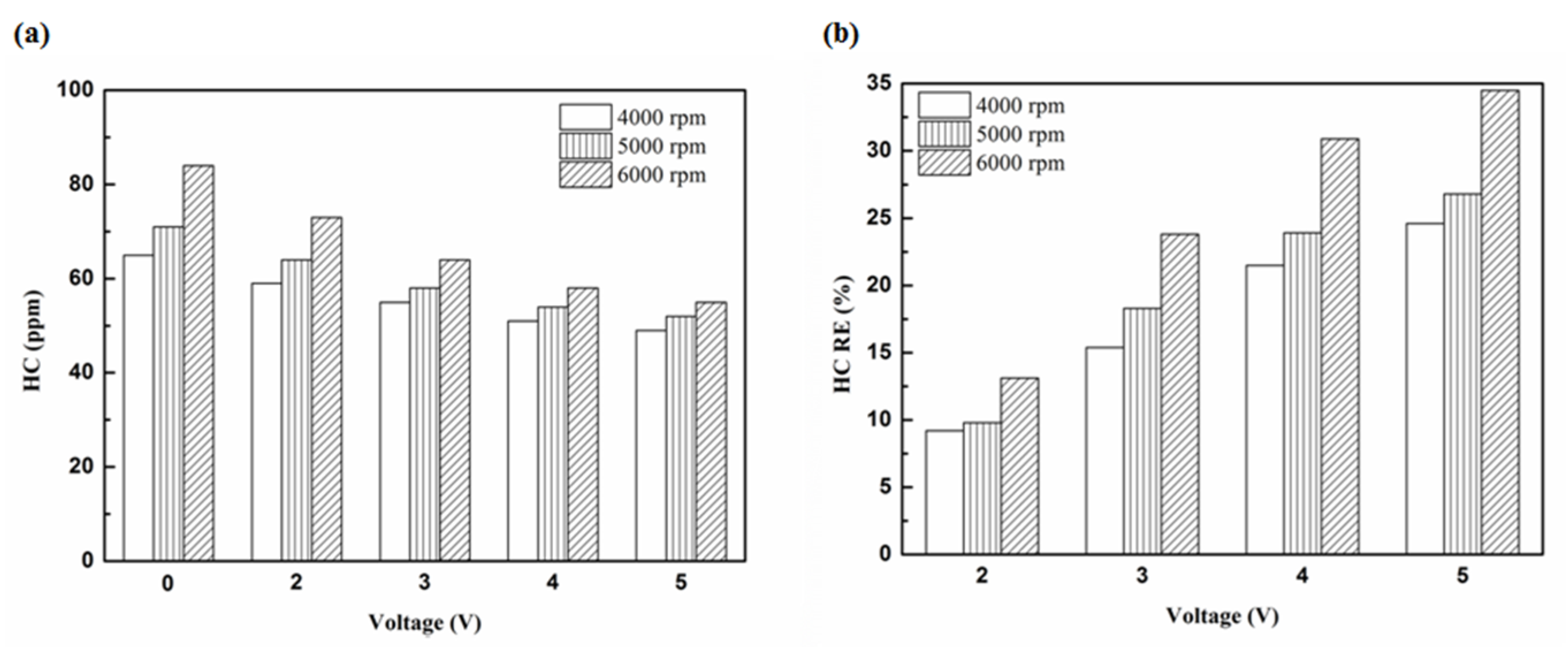
Figure 6.
The HC (a) emission values and (b) RE using iridium alloy in the front of the exhaust pipe.
Figure 6.
The HC (a) emission values and (b) RE using iridium alloy in the front of the exhaust pipe.
Figure 7.
The HC (a) emission values and (b) RE using platinum in the rear of the exhaust pipe.
Figure 7.
The HC (a) emission values and (b) RE using platinum in the rear of the exhaust pipe.
Figure 8.
The HC (a) emission values and (b) RE using iridium alloy in the rear of the exhaust pipe.
Figure 8.
The HC (a) emission values and (b) RE using iridium alloy in the rear of the exhaust pipe.
Figure 9.
The HC (a) emission values and (b) RE of control voltage 5 V using platinum.
Figure 9.
The HC (a) emission values and (b) RE of control voltage 5 V using platinum.
Figure 10.
The HC (a) emission values and (b) RE of control voltage 5 V using iridium alloy.
Figure 10.
The HC (a) emission values and (b) RE of control voltage 5 V using iridium alloy.
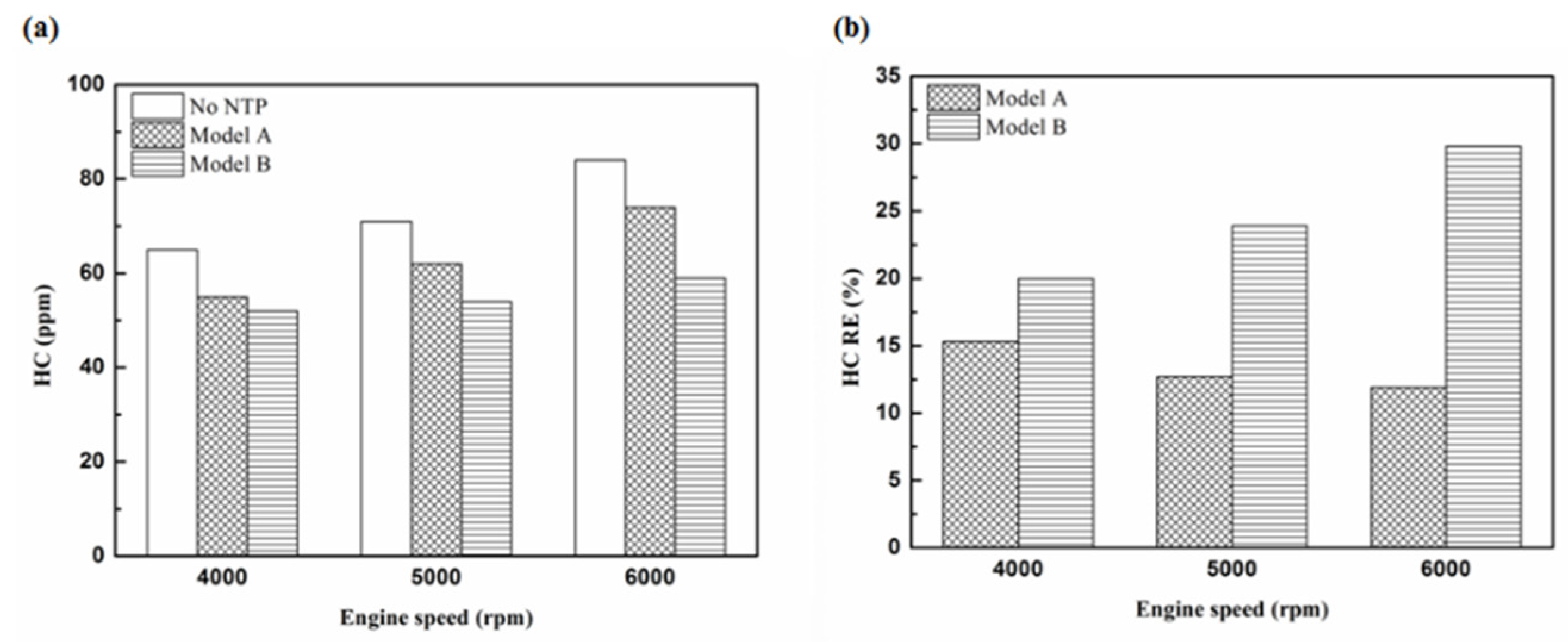
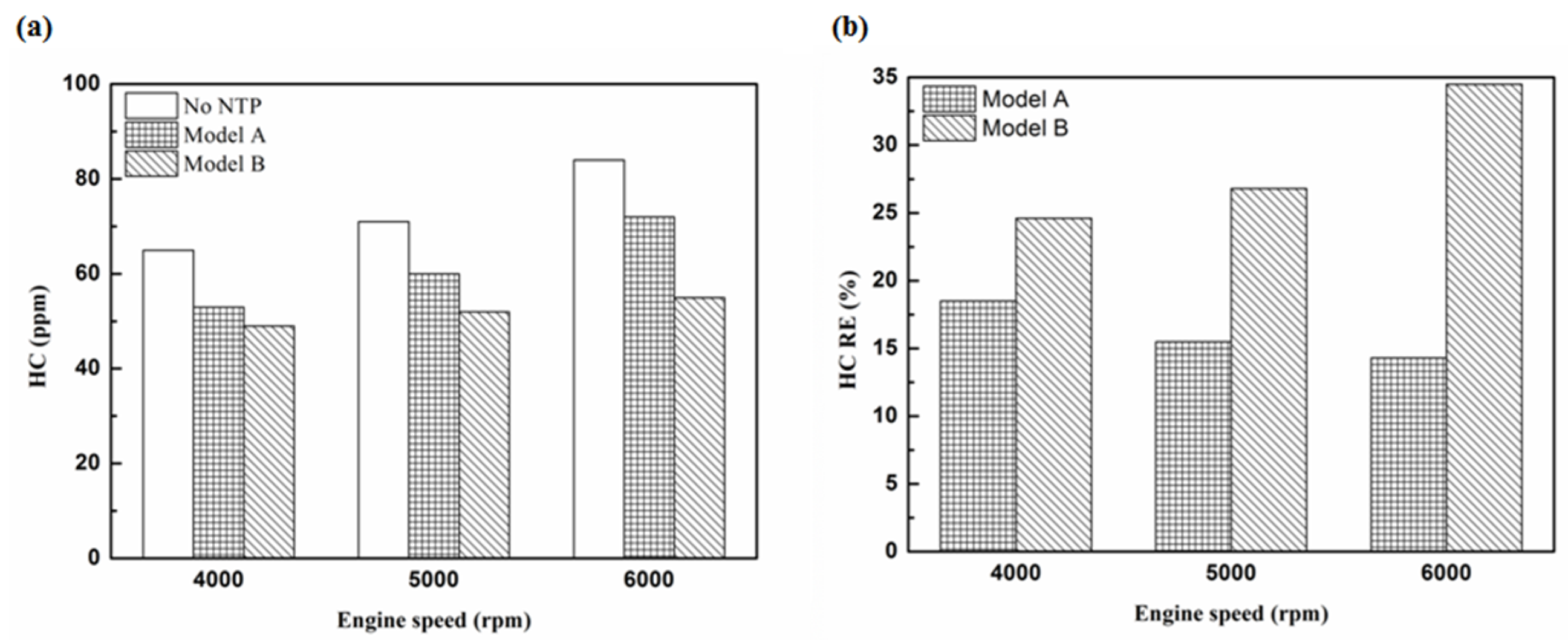
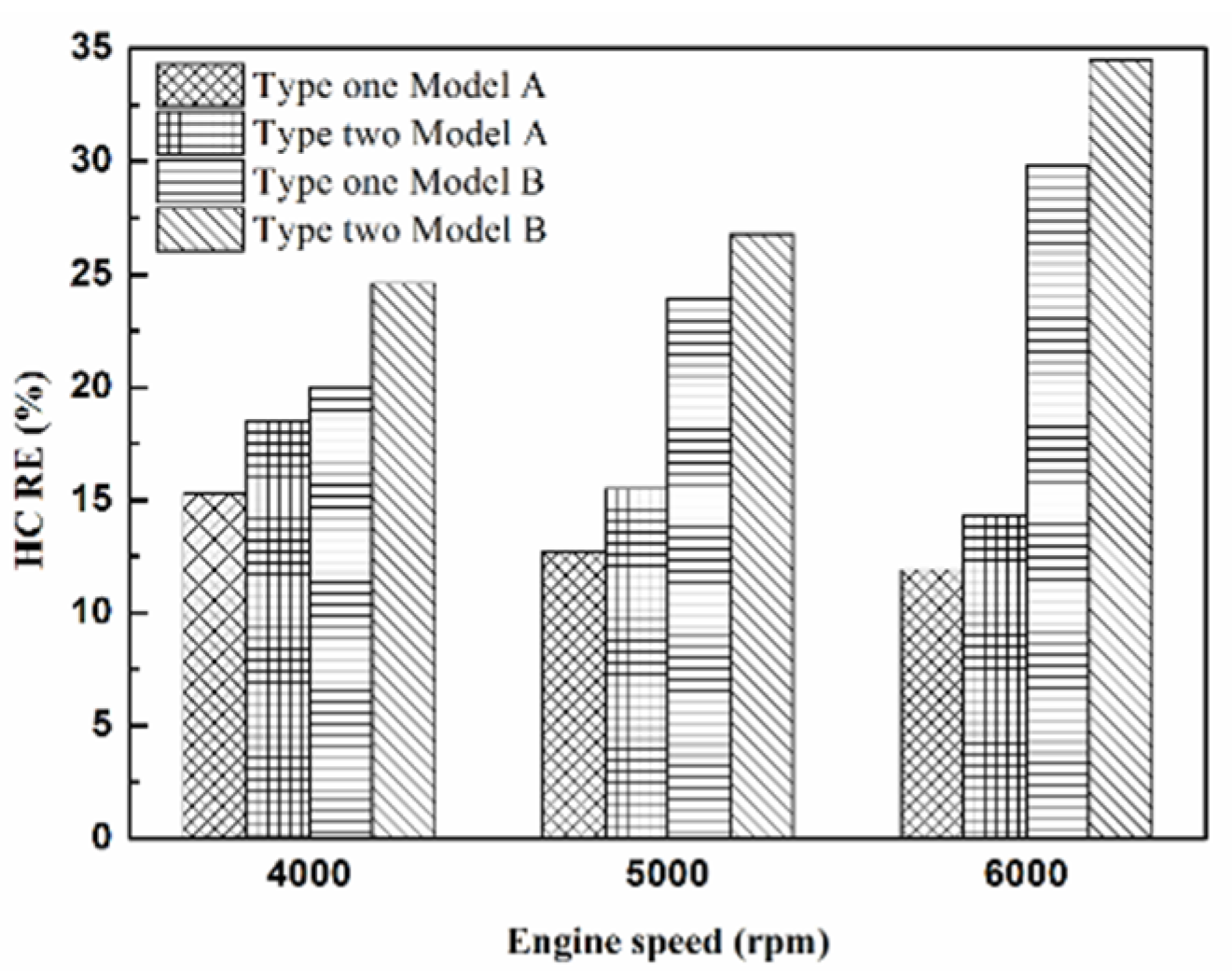
Figure 11.
The HC RE at control voltage 5 V.
Figure 11.
The HC RE at control voltage 5 V.
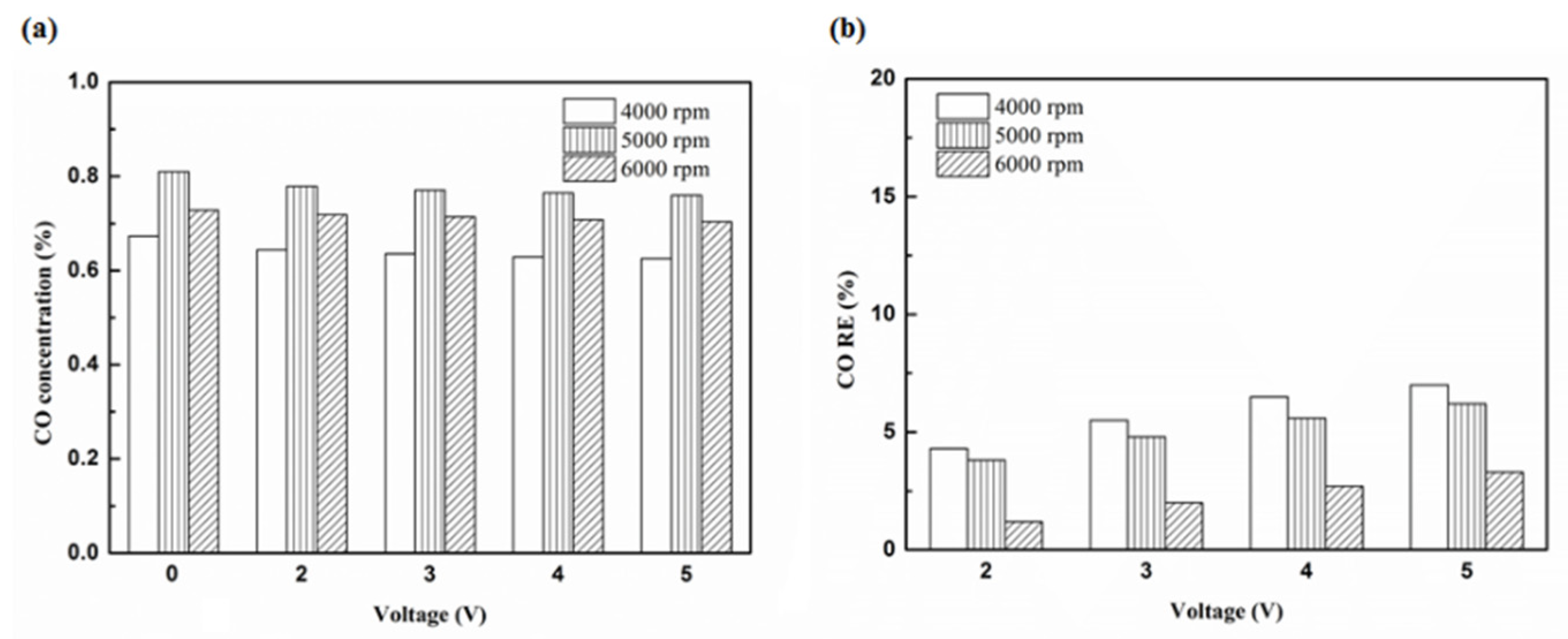
Figure 12.
The CO (a) emission values and (b) RE using platinum in the front of the exhaust pipe.
Figure 12.
The CO (a) emission values and (b) RE using platinum in the front of the exhaust pipe.
Figure 13.
The CO (a) emission values and (b) RE using iridium alloy in the front of the exhaust pipe.
Figure 13.
The CO (a) emission values and (b) RE using iridium alloy in the front of the exhaust pipe.
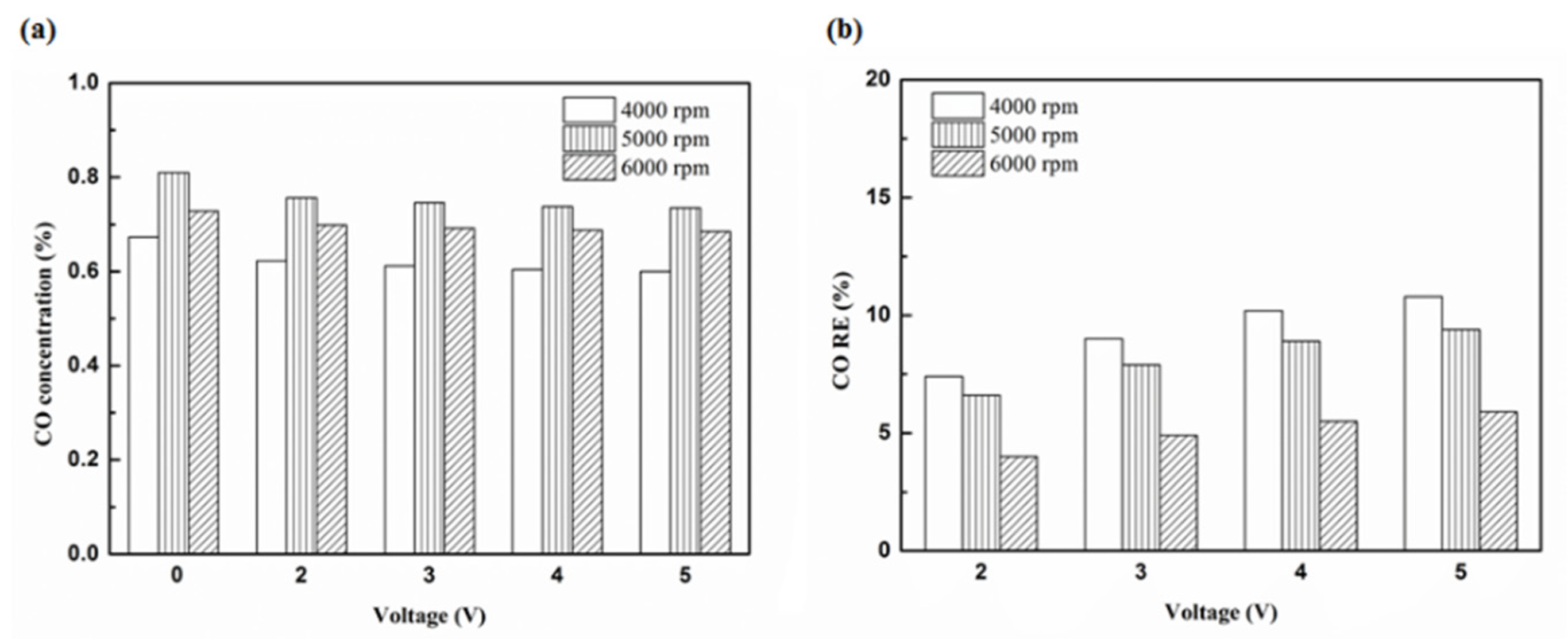
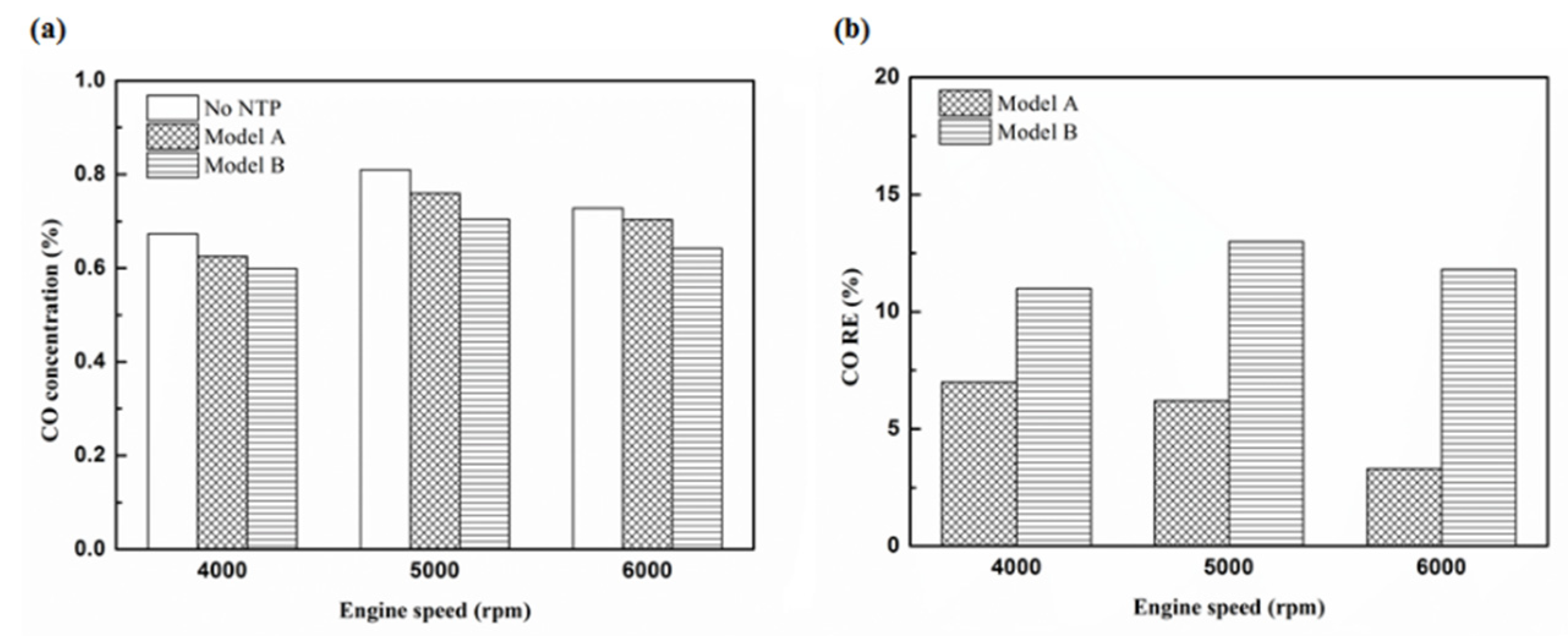
Figure 14.
The CO (a) emission values and (b) RE using platinum in the rear of the exhaust pipe.
Figure 14.
The CO (a) emission values and (b) RE using platinum in the rear of the exhaust pipe.
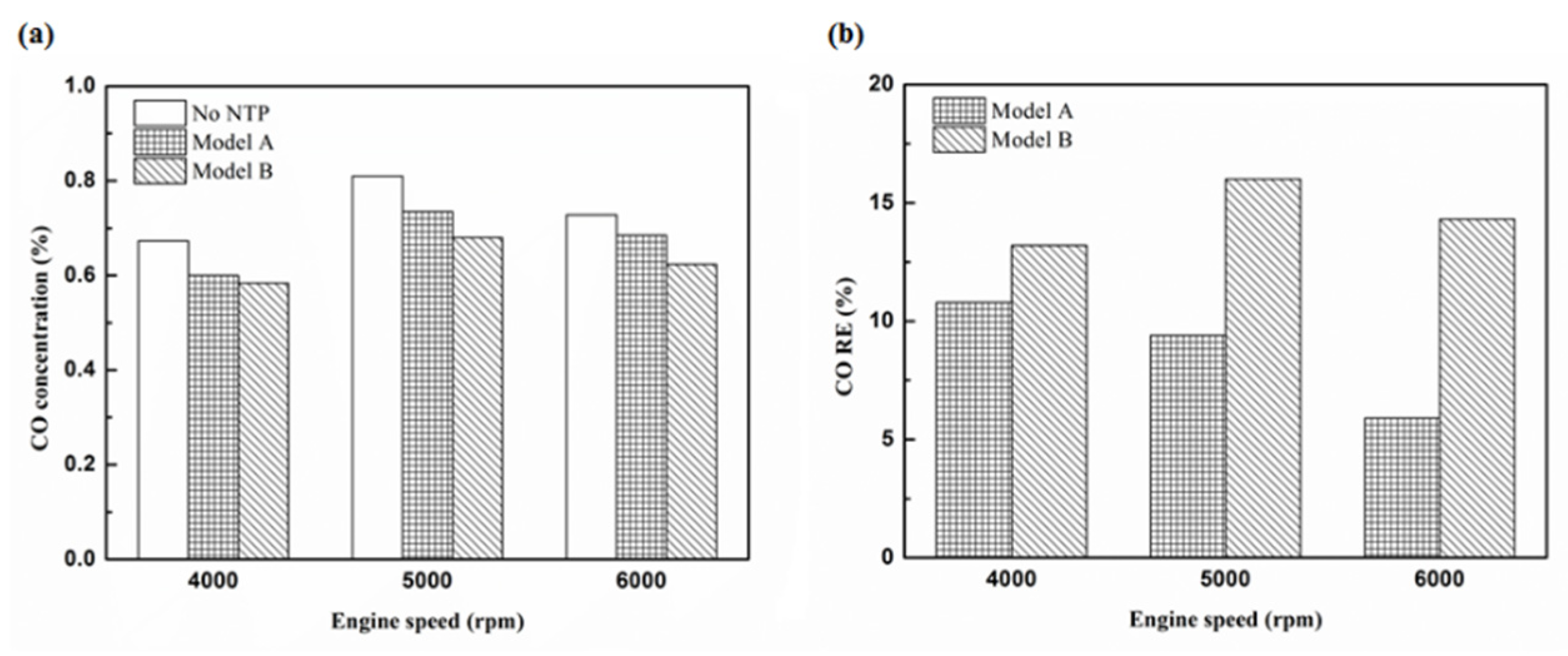
Figure 15.
The CO (a) emission values and (b) RE using iridium alloy in the rear of the exhaust pipe.
Figure 15.
The CO (a) emission values and (b) RE using iridium alloy in the rear of the exhaust pipe.
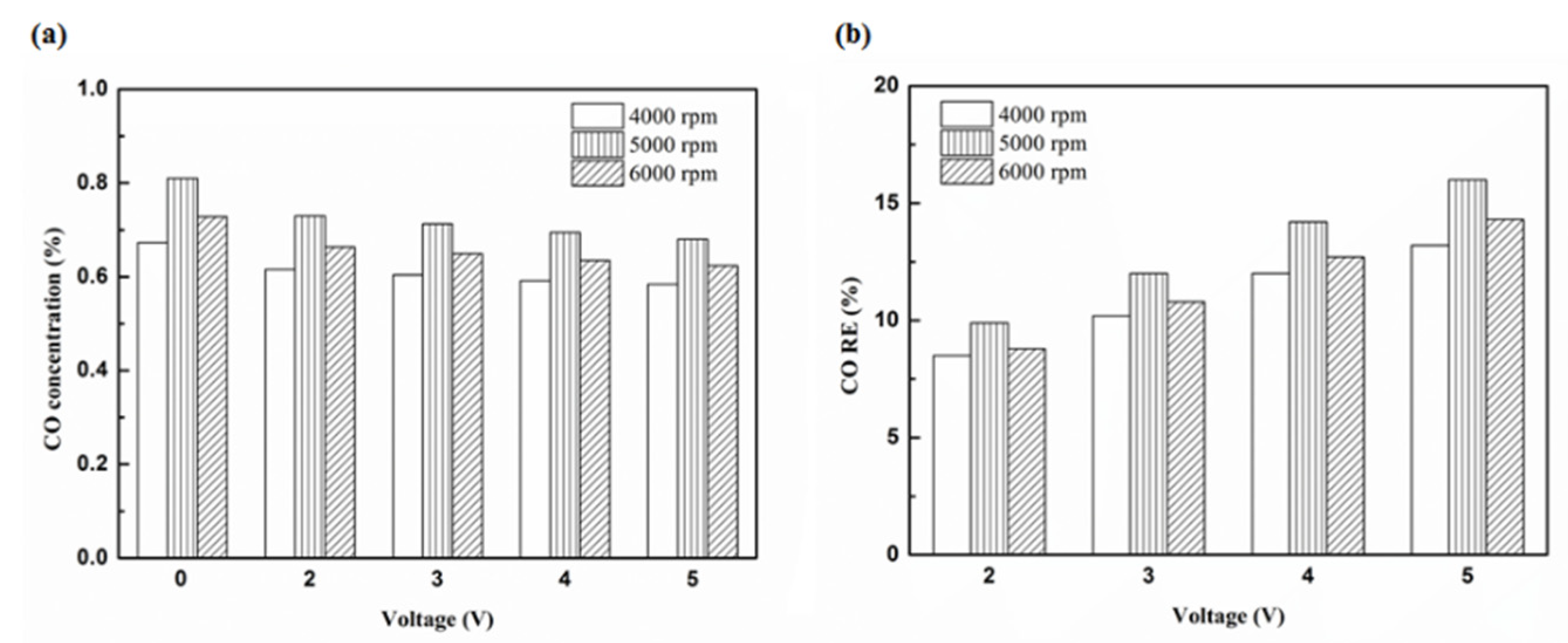
Figure 16.
The CO (a) emission values and (b) RE of control voltage 5 V using platinum.
Figure 16.
The CO (a) emission values and (b) RE of control voltage 5 V using platinum.
Figure 17.
The CO (a) emission values and (b) RE of control voltage 5 V using iridium alloy.
Figure 17.
The CO (a) emission values and (b) RE of control voltage 5 V using iridium alloy.
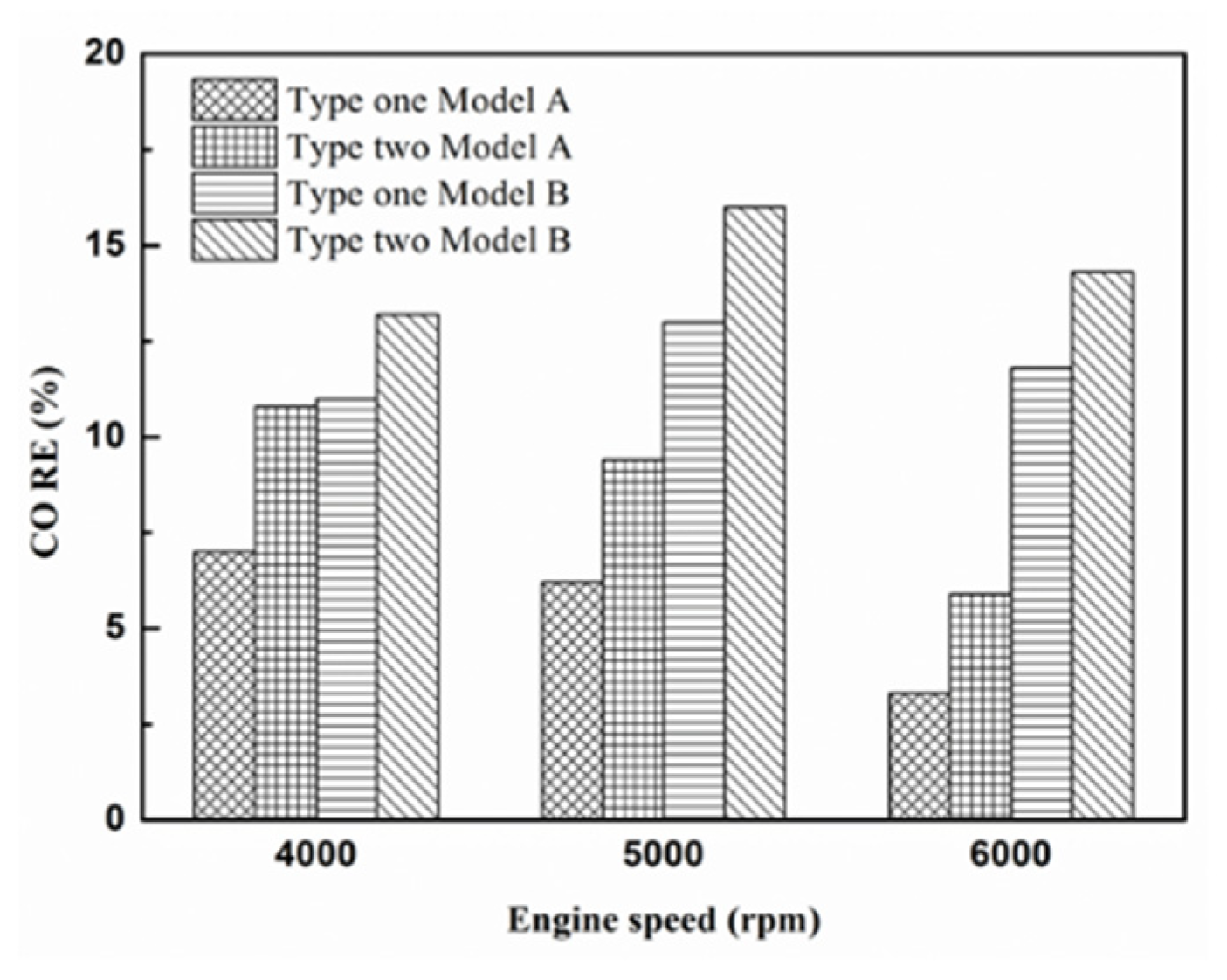
Figure 18.
The CO RE at control voltage 5 V.
Figure 18.
The CO RE at control voltage 5 V.
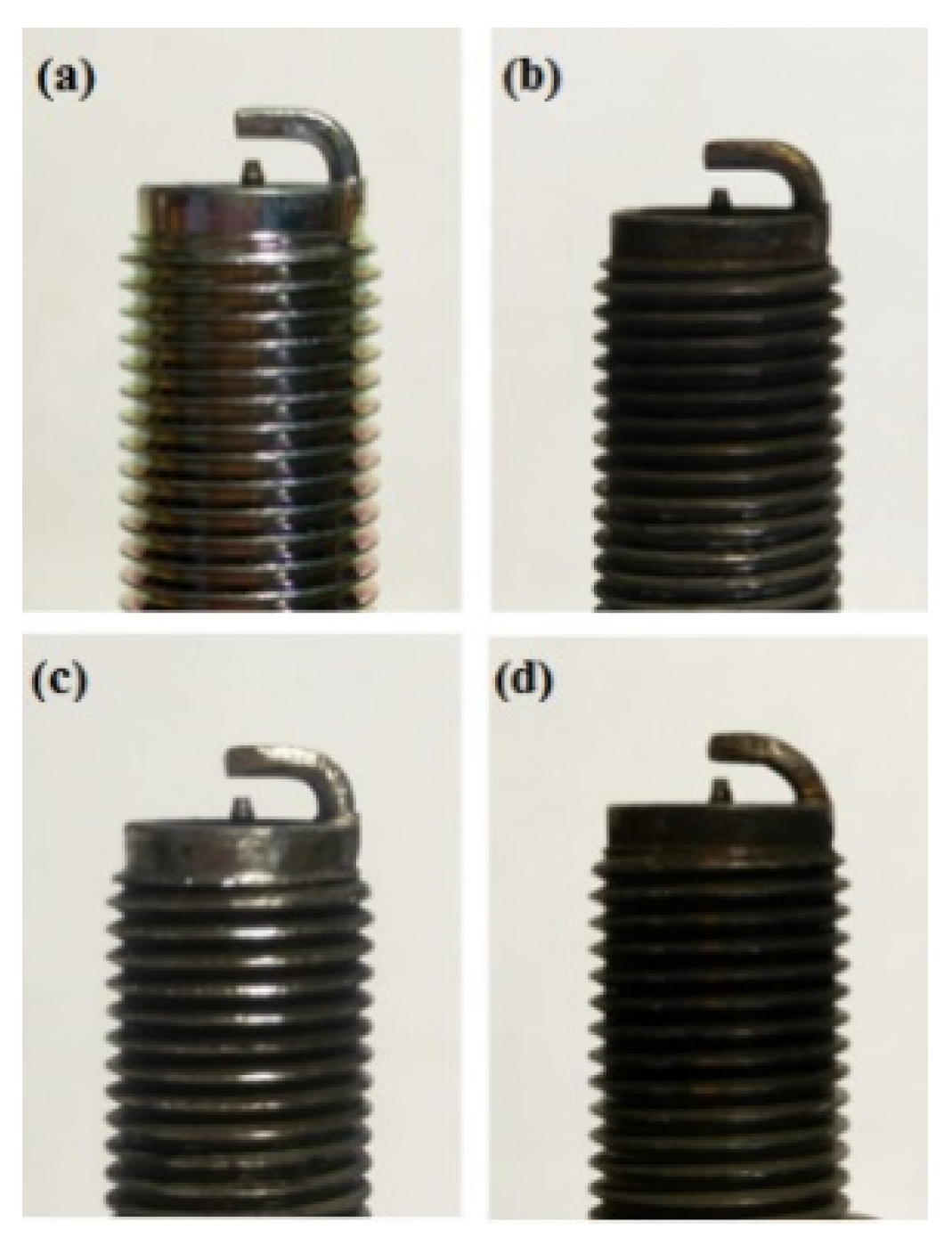
Figure 19.
A photographic view of NTP reactor (a) platinum and (c) iridium alloy before this experiment and (b) platinum and (d) iridium alloy after this experiment.
Figure 19.
A photographic view of NTP reactor (a) platinum and (c) iridium alloy before this experiment and (b) platinum and (d) iridium alloy after this experiment.
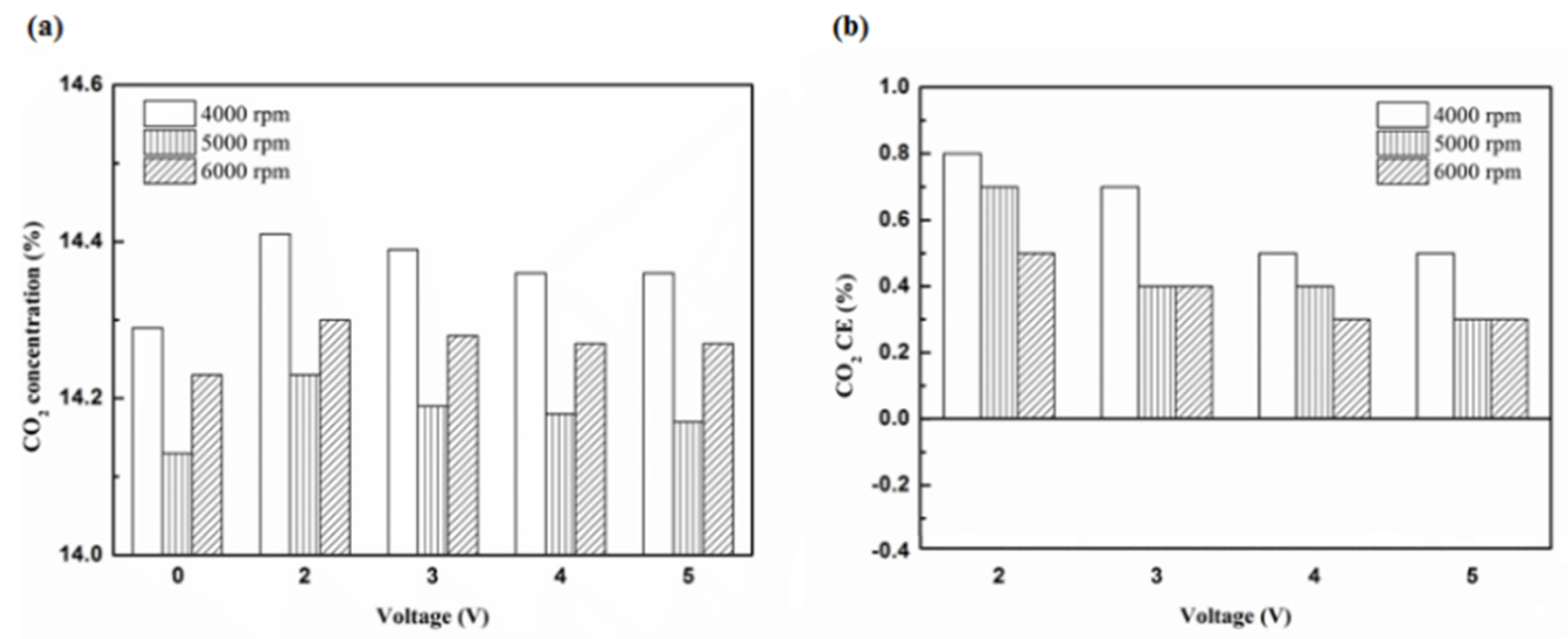
Figure 20.
The CO2 (a) emission values and (b) CE at platinum in the front of the exhaust pipe.
Figure 20.
The CO2 (a) emission values and (b) CE at platinum in the front of the exhaust pipe.
Figure 21.
The CO2 (a) emission values and (b) CE using iridium alloy in the front of the exhaust pipe.
Figure 21.
The CO2 (a) emission values and (b) CE using iridium alloy in the front of the exhaust pipe.
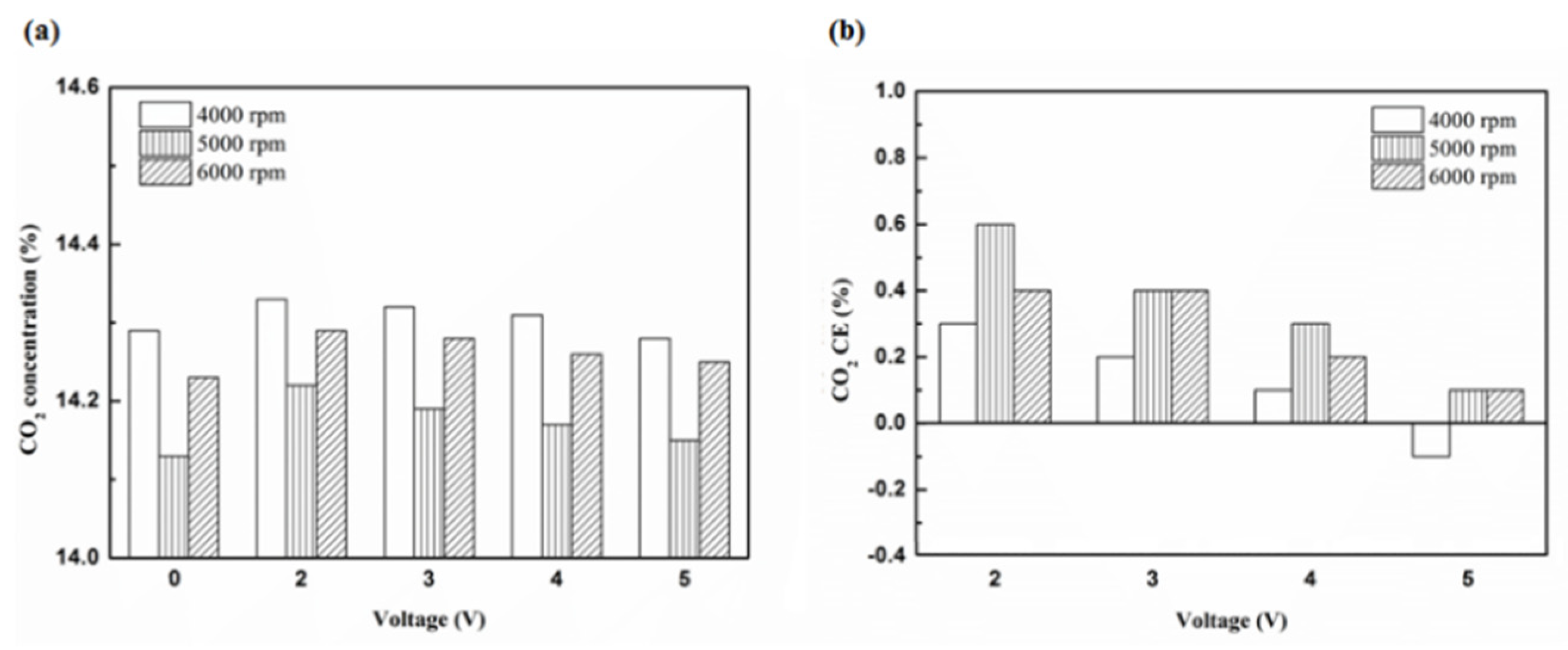
Figure 22.
The CO2 (a) emission values and (b) CE using platinum in the rear of the exhaust pipe.
Figure 22.
The CO2 (a) emission values and (b) CE using platinum in the rear of the exhaust pipe.
Figure 23.
The CO2 (a) emission values and (b) CE using iridium alloy in the rear of the exhaust pipe.
Figure 23.
The CO2 (a) emission values and (b) CE using iridium alloy in the rear of the exhaust pipe.
Figure 24.
The CO2 (a) emission values and (b) CE of control voltage 5 V using platinum.
Figure 24.
The CO2 (a) emission values and (b) CE of control voltage 5 V using platinum.
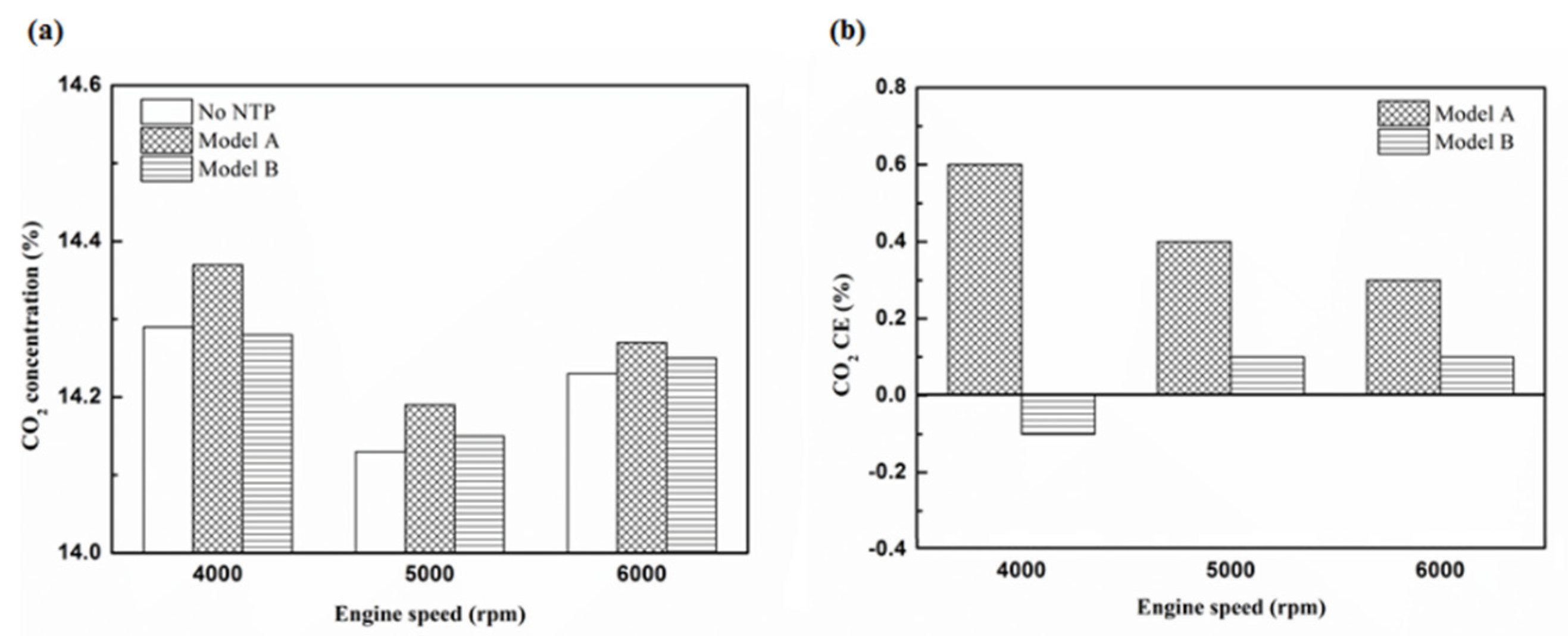
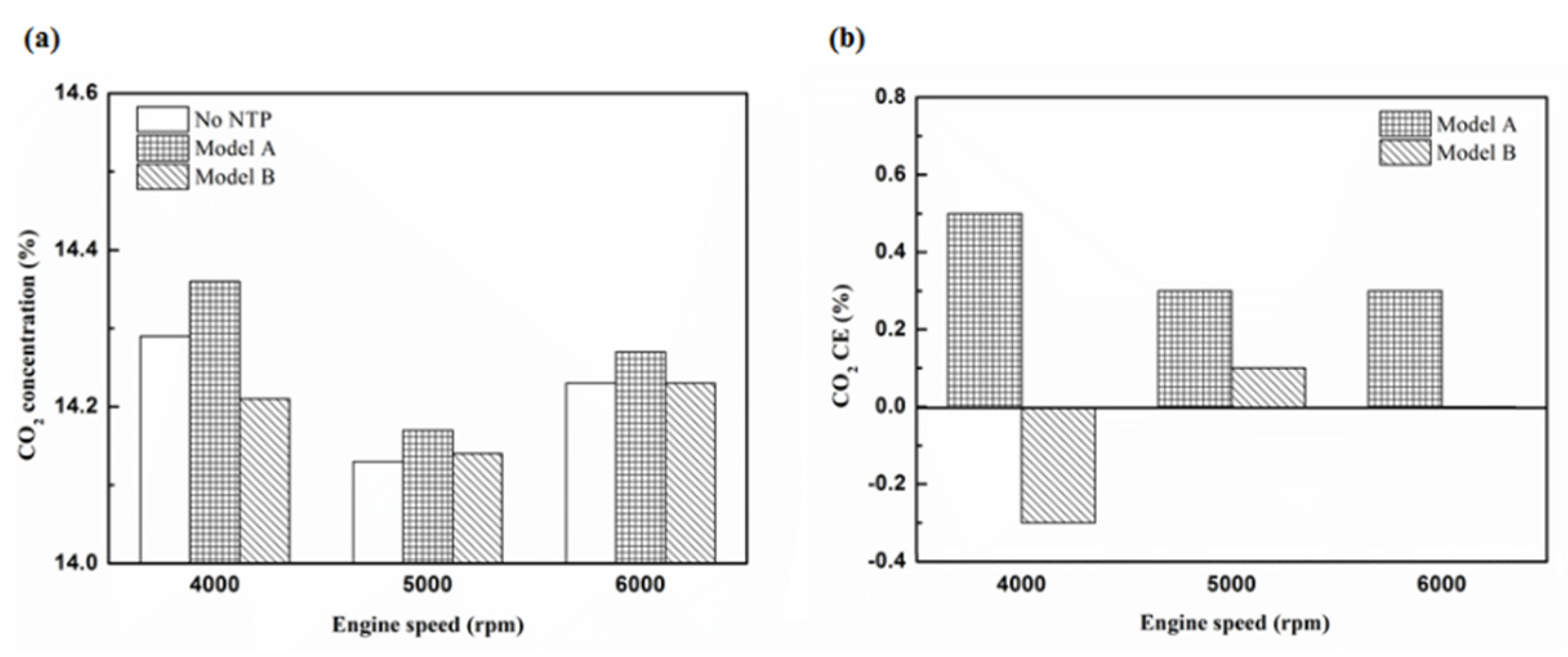
Figure 25.
The CO2 (a) emission values and (b) CE of control voltage 5 V using iridium alloy.
Figure 25.
The CO2 (a) emission values and (b) CE of control voltage 5 V using iridium alloy.
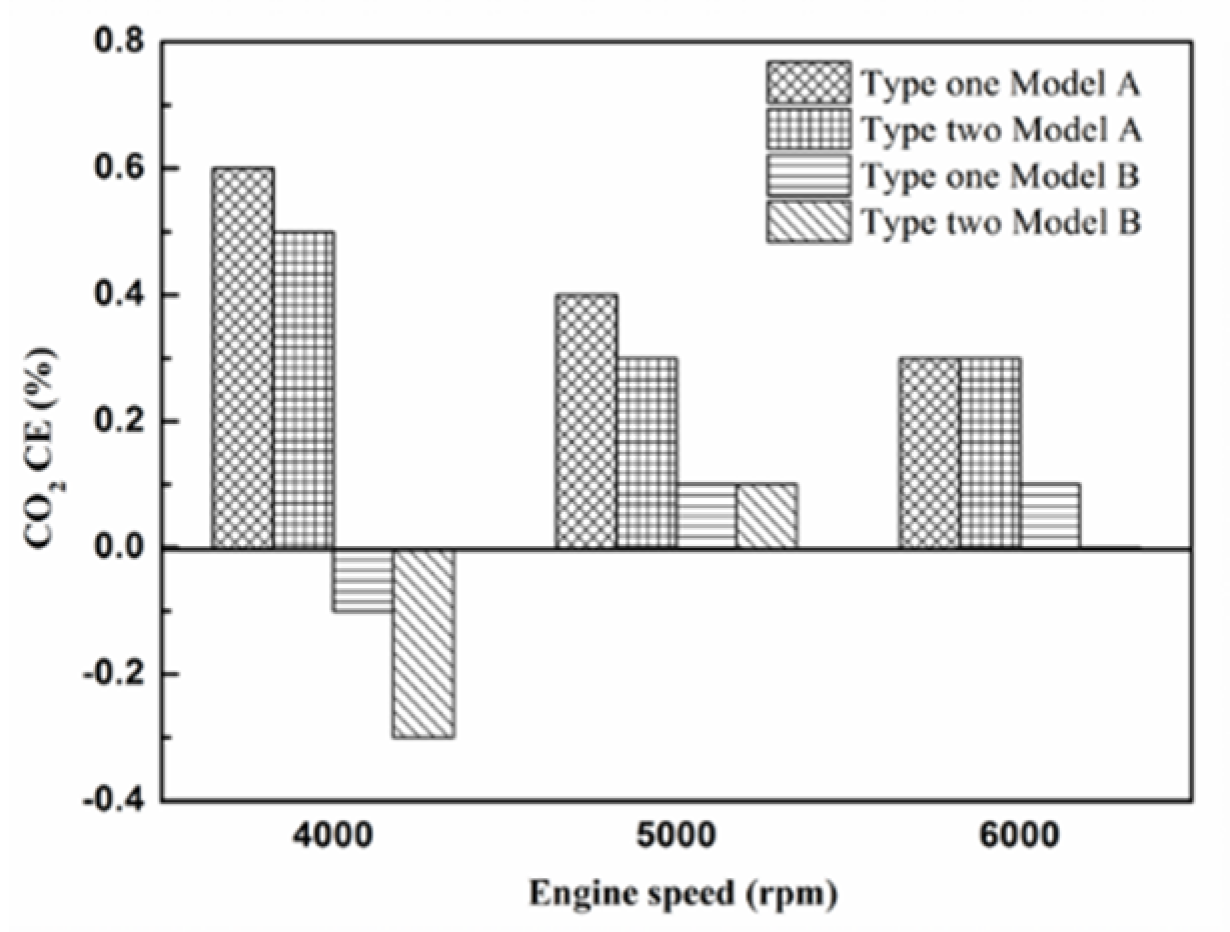
Figure 26.
The CO2 CE at control voltage 5 V.
Figure 26.
The CO2 CE at control voltage 5 V.
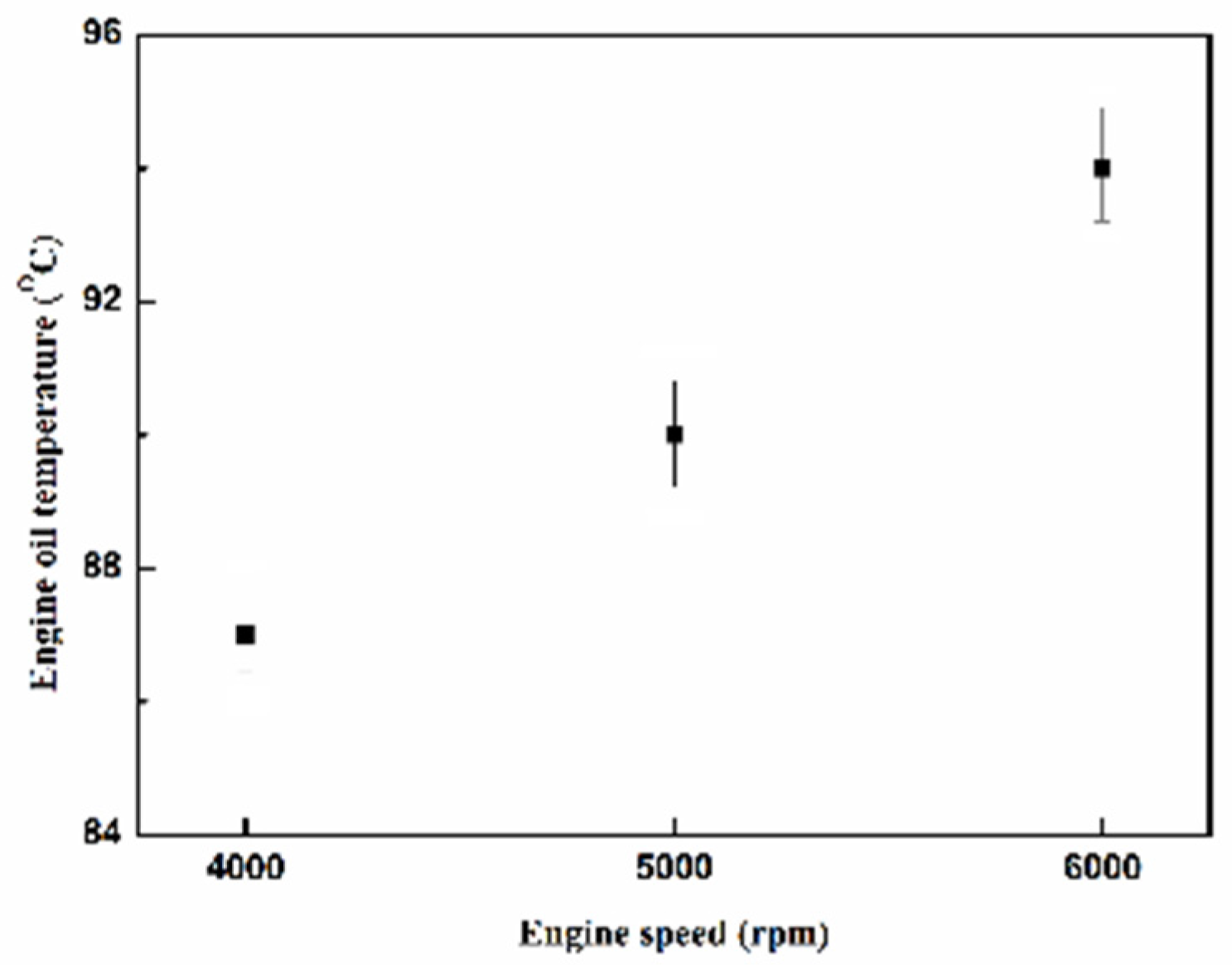
Figure 27.
The oil temperature of the engine at different engine speeds.
Figure 27.
The oil temperature of the engine at different engine speeds.
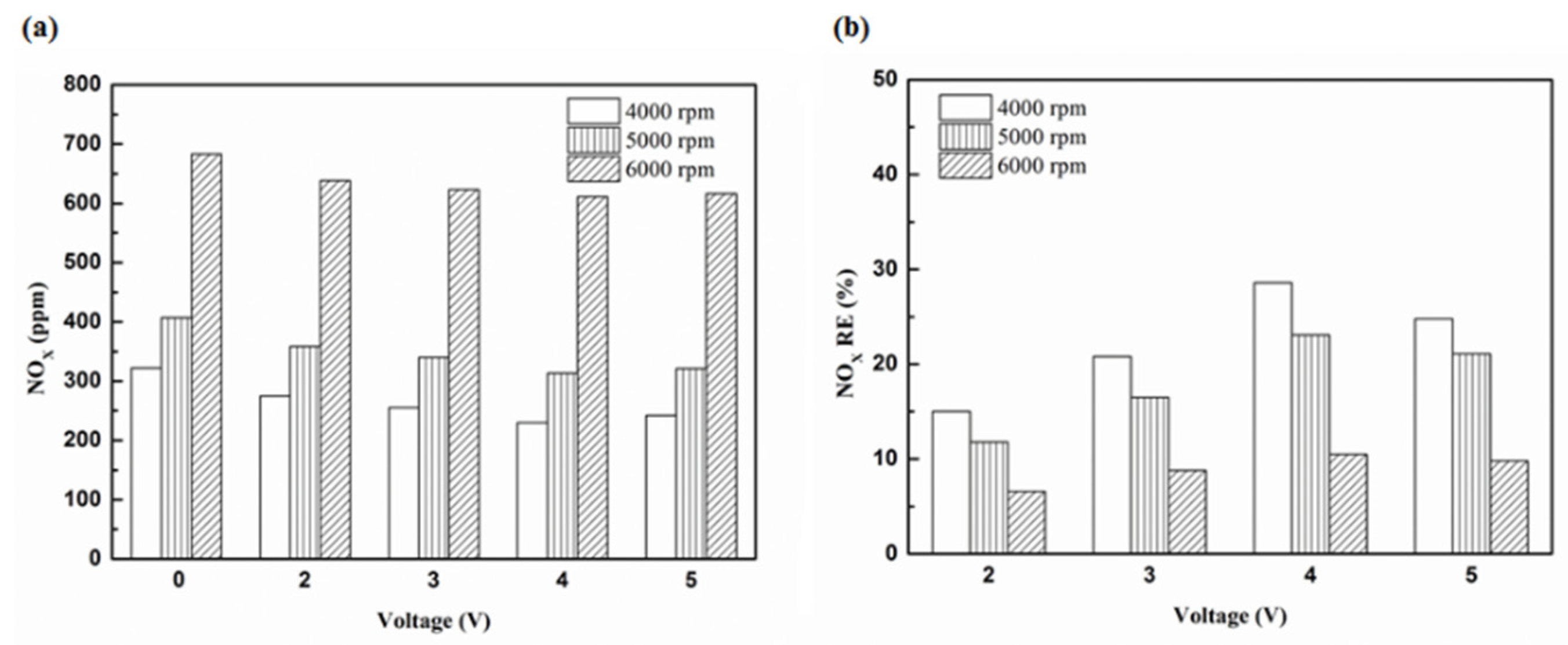
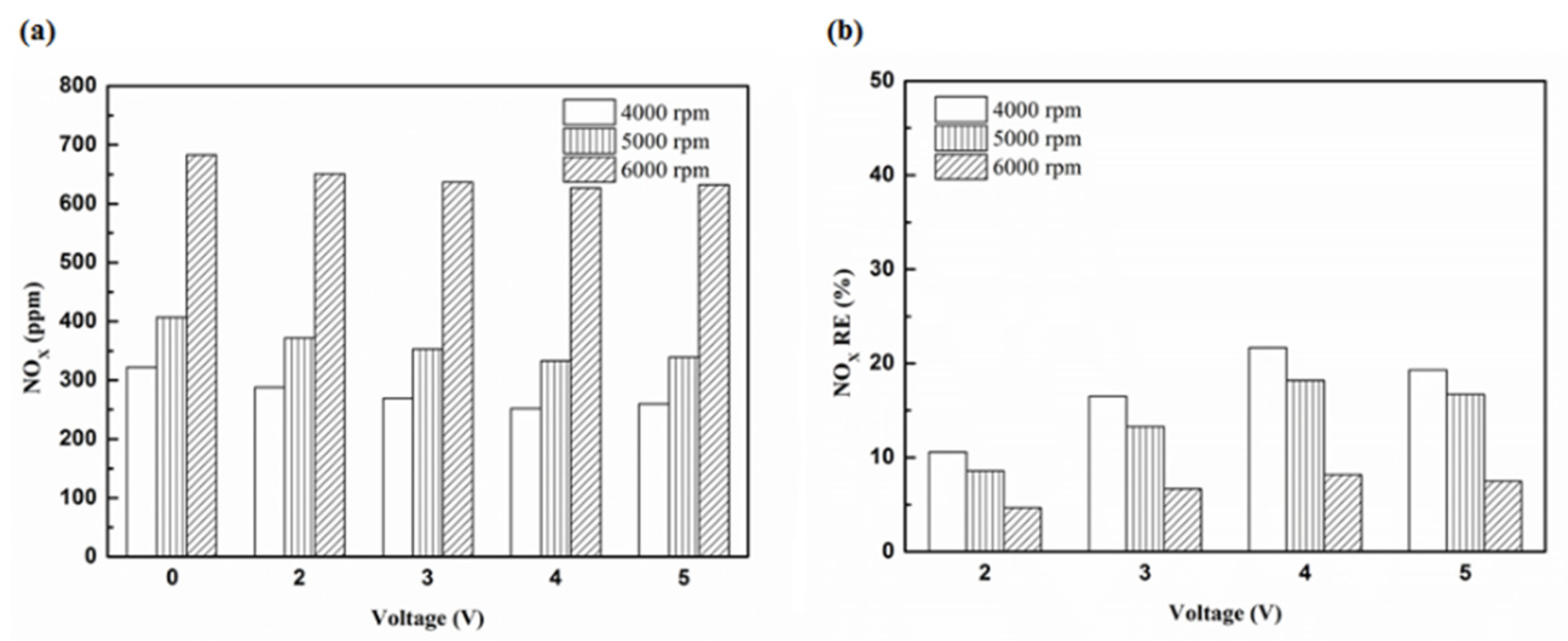
Figure 28.
The NOx (a) emission values and (b) RE using platinum in the front of the exhaust pipe.
Figure 28.
The NOx (a) emission values and (b) RE using platinum in the front of the exhaust pipe.
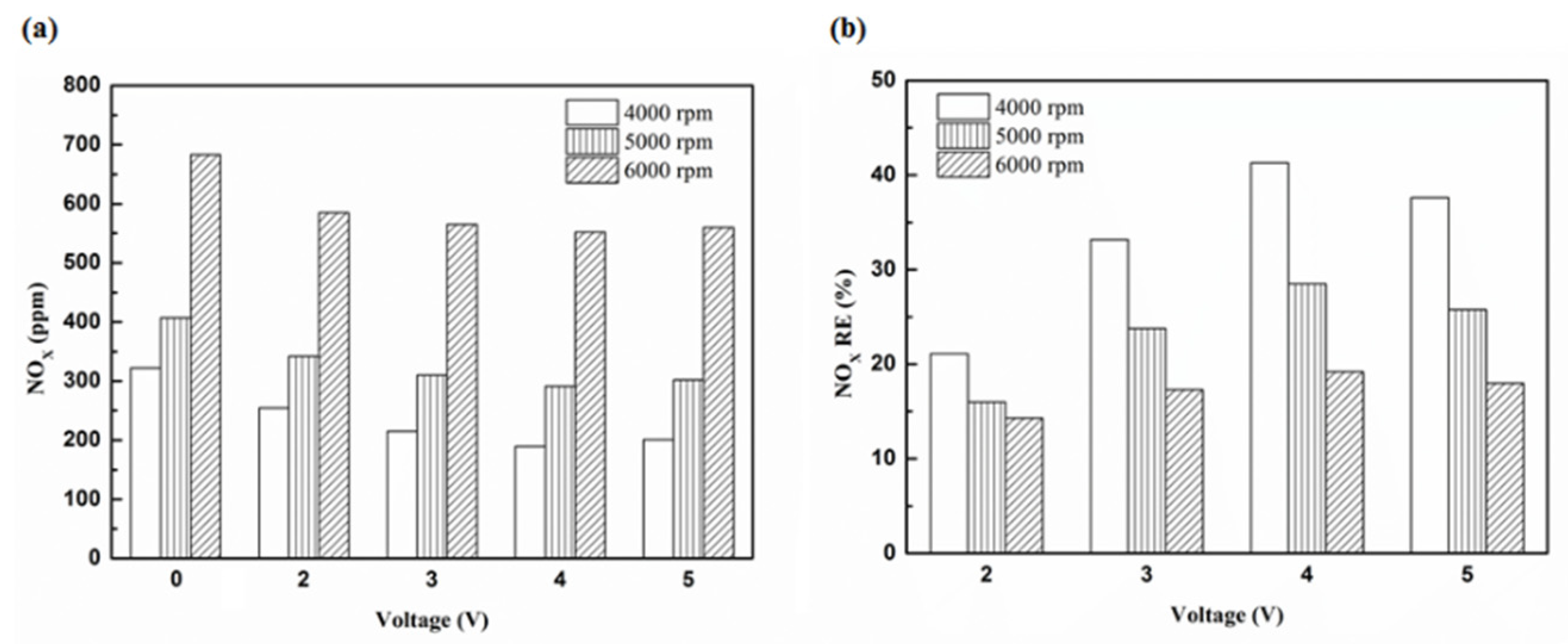
Figure 29.
The NOx (a) emission values and (b) RE using iridium alloy in the front of the exhaust pipe.
Figure 29.
The NOx (a) emission values and (b) RE using iridium alloy in the front of the exhaust pipe.
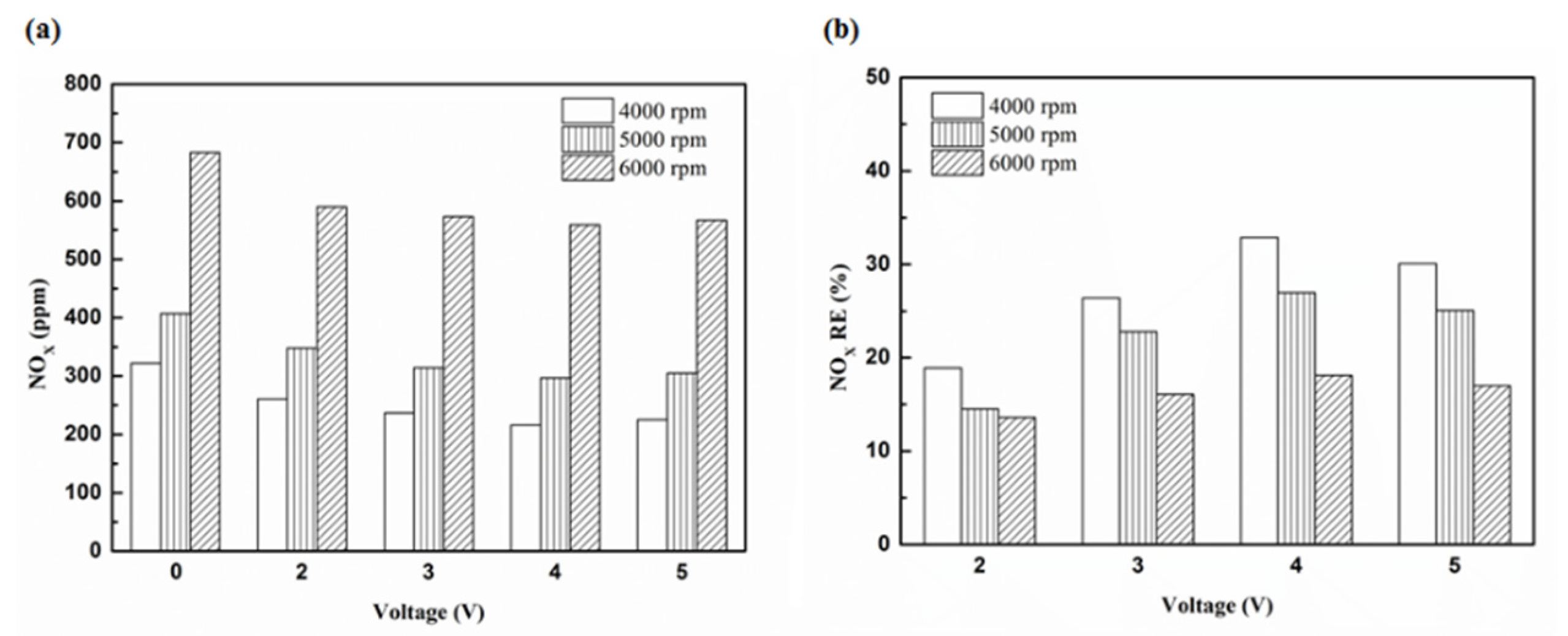
Figure 30.
The NOx (a) emission values and (b) RE using platinum and in the rear of the exhaust pipe.
Figure 30.
The NOx (a) emission values and (b) RE using platinum and in the rear of the exhaust pipe.
Figure 31.
The NOx (a) emission values and (b) RE using iridium alloy in the rear of the exhaust pipe.
Figure 31.
The NOx (a) emission values and (b) RE using iridium alloy in the rear of the exhaust pipe.
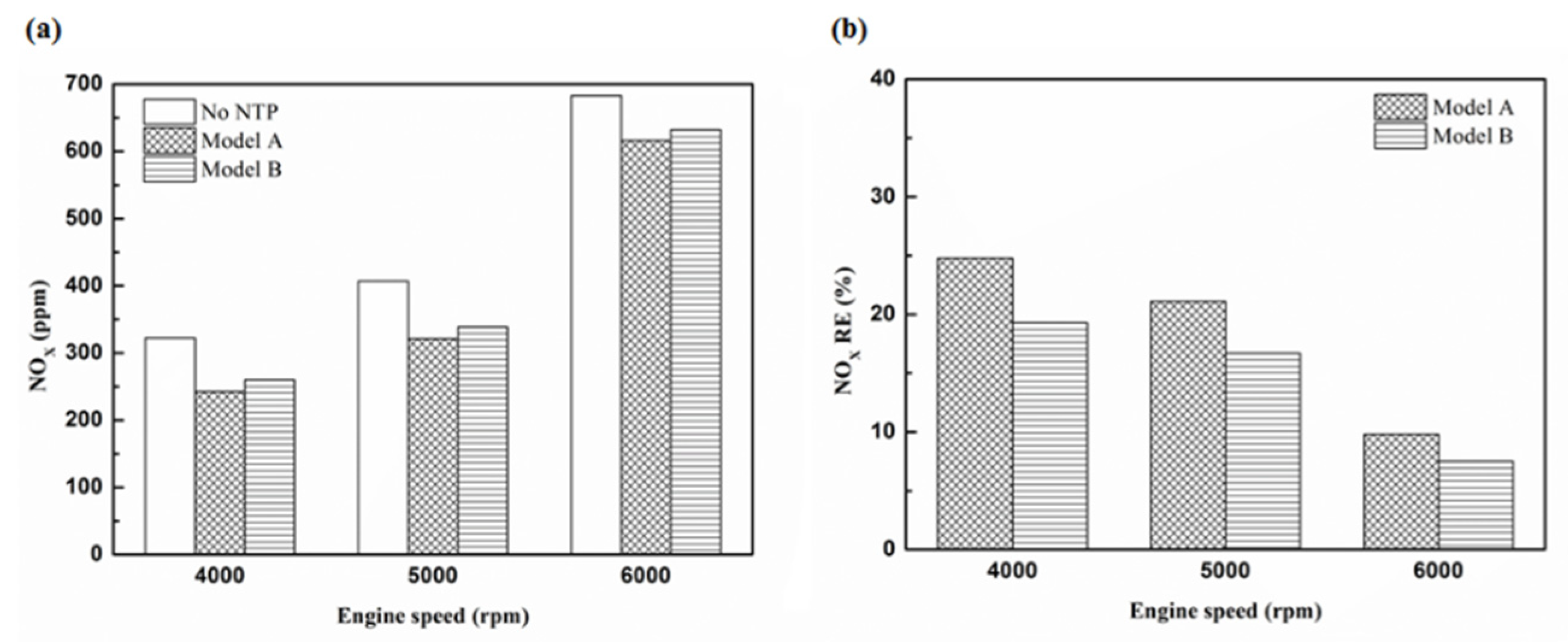
Figure 32.
The NOx (a) emission values and (b) RE of control voltage 5 V using platinum.
Figure 32.
The NOx (a) emission values and (b) RE of control voltage 5 V using platinum.
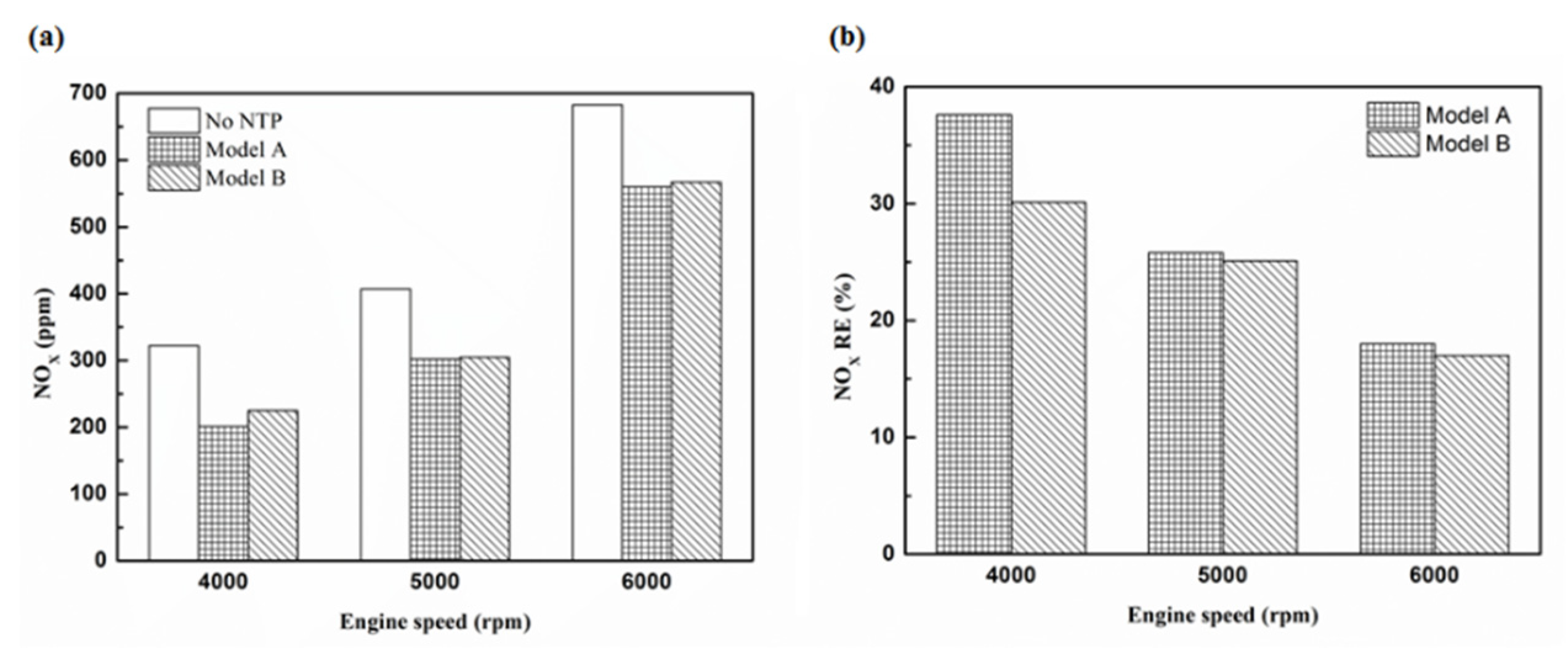
Figure 33.
The NOx (a) emission values and (b) RE of control voltage 5 V using iridium alloy.
Figure 33.
The NOx (a) emission values and (b) RE of control voltage 5 V using iridium alloy.
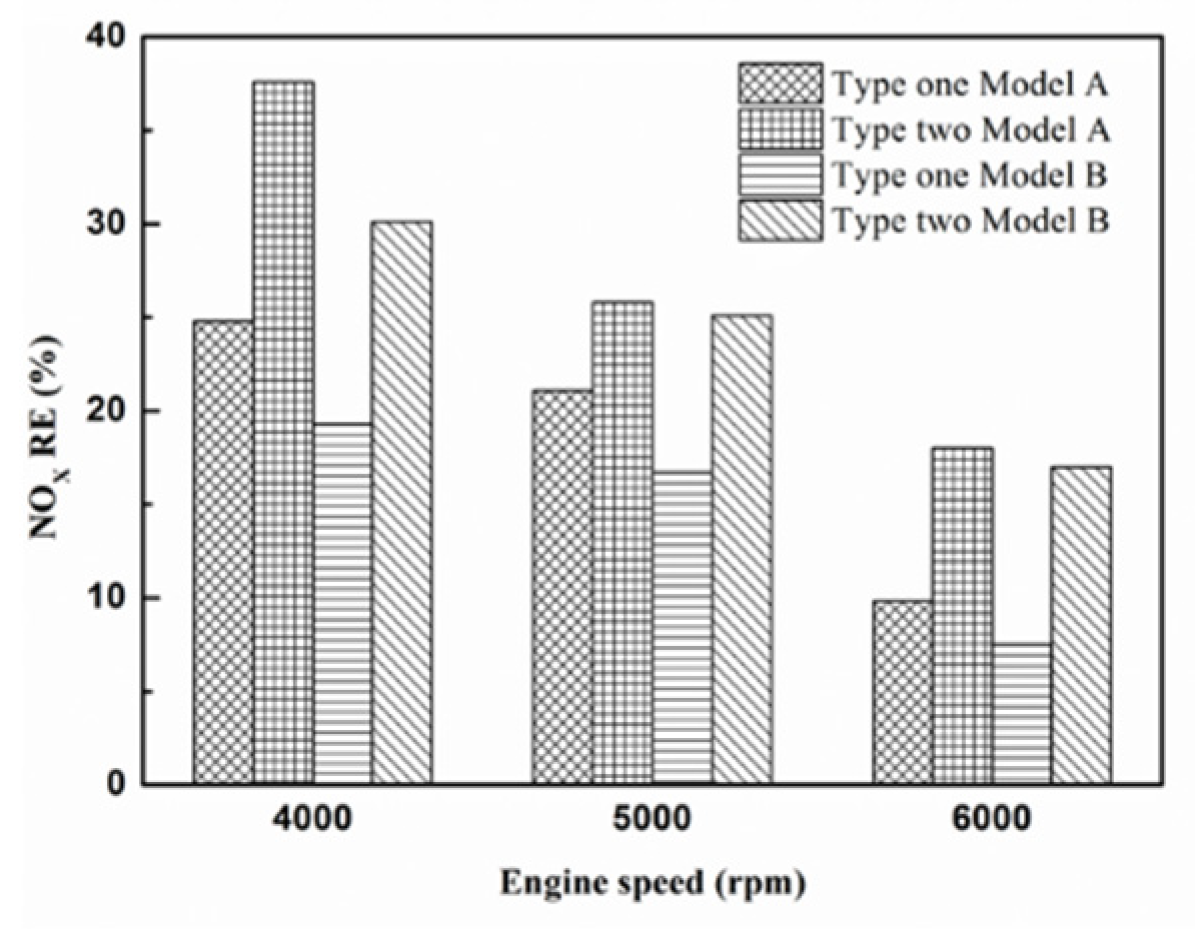
Figure 34.
The NOx RE at control voltage 5 V.
Figure 34.
The NOx RE at control voltage 5 V.
Table 1.
The engine specification of motorcycle.
Table 1.
The engine specification of motorcycle.
| Parameter | Value |
|---|
| Model | GP125 |
| Stoke | four-stoke |
| Engine type | Single cylinder |
| Displacement (c.c.) | 124.6 |
| Bore × Stoke (mm) | 52.4 × 57.8 |
| Compression ratio | 9.9:1 |
| Fuel | Unleaded gasoline |
| Max power (kw/rpm) | 7.8/7500 |
| Max torque (N-m/rpm) | 10.3/5500 |
| Wheelbase (mm) | 1220 |
| Air/fuel ratio (A/F) | 13.7 |
Table 2.
The control conditions of engine.
Table 2.
The control conditions of engine.
| Parameter | Value |
|---|
| Engine speed (rpm) | 4000, 5000, 6000 |
| Fuel octane rating | 95 |
| Engine temperature (°C) | 100–110 |
| Intake air temperature (°C) | 22–26 |
Table 3.
The types of NTP reactor.
Table 3.
The types of NTP reactor.
| Parameter | Value |
|---|
| Type One | Type Two |
|---|
| Model | NGK-CR8EGP | NGK-CR8EIX |
| Material | Platinum | Iridium alloy |
| Discharging Gap (before used) (mm) | 0.7 | 0.8 |
| Discharging Gap (after used) (mm) | 0.7 | 0.7 |
| Center electrode diameter (mm) | 0.8 | 0.8 |
| Outer electrode diameter (mm) | 2.20 | 1.85 |
Table 4.
The specification of NTP control device.
Table 4.
The specification of NTP control device.
| Parameter | Value |
|---|
| Model | DP-30032 |
| Main output voltage (V) | 0–30 |
| Main output current (A) | 0–3 |
| Fixed output voltage (V) | 2.5/3.3/5 |
| Fixed output current (A) | 3 |
| Resolution (mV/mA) | 100/10 |
| Precision | ±(1% reading + 2 digits) |
| Power supply (V, Hz) | AC110/220 ± 10% selectable, 50/60 |
| Dimensions (mm) | 250W × 150H × 310D |
Table 5.
The range of each exhaust gas.
Table 5.
The range of each exhaust gas.
| Parameter | Value |
|---|
| Measuring Range | Tolerance |
|---|
| HC (ppm) | 0–2000 | ±12 |
| CO (%) | 0–10 | ±0.06 |
| CO2 (%) | 0–20 | ±0.5 |
| NOx (ppm) | 0–5000 | ±25 |
Table 6.
The maximum and minimum values of HC removal efficiency at control voltage 5 V.
Table 6.
The maximum and minimum values of HC removal efficiency at control voltage 5 V.
| Parameter | HC RE Value |
|---|
| Maximum | Minimum |
|---|
| Platinum and in the front of the exhaust pipe (Type one and model A) | 15.3% | 11.9% |
| Iridium alloy and in the front of the exhaust pipe (Type two and model A) | 18.5% | 14.3% |
| Platinum and in the rear of the exhaust pipe (Type one and model B) | 29.8% | 20% |
| Iridium alloy and in the rear of the exhaust pipe (Type two and model B) | 34.5% | 24.6% |
Table 7.
The maximum and minimum values of CO removal efficiency at control voltage 5 V.
Table 7.
The maximum and minimum values of CO removal efficiency at control voltage 5 V.
| Parameter | CO RE Value |
|---|
| Maximum | Minimum |
|---|
| Platinum and in the front of the exhaust pipe (Type one and model A) | 7% | 3.3% |
| Iridium alloy and in the front of the exhaust pipe (Type two and model A) | 10.8% | 5.9% |
| Platinum and in the rear of the exhaust pipe (Type one and model B) | 13% | 10.1% |
| Iridium alloy and in the rear of the exhaust pipe (Type two and model B) | 16% | 13.2% |
Table 8.
The maximum and minimum values of CO2 conversion efficiency at control voltage 5 V.
Table 8.
The maximum and minimum values of CO2 conversion efficiency at control voltage 5 V.
| Parameter | CO2 CE Value |
|---|
| Maximum | Minimum |
|---|
| Platinum and in the front of the exhaust pipe (Type one and model A) | 0.6% | 0.3% |
| Iridium alloy and in the front of the exhaust pipe (Type two and model A) | 0.5% | 0.3% |
| Platinum and in the rear of the exhaust pipe (Type one and model B) | 0.1% | −0.1% |
| Iridium alloy and in the rear of the exhaust pipe (Type two and model B) | 0% | −0.3% |
Table 9.
The maximum and minimum values of NOx removal efficiency at control voltage 5 V.
Table 9.
The maximum and minimum values of NOx removal efficiency at control voltage 5 V.
| Parameter | NOx RE Value |
|---|
| Maximum | Minimum |
|---|
| Platinum and in the front of the exhaust pipe (Type one and model A) | 24.8% | 9.8% |
| Iridium alloy and in the front of the exhaust pipe (Type two and model A) | 37.6% | 18% |
| Platinum and in the rear of the exhaust pipe (Type one and model B) | 19.3% | 7.5% |
| Iridium alloy and in the rear of the exhaust pipe (Type two and model B) | 30.1% | 17% |