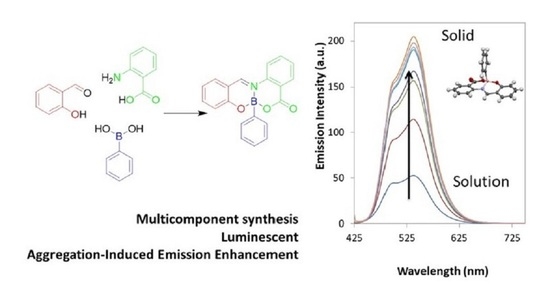
Multicomponent Synthesis of Luminescent Iminoboronates
Abstract
1. Introduction
2. Results and Discussion
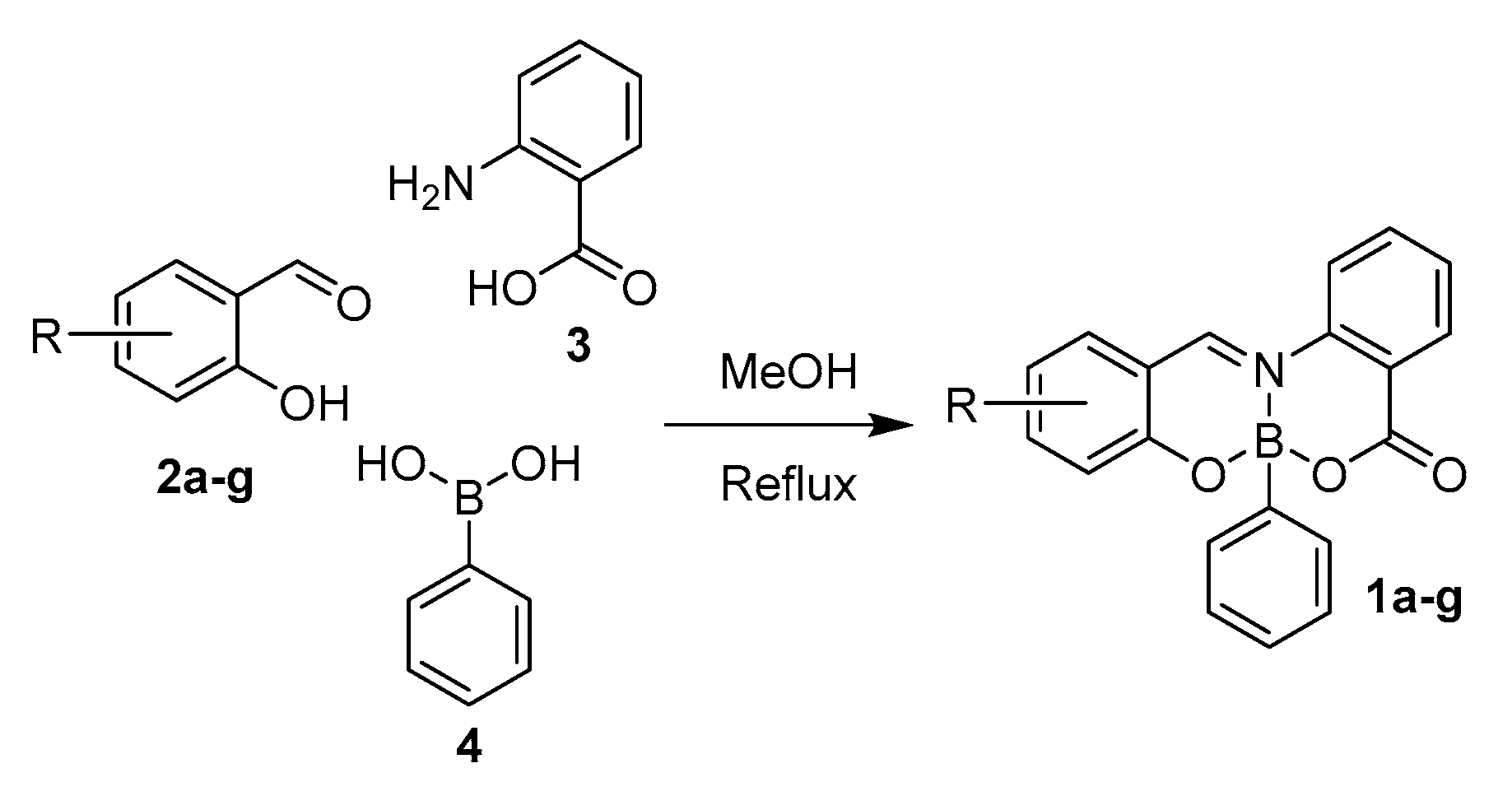
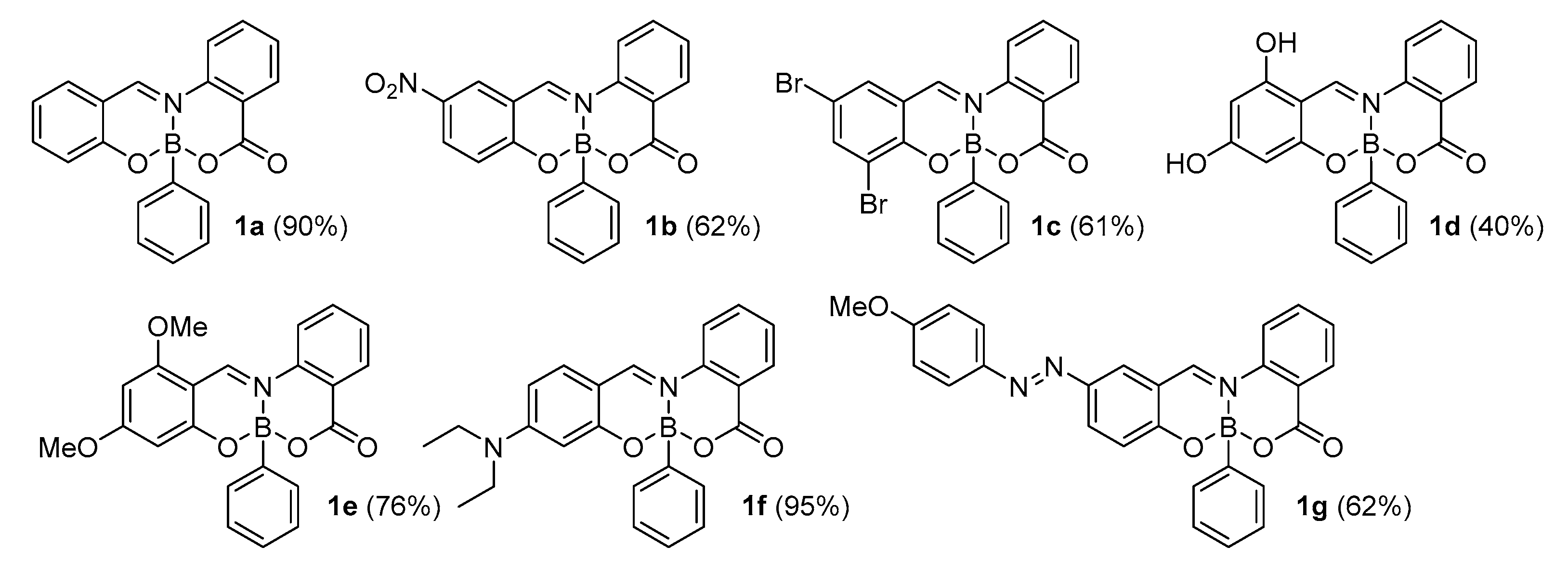
2.1. Synthesis
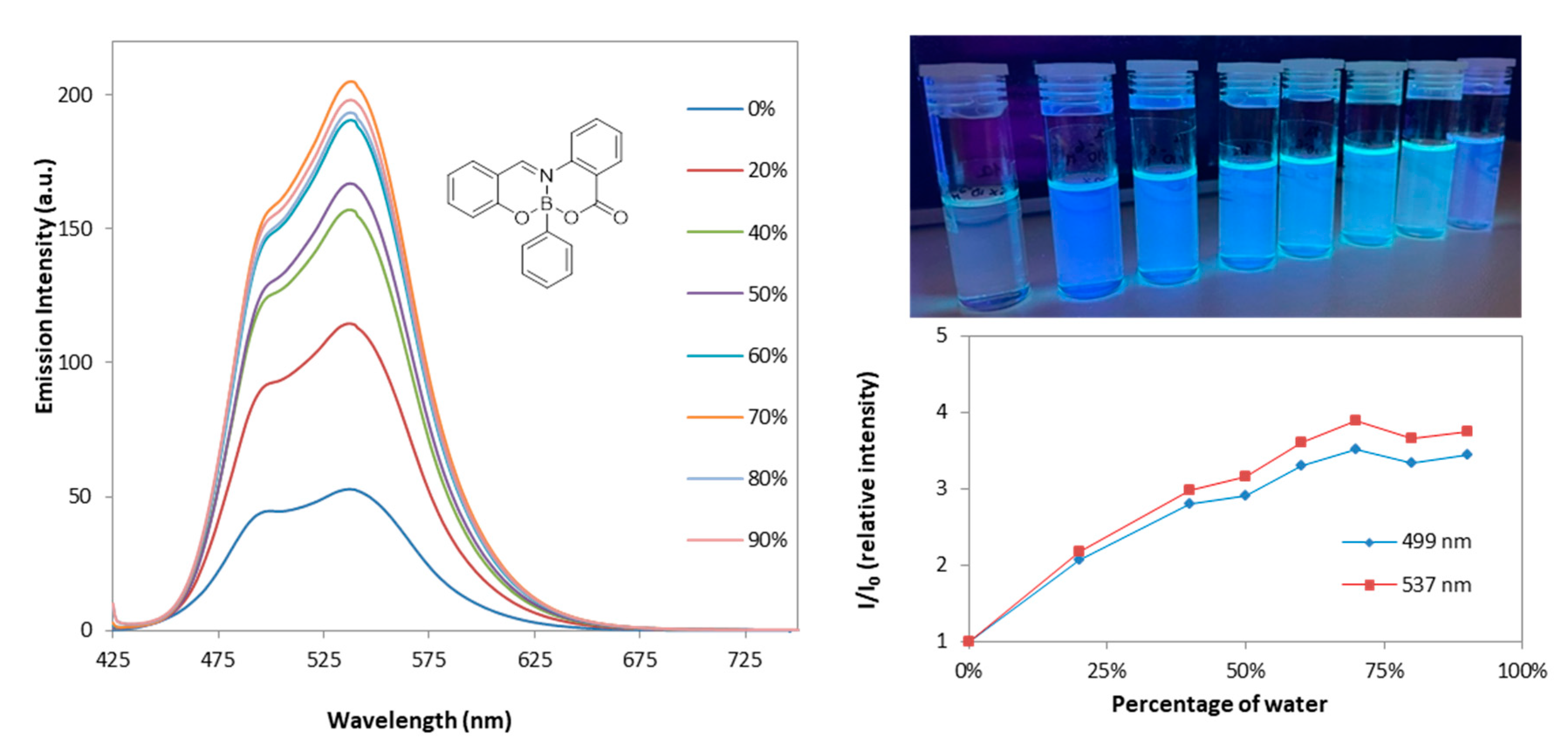
2.2. Photophysical Properties
2.3. Crystal Structures
3. Materials and Methods
3.1. 7-Phenyl-5H,7H-7λ4,14λ4-benzo[d]benzo[5,6][1,3,2]oxazaborinino[2,3-b][1,3,2]oxazaborinin-5-one 1a
3.2. 11-Nitro-7-phenyl-5H,7H-7λ4,14λ4-benzo[d]benzo[5,6][1,3,2]oxazaborinino[2,3-b][1,3,2]oxazaborinin-5-one 1b
3.3. 9,11-Dibromo-7-phenyl-5H,7H-7λ4,14λ4-benzo[d]benzo[5,6][1,3,2]oxazaborinino[2,3-b][1,3,2]oxazaborinin-5-one 1c
3.4. 10,12-Dihydroxy-7-phenyl-5H,7H-7λ4,14λ4-benzo[d]benzo[5,6][1,3,2]oxazaborinino[2,3-b][1,3,2]oxazaborinin-5-one 1d
3.5. 10,12-Dimethoxy-7-phenyl-5H,7H-7λ4,14λ4-benzo[d]benzo[5,6][1,3,2]oxazaborinino[2,3-b][1,3,2]oxazaborinin-5-one 1e
3.6. 10-(Diethylamino)-7-phenyl-5H,7H-7λ4,14λ4-benzo[d]benzo[5,6][1,3,2]oxazaborinino[2,3-b][1,3,2]oxazaborinin-5-one 1f
3.7. (E)-11-[(4-Methoxyphenyl)diazenyl]-7-phenyl-5H,7H-7λ4,14λ4-benzo[d]benzo[5,6][1,3,2]oxazaborinino[2,3-b][1,3,2]oxazaborinin-5-one 1g
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frath, D.; Massue, J.; Ulrich, G.; Ziessel, R. Luminescent materials: Locking p-conjugated and heterocyclic ligands with Boron(III). Angew. Chem. Int. Ed. 2014, 53, 2290–2310. [Google Scholar] [CrossRef]
- Loudet, A.; Burgess, K. BODIPY dyes and their derivatives: Syntheses and spectroscopic properties. Chem Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef] [PubMed]
- Guieu, S.; Pinto, J.; Silva, V.L.M.; Rocha, J.; Silva, A.M.S. Synthesis, post-modification and fluorescence properties of boron-diketonate complexes. Eur. J. Org. Chem. 2015, 2015, 3423–3426. [Google Scholar] [CrossRef]
- Costa, L.D.; Guieu, S.; Rocha, J.; Silva, A.M.S.; Tomé, A.C. Porphyrin-boron diketonate dyads. New J. Chem. 2017, 41, 2186–2192. [Google Scholar] [CrossRef]
- Vaz, P.A.A.M.; Rocha, J.; Silva, A.M.S.; Guieu, S. Difluoroborate complexes based on 2′-hydroxyphenones as solid-state fluorophores. Dyes Pigments 2021, 184, 108720. [Google Scholar] [CrossRef]
- Yoshii, R.; Nagai, A.; Tanaka, K.; Chujo, Y. Highly emissive boron ketoiminate derivatives as a new class of Aggregation-Induced Emission fluorophores. Chem. Eur. J. 2013, 19, 4506–4512. [Google Scholar] [CrossRef] [PubMed]
- Frath, D.; Azizi, S.; Ulrich, G.; Retailleau, P.; Ziessel, R. Facile synthesis of highly fluorescent boranil complexes. Org. Lett. 2011, 13, 3414–3417. [Google Scholar] [CrossRef]
- Guieu, S.; Cardona, F.; Rocha, J.; Silva, A.M.S. Luminescent bi-metallic fluoroborate derivatives of bulky salen ligands. New J. Chem. 2014, 38, 5411–5414. [Google Scholar] [CrossRef]
- Cardona, F.; Rocha, J.; Silva, A.M.S.; Guieu, S. Δ1-Pyrroline based boranyls: Synthesis, crystal structures and luminescent properties. Dyes Pigments 2014, 111, 16–20. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef]
- Vaz, P.A.A.M.; Rocha, J.; Silva, A.M.S.; Guieu, S. Aggregation-induced emission enhancement of chiral boranils. New J. Chem. 2018, 42, 18166–18171. [Google Scholar] [CrossRef]
- Duan, W.; Liu, Q.; Huo, Y.; Cui, J.; Gong, S.; Liu, Z. AIE-active boron complexes based on benzothiazole–hydrazone chelates. Org. Biomol. Chem. 2018, 16, 4977–4984. [Google Scholar] [CrossRef] [PubMed]
- Potopnyk, M.A.; Lytvyn, R.; Danyliv, Y.; Ceborska, M.; Bezvikonnyi, O.; Volyniuk, D.; Gražulevičius, J.V. N,O π-Conjugated 4-substituted 1,3-thiazole BF2 complexes: Synthesis and photophysical properties. J. Org. Chem. 2018, 83, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Xu, D.; Wang, Y.; Zhang, C.; Yang, Y.; Liu, X.; Han, A.; Wang, Y. Aggregation-induced emission and mechanofluorochromism of tetraphenylbutadiene modified β-ketoiminate boron complexes. Dyes Pigments 2018, 150, 165–173. [Google Scholar] [CrossRef]
- Zhao, N.; Ma, C.; Yang, W.; Yin, W.; Wei, J.; Li, N. Facile construction of boranil complexes with aggregation-induced emission characteristics and their specific lipid droplet imaging applications. Chem. Commun. 2019, 55, 8494–8497. [Google Scholar] [CrossRef]
- Fang, G.; Wang, H.; Bian, Z.; Sun, J.; Liu, A.; Fang, H.; Liu, B.; Yao, Q.; Wu, Z. Recent development of boronic acid-based fluorescent sensors. RSC Adv. 2018, 8, 29400–29427. [Google Scholar] [CrossRef]
- Zhang, H.; Huo, C.; Ye, K.; Zhang, P.; Tian, W.; Wang, Y. Synthesis, structures, and luminescent properties of phenol-pyridyl boron complexes. Inorg. Chem. 2006, 45, 2788–2794. [Google Scholar] [CrossRef]
- Alcaide, M.M.; Santos, F.M.F.; Pais, V.F.; Carvalho, J.I.; Collado, D.; Pérez-Inestrosa, E.; Arteaga, J.F.; Boscá, F.; Gois, P.M.P.; Pischel, U. Electronic and functional scope of boronic acid derived salicylidenehydrazone (BASHY) complexes as fluorescent dyes. J. Org. Chem. 2017, 82, 7151–7158. [Google Scholar] [CrossRef]
- Jiménez, V.G.; Santos, F.M.F.; Castro-Fernández, S.; Cuerva, J.M.; Gois, P.M.P.; Pischel, U.; Campaña, A.G. Circularly polarized luminescence of boronic acid-derived salicylidenehydrazone complexes containing chiral boron as stereogenic unit. J. Org. Chem. 2018, 83, 14057–14062. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, S.; Tan, J.; Zhang, X. Unique fluorescence of boronic acid derived salicylidenehydrazone complexes with two perpendicular ICT: Solvent effect on PET process. Dyes Pigments 2018, 155, 186–193. [Google Scholar] [CrossRef]
- Christou, V.; Watkins, S.E.; McCann, R.; Redshaw, C.; Elsegood, M.R.J. Synthesis, structures and luminescent behaviour of tridentate salicylaldiminato-type borate complexes. Inorg. Chim. Acta 2010, 363, 1173–1178. [Google Scholar] [CrossRef]
- Montalbano, F.; Candeias, N.R.; Veiros, L.F.; André, V.; Duarte, M.T.; Bronze, M.R.; Moreira, R.; Gois, P.M.P. Four-component assembly of chiral N-B heterocycles with a natural product-like framework. Org. Lett. 2012, 14, 988–991. [Google Scholar] [CrossRef] [PubMed]
- Montalbano, F.; Cal, P.M.S.D.; Carvalho, M.A.B.R.; Gonçalves, L.M.; Lucas, S.D.; Guedes, R.C.; Veiros, L.F.; Moreira, R.; Gois, P.M.P. Discovery of new heterocycles with activity against human neutrophile elastase based on a boron promoted one-pot assembly reaction. Org. Biomol. Chem. 2013, 11, 4465–4472. [Google Scholar] [CrossRef] [PubMed]
- Adib, M.; Sheikhi, E.; Bijanzadeh, H.R.; Zhu, L.-G. Microwave-assisted reaction between 2-aminobenzoic acids, 2-hydroxybenzaldehydes, and arylboronic acids: A one-pot three-component synthesis of bridgehead bicyclo[4.4.0]boron heterocycles. Tetrahedron 2012, 68, 3377–3383. [Google Scholar] [CrossRef]
- Santos, F.M.F.; Rosa, J.N.; Candeias, N.R.; Parente Carvalho, C.; Matos, A.I.; Ventura, A.E.; Florindo, H.F.; Silva, L.C.; Pischel, U.; Gois, P.M.P. A three-component assembly promoted by boronic acids delivers a modular fluorophore platform (BASHY dyes). Chem. Eur. J. 2016, 22, 1631–1637. [Google Scholar] [CrossRef]
- Crosby, G.A.; Demas, J.N. Measurement of photoluminescence quantum yields. Review. J. Phys. Chem. 1971, 75, 991–1024. [Google Scholar] [CrossRef]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc. Perkin Trans. 2 1987, 12, 1–19. [Google Scholar] [CrossRef]
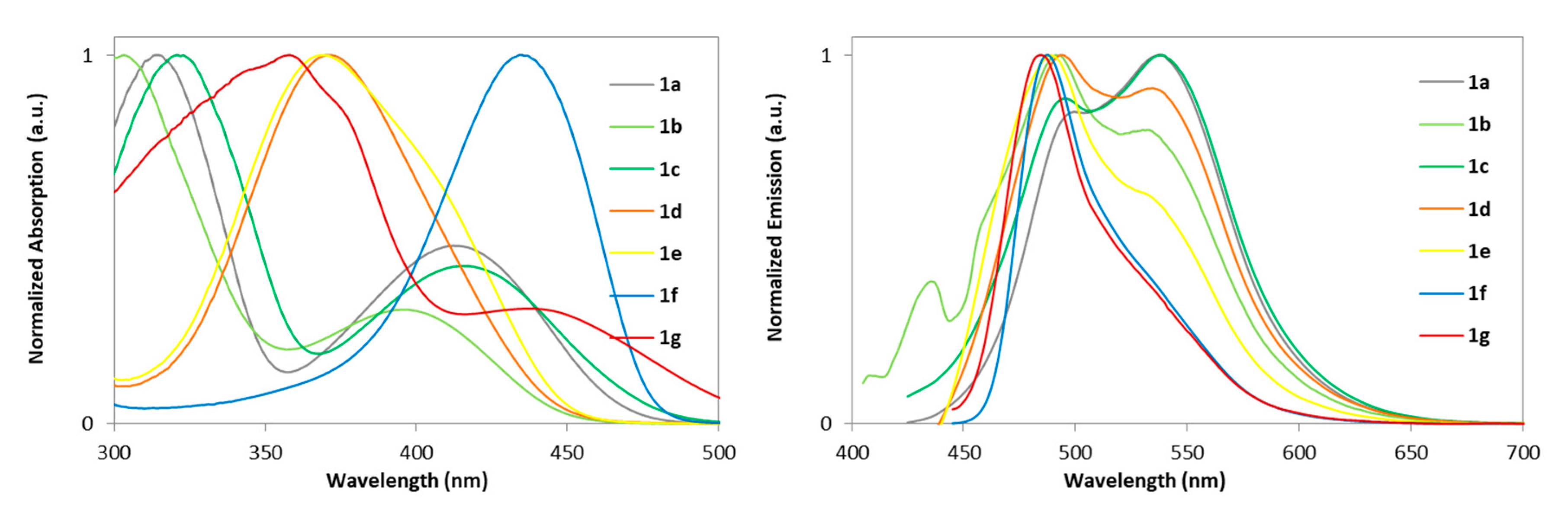

| Complex | THF Solution | |||
|---|---|---|---|---|
| λmax abs | Log ε | λmax em1 | Φ2 (%) | |
| 1a | 412 | 3.92 | 537 | 0.7 |
| 1b | 396 | 3.90 | 491 | 0.2 |
| 1c | 415 | 3.80 | 538 | 0.4 |
| 1d | 371 | 4.44 | 494 | 0.5 |
| 1e | 368 | 4.43 | 489 | 0.3 |
| 1f | 434 | 4.72 | 487 | 6.0 |
| 1g | 445 | 3.94 | 480 | 0.1 |
Sample Availability: Samples of the compounds 1a–g are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guieu, S.; Esteves, C.I.C.; Rocha, J.; Silva, A.M.S. Multicomponent Synthesis of Luminescent Iminoboronates. Molecules 2020, 25, 6039. https://doi.org/10.3390/molecules25246039
Guieu S, Esteves CIC, Rocha J, Silva AMS. Multicomponent Synthesis of Luminescent Iminoboronates. Molecules. 2020; 25(24):6039. https://doi.org/10.3390/molecules25246039
Chicago/Turabian StyleGuieu, Samuel, Cátia I. C. Esteves, João Rocha, and Artur M. S. Silva. 2020. "Multicomponent Synthesis of Luminescent Iminoboronates" Molecules 25, no. 24: 6039. https://doi.org/10.3390/molecules25246039
APA StyleGuieu, S., Esteves, C. I. C., Rocha, J., & Silva, A. M. S. (2020). Multicomponent Synthesis of Luminescent Iminoboronates. Molecules, 25(24), 6039. https://doi.org/10.3390/molecules25246039