A Compressive Review about Taxol®: History and Future Challenges
Abstract
1. Introduction
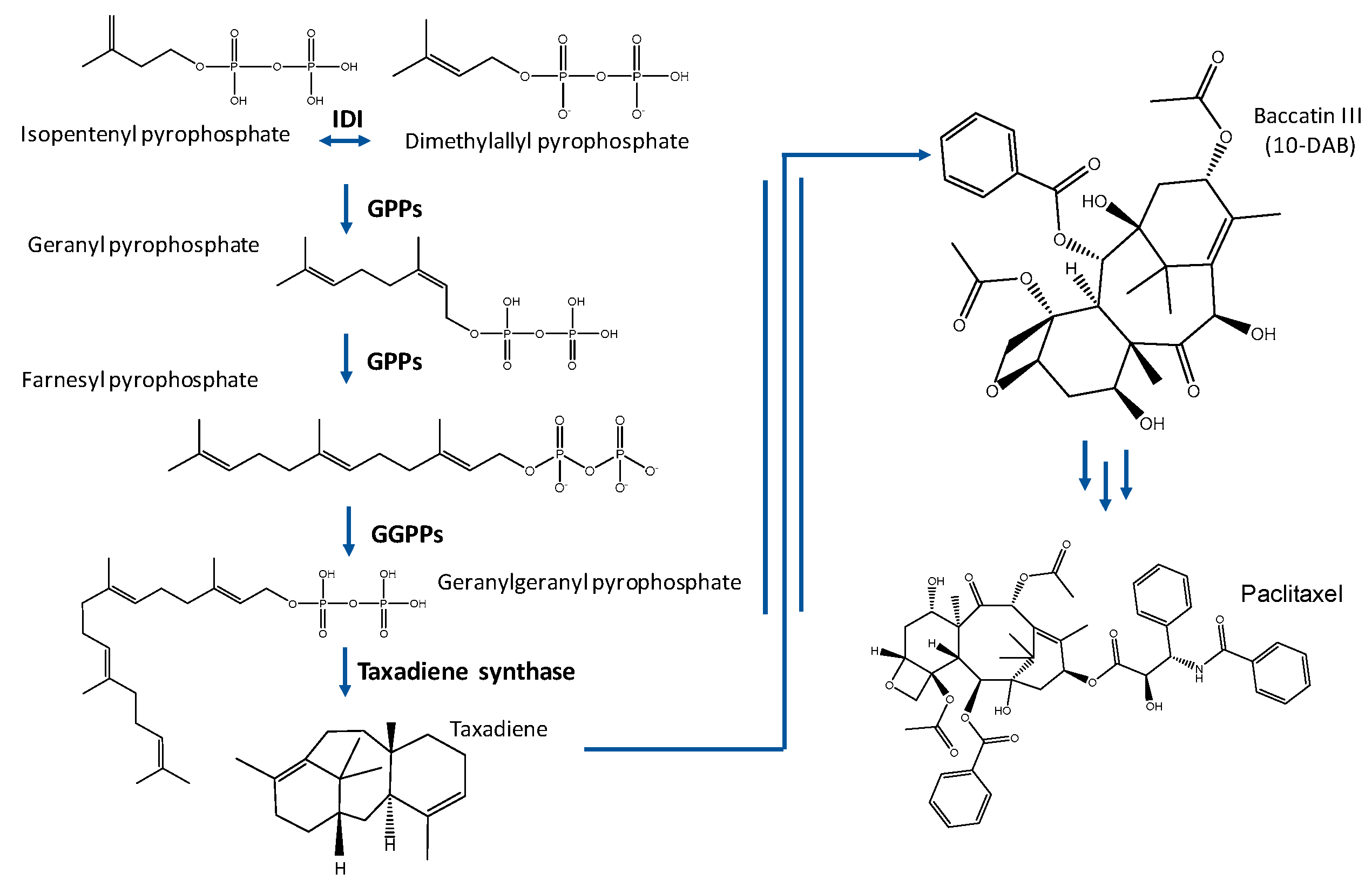
1.1. Taxol® Biosynthesis
1.2. Taxol® Production
1.3. Taxol®: Formulations and Bioavailability
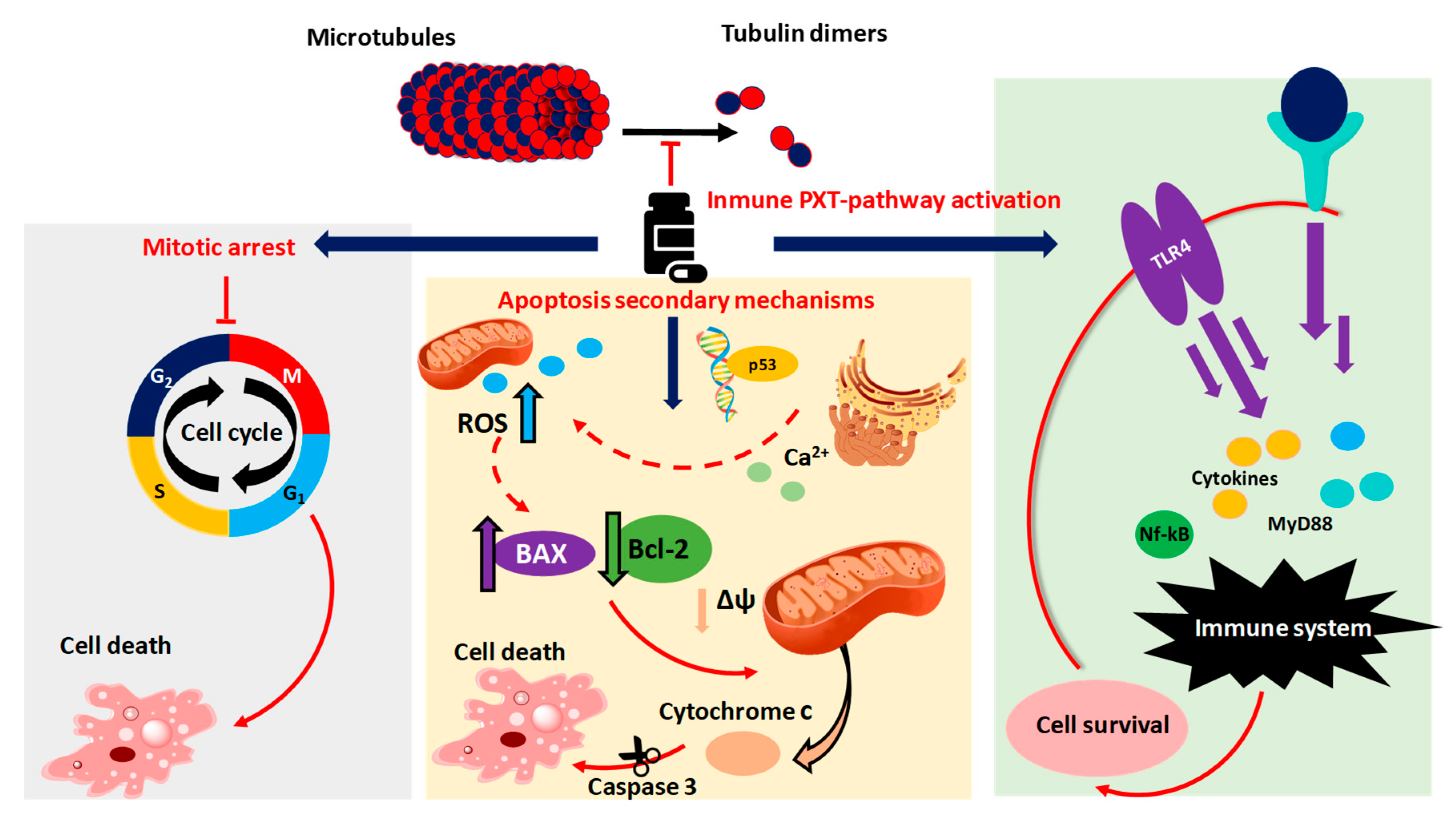
1.4. Taxol® Antitumoral Mechanism
2. The Fight against Drug Resistance
3. RNA-Based Therapies
4. Conclusions and Future Challenges
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PTX | Paclitaxel |
| NCI | National Cancer Institute |
| T. brevifolia | Taxus brevifolia |
| BMS | Bristol-Myers Squibb |
| FDA | Food and Drug Administration |
| EMA | European Medicines Agency |
| NSCLC | Non-small-cell lung carcinoma |
| PDAC | Pancreatic ductal adenocarcinoma |
| MVA | Mevalonate |
| MEP | methylerythritol phosphate |
| IPP | Isopentenyl pyrophosphate |
| DMAPP | Dimethylalyl pyrophosphate |
| taxadiene | Taxa-4(5),11(12)-diene |
| IDI | Isopentenyldiphosphate isomerase |
| GPPS | Geranylpyrophosphate synthase |
| GGPPS | Geranylgeranylpyrophosphate synthase |
| 10-DAB | 10-deacetyl baccatin III |
| E. coli | Escherichia coli |
| S. cerevisiae | Sacharomyces cerevisiae |
| B. subtillis | Bacillus subtilis |
| CrEL | Cremophor EL |
| MTD | Maximum authorized dose |
| ROS | Reactive-oxygen species |
| GSH | Glutathione |
| PEG | polyethylenglycoly |
| AUC | Area under the concentration–time curve |
| P-gp | P-glycoprotein |
| mPEG-PDLLA | Monomethoxy-poly (ethylene glycol)-block-poly(d,l-lactide) |
| PVP-b-PNIPAAM | poly-(vinylpyrrolidone)-b–poly-(N-isopropyl acryl-amide) |
| WHO | Word Health Organization |
| MTA | Microtubule Targeting Agent |
| ER | Endoplasmic reticulum |
| Bcl-2 | B-cell Leukemia 2 |
| BAX | Bcl-2-associated X protein |
| MMP | Mitochondrial membrane potential |
| ATG5 | Autophagy protein 5 |
| TLR4 | Toll-like receptor 4 |
| PAMPs | Pathogen-associated molecular patterns |
| HIF-1 | Hypoxia-inducible factor 1 |
| KRT17 | keratin 17 |
| FNDC5 | Fibronectin type III domain-containing protein 5 |
| MDR1 | Multidrug resistance-associated protein 1 |
| ABC | ATP-binding cassette |
| RTK | Receptor tyrosine kinase |
| TKIs | Inhibitors of receptor tyrosine kinases |
| MUC-1 | Mucin 1 |
| PFL | Penfluridol |
| FoxM1 | Forkhead protein 1 |
| PHB1 | Prohibitin 1 |
| SSTA | Somatostatin analogue |
| SSTR | Somatostatin receptor |
| MAPK | Mitogen-Activated Protein Kinase |
| LdA | Lactate dehydrogenase |
| Pdk2 | Pyruvate dehydrogenase kinase 2 |
| PN | Peripheral neuropathy (PN) |
| lncRNA | Long noncoding RNA |
| EGOT | Eosinophil granule ontogeny transcript |
| FER1L4 | Fer-1-like family member 4 |
| LINC-PINT | lncRNA intergenic non-protein-coding RNA p53-induced transcript |
| UCA1 | Urothelial Carcinoma-Associated 1 |
| ABCB1 | ATP Binding Cassette Subfamily B Member |
| LINC01118 | Long Intergenic Non-Coding RNA 1118 |
| lncRNA SDHAP1 | LnRNA succinate dehydrogenase complex flavoprotein subunit A pseudogene 1 |
| EIF4G2 | Eukaryotic translation initiation factor 4 gamma 2 |
| CCAT1 | Colon cancer-associated transcript 1 |
| FSCN1 | Fascin1 |
| CAAs | Cancer-associated adipocytes |
| CAFs | Cancer-associated fibroblasts |
| (CTSL | Cathepsin L |
| SIK2 | Salt-inducible kinase 2 |
| IKBKB | IκB kinase β |
| E2F5 | E2F transcription factor 5 |
| MCT1 | Monocarboxylic acid solute transporter 1 |
| KEAP1 | Kelch-like ECH-associated protein 1 |
| ITGB1 | Upregulation of integrin beta-1 |
| ITPR1 | Inositol 1,4,5-Trisphosphate Receptor Type 1 |
References
- Wang, Y.; Tang, K. A new endophytic Taxol- and Baccatin III-producing Fungus isolated from Taxus Chinensis Var. Mairei. Afr. J. Biotechnol. 2011, 10, 16379–16386. [Google Scholar] [CrossRef]
- Stahlhut, R.; Park, G.; Petersen, R.; Ma, W.; Hylands, P. The occurrence of the anti-cancer diterpene Taxol in Podocarpus Gracilior Pilger (Podocarpaceae). Biochem. Syst. Ecol. 1999, 27, 613–622. [Google Scholar] [CrossRef]
- Service, R.F. Hazel trees offer new source of cancer drug. Science 2000, 288, 27–28. [Google Scholar] [CrossRef] [PubMed]
- Arnst, J. When Taxol met Tubulin. J. Biol. Chem. 2020, 295, 13994–13995. [Google Scholar] [CrossRef]
- Walsh, V.; Goodman, J. From Taxol to Taxol®: The changing identities and ownership of an anti-cancer drug. Med. Anthr. Cross Cult. Stud. Health Illn. 2002, 21, 307–336. [Google Scholar] [CrossRef]
- McPhail, A.T.; Taylor, H.L.; Wall, M.E.; Coggon, P.; Wani, M.C. Plant antitumor agents. VI. The isolation and structure of Taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 1971, 243, 2325–2327. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Yang, Z.; Llu, J.J.; Ueno, H.; Nantermet, P.G.; Guy, R.K.; Claiborne, C.F.; Renaud, J.; Couladouros, E.A.; Paulvannan, K.; et al. Total synthesis of Taxol. Nature 1994, 367, 630–634. [Google Scholar] [CrossRef]
- Sofias, A.M.; Dunne, M.; Storm, G.; Allen, C. The battle of “Nano” Paclitaxel. Adv. Drug Deliv. Rev. 2017, 122, 20–30. [Google Scholar] [CrossRef]
- Berenson, A. Hope, at $4,200 a Dose. The New York Times, 1 October 2006. [Google Scholar]
- Weaver, B.A. How Taxol/Paclitaxel kills cancer cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef]
- Singla, A.K.; Garg, A.; Aggarwal, D. Paclitaxel and its formulations. Int. J. Pharm. 2002, 235, 179–192. [Google Scholar] [CrossRef]
- Barkat, M.A.; Beg, S.; Pottoo, F.H.; Ahmad, F.J. Nanopaclitaxel therapy: An evidence based review on the battle for next-generation formulation challenges. Nanomedicine 2019, 14, 1323–1341. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, H.; Hsiao, C.H.; Chow, D.S.L.; Koay, E.J.; Kang, Y.; Wen, X.; Huang, Q.; Ma, Y.; Bankson, J.A.; et al. Simultaneous inhibition of hedgehog signaling and tumor proliferation remodels stroma and enhances pancreatic cancer therapy. Biomaterials 2018, 159, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Kocher, H.M.; Basu, B.; Froeling, F.E.M.; Sarker, D.; Slater, S.; Carlin, D.; de Souza, N.M.; De Paepe, K.N.; Goulart, M.R.; Hughes, C.; et al. Phase I clinical trial repurposing all-trans retinoic acid as a stromal targeting agent for pancreatic cancer. Nat. Commun. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Wall, M.E. Camptothecin and Taxol: Discovery to clinic. Med. Res. Rev. 1998, 18, 299–314. [Google Scholar] [CrossRef]
- Walsh, V.; Goodman, J. The billion dollar molecule: Taxol in historical and theoretical perspective. CLIO Med. 2002, 66, 245–267. [Google Scholar] [CrossRef]
- Bian, G.; Deng, Z.; Liu, T. Strategies for terpenoid overproduction and new terpenoid discovery. Curr. Opin. Biotechnol. 2017, 48, 234–241. [Google Scholar] [CrossRef]
- Erb, T.J.; Evans, B.S.; Cho, K.; Warlick, B.P.; Sriram, J.; Wood, B.M.; Imker, H.J.; Sweedler, J.V.; Tabita, F.R.; Gerlt, J.A. A RubisCO like protein links SAM metabolism with Isoprenoid Biosynthesis. Nat. Chem. Biol. 2012, 8, 926–932. [Google Scholar] [CrossRef]
- Bach, T.J.; Boronat, A.; Campos, N.; Ferrer, A.; Vollack, K.U. Mevalonate biosynthesis in plants. Crit. Rev. Biochem. Mol. Biol. 1999, 34, 107–122. [Google Scholar] [CrossRef]
- Rohmer, M.; Seemann, M.; Horbach, S.; Bringer-Meyer, S.; Sahm, H. Glyceraldehyde 3-Phosphate and Pyruvate as precursors of Isoprenic units in an alternative non-mevalonate pathway for Terpenoid biosynthesis. J. Am. Chem. Soc. 1996, 118, 2564–2566. [Google Scholar] [CrossRef]
- Rohmer, M.; Knani, M.; Simonin, P.; Sutter, B.; Sahm, H. Isoprenoid biosynthesis in bacteria: A novel pathway for the early steps leading to Isopentenyl Diphosphate. Biochem. J. 1993, 295, 517–524. [Google Scholar] [CrossRef]
- Rodrı, M. Elucidation of the Methylerythritol Phosphate pathway for Isoprenoid Biosynthesis in bacteria and Plastids. Plant Physiol. 2002, 130, 1079–1089. [Google Scholar] [CrossRef]
- Chang, W.; Song, H.; Liu, H.; Pinghua, L. Current development in Isoprenoid Biosynthesis and regulation. Curr. Opin. Chem. Biol. 2014, 17, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Kaspera, R.; Croteau, R. Cytochrome P450 Oxygenases of Taxol Biosynthesis. Phytochem. Rev. 2006, 5, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.C.; Gong, T.; Zhu, P. Advances in exploring alternative Taxol sources. RSC Adv. 2016, 6, 48800–48809. [Google Scholar] [CrossRef]
- Sabzehzari, M.; Zeinali, M.; Naghavi, M.R. Alternative sources and metabolic engineering of Taxol: Advances and future perspectives. Biotechnol. Adv. 2020, 43, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hezari, M.; Croteau, R. Taxol Biosynthesis: An update. Planta Med. 1997, 63, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, S.; Chen, X.; Liu, M.; Mao, C.; Fang, X. Overexpression of MiR-203 sensitizes Paclitaxel (Taxol)-resistant colorectal cancer cells through targeting the salt-inducible Kinase 2 (SIK2). Tumor Biol. 2016, 37, 12231–12239. [Google Scholar] [CrossRef]
- Baloglu, E.; Kingston, D.G.I. A new Semisynthesis of Paclitaxel from Baccatin III. J. Nat. Prod. 1999, 62, 1068–1071. [Google Scholar] [CrossRef]
- Li, D.; Fu, D.; Zhang, Y.; Ma, X.; Gao, L.; Wang, X.; Zhou, D.; Zhao, K. Isolation, purification, and identification of Taxol and related Taxanes from Taxol-producing Fungus Aspergillus Niger Subsp. Taxi. J. Microbiol. Biotechnol. 2017, 27, 1379–1385. [Google Scholar] [CrossRef]
- Liu, X.; Ding, W.; Jiang, H. Engineering microbial cell factories for the production of plant natural products: From design principles to industrial-scale production. Microb. Cell Fact. 2017, 16, 1–9. [Google Scholar] [CrossRef]
- Du, J.; Shao, Z.; Zhao, H. Engineering microbial factories for synthesis of value-added products. J. Ind. Microbiol. Biotechnol. 2011, 38, 873–890. [Google Scholar] [CrossRef] [PubMed]
- Ajikumar, P.K.; Xiao, W.-H.; Tyo, K.E.J.; Wang, Y.; Stephanopoulos, F.G. Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli. Science 2010, 330, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Engels, B.; Dahm, P.; Jennewein, S. Metabolic engineering of Taxadiene Biosynthesis in yeast as a first step towards Taxol (Paclitaxel) production. Metab. Eng. 2008, 10, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.Z.; Yan, H.F.; Li, L.F.; Zhai, F.; Shang, L.Q.; Yin, Z.; Yuan, Y.J. Biosynthesis of Taxadiene in Saccharomyces cerevisiae: Selection of Geranylgeranyl Diphosphate Synthase directed by a computer-aided docking strategy. PLoS ONE 2014, 9, e109348. [Google Scholar] [CrossRef]
- Abdallah, I.I.; Pramastya, H.; Van Merkerk, R.; Sukrasno; Quax, W.J. Metabolic engineering of Bacillus subtilis toward Taxadiene Biosynthesis as the first committed step for Taxol production. Front. Microbiol. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Walters, B.; Giaw, C.; Sagliani, K.; Shankar, S.; Stephanopoulos, G. Overcoming heterologous protein interdependency to optimize P450-mediated Taxol precursor synthesis in Escherichia coli. Proc. Natl. Acad. Sci. USA 2016, 113, 3209–3214. [Google Scholar] [CrossRef]
- Yan, L.; Zhao, H.; Zhao, X.; Xu, X.; Di, Y.; Jiang, C.; Shi, J.; Shao, D. Production of Bioproducts by Endophytic Fungi: Chemical ecology, biotechnological applications, bottlenecks, and solutions. Appl. Microbiol. Biotechnol. 2018, 102, 6279–6298. [Google Scholar] [CrossRef]
- Field, K.J.; Pressel, S.; Duckett, J.G.; Rimington, W.R.; Bidartondo, M.I. Symbiotic options for the conquest of land. Trends Ecol. Evol. 2015, 30, 477–486. [Google Scholar] [CrossRef]
- Das, A.; Rahman, M.I.; Ferdous, A.S.; Amin, A.; Rahman, M.M.; Nahar, N.; Uddin, M.A.; Islam, M.R.; Khan, H. An Endophytic Basidiomycete, Grammothele Lineata, isolated from Corchorus Olitorius, PRODUCES Paclitaxel that shows cytotoxicity. PLoS ONE 2017, 12, e0178612. [Google Scholar] [CrossRef]
- Gill, H.; Vasundhara, M. Isolation of Taxol producing Endophytic Fungus Alternaria Brassicicola from Non-Taxus medicinal plant Terminalia arjuna. World J. Microbiol. Biotechnol. 2019, 35, 1–8. [Google Scholar] [CrossRef]
- El-Sayed, A.S.A.; Ali, D.M.I.; Yassin, M.A.; Zayed, R.A.; Ali, G.S. Sterol Inhibitor “Fluconazole” enhance the Taxol yield and molecular expression of its encoding genes cluster from Aspergillus flavipes. Process Biochem. 2019, 76, 55–67. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, T.; Chen, T.; Xie, C.; Zhang, Q. Sesquiterpenoids isolated from the flower of Inula Japonica as potential antitumor leads for intervention of Paclitaxel-resistant non-small-cell lung cancer. Bioorg. Chem. 2020, 101, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Naik, B.S. Developments in Taxol production through Endophytic Fungal biotechnology: A review. Orient. Pharm. Exp. Med. 2018, 19, 1–13. [Google Scholar] [CrossRef]
- Zhao, K.; Yu, L.; Jin, Y.; Ma, X.; Liu, D.; Wang, X.; Wang, X. Advances and prospects of Taxol Biosynthesis by Endophytic Fungi. Shengwu Gongcheng Xuebao/Chin. J. Biotechnol. 2016, 32, 1038–1051. [Google Scholar] [CrossRef]
- El-Sayed, A.S.A.; Fathalla, M.; Yassin, M.A.; Zein, N.; Morsy, S.; Sitohy, M.; Sitohy, B. Conjugation of Aspergillus Flavipes Taxol with Porphyrin increases the Anticancer Activity of Taxol and Ameliorates its Cytotoxic effects. Molecules 2020, 25, 263. [Google Scholar] [CrossRef]
- Qiao, W.; Ling, F.; Yu, L.; Huang, Y.; Wang, T. Enhancing Taxol production in a novel Endophytic Fungus, Aspergillus aculeatinus Tax-6, isolated from Taxus chinensis Var. Mairei. Fungal Biol. 2017, 121, 1037–1044. [Google Scholar] [CrossRef]
- El-maali, N.A.; Mohrram, A.M.; El-kashef, H.; Gamal, K. Novel resources of Taxol from Endophytic and Entomopathogenic Fungi: Isolation, characterization and LC-triple mass spectrometric quantification. Talanta 2018, 190, 466–474. [Google Scholar] [CrossRef]
- Suresh, G.; Kokila, D.; Suresh, T.C.; Kumaran, S.; Velmurugan, P.; Vedhanayakisri, K.A.; Sivakumar, S.; Ravi, A.V. Mycosynthesis of anticancer drug Taxol by Aspergillus oryzae, an Endophyte of Tarenna asiatica, characterization, and its activity against a human lung cancer cell line. Biocatal. Agric. Biotechnol. 2020, 24, 1–9. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, B.; Thakur, V.; Thakur, A.; Thakur, N.; Pandey, D.; Chand, D. Hyper-Production of Taxol from Aspergillus fumigatus, an Endophytic Fungus isolated from Taxus Sp. of the northern Himalayan region. Biotechnol. Rep. 2019, 24, e00395. [Google Scholar] [CrossRef]
- Lyseng-Williamson, K.A.; Fenton, C. Docetaxel: A Review of its use in metastatic breast cancer. Drugs 2005, 65, 2513–2531. [Google Scholar] [CrossRef]
- Bernabeu, E.; Cagel, M.; Lagomarsino, E.; Moretton, M.; Chiappetta, D.A. Paclitaxel: What has been done and the challenges remain ahead. Int. J. Pharm. 2017, 526, 474–495. [Google Scholar] [CrossRef] [PubMed]
- Gelderblom, H.; Verweij, J.; Nooter, K.; Sparreboom, A. Cremophor EL: The drawbacks and advantages of vehicle selection for drug formulation. Eur. J. Cancer 2001, 37, 1590–1598. [Google Scholar] [CrossRef]
- Choudhury, H.; Gorain, B.; Tekade, R.K.; Pandey, M.; Karmakar, S.; Pal, T.K. Safety against Nephrotoxicity in Paclitaxel treatment: Oral nanocarrier as an effective tool in preclinical evaluation with marked In Vivo antitumor activity. Regul. Toxicol. Pharm. 2017, 91, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Borgå, O.; Henriksson, R.; Bjermo, H.; Lilienberg, E.; Heldring, N.; Loman, N. Maximum tolerated dose and pharmacokinetics of Paclitaxel micellar in patients with recurrent malignant solid tumours: A dose-escalation study. Adv. Ther. 2019, 36, 1150–1163. [Google Scholar] [CrossRef]
- Yang, N.; Wang, C.; Wang, J.; Wang, Z.; Huang, D.; Yan, M.; Kamran, M.; Liu, Q.; Xu, B.L. Aurora Kinase A stabilizes FOXM1 to enhance Paclitaxel resistance in triple-negative breast cancer. J. Cell. Mol. Med. 2019, 23, 6442–6453. [Google Scholar] [CrossRef]
- Chowdhury, M.R.; Moshikur, R.M.; Wakabayashi, R.; Tahara, Y.; Kamiya, N.; Moniruzzaman, M.; Goto, M. In Vivo biocompatibility, pharmacokinetics, antitumor efficacy, and hypersensitivity evaluation of ionic liquid-mediated Paclitaxel formulations. Int. J. Pharm. 2019, 565, 219–226. [Google Scholar] [CrossRef]
- Chowdhury, M.R.; Moshikur, R.M.; Wakabayashi, R.; Tahara, Y.; Kamiya, N.; Moniruzzaman, M.; Goto, M. Ionic-liquid-based Paclitaxel preparation: A new potential formulation for cancer treatment. Mol. Pharm. 2018, 15, 2484–2488. [Google Scholar] [CrossRef]
- Chung, H.J.; Kim, H.J.; Hong, S.T. Tumor-specific delivery of a Paclitaxel-loading HSA-Haemin nanoparticle for cancer treatment. Nanomed. Nanotechnol. Biol. Med. 2020, 23, 1–11. [Google Scholar] [CrossRef]
- Ye, L.; He, J.; Hu, Z.; Dong, Q.; Wang, H.; Fu, F.; Tian, J. antitumor effect and toxicity of lipusu in rat ovarian cancer xenografts. Food Chem. Toxicol. 2013, 52, 200–206. [Google Scholar] [CrossRef]
- Ma, W.W.; Lam, E.T.; Dy, G.K.; Diamond, J.R.; Zhao, Y.; Bui, L.A.; Fetterly, G.J.; Abramowitz, W.; Harning, R.; Pencheva, P.; et al. A Pharmacokinetic and dose-escalating study of Paclitaxel injection concentrate for nano-dispersion (PICN) Alone and with Carboplatin in patients with advanced solid tumors. J. Clin. Oncol. 2013, 31, 2557. [Google Scholar] [CrossRef]
- Micha, J.P.; Goldstein, B.H.; Birk, C.L.; Rettenmaier, M.A.; Brown, J.V. Abraxane in the treatment of ovarian cancer: The absence of hypersensitivity reactions. Gynecol. Oncol. 2006, 100, 437–438. [Google Scholar] [CrossRef] [PubMed]
- Ingle, S.G.; Pai, R.V.; Monpara, J.D.; Vavia, P.R. Liposils: An effective strategy for stabilizing Paclitaxel loaded liposomes by surface coating with silica. Eur. J. Pharm. Sci. 2018, 122, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Abriata, J.P.; Turatti, R.C.; Luiz, M.T.; Raspantini, G.L.; Tofani, L.B.; do Amaral, R.L.F.; Swiech, K.; Marcato, P.D.; Marchetti, J.M. Development, characterization and biological In Vitro assays of Paclitaxel-loaded PCL polymeric nanoparticles. Mater. Sci. Eng. C 2019, 96, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Fu, S.; Peng, Q.; Han, Y.W.; Xie, J.; Zan, N.; Chen, Y.; Fan, J. Paclitaxel-loaded polymeric nanoparticles combined with Chronomodulated chemotherapy on lung cancer: In Vitro and In Vivo evaluation. Int. J. Pharm. 2017, 516, 313–322. [Google Scholar] [CrossRef]
- Dranitsaris, G.; Yu, B.; Wang, L.; Sun, W.; Zhou, Y.; King, J.; Kaura, S.; Zhang, A.; Yuan, P. Abraxane® versus Taxol® for patients with advanced breast cancer: A prospective time and motion analysis from a chinese health care perspective. J. Oncol. Pharm. Pr. 2016, 22, 205–211. [Google Scholar] [CrossRef]
- Pei, Q.; Hu, X.; Liu, S.; Li, Y.; Xie, Z.; Jing, X. Paclitaxel dimers assembling nanomedicines for treatment of cervix carcinoma. J. Control. Release 2017, 254, 23–33. [Google Scholar] [CrossRef]
- Thomas, F.C.; Taskar, K.; Rudraraju, V.; Goda, S.; Thorsheim, H.R.; Gaasch, J.A.; Palmieri, D.; Steeg, P.S.; Lockman, P.R.; Smith, Q.R. Uptake of ANG1005, a novel Paclitaxel derivative, Through the blood-brain barrier into brain and experimental brain metastases of breast cancer. Pharm. Res. 2009, 26, 2486–2494. [Google Scholar] [CrossRef]
- Régina, A.; Demeule, M.; Ché, C.; Lavallée, I.; Poirier, J.; Gabathuler, R.; Béliveau, R.; Castaigne, J.P. Antitumour activity of ANG1005, a conjugate between Paclitaxel and the new brain delivery vector Angiopep-2. Br. J. Pharm. 2008, 155, 185–197. [Google Scholar] [CrossRef]
- Maya, S.; Kumar, L.G.; Sarmento, B.; Sanoj Rejinold, N.; Menon, D.; Nair, S.V.; Jayakumar, R. Cetuximab Conjugated O-Carboxymethyl Chitosan nanoparticles for targeting EGFR overexpressing cancer cells. Carbohydr. Polym. 2013, 93, 661–669. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.M.; Chen, Y.; Chen, J.T.; Liu, Y. Polysaccharide-based noncovalent assembly for targeted delivery of taxol. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Zhou, L.; Lv, F.; Liu, L.; Shen, G.; Yan, X.; Bazan, G.C.; Wang, S. Cross-linking of Thiolated Paclitaxel–Oligo(p-Phenylene Vinylene) conjugates aggregates inside tumor cells leads to “chemical locks” that increase drug efficacy. Adv. Mater. 2018, 30, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhang, G.; Li, R.; Guan, M.; Wang, X.; Zou, T.; Zhang, Y.; Wang, C.; Shu, C.; Hong, H.; et al. Biodegradable, hydrogen peroxide, and Glutathione dual responsive nanoparticles for potential programmable Paclitaxel release. J. Am. Chem. Soc. 2018, 140, 7373–7376. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Sun, J.; Liu, D.; Sun, B.; Miao, L.; Musetti, S.; Li, J.; Han, X.; Du, Y.; Li, L.; et al. Self-assembled redox dual-responsive prodrug-nanosystem formed by single Thioether-bridged Paclitaxel-fatty acid conjugate for cancer chemotherapy. Nano Lett. 2016, 16, 5401–5408. [Google Scholar] [CrossRef] [PubMed]
- Pei, Q.; Hu, X.; Zhou, J.; Liu, S.; Xie, Z. Glutathione-responsive Paclitaxel Dimer Nanovesicles with high drug content. Biomater. Sci. 2017, 5, 1517–1521. [Google Scholar] [CrossRef] [PubMed]
- Xia, R.; Pei, Q.; Wang, J.; Wang, Z.; Hu, X.; Xie, Z. Redox responsive Paclitaxel dimer for programmed drug release and selectively killing cancer cells. J. Colloid Interface Sci. 2020, 580, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Gillies, E.R.; Goodwin, A.P.; Fréchet, J.M.J. Acetals as PH-sensitive linkages for drug delivery. Bioconjug. Chem. 2004, 15, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Zhou, Q.; Xiang, J.; Liu, F.; Zhou, Z.; Shen, Y. Self-assembly of oxidation-responsive Polyethylene Glycol-Paclitaxel prodrug for cancer chemotherapy. J. Control. Release 2020, 321, 529–539. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhou, X.; Jia, L.; Ma, C.; Song, R.; Deng, Y.; Hu, X.; Sun, W. Acetal-linked Paclitaxel polymeric prodrug based on functionalized MPEG-PCL Diblock polymer for PH-triggered drug delivery. Polymers 2017, 9, 698. [Google Scholar] [CrossRef]
- Huang, D.; Zhuang, Y.; Shen, H.; Yang, F.; Wang, X.; Wu, D. Acetal-linked PEGylated Paclitaxel prodrugs forming free-Paclitaxel-loaded PH-responsive micelles with high drug loading capacity and improved drug delivery. Mater. Sci. Eng. C 2018, 82, 60–68. [Google Scholar] [CrossRef]
- Shu, X.; Zhu, Z.; Cao, D.; Zheng, L.; Wang, F.; Pei, H.; Wen, J.; Yang, J.; Li, D.; Bai, P.; et al. PEG-derivatized Birinapant as a Nanomicellar carrier of Paclitaxel delivery for cancer therapy. Colloids Surf. B Biointerfaces 2019, 182, 1–10. [Google Scholar] [CrossRef]
- Mu, J.; Zhong, H.; Zou, H.; Liu, T.; Yu, N.; Zhang, X.; Xu, Z.; Chen, Z.; Guo, S. Acid-sensitive PEGylated Paclitaxel prodrug nanoparticles for cancer therapy: Effect of PEG length on antitumor efficacy. J. Control. Release 2020, 326, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteom. 2010, 73, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of Exosome-encapsulated Paclitaxel to overcome MDR in cancer cells. Nanomedicine 2016, 12, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Yuan, D.; Deygen, I.; Klyachko, N.L.; Kabanov, A.V.; Batrakova, E.V. Engineering macrophage-derived exosomes for targeted Paclitaxel delivery to pulmonary metastases: In Vitro and In Vivo evaluations. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Britten, C.D.; Baker, S.D.; Denis, L.J.; Johnson, T.; Drengler, R.; Siu, L.L.; Duchin, K.; Kuhn, J.; Rowinsky, E.K. Oral Paclitaxel and concurrent Cyclosporin A: Targeting clinically relevant systemic exposure to paclitaxel. Clin. Cancer Res. 2000, 6, 3459–3468. [Google Scholar] [PubMed]
- Jang, Y.; Ko, M.K.; Park, Y.E.; Hong, J.W.; Lee, I.H.; Chung, H.J.; Chung, H. Effect of Paclitaxel content in the DHP107 oral formulation on oral bioavailability and antitumor activity. J. Drug Deliv. Sci. Technol. 2018, 48, 183–192. [Google Scholar] [CrossRef]
- Pandita, D.; Ahuja, A.; Lather, V.; Benjamin, B.; Dutta, T.; Velpandian, T.; Khar, R.K. Development of Lipid-based nanoparticles for enhancing the oral bioavailability of Paclitaxel. AAPS Pharmscitech 2011, 12, 712–722. [Google Scholar] [CrossRef]
- Lee, E.; Lee, J.; Lee, I.H.; Yu, M.; Kim, H.; Chae, S.Y.; Jon, S. Conjugated Chitosan as a novel platform for oral delivery of Paclitaxel. J. Med. Chem. 2008, 51, 6442–6449. [Google Scholar] [CrossRef]
- Du, X.; Yin, S.; Xu, L.; Ma, J.; Yu, H.; Wang, G.; Li, J. Polylysine and Cysteine functionalized Chitosan Nanoparticle as an efficient platform for oral delivery of Paclitaxel. Carbohydr. Polym. 2020, 229, 1–11. [Google Scholar] [CrossRef]
- Zhang, M.; Asghar, S.; Jin, X.; Hu, Z.; Ping, Q.; Chen, Z.; Shao, F.; Xiao, Y. The enhancing effect of N-Acetylcysteine modified Hyaluronic Acid-Octadecylamine Micelles on the oral absorption of Paclitaxel. Int. J. Biol. Macromol. 2019, 138, 636–647. [Google Scholar] [CrossRef]
- Agrawal, A.K.; Aqil, F.; Jeyabalan, J.; Spencer, W.A.; Beck, J.; Gachuki, B.W.; Alhakeem, S.S.; Oben, K.; Munagala, R.; Bondada, S.; et al. Milk-derived Exosomes for oral delivery of Paclitaxel. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Gursoy, R.N.; Lambert, G.; Benita, S. Enhanced oral absorption of Paclitaxel in a novel self-microemulsifying drug delivery system with or without concomitant use of P-Glycoprotein inhibitors. Pharm. Res. 2004, 21, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Ezrahi, S.; Aserin, A.; Garti, N. Basic principles of drug delivery systems—The Case of Paclitaxel. Adv. Colloid Interface Sci. 2019, 263, 95–130. [Google Scholar] [CrossRef] [PubMed]
- Weidner, L.D.; Fung, K.L.; Kannan, P.; Moen, J.K.; Kumar, J.S.; Mulder, J.; Innis, R.B.; Gottesman, M.M.; Hall, M.D. Tariquidar is an inhibitor and not a substrate of human and mouse P-Glycoprotein. Drug Metab. Dispos. 2016, 44, 275–282. [Google Scholar] [CrossRef]
- Xia, D.; Yu, H.; Tao, J.; Zeng, J.; Zhu, Q.; Zhu, C.; Gan, Y. Supersaturated polymeric micelles for oral Cyclosporine A delivery: The role of Soluplus-Sodium Dodecyl Sulfate complex. Colloids Surf. B Biointerfaces 2016, 141, 301–310. [Google Scholar] [CrossRef]
- El-Araby, M.E.; Omar, A.M.; Khayat, M.T.; Assiri, H.A.; Al-Abd, A.M. Molecular mimics of classic P-Glycoprotein inhibitors as multidrug resistance suppressors and their synergistic effect on Paclitaxel. PLoS ONE 2017, 12, e0168938. [Google Scholar] [CrossRef]
- Chen, T.; Tu, L.; Wang, G.; Qi, N.; Wu, W.; Zhang, W.; Feng, J. Multi-Functional Chitosan Polymeric Micelles as oral Paclitaxel delivery systems for enhanced bioavailability and anti-tumor efficacy. Int. J. Pharm. 2020, 578, 1–10. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Tjulandin, S.; Davidson, N.; Shaw, H.; Desai, N.; Bhar, P.; Hawkins, M.; O’Shaughnessy, J. Phase III trial of nanoparticle albumin-bound Paclitaxel compared with Polyethylated castor oil-based Paclitaxel in women with breast cancer. J. Clin. Oncol. 2005, 23, 7794–7803. [Google Scholar] [CrossRef]
- European Medicines Agency. Assesment Report for Abraxane. 2007. Available online: https://www.ema.europa.eu/en/documents/assessment-report/abraxane-epar-public-assessment-report_en.pdf (accessed on 20 November 2020).
- Park, I.H.; Sohn, J.H.; Kim, S.B.; Lee, K.S.; Chung, J.S.; Lee, S.H.; Kim, T.Y.; Jung, K.H.; Cho, E.K.; Kim, Y.S.; et al. An open-label, randomized, parallel, phase III trial evaluating the efficacy and safety of Polymeric Micelle-formulated Paclitaxel compared to conventional Cremophor EL-based Paclitaxel for recurrent or metastatic HER2-negative breast cancer. Cancer Res. Treat. 2017, 49, 569–577. [Google Scholar] [CrossRef]
- Desai, N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012, 14, 282–295. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, X.E.; Gao, L.L. A clinical study on the premedication of Paclitaxel Liposome in the treatment of solid tumors. Biomed. Pharm. 2009, 63, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Ranade, A.A.; Joshi, D.A.; Phadke, G.K.; Patil, P.P.; Kasbekar, R.B.; Apte, T.G.; Dasare, R.R.; Mengde, S.D.; Parikh, P.M.; Bhattacharyya, G.S.; et al. Clinical and economic implications of the use of nanoparticle Paclitaxel (Nanoxel) in India. Ann. Oncol. 2013, 24, v6–v12. [Google Scholar] [CrossRef] [PubMed]
- Giodini, L.; Re, F.L.; Campagnol, D.; Marangon, E.; Posocco, B.; Dreussi, E.; Toffoli, G. Nanocarriers in cancer clinical practice: A pharmacokinetic issue. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 583–599. [Google Scholar] [CrossRef]
- European Medicines Agency. Apealea Assessment Report. 2018. Available online: https://www.ema.europa.eu/en/documents/assessment-report/apealea-epar-public-assessment-report_en.pdf. (accessed on 20 November 2020).
- U.S. Food and Drug Administration, (FDA). Taxol Approval; U.S. Department of Health and Human Services: Washington, DC, USA, 1998.
- Barbuti, A.M.; Chen, Z.S. Paclitaxel through the ages of anticancer therapy: Exploring its role in chemoresistance and radiation therapy. Cancers 2015, 7, 2360–2371. [Google Scholar] [CrossRef] [PubMed]
- Gornstein, E.; Schwarz, T.L. The Paradox of Paclitaxel neurotoxicity: Mechanisms and unanswered questions. Neuropharmacology 2014, 76, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L. Microtubules as drug receptors: Pharmacological properties of microtubule protein. Ann. N. Y. Acad. Sci. 1975, 253, 213–231. [Google Scholar] [CrossRef]
- Zhang, D.; Kanakkanthara, A. Beyond the Paclitaxel and Vinca Alkaloids: Next generation of plant-derived microtubule-targeting agents with potential anticancer activity. Cancers 2020, 12, 1721. [Google Scholar] [CrossRef]
- Ganguly, A.; Yang, H.; Cabral, F. Paclitaxel dependent cell lines reveal a novel drug activity. Mol. Cancer 2010, 9, 1–9. [Google Scholar] [CrossRef]
- Rieder, C.L.; Medema, R.H. No way out for tumor cells. Cancer Cell 2009, 16, 274–275. [Google Scholar] [CrossRef]
- Ren, X.; Zhao, B.; Chang, H.; Xiao, M.; Wu, Y.; Liu, Y. Paclitaxel Suppresses Proliferation and Induces Apoptosis through regulation of ROS and the AKT/MAPK signaling pathway in canine mammary gland tumor cells. Mol. Med. Rep. 2018, 17, 8289–8299. [Google Scholar] [CrossRef]
- Strobel, T.; Swanson, L.; Korsmeyer, S.; Cannistra, S.A. BAX enhances Paclitaxel-induced Apoptosis through a P53-Independent pathway. Proc. Natl. Acad. Sci. USA 1996, 93, 14094–14099. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yin, L.; Wu, L.; Zhu, Y.; Wang, X. Paclitaxel inhibits proliferation and promotes apoptosis through regulation ROS and endoplasmic reticulum stress in osteosarcoma cell. Mol. Cell. Toxicol. 2020, 16, 377–384. [Google Scholar] [CrossRef]
- Csordás, G.; Hajnóczky, G. SR/ER-Mitochondrial local communication: Calcium and ROS. Biochim. Biophys. Acta 2009, 1787, 1352–1362. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Ferlini, C.; Cicchillitti, L.; Raspaglio, G.; Bartollino, S.; Cimitan, S.; Bertucci, C.; Mozzetti, S.; Gallo, D.; Persico, M.; Fattorusso, C.; et al. Paclitaxel directly binds to Bcl-2 and functionally mimics activity of Nur77. Cancer Res. 2009, 69, 6906–6914. [Google Scholar] [CrossRef]
- Mikuła-Pietrasik, J.; Witucka, A.; Martyna, P.; Uruski, P.; Begier-Krasińska, B.; Niklas, A.; Tykarski, A.; Krzysztof, K. Comprehensive review on how platinum- and Taxane-based chemotherapy of ovarian cancer affects biology of normal cells. Cell. Mol. Life Sci. 2019, 76, 681–697. [Google Scholar] [CrossRef]
- Yang, M.; Wang, B.; Gao, J.; Zhang, Y.; Xu, W.; Tao, L. Spinosad induces programmed cell death involves mitochondrial dysfunction and Cytochrome C release in Spodoptera Frugiperda Sf9 Cells. Chemosphere 2017, 169, 155–161. [Google Scholar] [CrossRef]
- Suh, D.H.; Kim, M.K.; Kim, H.S.; Chung, H.H.; Song, Y.S. Mitochondrial permeability transition pore as a selective target for anti-cancer therapy. Front. Oncol. 2013, 3, 1–11. [Google Scholar] [CrossRef]
- Eom, S.Y.; Hwang, S.H.; Yeom, H.; Lee, M. An ATG5 knockout promotes Paclitaxel resistance in V-Ha-Ras-transformed NIH 3T3 cells. Biochem. Biophys. Res. Commun. 2019, 513, 234–241. [Google Scholar] [CrossRef]
- Bai, Z.; Ding, N.; Ge, J.; Wang, Y.; Wang, L.; Wu, N.; Wei, Q.; Xu, S.; Liu, X.; Zhou, G. Esomeprazole overcomes Paclitaxel-resistance and enhances anticancer effects of Paclitaxel by inducing autophagy in A549/Taxol Cells. Cell Biol. Int. 2020. [Google Scholar] [CrossRef]
- Yu, Y.F.; Hu, P.C.; Wang, Y.; Xu, X.L.; Rushworth, G.M.; Zhang, Z.; Wei, L.; Zhang, J.W. Paclitaxel induces autophagy in gastric cancer BGC823 Cells. Ultrastruct. Pathol. 2017, 41, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, W.; Peng, X.; Xie, J.; Li, H.; Tan, G. Inhibition of Autophagy results in a reversal of Taxol resistance in Nasopharyngeal Carcinoma by enhancing Taxol-induced caspase-dependent Apoptosis. Am. J. Transl. Res. 2017, 9, 1934–1942. [Google Scholar] [PubMed]
- Tan, Q.; Joshua, A.M.; Wang, M.; Bristow, R.G.; Wouters, B.G.; Allen, C.J.; Tannock, I.F. Up-regulation of autophagy is a mechanism of resistance to chemotherapy and can be inhibited by Pantoprazole to increase drug sensitivity. Cancer Chemother. Pharm. 2017, 79, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Vanzo, R.; Bartkova, J.; Merchut-Maya, J.M.; Hall, A.; Bouchal, J.; Dyrskjøt, L.; Frankel, L.B.; Gorgoulis, V.; Maya-Mendoza, A.; Jäättelä, M.; et al. Autophagy role(s) in response to Oncogenes and DNA replication stress. Cell Death Differ. 2020, 27, 1134–1153. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Haskill, J.S.; Mukaida, N.; Matsushima, K.; Ting, J.P.-Y. Identification of tumor-specific Paclitaxel (Taxol) -responsive regulatory elements in the Interleukin-8 Promoter. Mol. Cell. Biol. 1997, 17, 5097–5105. [Google Scholar] [CrossRef] [PubMed]
- Pusztai, L.; Mendoza, T.R.; Reuben, J.M.; Martinez, M.M.; Willey, J.S.; Lara, J.; Syed, A.; Fritsche, H.A.; Bruera, E.; Booser, D.; et al. Changes in plasma levels of inflammatory Cytokines in response to Paclitaxel chemotherapy. Cytokine 2004, 25, 94–102. [Google Scholar] [CrossRef]
- White, C.M.; Martin, B.K.; Lee, L.F.; Haskill, J.S.; Ting, J.P.Y. Effects of Paclitaxel on Cytokine Synthesis by unprimed human monocytes, T Lymphocytes, and breast cancer cells. Cancer Immunol. Immunother. 1998, 46, 104–112. [Google Scholar] [CrossRef]
- Wang, A.C.; Su, Q.B.; Wu, F.X.; Zhang, X.L.; Liu, P.S. Role of TLR4 for Paclitaxel chemotherapy in human Epithelial ovarian cancer cells. Eur. J. Clin. Invest. 2009, 39, 157–164. [Google Scholar] [CrossRef]
- Rajput, S.; Volk-Draper, L.D.; Ran, S. TLR4 Is a Novel determinant of the response to Paclitaxel in breast cancer. Mol. Cancer 2013, 12, 1–22. [Google Scholar] [CrossRef]
- Carpenter, S.; O’Neill, L.A.J. How important are toll-like receptors for Antimicrobial responses? Cell. Microbiol. 2007, 9, 1891–1901. [Google Scholar] [CrossRef]
- Liao, S.J.; Zhou, Y.H.; Yuan, Y.; Li, D.; Wu, F.H.; Wang, Q.; Zhu, J.H.; Yan, B.; Wei, J.J.; Zhang, G.M.; et al. Triggering of toll-like receptor 4 on metastatic breast cancer cells promotes Avβ3-mediated adhesion and invasive migration. Breast Cancer Res. Treat. 2012, 133, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Wu, C.L.; Shiau, A.L. Toll-like receptor 4 signaling promotes tumor growth. J. Immunother. 2010, 33, 73–82. [Google Scholar] [CrossRef] [PubMed]
- González-Reyes, S.; Marín, L.; González, L.; González, L.O.; Del Casar, J.M.; Lamelas, M.L.; González-Quintana, J.M.; Vizoso, F.J. Study of TLR3, TLR4 and TLR9 in breast carcinomas and their association with metastasis. BMC Cancer 2010, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Silasi, D.A.; Alvero, A.B.; Illuzzi, J.; Kelly, M.; Chen, R.; Fu, H.H.; Schwartz, P.; Rutherford, T.; Azodi, M.; Mor, G. MyD88 predicts chemoresistance to Paclitaxel in Epithelial ovarian cancer. Yale J. Biol. Med. 2006, 79, 153–163. [Google Scholar] [PubMed]
- González-Reyes, S.; Fernández, J.M.; González, L.O.; Aguirre, A.; Suárez, A.; González, J.M.; Escaff, S.; Vizoso, F.J. Study of TLR3, TLR4, and TLR9 in prostate Carcinomas and their association with biochemical recurrence. Cancer Immunol. Immunother. 2011, 60, 217–226. [Google Scholar] [CrossRef]
- Wanderley, C.W.; Colón, D.F.; Luiz, J.P.M.; Oliveira, F.F.; Viacava, P.R.; Leite, C.A.; Pereira, J.A.; Silva, C.M.; Silva, C.R.; Silva, R.L.; et al. Paclitaxel reduces tumor growth by reprogramming tumor-associated Macrophages to an M1 profile in a TLR4-dependent manner. Cancer Res. 2018, 78, 5891–5900. [Google Scholar] [CrossRef]
- Son, S.; Shim, D.W.; Hwang, I.; Park, J.H.; Yu, J.W. Chemotherapeutic agent Paclitaxel mediates priming of NLRP3 inflammasome activation. Front. Immunol. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Xu, J.H.; Hu, S.L.; Shen, G.D.; Shen, G. Tumor suppressor genes and their underlying interactions in Paclitaxel resistance in cancer therapy. Cancer Cell Int. 2016, 16, 1–10. [Google Scholar] [CrossRef]
- Wu, H.; Chen, S.; Yu, J.; Li, Y.; Zhang, X.; Yang, L.; Zhang, H.; Hou, Q.; Jiang, M.; Brunicardi, F.C.; et al. Single-cell transcriptome analyses reveal molecular signals to intrinsic and acquired Paclitaxel resistance in Esophageal Squamous cancer cells. Cancer Lett. 2018, 420, 156–167. [Google Scholar] [CrossRef]
- Fan, G.H.; Zhu, T.Y.; Huang, J. FNDC5 promotes Paclitaxel sensitivity of non-small cell lung cancers via inhibiting MDR1. Cell. Signal. 2020, 72, 109665. [Google Scholar] [CrossRef]
- Li, J.; Chen, Q.; Deng, Z.; Chen, X.; Liu, H.; Tao, Y.; Wang, X.; Lin, S.; Liu, N. KRT17 Confers Paclitaxel-induced resistance and migration to cervical cancer cells. Life Sci. 2019, 224, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Szakács, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Němcová-Fürstová, V.; Kopperová, D.; Balušíková, K.; Ehrlichová, M.; Brynychová, V.; Václavíková, R.; Daniel, P.; Souček, P.; Kovář, J. Characterization of acquired Paclitaxel resistance of breast cancer cells and involvement of ABC transporters. Toxicol. Appl. Pharm. 2016, 310, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.C.; Chen, C.Y.; Wu, Y.C.; Huang, C.F.; Huang, Y.C.; Chen, Y.C.; Chang, C.S. Synthesis and biological evaluation of Thiophenylbenzofuran derivatives as potential P-Glycoprotein inhibitors. Eur. J. Med. Chem. 2020, 201, 1–11. [Google Scholar] [CrossRef]
- Wang, B.; Li, S.; Meng, X.; Shang, H.; Guan, Y. Inhibition of Mdr1 by G-Quadruplex Oligonucleotides and reversal of Paclitaxel resistance in human ovarian cancer cells. Tumor Biol. 2015, 36, 6433–6443. [Google Scholar] [CrossRef]
- Zheng, A.W.; Chen, Y.Q.; Zhao, L.Q.; Feng, J.G. Myricetin induces apoptosis and enhances chemosensitivity in ovarian cancer cells. Oncol. Lett. 2017, 13, 4974–4978. [Google Scholar] [CrossRef]
- Hynes, N.E.; MacDonald, G. ErbB Receptors and signaling pathways in cancer. Curr. Opin. Cell Biol. 2009, 21, 177–184. [Google Scholar] [CrossRef]
- Harari, P.M. Epidermal growth factor receptor inhibition strategies in oncology. Endocr. Relat. Cancer 2004, 11, 689–708. [Google Scholar] [CrossRef]
- Lv, Y.; Cang, W.; Li, Q.; Liao, X.; Zhan, M.; Deng, H.; Li, S.; Jin, W.; Pang, Z.; Qiu, X.; et al. Erlotinib Overcomes Paclitaxel-resistant cancer stem cells by blocking the EGFR-CREB/GRβ-IL-6 Axis in MUC1-positive cervical cancer. Oncogenesis 2019, 8, 1–12. [Google Scholar] [CrossRef]
- Gupta, N.; Gupta, P.; Srivastava, S.K. Penfluridol Overcomes Paclitaxel resistance in metastatic breast cancer. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Aldonza, M.B.D.; Ku, J.; Hong, J.Y.; Kim, D.; Yu, S.J.; Lee, M.S.; Prayogo, M.C.; Tan, S.; Kim, D.; Han, J.; et al. Prior acquired resistance to Paclitaxel relays diverse EGFR-targeted therapy persistence mechanisms. Sci. Adv. 2020, 6, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhang, X.; Jiang, L.; Zhang, L.; Xiang, M.; Ren, H. FoxM1 Induced Paclitaxel resistance via activation of the FoxM1/PHB1/RAF-MEK-ERK pathway and enhancement of the ABCA2 transporter. Mol. Oncolytics 2019, 14, 196–212. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, X.Y.; Chen, X.; Fan, L.L.; Ren, M.L.; Wu, Y.P.; Chanda, K.; Jiang, S.W. Synthetic Paclitaxel-Octreotide conjugate reverses the resistance of Paclitaxel in A2780/Taxol ovarian cancer cell line. Oncol. Rep. 2017, 37, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.L.; Chen, X.; Zhang, X.Y.; Li, Z.M.; Fan, X.M.; Shen, Y. Octreotide-Paclitaxel conjugate reverses Paclitaxel resistance by P38 mitogen-activated protein Kinase (MAPK) signaling pathway in A2780/Taxol human ovarian cancer cells. Med. Sci. Monit. 2020, 26, 1–9. [Google Scholar] [CrossRef]
- Zaal, E.A.; Berkers, C.R. The influence of metabolism on drug response in cancer. Front. Oncol. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Zhou, M.; Zhao, Y.; Ding, Y.; Liu, H.; Liu, Z.; Fodstad, O.; Riker, A.I.; Kamarajugadda, S.; Lu, J.; Owen, L.B.; et al. Warburg effect in chemosensitivity: Targeting Lactate Dehydrogenase-A Re-Sensitizes Taxol-resistant cancer cells to Taxol. Mol. Cancer 2010, 9, 1–12. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, A.; Zhou, X.; Wang, F. Suppression of Pyruvate Dehydrogenase Kinase-2 Re-Sensitizes Paclitaxel-resistant human lung cancer cells to Paclitaxel. Oncotarget 2017, 8, 52642–52650. [Google Scholar] [CrossRef]
- Drukman, S.; Kavallaris, M. Microtubule alterations and resistance to Tubulin-binding agents (Review). Int. J. Oncol. 2002, 21, 621–628. [Google Scholar] [CrossRef]
- Borys, F.; Joachimiak, E.; Krawczyk, H.; Fabczak, H. Intrinsic and extrinsic factors affecting Microtubule dynamics in normal and cancer cells. Molecules 2020, 25, 3705. [Google Scholar] [CrossRef]
- Parker, A.L.; Teo, W.S.; McCarroll, J.A.; Kavallaris, M. An emerging role for Tubulin Isotypes in modulating cancer biology and chemotherapy resistance. Int. J. Mol. Sci. 2017, 18, 1434. [Google Scholar] [CrossRef]
- Christoph, D.C.; Kasper, S.; Gauler, T.C.; Loesch, C.; Engelhard, M.; Theegarten, D.; Poettgen, C.; Hepp, R.; Peglow, A.; Loewendick, H.; et al. Βv-Tubulin expression is associated with outcome following Taxane-based chemotherapy in non-small cell lung cancer. Br. J. Cancer 2012, 107, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Akasaka, K.; Maesawa, C.; Shibazaki, M.; Maeda, F.; Takahashi, K.; Akasaka, T.; Masuda, T. Loss of class III Β-Tubulin induced by Histone Deacetylation is associated with Chemosensitivity to Paclitaxel in malignant Melanoma cells. J. Investig. Derm. 2009, 129, 1516–1526. [Google Scholar] [CrossRef] [PubMed]
- Roque, D.M.; Bellone, S.; English, D.P.; Buza, N.; Cocco, E.; Gasparrini, S.; Bortolomai, I.; Ratner, E.; Silasi, D.A.; Azodi, M.; et al. Tubulin-β-III overexpression by uterine serous carcinomas is a marker for poor overall survival after platinum/Taxane chemotherapy and sensitivity to Epothilones. Cancer 2013, 119, 2582–2592. [Google Scholar] [CrossRef] [PubMed]
- Öztop, S.; Işik, A.; Güner, G.; Gürdal, H.; Karabulut, E.; Yilmaz, E.; Akyol, A. Class III β-Tubulin expression in colorectal Neoplasms is a potential predictive biomarker for Paclitaxel response. Anticancer Res. 2019, 39, 655–662. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, Y.; Wu, B.; Kurie, J.M.; Pertsemlidis, A. The MiR-195 Axis regulates Chemoresistance through TUBB and lung cancer progression through BIRC5. Mol. Oncolytics 2019, 14, 288–298. [Google Scholar] [CrossRef]
- Kavallaris, M.; Kuo, D.Y.S.; Burkhart, C.A.; Regl, D.L.; Norris, M.D.; Haber, M.; Horwitz, S.B. Taxol-resistant Epithelial ovarian tumors are associated with altered expression of specific β-Tubulin Isotypes. J. Clin. Investig. 1997, 100, 1282–1293. [Google Scholar] [CrossRef]
- Cuihua, L.; Jing, Z.; Song, H.; Chunhua, W.; Aidong, S.; Yingying, W.; Litao, Y.; Guoliang, L.; Ken, C.; Jing, S.; et al. Increased a-Tubulin1b Expression Indicates Poor Prognosis and Resistance to Chemotherapy in Hepatocellular Carcinoma. Dig. Dis. Sci. 2013, 58, 2713–2720. [Google Scholar] [CrossRef]
- Banerjee, A. Increased Levels of Tyrosinated α-, ΒIII-, and ΒIV-Tubulin Isotypes in Paclitaxel-resistant MCF-7 breast cancer cells. Biochem. Biophys. Res. Commun. 2002, 293, 598–601. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, H.; Wang, X.; Patterson, J.; Winter, P.; Graham, K.; Ghosh, S.; Lee, J.C.; Katsetos, C.D.; Mackey, J.R.; et al. Novel mutations involving ΒI-, ΒIIA-, or ΒIVB-Tubulin Isotypes with Functional resemblance to ΒIII-Tubulin in breast cancer. Protoplasma 2017, 254, 1163–1173. [Google Scholar] [CrossRef]
- Berrieman, H.K.; Lind, M.J.; Cawkwell, L. Do β-Tubulin mutations have a role in resistance to chemotherapy? Lancet Oncol. 2004, 5, 158–164. [Google Scholar] [CrossRef]
- Chowdhury, P.; Nagesh, P.K.B.; Hatami, E.; Wagh, S.; Dan, N.; Tripathi, M.K.; Khan, S.; Hafeez, B.B.; Meibohm, B.; Chauhan, S.C.; et al. Tannic acid-inspired Paclitaxel nanoparticles for enhanced anticancer effects in breast cancer cells. J. Colloid Interface Sci. 2019, 535, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, H.; Wang, S.; Gai, C.; Cui, X.; Xu, Z.; Li, W.; Zhang, W. Targeted delivery of Quercetin by nanoparticles based on Chitosan sensitizing Paclitaxel-resistant lung cancer cells to Paclitaxel. Mater. Sci. Eng. C 2021, 119, 111442. [Google Scholar] [CrossRef]
- Meng, J.; Guo, F.; Xu, H.; Liang, W.; Wang, C.; Yang, X.D. Combination Therapy using co-encapsulated Resveratrol and Paclitaxel in Liposomes for drug resistance reversal in breast cancer cells In Vivo. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Zhang, B.; Tian, L.; Xie, J.; Chen, G.; Wang, F. Targeting MiRNAs by Natural products: A new way for cancer therapy. Biomed. Pharm. 2020, 130, 1–9. [Google Scholar] [CrossRef] [PubMed]
- He, D.X.; Gu, F.; Gao, F.; Hao, J.J.; Gong, D.; Gu, X.T.; Mao, A.Q.; Jin, J.; Fu, L.; Ma, X. Genome-wide profiles of Methylation, MicroRNAs, and gene expression in chemoresistant breast cancer. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.A.; Huang, Z.A.; You, Z.H.; Zhu, Z.; Huang, W.Z.; Guo, J.X.; Yu, C.Q. Predicting LncRNA-MiRNA Interaction via graph convolution auto-encoder. Front. Genet. 2019, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.-C.; Ni, J.-J.; Cui, W.-Y.; Wang, B.-Y.; Zhuo, W. Emerging Roles of LncRNA in Cancer and therapeutic opportunities. Am. J. Cancer Res. 2019, 9, 1354–1366. [Google Scholar]
- Xu, J.; Wu, J.; Fu, C.; Teng, F.; Liu, S.; Dai, C.; Shen, R.; Jia, X. Multidrug resistant LncRNA profile in chemotherapeutic sensitive and resistant ovarian cancer cells. J. Cell. Physiol. 2018, 233, 5034–5043. [Google Scholar] [CrossRef]
- Abildgaard, C.; Do Canto, L.M.; Steffensen, K.D.; Rogatto, S.R. Long non-coding RNAs involved in resistance to chemotherapy in ovarian cancer. Front. Oncol. 2020, 9, 1–17. [Google Scholar] [CrossRef]
- Bomane, A.; Gonçalves, A.; Ballester, P.J. Paclitaxel Response can be predicted with interpretable multi-variate classifiers exploiting DNA-Methylation and MiRNA data. Front. Genet. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Li, L.; Wu, C.; Zhao, Y. MiRNA-34a Enhances the sensitivity of gastric cancer cells to treatment with Paclitaxel by targeting E2F5. Oncol. Lett. 2017, 13, 4837–4842. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, Y.F.; Han, M.L.; Xiong, Y.J.; Wang, L.; Fei, Y.; Shen, X.; Zhu, Y.; Liang, Z.Q. A MiRNA-200c/Cathepsin L feedback loop determines Paclitaxel resistance in human lung cancer A549 cells In Vitro through regulating Epithelial-Mesenchymal transition. Acta Pharm. Sin. 2018, 39, 1034–1047. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Xie, Z.; Peng, Q. MiRNA-107 Enhances chemosensitivity to Paclitaxel by targeting antiapoptotic factor Bcl-w in non small cell lung cancer. Am. J. Cancer Res. 2017, 7, 1863–1873. [Google Scholar] [PubMed]
- Hou, L.; Zhao, Y.; Song, G.Q.; Ma, Y.H.; Jin, X.H.; Jin, S.L.; Fang, Y.H.; Chen, Y.C. interfering cellular lactate homeostasis overcomes Taxol resistance of breast cancer cells through the MicroRNA-124-mediated lactate transporter (MCT1) inhibition. Cancer Cell Int. 2019, 19, 1–12. [Google Scholar] [CrossRef]
- Huang, L.; Hu, C.; Chao, H.; Wang, R.; Lu, H.; Li, H.; Chen, H. MiR-29c Regulates resistance to Paclitaxel in Nasopharyngeal cancer by targeting ITGB1. Exp. Cell Res. 2019, 378, 1–10. [Google Scholar] [CrossRef]
- Duan, F.G.; Wang, M.F.; Cao, Y.B.; Li, D.; Li, R.Z.; Fan, X.X.; Khan, I.; Lai, H.L.; Zhang, Y.Z.; Hsiao, W.W.L.; et al. MicroRNA-421 Confers Paclitaxel resistance by binding to the KEAP1 3′UTR and predicts poor survival in non-small cell lung cancer. Cell Death Dis. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Sha, L.Y.; Zhang, Y.; Wang, W.; Sui, X.; Liu, S.K.; Wang, T.; Zhang, H. MiR-18a Upregulation decreases dicer expression and confers Paclitaxel resistance in triple negative breast cancer. Eur. Rev. Med. Pharm. Sci. 2016, 20, 2201–2208. [Google Scholar]
- Liu, X.; Du, P.; Han, L.; Zhang, A.; Jiang, K.; Zhang, Q. Effects of MiR-200a and FH535 combined with Taxol on proliferation and invasion of gastric cancer. Pathol. Res. Pr. 2018, 214, 442–449. [Google Scholar] [CrossRef]
- Hejazi, M.; Baghbani, E.; Amini, M.; Rezaei, T.; Aghanejad, A.; Mosafer, J.; Mokhtarzadeh, A.; Baradaran, B. MicroRNA-193a and Taxol combination: A new strategy for treatment of colorectal cancer. J. Cell. Biochem. 2020, 121, 1388–1399. [Google Scholar] [CrossRef]
- Xu, S.; Wang, P.; Zhang, J.; Wu, H.; Sui, S.; Zhang, J.; Wang, Q.; Qiao, K.; Yang, W.; Xu, H.; et al. Ai-LncRNA EGOT enhancing autophagy sensitizes Paclitaxel cytotoxicity via upregulation of ITPR1 expression by RNA-RNA and RNA-protein interactions in human cancer. Mol. Cancer 2019, 18, 1–18. [Google Scholar] [CrossRef]
- Liu, S.; Zou, B.; Tian, T.; Luo, X.; Mao, B.; Zhang, X.; Lei, H. Overexpression of the LncRNA FER1L4 inhibits Paclitaxel tolerance of ovarian cancer cells via the regulation of the MAPK signaling pathway. J. Cell. Biochem. 2019, 120, 7581–7589. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, S.; Fu, C.; Wang, X.; Zhang, M.; Liu, G.; Dai, C.; Gong, Z.; Xu, H.; Fu, Z.; et al. LncRNA KB-1471A8.2 Overexpression suppresses cell proliferation and migration and antagonizes the Paclitaxel resistance of ovarian cancer cells. Cancer Biother. Radiopharm. 2019, 34, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Zheng, W.; Zhang, M.; Dong, X.; Zhao, Y.; Wang, S.; Jiang, H.; Zheng, X. LncRNA NONHSAT141924 promotes Paclitaxel chemotherapy resistance through P-CREB/Bcl-2 apoptosis signaling pathway in breast cancer. J. Cancer 2020, 11, 3645–3654. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, M.; Zou, L.; Xia, J.; Huang, J.; Deng, Q.; Xu, R. Long non-coding RNA LINC-PINT attenuates Paclitaxel resistance in triple-negative breast cancer cells via targeting the RNA-binding protein NONO. Acta Biochim. Biophys. Sin. 2020, 52, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Feng, D.; Li, M.; Zhou, J.; Li, X.; Zhao, D.; Hao, B.; Li, D.; Ding, K. Transcriptomic analysis of MRNA-LncRNA-MiRNA interactions in hepatocellular carcinoma. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tay, Y.; Rinn, J.; Pandolfi, P.P. The multilayered complexity of CeRNA crosstalk and competition. Nature 2014, 505, 344–352. [Google Scholar] [CrossRef]
- Yoon, J.H.; Abdelmohsen, K.; Gorospe, M. Functional interactions among MicroRNAs and long noncoding RNAs. Semin. Cell Dev. Biol. 2014, 34, 9–14. [Google Scholar] [CrossRef]
- Wang, J.; Ye, C.; Liu, J.; Hu, Y. UCA1 Confers Paclitaxel resistance to ovarian cancer through MiR-129/ABCB1 Axis. Biochem. Biophys. Res. Commun. 2018, 501, 1034–1040. [Google Scholar] [CrossRef]
- Shi, C.; Wang, M. LINC01118 modulates Paclitaxel resistance of epithelial ovarian cancer by regulating MiR-134/ABCC1. Med. Sci. Monit. 2018, 24, 8831–8839. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, A.; Zhang, Z. LncRNA SDHAP1 confers Paclitaxel resistance of ovarian cancer by regulating EIF4G2 expression via MiR-4465. J. Biochem. 2020, 168, 171–181. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, T.; Yang, Z.; Jiang, C.; Seng, J. Long non-coding RNA FTH1P3 activates Paclitaxel resistance in breast cancer through MiR-206/ABCB1. J. Cell. Mol. Med. 2018, 22, 4068–4075. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, X.; Wei, P.; Yang, J.; Sun, J. Knockdown of LncRNA CCAT1 enhances sensitivity of Paclitaxel in prostate cancer via regulating MiR-24-3p and FSCN1. Cancer Biol. 2020, 21, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Au Yeung, C.L.; Co, N.N.; Tsuruga, T.; Yeung, T.L.; Kwan, S.Y.; Leung, C.S.; Li, Y.; Lu, E.S.; Kwan, K.; Wong, K.K.; et al. Exosomal transfer of stroma-derived MiR21 confers Paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.C.; Guo, S.C. Role of extracellular vesicles in tumour microenvironment. Cell Commun. Signal. 2020, 18, 1–24. [Google Scholar] [CrossRef]
- Tang, X.; Jin, L.; Cao, P.; Cao, K.; Huang, C.; Luo, Y.; Ma, J.; Shen, S.; Tan, M.; Li, X.; et al. MicroRNA-16 sensitizes breast cancer cells to Paclitaxel through suppression of IKBKB expression. Oncotarget 2016, 7, 23668–23683. [Google Scholar] [CrossRef]

| Product | Microbial Host | Maximum Yield | Reference |
|---|---|---|---|
| Taxadiene | E. coli | 1000 mg/L | [33] |
| S. cerevisiae | 72.8 mg/L | [35] | |
| B. subtilis | 17.8 mg/L | [36] | |
| Oxygenated taxanes | E. coli | 570 mg/L | [37] |
| Commercial Name | Formulation | Company | Delivery System (Nanoparticle Diameter) | Status | Cancer Type tXarget | Advantages | Common Dose/MTD (mg/m2) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Abraxane® (nab-PTX) | Paclitaxel and albumin. | Celgene Abraxis Abraxis  | Albumin-bound paclitaxel nanoparticles (130 nm) | Approved internationally (FDA in 2005, EMA in 2008). | Breast cancer, NSCLC, and pancreatic cancer. | Lower toxicity. High MTD. | 260/300 | [8,12,99,100] |
| Cynviloq™ (Genexol-PM®) | Paclitaxel and mPEG-PDLLA. | Samyang  Nantpharma Nantpharma | Polymeric micelles (25 nm) | Approved in South Korea in 2007. | Breast cancer, NSCLC, ovarian cancer, pancreatic cancer, and bladder cancer. | Lower toxicity. The absence of albumin reduces the risk of microbial growth. High MTD. | 260/390 | [8,12,52,101,102] |
| Lipusu® | Paclitaxel, lecithin, and cholesterol. | Luye Pharma | Liposome (400 nm) | Approved in China in 2006. | Ovarian cancer and NSCLC (approved). Breast cancer (clinical trial). | Lower toxicity. | 175/no data | [8,12,52,103] |
| PICN | Paclitaxel, polyvinyl-pyrrolidone, cholesteryl sulfate, and caprylic. | Sun Pharma  | Polymeric lipid-nanoparticles (100 nm) | Approved in India in 2014. | Breast cancer (approved). Ovarian cancer and bladder cancer (clinical trial). | Lower toxicity. High MTD. | 260, 95/325 | [8,12,52,102] |
| Nanoxel® | Paclitaxel and PVP-b-PNIPAAM. | Dabur  | Polymeric micelles (80–100 nm) | Approved in India in 2008. | Breast cancer, NSCLC, ovarian cancer, and AIDS-related Kaposi’s sarcoma (approved). | The carrier is pH-sensitive (tumor targeted drug). High MTD. Lower toxicity. | 300/375 | [12,52,104,105] |
| DHP-107 (Liporaxel®) | Paclitaxel, monoolein, tricaprylin, and Tween 80. | Daehwa  | Emulsion (oral administration) | Approved in South Korea in 2016. | Gastric cancer. | Oral administration. High MTD. | 200/600 | [8,12,87] |
| (Apealea®) Paclical® | Paclitaxel, N-tr-Lc methyl ester, and N.13cr-Lc methyl ester. | Oasmia  | Polymeric micelles (20–60 nm) | Approved in Russian Federation in 2015. | Ovarian cancer (approved). | The carrier is rapidly metabolized. | 260/250 | [8,12,52,106] |
| RNA | Metabolic Target | Differential Expression Found in PTX-Resistant Cells | Reference |
|---|---|---|---|
| 200c | Cathepsin L (CTSL) | Downregulation | [186] |
| 203 | Salt-inducible kinase 2 (SIK2) | Downregulation | [28] |
| 18a | Endoribonuclease Dicer | Upregulated | [191] |
| 16 | IκB kinase β (IKBKB) | Downregulation | [209] |
| 107 | Antiapoptotic factor Bcl-w | Downregulation | [187] |
| 34a | E2F transcription factor 5 (E2F5) | Downregulation | [185] |
| 124 | Monocarboxylic acid solute transporter 1 (MCT1) | Downregulated | [188] |
| 421 | Kelch-like ECH-associated protein 1 (KEAP1) | Upregulation | [190] |
| 29c | Upregulation of integrin beta-1 (ITGB1). | Downregulation | [189] |
| EGOT | Inositol 1,4,5-Trisphosphate Receptor Type 1 (ITPR1) | Downregulated | [194] |
| UCA1 | miRNA129/ABCB1 | Upregulated | [202] |
| FER1L4 | MAPK | Downregulated | [195] |
| LINC01118 | miRNA134/ABCB1 | Upregulated | [203] |
| KB-1471A8.2 | Cyclin-dependent kinase 4 (CDK4) | Downregulated | [196] |
| SDHAP1 | miR-4465/EIF4G2 | Upregulated | [204] |
| NONHSAT141924 | p-CREB/Bcl-2 | Upregulated | [197] |
| LINC-PINT | NONO (a non-POU-domain-containing, octamer-binding protein) | Downregulated | [198] |
| FTH1P3 | miR-206/ABCB1 | Upregulated | [205] |
| CCAT1 | miR-24-3p/FSCN1 | Upregulated | [206] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallego-Jara, J.; Lozano-Terol, G.; Sola-Martínez, R.A.; Cánovas-Díaz, M.; de Diego Puente, T. A Compressive Review about Taxol®: History and Future Challenges. Molecules 2020, 25, 5986. https://doi.org/10.3390/molecules25245986
Gallego-Jara J, Lozano-Terol G, Sola-Martínez RA, Cánovas-Díaz M, de Diego Puente T. A Compressive Review about Taxol®: History and Future Challenges. Molecules. 2020; 25(24):5986. https://doi.org/10.3390/molecules25245986
Chicago/Turabian StyleGallego-Jara, Julia, Gema Lozano-Terol, Rosa Alba Sola-Martínez, Manuel Cánovas-Díaz, and Teresa de Diego Puente. 2020. "A Compressive Review about Taxol®: History and Future Challenges" Molecules 25, no. 24: 5986. https://doi.org/10.3390/molecules25245986
APA StyleGallego-Jara, J., Lozano-Terol, G., Sola-Martínez, R. A., Cánovas-Díaz, M., & de Diego Puente, T. (2020). A Compressive Review about Taxol®: History and Future Challenges. Molecules, 25(24), 5986. https://doi.org/10.3390/molecules25245986






