How the Supramolecular Nature of Lignohumate Affects Its Diffusion in Agarose Hydrogel
Abstract
:1. Introduction
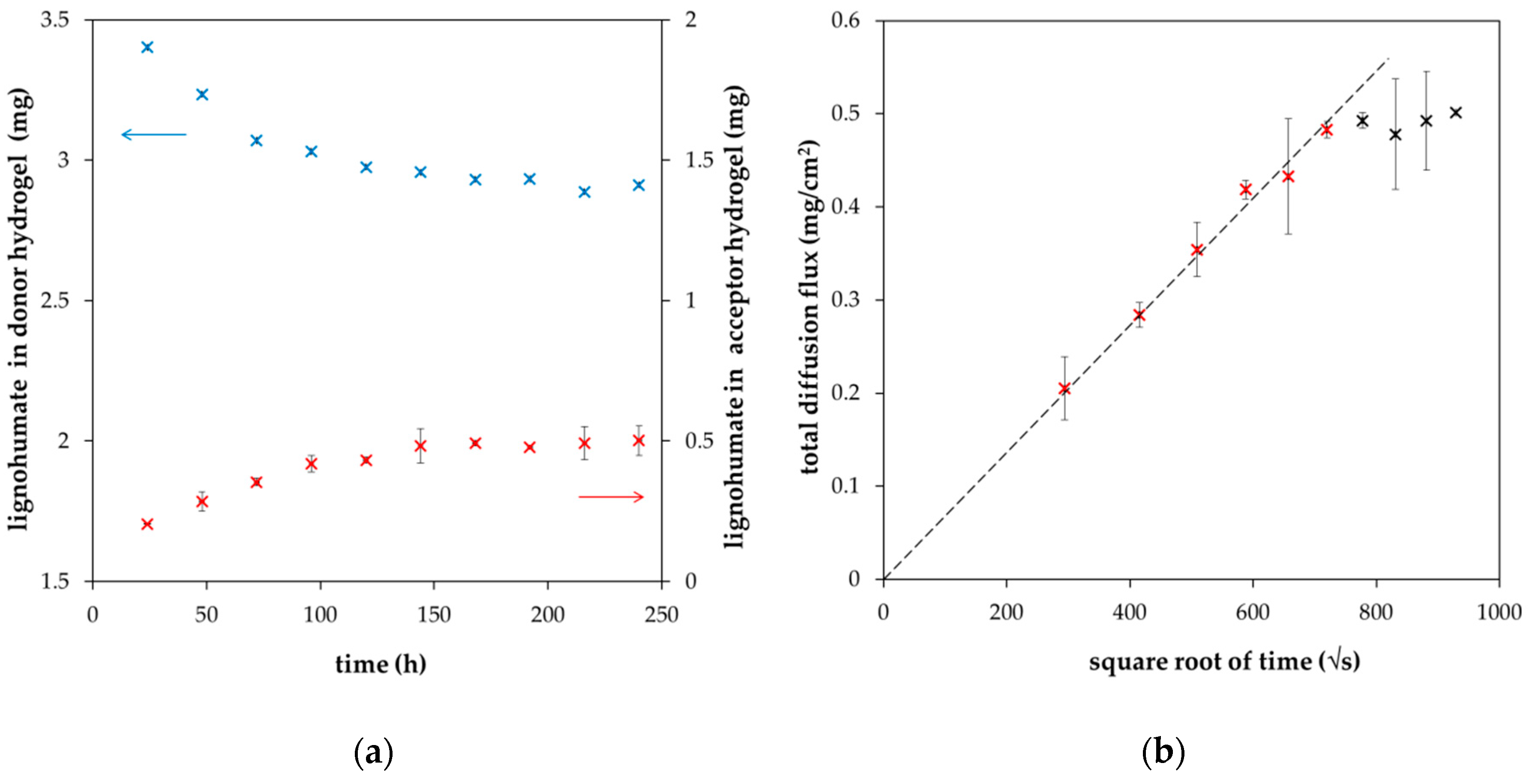
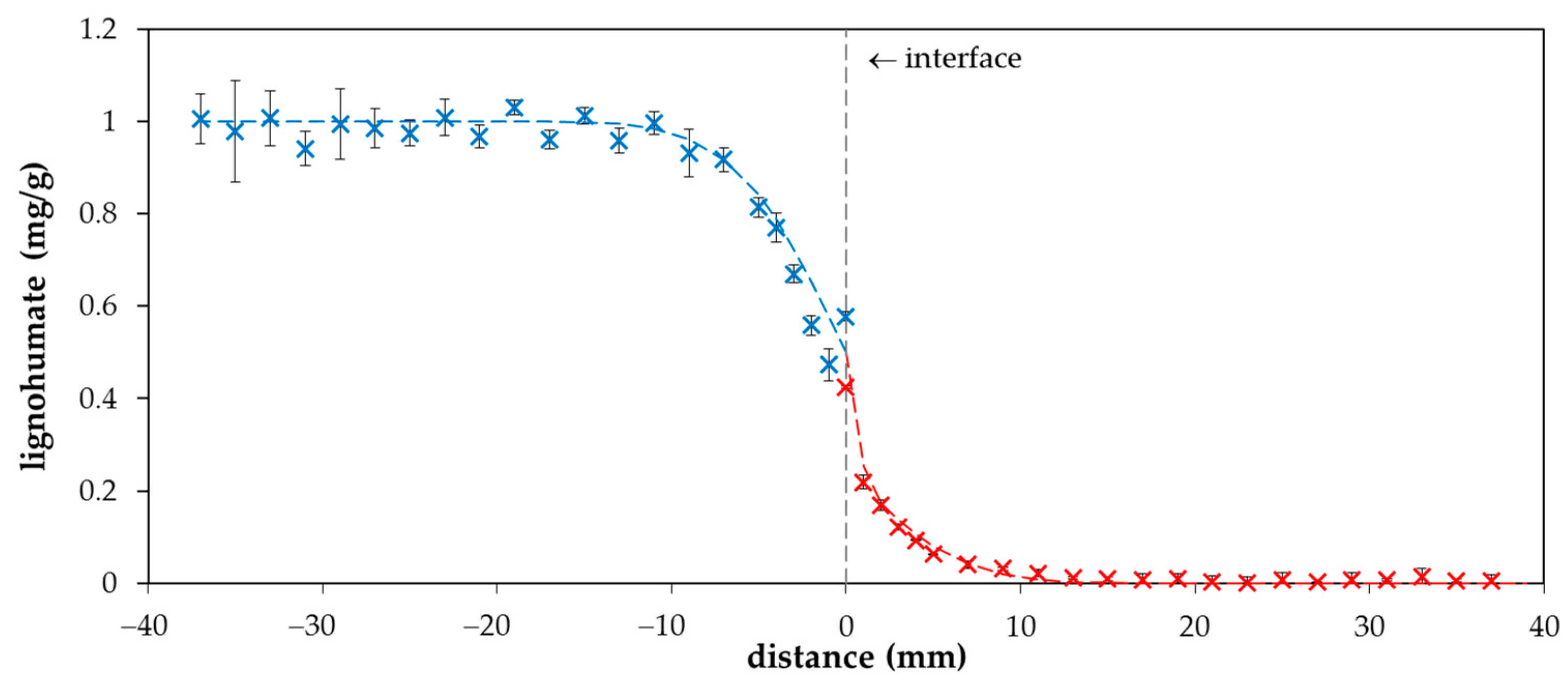
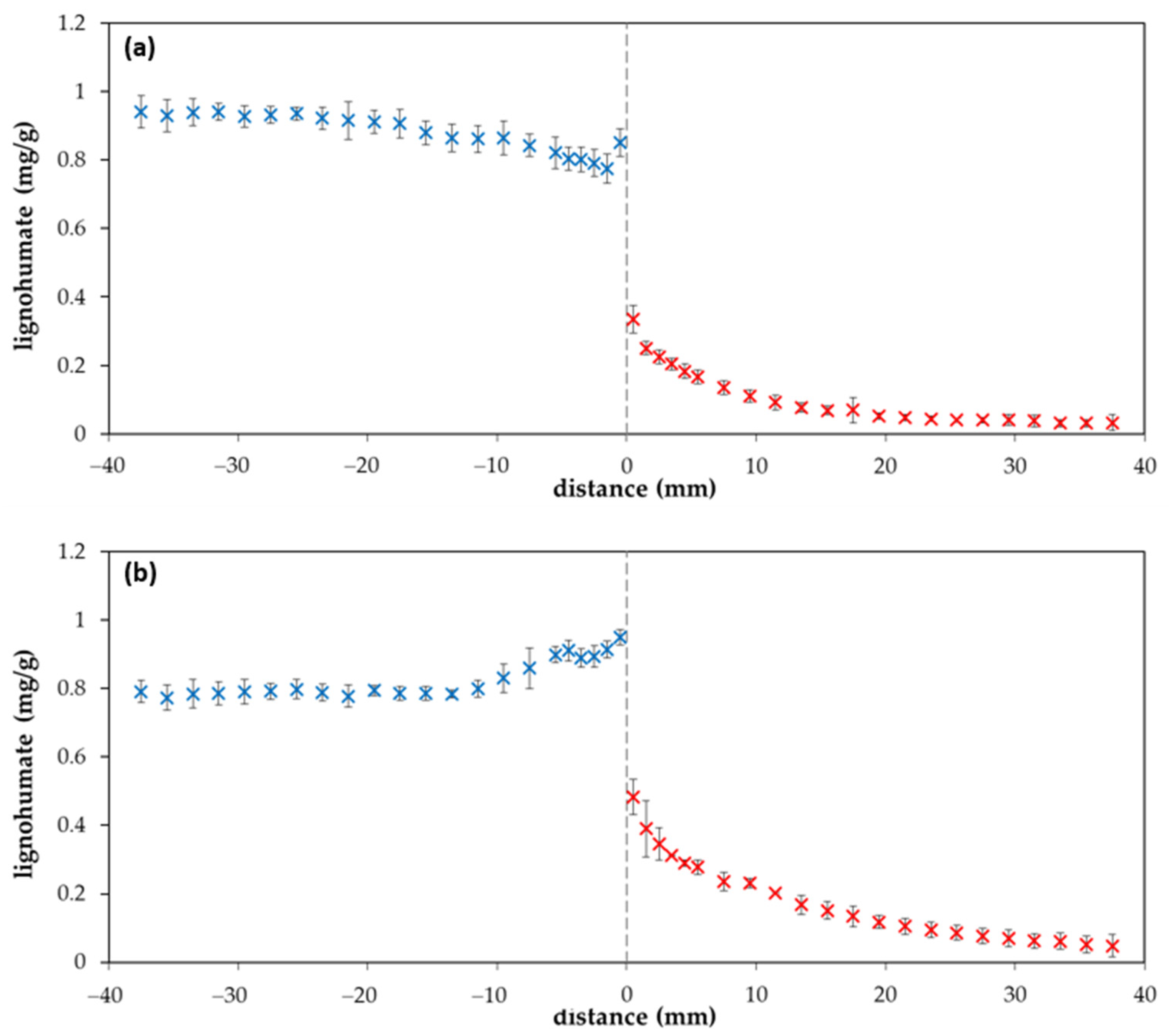
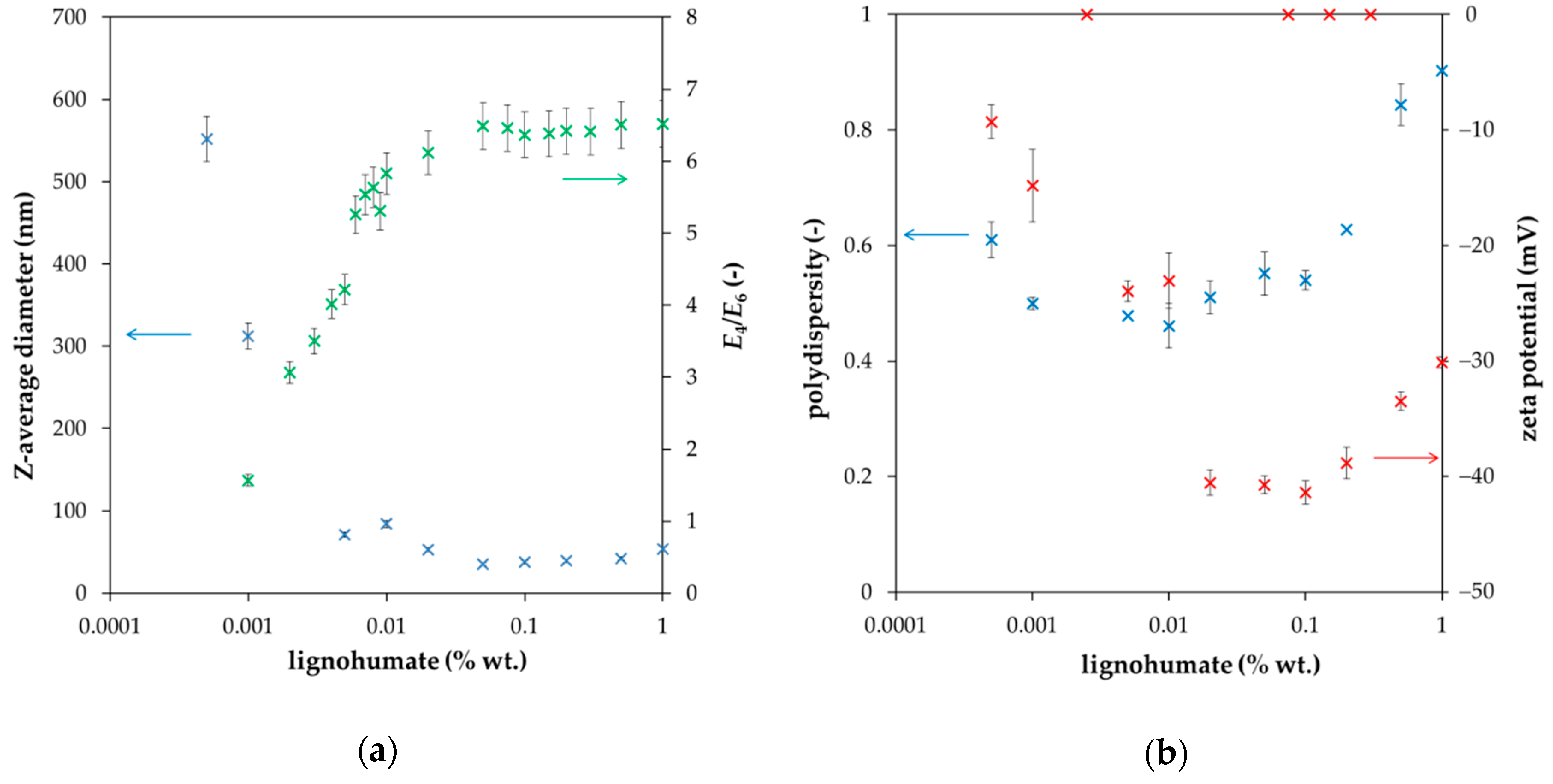
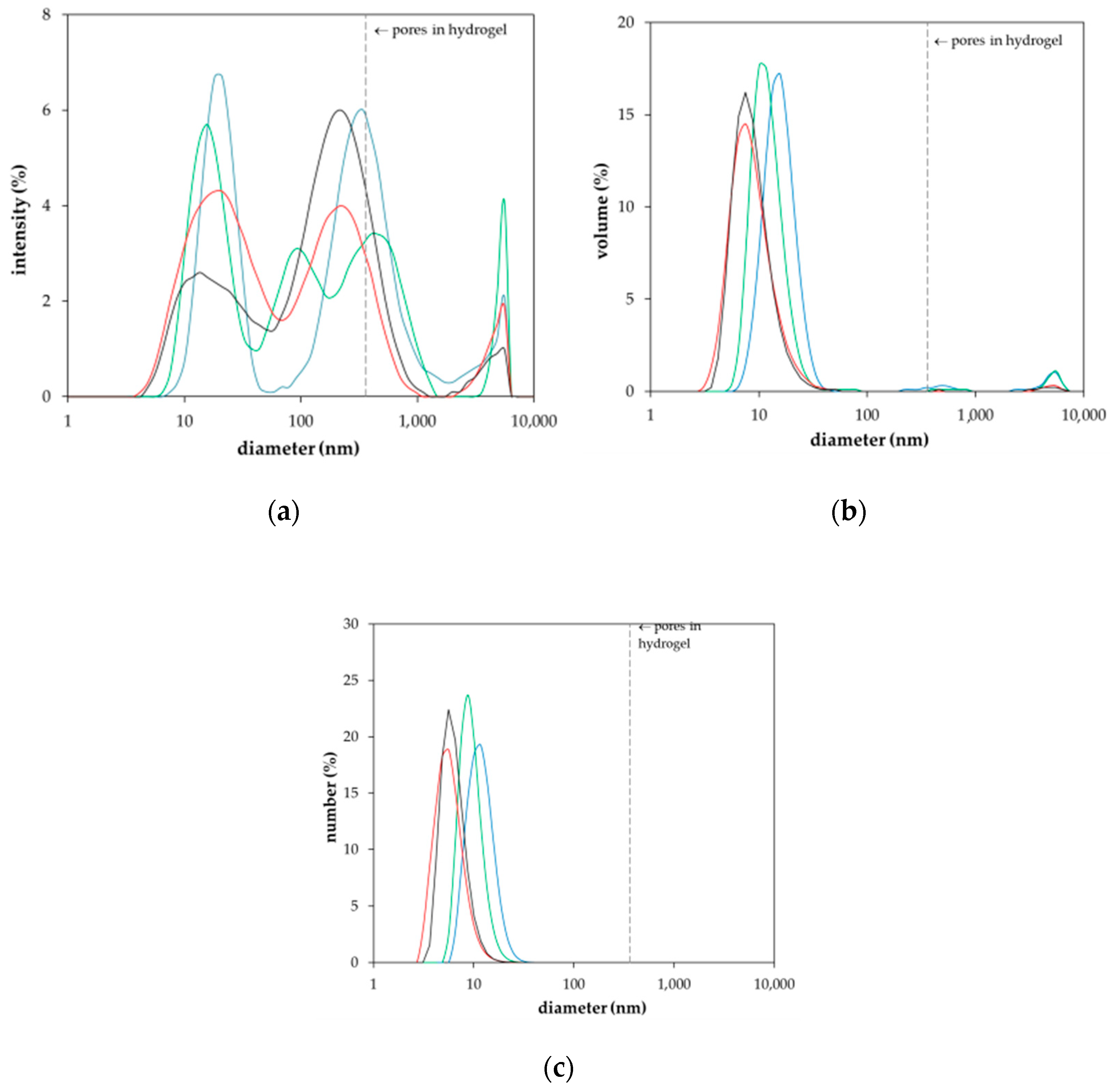
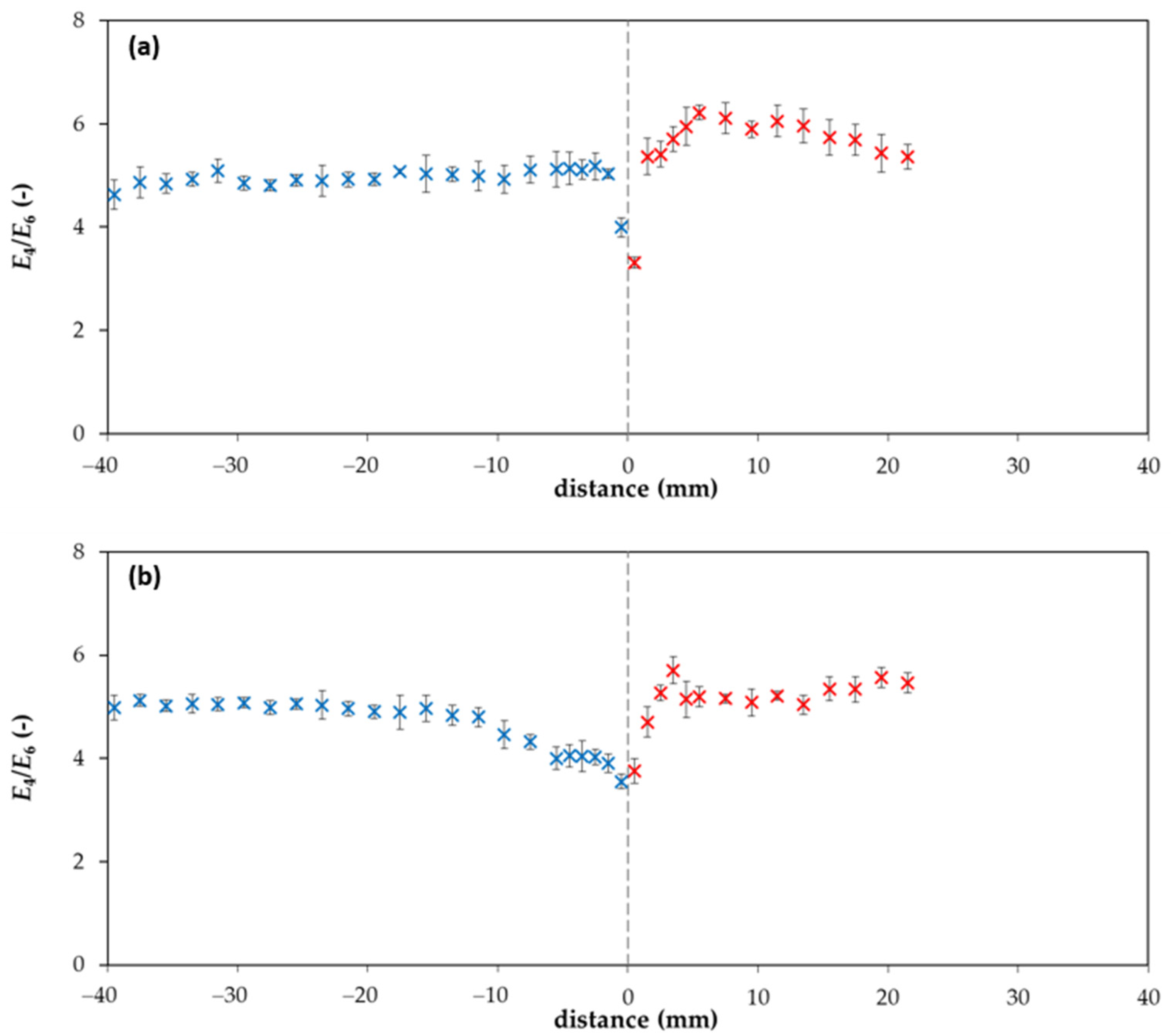
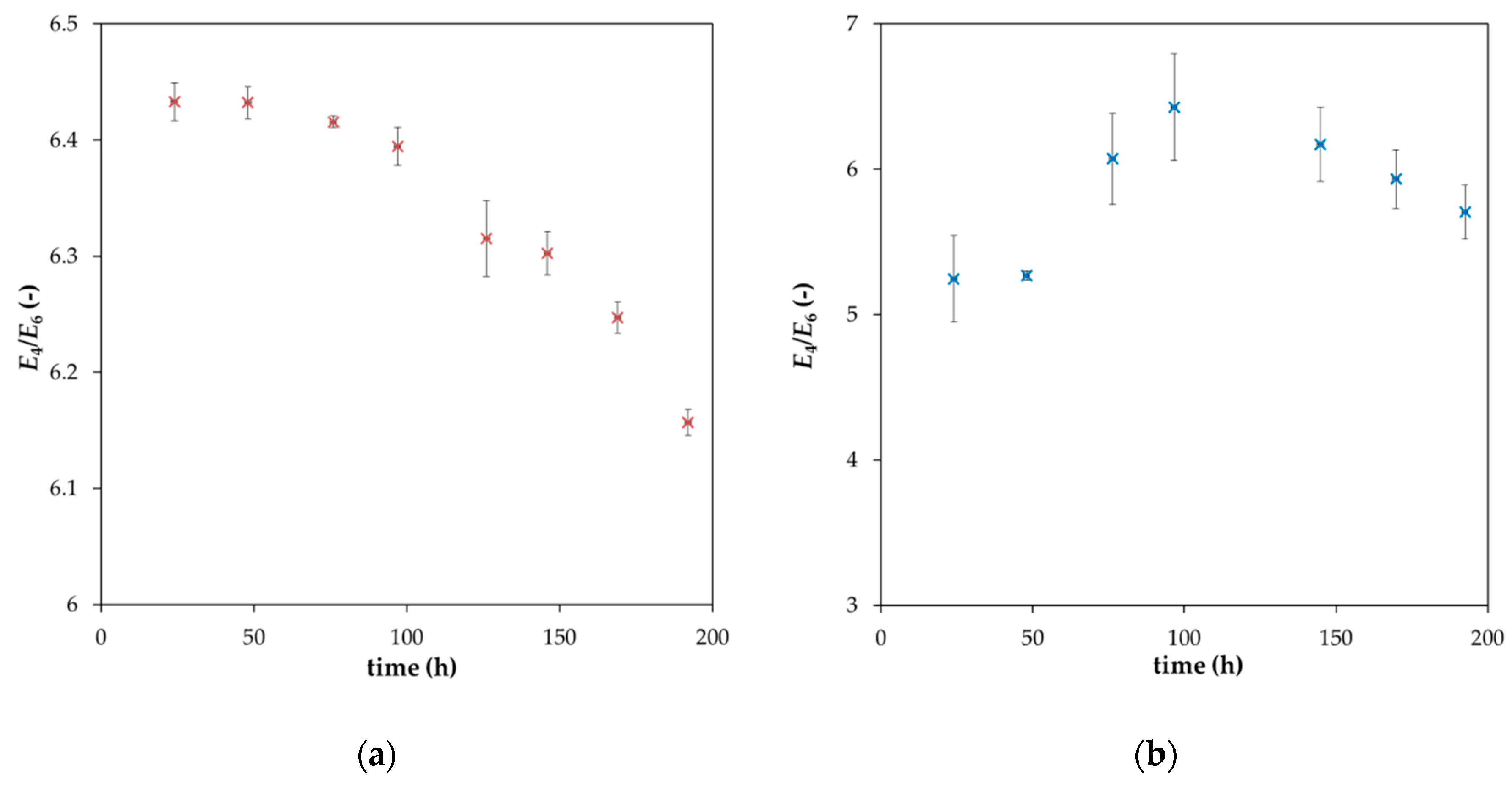
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Hydrogels
3.3. Diffusion Experiments
3.3.1. Diffusion Couple
3.3.2. Diffusion from Solution into Hydrogel
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Novák, F.; Šestauberová, M.; Hrabal, R. Structural features of lignohumic acids. J. Mol. Struct. 2015, 1093, 179–185. [Google Scholar] [CrossRef]
- Holub, P.; Klema, K.; Tuma, I.; Vavríková, J.; Surá, K.; Veselá, B.; Urban, O.; Záhora, J. Application of organic carbon affects mineral nitrogen uptake by winter wheat and leaching in subsoil: Proximal sensing as a tool for agronomic practice. Sci. Total Environ. 2020, 717, 137058. [Google Scholar] [CrossRef] [PubMed]
- Vuorinen, I.; Hamberg, L.; Müller, M.; Seiskari, P.; Pennanen, T. Development of growth media for solid substrate propagation of ectomycorrhiza fungi for inoculation of Norway spruce (Picea abies) seedlings. Mycorrhiza 2015, 25, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Adani, F.; Genevini, P.; Zaccheo, P.; Zocchi, G. The effect of commercial humic acid on tomato plant growth and mineral nutrition. J. Plant Nutr. 1998, 21, 561–575. [Google Scholar] [CrossRef]
- Arancon, N.Q.; Edwards, C.A.; Bierman, P.; Welch, C.; Metzger, J.D. Influences of vermicomposts on field strawberries: 1. Effects on growth and yields. Bioresour. Technol. 2004, 93, 145–153. [Google Scholar] [CrossRef]
- Arancon, N.Q.; Edwards, C.A.; Bierman, P.; Metzger, J.D.; Lucht, C. Effects of vermicomposts produced from cattle manure, food waste and paper waste on the growth and yield of peppers in the field. Pedobiologia 2005, 49, 297–306. [Google Scholar] [CrossRef]
- Arancon, N.Q.; Edwards, C.A.; Bierman, P. Influences of vermicomposts on field strawberries: 2. Effects on soil microbiological and chemical properties. Bioresour. Technol. 2006, 97, 831–840. [Google Scholar] [CrossRef]
- Smilková, M.; Smilek, J.; Kalina, M.; Sedláček, P.; Pekař, M.; Klučáková, M. A simple technique for assessing of the cuticular diffusion of humic acid biostimulants. Plant Methods 2019, 15, 83. [Google Scholar] [CrossRef]
- Pozdnyakov, L.A.; Stepanov, A.L.; Gasanov, M.E.; Semenov, M.V.; Yakimenko, O.S.; Suada, I.K.; Rai, I.N.; Shchegolkova, N.M. Effect of lignohumate on soil biological activity on the Bali Island, Indonesia. Eurasian Soil Sci. 2020, 53, 653–656. [Google Scholar] [CrossRef]
- Vialykh, E.A.; Salahub, D.R.; Achari, G. Metal ion binding by humic substances as emergent functions of labile supramolecular assemblies. Environ. Chem. 2020, 17, 252–265. [Google Scholar] [CrossRef]
- Piccolo, A. The supramolecular structure of humic substances. Soil Sci. 2001, 166, 810–832. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Puebla, R.A.; Valenzuela-Calahorro, C.; Garrido, J.J. Theoretical study on fulvic acid structure, conformation and aggregation: A molecular modelling approach. Sci. Total Environ. 2006, 358, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Klučáková, M.; Věžníková, K. The role of concentration and solvent character in the molecular organization of humic acids. Molecules 2016, 21, 1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klučáková, M.; Smilek, J.; Sedláček, P. How humic acids affect the rheological and transport properties of hydrogels. Molecules 2019, 24, 1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christl, I.; Metzger, A.; Heidmann, I.; Kretzschmar, R. Effect of humic and fulvic acid concentrations and ionic strength on copper and lead binding. Environ. Sci. Technol. 2005, 39, 5319–5326. [Google Scholar] [CrossRef] [PubMed]
- Conte, P.; Piccolo, A. Conformational arrangement of dissolved humic substances. Influence of solution composition on association of humic molecules. Environ. Sci. Technol. 1999, 33, 1682–1690. [Google Scholar] [CrossRef]
- Picollo, A.; Nardi, S.; Concheri, G. Micelle-like conformation of humic substances as revealed by size exclusion chromatography. Chemosphere 1996, 33, 595–602. [Google Scholar] [CrossRef]
- Simpson, A.J.; Kingery, W.L.; Hayes, M.H.B.; Spraul, M.; Humpfer, E.; Dvortsak, P.; Kerssebaum, R.; Godejohann, M.; Hofman, M. Molecular structure and associations of humic substances in the terrestrial environment. Naturwissenschaften 2002, 89, 84–88. [Google Scholar] [CrossRef]
- Simpson, A.J. Determining the molecular weight, aggregation, structures and interactions of natural organic matter using diffusion ordered spectroscopy. Magn. Reson. Chem. 2002, 40, S72–S82. [Google Scholar] [CrossRef]
- Tarasevich, Y.I.; Dolenko, S.A.; Trifonova, M.Y.; Alexeenko, E.Y. Association and colloid-chemical properties of humic acids in aqueous solutions. Colloid J. 2013, 75, 207–213. [Google Scholar] [CrossRef]
- Baalousha, M.; Motelica-Heino, M.; Galaup, S.; LeCoustumer, P. Supramolecular structure of humic acids by TEM with improved sample preparation and staining. Microsc. Res. Tech. 2005, 66, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T. Humic supramolecular structures have polar surfaces and unipolar cores in native soil. Chemosphere 2017, 183, 437–443. [Google Scholar] [CrossRef]
- Springer, U. Der heutige Stand der Humusuntersuchungsmethodik mit besonderer Berücksichtigung der Trennung, Bestimmung und Charakterisierung der Huminsäuretypen und ihre Anwendung auf charakteristische Humusformen. Bodenkd. Pflanenernähr. 1938, 6, 312–373. (In German) [Google Scholar] [CrossRef]
- Enev, V.; Pospíšilová, L.; Klučáková, M.; Liptaj, T.; Doskočil, L. Spectral characterization of selected natural humic substances. Soil Water Res. 2014, 9, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Klučáková, M. Agarose hydrogels enraged by humic acids as the complexation agent. Polymers 2020, 12, 687. [Google Scholar]
- Crank, J. The Mathematics of Diffusion; Clarendon Press: Oxford, UK, 1956; pp. 1–61. [Google Scholar]
- Cussler, E.L. Diffusion Mass: Transfer in Fluid Systems, 2nd ed.; Cambridge University Press: Cambridge, UK, 1984; pp. 13–35. [Google Scholar]
- Klučáková, M.; Pekař, M. Transport of copper (II) ions in humic gel—New results from diffusion couple. Colloid. Surf. A 2009, 349, 96–101. [Google Scholar] [CrossRef]
- Cornel, P.K.; Summers, R.S.; Roberts, P.V. Diffusion of humic acids in dilute aqueous solution. J. Colloid Interface Sci. 1985, 110, 149–164. [Google Scholar] [CrossRef]
- Pinheiro, J.P.; Mota, A.M.; Simoes Goncalves, M.L.S.; van Leeuwen, H.P. The pH effect in the diffusion coefficient of humic matter: Influence in speciation studies using voltammetric techniques. Colloid. Surf. A 1998, 137, 165–170. [Google Scholar] [CrossRef]
- Lead, J.R.; Wilkinson, K.J.; Starchev, K.; Canonica, S.; Buffle, J. Determination of diffusion coefficients of humic substances by fluorescence correlation spectroscopy: Role of solution conditions. Environ. Sci. Technol. 2000, 34, 1365–1369. [Google Scholar] [CrossRef]
- Lead, J.R.; Wilkinson, K.J. Measurement of diffusion coefficients of humic substances in a hydrogel and in water by fluorescence correlation spectroscopy. WIT Trans. Ecol. Environ. 2001, 49, 487–494. [Google Scholar]
- Lead, J.R.; Starchev, K.; Wilkinson, K.J. Diffusion coefficients of humic substances in agarose gel and in water. Environ. Sci. Technol. 2003, 37, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, J.P.; Mota, A.M.; d’Oliveira, J.M.R.; Martinho, J.M.G. Dynamic properties of humic matter by dynamic light scattering and voltammetry. Anal. Chim. Acta 1996, 329, 15–24. [Google Scholar] [CrossRef]
- Aymard, P.; Martin, D.R.; Plucknett, K.; Foster, T.J.; Clark, A.H.; Norton, I.T. Influence of thermal history on the structural and mechanical properties of agarose gels. Biopolymers 2001, 59, 131–144. [Google Scholar] [CrossRef]
- Sedláček, P.; Smilek, J.; Klučáková, M. How interactions with polyelectrolytes affect mobility of low molecular ions—Results from diffusion cells. React. Funct. Polym. 2013, 73, 1500–1509. [Google Scholar] [CrossRef]
- Chen, Y.; Senesi, N.; Schnitzer, M. Information provided on humic substances by E4/E6 ratios. Soil Sci. Soc. Am. J. 1977, 41, 352–358. [Google Scholar] [CrossRef]
- Chen, J.; Gu, B.; LeBoeuf, E.J.; Pan, H.; Dai, S. Spectroscopic characterization of the structural and functional properties of natural organic matter fractions. Chemosphere 2002, 48, 59–68. [Google Scholar] [CrossRef]
- Fuentes, M.; González-Gaitano, G.; García-Mina, J.M. The usefulness of UV–visible and fluorescence spectroscopies to study the chemical nature of humic substances from soils and composts. Org. Geochem. 2006, 37, 1949–1959. [Google Scholar] [CrossRef]
- Klučáková, M.; Kalina, M. Composition, particle size, charge and colloidal stability of pH-fractionated humic acids. J. Soil. Sediment. 2015, 15, 1900–1908. [Google Scholar] [CrossRef]
- Baalousha, M.; Motelica-Heino, M.; Le Coustumer, P. Conformation and size of humic substances: Effects of major cation concentration and type, pH, salinity, and residence time. Colloid. Surf. A 2006, 272, 48–55. [Google Scholar] [CrossRef]
- Klučáková, M. Size and charge evaluation of standard humic and fulvic acids as crucial factors to determine their environmental behavior and impact. Front. Chem. 2018, 6, 1–8. [Google Scholar] [CrossRef]
- Fageria, N.K.; Barbosa Filho, M.P.; Moreira, A.; Guimaraes, C.M. Foliar fertilization of crop plants. J. Plant Nutr. 2009, 32, 1044–1064. [Google Scholar] [CrossRef]
- Gladkov, O.A.; Poloskin, R.B.; Polyakov, Y.J.; Sokolova, I.V.; Sorokin, N.I.; Glebov, A.V. Method for Producing Humic Acid Salts. U.S. Patent 7198805B2, 3 April 2007. [Google Scholar]
- Klučáková, M.; Věžníková, K. Micro-organization of humic acids in aqueous solutions. J. Mol. Struct. 2017, 1144, 33–40. [Google Scholar] [CrossRef]
- Reid, P.M.; Wilkinson, A.E.; Tipping, E.; Jones, M.N. Aggregation of humic substances in aqueous media as determined by light-scattering methods. J. Soil Sci. 1991, 42, 259–270. [Google Scholar] [CrossRef]
- Sedláček, P.; Smilek, J.; Klučáková, M. How interactions with polyelectrolytes affect mobility of ionic dyes in hydrogels. 2. Non-stationary diffusion experiments. React. Funct. Polym. 2014, 75, 41–50. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds (agarose, lignohumate) are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klučáková, M.; Kalina, M.; Enev, V. How the Supramolecular Nature of Lignohumate Affects Its Diffusion in Agarose Hydrogel. Molecules 2020, 25, 5831. https://doi.org/10.3390/molecules25245831
Klučáková M, Kalina M, Enev V. How the Supramolecular Nature of Lignohumate Affects Its Diffusion in Agarose Hydrogel. Molecules. 2020; 25(24):5831. https://doi.org/10.3390/molecules25245831
Chicago/Turabian StyleKlučáková, Martina, Michal Kalina, and Vojtěch Enev. 2020. "How the Supramolecular Nature of Lignohumate Affects Its Diffusion in Agarose Hydrogel" Molecules 25, no. 24: 5831. https://doi.org/10.3390/molecules25245831
APA StyleKlučáková, M., Kalina, M., & Enev, V. (2020). How the Supramolecular Nature of Lignohumate Affects Its Diffusion in Agarose Hydrogel. Molecules, 25(24), 5831. https://doi.org/10.3390/molecules25245831





