Investigation of the Phytochemical Composition, Antioxidant Activity, and Methylglyoxal Trapping Effect of Galega officinalis L. Herb In Vitro
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition of G. officinalis Extracts
2.1.1. Characterization of Quinazoline Alkaloids and Guanidines
2.1.2. Characterization of Phenolic Acids
2.1.3. Characterization of Flavonoids
2.2. Quantification of Polyphenols and Guanidines
2.3. In Vitro Studies
2.3.1. Non-Enzymatic Antioxidant Activity
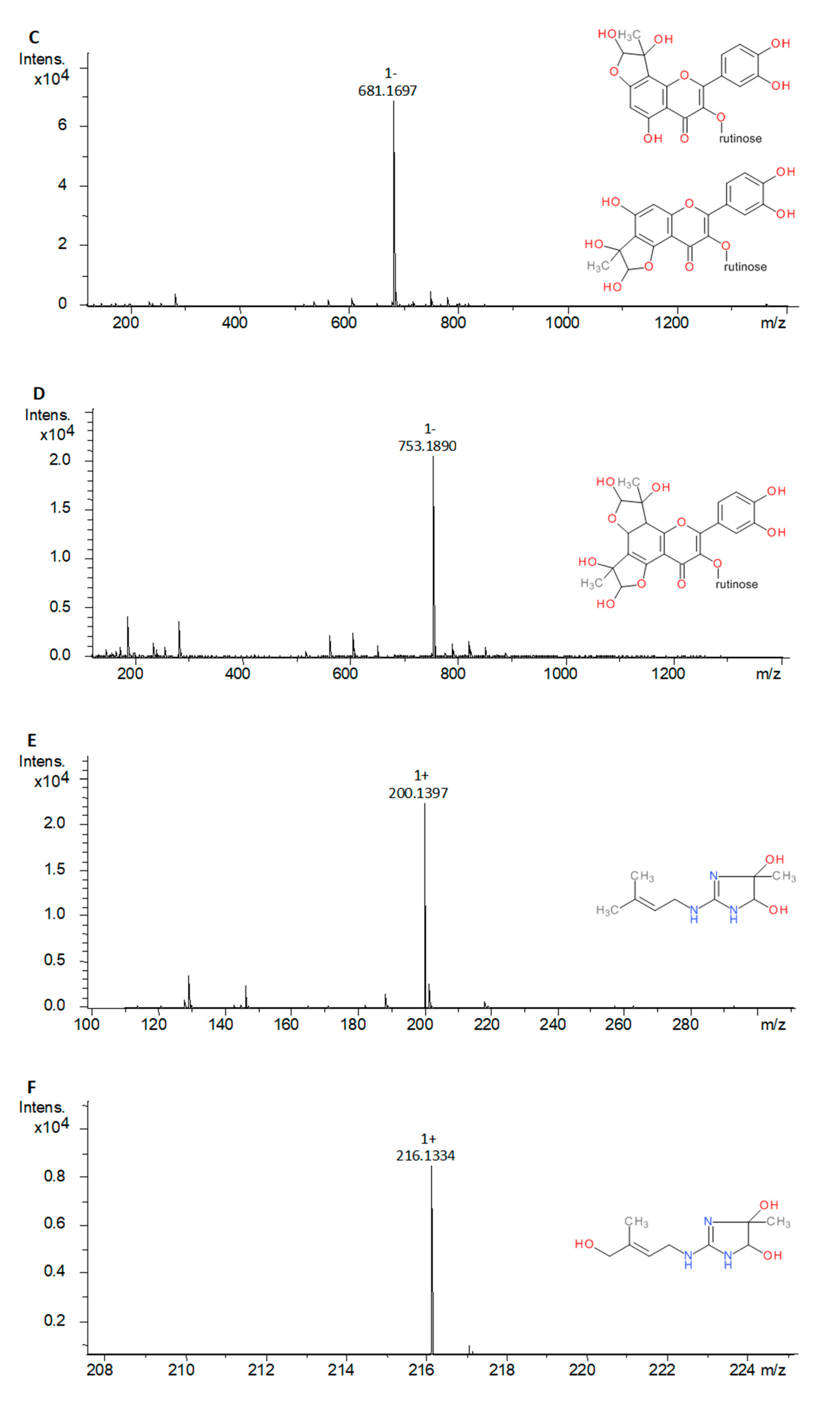
2.3.2. Methylglyoxal Trapping Capacity
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals and Standards
3.3. Preparation of Extracts
3.4. UHPLC-DAD and UHPLC-ESI-MS Analyses
3.5. Validation of Chromatographic Methods and Quantification
3.6. In Vitro Studies
3.6.1. DPPH Radical Scavenging Assay
3.6.2. ABTS Radical Scavenging Assay
3.6.3. Methylglyoxal Trapping Assay
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| T2D | type 2 diabetes mellitus |
| MGO | methylglyoxal |
| G. officinalis | Galega officinalis |
| tR | retention time |
| λmax | absorbance maximum in UV-Vis spectrum |
| ROS | reactive oxygen species |
| RCS | reactive carbonyl species |
| AGEs | advanced glycation end products |
| AOPPs | advanced oxidation end products |
| UHPLC | ultra high-performance liquid chromatography |
| DAD | diode array detector |
| ESI-MS | electrospray ionization mass spectrometry |
| ABTS | 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) |
| DPPH | 2,2-diphenyl-1-(2,4,6-trinitrophenyl)hydrazyl |
| HCAs | hydroxycinnamic acids |
| IC50 | the half maximal inhibitory concentration |
| EIC | extracted ion chromatogram |
| DER | drug extract ratio |
References
- Farzaei, F.; Morovati, M.R.; Farjadmand, F.; Farzaei, M.H. A Mechanistic review on medicinal plants used for diabetes mellitus in traditional Persian medicine. J. Evid. Based Complement. Altern. Med. 2017, 22, 944–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delaviz, H.; Mohammadi, J.; Ghalamfarsa, G.; Mohammadi, B.; Farhadi, N. A review study on phytochemistry and pharmacology applications of Juglans regia plant. Pharmacogn. Rev. 2017, 11, 145. [Google Scholar] [PubMed] [Green Version]
- Witters, L.A. The blooming of the French lilac. J. Clin. Investig. 2001, 108, 1105–1107. [Google Scholar] [CrossRef] [PubMed]
- Tabares, F.P.; Jaramillo, J.V.B.; Ruiz-Cortés, Z.T. Pharmacological Overview of Galactogogues. Vet. Med. Int. 2014, 2014, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Nagalievska, M.; Sabadashka, M.; Hachkova, H.; Sybirna, N. Galega officinalis extract regulate the diabetes mellitus related violations of proliferation, functions and apoptosis of leukocytes. BMC Complement. Altern. Med. 2018, 18, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Keeler, R.F.; Johnson, A.E.; Stuart, L.D. Toxicosis from and possible adaptation to Galega officinalis in sheep and the relationship to verbesina encelioides toxicosis. Vet. Hum. Toxicol. 1986, 28, 309–315. [Google Scholar]
- Anadón, A.; Martínez-Larrañaga, M.R.; Ares, I.; Martínez, M.A. Poisonous Plants of the Europe. In Veterinary Toxicology: Basic and Clinical Principles, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2018; Chapter 62; pp. 891–909. [Google Scholar] [CrossRef]
- Vronsk, L.V.; Timoftevich, N.; Ezhned, M.A.; Barchuk, O. The otherview of of medicinal plants that express hypoglycemic activity. Pharm. Rev. 2014, 2, 142–148. [Google Scholar] [CrossRef]
- British Herbal Medicine Association. British Herbal Pharmacopoeia; The Association: London, UK, 1976; p. 93. [Google Scholar]
- Youngken, H. Textbook of Pharmacognosy, 6th ed.; The Blakiston Company: Philadelphia, PA, USA, 1958; p. 56. [Google Scholar]
- Pulito, C.; Sanli, T.; Rana, P.; Muti, P.; Blandino, G.; Strano, S. Metformin: On ongoing journey across diabetes, cancer therapy and prevention. Metabolites 2013, 3, 1051–1075. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, C. Studies in metabolic changes induced by the administration of guanidine bases. J. Biol. Chem. 1918, 33, 253–265. [Google Scholar] [CrossRef]
- Bretzel, R.G.; Voigt, K.; Schatz, H. The United Kingdom Prospective Diabetes Study (UKPDS). Implications for the pharmacotherapy of type 2 diabetes mellitus. Exp. Clin. Endocrinol. Diabetes 1998, 106, 369–372. [Google Scholar] [CrossRef]
- Kender, Z.; Fleming, T.; Kopf, S.; Torzsa, P.; Grolmusz, V.; Herzig, S.; Schleicher, E.; Rácz, K.; Reismann, P.; Nawroth, P.P. Effect of metformin on methylglyoxal metabolism in patients with type 2 diabetes. Exp. Clin. Endocrinol. Diabetes 2014, 122, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Randell, E.; Vasdev, S.; Gill, V.; Gadag, V.; Newhook, L.A.; Grant, M.; Hagerty, D. Plasma methylglyoxal and glyoxal are elevated and related to early membrane alteration in young, complication-free patients with Type 1 diabetes. Mol. Cell. Biochem. 2007, 305, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.; Matafome, P.; Santos-Silva, D.; Sena, C.; Seiça, R. Reduction of methylglyoxal-induced glycation by pyridoxamine improves adipose tissue microvascular lesions. J. Diabetes Res. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shojaee, S.S.; Vahdati, A.; Assaei, R.; Sepehrimanesh, M. Effect of Galega officinalis leaf powder and Trigonella foenum-graecum seed powder on blood glucose levels and weight gain in a diabetes mellitus rat model. Comp. Clin. Path. 2013, 24, 145–148. [Google Scholar] [CrossRef]
- Pundarikakshudu, K.; Patel, J.K.; Bodar, M.S.; Deans, S.G. Anti-bacterial activity of Galega officinalis L. (Goat’s Rue). J. Ethnopharmacol. 2001, 77, 111–112. [Google Scholar] [CrossRef]
- Atanasov, A.T.; Spasov, V. Inhibiting effect of desalted extract from Galega officinalis L. on platelet aggregation. Folia Med. 1999, 41, 46–50. [Google Scholar]
- Atanasov, A.T.; Chorbanov, B.P.; Dimitrov, B.D. Anti-aggregation activity of crude water extract of Galega officinalis L. fractionated on Sephadex G-25 and Sepharose 4B. Folia Med. 2002, 44, 45–49. [Google Scholar]
- Seyd-Hosein, A.-E.; Majid Shokoohi, A.A.; Asghar, R.; Hamed Shoorei, H.K. Protective effect of Galega officinalis extract on streptozotocin-induced kidney damage and biochemical factor in diabetic rats. Crescent J. Med. Biol. Sci. 2017, 4, 108–114. [Google Scholar]
- Kim, J.H.; Kim, M., II; Syed, A.S.; Jung, K.; Kim, C.Y. Rapid identification of methylglyoxal trapping constituents from onion peels by pre-column incubation method. Nat. Prod. Sci. 2017, 23, 247–252. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zheng, T.; Sang, S.; Lv, L. Quercetin inhibits advanced glycation end product formation by trapping methylglyoxal and glyoxal. J. Agric. Food Chem. 2014, 62, 12152–12158. [Google Scholar] [CrossRef]
- Oldham, M.; Ransom, C.V.; Ralphs, M.H.; Gardner, D.R. Galegine content in goatsrue (Galega officinalis) varies by plant part and phenological growth stage. Weed Sci. 2011, 59, 349–352. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, S.; Reddy, T.J.; Rameshkumar, K.B.; Kumar, B. Screening of tricyclic quinazoline alkaloids in the alkaloidal fraction of Adhatoda beddomei and Adhatoda vasica leaves by high-performance liquid chromatography/electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2015, 29, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Shi, X.; Yang, Y.; Cheng, X.; Liu, Q.; Han, H.; Yang, B.; He, C.; Wang, Y.; Jiang, B.; et al. In vitro and in vivo metabolism and inhibitory activities of vasicine, a potent acetylcholinesterase and butyrylcholinesterase inhibitor. PLoS ONE 2015, 10, e0129759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamagaki, T.; Watanabe, T. Hydrogen radical removal causes complex overlapping Isotope patterns of aromatic carboxylic acids in negative-ion matrix-assisted laser desorption/ionization mass spectrometry. Mass Spectrom. 2012, 1, A0005. [Google Scholar] [CrossRef] [Green Version]
- Hvattum, E.; Ekeberg, D. Study of the collision-induced radical cleavage of flavonoid glycosides using negative electrospray ionization tandem quadrupole mass spectrometry. J. Mass Spectrom. 2003, 38, 43–49. [Google Scholar] [CrossRef]
- Cuyckens, F.; Claeys, M. Determination of the glycosylation site in flavonoid mono-O-glycosides by collision-induced dissociation of electrospray-generated deprotonated and sodiated molecules. J. Mass Spectrom. 2005, 40, 364–372. [Google Scholar] [CrossRef]
- Davis, B.D.; Brodbelt, J.S. An investigation of the hemolytic saccharide cleavage of deprotonated flavonol 3-O-glycosides in a quadrupole ion trap mass spectrometer. J. Mass Spectrom. 2008, 43, 1045–1052. [Google Scholar] [CrossRef]
- Hirose, Y.; Fujita, T.; Ishii, T.; Ueno, N. Antioxidative properties and flavonoid composition of Chenopodium quinoa seeds cultivated in Japan. Food Chem. 2010, 119, 1300–1306. [Google Scholar] [CrossRef]
- Nahrstedt, A.; Hungeling, M.; Petereit, F. Flavonoids from Acalypha indica. Fitoterapia 2006, 77, 484–486. [Google Scholar] [CrossRef]
- Owis, A.I.; El-Hawary, M.S.; El Amir, D.; Aly, O.M.; Abdelmohsen, U.R.; Kamel, M.S. Molecular docking reveals the potential of Salvadora persica flavonoids to inhibit COVID-19 virus main protease. RSC Adv. 2020, 10, 19570. [Google Scholar] [CrossRef]
- Champavier, Y.; Allais, D.P.; Chulia, A.J.; Kaouadji, M. Acetylated and non-acetylated flavonol triglycosides from Galega officinalis. Chem. Pharm. Bull. 2000, 48, 281–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peiretti, P.G.; Gai, F. Fatty acid and nutritive quality of chia (Salvia hispanica L.) seeds and plant during growth. Anim. Feed Sci. Technol. 2009, 148, 267–275. [Google Scholar] [CrossRef]
- Barchuk, O.Z.; Lysiuk, R.M.; Denys, A.I.; Zaliska, O.M.; Smalyuh, O.G. Experimental study of goat’s rue ( Galega Officinalis L.) herb and its liquid extracts. Pharma Innov. J. 2017, 6, 393–397. [Google Scholar]
- Vlassara, H.; Palace, M.R. Diabetes and advanced glycation endproducts. J. Intern. Med. 2002, 251, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Maessen, D.E.M.; Stehouwer, C.D.A.; Schalkwijk, C.G. The role of methylglyoxal and the glyoxalase system in diabetes and other age-related diseases. Clin. Sci. 2015, 128, 839–861. [Google Scholar] [CrossRef] [PubMed]
- Piwowar, A.; Rorbach-Dolata, A.; Fecka, I. The antiglycoxidative ability of selected phenolic compounds—An in vitro study. Molecules 2019, 24, 2689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiveron, A.P.; Melo, P.S.; Bergamaschi, K.B.; Vieira, T.M.F.S.; Regitano-d’Arce, M.A.B.; Alencar, S.M. Antioxidant activity of brazilian vegetables and its relation with phenolic composition. Int. J. Mol. Sci. 2012, 13, 8943–8957. [Google Scholar] [CrossRef] [Green Version]
- Giuffrè, A.M. Bergamot (Citrus bergamia, Risso): The Effects of cultivar and harvest date on functional properties of juice and cloudy juice. Antioxidants 2019, 8, 221. [Google Scholar] [CrossRef] [Green Version]
- Zapata, M.; Chaparro, D.; Rojano, B.; Alzate, A.; Restrepo, L.; Maldonado, M. Effect of storage time on physicochemical, sensorial, and antioxidant characteristics, and composition of mango (cv. Azúcar) juice. Emir. J. Food Agric. 2017, 29, 367–377. [Google Scholar] [CrossRef] [Green Version]
- Vergun, O.; Shymanska, O.; Rakhmetov, D.; Grygorieva, O.; Ivanišová, E.; Brindza, J. Parameters of antioxidant activity of Galega officinalis L. and Galega orientalis Lam. (Fabaceae Lindl.) plant raw material. Potravin. Slovak J. Food Sci. 2020, 14, 125–134. [Google Scholar] [CrossRef]
- Calderon Moreno, R.; Navas-Acien, A.; Escolar, E.; Nathan, D.M.; Newman, J.; Schmedtje, J.F.; Diaz, D.; Lamas, G.A.; Fonseca, V. Potential role of metal chelation to prevent the cardiovascular complications of diabetes. J. Clin. Endocrinol. Metab. 2019, 104, 2931–2941. [Google Scholar] [CrossRef] [PubMed]
- Duraipandiyan, V.; Balachandran, C.; Ignacimuthu, S.; Sankar, C.; Balakrishna, K. Properties of vasicine acetate synthesized from vasicine isolated from Adhatoda vasica L. BioMed Res. Int. 2015, 2015, 7–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhuiyan, M.N.I.; Mitsuhashi, S.; Sigetomi, K.; Ubukata, M. Quercetin inhibits advanced glycation end product formation via chelating metal ions, trapping methylglyoxal, and trapping reactive oxygen species. Biosci. Biotechnol. Biochem. 2017, 81, 882–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gugliucci, A.; Bastos, D.H.M.; Schulze, J.; Souza, M.F.F. Caffeic and chlorogenic acids in Ilex paraguariensis extracts are the main inhibitors of AGE generation by methylglyoxal in model proteins. Fitoterapia 2009, 80, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Kim, H.Y.; Zuo, G.; Wang, Z.; Lee, J.Y.; Lim, S.S. Anti-glycation, carbonyl trapping and anti-inflammatory activities of chrysin derivatives. Molecules 2018, 23, 1752. [Google Scholar] [CrossRef] [Green Version]
- Shao, X. Scavenging Effects of Dietary Flavonoids on Reactive Dicaronyl Species and Their Possible Implications on the Inhibition of the Formation of Advanced Glycation-End Products. Ph.D. Thesis, The State University of New Jersey, New Brunswick, NJ, USA, 2010. [Google Scholar]
- Van Den Eynde, M.D.G.; Geleijnse, J.M.; Scheijen, J.L.J.M.; Hanssen, N.M.J.; Dower, J.I.; Afman, L.A.; Stehouwer, C.D.A.; Hollman, P.C.H.; Schalkwijk, C.G. Quercetin, but not epicatechin, decreases plasma concentrations of methylglyoxal in adults in a randomized, double-blind, placebo-controlled, crossover trial with pure flavonoids. J. Nutr. 2018, 148, 1911–1916. [Google Scholar] [CrossRef]
- Thornalley, P.J. Use of aminoguanidine (pimagedine) to prevent the formation of advanced glycation endproducts. Arch. Biochem. Biophys. 2003, 149, 31–40. [Google Scholar] [CrossRef]
- Kinsky, O.R.; Hargraves, T.L.; Anumol, T.; Jacobsen, N.E.; Dai, J.; Snyder, S.A.; Monks, T.J.; Lau, S.S. Metformin scavenges methylglyoxal to form a novel imidazolinone metabolite in humans. Chem. Res. Toxicol. 2016, 29, 227–234. [Google Scholar] [CrossRef] [Green Version]
- Lo, T.W.C.; Westwood, M.E.; McLellan, A.C.; Selwood, T.; Thornalley, P.J. Binding and Modification of Proteins by Methylglyoxal under Physiological Conditions. J. Biol. Chem. 1994, 269, 32299–32305. [Google Scholar]
- Yeh, W.J.; Hsia, S.M.; Lee, W.H.; Wu, C.H. Polyphenols with antiglycation activity and mechanisms of action: A review of recent findings. J. Food Drug Anal. 2017, 25, 84–92. [Google Scholar] [CrossRef]
- Mooney, M.H.; Fogarty, S.; Stevenson, C.; Gallagher, A.M.; Palit, P.; Hawley, S.A.; Hardie, D.G.; Coxon, G.D.; Waigh, R.D.; Tate, R.J.; et al. Mechanisms underlying the metabolic actions of galegine that contribute to weight loss in mice. Br. J. Pharmacol. 2008, 153, 1669–1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rachana, R.; Basu, S.; Pant, M.; Priyanka, K.M.; Sonam, S. Review & Future Perspectives of Using Vasicine, and Related Compounds. Indo Glob. J. Pharm. Sci. 2011, 1, 85–98. [Google Scholar]
- Rachana, R.; Pant, M.; Basu, S. Cytoprotective activity of Adhatoda vasica extract and vasicine against tobacco smoke induced cytotoxicity. J. Pharm. Technol. Res. Manag. 2013, 1, 109–117. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, L.; Ding, M.; Li, M. Protective effect of vasicine against myocardial infarction in rats via modulation of oxidative stress, inflammation, and the PI3K/AKT pathway. Drug Des. Devel. Ther. 2019, 13, 3773–3784. [Google Scholar] [CrossRef] [Green Version]
- Wakhloo, R.L.; Kaul, G.; Gupta, O.P.; Atal, C.K. Safety of vasicine hydrochloride in human volunteers. Indian J. Pharmacol. 1980, 12, 129–131. [Google Scholar]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Chen, L.; Kang, Y.H. Antioxidant and enzyme inhibitory activities of plebeian herba (Salvia plebeia R. Br.) under different cultivation conditions. J. Agric. Food Chem. 2014, 62, 2190–2197. [Google Scholar] [CrossRef]
- Sang, S.; Shao, X.; Bai, N.; Lo, C.Y.; Yang, C.S.; Ho, C.T. Tea polyphenol (-)-epigallocatechin-3-gallate: A new trapping agent of reactive dicarbonyl species. Chem. Res. Toxicol. 2007, 20, 1862–1870. [Google Scholar] [CrossRef]
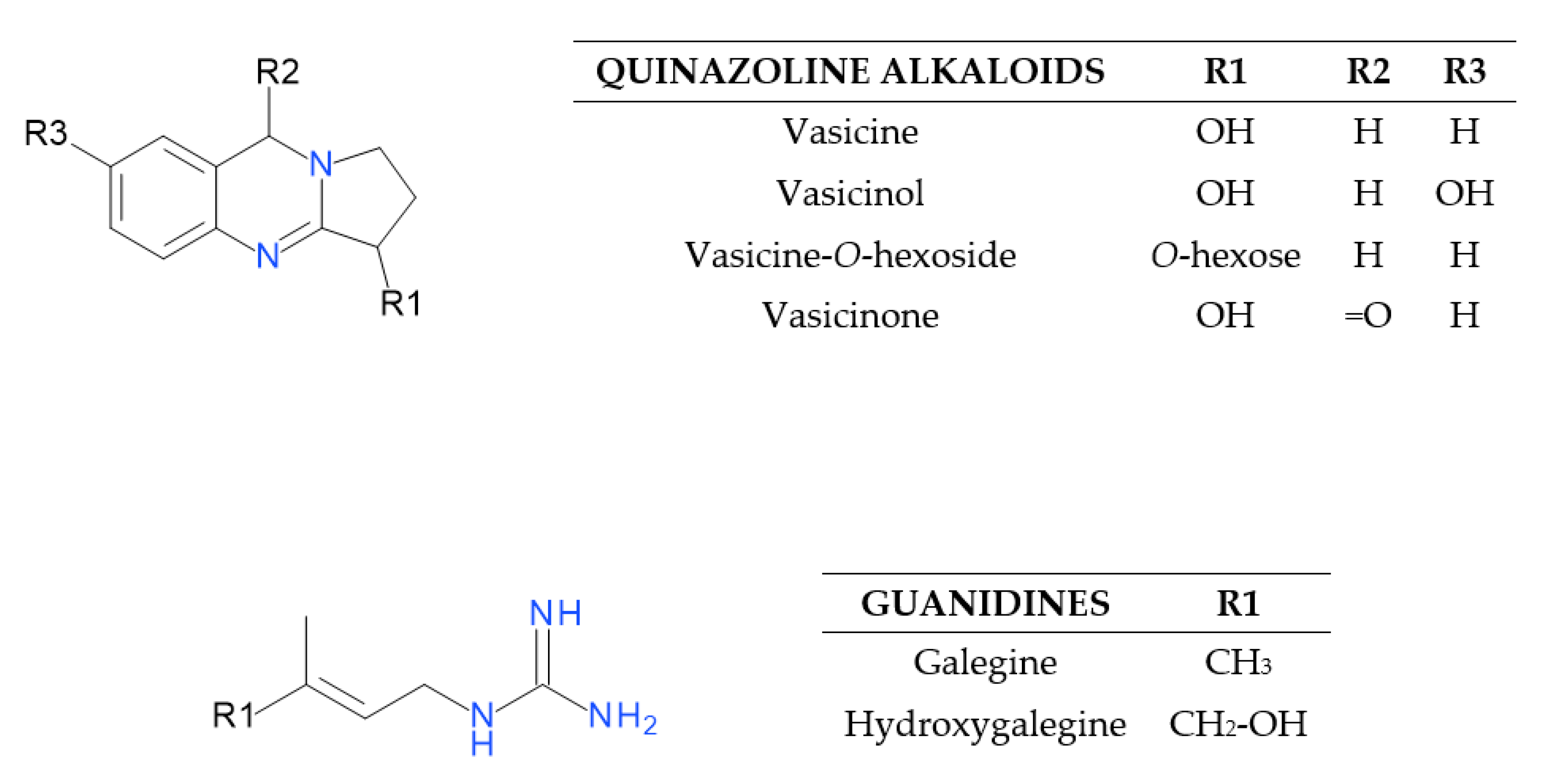
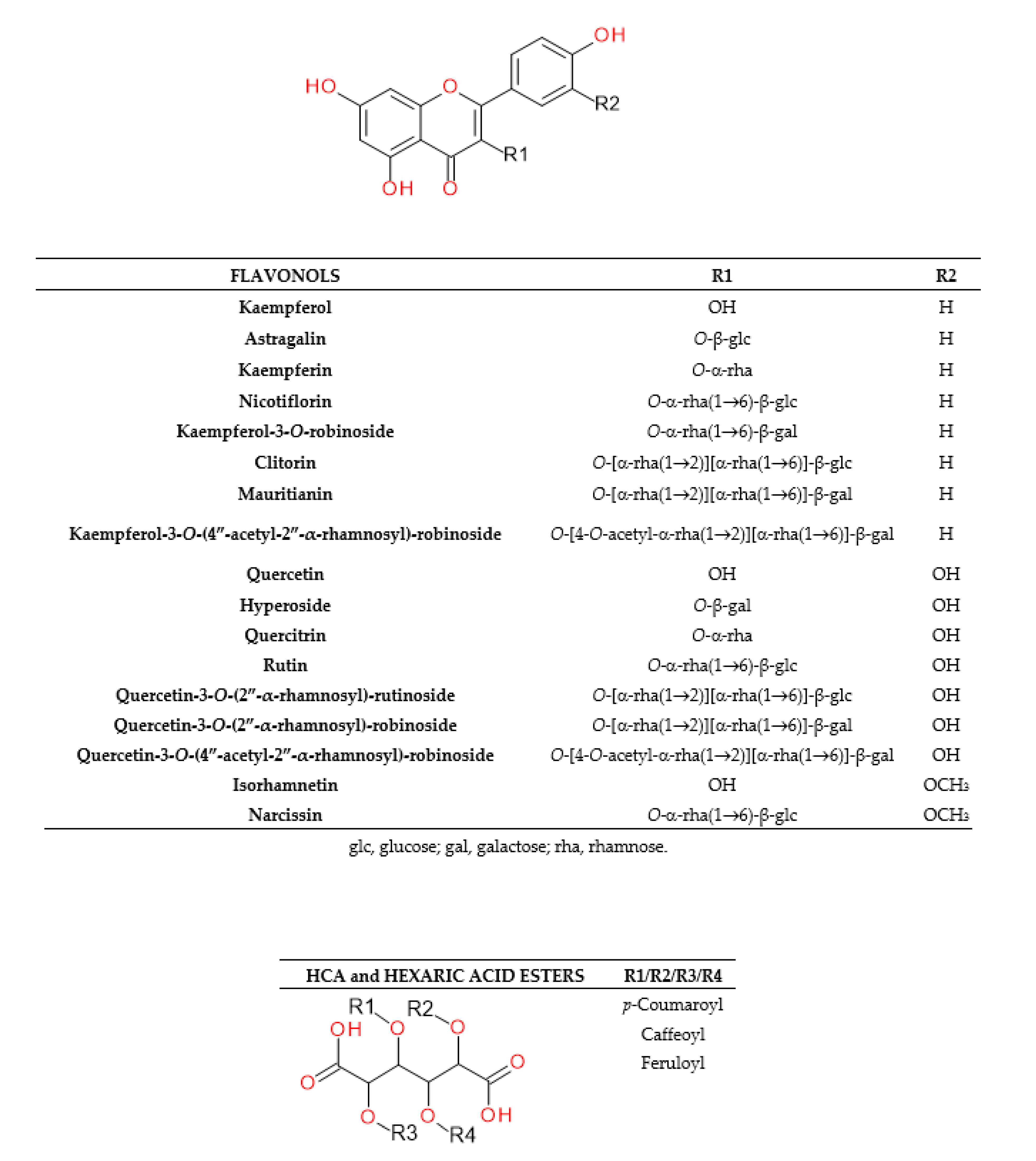
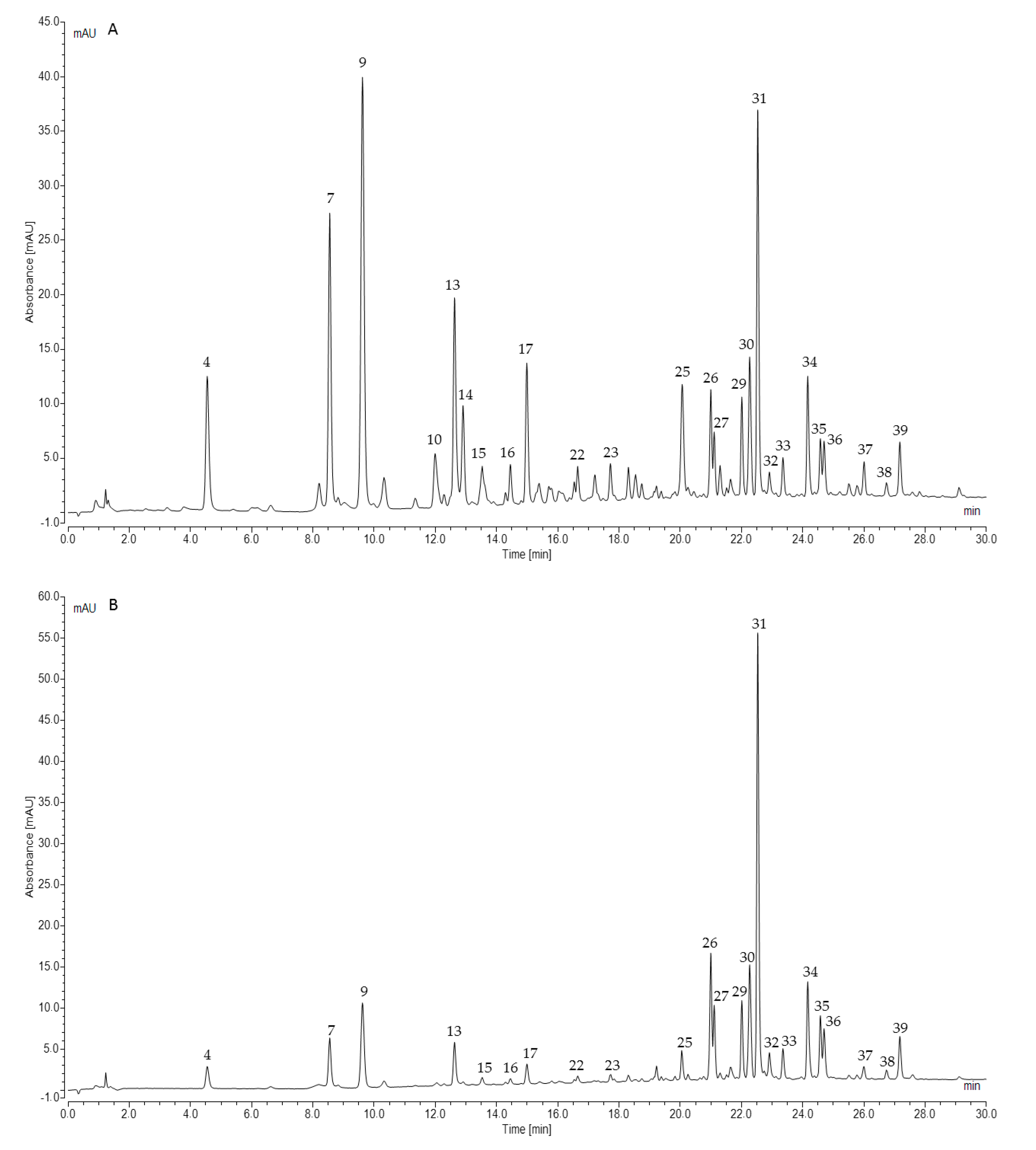

| Peak No. | tR [min] | λmax [nm] | [M – H]− (m/z) | Error [ppm] | MS/MS (m/z) | Identification Proposal |
|---|---|---|---|---|---|---|
| [M + H]+ (m/z) | ||||||
| 1 | 0.89 | 200 | 209.0309 | −1.6 | 165 [M − 44/CO2 − H]− | Hexaric acid |
| 2 | 1.28 | 250 | 191.0203 | −1.8 | 111 [M − 44/CO2 − 36/2xH2O − H]− | Hexaric acid monolactone |
| 3 | 1.49 | – | 144.1132 | −1.2 | - | Hydroxygalegine |
| 4 | 4.32 | 325 | 371.0618 | 0.4 | 209 [M − 162/caffeoyl − H]−, 191 [209 − 18/H2O − H]−, 147 [209 − 18/H2O − 44/CO2 − H]−, 179 [CA − H]−, 135 [CA − 44/CO2 − H]−, 129 [209 − 44/CO2 − 36/2xH2O − H]−, 111 [209 − 44/CO2 − 54/3xH2O − H]− | Monocaffeoylhexaric acid 1 |
| 5 | 5.48 | – | 205.0977 | −2.9 | 187 [M − 18/H2O + H]+ | Vasicinol |
| 6 | 7.5 | – | 128.1185 | −2.2 | - | Galegine |
| 7 | 8.34 | 325 | 371.0613 | 1.8 | 209 [M − 162/caffeoyl − H]−, 191 [209 − 18/H2O − H]−, 147 [209 − 18/H2O − 44/CO2 − H]−, 179 [CA − H]−, 135 [CA − 44/CO2 − H]−, 129[209 − 44/CO2 − 36/2xH2O − H]−, 111 [209 − 44/CO2 − 54/3xH2O − H]− | Monocaffeoylhexaric acid 2 |
| 8 | 8.74 | 206, 285 | 189.1029 | −3.5 | 171 [M − 18/H2O + H]+, 144 [M − 18/H2O − 27/CHN + H]+, 118 [M − 18/H2O − 27/CHN − 26/C2H2 + H]+ | Vasicine |
| 9 | 9.49 | 325 | 371.0621 | −0.1 | 209 [M − 162/caffeoyl − H]−, 191 [209 − 18/H2O − H]−, 147 [209 − 18/H2O − 44/CO2 − H]−, 179 [CA − H]−, 135 [CA − 44/CO2 − H]−, 129[209 − 44/CO2 − 36/2xH2O − H]−, 111 [209 − 44/CO2 − 54/3xH2O − H]− | Monocaffeoylhexaric acid 3 |
| 10 | 11.84 | 313 | 355.0671 | 0.9 | 209 [M − 146/coumaroyl − H]−, 191 [209 − 18/H2O − H]−, 147 [209 − 18/H2O − 44/CO2 − H]−, 163 [CuA − H]−, 129 [209 − 44/CO2 − 36/2xH2O − H]−, 119 [CuA − 4/CO2 − H]−, 111 [209 − 44/CO2 − 54/3xH2O − H]− | Monocoumaroylhexaricacid 1 |
| 11 | 11.92 | 285 | 285.0617 | 0.6 | 152/153 a [M − 132/pentose − H]−, 108/109 a [PA − 44/CO2 − H]− | Protocatechuic acid O-pentoside |
| 12 | 12.31 | 208 | 351.1565 | −0.2 | 189 [M − 162/hexose + H]+, 171 [M − 162/hexose − 18/H2O + H]+ | Vasicine-O-hexoside |
| 13 | 12.43 | 325 | 371.0614 | 1.6 | 209 [M − 162/caffeoyl − H]−, 191 [209 − 18/H2O − H]−, 147 [209 − 18/H2O − 44/CO2 − H]−, 179 [CA − H]−, 135 [CA − 44/CO2 − H]−, 129[209 − 44/CO2 − 36/2xH2O − H]−, 111 [209 − 44/CO2 − 54/3xH2O − H]− | Monocaffeoylhexaric acid 4 |
| 14 | 12.71 | 314 | 355.0671 | 0.4 | 209 [M − 146/coumaroyl − H]−, 191 [209 − 18/H2O − H]−, 147 [209 − 18/H2O − 44/CO2 − H]−, 163 [CuA–H]−, 129 [209 − 44/CO2 − 36/2xH2O − H]−, 119 [CuA − 44/CO2 − H]−, 111 [209 − 44/CO2 − 54/3xH2O − H]− | Monocoumaroylhexaricacid 2 |
| 15 | 13.24 | 325 | 385.0765 | 0.9 | 209 [M − 176/feruloyl − H]−, 193 [FeA − 191 − H]−, 191 [M − 193 − H or 209 − 18/H2O − H]−, 147 [209 − 18/H2O − 44/CO2 − H]−, 111 [209 − 44/CO2 − 54/3xH2O − H]− | Monoferuloylhexaric acid 1 |
| 16 | 14.3 | 325 | 385.0775 | 1.0 | 209 [M − 176/feruloyl − H]−, 193 [FeA − H]−, 191 [M − 193 − H or 209 − 18/H2O − H]−, 147 [209 − 18/H2O − 44/CO2 − H]−, 111 [209 − 44/CO2 − 54/3xH2O − H]− | Monoferuloylhexaric acid 2 |
| 17 | 14.82 | 325 | 385. 0773 | 0.7 | 209 [M − 176/feruloyl − H]−, 193 [FeA − H]−, 191 [M − 193 − H or 209 − 18/H2O − H]−, 149 [194v − 44/CO2 − H]−, 147 [209 − 18/H2O − 44/CO2 − H]−, 111 [209 − 44/CO2 − 54/3xH2O − H]− | Monoferuloylhexaric acid 3 |
| 18 | 15.1 | 315 | 325.0922 | 1.5 | 163 [M − 162/hexose − H]−, 119 [CuA − 44/CO2 − H]− | Coumaric acid O-hexoside |
| 19 | 15.12 | 285 | 417.1050 | −1.6 | 152/153 a [M − 264/2 pentose − H]−, 108/109 a [PA − 44/CO2 − H]− | Protocatechuic acid O-di-pentoside |
| 20 | 15.21 | 312 | 355.0665 | 1.2 | 209 [M − 146/coumaroyl − H]−, 191 [209 − 146–18/H2O − H]−, 147 [209 − 18 − 44/CO2 − H]−, 163 [CuA − H]−, 129 [209 − 44/CO2 − 36/2xH2O − H]−, 119 [CuA − 44/CO2 − H]−, 111 [209 − 44/CO2 − 54/3xH2O − H]− | Monocoumaroylhexaricacid 3 |
| 21 | 15.58 | 211 | 203.0825 | −4.2 | 185 [M–18/H2O+H]+ | Vasicinone |
| 22 | 16.54 | 325 | 385.0753 | 1.3 | 209 [M − 176/feruloyl − H]−, 193 [385 − 191 − H]−, 191 [M − 193 − H]−, 147 [191 − 44/CO2 − H]−, 111 [209 − 44/CO2 − 54/3xH2O − H]− | Monoferuloylhexaric acid 4 |
| 23 | 17.59 | 325 | 353.0512 | 1.8 | 191 [M − 162/caffeoyl − H]−, 179 [CA − H]−, 111 [QA − 44/CO2 − 36/2xH2O − H]− | Chlorogenicacid S |
| 24 | 18.39 | 312 | 163.0401 | −0.1 | 119 [M − 44/CO2 − H]− | p-Coumaricacid S |
| 25 | 19.98 | 290 | 465.1030 | 1.8 | 303 [M − 162/hexose − H]−, 285 [M − 162/hexose − 18/H2O − H]− | Taxifolin-3-O-hexoside |
| 26 | 20.94 | 254, 354 | 755.2036 | 1.2 | 609 [M − 146/deoxyhexose − H]−, 300/301 a [M − 2 × 146/dideoxyhexose − 162/hexose − H]− | Quercetin-3-O-dideoxyhexosyl– hexoside 1 |
| 757.2224 | 1.3 | 611[M − 146/deoxyhexose + H]+, 303 [M − 2 × 146/dideoxyhexose − 162/Hexose + H]+ | ||||
| 27 | 21.06 | 254, 354 | 755.2038 | 0.4 | 609 [M − 146/deoxyhexose − H]−, 300/301 a [M – 2 × 146/dideoxyhexose – 162/Hexose – H]− | Quercetin-3-O-dideoxyhexosyl- hexoside 2 |
| 757.2215 | 0.4 | 465 [M–2 × 146/dideoxyhexose + H]+, 303 [M – 2 × 146/dideoxyhexose – 162/Hexose + H]+ | ||||
| 28 | 21.61 | 265, 344 | 447.0942 | −0.7 | 285 [M − 162/glucose – H]− | Kaempferol-3-O-glucoside (astragalin S) |
| 29 | 21.97 | 265, 344 | 739.2088 | 0.5 | 284/285 a [M – 2 × 146/dideoxyhexose − 162/hexose–H]− | Kaempferol-3-O-dideoxyhexosyl- hexoside 1 |
| 30 | 22.21 | 265, 344 | 739.2083 | 1.0 | 284/285 a [M – 2 × 146/dideoxyhexose − 162/hexose − H]− | Kaempferol-3-O-dideoxyhexosyl- hexoside 2 |
| 31 | 22.47 | 255, 353 | 609.1416 | −0.2 | 300/301 a [M − 308/rutinose − H]− | Quercetin-3-O-rutinoside (rutin S) |
| 611.1634 | 0,2 | 303 [M − 308/rutinose + H]+ | ||||
| 32 | 22.89 | 255, 353 | 463.0870 | 1.8 | 300/301 a [M − 162/galactose − H]− | Quercetin-3-O-galactoside (hyperoside S) |
| 465.1037 | −1.7 | 303 [M − 162 + H/galactose]+ | ||||
| 33 | 23.32 | 263, 368 | 593.1495 | 3.1 | 284/285 a [M − 308/rutinose − H]− | Kaempferol-3-O-deoxyhexosyl- hexoside 1 (nicotiflorin S) |
| 595.1681 | −3.4 | 287 [M − 308/rutinose + H]+ | ||||
| 34 | 24.12 | 263, 368 | 593.1505 | 1.0 | 284/285 a [M − 308/deoxyhexose-hexose − H]− | Kaempferol-3-O-deoxyhexosyl- hexoside 2 |
| 595.1683 | −3.8 | 287 [M − 308/deoxyhexose-hexose + H]+ | ||||
| 35 | 24.53 | 255, 353 | 623.1608 | 2.7 | 315 [M − 308/rutinose − H]−, 300 [M − 308/rutinose − 15/Me• − H]−• | Isorhamnetin-3-O-rutinoside (narcissin S) |
| 625.1787 | −4.0 | 317 [M − 308/rutinose + H]+, 300 [M − 308/rutinose − 15/Me• + H]+• | ||||
| 36 | 24.66 | 254, 348 | 447.0924 | 2.9 | 300/301 a [M − 146/rhamnose − H]− | Quercetrin-3-O-rhamnoside (quercitrin S) |
| 37 | 25.96 | 255, 353 | 797.2135 | 2.5 | 300/301 a [M – 2 × 146/dideoxyhexose − 162/hexose − 42/acetyl − H]− | Quercetin-3-O-acetyl- dideoxyhexosyl-hexoside |
| 38 | 26.69 | 263, 368 | 431.0973 | 2.2 | 284/285 a [M − 146/deoxyhexose − H]− | Kaempferol-3-O-deoxyhexoside |
| 39 | 27.13 | 264, 368 | 781.2192 | −0.1 | 284/285 a [M – 2 × 146/dideoxyhexose − 162/hexose − 42/acetyl − H]− | Kaempferol-3-O-acetyl- dideoxyhexosyl-hexoside |
| 783.2373 | −2.7 | 287 [M – 2 × 146/dideoxyhexose − 162/hexose − 42/acetyl + H]+ |
| Compound | tR [min] | Gof1 | Gof2 | Gof3 | Average |
|---|---|---|---|---|---|
| Content [mg/g] of DW | |||||
| Flavonoids | |||||
| Taxifolin-3-O-hexoside (25) a | 19.98 | 1.67 ± 0.20 | 0.26 ± 0.01 | 0.03 ± 0.00 | 0.68 ± 0.65 |
| Quercetin-derivative (26) b | 20.94 | 0.59 ± 0.01 | 0.91 ± 0.02 | 0.74 ± 0.03 | 0.75 ± 0.13 |
| Quercetin-derivative (27) b | 21.06 | 0.32 ± 0.01 | 0.47 ± 0.01 | 0.32 ± 0.01 | 0.37 ± 0.07 |
| Clitorin (29) b | 21.97 | 0.32 ± 0.01 | 0.47 ± 0.01 | 0.28 ± 0.02 | 0.35 ± 0.08 |
| Mauritianin (30) b | 22.21 | 0.67 ± 0.01 | 0.82 ± 0.02 | 0.47 ± 0.01 | 0.65 ± 0.15 |
| Rutin (31) | 22.47 | 1.70 ± 0.04 | 3.31 ± 0.07 | 2.17 ± 0.08 | 2.43 ± 0.69 |
| Hyperoside (32) | 22.89 | 0.08 ± 0.01 | 0.20 ± 0.01 | 0.10 ± 0.01 | 0.13 ± 0.05 |
| Nicotiflorin (33) b | 23.32 | 0.14 ±0.01 | 0.20 ± 0.00 | 0.13 ± 0.01 | 0.15 ± 0.03 |
| Kaempferol-3-O-robinoside (34) b | 24.12 | 0.39 ± 0.19 | 0.65 ± 0.01 | 0.44 ± 0.04 | 0,49 ± 0.06 |
| Narissin (35) b | 24.53 | 0.23 ± 0.01 | 0.35 ± 0.01 | 0.13 ± 0.01 | 0.24 ± 0.09 |
| Quercitrin (36) b | 24.66 | 0.13 ± 0,01 | 0.44 ± 0.02 | 0.02 ± 0.01 | 0.20 ± 0.19 |
| Kaempferol-derivative (39) b | 27.13 | 0.20 ±0.01 | 0.26 ± 0.01 | 0.14 ± 0.01 | 0.19 ± 0.05 |
| Sum of flavonoids | 6.44 ± 0.52 | 8.34 ± 0.20 | 4.97 ± 0.24 | 6.63 ± 2.24 | |
| Hydroxycinnamic acids | |||||
| Monocaffeoylhexaric acid isomer 1 (4) c | 4.32 | 0.39 ± 0.03 | 0.57 ± 0.02 | 0.59 ± 0.03 | 0.52 ± 0.09 |
| Monocaffeoylhexaric acid isomer 2 (7) c | 8.34 | 0.75 ± 0.02 | 1.05 ± 0.04 | 1.02 ± 0.03 | 0.95 ± 0.14 |
| Monocaffeoylhexaric acid isomer 3 (9) c | 9.49 | 1.12 ± 0.05 | 1.58 ± 0.05 | 1.64 ± 0.22 | 1.46 ± 0.26 |
| Monocaffeoylhexaric acid isomer 4 (13) c | 12.43 | 0.38 ±0.03 | 0.49 ± 0.02 | 0.48 ± 0.02 | 0.46 ± 0.05 |
| Monocoumaroylhexaric acid isomer 1 (10) d | 11.84 | 0.20 ± 0.03 | 0.20 ± 0.01 | 0.27 ± 0.01 | 0.22 ± 0.03 |
| Monocoumaroylhexaric acid isomer 2 (14) d | 12.71 | 0.35 ± 0.03 | 0.47 ± 0.04 | 0.36 ± 0.01 | 0.39 ± 0.06 |
| Monoferuloylhexaric acid isomer 1 (15) e | 13.24 | 0.20 ±0.01 | 0.25 ± 0.01 | 0.25 ± 0.01 | 0.23 ± 0.02 |
| Monoferuloylhexaric acid isomer 2 (16) e | 14.30 | 0.12 ± 0.01 | 0.14 ± 0.01 | 0.16 ± 0.01 | 0.14 ± 0.01 |
| Monoferuloylhexaric acid isomer 3 (17) e | 14.82 | 0.32 ± 0.02 | 0.45 ± 0.06 | 0.47 ± 0.02 | 0.42 ± 0.07 |
| Monocoumaroylhexaric acid isomer 3 (20) d | 15.21 | 0.09 ± 0.01 | 0.07 ± 0.01 | 0.12 ± 0.03 | 0.09 ± 0.02 |
| Monoferuloylhexaric acid isomer 4 (22) e | 16.54 | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.06 ± 0.01 |
| Chlorogenic acid (23) | 17.59 | 0.09 ± 0.01 | 0.12 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 |
| Sum of hydroxycinnamic acids | 4.27 ± 0.26 | 5.45 ± 0.29 | 5.53 ± 0.41 | 5.04 ± 0.77 | |
| Sum of polyphenols | 10.71 ± 0.78 | 13.79 ± 0.49 | 10.5 ± 0.65 | 11.67 ± 3.01 | |
| Guanidines | |||||
| Hydroxygalegine (3) f | 1.49 | 1.98 ± 0.03 | 1.11 ± 0.06 | 1.95 ± 0.04 | 1.68 ± 0.12 |
| Galegine (6) | 7.50 | 4.28 ± 0.04 | 9.38 ± 0.40 | 6.08 ± 0.17 | 6.58 ± 0.61 |
| Sum of guanidines | 6.26 ± 0.07 | 10.49 ± 0.46 | 8.03 ± 0.21 | 8.26 ± 0.73 | |
| Sample | DPPH | ABTS | ||||
|---|---|---|---|---|---|---|
| IC50 [µg/mL] | IC50 [μM] | % of Inhibition a | IC50 [µg/mL] | IC50 [μM] | % of Inhibition b | |
| Aq. methanol (1:1) c | 11.72 d | - | 73.00 | 0.94 d | - | 85.31 |
| Water infusion c | 12.97 d | - | 68.80 | 1.06 d | - | 82.34 |
| Chlorogenic acid | 27.60 | 77.90 | 33.08 | 2.62 | 7.41 | 37.00 |
| Rutin | 22.29 | 36.51 | 42.91 | 4.07 | 6.66 | 31.02 |
| Quercetin | 8.49 | 28.08 | 91.76 | 1.25 | 4.13 | 87.14 |
| Galegine sulfate | 1656.07 | 13,020.31 | 0.90 | 42.52 | 334.32 | 0 |
| Metformin hydrochloride | 0 | 0 | 0 | 0 | 0 | 0 |
| Gallic acid | 2.93 | 17.25 | >100 | - | - | - |
| Trolox | - | - | - | 1.48 | 5.90 | 61.37 |
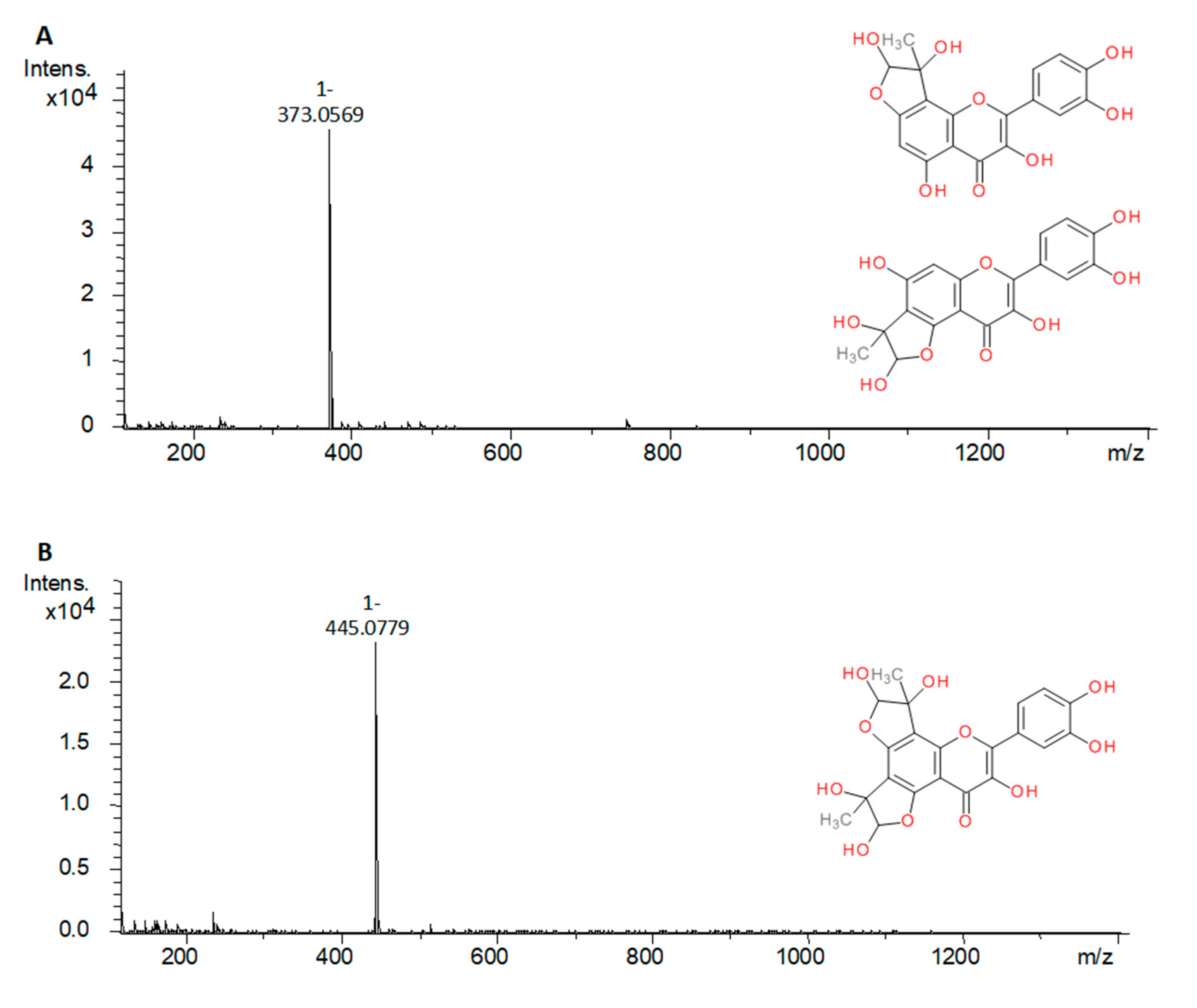
| Compound | Source | Peak | Mono-MGO Adduct (m/z) | di-MGO Adduct (m/z) |
|---|---|---|---|---|
| Chlorogenic acid | S | - | n.d. | n.d. |
| Rutin | S | a | 681.1682 [M − H]− | 753.1892 [M − H]− |
| b | 681.1695 [M − H]− | 753.1890 [M − H]− | ||
| c | 681.1683 [M − H]− | 753.1885 [M − H]− | ||
| Inf | a | 681.1684 [M − H]− | n.d. | |
| Quercetin | S | a | 373.0569 [M − H]− | 445.0779 [M − H]− |
| b | 373.0564 [M − H]− | n.d. | ||
| Galegine sulfate | S | a | 200.1367 [M + H]+ | n.d. |
| b | 200.1364 [M + H]+ | n.d. | ||
| c | 200.1364 [M + H]+ | n.d. | ||
| Galegine | Inf | a | 200.1397 [M + H]+ | n.d. |
| b | 200.1386 [M + H]+ | n.d. | ||
| c | 200.1388 [M + H]+ | n.d. | ||
| Hydroxygalegine | Inf | a | 216.1334 [M + H]+ | n.d. |
| Metformin hydrochloride | S | a | 202.1282 [M + H]+ | n.d. |
| Compound | Method | Λ [nm] | Linear Equation | R2 | Range [µg/mL] | LOD [µg/mL] | LOQ [µg/mL] |
|---|---|---|---|---|---|---|---|
| Chlorogenic acid | UHPLC-DAD | 320 | y = 0.0051x – 0.0009 | 0.9999 | 10–250 | 0.16 | 0.50 |
| Caffeic acid | UHPLC-DAD | 320 | y = 0.00301x + 0.00047 | 0.9999 | 10–250 | 0.56 | 1.87 |
| p–Coumaric acid | UHPLC-DAD | 320 | y = 0.00250x − 0.00003 | 0.9999 | 10–250 | 0.57 | 1.90 |
| Ferulic acid | UHPLC-DAD | 320 | y = 0.00321x + 0.00018 | 0.9999 | 10–250 | 0.48 | 1.61 |
| Hyperoside | UHPLC-DAD | 360 | y = 0.00612x + 0.00007 | 0.9999 | 10–400 | 0.11 | 0.36 |
| Rutin | UHPLC-DAD | 360 | Y = 0.0097x − 0.000007 | 0.9999 | 10–400 | 0.16 | 0.50 |
| Taxifolin | UHPLC-DAD | 280 | y = 0.02357x + 0.00163 | 0.9999 | 10–400 | 0.08 | 0.28 |
| Galegine sulfate | UHPLC-ESI-MS | [M + H]+ | y = 1.00029x − 0.00008 | 0.9985 | 10–400 | 0.01 | 0.03 |
Sample Availability: Samples of the plants and compounds are available from authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bednarska, K.; Kuś, P.; Fecka, I. Investigation of the Phytochemical Composition, Antioxidant Activity, and Methylglyoxal Trapping Effect of Galega officinalis L. Herb In Vitro. Molecules 2020, 25, 5810. https://doi.org/10.3390/molecules25245810
Bednarska K, Kuś P, Fecka I. Investigation of the Phytochemical Composition, Antioxidant Activity, and Methylglyoxal Trapping Effect of Galega officinalis L. Herb In Vitro. Molecules. 2020; 25(24):5810. https://doi.org/10.3390/molecules25245810
Chicago/Turabian StyleBednarska, Katarzyna, Piotr Kuś, and Izabela Fecka. 2020. "Investigation of the Phytochemical Composition, Antioxidant Activity, and Methylglyoxal Trapping Effect of Galega officinalis L. Herb In Vitro" Molecules 25, no. 24: 5810. https://doi.org/10.3390/molecules25245810
APA StyleBednarska, K., Kuś, P., & Fecka, I. (2020). Investigation of the Phytochemical Composition, Antioxidant Activity, and Methylglyoxal Trapping Effect of Galega officinalis L. Herb In Vitro. Molecules, 25(24), 5810. https://doi.org/10.3390/molecules25245810






