Sensitive Analysis of Idarubicin in Human Urine and Plasma by Liquid Chromatography with Fluorescence Detection: An Application in Drug Monitoring
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of the Sample Preparation
2.2. Optimization of LC Parameters
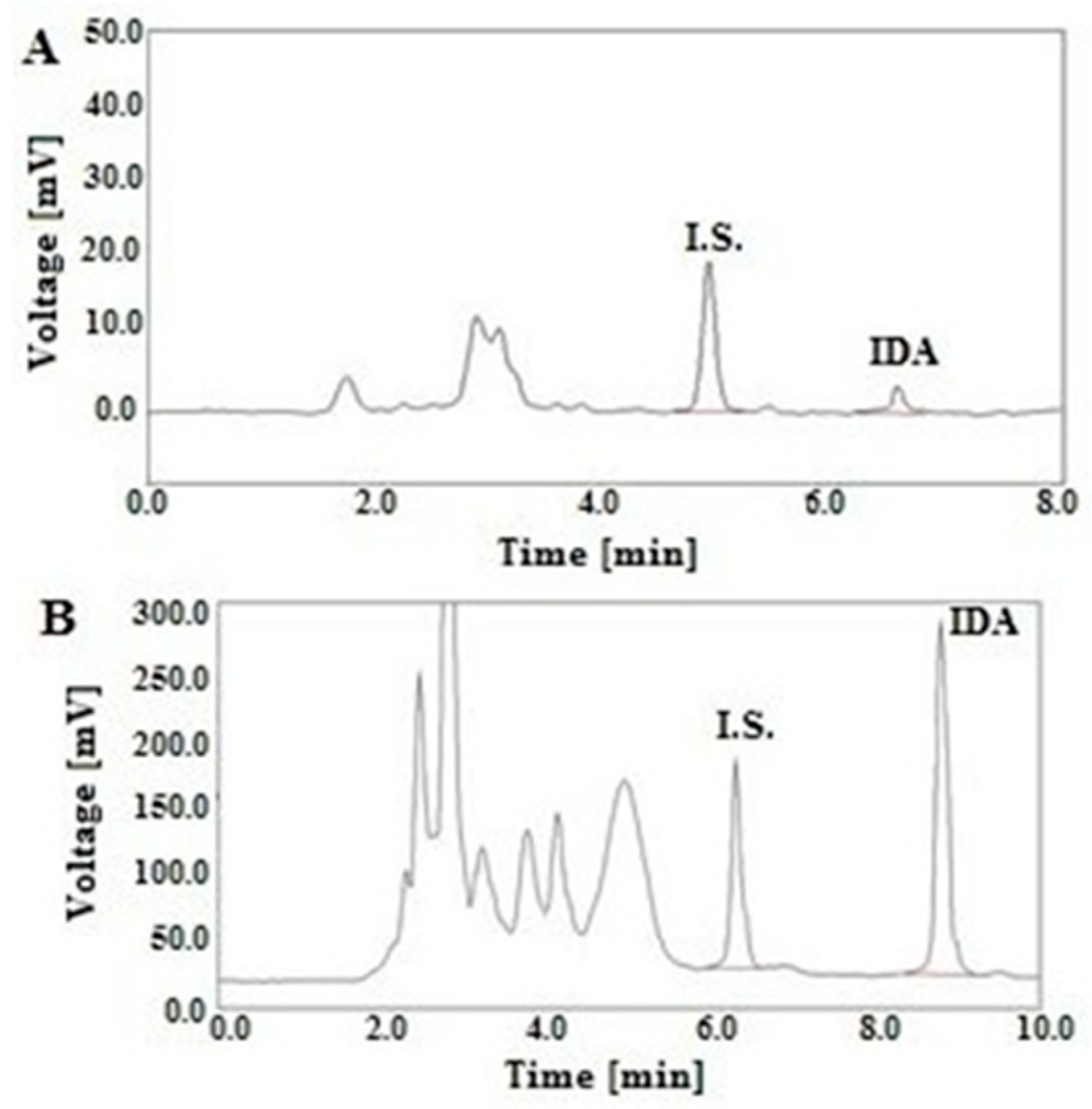
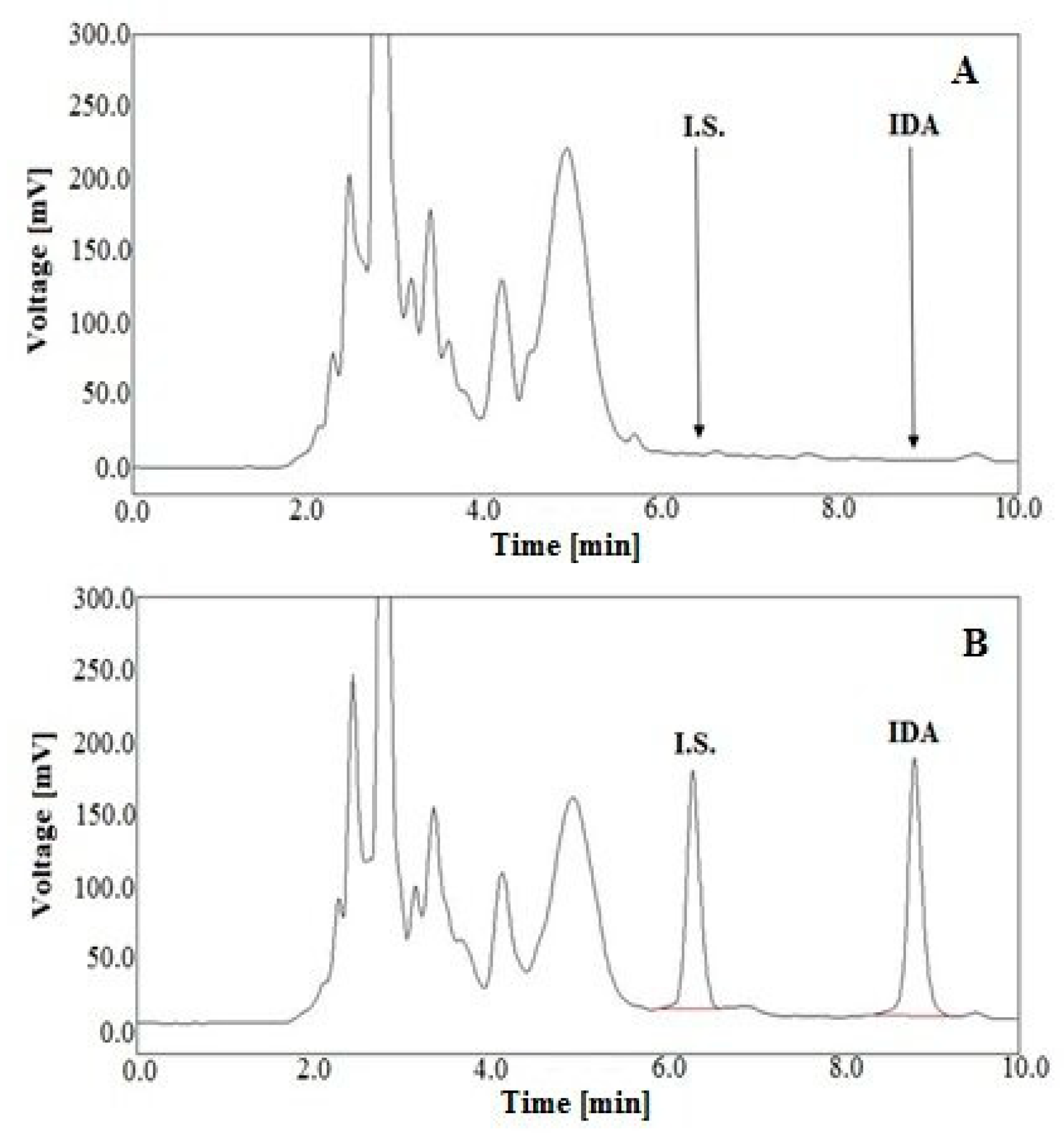
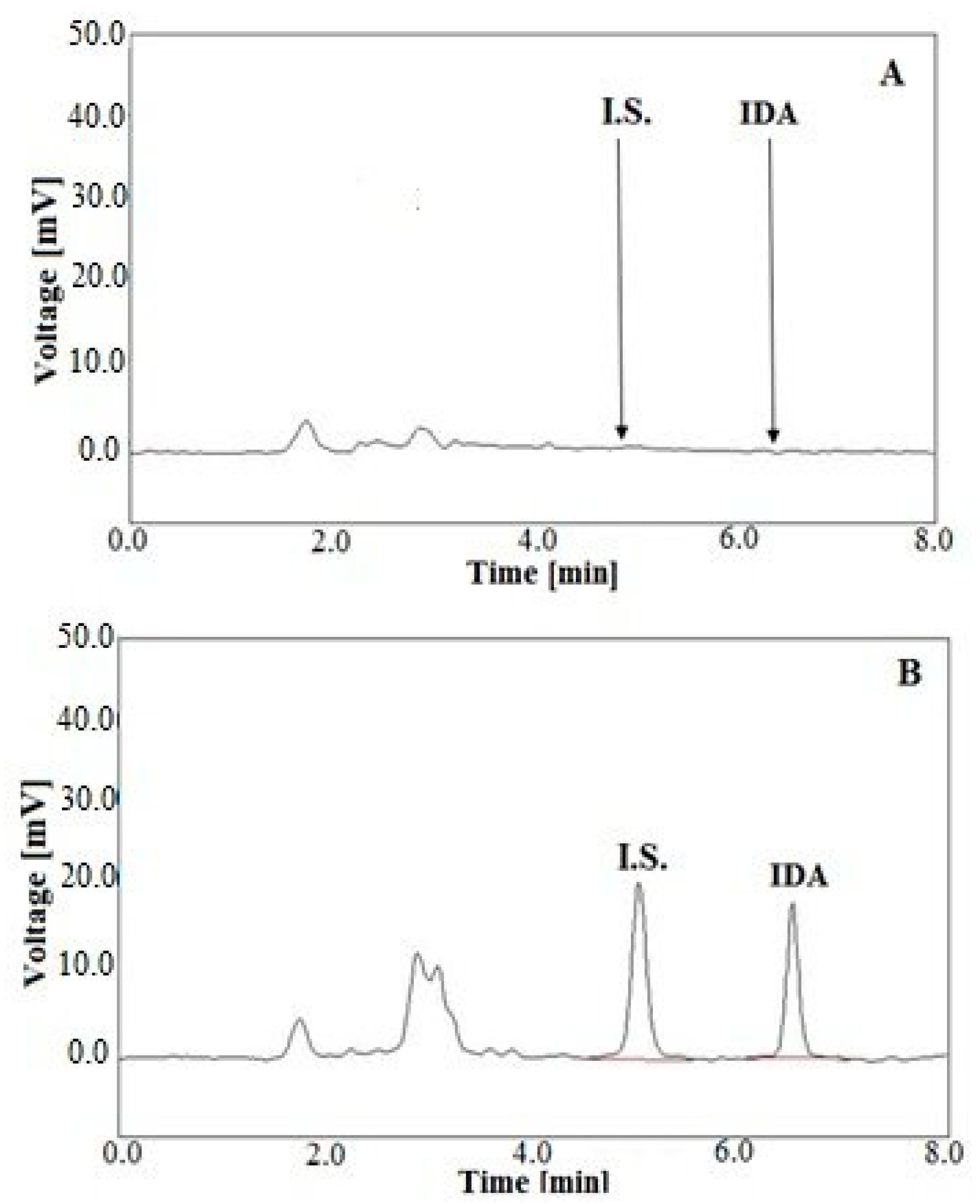
2.3. Validation of Analytical Method
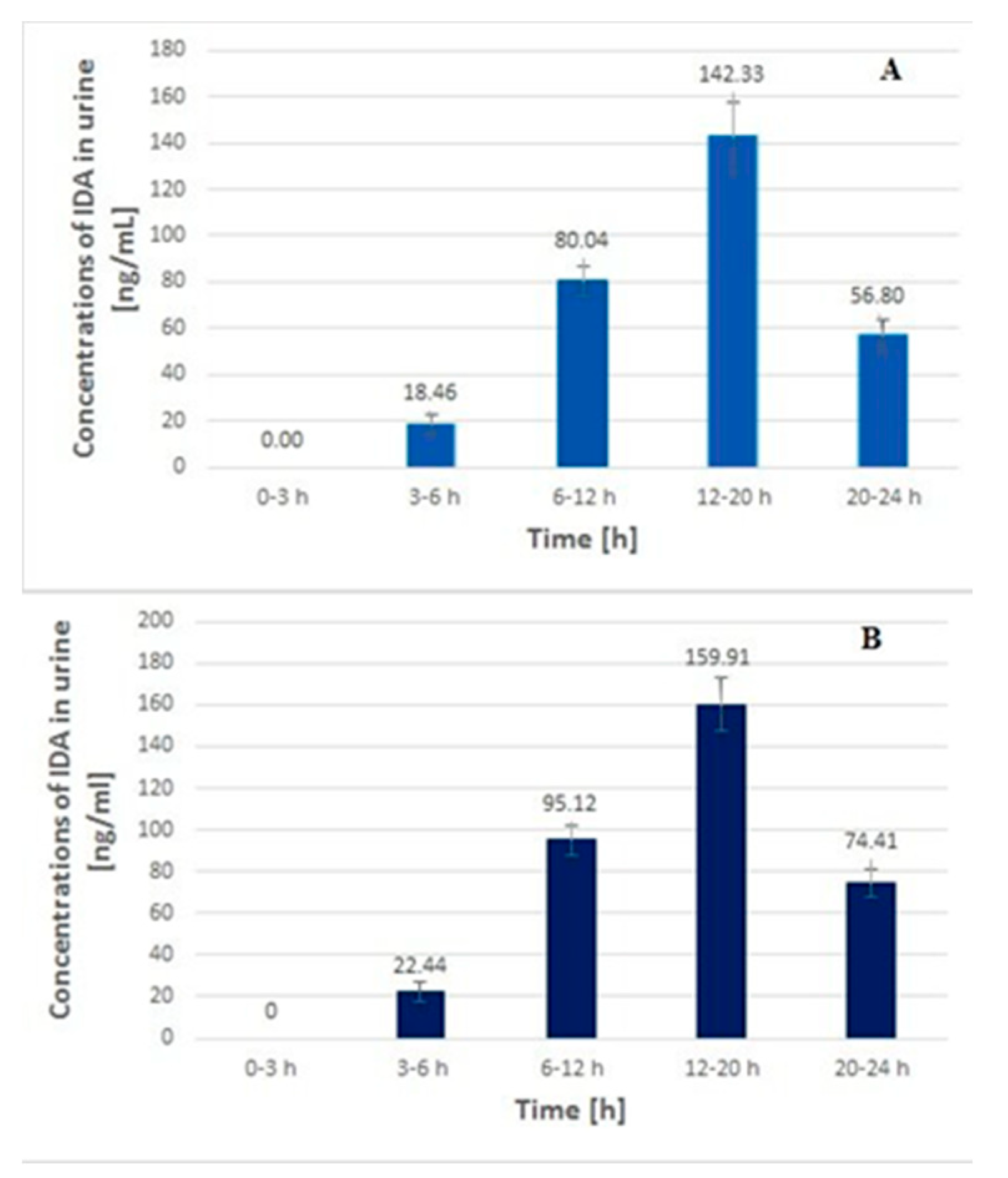
2.4. Application to Real Samples
3. Materials and Methods
3.1. Reagents
3.2. Chromatographic Conditions
3.3. Standard Solutions
3.4. Plasma and Urine Standards
3.5. Sample Preparation
3.6. Validation of Analytical Method
3.6.1. Calibration Curve
3.6.2. Linearity
3.6.3. Selectivity
3.6.4. Limit of Detection and Limit of Quantification
3.6.5. Accuracy and Precision
3.6.6. Stability Study
3.7. Method Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Danesi, R.; Fogli, S.; Gennari, A.; Conte, P.; Del Tacca, M. Pharmacokinetic-Pharmacodynamic Relationships of the Anthracycline Anticancer Drugs. Clin. Pharmacokinet. 2002, 41, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Toffoli, G.; Sorio, R.; Aita, P.; Crivellari, D.; Corona, G.; Bearz, A.; Robieux, I.; Colussi, A.M.; Stocco, F.; Boiocchi, M. Dose-finding and Pharmacologic Study of Chronic Oral Idarubicin Therapy in Metastatic Breast Cancer Patients. Clin. Cancer Res. 2000, 6, 2279–2287. [Google Scholar]
- Kalska-Kowalska, A.; Cendrowska, I.; Beczkowicz, H.; Cholewinska, A.; Zagrodzka, J.; Ramza, J.; Grynkiewicz, G. Remarks on the USP Recommendations for Quantitative Determination of Idarubicin Hydrochloride by HPLC. Chem. Anal. 2000, 45, 73. [Google Scholar]
- Crivellari, D.; Lombardi, D.; Spazzapan, S.; Veronesi, A.; Toffoli, G. New oral drugs in older patients: A review of idarubicin in elderly patients. Crit. Rev. Oncol. Hematol. 2004, 49, 153–163. [Google Scholar] [CrossRef]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular Advances and Pharmacologic Developments in Antitumor Activity and Cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef]
- Schleyer, E.; Kuhn, S.; Ruhrs, H.; Unterhalt, M.; Kaufmann, C.C.; Kern, W.; Braess, J.; Straubel, G.; Hiddemann, W. Oral idarubicin pharmacokinetics—Correlation of trough level with idarubicin area under curve. Leukemia 1997, 11, 15–21. [Google Scholar]
- Stewart, D.J.; Grewaal, D.; Greens, R.M.; Verma, S.; Maroun, J.A.; Redmond, D.; Robillard, L.; Gupta, S. Bioavailability and pharmacology of oral idarubicin. Cancer Chemother. Pharmacol. 1991, 27, 308–314. [Google Scholar] [CrossRef]
- Schleyer, E.; Budeus, S.; Reinhardt, J.; Rolff, C.; Buchner, T.; Hiddemann, W. Idarubicin Pharmacokinetics. In Acute Leukemias. Haematology and Blood Transfusion/Hämatologie und Bluttransfusion; Springer: Berlin/Heidelberg, Germany, 1992; pp. 617–624. [Google Scholar] [CrossRef]
- Tedeschi, A.; Montillo, M.; Strocchi, E.; Cafro, A.M.; Tresoldi, E.; Intropido, L.; Nichelatti, M.; Marbello, L.; Baratč, C.; Camaggi, C.M.; et al. High-dose idarubicin in combination with Ara-C in patients with relapsed or refractory acute lymphoblastic leukemia: A pharmacokinetic and clinical study. Cancer Chemother. Pharmacol. 2007, 59, 771–779. [Google Scholar] [CrossRef]
- Boogerd, W.; Tjahja, I.S.; Van De Sandt, M.M.; Beijnen, J.H. Penetration of idarubicin into malignant brain tumor tissue. J. Neurooncol. 1999, 44, 65–69. [Google Scholar] [CrossRef]
- Siepermann, M.; Koscielniak, E.; Dantonello, T.; Klee, D.; Boos, J.; Krefeld, B.; Borkhardt, A.; Hoehn, T.; Asea, A.; Wessalowski, R. Oral Low-Dose Chemotherapy: Successful Treatment of an Alveolar Rhabdomyosacoma during Pregnancy. Pediatr. Blood Cancer 2012, 58, 104–106. [Google Scholar] [CrossRef][Green Version]
- Dreyer, Z.E.; Kadota, R.P.; Stewart, C.F.; Friedman, H.S.; Mahoney, D.H.; Kun, L.K.; McCluggage, C.W.; Burger, P.C.; Kepner, J.; Heideman, R.L. Phase 2 study of idarubicin in pediatric brain tumors: Pediatric Oncology Group study POG 9237. Neuro-oncology 2003, 4, 261–267. [Google Scholar] [CrossRef]
- Klingebiel, T.; Boos, J.; Beske, F.; Hallmen, E.; Int-Veen, C.; Dantonello, T.; Treumer, J.; Gadner, H.; Marky, I.; Kazanowska, B.; et al. Treatment of Children with Metastatic Soft Tissue Sarcoma with Oral Maintenance Compared to High Dose Chemotherapy: Report of the HD CWS-96 Trial. Pediatr. Blood Cancer 2008, 50, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Sottani, C.; Tranfo, G.; Bettinelli, M.; Faranda, P.; Spagnoli, M.; Minoia, C. Trace determination of anthracyclines in urine: A new high-performance liquid chromatography/tandem mass spectrometry method for assessing exposure of hospital personnel. Rapid Commun. Mass Spectrom. 2004, 18, 2426–2436. [Google Scholar] [CrossRef] [PubMed]
- Fogli, S.; Danesi, R.; Innocenti, F.; Di Paolo, A.; Bocci, G.; Barbara, C.; Del Tacca, M. An Improved HPLC Method for Therapeutic Drug Monitoring of Daunorubicin, Idarubicin, Doxorubicin, Epirubicin, and Their 13-Dihydro Metabolites in Human Plasma. Ther. Drug Monit. 1999, 21, 367–375. [Google Scholar] [CrossRef]
- Maudens, K.E.; Stove, C.P.; Cocquyt, V.F.J.; Denys, H.; Lambert, W.E. Development and validation of a liquid chromatographic method for the simultaneous determination of four anthracyclines and their respective 13-S-dihydro metabolites in plasma and saliva. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 3907–3915. [Google Scholar] [CrossRef]
- Lachâtre, F.; Marquet, P.; Ragot, S.; Gaulier, J.M.; Cardot, P.; Dupuy, J.L. Simultaneous determination of four anthracyclines and three metabolites in human serum by liquid chromatography-electrospray mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 2000, 738, 281–291. [Google Scholar] [CrossRef]
- Camaggi, C.M.; Carisi, P.; Strocchi, E.; Pannuti, F. High-performance liquid chromatographic analysis of idarubicin and fluorescent metabolites in biological fluids. Cancer Chemother. Pharmacol. 1992, 30, 303–306. [Google Scholar] [CrossRef]
- Kuhlmann, O.; Hofmann, S.; Weiss, M. Determination of idarubicin and idarubicinol in rat plasma using reversed-phase high-performance liquid chromatography and fluorescence detection. J. Chromatogr. B 1999, 728, 279–282. [Google Scholar] [CrossRef]
- Looby, M.; Linke, R.; Weiss, M. Pharmacokinetics and tissue distribution of idarubicin and its active metabolite idarubicinol in the rabbit. Cancer Chemother. Pharmacol. 1997, 39, 554–556. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhang, L.; Zhou, T.; Fang, Y. Determination of daunorubicin in human urine by capillary zone electrophoresis with amperometric detection. Anal. Chim. Acta 2000, 1, 15–19. [Google Scholar] [CrossRef]
- Hempel, G.; Haberl, S.; Schulze-Westhoff, P.; Mohhng, N.; Blaschke, G.; Boos, J. Determination of idarubicin and idarubicinol in plasma by capillary electrophoresis. J. Chromatogr. B 1997, 698, 287–292. [Google Scholar] [CrossRef]
- Chandra, P.; Zaidi, S.A.; Noh, H.B.; Shim, Y.B. Separation and simultaneous detection of anticancer drugs in a microfluidic device with an amperometric biosensor. Biosens. Bioelectron. 2011, 28, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Arkan, E.; Paimarda, G.; Moradi, K. A novel electrochemical sensor based on electrospun TiO2 nanoparticles/carbon nanofibers for determination of Idarubicin in biological samples. J. Electroanal. Chem. 2017, 801, 480–487. [Google Scholar] [CrossRef]
- Dehdashtian, S.; Behbahanian, N.; Taherzadeh, K.M.; Hashemi, B. Development of electrochemical sensor based on multiwall carbon nanotube for determination of anticancer drug idarubicin in biological samples. Adv. Nanochem. 2019, 1, 22–28. [Google Scholar] [CrossRef]
- Reid, J.M.; Pendergrass, T.W.; Krailo, M.D.; Hammond, G.D.; Ames, M.M. Plasma Pharmacokinetics and Cerebrospinal Fluid Concentrations of Idarubicin and Idarubicinol in Pediatric Leukemia Patients: A Childrens Cancer Study Group Report. Cancer Res. 1990, 20, 6525–6528. [Google Scholar]
- US Food Drug Administration. FDA Guidance for Industry: Bioanalytical Method Validation, US Department of Health and Human, Services Food and Drug Administration. 2013. Available online: https://www.fda.gov/files/drugs/published/BioanalyticalMethod-Validation-Guidance-for-Industry.pdf (accessed on 23 August 2019).
- European Medicines Agency. Guide to Bioanalytical Method Validation, Committee for Medicinal Products for Human Use and European Medicines Agency. 2011. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guidelinebioanaly (accessed on 23 October 2019).
- Maliszewska, O.; Plenis, A.; Oledzka, I.; Kowalski, P.; Miekus, N.; Bień, E.; Krawczyk, M.A.; Adamkiewicz-Drozynska, E.; Bączek, T. Optimization of LC method for the quantification of doxorubicin in plasma and urine samples in view of pharmacokinetic, biomedical and drug monitoring therapy studies. J. Pharm. Biomed. Anal. 2018, 158, 376–385. [Google Scholar] [CrossRef]
- Treder, N.; Maliszewska, O.; Olędzka, I.; Kowalski, P.; Miękus, N.; Bączek, T.; Bień, E.; Krawczyk, M.A.; Adamkiewicz-Drożynska, E.; Plenis, A. Development and validation of a high-performance liquid chromatographic method with a fluorescence detector for the analysis of epirubicin in human urine and plasma, and its application in drug monitoring. J. Chromatogr. B 2020, 1136, 121910. [Google Scholar] [CrossRef]
- Loubrock, N.; Hempel, G.; Schulze-Westhoff, P.; Wurthwein, G.; Flege, S.; Boos, J. The stability of doxorubicin and idarubicin in plasma and whole blood. Chromatographia 2000, 52, 9–13. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

| Deproteinizing Method/Liquid-Liquid Extraction | The Absolute Recovery of IDA (%) | |||||
| Urine | Plasma | |||||
| ACN | 45.3 ± 4.6 | - | ||||
| DCHM | 26.7 ± 2.2 | - | ||||
| DCHM + 50 µL of 1 M NaOH | 37.2 ± 4.2 | - | ||||
| Chloroform: MeOH (4:1, v/v) | 33.2 ± 3.9 | - | ||||
| Chloroform: MeOH (4:1, v/v) + 50 µL of 1 M NaOH | 25.0 ± 4.9 | - | ||||
| Chloroform: 1-propranol (4:1, v/v) | 71.1 ± 6.7 | 69. 2 ± 6.3 | ||||
| Chloroform: 2-propranol (4:1, v/v) | 92.8 ± 9.9 | 89.1 ± 8.8 | ||||
| Chloroform: 2-propranol (4:1, v/v) + 50 µL of 1 M NaOH | 73.5 ± 8.6 | 75.1 ± 7.1 | ||||
| Chloroform: 2-propranol (4:1, v/v) + 100 µL of phosphate buffer (pH 8.5) | 72.7 ± 6.3 | 75.8 ± 6.2 | ||||
| Chloroform: 2-propranol (4:1, v/v) + 100 µL of phosphate buffer (pH 8.5) + ultrasonication | 23.6 ± 2.9 | - | ||||
| Solid Phase Extraction (SPE) | ||||||
| SPE Cartridge | Sample Matrix Modification | Washing Agent | Eluting Solvent | The Absolute Recovery of IDA (%) | ||
| Urine | Plasma | |||||
| C18 40 mg | 900 µL of H2O + 100 µL of phosphate buffer (pH 8.5) | Water | MeOH | 39.2 ± 1.3 | - | |
| 0.1 M HCl | 94.6 ± 5.9 | 97.1 ± 5.5 | ||||
| HLB 30 mg | 900 µL of H2O + 100 µL of phosphate buffer (pH 8.5) | Water | MeOH | 46.3 ± 4.5 | - | |
| 950 µL of H2O + 50 µL of 1 M NaOH | 56.2 ± 4.8 | 52.2 ± 4.9 | ||||
| 0.1 M HCl | 95.2 ± 3.7 | 99.4 ± 4.0 | ||||
| 0.1 M HCl + ultrasonication | 95.0 ± 7.1 | 98.5 ± 8.8 | ||||
| 0.1 M HCl | ACN:MeOH (1:1, v/v) | 89.2 ± 5.9 | 94.6 ± 6.1 | |||
| DCHM:2-propranol (1:1, v/v) | 92.1 ± 3.8 | 95.9 ± 4.9 | ||||
| Solid Phase Microextraction (SPME) | ||||||
| SPME Cartridge | Eluting Solvent | Washing Agent | Desorbing Solvent | The Absolute Recovery of IDA (%) | ||
| Urine | Plasma | |||||
| C18 | 0.1 M HCl | Water | MeOH | 36.7 ± 1.9 | - | |
| ACN: MeOH (1:1, v/v) | 37.9 ± 2.6 | - | ||||
| ACN: H2O (1:1, v/v) | 51.6 ± 4.7 | 52. 2 ± 5.3 | ||||
| Chloroform: 2-propranol (4:1, v/v) | 52.2 ± 5.7 | 54.8 ± 5.5 | ||||
| DCHM: 2-propranol (4:1, v/v) | 52.0 ± 2.9 | 55.3 ± 3.4 | ||||
| DCHM: 2-propranol (1:1, v/v) | 58.5± 2.7 | 59.2 ± 3.1 | ||||
| Urine | Plasma | |
|---|---|---|
| Linearity | 0.25–200 ng/mL | 0.1–50 ng/mL |
| Equation parameter | ||
| Slope | 0.0109 ± 0.00007 | 0.061 ± 0.0003 |
| Intercept | 0.0025 ± 0.0068 | 0.0041 ± 0.0069 |
| Standard error | 0.0139 | 0.0150 |
| Correlation coefficient (R2) | 0.9997 | 0.9998 |
| LOD (ng/mL) | 0.125 | 0.05 |
| Urine | Plasma | |||||||
|---|---|---|---|---|---|---|---|---|
| Intra-Day (n = 6) | ||||||||
| Concentration (ng/mL) | Precisio RSD (%) | Accuracy (%) | Concentration (ng/mL) | Precision RSD (%) | Accuracy (%) | |||
| Spiked (ng/mL) | Found (Mean ± SD) | Spiked (ng/mL) | Found (Mean ± SD) | |||||
| LLQC | 0.25 | 0.26 ± 0.02 | 8.82 | 103.4 | 0.1 | 0.11 ± 0.01 | 9.09 | 110.0 |
| LQC | 50 | 49.16 ± 2.13 | 4.32 | 99.3 | 0.5 | 0.48 ± 0.03 | 6.38 | 96.4 |
| MQC | 100 | 97.63 ± 2.88 | 2.95 | 97.6 | 5 | 4.96 ± 0.20 | 4.00 | 99.2 |
| HQC | 150 | 152.22 ± 2.28 | 1.50 | 101.5 | 15 | 14.58 ± 0.42 | 2.90 | 97.2 |
| Inter-day (n = 6) | ||||||||
| LLQC | 0.25 | 0.26 ± 0.03 | 9.78 | 105.2 | 0.1 | 0.11 ± 0.01 | 9.09 | 110.0 |
| LQC | 50 | 49.79 ± 2.02 | 4.06 | 99.6 | 0.5 | 0.53 ± 0.05 | 9.50 | 106.4 |
| MQC | 100 | 100.42 ± 4.04 | 4.02 | 100.4 | 5 | 4.95 ± 0.07 | 1.48 | 99.1 |
| HQC | 150 | 149.79 ± 2.02 | 1.35 | 99.9 | 15 | 15.02 ± 0.02 | 0.15 | 100.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maliszewska, O.; Treder, N.; Olędzka, I.; Kowalski, P.; Miękus, N.; Bączek, T.; Rodzaj, W.; Bień, E.; Krawczyk, M.A.; Plenis, A. Sensitive Analysis of Idarubicin in Human Urine and Plasma by Liquid Chromatography with Fluorescence Detection: An Application in Drug Monitoring. Molecules 2020, 25, 5799. https://doi.org/10.3390/molecules25245799
Maliszewska O, Treder N, Olędzka I, Kowalski P, Miękus N, Bączek T, Rodzaj W, Bień E, Krawczyk MA, Plenis A. Sensitive Analysis of Idarubicin in Human Urine and Plasma by Liquid Chromatography with Fluorescence Detection: An Application in Drug Monitoring. Molecules. 2020; 25(24):5799. https://doi.org/10.3390/molecules25245799
Chicago/Turabian StyleMaliszewska, Olga, Natalia Treder, IIona Olędzka, Piotr Kowalski, Natalia Miękus, Tomasz Bączek, Wojciech Rodzaj, Ewa Bień, Małgorzata Anna Krawczyk, and Alina Plenis. 2020. "Sensitive Analysis of Idarubicin in Human Urine and Plasma by Liquid Chromatography with Fluorescence Detection: An Application in Drug Monitoring" Molecules 25, no. 24: 5799. https://doi.org/10.3390/molecules25245799
APA StyleMaliszewska, O., Treder, N., Olędzka, I., Kowalski, P., Miękus, N., Bączek, T., Rodzaj, W., Bień, E., Krawczyk, M. A., & Plenis, A. (2020). Sensitive Analysis of Idarubicin in Human Urine and Plasma by Liquid Chromatography with Fluorescence Detection: An Application in Drug Monitoring. Molecules, 25(24), 5799. https://doi.org/10.3390/molecules25245799







