Synthesis and Biological Evaluation of Halogenated E-Stilbenols as Promising Antiaging Agents
Abstract
1. Introduction
2. Results
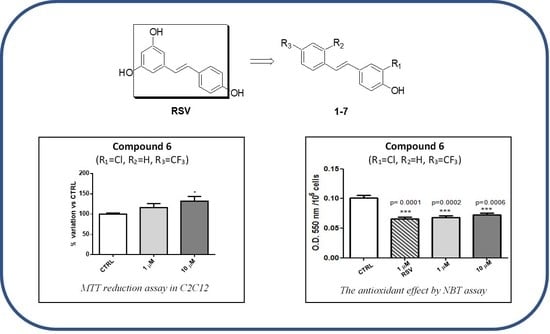
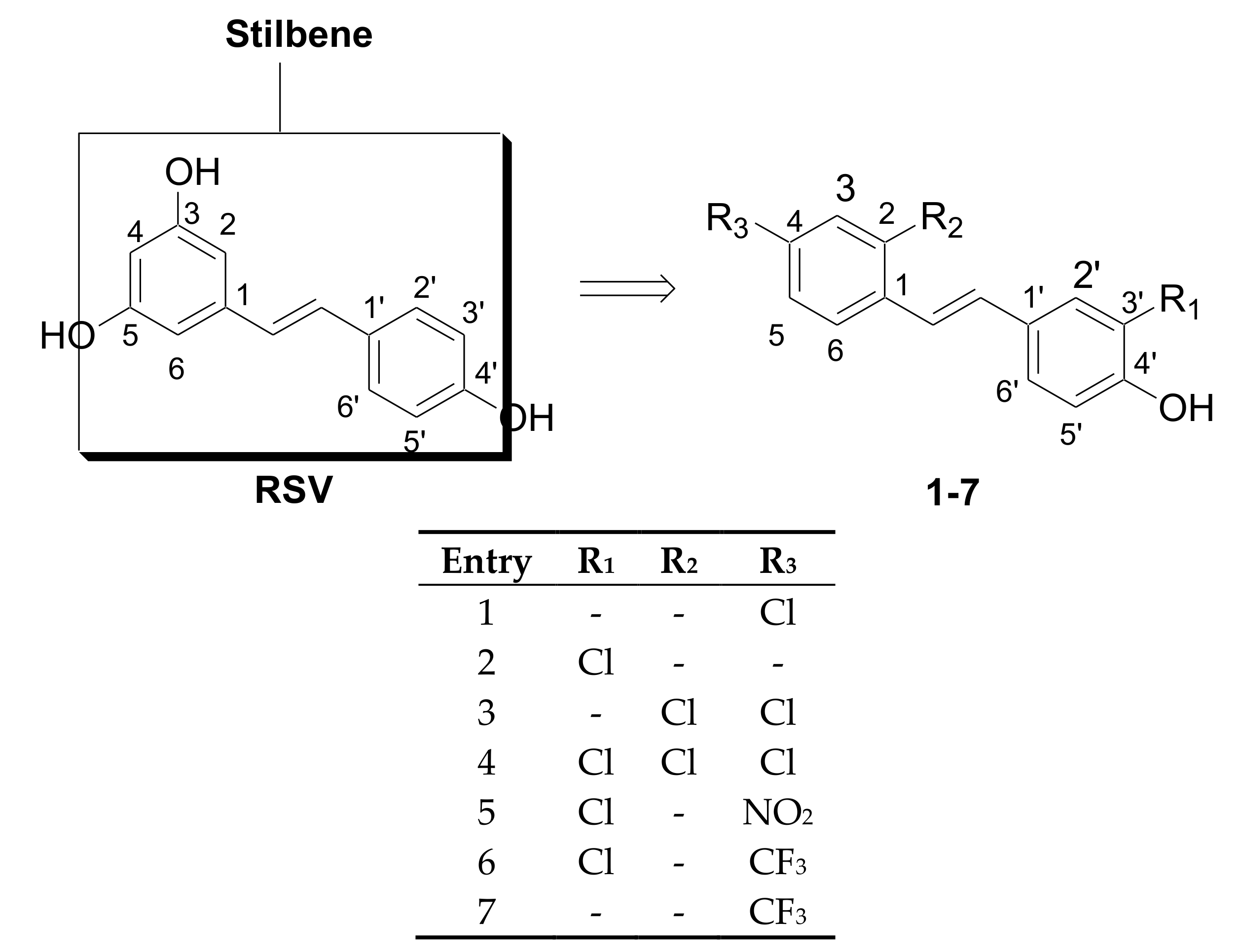
2.1. Chemistry
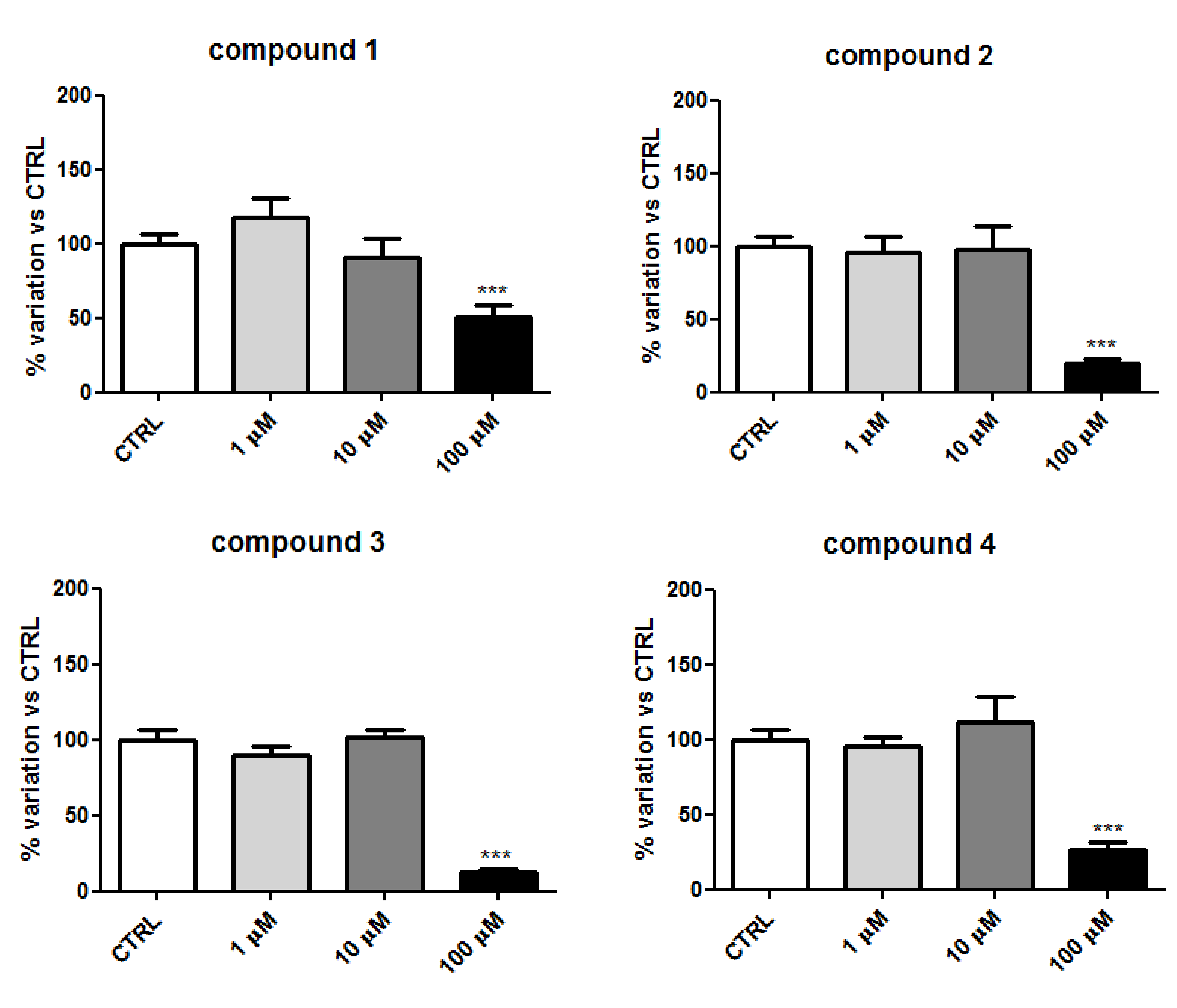
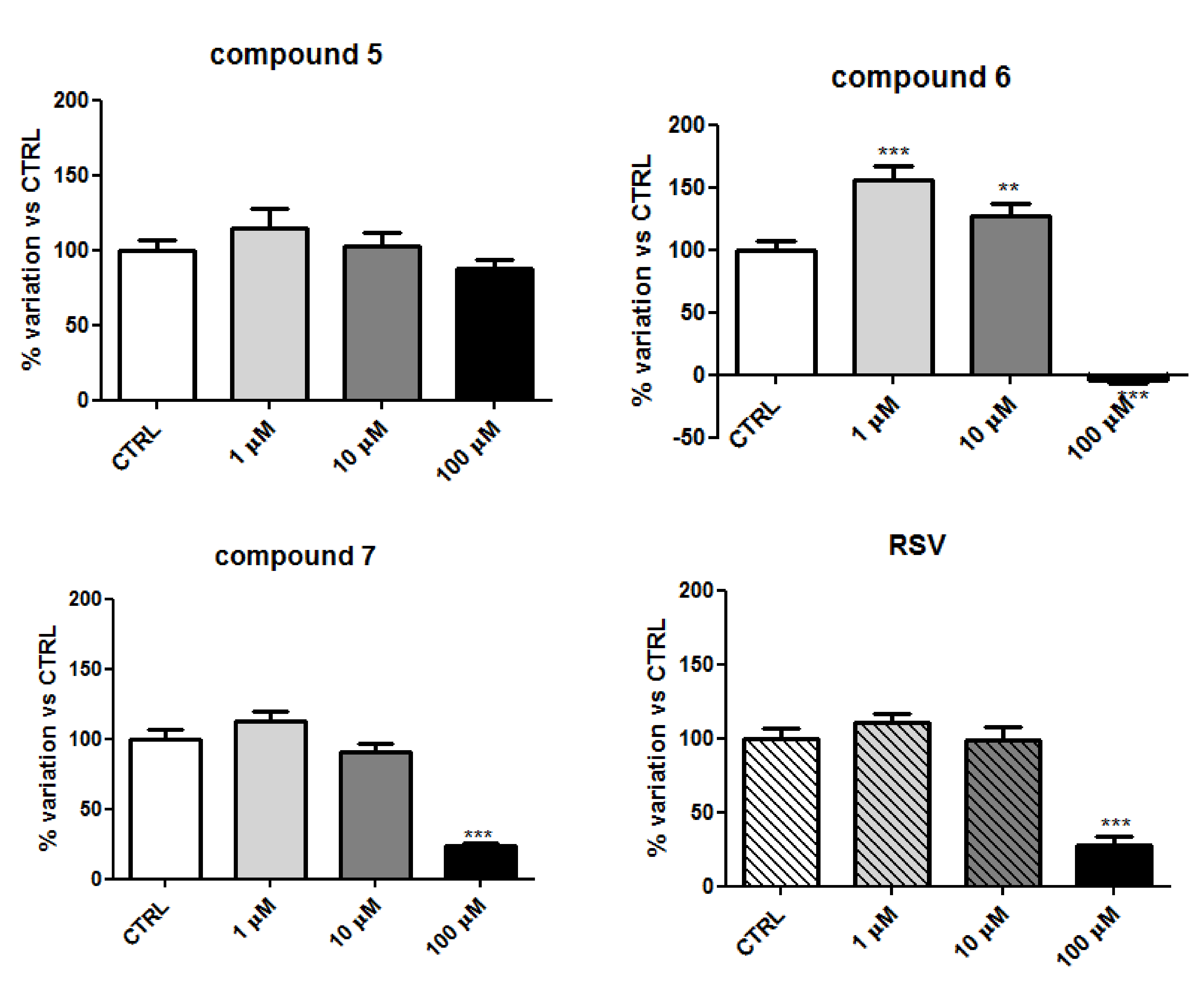
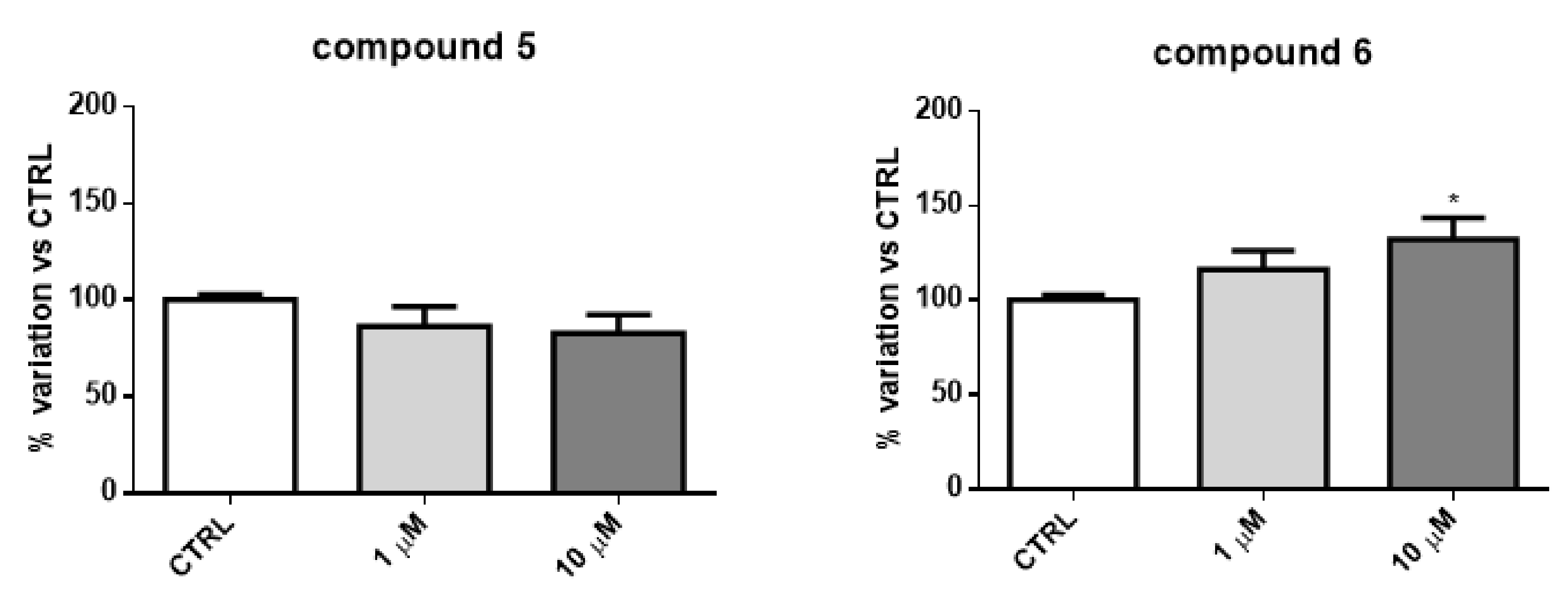
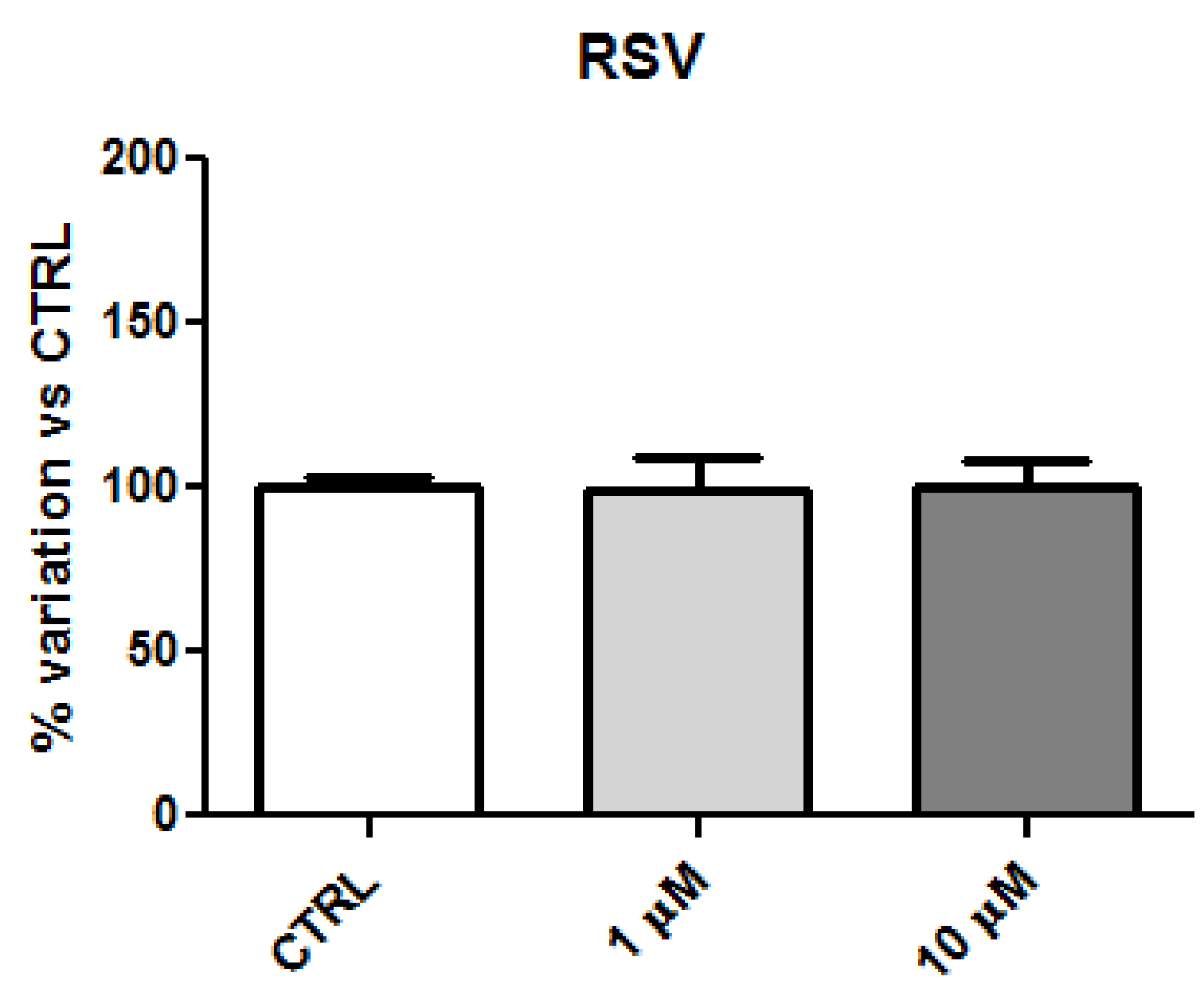
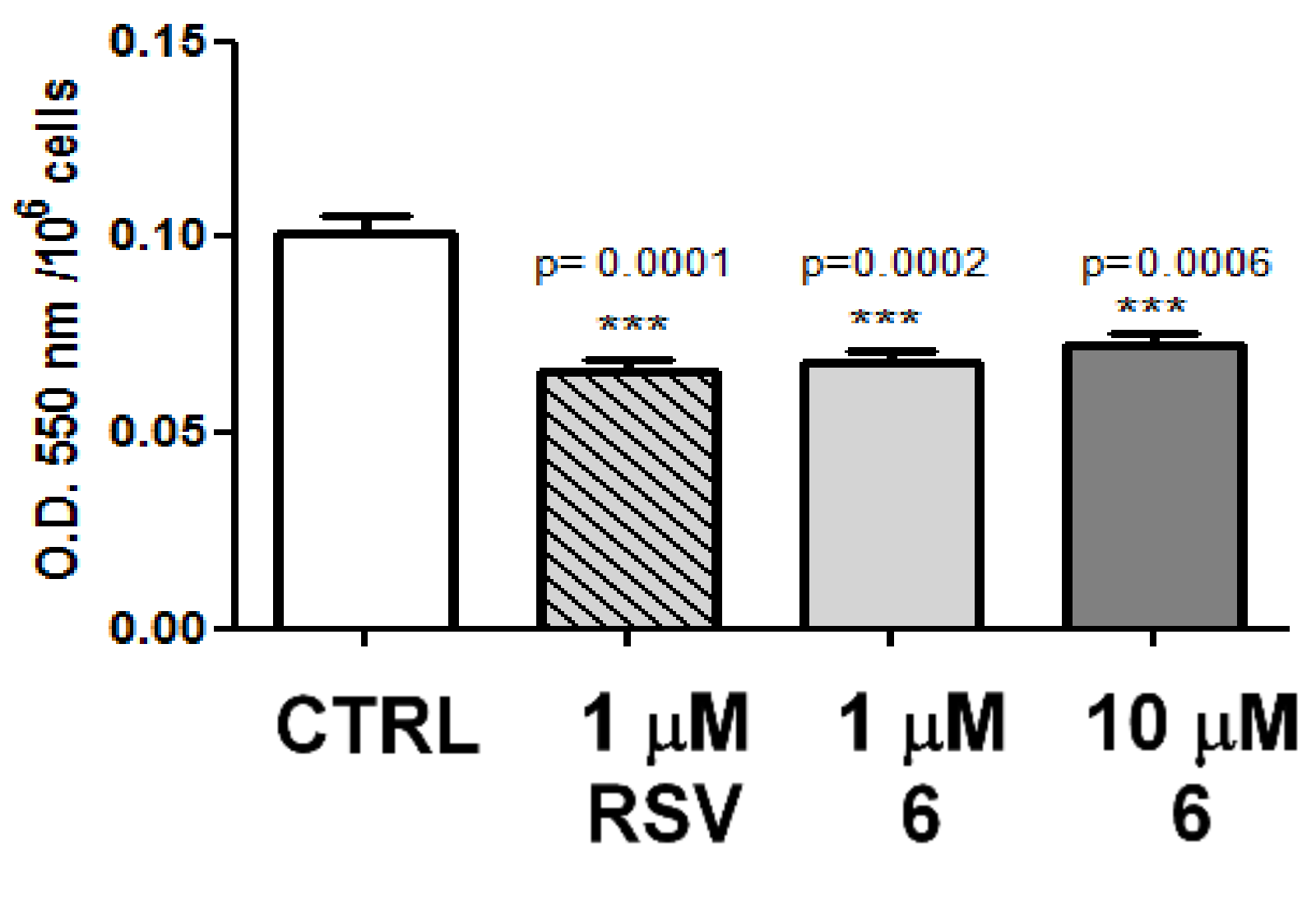
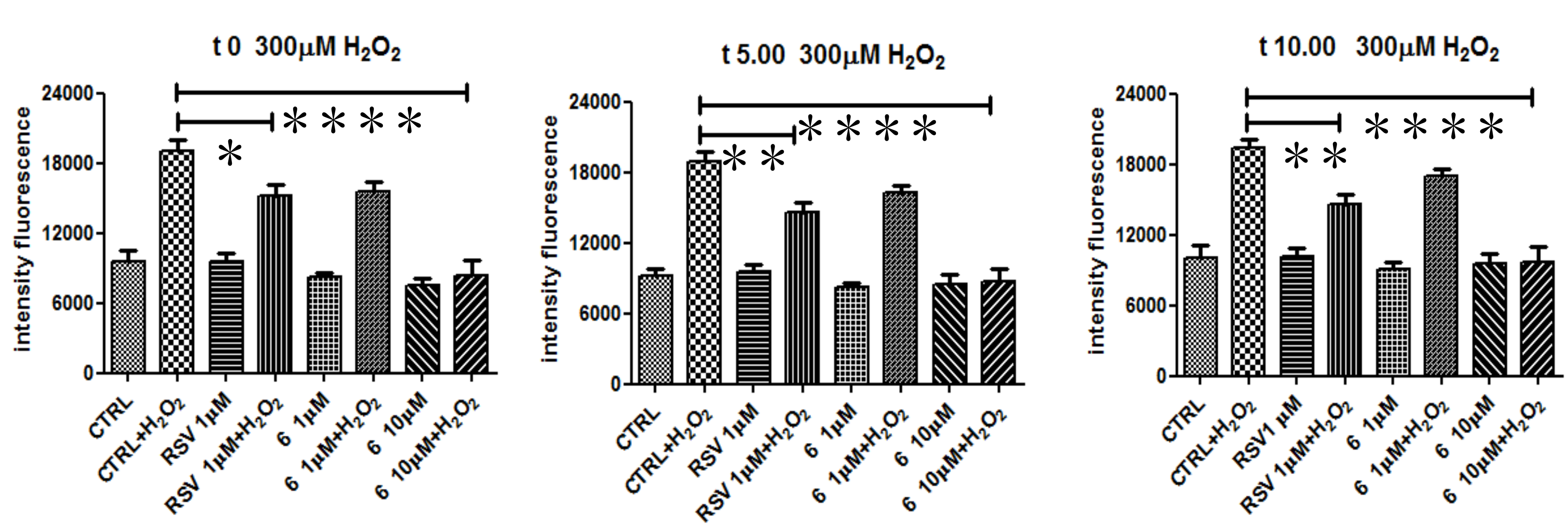
2.2. Biology
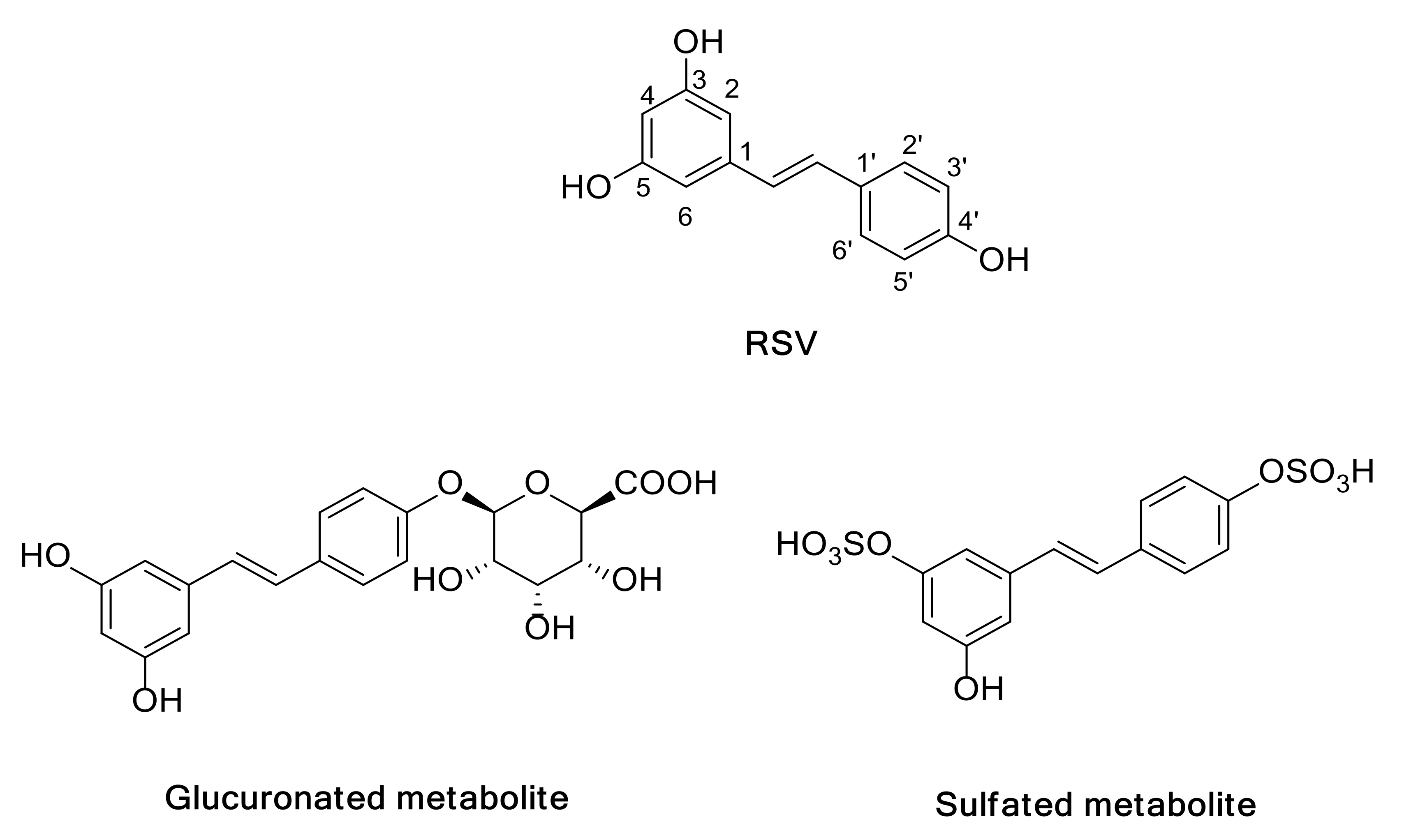
2.3. Log P Analysis
2.4. Discussion
3. Materials and Methods
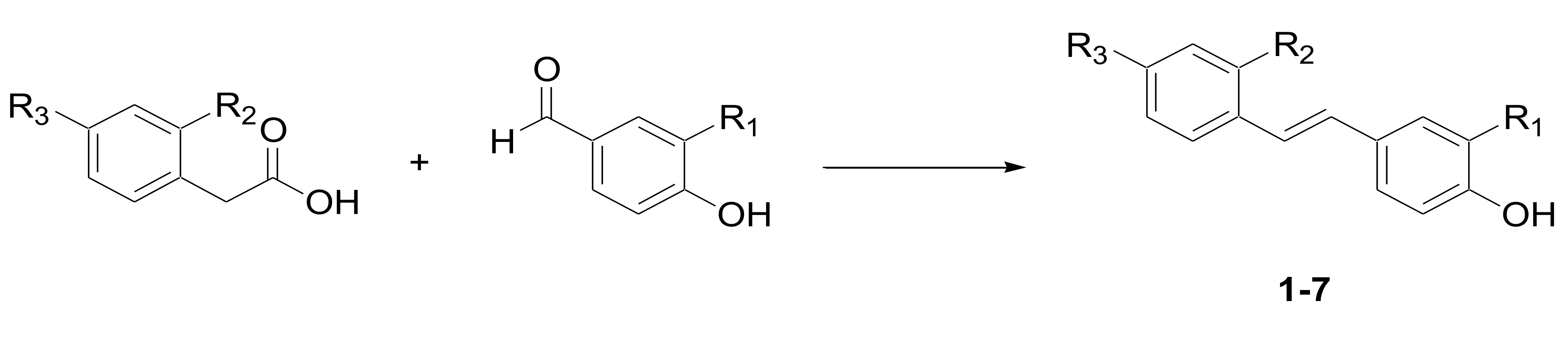
3.1. Chemistry
General Procedure for the Preparation of Phenols 1–7
3.2. Biology
3.2.1. Cell Culture Medium Preparation
3.2.2. MTT Assay
3.2.3. NBT Assay
3.2.4. Measurement of Intracellular ROS
3.2.5. Statistical Analysis
3.3. Log P Studies
3.3.1. Sample Preparation
3.3.2. Chromatographic Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Resveratrol | RSV |
| structure-activity relationship | SAR |
| type 2 diabetes | T2D |
| age-related macular degeneration | AMD |
| Alzheimer’s disease | AD |
| Parkinson’s disease | PD |
| reactive oxygen species | ROS |
| glucose restriction | GR |
| xanthine oxidase | XO |
References
- Vasefi, M.; Ghaboolian-Zare, E.; Abedelwahab, H.; Osu, A. Environmental toxins and Alzheimer’s disease progression. Neurochem. Int. 2020, 141, 104852. [Google Scholar] [CrossRef] [PubMed]
- Gallart-Palau, X.; Serra, A.; Gao, Y.; Ho, H.H.; Richards, A.M.; Kandiah, N.; Chen, C.P.; Kalaria, R.N.; Sze, S.K. Degenerative protein modifications in the aging vasculature and central nervous system: A problem shared is not always halved. Ageing Res. Rev. 2019, 53, 100909. [Google Scholar] [CrossRef]
- Harman, D. Aging: A Theory Based on Free Radical and Radiation Chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P.; Rahman, H.S. Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front. Pharmacol. 2018, 9, 1162–1190. [Google Scholar] [CrossRef]
- Cipolla, C.M.; Lodhi, I.J. Peroxisomal dysfunction in age-related diseases. Trends Endocrinol. Metab. 2017, 28, 297–308. [Google Scholar] [CrossRef]
- Jo, D.S.; Cho, D.-H. Peroxisomal dysfunction in neurodegenerative diseases. Arch. Pharmacal Res. 2019, 42, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.J.; Martinez, P.F.; Pagan, L.U.; Damatto, R.L.; Cezar, M.D.M.; Lima, A.R.R.; Okoshi, K.; Okoshi, M.P. Skeletal muscle aging: Influence of oxidative stress and physical exercise. Oncotarget 2017, 8, 20428–20440. [Google Scholar] [CrossRef]
- Gandhi, S.; Abramov, A.Y. Mechanism of oxidative stress in neurodegeneration. Oxid. Med. Cell. Longev. 2012, 2012, 428010. [Google Scholar] [CrossRef] [PubMed]
- Cătană, C.-S.; Atanasov, A.G.; Berindan-Neagoe, I. Natural products with anti-aging potential: Affected targets and molecular mechanisms. Biotechnol. Adv. 2018, 36, 1649–1656. [Google Scholar] [CrossRef]
- Devore, E.E.; Kang, J.H.; Breteler, M.M.; Grodstein, F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann. Neurol. 2012, 72, 135–143. [Google Scholar] [CrossRef]
- Lee, C.; Longo, V. Dietary restriction with and without caloric restriction for healthy aging. F1000 Research 2016, 5, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.J.; Crozier, A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.-J.; Qian, Y.-P.; Liu, X.-D.; Dai, F.; Shang, X.-L.; Jia, W.-Q.; Liu, Q.; Fang, J.-G.; Zhou, B. Radical-scavenging activity and mechanism of resveratrol-oriented analogues: Influence of the solvent, radical, and substitution. J. Org. Chem. 2009, 74, 5025–5031. [Google Scholar] [CrossRef]
- De Filippis, B.; Ammazzalorso, A.; Fantacuzzi, M.; Giampietro, L.; Maccallini, C.; Amoroso, R. Anticancer activity of stilbene-based derivatives. ChemMedChem 2017, 12, 558–570. [Google Scholar] [CrossRef]
- De Filippis, B.; De Lellis, L.; Florio, R.; Ammazzalorso, A.; Amoia, P.; Fantacuzzi, M.; Giampietro, L.; Maccallini, C.; Amoroso, R.; Veschi, S. Synthesis and cytotoxic effects on pancreatic cancer cells of resveratrol analogs. Med. Chem. Res. 2019, 28, 984–991. [Google Scholar] [CrossRef]
- Singh, C.K.; Ndiaye, M.A.; Ahmad, N. Resveratrol and cancer: Challenges for clinical translation. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2015, 1852, 1178–1185. [Google Scholar] [CrossRef]
- Patil, P.; Thakur, A.; Sharma, A.; Flora, S.J.S. Natural products and their derivatives as multifunctional ligands against Alzheimer’s disease. Drug Dev. Res. 2020, 81, 165–183. [Google Scholar] [CrossRef]
- Jayaprakash, J.S.; Gowda, D.V.; Kulkarni, P.K. Therapeutic application of Resveratrol in human diseases. Int. J. Res. Pharm. Sci. 2020, 11, 1447–1456. [Google Scholar] [CrossRef]
- Lutz, M.; Fuentes, E.; Ávila, F.; Alarcón, M.; Palomo, I. Roles of phenolic compounds in the reduction of risk factors of cardiovascular diseases. Molecules 2019, 24, 366. [Google Scholar] [CrossRef]
- Reinisalo, M.; Kårlund, A.; Koskela, A.; Kaarniranta, K.; Karjalainen, R.O. Polyphenol stilbenes: Molecular mechanisms of defence against oxidative stress and aging-related diseases. Oxid. Med. Cell. Longev. 2015, 2015, 340520. [Google Scholar] [CrossRef] [PubMed]
- Olas, B.; Zbikowska, H.; Wachowicz, B.; Krajewski, T.; Buczyński, A.; Magnuszewska, A. Inhibitory effect of resveratrol on free radical generation in blood platelets. Acta Biochim. Pol. 1999, 46, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Daiber, A.; Förstermann, U.; Li, H. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1633–1646. [Google Scholar] [CrossRef] [PubMed]
- Orallo, F. Comparative studies of the antioxidant effects of cis-and trans-resveratrol. Curr. Med. Chem. 2006, 13, 87–98. [Google Scholar] [CrossRef]
- Salucci, S.; Falcieri, E. Polyphenols and their potential role in preventing skeletal muscle atrophy. Nutr. Res. 2020, 74, 10–22. [Google Scholar] [CrossRef]
- Dugdale, H.F.; Hughes, D.C.; Allan, R.; Deane, C.S.; Coxon, C.R.; Morton, J.P.; Stewart, C.E.; Sharples, A.P. The role of resveratrol on skeletal muscle cell differentiation and myotube hypertrophy during glucose restriction. Mol. Cell. Biochem. 2018, 444, 109–123. [Google Scholar] [CrossRef]
- Kaminski, J.; Lançon, A.; Aires, V.; Limagne, E.; Tili, E.; Michaille, J.-J.; Latruffe, N. Resveratrol initiates differentiation of mouse skeletal muscle-derived C2C12 myoblasts. Biochem. Pharmacol. 2012, 84, 1251–1259. [Google Scholar] [CrossRef]
- Montesano, A.; Luzi, L.; Senesi, P.; Mazzocchi, N.; Terruzzi, I. Resveratrol promotes myogenesis and hypertrophy in murine myoblasts. J. Transl. Med. 2013, 11, 310–325. [Google Scholar] [CrossRef]
- Liu, J.; Peng, Y.; Wang, X.; Fan, Y.; Qin, C.; Shi, L.; Tang, Y.; Cao, K.; Li, H.; Long, J.; et al. Mitochondrial dysfunction launches dexamethasone-induced skeletal muscle Atrophy via AMPK/FOXO3 signaling. Mol. Pharm. 2016, 13, 73–84. [Google Scholar] [CrossRef]
- Haramizu, S.; Asano, S.; Butler, D.C.; Stanton, D.A.; Hajira, A.; Mohamed, J.S.; Alway, S.E. Dietary resveratrol confers apoptotic resistance to oxidative stress in myoblasts. J. Nutr. Biochem. 2017, 50, 103–115. [Google Scholar] [CrossRef]
- Di Filippo, E.S.; Mancinelli, R.; Pietrangelo, T.; La Rovere, R.M.; Quattrocelli, M.; Sampaolesi, M.; Fulle, S. Myomir dysregulation and reactive oxygen species in aged human satellite cells. Biochem. Biophys. Res. Commun. 2016, 473, 462–470. [Google Scholar] [CrossRef]
- Fulle, S.; Sancilio, S.; Mancinelli, R.; Gatta, V.; Di Pietro, R. Dual role of the caspase enzymes in satellite cells from aged and young subjects. Cell Death Dis. 2013, 4, e955. [Google Scholar] [CrossRef]
- Salminen, A.; Braun, T.; Buchberger, A.; Jurs, S.; Winter, B.; Arnold, H.H. Transcription of the muscle regulatory gene Myf4 is regulated by serum components, peptide growth factors and signaling pathways involving G proteins. J. Cell Biol. 1991, 115, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Momken, I.; Stevens, L.; Bergouignan, A.; Desplanches, D.; Rudwill, F.; Chery, I.; Zahariev, A.; Zahn, S.; Stein, T.P.; Sebedio, J.L. Resveratrol prevents the wasting disorders of mechanical unloading by acting as a physical exercise mimetic in the rat. FASEB J. 2011, 25, 3646–3660. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.; Mock, C.; Liang, D. Pre-formulation studies of resveratrol. Drug Dev. Ind. Pharm. 2015, 41, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wang, L.; Zhu, M.; Zhang, L.; Yan, L. Properties and molecular mechanisms of resveratrol: A review. Pharm. Int. J. Pharm. Sci. 2015, 70, 501–506. [Google Scholar]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef]
- Karki, S.S.; Bhutle, S.R.; Pedgaonkar, G.S.; Zubaidha, P.; Shaikh, R.M.; Rajput, C.G.; Shendarkar, G.S. Synthesis and biological evaluation of some stilbene-based analogues. Med. Chem. Res. 2011, 20, 1158–1163. [Google Scholar] [CrossRef]
- Queiroz, A.N.; Gomes, B.A.; Moraes, W.M., Jr.; Borges, R.S. A theoretical antioxidant pharmacophore for resveratrol. Eur. J. Med. Chem. 2009, 44, 1644–1649. [Google Scholar] [CrossRef]
- Stivala, L.A.; Savio, M.; Carafoli, F.; Perucca, P.; Bianchi, L.; Maga, G.; Forti, L.; Pagnoni, U.M.; Albini, A.; Prosperi, E.; et al. Specific structural determinants are responsible for the antioxidant activity and the cell cycle effects of resveratrol. J. Biol. Chem. 2001, 276, 22586–22594. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, J.; Rangarajan, M.; Shao, Y.; LaVoie, E.J.; Huang, T.-C.; Ho, C.-T. Antioxidative phenolic compounds from sage (Salvia officinalis). J. Agric. Food Chem. 1998, 46, 4869–4873. [Google Scholar] [CrossRef]
- Garcia, G.X.; Larsen, S.W.; Pye, C.; Galbreath, M.; Isovitsch, R.; Fradinger, E.A. The functional group on (E)-4,4′–disubstituted stilbenes influences toxicity and antioxidative activity in differentiated PC-12 cells. Bioorg. Med. Chem. Lett. 2013, 23, 6355–6359. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, S.; Sprinz, H.; Brede, O. Efficiency and mechanism of the antioxidant action of trans-resveratrol and its analogues in the radical liposome oxidation. Arch. Biochem. Biophys. 2001, 391, 79–89. [Google Scholar] [CrossRef]
- Leporini, L.; Giampietro, L.; Amoroso, R.; Ammazzalorso, A.; Fantacuzzi, M.; Menghini, L.; Maccallini, C.; Ferrante, C.; Brunetti, L.; Orlando, G. In vitro protective effects of resveratrol and stilbene alkanoic derivatives on induced oxidative stress on C2C12 and MCF7 cells. J. Biol. Regul. Homeost. Agents 2017, 31, 589–601. [Google Scholar]
- Nawaz, W.; Zhou, Z.; Deng, S.; Ma, X.; Ma, X.; Li, C.; Shu, X. Therapeutic Versatility of Resveratrol Derivatives. Nutrients 2017, 9, 1188. [Google Scholar] [CrossRef]
- De Filippis, B.; Agamennone, M.; Ammazzalorso, A.; Bruno, I.; D’Angelo, A.; Di Matteo, M.; Fantacuzzi, M.; Giampietro, L.; Giancristofaro, A.; Maccallini, C. PPARα agonists based on stilbene and its bioisosteres: Biological evaluation and docking studies. MedChemComm 2015, 6, 1513–1517. [Google Scholar] [CrossRef]
- Francioso, A.; Mastromarino, P.; Masci, A.; d’Erme, M.; Mosca, L. Chemistry, stability and bioavailability of resveratrol. Med. Chem. 2014, 10, 237–245. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Wang, P.; Sang, S. Metabolism and pharmacokinetics of resveratrol and pterostilbene. Biofactors 2018, 44, 16–25. [Google Scholar] [CrossRef]
- Orgován, G.; Gonda, I.; Noszál, B. Biorelevant physicochemical profiling of (E)-and (Z)-resveratrol determined from isomeric mixtures. J. Pharm. Biomed. Anal. 2017, 138, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, R.; Pietrangelo, T.; Burnstock, G.; Fanò, G.; Fulle, S. Transcriptional profile of GTP-mediated differentiation of C2C12 skeletal muscle cells. Purinergic Signal. 2012, 8, 207–221. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fornasari, E.; Marinelli, L.; Di Stefano, A.; Eusepi, P.; Turkez, H.; Fulle, S.; Di Filippo, E.S.; Scarabeo, A.; Di Nicola, S.; Cacciatore, I. Synthesis and Antioxidant Properties of Novel Memantine Derivatives. Cent. Nerv. Syst. Agents Med. Chem. (Former. Curr. Med. Chem. Cent. Nerv. Syst. Agents). [CrossRef] [PubMed]
- Alway, S.E.; McCrory, J.L.; Kearcher, K.; Vickers, A.; Frear, B.; Gilleland, D.L.; Bonner, D.E.; Thomas, J.M.; Donley, D.A.; Lively, M.W. Resveratrol enhances exercise-induced cellular and functional adaptations of skeletal muscle in older men and women. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2017, 72, 1595–1606. [Google Scholar] [CrossRef]
- Silva, P.; Sureda, A.; Tur, J.A.; Andreoletti, P.; Cherkaoui-Malki, M.; Latruffe, N. How efficient is resveratrol as an antioxidant of the Mediterranean diet, towards alterations during the aging process? Free Radic. Res. 2019, 53, 1101–1112. [Google Scholar] [CrossRef]
- Di Stefano, A.; Marinelli, L.; Eusepi, P.; Ciulla, M.; Fulle, S.; Di Filippo, E.S.; Magliulo, L.; Di Biase, G.; Cacciatore, I. Synthesis and Biological Evaluation of Novel Selenyl and Sulfur-l-Dopa Derivatives as Potential Anti-Parkinson’s Disease Agents. Biomolecules 2019, 9, 239. [Google Scholar] [CrossRef]
- La Rovere, R.M.L.; Quattrocelli, M.; Pietrangelo, T.; Di Filippo, E.S.; Maccatrozzo, L.; Cassano, M.; Mascarello, F.; Barthélémy, I.; Blot, S.; Sampaolesi, M. Myogenic potential of canine craniofacial satellite cells. Front. Aging Neurosci. 2014, 6, 90–103. [Google Scholar] [CrossRef]
- Pietrangelo, T.; Puglielli, C.; Mancinelli, R.; Beccafico, S.; Fanò, G.; Fulle, S. Molecular basis of the myogenic profile of aged human skeletal muscle satellite cells during differentiation. Exp. Gerontol. 2009, 44, 523–531. [Google Scholar] [CrossRef]
- Epifanio, I.; Genovese, S.; Carlucci, G.; Epifano, F.; Locatelli, M. Secondary plant metabolites LogP determination: The case of boropinic and geraniloxyferulic acids. Curr. Bioact. Compd. 2015, 11, 131–141. [Google Scholar] [CrossRef]
- Kempińska, D.; Chmiel, T.; Kot-Wasik, A.; Mróz, A.; Mazerska, Z.; Namieśnik, J. State of the art and prospects of methods for determination of lipophilicity of chemical compounds. TrAC Trends Anal. Chem. 2019, 113, 54–73. [Google Scholar] [CrossRef]
- Linciano, P.; De Filippis, B.; Ammazzalorso, A.; Amoia, P.; Cilurzo, F.; Fantacuzzi, M.; Giampietro, L.; Maccallini, C.; Petit, C.; Amoroso, R. Druggability profile of stilbene-derived PPAR agonists: Determination of physicochemical properties and PAMPA study. MedChemComm 2019, 10, 1892–1899. [Google Scholar] [CrossRef] [PubMed]
- Wesołowska, O.; Ku&żdżał, M.; Štrancar, J.; Michalak, K. Interaction of the chemopreventive agent resveratrol and its metabolite, piceatannol, with model membranes. Biochim. Biophys. Acta (BBA) Biomembr. 2009, 1788, 1851–1860. [Google Scholar]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, E.; Somoza, V. Metabolism and bioavailability of trans-resveratrol. Mol. Nutr. Food Res. 2005, 49, 472–481. [Google Scholar] [CrossRef] [PubMed]

| % MeOH | 80 | 75 | 70 | 65 | 60 | 50 | 40 | 30 | 25 | 20 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | 2.230 | 3.007 | 4.485 | 7.441 | 13.529 | |||||
| 2. | 2.615 | 3.412 | 5.353 | 9.004 | 15.779 | |||||
| 3. | 4.006 | 6.433 | 11.254 | 39.949 | 173.677 | |||||
| 4. | 5.719 | 9.004 | 17.887 | 36.844 | 76.574 | |||||
| 5. | 2.368 | 3.091 | 4.821 | 7.906 | 13.950 | |||||
| 6. | 3.075 | 4.956 | 8.689 | 16.674 | 35.018 | |||||
| 7. | 2.430 | 3.578 | 5.667 | 10.032 | 19.349 | |||||
| RSV | 2.553 | 5.792 | 16.230 | 30.301 | 57.921 |
| % MeOH | 80 | 75 | 70 | 65 | 60 | 50 | 40 | 30 | 25 | 20 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | 0.7490 | 1.3584 | 2.5176 | 4.8361 | 9.6110 | |||||
| 2. | 1.0510 | 1.6761 | 3.1984 | 6.0620 | 11.3757 | |||||
| 3. | 2.1420 | 4.0455 | 7.8267 | 30.3325 | 135.2173 | |||||
| 4. | 3.4855 | 6.0620 | 13.0290 | 27.8973 | 59.0580 | |||||
| 5. | 0.8573 | 1.4243 | 2.7812 | 5.2008 | 9.9412 | |||||
| 6. | 1.4118 | 2.8871 | 5.8149 | 12.0776 | 26.4651 | |||||
| 7. | 0.9059 | 1.8063 | 3.4447 | 6.8682 | 14.1757 | |||||
| RSV | 1.0024 | 3.5427 | 11.7294 | 22.7655 | 44.4282 |
| % MeOH | 80 | 75 | 70 | 65 | 60 | 50 | 40 | 30 | 25 | 20 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | −0.1255 | 0.1330 | 0.4010 | 0.6845 | 0.9828 | |||||
| 2. | 0.0216 | 0.2243 | 0.5049 | 0.7826 | 1.0560 | |||||
| 3. | 0.3308 | 0.6070 | 0.8936 | 1.4819 | 2.1310 | |||||
| 4. | 0.5423 | 0.7826 | 1.1149 | 1.4456 | 1.7713 | |||||
| 5. | −0.0669 | 0.1536 | 0.4442 | 0.7161 | 0.9974 | |||||
| 6. | 0.1498 | 0.4605 | 0.7645 | 1.0820 | 1.4227 | |||||
| 7. | −0.0429 | 0.2568 | 0.5372 | 0.8368 | 1.1515 | |||||
| RSV | 0.0010 | 0.5493 | 1.0693 | 1.3573 | 1.6477 |
| Compound | Log P * ± SD | Log P # |
|---|---|---|
| 1. | 4.29 ± 0.07 | 4.88 |
| 2. | 4.20 ± 0.12 | 4.85 |
| 3. | 5.11 ± 0.07 | 5.59 |
| 4. | 5.50 ± 0.14 | 6.27 |
| 5. | 4.22 ± 0.09 | 4.59 |
| 6. | 5.21 ± 0.06 | 5.73 |
| 7. | 4.70 ± 0.05 | 5.05 |
| RSV | 2.47 ± 0.11 | 2.83 |
| FLOW RATE (mL/min) | % A H2O Milli-Q | % B Methanol |
|---|---|---|
| 1 | 20 | 80 |
| 1 | 25 | 75 |
| 1 | 30 | 70 |
| 1 | 35 | 65 |
| 1 | 40 | 60 |
| 1 | 50 | 50 |
| 1 | 60 | 40 |
| 1 | 70 | 30 |
| 1 | 75 | 25 |
| 1 | 80 | 20 |
Sample Availability: Samples of the compounds 1–7 are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Filippo, E.S.; Giampietro, L.; De Filippis, B.; Balaha, M.; Ferrone, V.; Locatelli, M.; Pietrangelo, T.; Tartaglia, A.; Amoroso, R.; Fulle, S. Synthesis and Biological Evaluation of Halogenated E-Stilbenols as Promising Antiaging Agents. Molecules 2020, 25, 5770. https://doi.org/10.3390/molecules25235770
Di Filippo ES, Giampietro L, De Filippis B, Balaha M, Ferrone V, Locatelli M, Pietrangelo T, Tartaglia A, Amoroso R, Fulle S. Synthesis and Biological Evaluation of Halogenated E-Stilbenols as Promising Antiaging Agents. Molecules. 2020; 25(23):5770. https://doi.org/10.3390/molecules25235770
Chicago/Turabian StyleDi Filippo, Ester Sara, Letizia Giampietro, Barbara De Filippis, Marwa Balaha, Vincenzo Ferrone, Marcello Locatelli, Tiziana Pietrangelo, Angela Tartaglia, Rosa Amoroso, and Stefania Fulle. 2020. "Synthesis and Biological Evaluation of Halogenated E-Stilbenols as Promising Antiaging Agents" Molecules 25, no. 23: 5770. https://doi.org/10.3390/molecules25235770
APA StyleDi Filippo, E. S., Giampietro, L., De Filippis, B., Balaha, M., Ferrone, V., Locatelli, M., Pietrangelo, T., Tartaglia, A., Amoroso, R., & Fulle, S. (2020). Synthesis and Biological Evaluation of Halogenated E-Stilbenols as Promising Antiaging Agents. Molecules, 25(23), 5770. https://doi.org/10.3390/molecules25235770