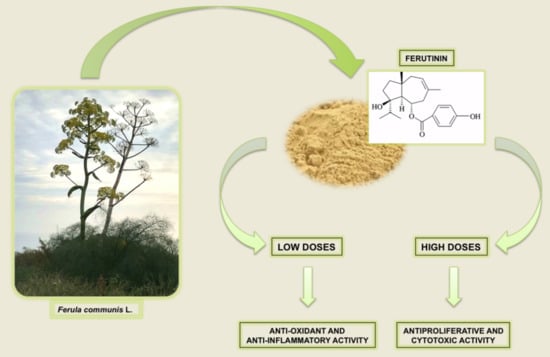
Ferula L. Plant Extracts and Dose-Dependent Activity of Natural Sesquiterpene Ferutinin: From Antioxidant Potential to Cytotoxic Effects
Abstract
1. Introduction
2. Ferula communis L.: Botanical Description and Chemical Composition
3. Antioxidant Potential of Low Doses of Ferutinin
4. Phytoestrogenic Activity of Ferutinin and the Hormone Replacement Therapy (HRT)
Phytoestrogenic and Ionophoric Activity of Ferutinin: Effects on Platelet Aggregation
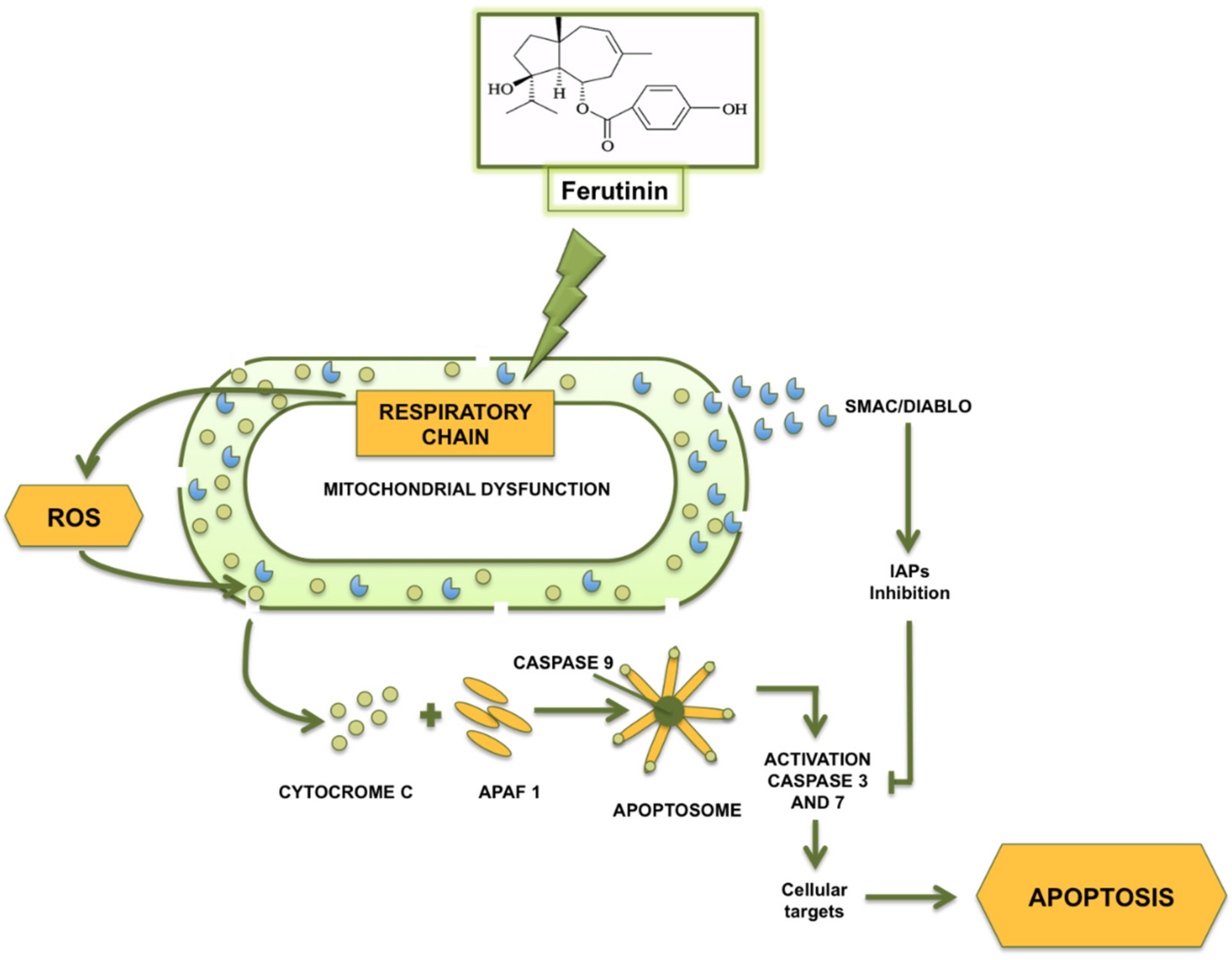
5. High Concentrations of Ferutinin: Pro-Oxidant and Cytotoxic Activity
6. Conclusions and Futures Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aye, M.M.; Aung, H.T.; Sein, M.M.; Armijos, C. A Review on the Phytochemistry Medicinal Properties and Pharmacological Activities of 15 Selected Myanmar Medicinal Plants. Molecules 2019, 24, 293. [Google Scholar] [CrossRef] [PubMed]
- Gliozzi, M.; Walker, R.; Mollace, V. Bergamot Polyphenols: Pleiotropic Players in the Treatment of Metabolic. J. Metabolic. Synd. 2014, 3, 2. [Google Scholar] [CrossRef]
- Oppedisano, F.; Macrì, R.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Bosco, F.; Nucera, S.; Zito, M.C.; Guarnieri, L.; et al. The Anti-Inflammatory and Antioxidant Properties of n-3 PUFAs: Their Role in Cardiovascular Protection. Biomedicines 2020, 25, 8. [Google Scholar] [CrossRef]
- Maridass, M.; De Britto, A.J. Origins of plant derived medicines. Ethnobot. Leafl. 2008, 12, 373–387. [Google Scholar]
- Tabassum, N.; Ahmad, F. Role of natural herbs in the treatment of hypertension. Pharmacogn. Rev. 2011, 5, 30–40. [Google Scholar] [CrossRef]
- Khan, T.; Ali, M.; Khan, A.; Nisar, P.; Jan, S.A.; Afridi, S.; Shinwari, Z.K. Anticancer Plants: A Review of the Active Phytochemicals, Applications in Animal Models, and Regulatory Aspects. Biomolecules 2019, 10, 47. [Google Scholar] [CrossRef]
- Chand, S.; Dave, R. In Vitro models for antioxidant activity evaluation and some medicinal plants possessing antioxidant properties: An overview. Afr. J. Microbiol. Res. 2009, 3, 981–996. [Google Scholar]
- Duthie, G.G.; Duthie, S.J.; Kyle, J.A. Plant polyphenols in cancer and heart disease: Implications as nutritional antioxidants. Nutr. Res. Rev. 2000, 13, 79–106. [Google Scholar] [CrossRef]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef]
- Maeda, H.; Dudareva, N. The shikimate pathway and aromatic amino Acid biosynthesis in plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef]
- Mollace, V.; Scicchitano, M.; Paone, S.; Casale, F.; Calandruccio, C.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Nucera, S.; et al. Hypoglycemic and Hypolipemic Effects of a New Lecithin Formulation of Bergamot Polyphenolic Fraction: A Double Blind, Randomized, Placebo-Controlled Study. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Musolino, V.; Gliozzi, M.; Nucera, S.; Carresi, C.; Maiuolo, J.; Mollace, R.; Paone, S.; Bosco, F.; Scarano, F.; Scicchitano, M.; et al. The effect of bergamot polyphenolic fraction on lipid transfer protein system and vascular oxidative stress in a rat model of hyperlipemia. Lipids Health Dis. 2019, 18, 115. [Google Scholar] [CrossRef] [PubMed]
- Musolino, V.; Gliozzi, M.; Bombardelli, E.; Nucera, S.; Carresi, C.; Maiuolo, J.; Mollace, R.; Paone, S.; Bosco, F.; Scarano, F.; et al. The synergistic effect of Citrus bergamia and Cynara cardunculus extracts on vascular inflammation and oxidative stress in non-alcoholic fatty liver disease. J. Tradit. Complement. Med. 2020, 10, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Oppedisano, F.; Muscoli, C.; Musolino, V.; Carresi, C.; Macrì, R.; Giancotta, C.; Bosco, F.; Maiuolo, J.; Scarano, F.; Paone, S.; et al. The Protective Effect of Cynara Cardunculus Extract in Diet-Induced NAFLD: Involvement of OCTN1 and OCTN2 Transporter Subfamily. Nutrients 2020, 12, 1435. [Google Scholar] [CrossRef] [PubMed]
- González-Burgos, E.; Gómez-Serranillos, M.P. Terpene compounds in nature: A review of their potential antioxidantactivity. Curr. Med. Chem. 2012, 19, 5319–5341. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, M.; Trewin, H.; Gawthrop, F.; Wagstaff, C. Sesquiterpenoids Lactones: Benefits to Plants and People. Int. J. Mol. Sci. 2013, 14, 12780–12805. [Google Scholar] [CrossRef]
- Jeena, K.; Liju, V.B.; Kuttan, R. A preliminary 13-week oral toxicity study of ginger oil in male and female Wistar rats. Int. J. Toxicol. 2011, 30, 662–670. [Google Scholar] [CrossRef]
- Ghareeb, D.A.; ElAhwany, A.M.D.; El-mallawany, S.M.; Saif, A.A. In vitro screening for anti-acetylcholiesterase, anti-oxidant, anti-glucosidase, anti-inflammatory and anti-bacterial effect of three traditional medicinal plants. Biotechnol. Biotechnol. Equip. 2014, 28, 1155–1164. [Google Scholar] [CrossRef]
- Ishnava, J.B.; Chauhan, M.B. Anticariogenic and phytochemical evaluation of Eucalyptus globules Labill. Saudi J. Biol. Sci. 2013, 20, 69–74. [Google Scholar] [CrossRef]
- Li-Weber, M.; Giaisi, M.; Treibe, M.K.; Krammer, P.H. The anti-inflammatory sesquiterpene lactone parthenolide suppresses IL-4 gene expression in peripheral blood T. Eur. J. Immunol. 2002, 32, 3587–3597. [Google Scholar] [CrossRef]
- Abramov, A.Y.; Zamaraeva, M.V.; Hagelgans, A.I.; Azimov, R.R.; Krasilnikov, O.V. Influence of plant terpenoids on the permeability of mitochondria and lipid bilayers. Biochim. Biophys. Acta 2001, 1512, 98–110. [Google Scholar] [CrossRef]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Gliozzi, M.; Scarano, F.; Musolino, V.; Carresi, C.; Scicchitano, M.; Ruga, S.; Zito, M.C.; Nucera, S.; Bosco, F.; Maiuolo, J.; et al. Role of TSPO/VDAC1 Upregulation and Matrix Metalloproteinase-2 Localization in the Dysfunctional Myocardium of Hyperglycaemic Rats. Int. J. Mol. Sci. 2020, 21, 7432. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xin, F.; Zhang, G.; Qu, H.; Yang, D.; Han, X. Recent Advances on Bioactive Constituents in Ferula. Drug Dev. Res. 2017, 78, 321–331. [Google Scholar] [CrossRef]
- Conti, F.; Abbate, G.; Alessandrini, A.; Blasi, C. (Eds.) An Annotated Checklist of the Italian Vascular Flora; Palombi Editori: Roma, Italy, 2005. [Google Scholar]
- Akaberi, M.; Iranshahy, M.; Iranshahi, M. Review of the traditional uses, phytochemistry, pharmacology and toxicology of giant fennel (Ferula communis L. subsp. communis). Iran J. Basic Med. Sci. 2015, 18, 1050–1062. [Google Scholar]
- Gault, G.; Lefebvre, S.; Benoit, E.; Lattard, V.; Grancher, D. Variability of ferulenol and ferprenin concentration in French giant fennel (Ferula sp.) leaves. Toxicon 2019, 165, 47–55. [Google Scholar] [CrossRef]
- Bergman, M.E.; Davis, B.; Phillips, M.A. Medically Useful Plant Terpenoids: Biosynthesis, Occurrence, and Mechanism of Action. Molecules 2019, 24, 3961. [Google Scholar] [CrossRef]
- Rubiolo, P.; Matteodo, M.; Riccio, G.; Ballero, M.; Christen, P.; Fleury-Souverain, S.; Veuthey, J.L.; Bicchi, C. Analytical discrimination of poisonous and nonpoisonous chemotypes of giant fennel (Ferula communis L.) through their biologically active and volatile fractions. J. Agric. Food Chem. 2006, 54, 7556–7563. [Google Scholar] [CrossRef]
- Miski, M.; Mabry, T.J. Daucane esters from Ferula communis subsp. communis. Phytochemistry 1985, 24, 1735–1741. [Google Scholar] [CrossRef]
- Nazari, Z.E.; Iranshahi, M. Biologically active sesquiterpene coumarins from Ferula species. Phytother Res 2011, 25, 315–323. [Google Scholar] [CrossRef]
- Iranshahi, M.; Rezaee, R.; Najaf Najafi, M.; Haghbin, A.; Kasaian, J. Cytotoxic activity of the genus Ferula (Apiaceae) and its bioactive constituents. Avicenna J. Phytomed. 2018, 8, 296–312. [Google Scholar] [PubMed]
- Appendino, G.; Spagliardi, P.; Cravotto, G.; Pocock, V.; Milligan, S. Daucane phytoestrogens: A structure-activity study. J. Nat. Prod. 2002, 65, 1612–1615. [Google Scholar] [CrossRef] [PubMed]
- Sattar, Z.; Iranshahi, M. Phytochemistry and Pharmacology of Ferula hermonis Boiss.—A Review. Drug Res. (Stuttg.) 2017, 67, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Macho, A.; Blanco-Molina, M.; Spagliardi, P.; Appendino, G.; Bremner, P.; Heinrich, M.; Fiebich, B.L.; Muñoz, E. Calcium ionophoretic and apoptotic effects of ferutinin in the human Jurkat T-cell line. Biochem. Pharm. 2004, 68, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Matin, M.M.; Nakhaeizadeh, H.; Bahrami, A.R.; Iranshahi, M.; Arghiani, N.; Rassouli, F.B. Ferutinin, an apoptosis inducing terpenoid from Ferula ovina. Asian Pac. J. Cancer Prev. 2014, 15, 2123–2128. [Google Scholar] [CrossRef] [PubMed]
- Al-Ja’fari, A.H.; Vila, R.; Freixa, B.; Costa, J.; Cañigueral, S. Antifungal compounds from the rhizome and roots of Ferula hermonis. Phytother. Res. 2013, 27, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Mamoci, E.; Cavoski, I.; Simeone, V.; Mondelli, D.; Al-Bitar, L.; Caboni, P. Chemical composition and in vitro activity of plant extracts from Ferula communis and Dittrichia viscosa against postharvest fungi. Molecules 2011, 16, 2609–2625. [Google Scholar] [CrossRef]
- Abourashed, E.A.; Galal, A.M.; Shibl, A.M. Antimycobacterial activity of ferutinin alone and in combination with antitubercular drugs against a rapidly growing surrogate of Mycobacterium tuberculosis. Nat. Prod. Res. 2001, 25, 1142–1149. [Google Scholar] [CrossRef]
- Kiokias, S.; Proestos, C.; Oreopoulou, V. Phenolic Acids of Plant Origin—A Review on Their Antioxidant Activity In Vitro (O/W Emulsion Systems) Along with Their in Vivo Health Biochemical Properties. Foods 2020, 9, 534. [Google Scholar] [CrossRef]
- Ornano, L.; Venditti, A.; Ballero, M.; Sanna, C.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Papa, F.; Vittori, S.; Maggi, F.; et al. Chemopreventive and antioxidant activity of the chamazulene-richessential oil obtained from Artemisia arborescens L. growing on the Isle of La Maddalena, Sardinia, Italy. Chem. Biodivers. 2013, 10, 1464–1474. [Google Scholar] [CrossRef]
- Geroushi, A.; Auzi, A.A.; Elhwuegi, A.S.; Elzawam, F.; Elsherif, A.; Nahar, L.; Sarker, S.D. Antiinflammatory sesquiterpenes from the root oil of Ferula hermonis. Phytother. Res. 2011, 25, 774–777. [Google Scholar] [CrossRef]
- Raafat, K.M. Exploration of the Protective Effects of Some Natural Compounds against Neurodegeneration Exploiting Glycine Receptors in vivo Model. Nat. Prod. Chem. Res. 2013, 1, 3. [Google Scholar] [CrossRef]
- Sepici-Dincel, A.; Acikgoz, S.; Cevik, C.; Sengelen, M.; Yesilada, E. Effects of in vivo antioxidant enzyme activities of myrtle oil in normoglycaemic and alloxan diabetic rabbits. J. Ethnopharmacol. 2007, 110, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Raafat, K.; El-Lakani, A. Acute and subchronic in-vivo effects of Ferula hermonis L. and Sambucus nigra L. and their potential active isolates in a diabetic mouse model of neuropathic pain. BMC Complement. Altern. Med. 2015, 15, 257. [Google Scholar] [CrossRef] [PubMed]
- Marjoribanks, J.; Farquhar, C.; Roberts, H.; Lethaby, A.; Lee, J. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst. Rev. 2017, 1, CD004143. [Google Scholar] [CrossRef] [PubMed]
- Tiosano, D.; Paris, F.; Grimaldi, M.; Georgescu, V.; Servant, N.; Hochberg, Z.; Balaguer, P.; Sultan, C. Evidence of ERalpha and ERbeta selectivity and partial estrogen agonism in traditional Chinese medicine. Reprod. Biol. Endocrinol. 2014, 12, 97. [Google Scholar] [CrossRef]
- Ferretti, M.; Bertoni, L.; Cavani, F.; Benincasa, M.; Sena, P.; Carnevale, G.; Zavatti, M.; Di Viesti, V.; Zanoli, P.; Palumbo, C. Structural and histomorphometric evaluations of ferutinin effects on the uterus of ovariectomized rats during osteoporosis treatment. Life Sci. 2012, 90, 161–168. [Google Scholar] [CrossRef]
- Oseni, T.; Patel, R.; Pyle, J.; Jordan, V.C. Selective estrogen receptor modulators and phytoestrogens. Planta Med. 2008, 74, 1656–1665. [Google Scholar] [CrossRef]
- Paterni, I.; Granchi, C.; Katzenellenbogen, J.A.; Minutolo, F. Estrogen Receptors Alpha (ERα) and Beta (ERβ): Subtype-Selective Ligands and Clinical Potential. Steroids 2014, 90, 13–29. [Google Scholar] [CrossRef]
- Ikeda, K.; Horie-Inoue, K.; Inoue, S. Identification of estrogen-responsive genes based on the DNA binding properties of estrogen receptors using high-throughput sequencing technology. Acta Pharmacol. Sin. 2015, 36, 24–31. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Arterburn, J.B. International Union of Basic and Clinical Pharmacology. XCVII. G Protein-Coupled Estrogen Receptor and Its Pharmacologic Modulators. Pharmacol. Rev. 2015, 67, 505–540. [Google Scholar] [CrossRef]
- Zanoli, P.; Zavatti, M.; Geminiani, E.; Corsi, L.; Baraldi, M. The phytoestrogen ferutinin affects female sexual behavior modulating ERalpha expression in the hypothalamus. Behav. Brain Res. 2009, 199, 283–287. [Google Scholar] [CrossRef]
- Messina, M.; McCaskill-Stevens, W.; Lampe, J.W. Addressing the soy and breast cancer relationship: Review, commentary, and workshop proceedings. J. Natl. Cancer Inst. 2006, 98, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Cavani, F.; Ferretti, M.; Carnevale, G.; Bertoni, L.; Zavatti, M.; Palumbo, C. Effects of different doses of ferutinin on bone formation/resorption in ovariectomized rats. J. Bone Miner. Metab. 2012, 30, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, C.; Ferretti, M.; Bertoni, L.; Cavani, F.; Resca, E.; Casolari, B.; Carnevale, G.; Zavatti, M.; Montanari, C.; Benelli, A.; et al. Influence of ferutinin on bone metabolism in ovariectomized rats. I: Role in preventing osteoporosis. J. Bone Miner. Metab. 2009, 27, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, M.; Bertoni, L.; Cavani, F.; Zavatti, M.; Resca, E.; Carnevale, G.; Benelli, A.; Zanoli, P.; Palumbo, C. Influence of ferutinin on bone metabolism in ovariectomized rats. II: Role in recovering osteoporosis. J. Anat. 2010, 217, 48–56. [Google Scholar] [CrossRef] [PubMed]
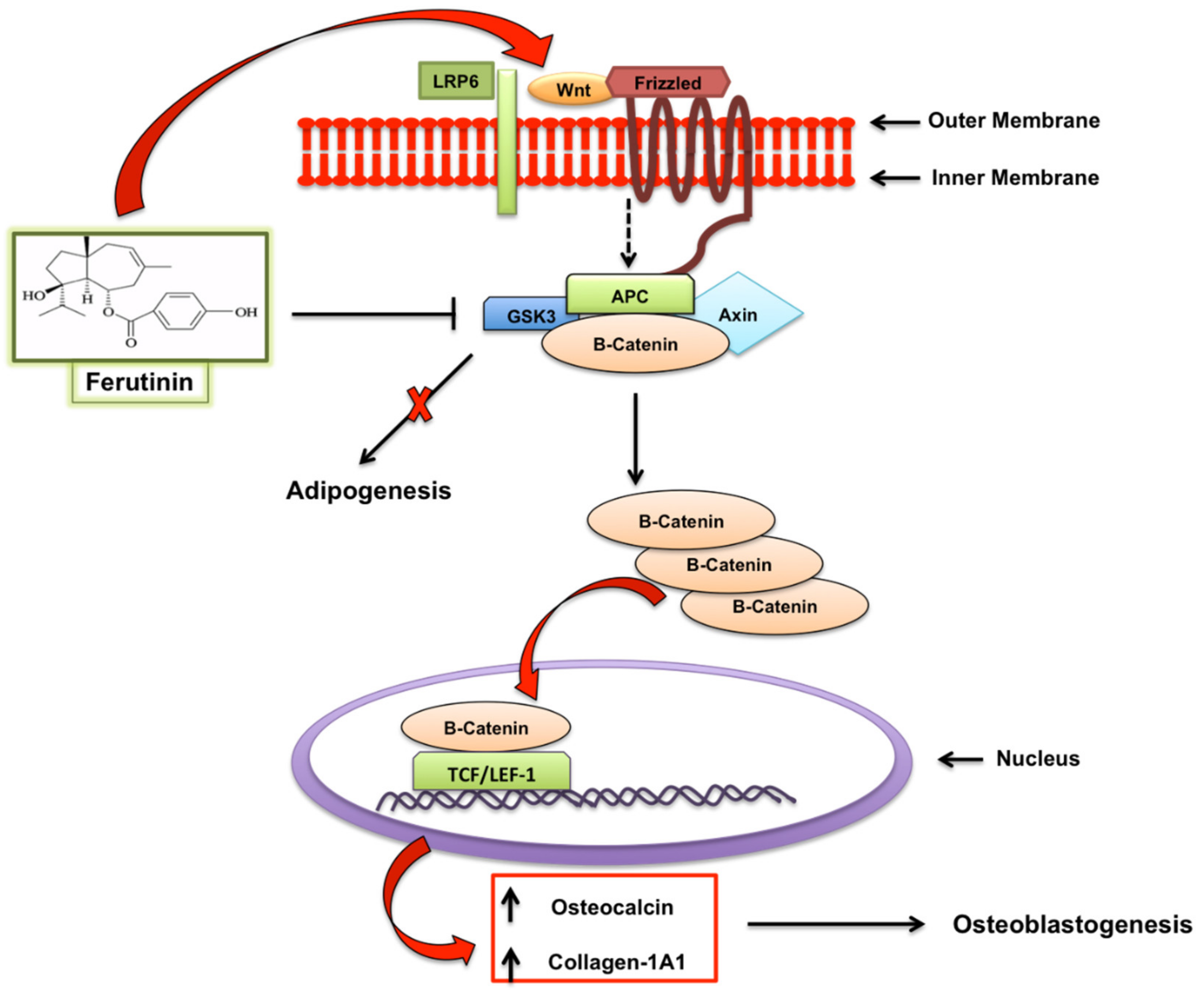
- Rolph, D.N.; Deb, M.; Kanji, S.; Greene, C.J.; Das, M.; Joseph, M.; Aggarwal, R.; Leblebicioglu, B.; Das, H. Ferutinin directs dental pulp-derived stem cells towards the osteogenic lineage by epigenetically regulating canonical Wnt signalling. Biochim. Biophys. Acta Mol. Basis Dis. 2018, S0925-4439, 30435–30436. [Google Scholar] [CrossRef]
- Zamaraeva, M.; Charishnikova, O.; Saidkhodjaev, A.; Isidorov, V.; Granosik, M.; Różalski, M.; Watała, C. Calcium mobilization by the plant estrogen ferutinin does not induce blood platelet aggregation. Pharmacol. Rep. 2010, 62, 1117–1126. [Google Scholar] [CrossRef]
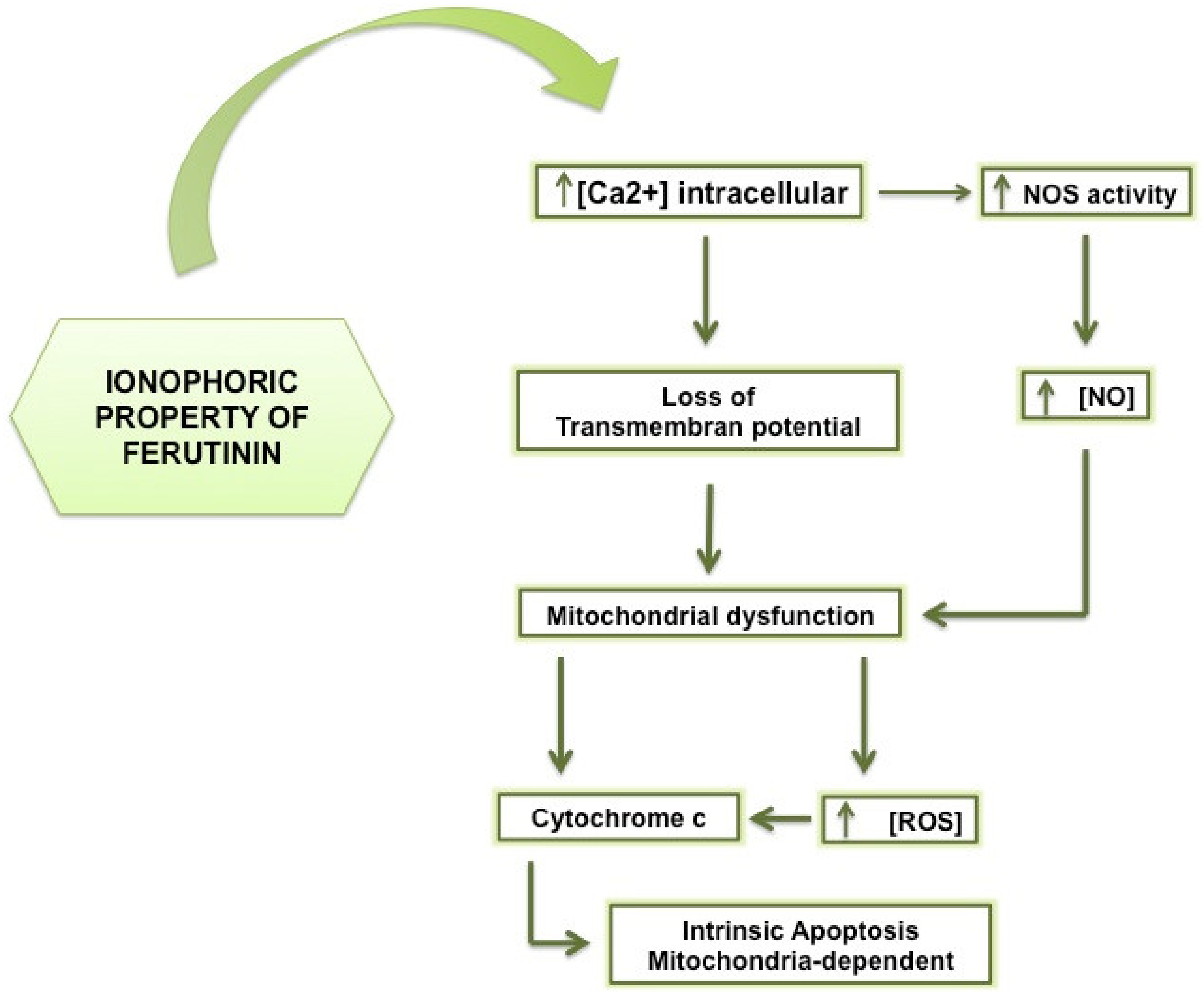
- Ilyich, T.; Charishnikova, O.; Sekowski, S.; Zamaraeva, M.; Cheshchevik, V.; Dremza, I.; Cheshchevik, N.; Kiryukhina, L.; Lapshina, E.; Zavodnik, I. Ferutinin Induces Membrane Depolarization, Permeability Transition Pore Formation, and Respiration Uncoupling in Isolated Rat Liver Mitochondria by Stimulation of Ca2+-Permeability. J. Membr. Biol. 2018, 251, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Nealen, M.L.; Vijayan, K.V.; Bolton, E.; Bray, P.F. Human platelets contain a glycosylated estrogen receptor. Circ. Res. 2001, 88, 438–442. [Google Scholar] [CrossRef]
- Zimmerman, M.A.; Budish, R.A.; Kashyap, S.; Lindsey, S.H. GPER novel membrane oestrogen receptor. Clin. Sci. 2016, 130, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.-K.; VerMeer, M.; Burgard, M.A.; Hassan, A.B.; Giles, J. Hetero-oligomeric Complex between the G Protein-coupled Estrogen Receptor 1 and the Plasma Membrane Ca2+-ATPase 4b. J. Biol. Chem. 2015, 290, 13293–13307. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, M.; Miller, V.M. Human platelets contain estrogen receptor alpha, caveolin-1 and estrogen receptor associated proteins. Platelets 2003, 14, 75–81. [Google Scholar] [CrossRef]
- Colman-Saizarbitoria, T.; Boutros, P.; Amesty, A.; Bahsas, A.; Mathison, Y.; Garrido, M.R.; Israel, A. Ferutinin stimulates nitric oxide synthase activity in median eminence of the rat. J. Ethnopharmacol. 2006, 106, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, M.; Severin, S.; Noirrit-Esclassan, E.; Arnal, J.F.; Payrastre, B.; Valéra, M.C. Effects of Estrogens on Platelets and Megakaryocytes. Int. J. Mol. Sci. 2019, 20, 3111. [Google Scholar] [CrossRef] [PubMed]
- Gurbel, P.A.; Kuliopulos, A.; Tantry, U.S. G-Protein–Coupled Receptors Signaling Pathways in New Antiplatelet Drug Development. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 500–512. [Google Scholar] [CrossRef]
- Sullivan, L.B.; Chandel, N.S. Mitochondrial reactive oxygen species and cancer. Cancer Metab. 2014, 2, 17. [Google Scholar] [CrossRef]
- Madungwe, N.B.; Feng, Y.; Lie, M.; Tombo, N.; Liu, L.; Kaya, F.; Bopassa, J.C. Mitochondrial inner membrane protein (mitofilin) knockdown induces cell death by apoptosis via an AIF-PARP-dependent mechanism and cell cycle arrest. Am. J. Physiol. Cell Physiol. 2018, 315, C28–C43. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef]
- Chien, K.J.; Yang, M.L.; Tsai, P.K.; Su, C.H.; Chen, C.H.; Horng, C.T.; Yeh, C.H.; Chen, W.Y.; Lin, M.L.; Chen, C.J.; et al. Safrole induced cytotoxicity, DNA damage, and apoptosis in macrophages via reactive oxygen species generation and Akt phosphorylation. Environ. Toxicol. Pharmacol. 2018, 64, 94–100. [Google Scholar] [CrossRef]
- Lee, H.N.; Jin, H.O.; Park, J.A.; Kim, J.H.; Kim, J.Y.; Kim, B.; Kim, W.; Hong, S.E.; Lee, Y.H.; Chang, Y.H.; et al. Heme oxygenase-1 determines the differential response of breast cancer and normal cells to piperlongumine. Mol. Cells 2015, 38, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Smithen, M.; Elustondo, P.A.; Winkfein, R.; Zakharian, E.; Abramov, A.Y.; Pavlov, E. Role of polyhydroxybutyrate in mitochondrial calcium uptake. Cell Calcium 2013, 54, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Ignatkov, V.I.; Akhmedkhodzhaeva, K.S.; Babichev, V.N. The effect of tefestrol on the secretion of luteinizing hormone from the hypophysis. Farmakol. Toksikol. 1990, 53, 37–38. [Google Scholar]
- Chauhan, N.S.; Sharma, V.; Dixit, V.K.; Thakur, M. A review on plants used for improvement of sexual performance and virility. Biomed. Res. Int. 2014, 2014, 868062. [Google Scholar] [CrossRef] [PubMed]
- Wink, D.A.; Mitchell, J.B. Chemical biology of nitric oxide: Insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radical. Biol. Med. 1998, 25, 434–456. [Google Scholar] [CrossRef]
- Mancardi, D.; Ridnour, L.A.; Thomas, D.D.; Katori, T.; Tocchetti, C.G.; Espey, M.G.; Miranda, K.M.; Paolocci, N.; Wink, D.A. The chemical dynamics of NO and reactive nitrogen oxides: A practical guide. Curr. Mol. Med. 2004, 4, 723–740. [Google Scholar] [CrossRef]
- González, R.; Molina-Ruiz, F.J.; Bárcena, J.A.; Padilla, C.A.; Muntané, J. Regulation of Cell Survival, Apoptosis, and Epithelial-to-Mesenchymal Transition by Nitric Oxide-Dependent Post-Translational Modifications. Antioxid. Redox Signal 2018, 29, 1312–1332. [Google Scholar] [CrossRef]
- Kim, P.K.; Zamora, R.; Petrosko, P.; Billiar, T.R. The regulatory role of nitric oxide in apoptosis. Int. Immunopharmacol. 2001, 1, 1421–1441. [Google Scholar] [CrossRef]
- Liu, X.; Kim, C.N.; Yang, J.; Jemmerson, R.; Wang, X. Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell 1996, 86, 147–157. [Google Scholar] [CrossRef]
- Kamm, A.; Przychodzen, P.; Kuban-Jankowska, A.; Jacewicz, D.; Dabrowska, A.M.; Nussberger, S.; Wozniak, M.; Gorska-Ponikowska, M. Nitric oxide and its derivatives in the cancer battlefield. Nitric. Oxide 2019, 93, 102–114. [Google Scholar] [CrossRef]
- Brown, G.C.; Borutaite, V. Nitric oxide, cytochrome c and mitochondria. Biochem. Soc. Symp. 1999, 66, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C.; Borutaite, V. Inhibition of mitochondrial respiratory complex I by nitric oxide, peroxynitrite and S-nitorsothiols. Bchim. Biophys Acta 2004, 1658, 44–49. [Google Scholar] [CrossRef]
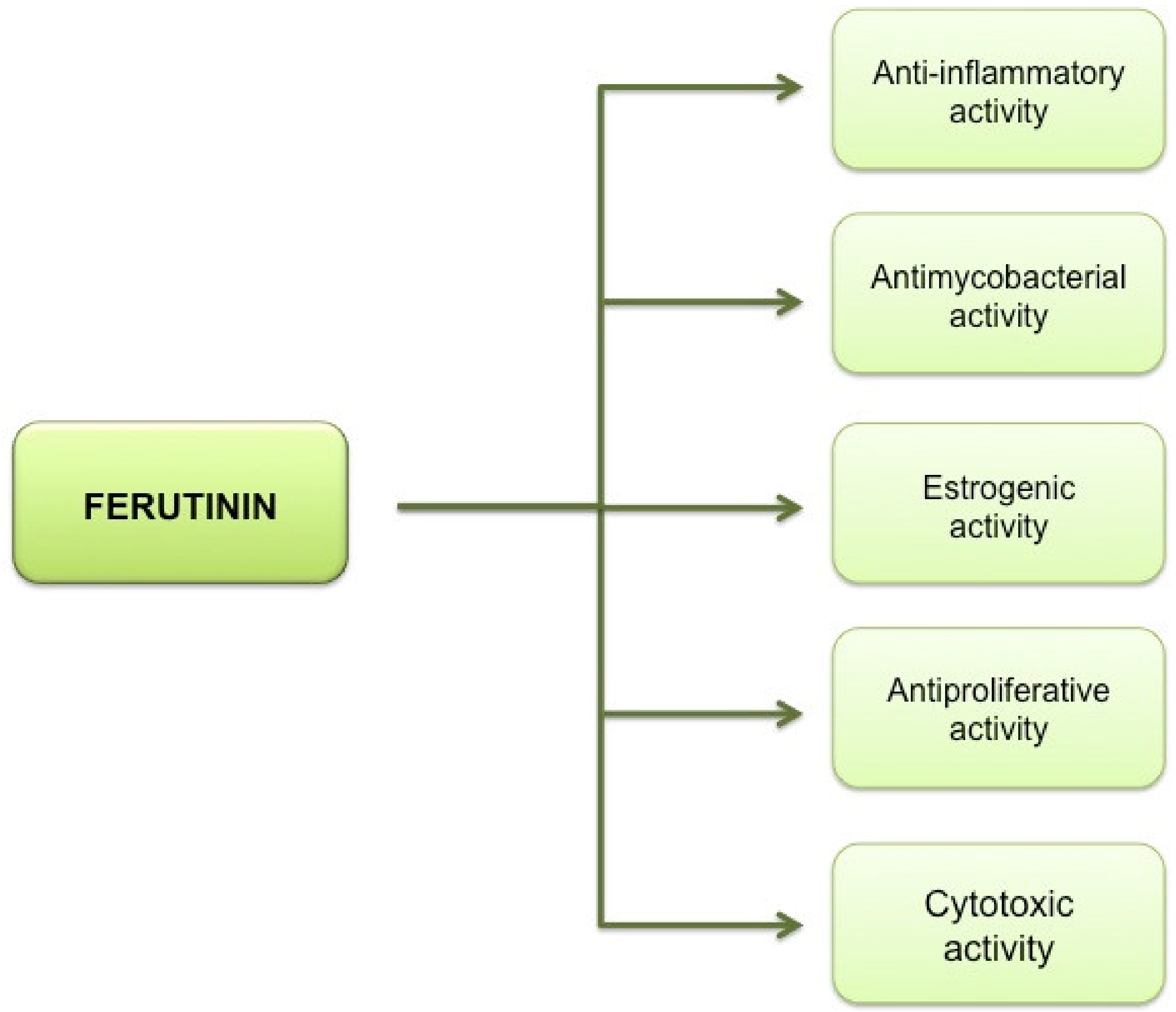
- Safi, R.; El-Sabban, M.; Najjar, F. Ferula hermonis: A Review of Current Use and Pharmacological Studies of its Sesquiterpene Ester Ferutinin. Curr. Drug Targets 2020, 21, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtseva, A.V.; Krasnov, G.S.; Dmitriev, A.A.; Alekseev, B.Y.; Kardymon, O.L.; Sadritdinova, A.F.; Fedorova, M.S.; Pokrovsky, A.V.; Melnikova, N.V.; Kaprin, A.D.; et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget 2016, 7, 44879–44905. [Google Scholar] [CrossRef] [PubMed]
- Burke, P.J. Mitochondria, Bioenergetics and Apoptosis in Cancer. Trends Cancer 2017, 3, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Zamaraeva, M.V.; Hagelgans, A.I.; Abramov, A.Y.; Ternovsky, V.I.; Merzlyak, P.G.; Tashmukhamedov, B.A.; Saidkhodzjaev, A.I. Ionophoretic properties of ferutinin. Cell Calcium 1997, 22, 235–241. [Google Scholar] [CrossRef]
- Bano, D.; Prehn, J.H.M. Apoptosis-Inducing Factor (AIF) in Physiology and Disease: The Tale of a Repented Natural Born Killer. EBioMedicine 2018, 30, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Budd, R.C. Activation-induced cell death. Curr. Opin. Immunol. 2001, 13, 356–362. [Google Scholar] [CrossRef]
- Shi, Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol. Cell 2002, 9, 459–470. [Google Scholar] [CrossRef]
- Shakeri, R.; Kheirollahi, A.; Davoodi, J. Apaf-1: Regulation and function in cell death. Biochimie 2017, 135, 111–125. [Google Scholar] [CrossRef]
- Würstle, M.L.; Laussmann, M.A.; Rehm, M. The central role of initiator caspase-9 in apoptosis signal transduction and the regulation of its activation and activity on the apoptosome. Exp. Cell Res. 2012, 318, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, R.; Hennebelle, T.; Joha, S.; Idziorek, T.; Preudhomme, C.; Quesnel, B.; Sahpaz, S.; Bailleul, F. Activity of elaeochytrin A from Ferula elaeochytris on leukemia cell lines. Phytochemistry 2008, 69, 2979–2983. [Google Scholar] [CrossRef] [PubMed]
- Arghiani, N.; Matin, M.M.; Bahrami, A.R.; Nakhaeizadeh, H.; Sazgarnia, A. Ferutinin: A Natural and Effective anti-tumour Compound. In Proceedings of the 1st International Nastaran Cancer Symposium-2015, Mashhad, Iran, 1 October 2015. [Google Scholar]
- Arghiani, N.; Matin, M.M.; Bahrami, A.R.; Iranshahi, M.; Sazgarnia, A.; Rassouli, F.B. Investigating anticancer properties of the sesquiterpene ferutinin on colon carcinoma cells, in vitro and in vivo. Life Sci. 2014, 109, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Lhuillier, A.; Fabre, N.; Cheble, E.; Oueida, F.; Maurel, S.; Valentin, A.; Fourasté, I.; Moulis, C. Daucane sesquiterpenes from Ferula hermonis. Nat. Prod. 2005, 68, 468–471. [Google Scholar] [CrossRef]
- Ferretti, M.; Cavani, F.; Manni, P.; Carnevale, G.; Bertoni, L.; Zavatti, M.; Palumbo, C. Ferutinin dose-dependent effects on uterus and mammary gland in ovariectomized rats. Histol. Histopathol. 2014, 29, 1027–1037. [Google Scholar] [CrossRef]
- Aydoğan, F.; Baykan, S.; Debeleç Bütüner, B. Cytotoxic Activity of Sesquiterpenoids Isolated from Endemic Ferula tenuissima Hub.-Mor & Peşmen. Turk. J. Pharm. Sci. 2019, 16, 476–480. [Google Scholar] [CrossRef]
- Gao, M.; Wong, S.Y.; Lau, P.M.; Kong, S.K. Ferutinin induces in vitro eryptosis/erythroptosis in human erythrocytes through membrane permeabilization and calcium influx. Chem. Res. Toxicol. 2013, 26, 1218–1228. [Google Scholar] [CrossRef]
| Cells Treated | IC 50 | Ref |
|---|---|---|
| MCF–7: Human breast adenocarcinoma cell line | 81 μM | [32] |
| TCC: Human Transitional Cell Carcinoma Cell line | 67 μM | [32] |
| CT26: Murine colorectal carcinoma cell line | 81 μM | [32] |
| HT29: Human colorectal adenocarcinoma cell line | 72 μM | [32] |
| K562R: Human chronic myeloid leukemia cell line | 25.3 μM | [93] |
| DA1-3b/M2BCR-ABL: Mouse leukemia cell line | 29.1 μM | [93] |
| NTERA2: Human teratocarcinoma cell line | 39 μM | [94] |
| KYSE30: Human oesophageal cancer cell line | 58 μM | [94] |
| PC3: Prostate cancer cell line | 16.7 μM | [98] |
| HFF3: Human foreskin fibroblast cell line | 98 μM | [32] |
| NIH/3T3: Mouse embryo fibroblast cell line | 136 μM | [32] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macrì, R.; Musolino, V.; Gliozzi, M.; Carresi, C.; Maiuolo, J.; Nucera, S.; Scicchitano, M.; Bosco, F.; Scarano, F.; Ruga, S.; et al. Ferula L. Plant Extracts and Dose-Dependent Activity of Natural Sesquiterpene Ferutinin: From Antioxidant Potential to Cytotoxic Effects. Molecules 2020, 25, 5768. https://doi.org/10.3390/molecules25235768
Macrì R, Musolino V, Gliozzi M, Carresi C, Maiuolo J, Nucera S, Scicchitano M, Bosco F, Scarano F, Ruga S, et al. Ferula L. Plant Extracts and Dose-Dependent Activity of Natural Sesquiterpene Ferutinin: From Antioxidant Potential to Cytotoxic Effects. Molecules. 2020; 25(23):5768. https://doi.org/10.3390/molecules25235768
Chicago/Turabian StyleMacrì, Roberta, Vincenzo Musolino, Micaela Gliozzi, Cristina Carresi, Jessica Maiuolo, Saverio Nucera, Miriam Scicchitano, Francesca Bosco, Federica Scarano, Stefano Ruga, and et al. 2020. "Ferula L. Plant Extracts and Dose-Dependent Activity of Natural Sesquiterpene Ferutinin: From Antioxidant Potential to Cytotoxic Effects" Molecules 25, no. 23: 5768. https://doi.org/10.3390/molecules25235768
APA StyleMacrì, R., Musolino, V., Gliozzi, M., Carresi, C., Maiuolo, J., Nucera, S., Scicchitano, M., Bosco, F., Scarano, F., Ruga, S., Zito, M. C., Guarnieri, L., Bombardelli, E., & Mollace, V. (2020). Ferula L. Plant Extracts and Dose-Dependent Activity of Natural Sesquiterpene Ferutinin: From Antioxidant Potential to Cytotoxic Effects. Molecules, 25(23), 5768. https://doi.org/10.3390/molecules25235768