Benefits and Detriments of Gadolinium from Medical Advances to Health and Ecological Risks
Abstract
:1. Introduction to Gadolinium
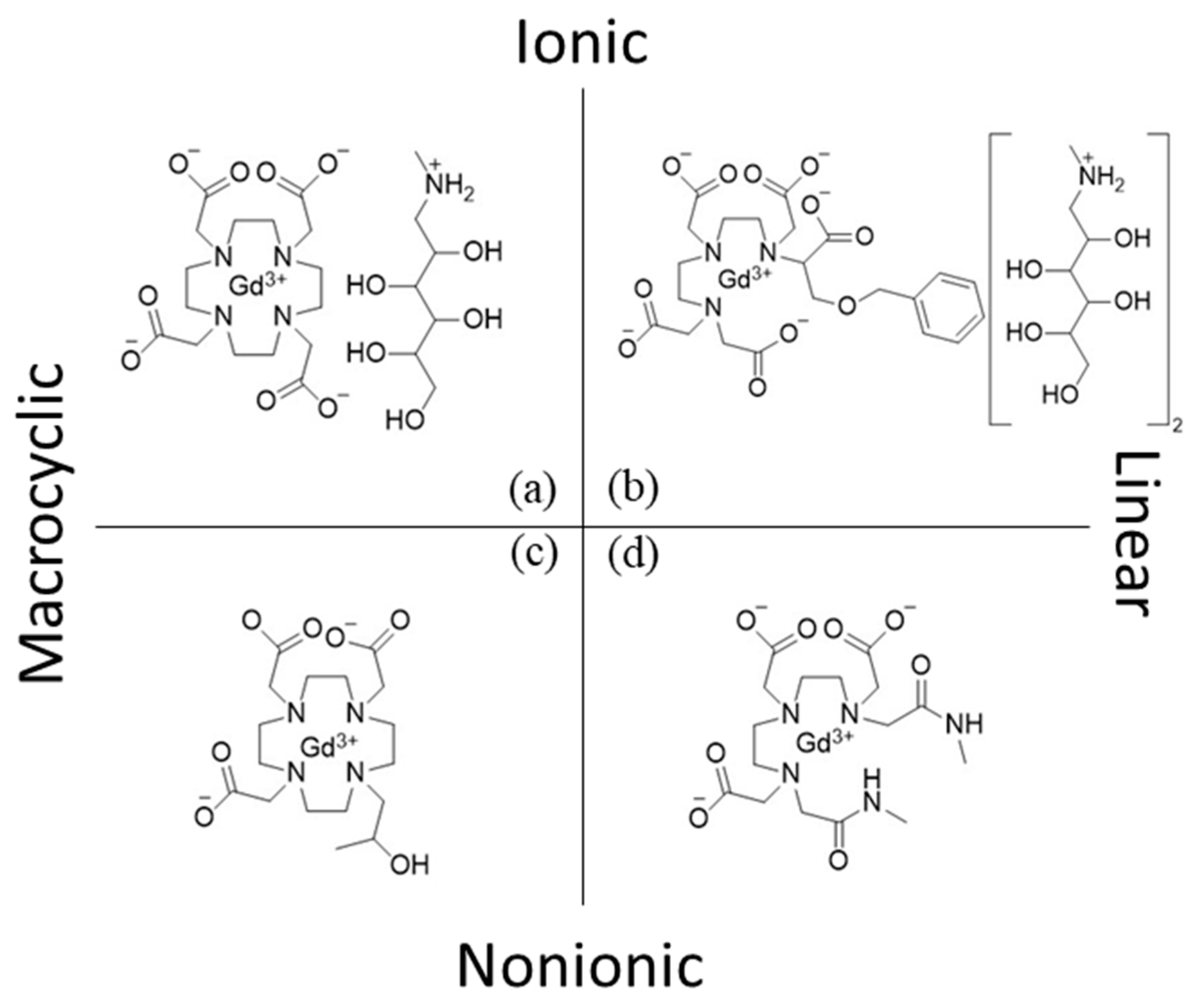
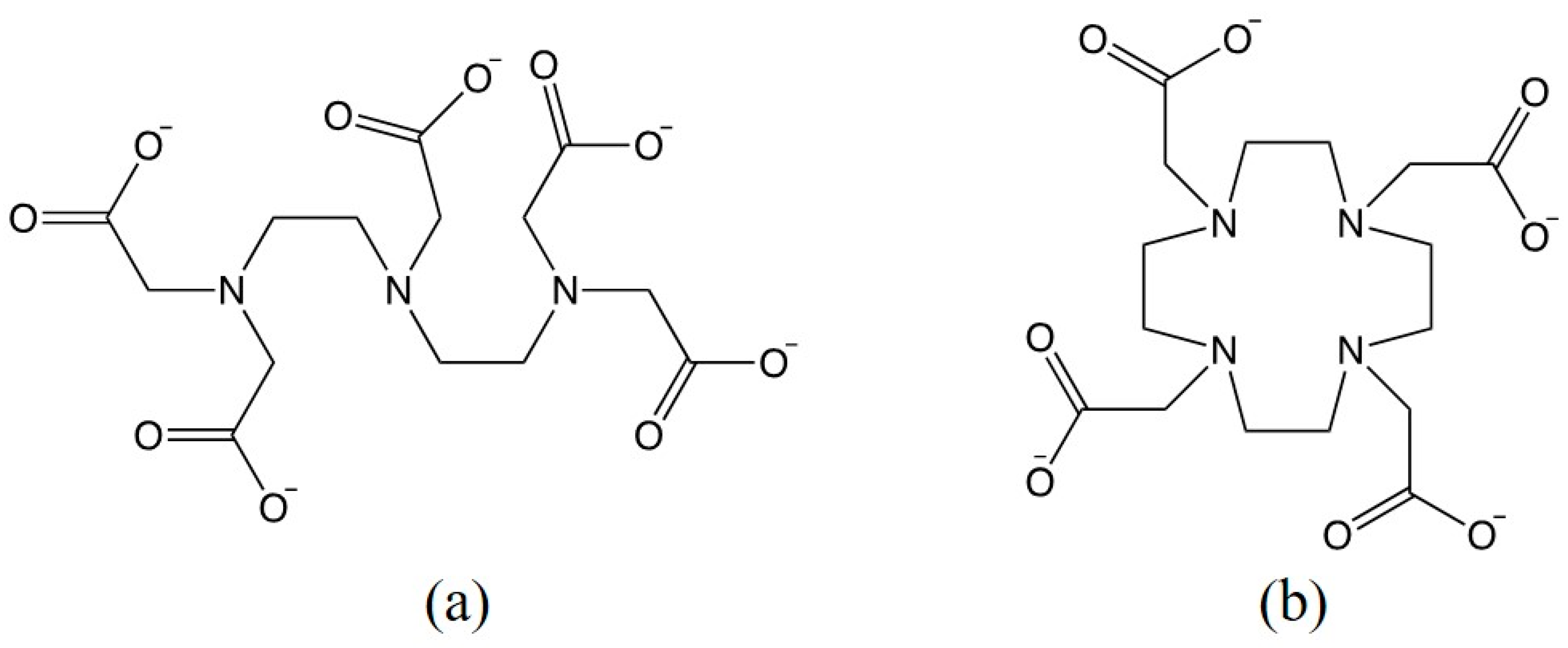
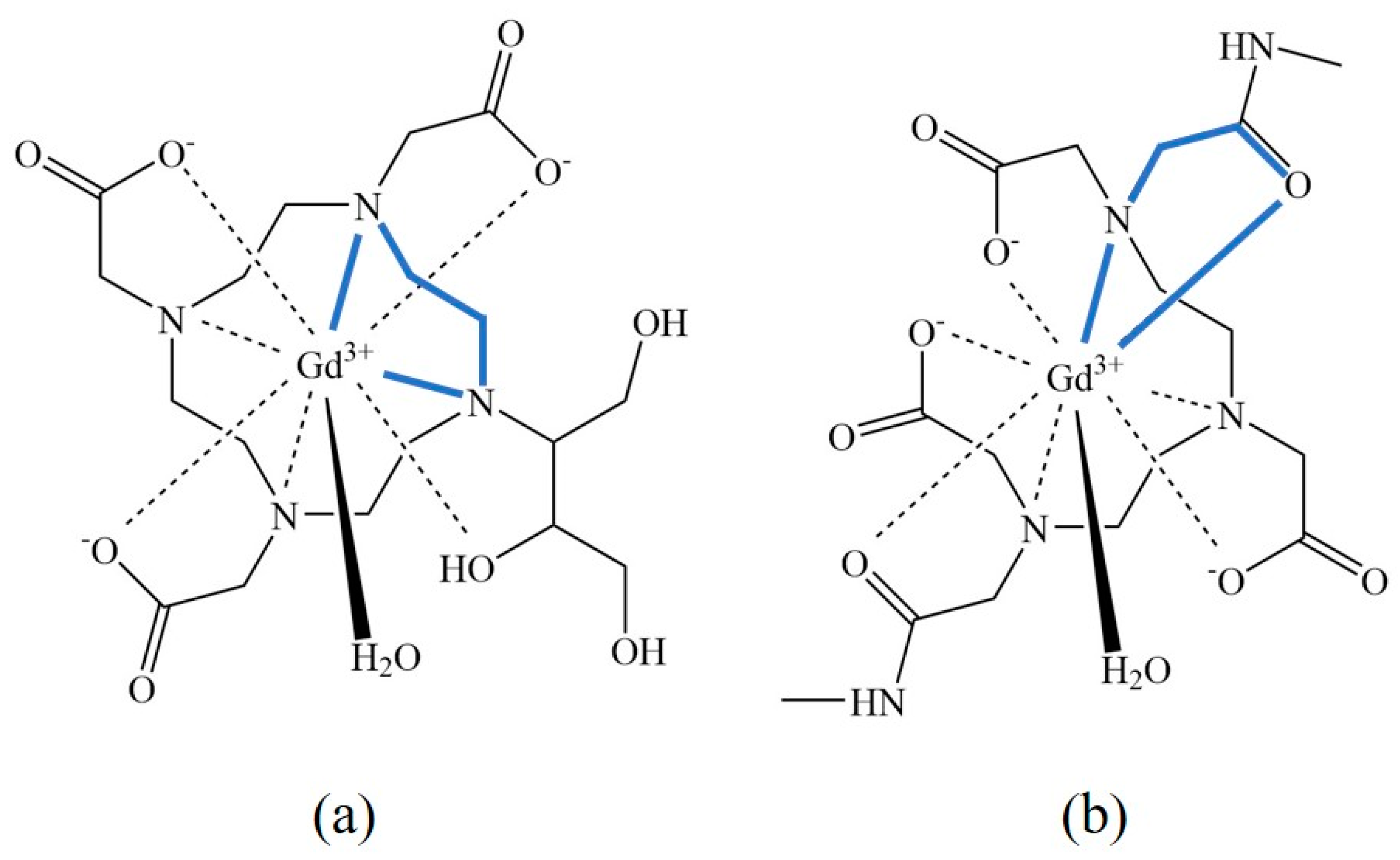
Classification of Gadolinium Chelating Compounds
2. Applications of Gadolinium
2.1. General Technological Applications of Gadolinium
2.2. Biomedical Application of Gadolinium
2.3. Therapeutic Applications
3. Gd in the Environment
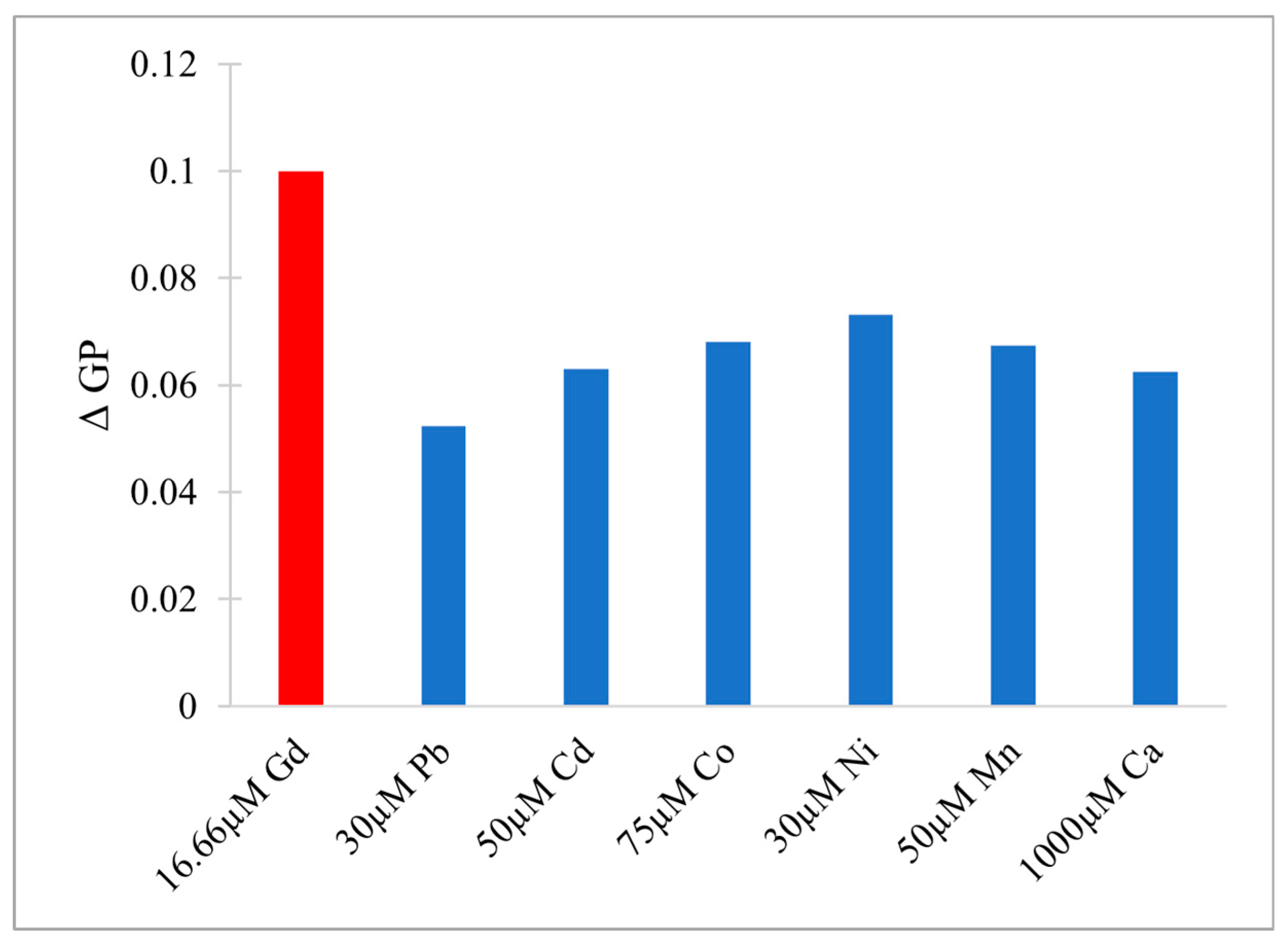
3.1. Toxicity and Ecological Impact of Gd
3.2. Remediation and Alternatives
4. Gadolinium in the Human Organism
4.1. Exposure and Uptake
4.2. Biodistribution and Excretion
4.3. GBCA Toxicity at the Organ Level
5. Gd Interactions at the Cellular and Molecular Level
5.1. Gd Interactions with Nucleic Acids and Carbohydrates
5.2. Effects on Proteins (Channels), Channels, Ca2+ Mimic
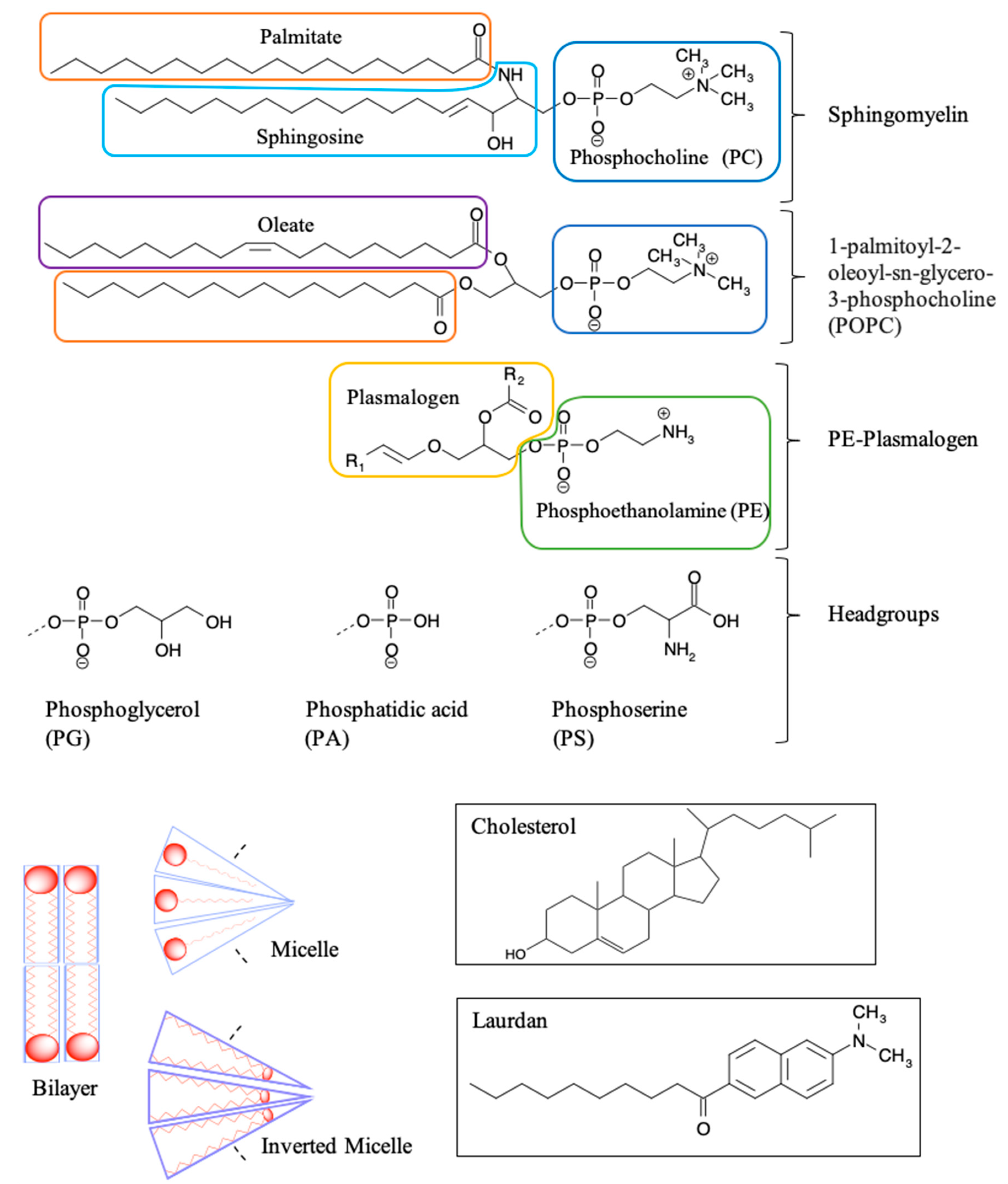
5.3. Introduction to Lipids and Model Membranes
Membrane Interactions of Gadolinium
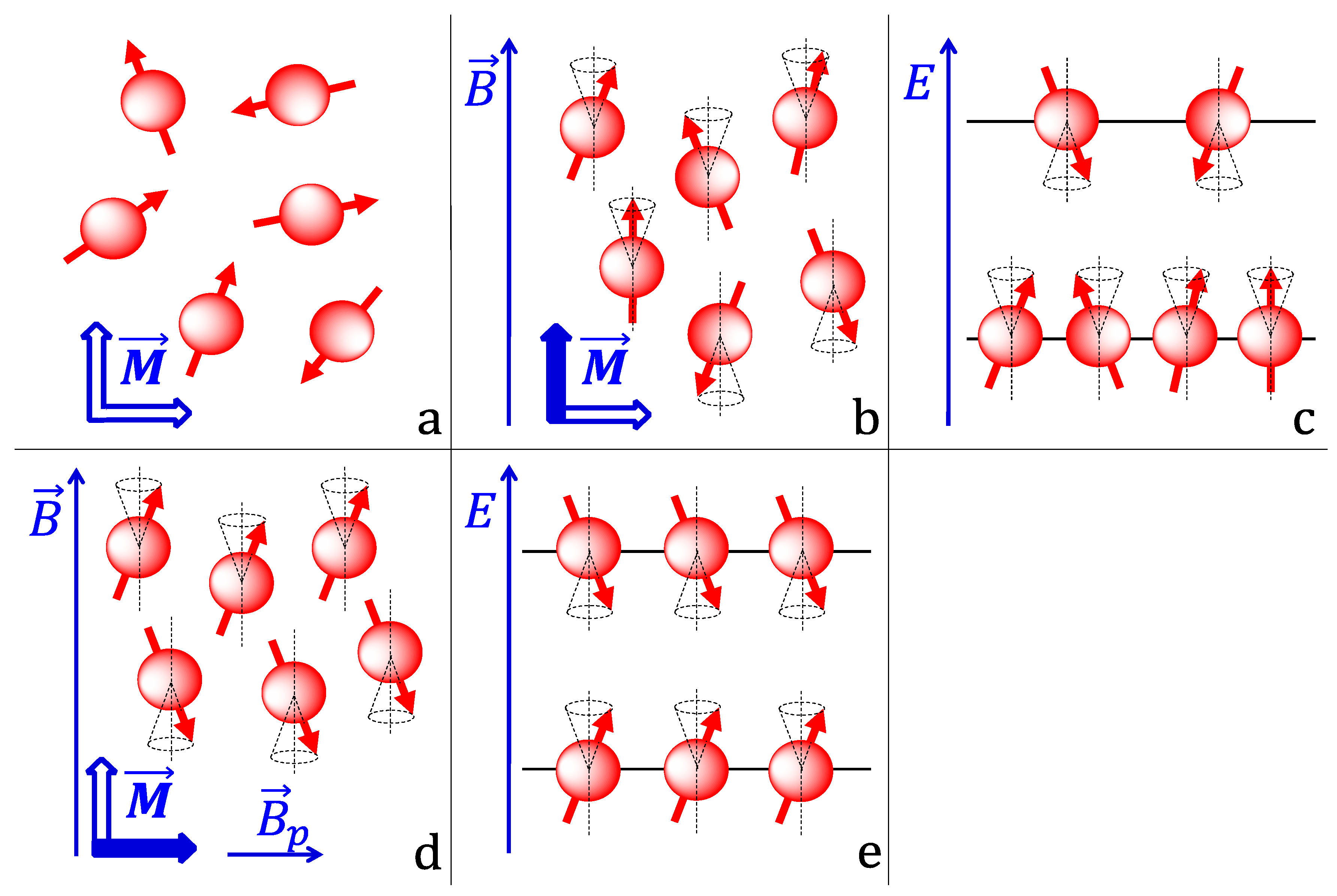
5.4. Gd3+ Effect on Membrane Potential
5.5. Introduction to Metal Membrane Interactions
Membrane Interactions of Gadolinium
6. Summary and Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Barbalace, K. Periodic Table of Elements: Gadolinium—Gd. EnvironmentalChemistry.com. Available online: https://environmentalchemistry.com/yogi/periodic/Gd.html (accessed on 11 August 2020).
- Rogowska, J.; Olkowska, E.; Ratajczyk, W.; Wolska, L. Gadolinium as a new emerging contaminant of aquatic environments. Environ. Toxicol. Chem. 2018, 37, 1523–1534. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries; U.S. Geological Survey: Reston, VA, USA, 2019; p. 133.
- Evans, C.H. Biochemistry of the Lanthanides; Springer: Pittsburgh, PA, USA, 1990. [Google Scholar]
- Bünzli, J.-C.G. Benefiting from the Unique Properties of Lanthanide Ions. Accounts Chem. Res. 2006, 39, 53–61. [Google Scholar] [CrossRef]
- Laing, M. Gadolinium: Central Metal of the Lanthanoids. J. Chem. Educ. 2009, 86, 188–189. [Google Scholar] [CrossRef]
- Sherry, A.D.; Caravan, P.; Lenkinski, R.E. Primer on gadolinium chemistry. J. Magn. Reson. Imaging 2009, 30, 1240–1248. [Google Scholar] [CrossRef] [Green Version]
- Darnall, D.W.; Birnbaum, E.R. Lanthanide ions activate α-amylase. Biochemistry 1973, 12, 3489–3491. [Google Scholar] [CrossRef]
- Evans, C.H. Interactions of Lanthanides with Tissues, Cells, and Cellular Organelles. In Biochemistry of the Lanthanides; Springer: Pittsburgh, PA, USA, 1990; pp. 211–283. [Google Scholar]
- Daumann, L.J. Essential and Ubiquitous: The Emergence of Lanthanide Metallobiochemistry. Angew. Chem. Int. Ed. 2019, 58, 12795–12802. [Google Scholar] [CrossRef]
- Rinard, P. Neutron Interactions with Matter. In Passive Nondestructive Assay of Nuclear Materials; Reilly, R.D., Ensslin, N., Smith, H., Eds.; US Nuclear Regulatory Commission: Washington, DC, USA, 1991; pp. 357–377. [Google Scholar]
- Evans, C.H. The Occurrence and Metabolism of Lanthanides. In Biochemistry of the Lanthanides; Springer: Pittsburgh, PA, USA, 1990; pp. 285–328. [Google Scholar]
- Sugimae, A. Atmospheric concentrations and sources of rare earth elements in the Osaka area, Japan. Atmos. Environ. 1980, 14, 1171–1175. [Google Scholar] [CrossRef]
- Bau, M.; Dulski, P. Anthropogenic origin of positive gadolinium anomalies in river waters. Earth Planet. Sci. Lett. 1996, 143, 245–255. [Google Scholar] [CrossRef]
- Rogosnitzky, M.; Branch, S. Gadolinium-based contrast agent toxicity: A review of known and proposed mechanisms. BioMetals 2016, 29, 365–376. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi, P.; Barbieri, M. Gadolinium as an Emerging Microcontaminant in Water Resources: Threats and Opportunities. Geosciences 2019, 9, 93. [Google Scholar] [CrossRef] [Green Version]
- Ringbom, A. Complexation in Analytical Chemistry; Interscience: New York, NY, USA, 1963. [Google Scholar]
- Ringbom, A. Treatise in Analytical Chemistry; Part 1; Kolthoff, I.M., Elving, P.J., Eds.; Interscience: New York, NY, USA, 1963; Volume 2. [Google Scholar]
- Weinmann, H.J.; Brasch, R.C.; Press, W.R.; Wesbey, G.E. Characteristics of gadolinium-DTPA complex: A potential NMR contrast agent. Am. J. Roentgenol. 1984, 142, 619–624. [Google Scholar] [CrossRef] [Green Version]
- Port, M.; Idée, J.-M.; Medina, C.; Robic, C.; Sabatou, M.; Corot, C. Efficiency, thermodynamic and kinetic stability of marketed gadolinium chelates and their possible clinical consequences: A critical review. BioMetals 2008, 21, 469–490. [Google Scholar] [CrossRef]
- Adding, L.C.; Bannenberg, G.L.; Gustafsson, L.E. Basic Experimental Studies and Clinical Aspects of Gadolinium Salts and Chelates. Cardiovasc. Drug Rev. 2001, 19, 41–56. [Google Scholar] [CrossRef]
- Caravan, P.; Lauffer, R.B. Contrast Agents: Basic Principles. In Clinical Magnetic Resonance Imaging; Edelman, R., Hesselink, J., Zlatkin, M., Crues, J., Eds.; Elsevier: North York, ON, Canada, 2005; pp. 357–375. [Google Scholar]
- Mann, J.S. Stability of Gadolinium Complexes In Vitro and In Vivo. J. Comput. Assist. Tomogr. 1993, 17, S19–S23. [Google Scholar] [CrossRef]
- White, D.H.; DeLearie, L.A.; Moore, D.A.; Wallace, R.A.; Dunn, T.J.; Cacheris, W.P.; Imura, H.; Choppin, G.R. The Thermodynamics of Complexation of Lanthanide (III) DTPA-Bisamide Complexes and Their Implication for Stability and Solution Structure Investigative Radiology. Investig. Radiol. 1991, 26, 226–228. [Google Scholar] [CrossRef]
- Meyer, D.; Schaefer, M.; Bonnemain, B. Gd-DOTA, A Potential MRI Contrast Agent Current Status of Physicochemical Knowledge. Investig. Radiol. 1988, 23, S232–S235. [Google Scholar] [CrossRef]
- Idée, J.M.; Port, M.; Robic, C.; Medina, C.; Sabatou, M.; Corot, C. Role of thermodynamic and kinetic parameters in gadolinium chelate stability. J. Magn. Reson. Imaging 2009, 30, 1249–1258. [Google Scholar] [CrossRef]
- Brücher, E. Kinetic Stabilities of Gadolinium(III) Chelates Used as MRI Contrast Agents. In Contrast Agents I Magnetic Resonance Imaging; Werner, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 103–122. [Google Scholar]
- Dumazert, J.; Coulon, R.; LeComte, Q.; Bertrand, G.H.V.; Hamel, M. Gadolinium for neutron detection in current nuclear instrumentation research: A review. Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2018, 882, 53–68. [Google Scholar] [CrossRef]
- Yang, M.; Liu, X.; Zhang, Z.; Song, Y.; Bai, L. Effect of Adding Rare Earth Elements Er and Gd on the Corrosion Residual Strength of Magnesium Alloy. Open Phys. 2019, 17, 373–380. [Google Scholar] [CrossRef] [Green Version]
- Lamastra, F.R.; Bianco, A.; Leonardi, F.; Montesperelli, G.; Nanni, F.; Gusmano, G. High density Gd-substituted yttrium iron garnets by coprecipitation. Mater. Chem. Phys. 2008, 107, 274–280. [Google Scholar] [CrossRef]
- Kuanr, B.K. Effect of rare-earth Gd3+ on instability threshold of YIG. J. Magn. Magn. Mater. 1997, 170, 40–48. [Google Scholar] [CrossRef]
- Bass, M. Handbook of Optics, Volume I—Fundamentals, Techniques, and Design, 2nd ed.; McGraw-Hill, Inc.: New York, NY, USA, 1995. [Google Scholar]
- Rim, K.T.; Koo, K.H.; Park, J.S. Toxicological Evaluations of Rare Earths and Their Health Impacts to Workers: A Literature Review. Saf. Health Work 2013, 4, 12–26. [Google Scholar] [CrossRef] [Green Version]
- Caravan, P.; Ellison, J.J.; McMurry, T.J.; Lauffer, R.B. Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. Chem. Rev. 1999, 99, 2293–2352. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Tahir, M.A.; Zhang, L.; Schäfer, C.G.; Xu, N. Gadolinium-containing polymer microspheres: A dual-functional theranostic agent for magnetic resonance imaging and cancer therapy. New J. Chem. 2019, 43, 5987–5995. [Google Scholar] [CrossRef]
- Šimečková, P.; Hubatka, F.; Kotouček, J.; Knötigová, P.T.; Mašek, J.; Slavík, J.; Kováč, O.; Neča, J.; Kulich, P.; Hrebík, D.; et al. Gadolinium labelled nanoliposomes as the platform for MRI theranostics: In vitro safety study in liver cells and macrophages. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Gao, T.; Xu, L.; Kuang, Y.; Xiong, D.; Gao, T. Gadolinium-based nanoscale MRI contrast agents for tumor imaging. J. Mater. Chem. B 2017, 5, 3431–3461. [Google Scholar] [CrossRef]
- Mishra, R.; Su, W.; Pohmann, R.; Pfeuffer, J.; Sauer, M.G.; Ugurbil, K.; Engelmann, J. Cell-Penetrating Peptides and Peptide Nucleic Acid-Coupled MRI Contrast Agents: Evaluation of Cellular Delivery and Target Binding. Bioconjug. Chem. 2009, 20, 1860–1868. [Google Scholar] [CrossRef]
- Heckl, S.; Pipkorn, R.; Waldeck, W.; Spring, H.; Jenne, J.; Von Der Lieth, C.-W.; Corban-Wilhelm, H.; Debus, J.; Braun, K. Intracellular visualization of prostate cancer using magnetic resonance imaging. Cancer Res. 2003, 63, 4766–4772. [Google Scholar]
- Nguyen, T.D.T.; Marasini, R.; Rayamajhi, S.; Aparicio, C.; Biller, D.; Aryal, S. Erythrocyte membrane concealed paramagnetic polymeric nanoparticle for contrast-enhanced magnetic resonance imaging. Nanoscale 2020, 12, 4137–4149. [Google Scholar] [CrossRef]
- Peters, T.; Grunewald, C.; Blaickner, M.; Ziegner, M.; Schütz, C.L.; Iffland, D.; Hampel, G.; Nawroth, T.; Langguth, P. Cellular uptake and in vitro antitumor efficacy of composite liposomes for neutron capture therapy. Radiat. Oncol. 2015, 10, 52. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Hashizume, M.; Hotta, Y.; Okahata, Y. Morphology and proliferation of B16 melanoma cells in the presence of lanthanoid and Al3+ ions. BioMetals 1998, 11, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, X.; Wang, K. Effects of Lanthanide Ions (La3+, Gd3+ and Yb3+) on Growth of Human Normal Liver Line 7701 and Human Cervical Carcinoma and the Effect of Cell Apoptosis Induced by Lanthanide. J. Chin. Rare Earth Soc. 2006, 24, 484–488. [Google Scholar]
- Fu, L.-J.; Li, J.-X.; Yang, X.-G.; Wang, K. Gadolinium-promoted cell cycle progression with enhanced S-phase entry via activation of both ERK and PI3K signaling pathways in NIH 3T3 cells. J. Biol. Inorg. Chem. 2009, 14, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fu, L.-J.; Li, J.-X.; Yang, X.-G.; Yang, X.D.; Wang, K. Gadolinium promoted proliferation and enhanced survival in human cervical carcinoma cells. BioMetals 2009, 22, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Long, X.-H.; Yang, P.-Y.; Liu, Q.; Yao, J.; Wang, Y.; He, G.-H.; Hong, G.-Y.; Ni, J.Z. Metabolomic profiles delineate potential roles for gadolinium chloride in the proliferation or inhibition of Hela cells. BioMetals 2011, 24, 663–677. [Google Scholar] [CrossRef] [PubMed]
- Ushikubo, S.; Aoyama, T.; Kamijo, T.; Wanders, R.J.A.; Rinaldo, P.; Vockley, J.; Hashimoto, T. Molecular Characterization of Mitochondrial Trifunctional Protein Deficiency: Formation of the Enzyme Complex Is Important for Stabilization of Both α- and β-Subunits. Am. J. Hum. Genet. 1996, 58, 979–988. [Google Scholar] [PubMed]
- Gardocki, M.E.; Jani, N.; Lopes, J.M. Phosphatidylinositol biosynthesis: Biochemistry and regulation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2005, 1735, 89–100. [Google Scholar] [CrossRef]
- Skwarek, L.C.; Boulianne, G.L. Great Expectations for PIP: Phosphoinositides as Regulators of Signaling During Development and Disease. Dev. Cell 2009, 16, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.-J.; Li, Y.; Tan, Y.-X.; Jiang, M.-R.; Tian, B.; Liu, Y.-K.; Shao, X.-X.; Ye, S.-L.; Wu, J.; Zeng, R.; et al. From Proteomic Analysis to Clinical Significance: Overexpression of Cytokeratin 19 Correlates with Hepatocellular Carcinoma Metastasis. Mol. Cell. Proteom. 2004, 3, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, M.A.; Borges, J.B.R.; De Almeida, D.C.G.; Curi, R. Metabolism of the microregions of human breast cancer. Cancer Lett. 2004, 216, 243–248. [Google Scholar] [CrossRef]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Vertuani, S.; Angusti, A.; Manfredini, S. The Antioxidants and Pro-Antioxidants Network: An Overview. Curr. Pharm. Des. 2004, 10, 1677–1694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashemy, S.I.; Ungerstedt, J.S.; Avval, F.Z.; Holmgren, A. Motexafin Gadolinium, a Tumor-selective Drug Targeting Thioredoxin Reductase and Ribonucleotide Reductase. J. Biol. Chem. 2006, 281, 10691–10697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, L.; Shi, Z.; Liu, H.; Yang, X.; Wang, K. Gadolinium induced apoptosis of human embryo liver L02 cell line by ROS-mediated AIF pathway. J. Rare Earths 2011, 29, 178–184. [Google Scholar] [CrossRef]
- Beneke, R.; Geisen, C.; Zevnik, B.; Bauch, T.; Müller, W.-U.; Küpper, J.-H.; Möröy, T. DNA Excision Repair and DNA Damage-Induced Apoptosis Are Linked to Poly(ADP-Ribosyl)ation but Have Different Requirements for P53. Mol. Cell. Biol. 2000, 20, 6695–6703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Candé, C.; Cecconi, F.; Dessen, P.; Kroemer, G. Apoptosis-inducing factor (AIF): Key to the conserved caspase-independent pathways of cell death? J. Cell Sci. 2002, 115, 4727–4734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, R.F.; Feinendegen, L.E. The quest to exploit the Auger effect in cancer radiotherapy—A reflective review. Int. J. Radiat. Biol. 2016, 92, 617–632. [Google Scholar] [CrossRef]
- Yokoya, A.; Ito, T. Photon-induced Auger effect in biological systems: A review. Int. J. Radiat. Biol. 2017, 93, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Saitoh, H.; Doan, T.L.H.; Shiro, A.; Nakai, K.; Komatsu, A.; Tsujimoto, M.; Yasuda, R.; Kawachi, T.; Tajima, T.; et al. Destruction of tumor mass by gadolinium-loaded nanoparticles irradiated with monochromatic X-rays: Implications for the Auger therapy. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Brünjes, R.; Hofmann, T. Anthropogenic gadolinium in freshwater and drinking water systems. Water Res. 2020, 182, 115966. [Google Scholar] [CrossRef] [PubMed]
- Kulaksız, S.; Bau, M. Anthropogenic gadolinium as a microcontaminant in tap water used as drinking water in urban areas and megacities. Appl. Geochem. 2011, 26, 1877–1885. [Google Scholar] [CrossRef]
- Zhu, Y.; Hoshino, M.; Yamada, H.; Itoh, A.; Haraguchi, H. Gadolinium Anomaly in the Distributions of Rare Earth Elements Observed for Coastal Seawater and River Waters around Nagoya City. Bull. Chem. Soc. Jpn. 2004, 77, 1835–1842. [Google Scholar] [CrossRef]
- McLennan, S.M. Chapter 7. Rare Earth Elements in Sedimentary Rocks: Influence of Provenance and Sedimentary Processes. In Geochemistry and Mineralogy of Rare Earth Elements; Walter de Gruyter GmbH: Berlin, Germany, 1989; pp. 169–200. [Google Scholar]
- De Boer, J.L.M.; Verweij, W.; Van Der Velde-Koerts, T.; Mennes, W. Levels of rare earth elements in Dutch drinking water and its sources. Determination by inductively coupled plasma mass spectrometry and toxicological implications. A pilot study. Water Res. 1996, 30, 190–198. [Google Scholar] [CrossRef]
- Kümmerer, K.; Helmers, E. Hospital Effluents as a Source of Gadolinium in the Aquatic Environment. Environ. Sci. Technol. 2000, 34, 573–577. [Google Scholar] [CrossRef]
- Roth, F.; Lessa, G.C.; Wild, C.; De Kikuchi, R.K.P.; Naumann, M.S. Impacts of a high-discharge submarine sewage outfall on water quality in the coastal zone of Salvador (Bahia, Brazil). Mar. Pollut. Bull. 2016, 106, 43–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhang, M.; Wang, X. Population Growth Responses of Tetrahymena shanghaiensis in Exposure to Rare Earth Elements. Biol. Trace Element Res. 2000, 75, 265–275. [Google Scholar] [CrossRef]
- Morroni, L.; Pinsino, A.; Pellegrini, D.; Regoli, F.; Matranga, V. Development of a new integrative toxicity index based on an improvement of the sea urchin embryo toxicity test. Ecotoxicol. Environ. Saf. 2016, 123, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, M.; Kuroda, R.; Muranaka, Y.; Uto, N.; Murai, J.; Kuroda, H. Asymmetric inhibition of spicule formation in sea urchin embryos with low concentrations of gadolinium ion. Dev. Growth Differ. 2010, 52, 735–746. [Google Scholar] [CrossRef]
- Martino, C.; Bonaventura, R.; Byrne, M.; Roccheri, M.; Matranga, V. Effects of exposure to gadolinium on the development of geographically and phylogenetically distant sea urchins species. Mar. Environ. Res. 2017, 128, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Gagné, F. Toxicity and Disruption of Quorum Sensing in Aliivibrio Fisheri by Environmental Chemicals: Impacts of Selected Contaminants and Microplastics. J. Xenobiotics 2017, 7, 7101. [Google Scholar] [CrossRef]
- Steinberg, S.M.; Poziomek, E.J.; Engelmann, W.H.; Rogers, K.R. A review of environmental applications of bioluminescence measurements. Chemosphere 1995, 30, 2155–2197. [Google Scholar] [CrossRef]
- Fuma, S.; Takeda, H.; Miyamoto, K.; Yanagisawa, K.; Inoue, Y.; Ishii, N.; Sugai, K.; Ishii, C.; Kawabata, Z. Ecological Evaluation of Gadolinium Toxicity Compared with Other Heavy Metals Using an Aquatic Microcosm. Bull. Environ. Contam. Toxicol. 2001, 66, 231–238. [Google Scholar] [CrossRef]
- Chieh, C. Water Treatment—Chemistry LibreTexts. Available online: https://chem.libretexts.org/Bookshelves/Environmental_Chemistry/Supplemental_Modules_Environmental_Chemistry)/Aquatic_Chemistry/Water_Treatment (accessed on 15 October 2020).
- Tyagi, V.; Chopra, A.; Durgapal, N.; Kumar, A. Evaluation of Daphnia magna as an indicator of Toxicity and Treatment efficacy of Municipal Sewage Treatment Plant. J. Appl. Sci. Environ. Manag. 2007, 11, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Villegas-Navarro, A.; Romero González, M.C.; Rosas López, E.; Domínguez Aguilar, R.; Sachetin Marçal, W. Evaluation of Daphnia magna as an indicator of toxicity and treatment efficacy of textile wastewaters. Environ. Int. 1999, 25, 619–624. [Google Scholar] [CrossRef]
- Elizalde-González, M.P.; García-Díaz, E.; González-Perea, M.; Mattusch, J. Removal of gadolinium-based contrast agents: Adsorption on activated carbon. Environ. Sci. Pollut. Res. 2017, 24, 8164–8175. [Google Scholar] [CrossRef]
- Verplanck, P.L.; Taylor, H.E.; Nordstrom, D.K.; Barber, L.B. Aqueous Stability of Gadolinium in Surface Waters Receiving Sewage Treatment Plant Effluent, Boulder Creek, Colorado. Environ. Sci. Technol. 2005, 39, 6923–6929. [Google Scholar] [CrossRef]
- Lawrence, M.G. Detection of anthropogenic gadolinium in the Brisbane River plume in Moreton Bay, Queensland, Australia. Mar. Pollut. Bull. 2010, 60, 1113–1116. [Google Scholar] [CrossRef]
- Oturan, M.A.; Aaron, J.-J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Niederste-Hollenberg, J.; Eckartz, K.; Peters, A.; Hillenbrand, T.; Maier, U.; Beer, M.; Reszt, A. Reducing the Emission of X-Ray Contrast Agents to the Environment: Decentralized Collection of Urine Bags and Its Acceptance. GAIA Ecol. Perspect. Sci. Soc. 2018, 27, 147–155. [Google Scholar] [CrossRef]
- Hu, F.; Zhao, Y.S. Inorganic nanoparticle-based T1 and T1/T2 magnetic resonance contrast probes. Nanoscale 2012, 4, 6235–6243. [Google Scholar] [CrossRef]
- Flacke, S.; Fischer, S.; Scott, M.J.; Fuhrhop, R.J.; Allen, J.S.; McLean, M.; Winter, P.; Sicard, G.A.; Gaffney, P.J.; Wickline, S.A.; et al. Novel MRI Contrast Agent for Molecular Imaging of Fibrin Implications for Detecting Vulnerable Plaques. Circulation 2001, 104, 1280–1285. [Google Scholar] [CrossRef] [Green Version]
- Rieter, W.J.; Kim, J.S.; Taylor, K.M.L.; An, H.; Lin, W.; Tarrant, T.; Lin, W. Hybrid Silica Nanoparticles for Multimodal Imaging. Angew. Chem. Int. Ed. 2007, 46, 3680–3682. [Google Scholar] [CrossRef]
- Rieter, W.J.; Taylor, K.M.L.; An, H.; Lin, W.; Lin, W. Nanoscale Metal−Organic Frameworks as Potential Multimodal Contrast Enhancing Agents. J. Am. Chem. Soc. 2006, 128, 9024–9025. [Google Scholar] [CrossRef] [Green Version]
- Richard, C.; Doan, B.-T.; Beloeil, J.-C.; Bessodes, M.; Tóth, É.; Scherman, D. Noncovalent Functionalization of Carbon Nanotubes with Amphiphilic Gd3+Chelates: Toward Powerful T1and T2MRI Contrast Agents. Nano Lett. 2008, 8, 232–236. [Google Scholar] [CrossRef]
- Sitharaman, B.; Kissell, K.R.; Hartman, K.B.; Tran, L.A.; Baikalov, A.; Rusakova, I.; Sun, Y.; Khant, H.A.; Ludtke, S.J.; Chiu, W.; et al. Superparamagnetic gadonanotubes are high-performance MRI contrast agents. Chem. Commun. 2005, 31, 3915–3917. [Google Scholar] [CrossRef]
- Bin Na, H.; Lee, J.H.; An, K.; Park, Y.I.; Park, M.; Lee, I.S.; Nam, D.-H.; Kim, S.T.; Kim, S.-H.; Kim, S.-W.; et al. Development of aT1 Contrast Agent for Magnetic Resonance Imaging Using MnO Nanoparticles. Angew. Chem. 2007, 119, 5493–5497. [Google Scholar] [CrossRef]
- Dubertret, M.N.B.; Skourides, P.; Norris, D.J.; Noireaux, V.; Brivanlou, A.H.; Libchaber, A. In Vivo Imaging of Quantum Dots Encapsulated in Phospholipid Micelles. Science 2002, 298, 1759–1762. [Google Scholar] [CrossRef] [Green Version]
- Gilad, A.A.; Walczak, P.; McMahon, M.T.; Hyon, B.N.; Jung, H.L.; An, K.; Hyeon, T.; Van Zijl, P.C.M.; Bulte, J.W.M. MR tracking of transplanted cells with “positive contrast” using manganese oxide nanoparticles. Magn. Reson. Med. 2008, 60, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-H.; Kim, B.H.; Bin Na, H.; Hyeon, T. Paramagnetic inorganic nanoparticles as T1 MRI contrast agents. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 196–209. [Google Scholar] [CrossRef]
- Kim, T.; Momin, E.; Choi, J.; Yuan, K.; Zaidi, H.; Kim, J.; Park, M.; Lee, N.; McMahon, M.T.; Quinones-Hinojosa, A.; et al. Mesoporous Silica-Coated Hollow Manganese Oxide Nanoparticles as PositiveT1Contrast Agents for Labeling and MRI Tracking of Adipose-Derived Mesenchymal Stem Cells. J. Am. Chem. Soc. 2011, 133, 2955–2961. [Google Scholar] [CrossRef]
- Choi, J.Y.; Lee, S.H.; Bin Na, H.; An, K.; Hyeon, T.; Seo, T.S. In vitro cytotoxicity screening of water-dispersible metal oxide nanoparticles in human cell lines. Bioprocess Biosyst. Eng. 2010, 33, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Ling, D.; Zhao, L.; Wang, S.; Liu, Y.; Bai, R.; Baik, S.; Zhao, Y.; Chen, C.; Hyeon, T. Parallel Comparative Studies on Mouse Toxicity of Oxide Nanoparticle- and Gadolinium-Based T1 MRI Contrast Agents. ACS Nano 2015, 9, 12425–12435. [Google Scholar] [CrossRef] [PubMed]
- Chambon, C.; Clement, O.; Le Blanche, A.; Schouman-Claeys, E.; Frija, G. Superparamagnetic iron oxides as positive MR contrast agents: In vitro and in vivo evidence. Magn. Reson. Imaging 1993, 11, 509–519. [Google Scholar] [CrossRef]
- Kim, B.H.; Lee, N.; Kim, H.; An, K.; Park, Y.I.; Choi, Y.; Shin, K.; Lee, Y.; Kwon, S.G.; Bin Na, H.; et al. Large-Scale Synthesis of Uniform and Extremely Small-Sized Iron Oxide Nanoparticles for High-ResolutionT1Magnetic Resonance Imaging Contrast Agents. J. Am. Chem. Soc. 2011, 133, 12624–12631. [Google Scholar] [CrossRef]
- Song, X.; Gong, H.; Yin, S.; Cheng, L.; Wang, C.; Li, Z.; Li, Y.; Wang, X.; Liu, G.; Liu, Z. Cancer Theranostics: Ultra-Small Iron Oxide Doped Polypyrrole Nanoparticles for In Vivo Multimodal Imaging Guided Photothermal Therapy. Adv. Funct. Mater. 2014, 24, 1193. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Dublin, A.B. Magnetic Resonance Imaging (MRI) Gadolinium; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Wang, L.; Liang, T. Geochemical fractions of rare earth elements in soil around a mine tailing in Baotou, China. Sci. Rep. 2015, 5, 12483. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Chen, Z.; Chen, Z.; Zhang, Y. A human health risk assessment of rare earth elements in soil and vegetables from a mining area in Fujian Province, Southeast China. Chemosphere 2013, 93, 1240–1246. [Google Scholar] [CrossRef] [Green Version]
- Adeel, M.; Lee, J.Y.; Zain, M.; Rizwan, M.; Nawab, A.; Ahmad, M.; Shafiq, M.; Yi, H.; Jilani, G.; Javed, R.; et al. Cryptic footprints of rare earth elements on natural resources and living organisms. Environ. Int. 2019, 127, 785–800. [Google Scholar] [CrossRef]
- Braun, M.; Zavanyi, G.; Laczovics, A.; Berényi, E.; Szabo, S. Can aquatic macrophytes be biofilters for gadolinium based contrasting agents? Water Res. 2018, 135, 104–111. [Google Scholar] [CrossRef] [Green Version]
- Lingott, J.; Lindner, U.; Telgmann, L.; Esteban-Fernández, D.; Jakubowski, N.; Panne, U. Gadolinium-uptake by aquatic and terrestrial organisms-distribution determined by laser ablation inductively coupled plasma mass spectrometry. Environ. Sci. Process. Impacts 2016, 18, 200–207. [Google Scholar] [CrossRef]
- Gwenzi, W.; Mangori, L.; Danha, C.; Chaukura, N.; Dunjana, N.; Sanganyado, E. Sources, behaviour, and environmental and human health risks of high-technology rare earth elements as emerging contaminants. Sci. Total. Environ. 2018, 636, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Kramsch, D.M.; Aspen, A.J.; Apstein, C.S. Suppression of experimental atherosclerosis by the Ca++-antagonist lanthanum. Possible role of calcium in atherogenesis. J. Clin. Investig. 1980, 65, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Aime, S.; Caravan, P. Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J. Magn. Reson. Imaging 2009, 30, 1259–1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, K. Clinical use of gadobutrol for contrast-enhanced magnetic resonance imaging of neurological diseases. Rep. Med. Imaging 2012, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Davenport, M.S. Choosing the Safest Gadolinium-based Contrast Medium for MR Imaging: Not So Simple after All. Radiology 2018, 286, 483–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLachlan, S.J.; Eaton, S.; De Simone, D.N. Pharmacokinetic behavior of gadoteridol injection. Investig. Radiol. 1992, 27, S12–S15. [Google Scholar] [CrossRef]
- Frenzel, T.; Lengsfeld, P.; Schirmer, H.; Hütter, J.; Weinmann, H.-J. Stability of Gadolinium-Based Magnetic Resonance Imaging Contrast Agents in Human Serum at 37 °C. Investig. Radiol. 2008, 43, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.; Ai, T.; Goerner, F.; Hu, X.; Runge, V.M.; Tweedle, M. MRI contrast agents: Basic chemistry and safety. J. Magn. Reson. Imaging 2012, 36, 1060–1071. [Google Scholar] [CrossRef]
- McDonald, R.J.; McDonald, J.S.; Kallmes, D.F.; Jentoft, M.E.; Murray, D.L.; Thielen, K.R.; Williamson, E.E.; Eckel, L.J. Intracranial Gadolinium Deposition after Contrast-enhanced MR Imaging. Radiology 2015, 275, 772–782. [Google Scholar] [CrossRef] [Green Version]
- Errante, Y.; Cirimele, V.; Mallio, C.A.; Di Lazzaro, V.; Zobel, B.B.; Quattrocchi, C.C. Progressive Increase of T1 Signal Intensity of the Dentate Nucleus on Unenhanced Magnetic Resonance Images Is Associated With Cumulative Doses of Intravenously Administered Gadodiamide in Patients With Normal Renal Function, Suggesting Dechelation. Investig. Radiol. 2014, 49, 685–690. [Google Scholar] [CrossRef]
- Gibby, W.A.; Gibby, K.A.; Gibby, W.A. Comparison of Gd DTPA-BMA (Omniscan) versus Gd HP-DO3A (ProHance) Retention in Human Bone Tissue by Inductively Coupled Plasma Atomic Emission Spectroscopy. Investig. Radiol. 2004, 39, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Darrah, T.H.; Prutsman-Pfeiffer, J.J.; Poreda, R.J.; Ellen Campbell, M.; Hauschka, P.V.; Hannigan, R.E. Incorporation of Excess Gadolinium into Human Bone from Medical Contrast Agents. Metallomics 2009, 1, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Cowper, S.E.; Robin, H.S.; Steinberg, S.M.; Su, L.D.; Gupta, S.; LeBoit, P.E. Scleromyxoedema-like Cutaneous Diseases in Renal-Dialysis Patients. Lancet 2000, 356, 1000–1001. [Google Scholar] [CrossRef]
- Wagner, B.; Drel, V.; Gorin, Y. Pathophysiology of Gadolinium-Associated Systemic Fibrosis. Am. J. Physiol. Physiol. 2016, 311, F1–F11. [Google Scholar] [CrossRef] [Green Version]
- Boyd, A.S.; Zic, J.A.; Abraham, J.L. Gadolinium Deposition in Nephrogenic Fibrosing Dermopathy. J. Am. Acad. Dermatol. 2007, 56, 27–30. [Google Scholar] [CrossRef]
- High, W.A.; Ayers, R.A.; Chandler, J.; Zito, G.; Cowper, S.E. Gadolinium Is Detectable within the Tissue of Patients with Nephrogenic Systemic Fibrosis. J. Am. Acad. Dermatol. 2007, 56, 21–26. [Google Scholar] [CrossRef]
- Leisman, S. Radiocontrast Toxicity. Adv. Chronic Kidney Dis. 2020, 27, 50–55. [Google Scholar] [CrossRef]
- Angeli, J.K.; Ramos, D.B.; Casali, E.A.; Souza, D.O.G.; Sarkis, J.J.F.; Stefanon, I.; Vassallo, D.V.; Fürstenau, C.R. Gadolinium Increases the Vascular Reactivity of Rat Aortic Rings. Braz. J. Med. Biol. Res. 2011, 44, 445–452. [Google Scholar] [CrossRef] [Green Version]
- Escalada, A.; Navarro, P.; Ros, E.; Aleu, J.; Solsona, C.; Martín-Satué, M. Gadolinium Inhibition of Ecto-Nucleoside Triphosphate Diphosphohydrolase Activity in Torpedo Electric Organ. Neurochem. Res. 2004, 29, 1711–1714. [Google Scholar] [CrossRef]
- Corot, C.; Idee, J.M.; Hentsch, A.M.; Santus, R.; Mallet, C.; Goulas, V.; Bonnemain, B.; Meyer, D. Structure-Activity Relationship of Macrocyclic and Linear Gadolinium Chelates: Investigation of Transmetallation Effect on the Zinc-Dependent Metallopeptidase Angiotensin-Converting Enzyme. J. Magn. Reson. Imaging 1998, 8, 695–702. [Google Scholar] [CrossRef]
- Gulani, V.; Calamante, F.; Shellock, F.G.; Kanal, E.; Reeder, S.B. Gadolinium Deposition in the Brain: Summary of Evidence and Recommendations. Lancet Neurol. 2017, 16, 564–570. [Google Scholar] [CrossRef]
- Vergauwen, E.; Vanbinst, A.-M.; Brussaard, C.; Janssens, P.; De Clerck, D.; Van Lint, M.; Houtman, A.C.; Michel, O.; Keymolen, K.; Lefevere, B.; et al. Central Nervous System Gadolinium Accumulation in Patients Undergoing Periodical Contrast MRI Screening for Hereditary Tumor Syndromes. Hered. Cancer Clin. Pract. 2018, 16, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lonser, R.R.; Glenn, G.M.; Walther, M.; Chew, E.Y.; Libutti, S.K.; Linehan, W.M.; Oldfield, E.H. Von Hippel-Lindau Disease. Lancet 2003, 361, 2059–2067. [Google Scholar] [CrossRef]
- Krueger, D.A.; Northrup, H.; International Tuberous Sclerosis Complex Consensus Group. Tuberous Sclerosis Complex Surveillance and Management: Recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr. Neurol. 2013, 49, 255–265. [Google Scholar] [CrossRef] [Green Version]
- Kanda, T.; Ishii, K.; Kawaguchi, H.; Kitajima, K.; Takenaka, D. High Signal Intensity in the Dentate Nucleus and Globus Pallidus on Unenhanced T1-Weighted MR Images: Relationship with Increasing Cumulative Dose of a Gadoliniumbased Contrast Material. Radiology 2014, 270, 834–841. [Google Scholar] [CrossRef]
- Kanda, T.; Nakai, Y.; Hagiwara, A.; Oba, H.; Toyoda, K.; Furui, S. Distribution and Chemical Forms of Gadolinium in the Brain: A Review. Br. J. Radiol. 2017, 90, 20170115. [Google Scholar] [CrossRef]
- Radbruch, A.; Haase, R.; Kieslich, P.J.; Weberling, L.D.; Kickingereder, P.; Wick, W.; Schlemmer, H.P.; Bendszus, M. No Signal Intensity Increase in the Dentate Nucleus on Unenhanced T1-Weighted Mr Images after More than 20 Serial Injections of Macrocyclic Gadolinium-Based Contrast Agents. Radiology 2017, 282, 699–707. [Google Scholar] [CrossRef] [Green Version]
- Jost, G.; Lenhard, D.C.; Sieber, M.A.; Lohrke, J.; Frenzel, T.; Pietsch, H. Signal Increase on Unenhanced T1-Weighted Images in the Rat Brain after Repeated, Extended Doses of Gadolinium-Based Contrast Agents Comparison of Linear and Macrocyclic Agents. Investig. Radiol. 2016, 51, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Robert, P.; Lehericy, S.; Grand, S.; Violas, X.; Fretellier, N.; Ideé, J.M.; Ballet, S.; Corot, C. T1-Weighted Hypersignal in the Deep Cerebellar Nuclei after Repeated Administrations of Gadolinium-Based Contrast Agents in Healthy Rats: Difference between Linear and Macrocyclic Agents. Investig. Radiol. 2015, 50, 473–480. [Google Scholar] [CrossRef] [Green Version]
- Welk, B.; McArthur, E.; Morrow, S.A.; MacDonald, P.; Hayward, J.; Leung, A.; Lum, A. Association between Gadolinium Contrast Exposure and the Risk of Parkinsonism. JAMA J. Am. Med. Assoc. 2016, 316, 96–98. [Google Scholar] [CrossRef] [Green Version]
- Williams, S.; Grimm, H. Gadolinium Toxicity: A Survey of the Chronic Effects of Retained Gadolinium from Contrast MRIs. Available online: www.GadoliniumToxicity.com (accessed on 4 October 2020).
- Łȩczkowska, A.; Vilar, R. Interaction of Metal Complexes with Nucleic Acids. Annu. Rep. Prog. Chem. Sect. A 2013, 109, 299–316. [Google Scholar] [CrossRef]
- Kohoutkova, V.; Babula, P.; Opatrilova, R.; Vrana, O.; Kizek, R.; Kohoutkova, V.; Babula, P. Analysis of DNA Modified by Cerium (III), Lanthanum (III) and Gadolinium (III) Ions by Using of Raman Spectroscopy. J. Biochem. Technol. 2014, 2, 100–101. [Google Scholar]
- Kim, S.H.; Shin, W.C.; Warrant, R.W. Heavy Metal Ion-Nucleic Acid Interaction. Methods Enzymol. 1985, 114, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Jack, A.; Landner, J.E.; Rhodes, D.; Brown, R.S.; Klug, A. A Crystallographic Study of Metal-Binding to Yeast Phenylalanine Transfer RNA. J. Mol. Biol. 1977, 111, 315–328. [Google Scholar] [CrossRef]
- Stout, C.D.; Mizuno, H.; Rao, S.T.; Swaminathan, P.; Rubin, J.; Brennan, T.; Sundaralingam, M. Crystal and Molecular Structure of Yeast Phenylalanyl Transfer RNA. Structure Determination, Difference Fourier Refinement, Molecular Conformation, Metal and Solvent Binding. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1978, 34, 1529–1544. [Google Scholar] [CrossRef]
- Angyal, S.J. Complexes of Carbohydrates with Metal Cations: I. Determination of the Extent of Complexing by N.M.R. Spectroscopy. Aust. J. Chem. 1972, 25, 1957–1966. [Google Scholar] [CrossRef]
- Beattie, J.K.; Terry Kelso, M. Equilibrium and Dynamics of the Binding of Calcium Ion to Sorbitol (D-Glucitol). Aust. J. Chem. 1981, 34, 2563–2568. [Google Scholar] [CrossRef]
- Dill, K.; Daman, M.E.; Batstone-Cunningham, R.L.; Lacombe, J.M.; Pavia, A.A. 13C-n.m.r.-Spectral Study of the Mode of Binding of Gd3+ to Various Glycopeptides. Carbohydr. Res. 1983, 123, 123–135. [Google Scholar] [CrossRef]
- Varki, A. Sialic Acids in Human Health and Disease. Trends Mol. Med. 2008, 14, 351–360. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Wuhrer, M.; Holst, S. Serum Sialylation Changes in Cancer. Glycoconj. J. 2018, 35, 139–160. [Google Scholar] [CrossRef] [Green Version]
- Dall’Olio, F.; Chiricolo, M. Sialyltransferases in Cancer. Glycoconj. J. 2001, 18, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Schauer, R. Achievements and Challenges of Sialic Acid Research. Glycoconj. J. 2000, 17, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Regueiro-Figueroa, M.; Djanashvili, K.; Esteban-Gómez, D.; Chauvin, T.; Tóth, É.; De Blas, A.; Rodríguez-Blas, T.; Platas-Iglesias, C. Molecular Recognition of Sialic Acid by Lanthanide(III) Complexes through Cooperative Two-Site Binding. Inorg. Chem. 2010, 49, 4212–4223. [Google Scholar] [CrossRef] [PubMed]
- Lacampagne, A.; Gannier, F.; Argibay, J.; Garnier, D.; Le Guennec, J.Y. The Stretch-Activated Ion Channel Blocker Gadolinium Also Blocks L-Type Calcium Channels in Isolated Ventricular Myocytes of the Guinea-Pig. Biochim. Biophys. Acta BBA Biomembr. 1994, 1191, 205–208. [Google Scholar] [CrossRef]
- Suzuki, S.; Toledo-Pereyra, L.H.; Rodriguez, F.; Lopez, F. Role of Kupffer Cells in Neutrophil Activation and Infiltration Following Total Hepatic Ischemia and Reperfusion. Circ. Shock 1994, 42, 204–209. [Google Scholar]
- Callery, M.P.; Kamei, T.; Flye, M.W. Kupffer Cell Blockade Increases Mortality During Intra-Abdominal Sepsis Despite Improving Systemic Immunity. Arch. Surg. 1990, 125, 36–41. [Google Scholar] [CrossRef]
- Palasz, A.; Czekaj, P. Toxicological and Cytophysiological Aspects of Lanthanides Action. Acta Biochim. Pol. 2000, 47, 1107–1114. [Google Scholar] [CrossRef] [Green Version]
- Decker, K. Biologically Active Products of Stimulated Liver Macrophages (Kupffer Cells). Eur. J. Biochem. 1990, 192, 245–261. [Google Scholar] [CrossRef]
- Rai, R.M.; Yang, S.Q.; Mcclain, C.; Karp, C.L.; Klein, A.S.; Diehl, A.M. Kupffer Cell Depletion by Gadolinium Chloride Enhances Liver Regeneration after Partial Hepatectomy in Rats. Am. J. Physiol. Gastrointest. Liver Physiol. 1996, 270, 33–36. [Google Scholar] [CrossRef]
- Rüttinger, D.; Vollmar, B.; Wanner, G.A.; Messmer, K. In Vivo Assessment of Hepatic Alterations Following Gadolinium Chloride-Induced Kupffer Cell Blockade. J. Hepatol. 1996, 25, 960–967. [Google Scholar] [CrossRef]
- Jarrar, D.; Wang, P.; Chaudry, I.H. Hepatocellular Dysfunction–Basic Considerations. In Surgical Treatment: Evidence-Based and Problem-Oriented; Holzheimer, R., Mannick, J., Eds.; Zuckschwerdt: Munich, Germany, 2001; pp. 1–7. [Google Scholar]
- Ryder, K.W.; Kaplan, J.E.; Saba, T.M. Serum Calcium and Hepatic Kupffer Cell Phagocytosis. Exp. Biol. Med. 1975, 149, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Razzak, Z.; Loyer, P.; Fautrel, A.; Gautier, J.C.; Corcos, L.; Turlin, B.; Beaune, P.; Guillouzo, A. Cytokines Down-Regulate Expression of Major Cytochrome P-450 Enzymes in Adult Human Hepatocytes in Primary Culture. Mol. Pharmacol. 1993, 44, 707–715. [Google Scholar] [PubMed]
- Badger, D.A.; Kuester, R.K.; Sauer, J.M.; Sipes, I.G. Gadolinium Chloride Reduces Cytochrome P450: Relevance to Chemical-Induced Hepatotoxicity. Toxicology 1997, 121, 143–153. [Google Scholar] [CrossRef]
- Kanda, T.; Oba, H.; Toyoda, K.; Kitajima, K.; Furui, S. Brain Gadolinium Deposition after Administration of Gadolinium-Based Contrast Agents. Jpn. J. Radiol. 2016, 34, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Barbieri, S.; Schroeder, C.; Froehlich, J.M.; Pasch, A.; Thoeny, H.C. High Signal Intensity in Dentate Nucleus and Globus Pallidus on Unenhanced T1-Weighted MR Images in Three Patients with Impaired Renal Function and Vascular Calcification. Contrast Media Mol. Imaging 2016, 11, 245–250. [Google Scholar] [CrossRef] [Green Version]
- Huettner, J.E.; Stack, E.; Wilding, T.J. Antagonism of Neuronal Kainate Receptors by Lanthanum and Gadolinium. Neuropharmacology 1998, 37, 1239–1247. [Google Scholar] [CrossRef]
- Chittajallu, R.; Vignes, M.; Dev, K.K.; Barnes, J.M.; Collingridge, G.L.; Henley, J.M. Regulation of Glutamate Release by Presynaptic Kainate Receptors in the Hippocampus. Nature 1996, 379, 78–81. [Google Scholar] [CrossRef]
- Castillo, P.E.; Malenka, R.C.; Nicoll, R.A. Kainate Receptors Mediate a Slow Postsynaptic Current in Hippocampal CA3 Neurons. Nature 1997, 388, 182–186. [Google Scholar] [CrossRef]
- Monaghan, D.T.; Bridges, R.J.; Cotman, C.W. The Excitatory Amino Acid Receptors: Their Classes, Pharmacology, and Distinct Properties in the Function of the Central Nervous System. Annu. Rev. Pharmacol. Toxicol. 1989, 29, 365–402. [Google Scholar] [CrossRef]
- Reichling, D.B.; MacDermott, A.B. Lanthanum Actions on Excitatory Amino Acid-gated Currents and Voltage-gated Calcium Currents in Rat Dorsal Horn Neurons. J. Physiol. 1991, 441, 199–218. [Google Scholar] [CrossRef]
- Sutresno, A.; Haryanto, F.; Viridi, S.; Arif, I. Influence Blocking by Gadolinium in Calcium Diffusion on Synapse Model: A Monte Carlo Simulation Study. J. Biomed. Phys. Eng. 2020, 10, 251–260. [Google Scholar] [CrossRef]
- Sachs, F. Stretch-Activated Ion Channels: What Are They? Physiology 2010, 25, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Millet, B.; Pickard, B.G. Gadolinium Ion Is an Inhibitor Suitable for Testing the Putative Role of Stretch-Activated Ion Channels in Geotropism and Thigmotropism. Biophys. J. 1988, 53, 115a. [Google Scholar]
- Yang, X.C.; Sachs, F. Block of Stretch-Activated Ion Channels in Xenopus Oocytes by Gadolinium and Calcium Ions. Science 1989, 243, 1068–1071. [Google Scholar] [CrossRef]
- Neher, E.; Steinbach, J.H. Local Anaesthetics Transiently Block Currents through Single Acetylcholine-receptor Channels. J. Physiol. 1978, 277, 153–176. [Google Scholar] [CrossRef]
- Dani, J.A. Ion-Channel Entrances Influence Permeation. Net Charge, Size, Shape, and Binding Considerations. Biophys. J. 1986, 49, 607–618. [Google Scholar] [CrossRef] [Green Version]
- Gustin, M.C.; Zhou, X.L.; Martinac, B.; Kung, C. A Mechanosensitive Ion Channel in the Yeast Plasma Membrane. Science 1988, 242, 762–765. [Google Scholar] [CrossRef]
- Alba, A.; Kano, M.; Chen, C.; Stanton, M.E.; Fox, G.D.; Herrup, K.; Zwingman, T.A.; Tonegawa, S. Deficient Cerebellar Long-Term Depression and Impaired Motor Learning in MGluR1 Mutant Mice. Cell 1994, 79, 377–388. [Google Scholar] [CrossRef]
- Hermans, E.; Challiss, R.A.J. Structural, Signalling and Regulatory Properties of the Group I Metabotropic Glutamate Receptors: Prototypic Family C G-Protein-Coupled Receptors. Biochem. J. 2001, 359, 465–484. [Google Scholar] [CrossRef]
- Tateyama, M.; Kubo, Y. Dual Signaling Is Differentially Activated by Different Active States of the Metabotropic Glutamate Receptor 1α. Proc. Natl. Acad. Sci. USA 2006, 103, 1124–1128. [Google Scholar] [CrossRef] [Green Version]
- Kovacs, G.; Danko, T.; Bergeron, M.J.; Balazs, B.; Suzuki, Y.; Zsembery, A.; Hediger, M.A. Heavy Metal Cations Permeate the TRPV6 Epithelial Cation Channel. Cell Calcium 2011, 49, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Südhof, T.C. The Synaptic Vesicle Cycle. Annu. Rev. Neurosci. 2004, 27, 509–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane Lipids: Where They Are and How They Behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Crane, J.M.; Tamm, L.K. Role of Cholesterol in the Formation and Nature of Lipid Rafts in Planar and Spherical Model Membranes. Biophys. J. 2004, 86, 2965–2979. [Google Scholar] [CrossRef] [Green Version]
- Singer, S.J.; Nicolson, G.L. The Fluid Mosaic Model of the Structure of Cell Membranes. Science 1972, 175, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Edidin, M. Lipids on the Frontier: A Century of Cell-Membrane Bilayers. Nat. Rev. Mol. Cell Biol. 2003, 4, 414–418. [Google Scholar] [CrossRef]
- Zachowski, A. Phospholipids in Animal Eukaryotic Membranes: Transverse Asymmetry and Movement. Biochem. J. 1993, 294, 1–14. [Google Scholar] [CrossRef]
- Kay, J.G.; Fairn, G.D. Distribution, Dynamics and Functional Roles of Phosphatidylserine within the Cell. Cell Commun. Signal. 2019, 17, 126. [Google Scholar] [CrossRef] [Green Version]
- Boettcher, J.M.; Davis-Harrison, R.L.; Clay, M.C.; Nieuwkoop, A.J.; Ohkubo, Y.Z.; Tajkhorshid, E.; Morrissey, J.H.; Rienstra, C.M. Atomic View of Calcium-Induced Clustering of Phosphatidylserine in Mixed Lipid Bilayers. Biochemistry 2011, 50, 2264–2273. [Google Scholar] [CrossRef] [Green Version]
- Cullis, P.R.; De Kruijff, B. Lipid Polymorphism and the Functional Roles of Lipids in Biological Membranes. Biochim. Biophys. Acta BBA Rev. Biomembr. 1979, 559, 399–420. [Google Scholar] [CrossRef]
- Lewis, R.N.A.H.; McElhaney, R.N. Membrane Lipid Phase Transitions and Phase Organization Studied by Fourier Transform Infrared Spectroscopy. Biochim. Biophys. Acta BBA Biomembr. 2013, 1828, 2347–2358. [Google Scholar] [CrossRef] [Green Version]
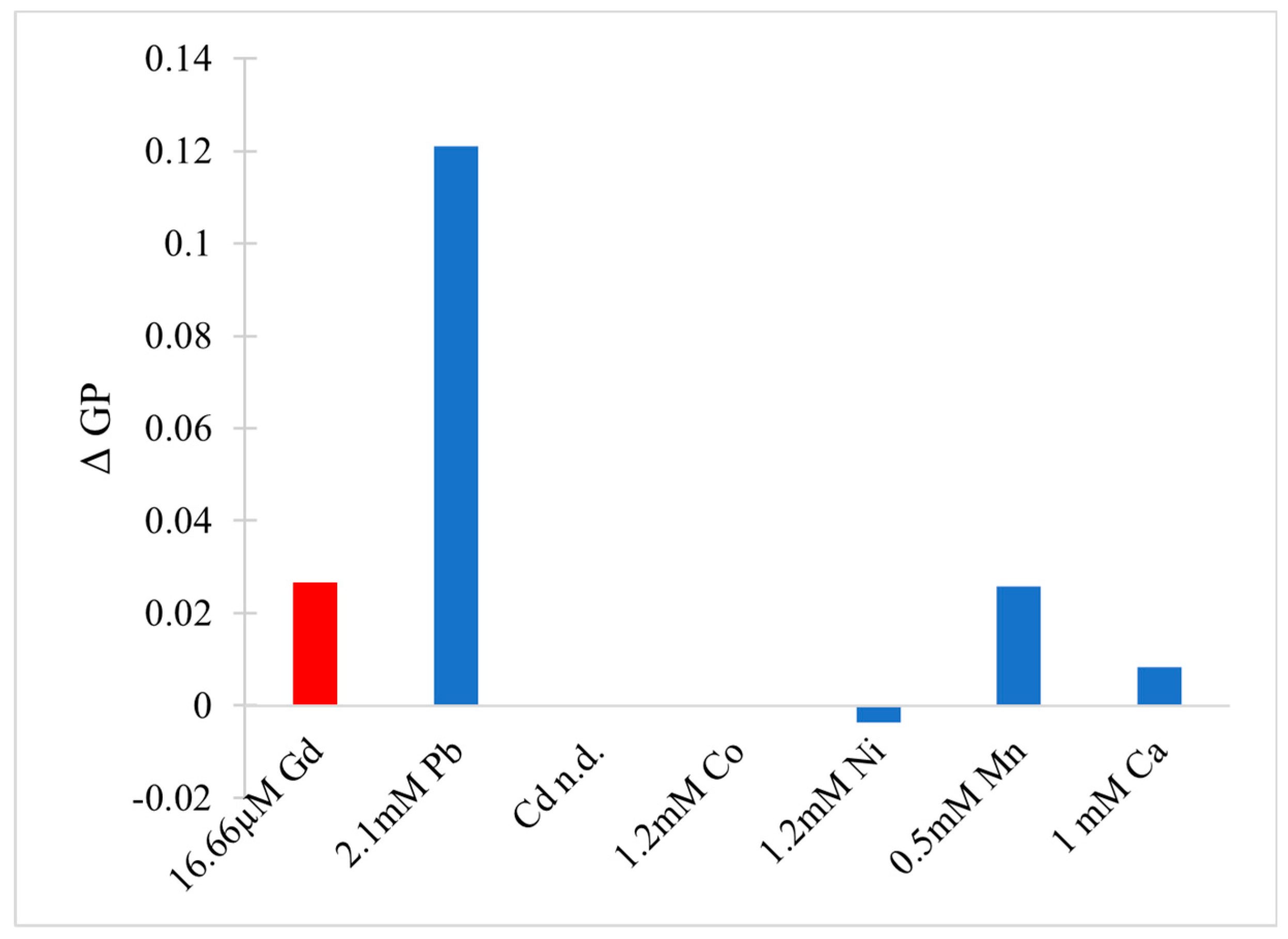
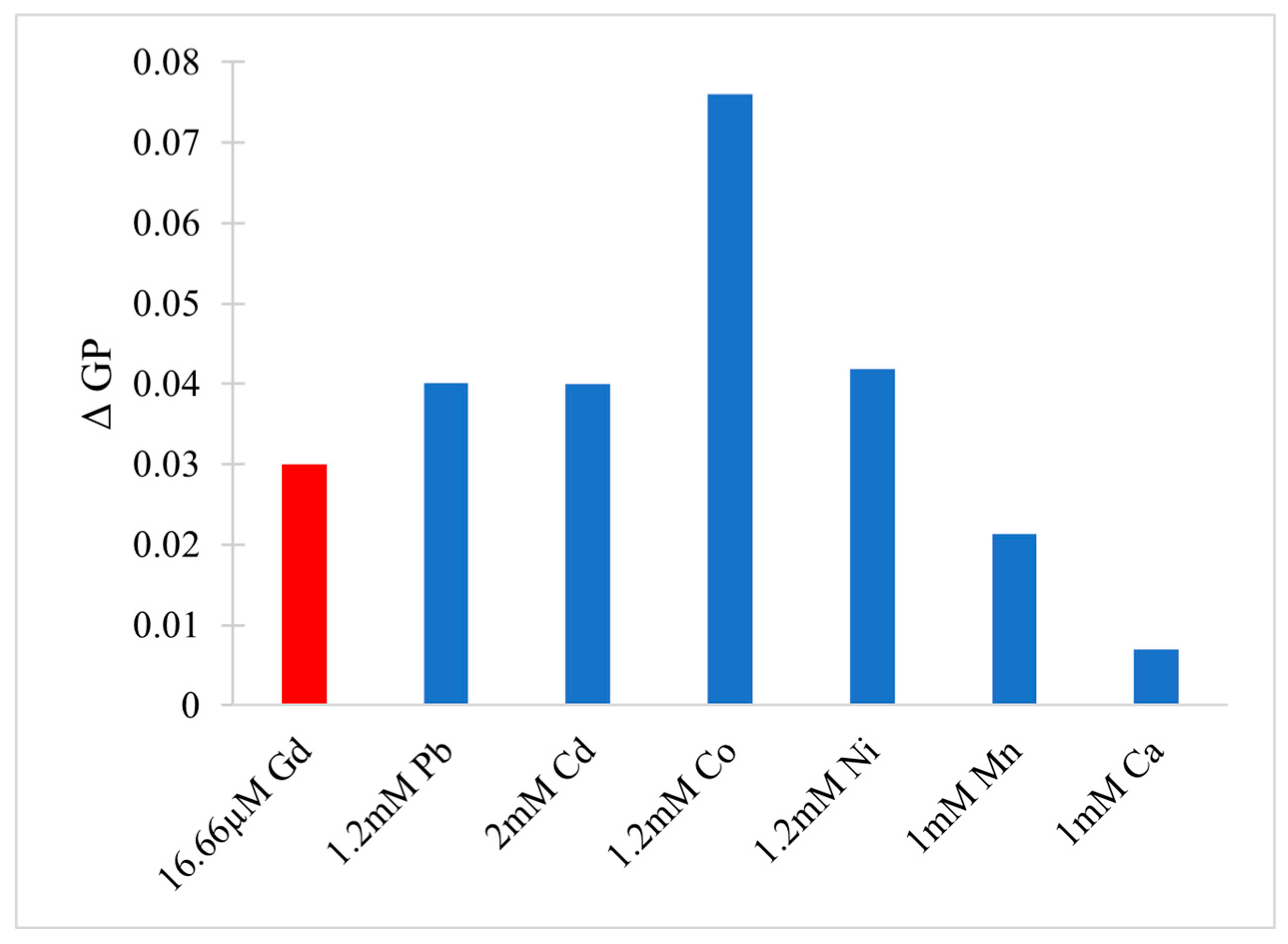
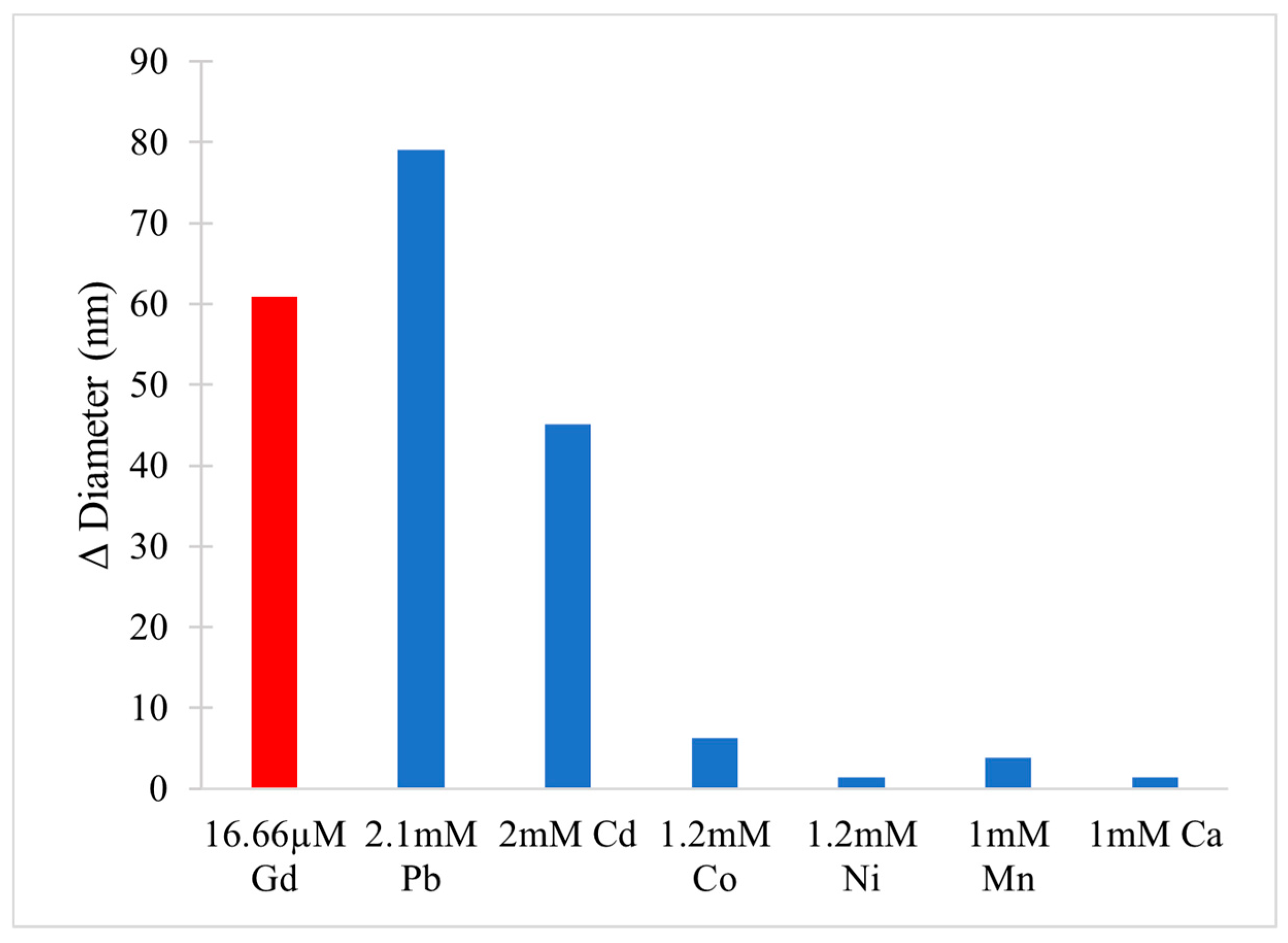
- Sule, K.; Umbsaar, J.; Prenner, E.J. Mechanisms of Co, Ni, and Mn Toxicity: From Exposure and Homeostasis to Their Interactions with and Impact on Lipids and Biomembranes. Biochim. Biophys. Acta BBA Biomembr. 2020, 1862, 183–250. [Google Scholar] [CrossRef]
- Evans, C.H. Membrane Interactions of Lanthanide Ions. In Biochemistry of the Lanthanides; Springer: New York, NY, USA, 1990; pp. 212–219. [Google Scholar]
- Payliss, B.J.; Hassanin, M.; Prenner, E.J. The Structural and Functional Effects of Hg(II) and Cd(II) on Lipid Model Systems and Human Erythrocytes: A Review. Chem. Phys. Lipids 2015, 193, 36–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.-M.; Zhang, Y.-F.; Ni, J.-Z.; Chen, J.-W.; Hwang, F. Effect of Lanthanide Ions on the Phase Behavior of Dipalmitoylphosphatidylcholine Multilamellar Liposomes. J. Inorg. Biochem. 1994, 53, 139. [Google Scholar] [CrossRef]
- Sabín, J.; Prieto, G.; Blanco, E.; Ruso, J.M.; Angelini, R.; Bordi, F.; Sarmiento, F. Interaction of Gadolinium with Phospholipids Bilayer Membranes: Photon Correlation Spectroscopy and DSC Study. J. Therm. Anal. Calorim. 2007, 87, 199–203. [Google Scholar] [CrossRef]
- Tanaka, T.; Tamba, Y.; Masum, S.M.; Yamashita, Y.; Yamazaki, M. La3+ and Gd3+ Induce Shape Change of Giant Unilamellar Vesicles of Phosphatidylcholine. Biochim. Biophys. Acta BBA Biomembr. 2002, 1564, 173–182. [Google Scholar] [CrossRef] [Green Version]
- Averbakh, A.; Pavlov, D.; Lobyshev, V.I. Effect of Gadolinium(III) Ions on the Phase Behaviour of Dimyristoylphosphatidyl Serine Multilamellar Liposomes. J. Therm. Anal. Calorim. 2000, 62, 101–110. [Google Scholar] [CrossRef]
- Gianulis, E.C.; Pakhomov, A.G. Gadolinium Modifies the Cell Membrane to Inhibit Permeabilization by Nanosecond Electric Pulses. Arch. Biochem. Biophys. 2015, 570, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Liu, M.; Li, R.; Wang, C.; Bai, C.; Wang, K. Gadolinium Induces Domain and Pore Formation of Human Erythrocyte Membrane: An Atomic Force Microscopic Study. Biochim. Biophys. Acta BBA Biomembr. 1999, 1421, 249–260. [Google Scholar] [CrossRef] [Green Version]
- Lopatina, L.P.; Waseem, T.W.; Fedorovich, S.V.; Konev, S.V. Lanthanides Induce Neurotransmitter Release from the Vesicular Pool in Rat Brain Synaptosomes. Biofizika 2005, 50, 131–134. [Google Scholar]
- Cosgrove, T. Colloid Science: Principles, Methods, and Applications; John Wiley & Sons: Chichester, UK, 2010. [Google Scholar]
- McLaughlin, S.; Mulrine, N.; Gresalfi, T.; Vaio, G.; McLaughlin, A. Adsorption of Divalent Cations to Bilayer Membranes Containing Phosphatidylserine. J. Gen. Physiol. 1981, 77, 445–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ermakov, Y.A.; Averbakh, A.Z.; Arbuzova, A.B.; Sukharev, S.I. Lipid and Cell Membranes in the Presence of Gadolinium and Other Ions with High Affinity to Lipids. 2. A Dipole Component of the Boundary Potential on Membranes with Different Surface Charges. Membr. Cell Biol. 1997, 12, 411–426. [Google Scholar]
- Ermakov, Y.A.; Averbakh, A.Z.; Sukharev, S.I. Lipid and Cell Membranes in the Presence of Gadolinium and Other Ions with High Affinity to Lipids. 1. Dipole and Diffuse Components of the Boundary Potential. Membr. Cell Biol. 1997, 11, 539–554. [Google Scholar] [PubMed]
- Hahne, H.C.H.; Kroontje, W. Significance of PH and Chloride Concentration on Behavior of Heavy Metal Pollutants: Mercury(II), Cadmium(II), Zinc(II), and Lead(II). J. Environ. Qual. 1973, 2, 444–450. [Google Scholar] [CrossRef]
- Kerek, E.; Hassanin, M.; Prenner, E.J. Inorganic Mercury and Cadmium Induce Rigidity in Eukaryotic Lipid Extracts While Mercury Also Ruptures Red Blood Cells. Biochim. Biophys. Acta BBA Biomembr. 2018, 1860, 710–717. [Google Scholar] [CrossRef]
- Gustafsson, J.P. Visual MINTEQ Chemical Equilibrium Software; KTH, Department of Sustainable Development, Environmental Science and Engineering: Stockholm, Sweden, 2016. [Google Scholar]
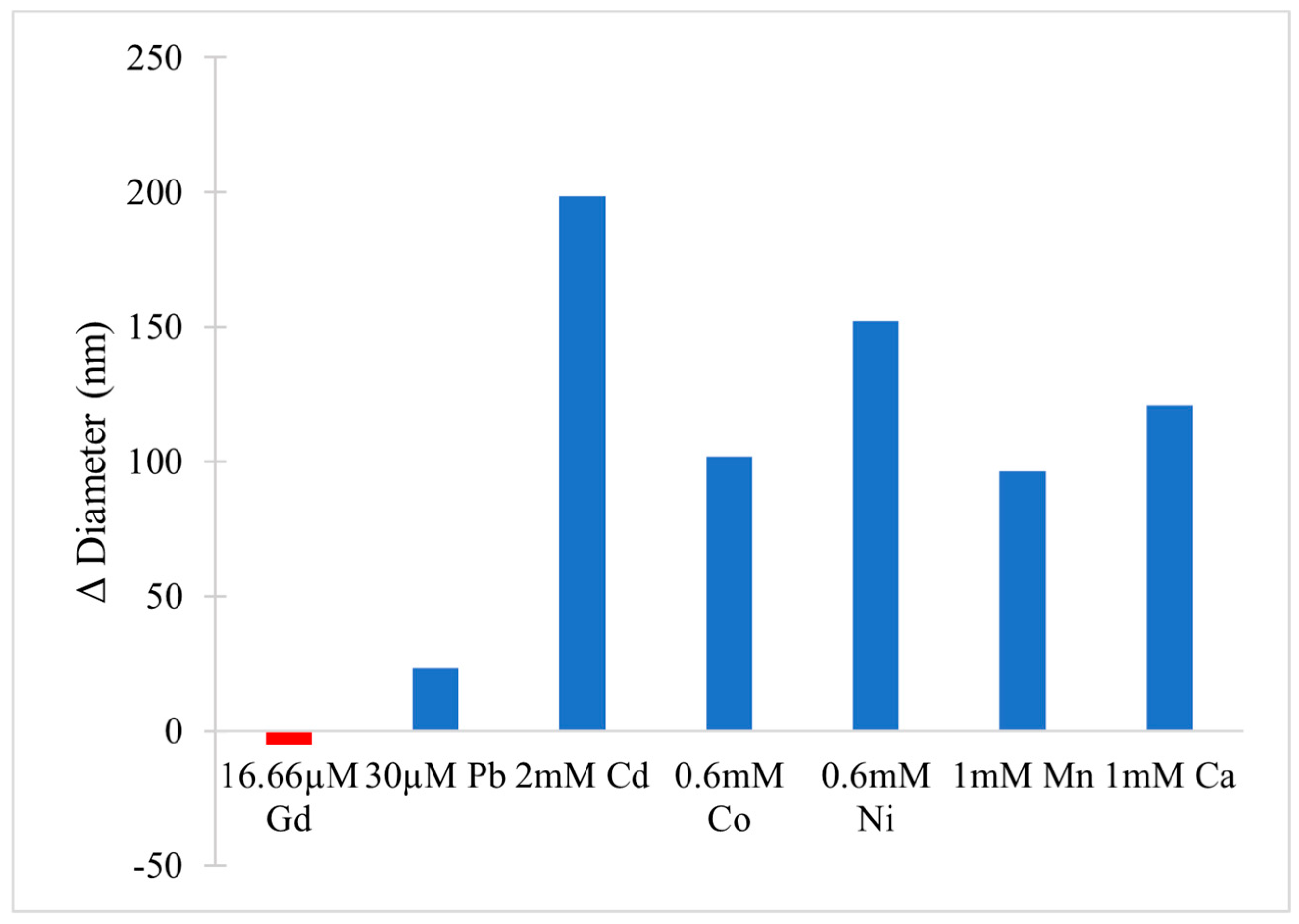
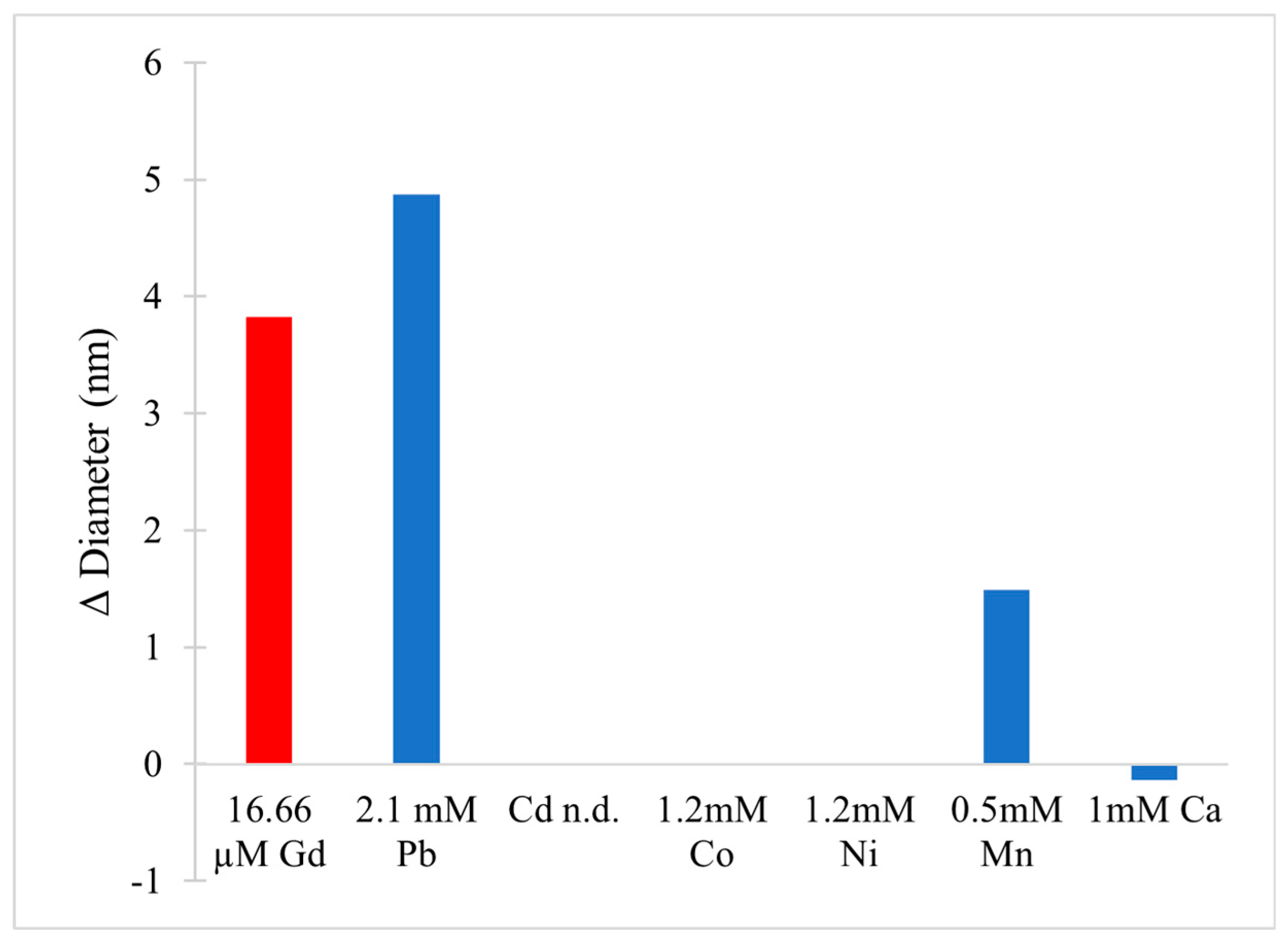
- Kerek, E.M.; Prenner, E.J. Inorganic Cadmium Affects the Fluidity and Size of Phospholipid Based Liposomes. Biochim. Biophys. Acta BBA Biomembr. 2016, 1858, 3169–3181. [Google Scholar] [CrossRef]
- Kerek, E.; Hassanin, M.; Zhang, W.; Prenner, E.J. Preferential Binding of Inorganic Mercury to Specific Lipid Classes and Its Competition with Cadmium. Biochim. Biophys. Acta BBA Biomembr. 2017, 1859, 1211–1221. [Google Scholar] [CrossRef]
- Parasassi, T.; De Stasio, G.; Ravagnan, G.; Rusch, R.M.; Gratton, E. Quantitation of Lipid Phases in Phospholipid Vesicles by the Generalized Polarization of Laurdan Fluorescence. Biophys. J. 1991, 60, 179–189. [Google Scholar] [CrossRef] [Green Version]
- ATSDR. Substance Priority List. Available online: https://www.atsdr.cdc.gov/spl/index.html#2019spl (accessed on 23 October 2020).
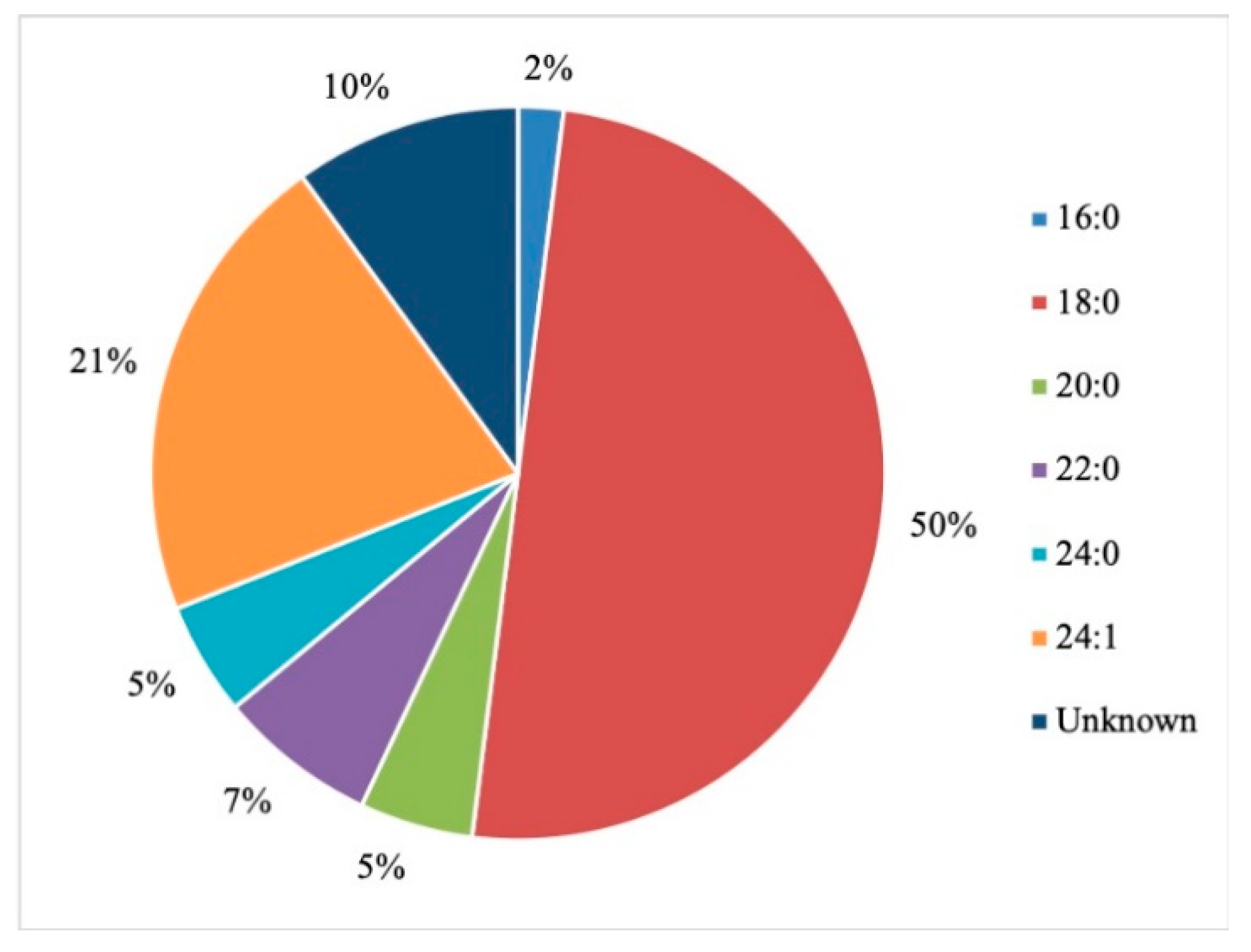
- Baba, T.; Campbell, J.L.; Le Blanc, J.C.Y.; Baker, P.R.S. In-Depth Sphingomyelin Characterization Using Electron Impact Excitation of Ions from Organics and Mass Spectrometry. J. Lipid Res. 2016, 57, 858–867. [Google Scholar] [CrossRef] [Green Version]
- Prenner, E.; Honsek, G.; Hönig, D.; Möbius, D.; Lohner, K. Imaging of the Domain Organization in Sphingomyelin and Phosphatidylcholine Monolayers. Chem. Phys. Lipids 2007, 145, 106–118. [Google Scholar] [CrossRef] [Green Version]
- Lingwood, D.; Simons, K. Lipid Rafts as a Membrane-Organizing Principle. Science 2010, 327, 46–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, M.T.; Hassanin, M.; Mahadeo, M.; Gailer, J.; Prenner, E.J. Hg- and Cd-Induced Modulation of Lipid Packing and Monolayer Fluidity in Biomimetic Erythrocyte Model Systems. Chem. Phys. Lipids 2013, 170–171, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Amoruso, M.A.; Witz, G.; Goldstein, B.D. Alteration of Erythrocyte Membrane Fluidity by Heavy Metal Cations. Toxicol. Ind. Health 1987, 3, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Suwalsky, M.; Villena, F.; Norris, B.; Cuevas, F.; Sotomayor, C.P. Cadmium-Induced Changes in the Membrane of Human Erythrocytes and Molecular Models. J. Inorg. Biochem. 2004, 98, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.K.; Probst, S.; Santoyo-Sánchez, M.P.; Al-Hamdani, W.; Diebels, I.; von Sivers, J.K.; Kerek, E.; Prenner, E.J.; Thévenod, F. Initial Autophagic Protection Switches to Disruption of Autophagic Flux by Lysosomal Instability during Cadmium Stress Accrual in Renal NRK-52E Cells. Arch. Toxicol. 2017, 91, 3225–3245. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, E.H.; Abreo, K.; Burgess, E.; Cannata, J.; Greger, J.L. Systemic Aluminum Toxicity: Effects on Bone, Hematopoietic Tissue, and Kidney. J. Toxicol. Environ. Health 1996, 48, 649–666. [Google Scholar] [CrossRef] [PubMed]
- Zatta, P.; Kiss, T.; Suwalsky, M.; Berthon, G. Aluminium(III) as a Promoter of Cellular Oxidation. Coord. Chem. Rev. 2002, 228, 271–284. [Google Scholar] [CrossRef]
- Verstraeten, S.V.; Oteiza, P.I. Sc3+, Ga3+, In3+, Y3+, and Be2+ Promote Changes in Membrane Physical Properties and Facilitate Fe2+-Initiated Lipid Peroxidation. Arch. Biochem. Biophys. 1995, 322, 284–290. [Google Scholar] [CrossRef]
- Verstraeten, S.V.; Aimo, L.; Oteiza, P.I. Aluminium and Lead: Molecular Mechanisms of Brain Toxicity. Arch. Toxicol. 2008, 82, 789–802. [Google Scholar] [CrossRef]
- Adonaylo, V.N.; Oteiza, P.I. Pb2+ Promotes Lipid Oxidation and Alterations in Membrane Physical Properties. Toxicology 1999, 132, 19–32. [Google Scholar] [CrossRef]
- Althumairy, D.; Murakami, H.A.; Zhang, D.; Barisas, B.G.; Roess, D.A.; Crans, D.C. Effects of Vanadium(IV) Compounds on Plasma Membrane Lipids Lead to G Protein-Coupled Receptor Signal Transduction. J. Inorg. Biochem. 2020, 203, 110873. [Google Scholar] [CrossRef] [PubMed]
- Levina, A.; Crans, D.C.; Lay, P.A. Speciation of Metal Drugs, Supplements and Toxins in Media and Bodily Fluids Controls in Vitro Activities. Coord. Chem. Rev. 2017, 352, 473–498. [Google Scholar] [CrossRef]
- Crans, D.C.; Woll, K.A.; Prusinskas, K.; Johnson, M.D.; Norkus, E. Metal Speciation in Health and Medicine Represented by Iron and Vanadium. Inorg. Chem. 2013, 52, 12262–12275. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unruh, C.; Van Bavel, N.; Anikovskiy, M.; Prenner, E.J. Benefits and Detriments of Gadolinium from Medical Advances to Health and Ecological Risks. Molecules 2020, 25, 5762. https://doi.org/10.3390/molecules25235762
Unruh C, Van Bavel N, Anikovskiy M, Prenner EJ. Benefits and Detriments of Gadolinium from Medical Advances to Health and Ecological Risks. Molecules. 2020; 25(23):5762. https://doi.org/10.3390/molecules25235762
Chicago/Turabian StyleUnruh, Colin, Nicolas Van Bavel, Max Anikovskiy, and Elmar J. Prenner. 2020. "Benefits and Detriments of Gadolinium from Medical Advances to Health and Ecological Risks" Molecules 25, no. 23: 5762. https://doi.org/10.3390/molecules25235762
APA StyleUnruh, C., Van Bavel, N., Anikovskiy, M., & Prenner, E. J. (2020). Benefits and Detriments of Gadolinium from Medical Advances to Health and Ecological Risks. Molecules, 25(23), 5762. https://doi.org/10.3390/molecules25235762