Detection of Fungi and Oomycetes by Volatiles Using E-Nose and SPME-GC/MS Platforms
Abstract
1. Introduction
2. Results
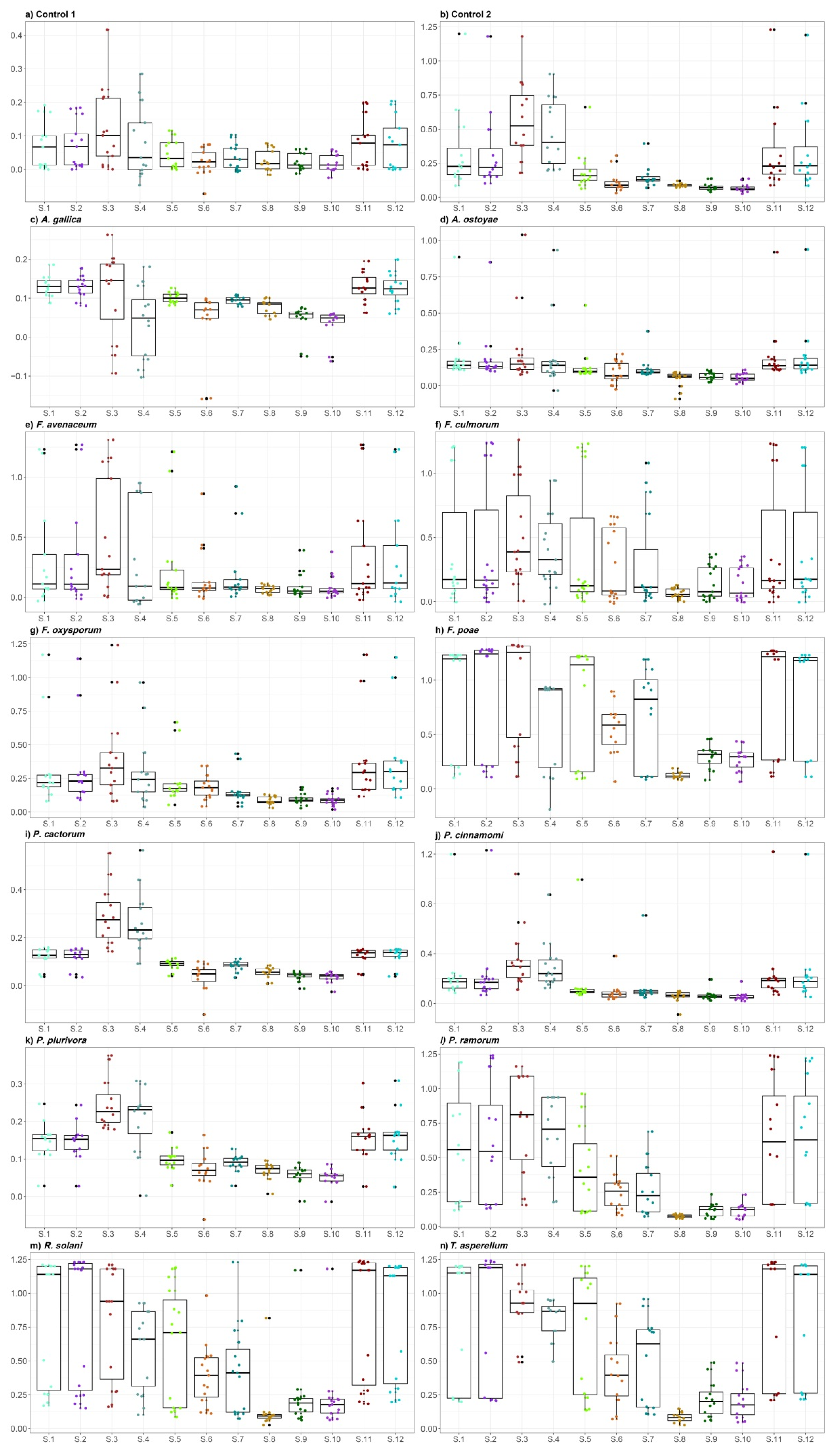
2.1. E-Nose Gas Detection
2.2. Determination of VOC Emitted by Fungi and Oomycetes
2.2.1. Control Treatments
2.2.2. Armillaria Species
2.2.3. Fusarium Species
2.2.4. Phytophthora Species
2.2.5. Rhizoctonia and Trichoderma Species
3. Discussion
3.1. Accurateness of Analyses Performed by E-Nose
3.2. Volatiles Identifying Fungal and Oomycete Species
3.2.1. Control Treatments
Empty Flask Control
PDA Medium Control
3.2.2. Armillaria Species
3.2.3. Fusarium Species
Fusarium avenaceum
Fusarium culmorum
Fusarium oxysporum
Fusarium poae
3.2.4. Phytophthora Species
Phytophthora cactorum
Phytophthora cinnamomi
Phytophthora plurivora
Phytophthora ramorum
3.2.5. Rhizoctonia solani
3.2.6. Trichoderma asperellum
3.2.7. Strategies for Improved Detection
4. Materials and Methods
4.1. Sample Material Preparation
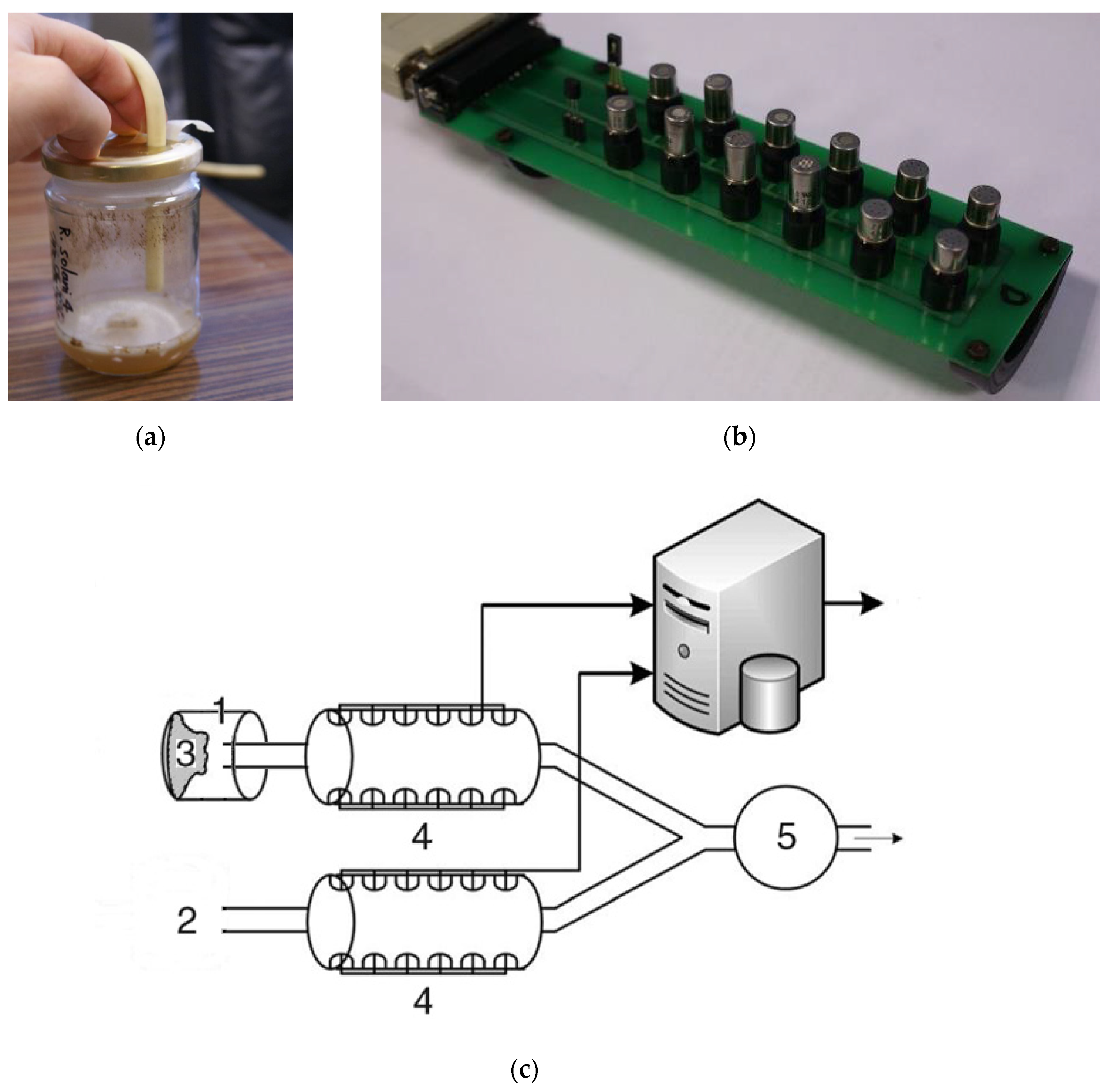
4.2. E-nose Device and Measurements
4.3. SPME-GC/MS Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schulz, S.; Dickschat, J.S. Bacterial volatiles: The smell of small organisms. Nat. Prod. Rep. 2007, 24, 814. [Google Scholar] [CrossRef]
- Garbeva, P.; Hordijk, C.; Gerards, S.; De Boer, W. Volatile-mediated interactions between phylogenetically different soil bacteria. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Pagans, E.; Font, X.; Sanchez, A. Emission of volatile organic compounds from composting of different solid wastes: Abatement by biofiltration. J. Hazard. Mater. 2006, 131, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Cordovez, V.; De Boer, W.; Raaijmakers, J.; Garbeva, P. Volatile affairs in microbial interactions. ISME J. 2015, 9, 2329–2335. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, A. The Chemistry and Biology of Volatiles; Wiley: Chichester, UK; Hoboken, NJ, USA, 2010; ISBN 978-0-470-77778-7. [Google Scholar]
- Korpi, A.; Järnberg, J.; Pasanen, A.-L. Microbial Volatile Organic Compounds. Crit. Rev. Toxicol. 2009, 39, 139–193. [Google Scholar] [CrossRef] [PubMed]
- Hung, R.; Lee, S.; Bennett, J.W. The effects of low concentrations of the enantiomers of mushroom alcohol (1-Octen-3-Ol) on Arabidopsis thaliana. Mycology 2014, 5, 73–80. [Google Scholar] [CrossRef]
- Zeringue, H.J.; Bhatnagar, D.; Cleveland, T.E. C(15)H(24) volatile compounds unique to aflatoxigenic strains of Aspergillus flavus. Appl. Environ. Microbiol. 1993, 59, 2264–2270. [Google Scholar] [CrossRef]
- Insam, H.; Seewald, M.S.A. Volatile organic compounds (VOCs) in soils. Biol. Fertil. Soils 2010, 46, 199–213. [Google Scholar] [CrossRef]
- Romoli, R.; Papaleo, M.C.; De Pascale, D.; Tutino, M.L.; Michaud, L.; LoGiudice, A.; Fani, R.; Bartolucci, G. GC–MS volatolomic approach to study the antimicrobial activity of the antarctic bacterium Pseudoalteromonas sp. TB41. Metabolomics 2014, 10, 42–51. [Google Scholar] [CrossRef]
- Lemfack, M.C.; Nickel, J.; Dunkel, M.; Preissner, R.; Piechulla, B. mVOC: A database of microbial volatiles. Nucleic Acids Res. 2014, 42, D744–D748. [Google Scholar] [CrossRef]
- Peñuelas, J.; Asensio, D.; Tholl, D.; Wenke, K.; Rosenkranz, M.; Piechulla, B.; Schnitzler, J.P. Biogenic volatile emissions from the soil: Biogenic volatile emissions from the soil. Plant Cell Environ. 2014, 37, 1866–1891. [Google Scholar] [CrossRef] [PubMed]
- Ortíz-Castro, R.; Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; López-Bucio, J. The role of microbial signals in plant growth and development. Plant Signal. Behav. 2009, 4, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Piechulla, B.; Degenhardt, J. The emerging importance of microbial volatile organic compounds: The emerging importance of microbial volatile organic compounds. Plant Cell Environ. 2014, 37, 811–812. [Google Scholar] [CrossRef] [PubMed]
- Kramer, R.; Abraham, W.-R. Volatile sesquiterpenes from fungi: What are they good for? Phytochem. Rev. 2012, 11, 15–37. [Google Scholar] [CrossRef]
- Peghaire, E.; Hamdache, S.; Galien, A.; Sleiman, M.; Ter Halle, A.; El Alaoui, H.; Kocer, A.; Richard, C.; Goupil, P. Inducing plant defense reactions in tobacco plants with phenolic-rich extracts from red maple leaves: A characterization of main active ingredients. Forests 2020, 11, 705. [Google Scholar] [CrossRef]
- Claeson, A.-S.; Sandström, M.; Sunesson, A.-L. Volatile organic compounds (VOCs) emitted from materials collected from buildings affected by microorganisms. J. Environ. Monit. 2007, 9, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Dalilla, C.R.; Mauricio, B.F.; Simone, C.B.; Silvia, B.; Sergio, F.P. Antimicrobial activity of volatile organic compounds and their effect on lipid peroxidation and electrolyte loss in Colletotrichum gloeosporioides and Colletotrichum acutatum mycelia. Afr. J. Microbiol. Res. 2015, 9, 1527–1535. [Google Scholar] [CrossRef]
- Šimpraga, M.; Ghimire, R.P.; Van Der Straeten, D.; Blande, J.D.; Kasurinen, A.; Sorvari, J.; Holopainen, T.; Adriaenssens, S.; Holopainen, J.K.; Kivimäenpää, M. Unravelling the functions of biogenic volatiles in boreal and temperate forest ecosystems. Eur. J. For. Res. 2019, 138, 763–787. [Google Scholar] [CrossRef]
- Wilkens, W.F.; Hartman, J.D. An Electronic Analog for the Olfactory Processes. J. Food Sci. 1964, 29, 372–378. [Google Scholar] [CrossRef]
- Gardner, J.W.; Bartlett, P.N. (Eds.) Sensors and Sensory Systems for An Electronic Nose; Kluwer Academic: Boston, MA, USA; Dordrecht, The Netherlands; New York, NY, USA, 1992. [Google Scholar] [CrossRef]
- Brudzewski, K.; Osowski, S.; Ulaczyk, J. Differential electronic nose of two chemo sensor arrays for odor discrimination. Sens. Actuators B Chem. 2010, 145, 24–249. [Google Scholar] [CrossRef]
- Persaud, K.; Dodd, G. Analysis of discrimination mechanisms in the mammalian olfactory system using a model nose. Nature 1982, 299, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.W. Pattern recognition in the Warwick Electronic Nose. In 8th Int Congress of European Chemoreception Research Organisation; University of Warwick: Warwick, UK, 1987. [Google Scholar]
- Di Pietrantonio, F.; Benetti, M.; Cannatà, D.; Verona, E.; Palla-Papavlu, A.; Fernández-Pradas, J.M.; Serra, P.; Staiano, M.; Varriale, A.; D’Auria, S. A surface acoustic wave bio-electronic nose for detection of volatile odorant molecules. Biosens. Bioelectron. 2015, 67, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.D. Diverse applications of electronic-nose technologies in agriculture and forestry. Sensors 2013, 13, 2295–2348. [Google Scholar] [CrossRef] [PubMed]
- Okorski, A.; Pszczółkowska, A.; Gorzkowska, A.; Okorska, S.; Głuszek, P. Fungi associated with conifer seedlings grown in forest nurseries under different systems. EEMJ 2019, 18, 1509–1517. [Google Scholar] [CrossRef]
- Jung, T.; Orlikowski, L.; Henricot, B.; Abad-Campos, P.; Aday, A.G.; Aguín Casal, O.; Bakonyi, J.; Cacciola, S.O.; Cech, T.; Chavarriaga, D.; et al. Widespread Phytophthora infestations in European nurseries put forest, semi-natural and horticultural ecosystems at high risk of Phytophthora diseases. For. Pathol. 2016, 46, 134–163. [Google Scholar] [CrossRef]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2017. [Google Scholar]
- Miyazawa, M.; Marumoto, S.; Kobayashi, T.; Yoshida, S.; Utsumi, Y. Determination of Characteristic Components in Essential Oils from Wisteria Braphybotrys Using Gas Chromatography—Olfactometry Incremental Dilution Technique, Rec. Nat. Prod. 2011, 5, 221–227. [Google Scholar]
- Adams, R.P.; Morris, J.A.; Pandey, R.N.; Schwarzbach, A.E. Cryptic speciation between Juniperus deltoides and Juniperus oxycedrus (Cupressaceae) in the Mediterranean. Biochem. Syst. Ecol. 2005, 33, 771–787. [Google Scholar] [CrossRef]
- Elmore, J.S.; Mottram, D.S.; Enser, M.; Wood, J.D. Effect of the polyunsaturated fatty acid composition of beef muscle on the profile of aroma volatiles. J. Agric. Food Chem. 1999, 47, 1619–1625. [Google Scholar] [CrossRef]
- Wang, Z.; Fingas, M.; Li, K. Fractionation of a light crude oil and identification and quantitation of aliphatic, aromatic, and biomarker compounds by GC-FID and GC-MS, Part II. J. Chromatogr. Sci. 1994, 32, 367–382. [Google Scholar] [CrossRef]
- Kotowska, U.; Zalikowski, M.; Isidorov, V.A. HS-SPME/GC-MS analysis of volatile and semi-volatile organic compounds emitted from municipal sewage sludge. Environ. Monit. Asses. 2012, 184, 2893–2907. [Google Scholar] [CrossRef]
- Bonaiti, C.; Irlinger, F.; Spinnler, H.E.; Engel, E. An iterative sensory procedure to select odor-active associations in complex consortia of microorganisms: Application to the construction of a cheese model. J. Dairy Sci. 2005, 88, 1671–1684. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Zenkevich, I.G.; Krajewska, U.; Dubis, E.N.; Jaroszynska, J.; Bal, K. Gas chromatographic analysis of essential oils with preliminary partition of components. Phytochem. Anal. 2001, 12, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.O.; Frisvad, J.C. Characterization of volatile metabolites from 47 Penicillium taxa. Mycol. Res. 1995, 99, 1153–1166. [Google Scholar] [CrossRef]
- Goodner, K.L. Practical retention index models of OV-101, DB-1, DB-5, and DB-Wax for flavor and fragrance compounds. LWT 2008, 41, 951–958. [Google Scholar] [CrossRef]
- Steinhaus, P.; Schieberle, P. Characterization of the key aroma compounds in soy sauce using approaches of molecular sensory science. J. Agric. Food Chem. 2007, 55, 6262–6269. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.J.; Margaria, C.A.; Shaw, P.E.; Goodner, K.L. Aroma active components in aqueous Kiwi fruit essence and Kiwi fruit puree by GC-MS and multidimensional GC/GC-O. J. Agric. Food Chem. 2002, 50, 5386–5390. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.-L.; Zhou, G.-H. Comparative study of volatile compounds in traditional Chinese Nanjing marinated duck by different extraction techniques. Int. J. Food Sci. Technol. 2007, 42, 543–550. [Google Scholar] [CrossRef]
- Jalali-Heravi, M.; Zekavat, B.; Sereshti, H. Characterization of essential oil components of Iranian geranium oil using gas chromatography-mass spectrometry combined with chemometric resolution techniques. J. Chromatogr. A 2006, 1114, 154–163. [Google Scholar] [CrossRef]
- Wilson, A.D.; Baietto, M. Applications and advances in electronic-nose technologies. Sensors 2009, 9, 5099–5148. [Google Scholar] [CrossRef]
- Wilson, A.D.; Baietto, M. Advances in Electronic-Nose Technologies Developed for Biomedical Applications. Sensors 2011, 11, 1105–1176. [Google Scholar] [CrossRef]
- Osowski, S.; Linh, T.H.; Brudzewski, K. Neuro-fuzzy TSK network for calibration of semiconductor sensor array for gas measurements. IEEE Trans. Instrum. Meas. 2004, 53, 630–637. [Google Scholar] [CrossRef]
- Brudzewski, K.; Osowski, S.; Markiewicz, T.; Ulaczyk, J. Classification of gasoline with supplement of bio-products by means of an electronic nose and SVM neural network. Sens. Actuators B Chem. 2006, 113, 135–141. [Google Scholar] [CrossRef]
- Brudzewski, K.; Osowski, S.; Pawlowski, W. Metal oxide sensor arrays for detection of explosives at sub-parts-per million concentration levels by the differential electronic nose. Sens. Actuators B Chem. 2012, 161, 528–533. [Google Scholar] [CrossRef]
- Brudzewski, K.; Osowski, S.; Golembiecka, A. Differential electronic nose and support vector machine for fast recognition of tobacco. Expert Syst. Appl. 2012, 39, 9886–9891. [Google Scholar] [CrossRef]
- Brudzewski, K.; Osowski, S.; Dwulit, A. Recognition of coffee using differential electronic nose. IEEE Trans. Instrum. Meas. 2012, 61, 1803–1810. [Google Scholar] [CrossRef]
- Weise, T.; Kai, M.; Gummesson, A.; Troeger, A.; Von Reuß, S.; Piepenborn, S.; Kosterka, F.; Sklorz, M.; Zimmermann, R.; Francke, W.; et al. Volatile organic compounds produced by the phytopathogenic bacterium Xanthomonas campestris pv. vesicatoria 85-10. Beilstein J. Org. Chem. 2012, 8, 579–596. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, W.; Zhu, N.; Mao, S.; Tu, K. Early detection and classification of pathogenic fungal disease in post-harvest strawberry fruit by electronic nose and gas chromatography–mass spectrometry. Food Res. Int. 2014, 62, 162–168. [Google Scholar] [CrossRef]
- Biondi, E.; Blasioli, S.; Galeone, A.; Spinelli, F.; Cellini, A.; Lucchese, C.; Braschi, I. Detection of potato brown rot and ring rot by electronic nose: From laboratory to real scale. Talanta 2014, 129, 422–430. [Google Scholar] [CrossRef]
- Rettinger, K.; Burschka, C.; Scheeben, P.; Fuchs, H.; Mosandl, A. Chiral 2-alkylbranched acids, esters and alcohols. Preparation and stereospecific flavour evaluation. Tetrahedron Asymmetry 1991, 2, 965–968. [Google Scholar] [CrossRef][Green Version]
- Bahrmann, H.; Hahn, H.-D.; Mayer, D.; Frey, G.D. 2-Ethylhexanol. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; ISBN 978-3-527-30673-2. [Google Scholar]
- Engels, H.W.; Weidenhaupt, H.J.; Abele, M.; Pieroth, M.; Hofmann, W. Rubber, 4. Chemicals and additives. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000. [Google Scholar] [CrossRef]
- Dickschat, J.S.; Brock, N.L.; Citron, C.A.; Tudzynski, B. Biosynthesis of sesquiterpenes by the fungus Fusarium verticillioides. ChemBioChem 2011, 12, 2088–2095. [Google Scholar] [CrossRef]
- Dickschat, J.S. Fungal volatiles–a survey from edible mushrooms to moulds. Nat. Prod. Rep. 2017, 34, 310–328. [Google Scholar] [CrossRef] [PubMed]
- Citron, C.A.; Gleitzmann, J.; Laurenzano, G.; Pukall, R.; Dickschat, J.S. Terpenoids are widespread in actinomycetes: A correlation of secondary metabolism and genome data. ChemBioChem 2012, 13, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Kuzuyama, T.; Komatsu, M.; Shin-ya, K.; Omura, S.; Cane, D.E.; Ikeda, H. Terpene synthases are widely distributed in bacteria. Proc. Nat. Acad. Sci. USA 2015, 112, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Yamagiwa, Y.; Inagaki, Y.; Ichinose, Y.; Toyoda, K.; Hyakumachi, M.; Shiraishi, T. Talaromyces wortmannii FS2 emits β-caryphyllene, which promotes plant growth and induces resistance. J. Gen. Plant Pathol. 2011, 77, 336–341. [Google Scholar] [CrossRef]
- Matsui, K.; Sasahara, S.; Akakabe, Y.; Kajiwara, T. Linoleic acid 10-hydroperoxide as an intermediate during formation of 1-octen-3-ol from linoleic acid in Lentinus decadetes. Biosci. Biotechnol. Biochem. 2003, 67, 2280–2282. [Google Scholar] [CrossRef]
- Suda, M. Short-step syntheses of (±)-bazzanene and (±) trichodiene. Tetrahedron Lett. 1982, 23, 427–428. [Google Scholar] [CrossRef]
- Zamir, L.O.; Gauthier, M.J.; Devor, K.A.; Nadeau, Y.; Sauriol, F. Trichodiene is a precursor to trichothecenes. J. Chem. Soc. Chem. Commun. 1989, 9, 598–600. [Google Scholar] [CrossRef]
- Lin, H.; Phelan, P.L. Comparison of volatiles from beetle-transmitted Ceratocystis fagacearum and four non-insect-dependent fungi. J. Chem. Ecol. 1992, 18, 1623–1632. [Google Scholar] [CrossRef]
- Wang, T.; Rabe, P.; Citron, C.A.; Dickschat, J.S. Halogenated volatiles from the fungus Geniculosporium and the actinomycete Streptomyces chartreusis. Beilstein J. Org. Chem. 2013, 9, 2767–2777. [Google Scholar] [CrossRef]
- Schalchli, H.; Hormazábal, E.; Becerra, J.; Briceño, G.; Hernández, V.; Rubilar, O.; Diez, M.C. Volatiles from white-rot fungi for controlling plant pathogenic fungi. Chem. Ecol. 2015, 31, 754–763. [Google Scholar] [CrossRef]
- Spinnler, H.E.; De Jong, E.; Mauvais, G.; Semon, E.; Le Quéré, J.L. Production of halogenated compounds by Bjerkandera adusta. Appl. Microbiol. Biotechnol. 1994, 42, 212–221. [Google Scholar] [CrossRef]
- Zawirska-Wojtasiak, R. Optical purity of (R)-(−)-1-octen-3-ol in the aroma of various species of edible mushrooms. Food Chem. 2004, 86, 113–118. [Google Scholar] [CrossRef]
- Thakeow, P.; Angeli, S.; Weißbecker, B.; Schütz, S. Antennal and behavioral responses of Cis boleti to fungal odor of Trametes gibbosa. Chem. Senses 2008, 33, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Mosandl, A.; Heusinger, G.; Gessner, M. Analytical and sensory differentiation of 1-octen-3-ol enantiomers. J. Agric. Food Chem. 1986, 34, 119–122. [Google Scholar] [CrossRef]
- Spiteller, P. Chemical defence strategies of higher fungi. Chem. A Eur. J. 2008, 14, 9100–9110. [Google Scholar] [CrossRef]
- Heddergott, C.; Calvo, A.M.; Latgé, J.P. The volatome of Aspergillus fumigatus. Eukaryot. Cell 2014, 13, 1014–1025. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Schrier, N.; Kalkhove, S.I.; Lugones, L.G.; Baars, J.J.; Zijlstra, C.; De Weerdt, M.; Wösten, H.A.B.; Bakker, P.A.H.M. Absence of induced resistance in Agaricus bisporus against Lecanicillium fungicola. Antonie Van Leeuwenhoek 2013, 103, 539–550. [Google Scholar] [CrossRef]
- Chitarra, G.S.; Abee, T.; Rombouts, F.M.; Posthumus, M.A.; Dijksterhuis, J. Germination of Penicillium paneum conidia is regulated by 1-octen-3-ol, a volatile self-inhibitor. Appl. Environ. Microbiol. 2004, 70, 2823–2829. [Google Scholar] [CrossRef]
- Nemčovič, M.; Jakubíková, L.; Ví-den, I.; Farkaš, V. Induction of conidiation by endogenous volatile compounds in Trichoderma spp. FEMS Microbiol. Lett. 2008, 284, 231–236. [Google Scholar] [CrossRef]
- Müller, A.; Faubert, P.; Hagen, M.; Zu Castell, W.; Polle, A.; Schnitzler, J.P.; Rosenkranz, M. Volatile profiles of fungi–chemotyping of species and ecological functions. Fungal Genet. Biol. 2013, 54, 25–33. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, J.L.; Liu, J.M.; Chen, R.D.; Xie, K.B.; Chen, D.W.; Feng, K.P.; Zhang, D.; Dai, J.G. Neural anti-inflammatory sesquiterpenoids from the endophytic fungus Trichoderma sp. Xy24. J. Asian Nat. Prod. Res. 2017, 19, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Takasu, K.; Mizutani, S.; Noguchi, M.; Makita, K.; Ihara, M. Total Synthesis of (±)-culmorin and (±)-longiborneol: An efficient construction of tricyclo [6.3. 0.03, 9] undecan-10-one by intramolecular double michael addition. J. Org. Chem. 2000, 65, 4112–4119. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.P.; Alexander, N.J.; Harris, L.J. CLM1 of Fusarium graminearum encodes a longiborneol synthase required for culmorin production. Appl. Environ. Microbiol. 2010, 76, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.O. Volatile flavour production by Penicillium caseifulvum. Int. Dairy J. 1998, 8, 883–887. [Google Scholar] [CrossRef]
- Brock, N.L.; Tudzynski, B.; Dickschat, J.S. Biosynthesis of sesqui-and diterpenes by the gibberellin producer Fusarium fujikuroi. ChemBioChem 2011, 12, 2667–2676. [Google Scholar] [CrossRef] [PubMed]
- Studt, L.; Janevska, S.; Niehaus, E.M.; Burkhardt, I.; Arndt, B.; Sieber, C.M.; Humpf, H.U.; Dickschat, J.S.; Tudzynski, B. Two separate key enzymes and two pathway-specific transcription factors are involved in fusaric acid biosynthesis in Fusarium fujikuroi. Environ. Microbiol. 2016, 18, 936–956. [Google Scholar] [CrossRef] [PubMed]
- Pollnitz, A.P.; Pardon, K.H.; Sefton, M.A. Quantitative analysis of 4-ethylphenol and 4-ethylguaiacol in red wine. J. Chromatogr. A 2000, 874, 101–109. [Google Scholar] [CrossRef]
- Martorell, N.; Martı, M.P.; Mestres, M.; Busto, O.; Guasch, J. Determination of 4-ethylguaiacol and 4-ethylphenol in red wines using headspace-solid-phase microextraction-gas chromatography. J. Chromatogr. A 2002, 975, 349–354. [Google Scholar] [CrossRef]
- Xiao, Z.; Xu, P. Acetoin metabolism in bacteria. Crit. Rev. Microbiol. 2007, 33, 127–140. [Google Scholar] [CrossRef]
- Bach, B.; Meudec, E.; Lepoutre, J.P.; Rossignol, T.; Blondin, B.; Dequin, S.; Camarasa, C. New insights into γ-aminobutyric acid catabolism: Evidence for γ-hydroxybutyric acid and polyhydroxybutyrate synthesis in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2009, 75, 4231–4239. [Google Scholar] [CrossRef]
- Singh, S.K.; Strobel, G.A.; Knighton, B.; Geary, B.; Sears, J.; Ezra, D. An endophytic Phomopsis sp. possessing bioactivity and fuel potential with its volatile organic compounds. Microb. Ecol. 2011, 61, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Strobel, G.; Singh, S.K.; Riyaz-Ul-Hassan, S.; Mitchell, A.M.; Geary, B.; Sears, J. An endophytic/pathogenic Phoma sp. from creosote bush producing biologically active volatile compounds having fuel potential. FEMS Microbiol. Lett. 2011, 320, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Fahlbusch, K.G.; Hammerschmidt, F.J.; Panten, J.; Pickenhagen, W.; Schatkowski, D.; Bauer, K.; Garbe, D.; Surburg, H. Flavors and fragrances. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000. [Google Scholar] [CrossRef]
- Lingappa, B.T.; Prasad, M.; Lingappa, Y.; Hunt, D.F.; Biemann, K. Phenethyl alcohol and tryptophol: Autoantibiotics produced by the fungus Candida albicans. Science 1969, 163, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Eshkol, N.; Sendovski, M.; Bahalul, M.; Katz-Ezov, T.; Kashi, Y.A.; Fishman, A. Production of 2-phenylethanol from by a stress tolerant Saccharomyces cerevisiae strain. J. Appl. Microbiol. 2009, 106, 534–542. [Google Scholar] [CrossRef]
- Rapior, S.; Cavalié, S.; Croze, P.; Andary, C.; Pélissier, Y.; Bessière, J.M. Volatile components of ten frozen mushrooms (Basidiomycetes). J. Essent. Oil Res. 1996, 8, 63–66. [Google Scholar] [CrossRef]
- Gao, F.; Daugulis, A.J. Bioproduction of the aroma compound 2-phenylethanol in a solid-liquid two-phase partitioning bioreactor system by Kluyveromyces marxianus. Biotechnol. Bioeng. 2009, 104, 332–339. [Google Scholar] [CrossRef]
- Hua, D.; Xu, P. Recent advances in biotechnological production of 2-phenylethanol. Biotechnol. Adv. 2011, 29, 654–660. [Google Scholar] [CrossRef]
- Drilling, K.; Dettner, K. Electrophysiological responses of four fungivorous coleoptera to volatiles of Trametes versicolor: Implications for host selection. Chemoecology 2009, 19, 109. [Google Scholar] [CrossRef]
- Wawrzyn, G.T.; Quin, M.B.; Choudhary, S.; López-Gallego, F.; Schmidt-Dannert, C. Draft genome of Omphalotus olearius provides a predictive framework for sesquiterpenoid natural product biosynthesis in Basidiomycota. Chem. Biol. 2012, 19, 772–783. [Google Scholar] [CrossRef]
- Ziegenbein, F.C.; Hanssen, H.P.; König, W.A. Chemical constituents of the essential oils of three wood-rotting fungi. Flavour Fragr. J. 2006, 21, 813–816. [Google Scholar] [CrossRef]
- Ziegenbein, F.C.; König, W.A.; Hanssen, H.P. Volatile metabolites from the wood-inhabiting fungi Bjerkandera adusta, Ganoderma applanatum, and Stereum hirsutum. J. Essent. Oil Res. 2010, 22, 116–118. [Google Scholar] [CrossRef]
- Costa, R.; De Grazia, S.; Grasso, E.; Trozzi, A. Headspace-solid-phase microextraction-gas chromatography as analytical methodology for the determination of volatiles in wild mushrooms and evaluation of modifications occurring during storage. J. Anal. Methods Chem. 2015, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dickschat, J.S.; Celik, E.; Brock, N.L. Volatiles from three genome sequenced fungi from the genus Aspergillus. Beilstein J. Org. Chem. 2018, 14, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Takigawa, H.; Kubota, H.; Sonohara, H.; Okuda, M.; Tanaka, S.; Fujikura, Y.; Ito, S. Novel Allylic Oxidation of α-Cedrene to sec-Cedrenol by a Rhodococcus Strain. Appl. Environ. Microbiol. 1993, 59, 1336–1341. [Google Scholar] [CrossRef] [PubMed]
- Matysik, S.; Herbarth, O.; Mueller, A. Determination of volatile metabolites originating from mould growth on wall paper and synthetic media. J. Microbiol. Methods 2008, 75, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Hamad, Y.K.; Abobakr, Y.; Salem, M.Z.; Ali, H.M.; Al-Sarar, A.S.; Al-Zabib, A.A. Activity of plant extracts/essential oils against three plant pathogenic fungi and mosquito larvae: GC/MS analysis of bioactive compounds. BioResources 2019, 14, 4489–4511. [Google Scholar] [CrossRef]
- Szmigielski, R.; Cieslak, M.; Rudziński, K.J.; Maciejewska, B. Identification of volatiles from Pinus silvestris attractive for Monochamus galloprovincialis using a SPME-GC/MS platform. Environ. Sci. Pollut. Res. 2012, 19, 2860–2869. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jeleń, H.H. Use of solid phase microextraction (SPME) for profiling fungal volatile metabolites. Lett. Appl. Microbiol. 2003, 36, 263–267. [Google Scholar] [CrossRef]
- Gardner, J.W.; Pearce, T.C.; Friel, S.; Bartlett, P.N.; Blair, N. A multisensor system for beer flavour monitoring using an array of conducting polymers and predictive classifiers. Sens. Actuators B Chem. 1994, 18, 240–243. [Google Scholar] [CrossRef]
- Stoppacher, N.; Kluger, B.; Zeilinger, S.; Krska, R.; Schuhmacher, R. Identification and profiling of volatile metabolites of the biocontrol fungus Trichoderma atroviride by HS-SPME-GC-MS. J. Microbiol. Methods 2010, 81, 187–193. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, G. A review of advances and new developments in the analysis of biological volatile organic compounds. Microchem. J. 2010, 95, 127–139. [Google Scholar] [CrossRef]
- Nowakowska, J.A.; Stocki, M.; Stocka, N.; Ślusarski, S.; Tkaczyk, M.; Caetano, J.M.; Tulik, M.; Hsiang, M.; Oszako, T. Interactions between Phytophthora cactorum, Armillaria gallica and Betula pendula Roth. Seedlings Subjected to Defoliation. Forests 2020, 11, 1107. [Google Scholar] [CrossRef]
- Senthilmohan, S.T.; McEwan, M.J.; Wilson, P.F.; Milligan, D.B.; Freeman, C.G. Real time analysis of breath volatiles using SIFT-MS in cigarette smoking. Redox Rep. 2001, 6, 185–187. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lindinger, W.; Hansel, A.; Jordan, A. On-line monitoring of volatile organic compounds at pptv levels by means of proton-transfer-reaction mass spectrometry (PTR-MS) medical applications, food control and environmental research. Int. J. Mass Spectrom. Ion Process. 1998, 173, 191–241. [Google Scholar] [CrossRef]
- Ezra, D.; Jasper, J.; Rogers, T.; Knighton, B.; Grimsrud, E.; Strobel, G. Proton transfer reaction-mass spectrometry as a technique to measure volatile emissions of Muscodor albus. Plant Sci. 2004, 166, 1471–1477. [Google Scholar] [CrossRef]
- Maenhaut, W.; Chi, X.; Wang, W.; Cafmeyer, J.; Yasmeen, F.; Vermeylen, R.; Szmigielska, K.; Janssens, I.; Claeys, M. Contribution from Selected Organic Species to PM2.5 Aerosol during a Summer Field Campaign at K.-Puszta, Hungary. Atmosphere 2017, 8, 221. [Google Scholar] [CrossRef]
- Osowski, S.; Siwek, K.; Grzywacz, T.; Brudzewski, K. Differential electronic nose in on-line dynamic measurements. Metrol. Meas. Syst. 2014, 21, 649–662. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 17 November 2020).
- Husson, F.; Josse, J.; Le, S.; Mazet, J. Factominer: Multivariate Exploratory Data Analysis and Data Mining. R Package Version 1.29. 2015. Available online: http://cran.r-project.org/package=FactoMineR (accessed on 17 November 2020).
- Ward, J.H. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Murtagh, F.; Legendre, P. Ward’s hierarchical agglomerative clustering method: Which algorithms implement Ward’s criterion? J. Classif. 2014, 31, 274–295. [Google Scholar] [CrossRef]
- Kaufman, L.; Rousseeuw, P.J. Finding Groups in Data: An Introduction to Cluster Analysis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Rousseeuw, P.; Struyf, A.; Hubert, M.; Studer, M.; Roudier, P.; Gonzalez, J. Package ‘Cluster’. Available online: https://cran.r-project.org/web/packages/cluster/index.html (accessed on 17 November 2020).

| tR 1 [min] | SI 2 % | RIExp 3 | RILit 4 | Ref. | Peak Area per Treatment | Name of Compound 5 |
|---|---|---|---|---|---|---|
| (1) Empty Container | ||||||
| 7.19 | 65 | 902 | 901 | [29] | 17,674,234 | Heptanal ++a |
| 10.05 | 80 | 1003 | 998 | [29] | 40,444,566 | Octanal ++a |
| 10.82 | 28 | 1029 | 1027 | [30] | 21,189,032 | 2-Ethyl-hexanol a |
| 13.05 | 79 | 1104 | 1100 | [29] | 76,538,207 | Nonanal ++a |
| 15.98 | 53 | 1206 | 1201 | [29] | 14,513,924 | Decanal ++a |
| 18.06 | − | 1281 | − | − | 14,724,741 | Unknown compound |
| 18.77 | − | 1307 | − | − | 13,630,063 | Unknown compound |
| 21.21 | 32 | 1400 | 1400 | [29] | 6,427,563 | Tetradecane ++a |
| 21.44 | 58 | 1409 | 1408 | [29] | 17,444,416 | Dodecanal ++a |
| 24.07 | 78 | 1516 | 1514 | [31] | 47,388,559 | Butylated Hydroxytoluene ++a |
| (2) PDA Medium | ||||||
| 4.77 | 50 | 804 | 801 | [29] | 19,939,473 | Hexanal +a |
| 5.48 | 57 | 834 | 828 | [29] | 11,223,384 | Furfural +a |
| 7.18 | 31 | 902 | 901 | [29] | 39,366,443 | Heptanal ++a |
| 8.80 | 72 | 959 | 952 | [29] | 60,063,839 | Benzaldehyde a |
| 9.74 | 87 | 992 | 984 | [29] | 31,027,584 | 2-Pentyl-furan b |
| 10.05 | 74 | 1003 | 998 | [29] | 11,751,703 | Octanal ++a |
| 10.81 | 44 | 1029 | 1027 | [30] | 32,462,061 | 2-Ethyl-hexanol a |
| 11.23 | 73 | 1043 | 1036 | [29] | 73,539,705 | Benzeneacetaldehyde ++a |
| 12.96 | 21 | 1101 | 1100 | [29] | 12,884,326 | Undecane +a |
| 13.04 | 80 | 1104 | 1100 | [29] | 41,840,277 | Nonanal ++a |
| 15.48 | − | 1189 | − | − | 4,687,953 | Unknown compound |
| 15.83 | 30 | 1201 | 1200 | [29] | 4,893,511 | Dodecane +a |
| 15.97 | 50 | 1206 | 1201 | [29] | 10,723,873 | Decanal ++a |
| 18.13 | − | 1284 | − | − | 5,876,115 | Unknown compound |
| 18.77 | − | 1307 | − | − | 4,815,975 | Unknown compound |
| 21.43 | 44 | 1409 | 1408 | [29] | 11,027,672 | Dodecanal ++a |
| 24.06 | 74 | 1515 | 1514 | [31] | 14,730,122 | Butylated Hydroxytoluene ++a |
| (3) A. gallica | ||||||
| 8.80 | 47 | 959 | n/a6 | − | 300,374,347 | (2E)-4,4-Dimethyl-2-pentenal c |
| 11.73 | 53 | 1060 | 1049 | [29] | 62,813,700 | (E)-2-Octen-1-al b |
| 20.87 | 10 | 1387 | n/a | - | 611,543,126 | 1,3,4,5,6,7-Hexahydro-2,5,5-trimethyl-2H-2,4a-ethanonaphthalene c |
| 23.89 | 26 | 1506 | 1506 | [29] | 74,690,325 | (Z)-α-Bisabolene +a |
| (4) A. ostoyae | ||||||
| 8.80 | 49 | 959 | n/a | - | 87,776,838 | (2E)-4,4-Dimethyl-2-pentenal c |
| 11.74 | 35 | 1060 | 1049 | [29] | 20,166,139 | (E)-2-Octen-1-al b |
| 18.49 | 21 | 1297 | 1293 | [29] | 8,070,650 | 2-Undecanone a |
| 20.83 | 43 | 1386 | n/a | - | 106,170,662 | 1,3,4,5,6,7-Hexahydro-2,5,5-trimethyl-2H-2,4a-ethanonaphthalene c |
| 22.49 | 59 | 1451 | 1440 | [29] | 43,908,793 | β-Barbatene a |
| 24.42 | 34 | 1531 | 1522 | [29] | 24,134,794 | δ-Cadinene +a |
| (5) F. avenaceum | ||||||
| 3.28 | 76 | 712 | 735 | [32] | 2,653,753 | 2,4-Dimethylfuran +b |
| 9.13 | 23 | 971 | 970 | [33] | 3,974,721 | Mesitilene +a |
| 10.91 | 76 | 1032 | 1029 | [29] | 9,540,610 | β-Phellandrene a |
| 21.59 | 35 | 1415 | 1407 | [29] | 11,961,247 | Longifolene a |
| 24.46 | 73 | 1532 | 1519 | [29] | 3,401,805 | β-Bazzanene +a |
| (6) F. culmorum | ||||||
| 1.62 | 77 | <500 | 448 | [34] | 163,586,144 | Ethanol a |
| 2.25 | 96 | 573 | 606 | [29] | 45,234,113 | Ethyl acetate +a |
| 3.55 | 41 | 728 | 731 | [29] | 6,085,804 | Isoamyl alcohol +a |
| 3.61 | 56 | 732 | 724 | [29] | 3,371,660 | 2-Methyl-butanol a |
| 10.91 | 18 | 1032 | 1029 | [29] | 177,256,946 | β-Phellandrene a |
| 21.80 | 43 | 1424 | 1408 | [29] | 20,993,355 | Acora-3,7(14)-diene +b |
| 22.23 | man.7 | 1441 | 1432 | [29] | 138,760,275 | β-Copaene +a |
| (7) F. oxysporum | ||||||
| 6.02 | 78 | 854 | 864 | [35] | 845,609 | 2-Furanmethanol +b |
| 7.96 | 59 | 930 | 927 | [36] | 2,621,568 | Hexyl formate +a |
| 8.86 | 79 | 961 | 952 | [29] | 49,523,292 | Benzaldehyde +a |
| 9.65 | 68 | 989 | 979 | [29] | 5,057,096 | 3-Octanone a |
| 10.27 | 71 | 1010 | 960 | [29] | 2,393,859 | Isoamyl propionate +a |
| 10.90 | 54 | 1032 | n/a | − | 63,835,818 | Heptyl formate +a |
| 21.98 | 33 | 1431 | 1419 | [29] | 4,969,490 | β-Cedrene a |
| 22.49 | 90 | 1451 | n/a | − | 23,065,220 | 3-Chloro-4-methoxy-benzaldehyde c |
| 22.77 | 20 | 1463 | n/a | − | 8,382,097 | 2,4-Dichloro-3-methoxy-1-benzene carbonyl chloride +c |
| 23.34 | 93 | 1486 | 864 | [35] | 2,740,362 | 3,4-Dimethoxy-benzaldehyde +a |
| (8) F. poae | ||||||
| 9.43 | 81 | 981 | 974 | [29] | 93,704,125 | 1-Octen-3-ol a |
| 9.79 | 36 | 994 | 988 | [29] | 166,758,547 | Myrcene +a |
| 13.39 | 59 | 1116 | 1122 | [37] | 100,643,564 | 1-Ethyl-4-methoxy-benzene b |
| 16.68 | 41 | 1231 | 1223 | [29] | 36,663,640 | Citronellol +a |
| 20.10 | 28 | 1358 | 1350 | [29] | 106,101,600 | α-Longipinene +a |
| 20.65 | 16 | 1379 | 1371 | [29] | 108,987,407 | Longicyclene +a |
| 21.43 | man. | 1409 | 1400 | [29] | 248,606,376 | β-Longipinene +b |
| 21.65 | 37 | 1418 | 1407 | [29] | 1,390,824,920 | Longifolene a |
| 21.81 | − | 1427 | − | − | 482,683,621 | Unknown sesquiterpene |
| 21.91 | 22 | 1428 | 1419 | [29] | 195,664,192 | β-Ylangene +a |
| 22.31 | − | 1444 | − | − | 954,381,082 | Unknown sesquiterpene |
| 22.55 | 55 | 1454 | 1440 | [29] | 129,567,010 | β-Barbatene +a |
| 22.68 | 16 | 1459 | 1449 | [29] | 117,848,857 | α-Himachalene +a |
| 22.75 | 50 | 1462 | 1454 | [29] | 143,907,373 | (E)-β-Farnesene +a |
| 23.02 | 20 | 1473 | 1466 | [29] | 116,555,130 | α-Acoradiene +a |
| 23.38 | 16 | 1487 | 1481 | [29] | 134,783,119 | γ-Himachalene +a |
| 23.98 | 43 | 1512 | n/a | − | 44,779,678 | 8-Isopropenyl-1,5-dimethyl-1,5-cyclodecadiene +c |
| 24.05 | 17 | 1515 | 1505 | [29] | 51,262,732 | β-Bisabolene +a |
| 25.03 | − | 1557 | − | − | 42,987,721 | Unknown sesquiterpene |
| 26.31 | 60 | 1612 | 1599 | [29] | 1,576,920,976 | Longiborneol +a |
| (9) P. cactorum | ||||||
| 1.71 | 86 | <500 | 500 | [34] | 5,827,788 | Acetone a |
| 3.75 | 97 | 740 | 744 | [38] | 4,459,515 | Dimethyl disulfide b |
| 4.12 | − | 763 | − | − | 4,321,348 | Unknown compound |
| 6.35 | 60 | 868 | 863 | [29] | 25,809,441 | 1-Hexanol +a |
| 9.15 | 58 | 971 | 959 | [29] | 5,111,166 | 1-Heptanol +a |
| 9.42 | 75 | 981 | 974 | [29] | 19,848,783 | 1-Octen-3-ol a |
| 9.65 | 76 | 989 | 979 | [29] | 12,627,315 | 3-Octanone a |
| 9.81 | 88 | 995 | 984 | [29] | 4,284,441 | 2-Pentyl-furana |
| 12.05 | 22 | 1071 | 1060 | [29] | 2,508,277 | 2-Octen-1-ol +a |
| 12.13 | 19 | 1073 | 1063 | [29] | 4,044,266 | 1-Octanol +a |
| (10) P. cinnamomi | ||||||
| 1.61 | 97 | <500 | 448 | [31] | 9,483,339 | Ethanol a |
| 9.43 | 56 | 981 | 974 | [29] | 2,215,655 | 1-Octen-3-ol a |
| 10.88 | 47 | 1031 | 1027 | [30] | 5,251,305 | 2-Ethyl-hexanol a |
| 18.13 | 85 | 1284 | 1282 | [39] | 1,001,882 | 4-Ethyl-2-methoxy-phenol +b |
| (11) P. plurivora | ||||||
| 1.71 | 84 | <500 | 500 | [34] | 4,481,938 | Acetone a |
| 3.20 | 64 | 707 | 711 | [40] | 2,268,583 | Acetoin +a |
| 6.37 | 36 | 869 | 863 | [29] | 1,803,069 | Hexanol +a |
| 7.51 | 76 | 914 | 933 | [41] | 3,627,373 | 4-Hydroxy-butanoic acid +b |
| 8.14 | 16 | 936 | 932 | [29] | 1,684,400 | α-Pinene +a |
| 10.35 | 14 | 1013 | 1008 | [29] | 1,103,050 | Δ-3-Carene +a |
| (12) P. ramorum | ||||||
| 1.16 | 98 | <500 | 448 | [34] | 20,885,799 | Ethanol a |
| 3.55 | 74 | 728 | 730 | [42] | 80,424,948 | 3-Methyl-butanol a |
| 3.61 | 74 | 732 | 724 | [29] | 26,123,097 | 2-Methyl-butanol a |
| 9.43 | 72 | 981 | 974 | [29] | 8,370,575 | 1-Octen-3-ol a |
| 9.65 | 70 | 989 | 979 | [29] | 11,144,929 | 3-Octanone a |
| 13.37 | 87 | 1116 | 1106 | [29] | 48,377,677 | 2-Phenylethanol +a |
| (13) R. solani | ||||||
| 1.71 | 87 | <500 | 500 | [34] | 3,565,392 | Acetone a |
| 9.42 | 74 | 981 | 974 | [29] | 24,589,405 | 1-Octen-3-ol a |
| 9.65 | 62 | 989 | 979 | [29] | 3,438,485 | 3-Octanone a |
| 9.90 | 75 | 998 | 988 | [29] | 6,372,403 | 3-Octanol +a |
| 15.56 | − | 1191 | - | - | 4,474,717 | Unknown compound |
| (14) T. asperellum | ||||||
| 1.71 | 82 | <500 | 500 | [34] | 3,920,210 | Acetonea |
| 3.56 | 62 | 729 | 730 | [41] | 7,870,509 | 3-Methyl-butanola |
| 3.62 | 45 | 733 | 724 | [29] | 4,424,960 | 2-Methyl-butanola |
| 3.76 | 98 | 741 | 744 | [38] | 11,829,339 | Dimethyl disulfideb |
| 7.26 | − | 905 | − | − | 153,721,663 | Unknown compound |
| 13.01 | − | 1103 | − | − | 20,319,451 | Unknown compound |
| 13.38 | 85 | 1116 | 1106 | [29] | 37,177,146 | 2-Phenyethanol +a |
| 20.08 | − | 1357 | − | − | 70,272,034 | Unknown sesquiterpene |
| 20.91 | 44 | 1389 | 1380 | [29] | 229,806,310 | Daucene +b |
| 21.81 | man. | 1424 | 1412 | [29] | 25,748,186 | 2-epi-β-Funebrene +b |
| 21.98 | 50 | 1431 | 1419 | [29] | 86,596,372 | β-Cedrenea |
| 23.93 | 28 | 1510 | 1500 | [29] | 118,470,969 | Isodaucene +b |
| 24.27 | 21 | 1524 | 1513 | [29] | 52,189,482 | γ-Cadinene +a |
| 24.63 | 40 | 1540 | 1530 | [29] | 194,592,295 | Dauca-4(11)-8-diene +a |
| 25.49 | − | 1576 | − | − | 8,894,971 | Unknown sesquiterpene |
| 27.81 | − | 1679 | − | − | 15,501,124 | Unknown sesquiterpene |
| 28.31 | 27 | 1701 | n/a | − | 15,437,838 | 1-Isopropyl-4,8-dimethylspiro [4.5]dec-8-en-7-one +c |
| 30.26 | − | 1793 | − | − | 21,104,266 | Unknown sesquiterpene |
| Treatment | Species | Reference from GenBank |
|---|---|---|
| Control | − | − |
| 1 | Armillaria gallica (Marxm. & Romagn.) 1987 | DQ115578 1 |
| 2 | Armillaria ostoyae (Romagn.) Herink 1973 | DQ115574 |
| 3 | Fusarium avenaceum (Fr.) Sacc. 1886 | MK560761 |
| 4 | Fusarium culmorum (Wm.G. Sm.) Sacc. 1892 | KP008988 |
| 5 | Fusarium oxysporum (Schltdl.) 1824 | MF162321 |
| 6 | Fusarium poae (Peck) Wollenw. 1913 | MF162318 |
| 7 | Phytophthora cactorum (Lebert & Cohn) J. Schröt. 1886 | KX242303 |
| 8 | Phytophthora cinnamomi Rands 1922 | KF682434 |
| 9 | Phytophthora plurivora T. Jung & T.I. Burgess 2009 | JX276032 |
| 10 | Phytophthora ramorum Werres, De Cock & Man in ‘t Veld 2001 | JF771575 |
| 11 | Rhizoctonia solani J.G. Kühn 1858 | KU901561 |
| 12 | Trichoderma asperellum Samuels, Lieckf. & Nirenberg 1999 | MT197117 |
Sample Availability: Samples of the compounds are not available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loulier, J.; Lefort, F.; Stocki, M.; Asztemborska, M.; Szmigielski, R.; Siwek, K.; Grzywacz, T.; Hsiang, T.; Ślusarski, S.; Oszako, T.; et al. Detection of Fungi and Oomycetes by Volatiles Using E-Nose and SPME-GC/MS Platforms. Molecules 2020, 25, 5749. https://doi.org/10.3390/molecules25235749
Loulier J, Lefort F, Stocki M, Asztemborska M, Szmigielski R, Siwek K, Grzywacz T, Hsiang T, Ślusarski S, Oszako T, et al. Detection of Fungi and Oomycetes by Volatiles Using E-Nose and SPME-GC/MS Platforms. Molecules. 2020; 25(23):5749. https://doi.org/10.3390/molecules25235749
Chicago/Turabian StyleLoulier, Jérémie, François Lefort, Marcin Stocki, Monika Asztemborska, Rafał Szmigielski, Krzysztof Siwek, Tomasz Grzywacz, Tom Hsiang, Sławomir Ślusarski, Tomasz Oszako, and et al. 2020. "Detection of Fungi and Oomycetes by Volatiles Using E-Nose and SPME-GC/MS Platforms" Molecules 25, no. 23: 5749. https://doi.org/10.3390/molecules25235749
APA StyleLoulier, J., Lefort, F., Stocki, M., Asztemborska, M., Szmigielski, R., Siwek, K., Grzywacz, T., Hsiang, T., Ślusarski, S., Oszako, T., Klisz, M., Tarakowski, R., & Nowakowska, J. A. (2020). Detection of Fungi and Oomycetes by Volatiles Using E-Nose and SPME-GC/MS Platforms. Molecules, 25(23), 5749. https://doi.org/10.3390/molecules25235749









