Synthesis of (Het)aryl 2-(2-hydroxyaryl)cyclopropyl Ketones
Abstract
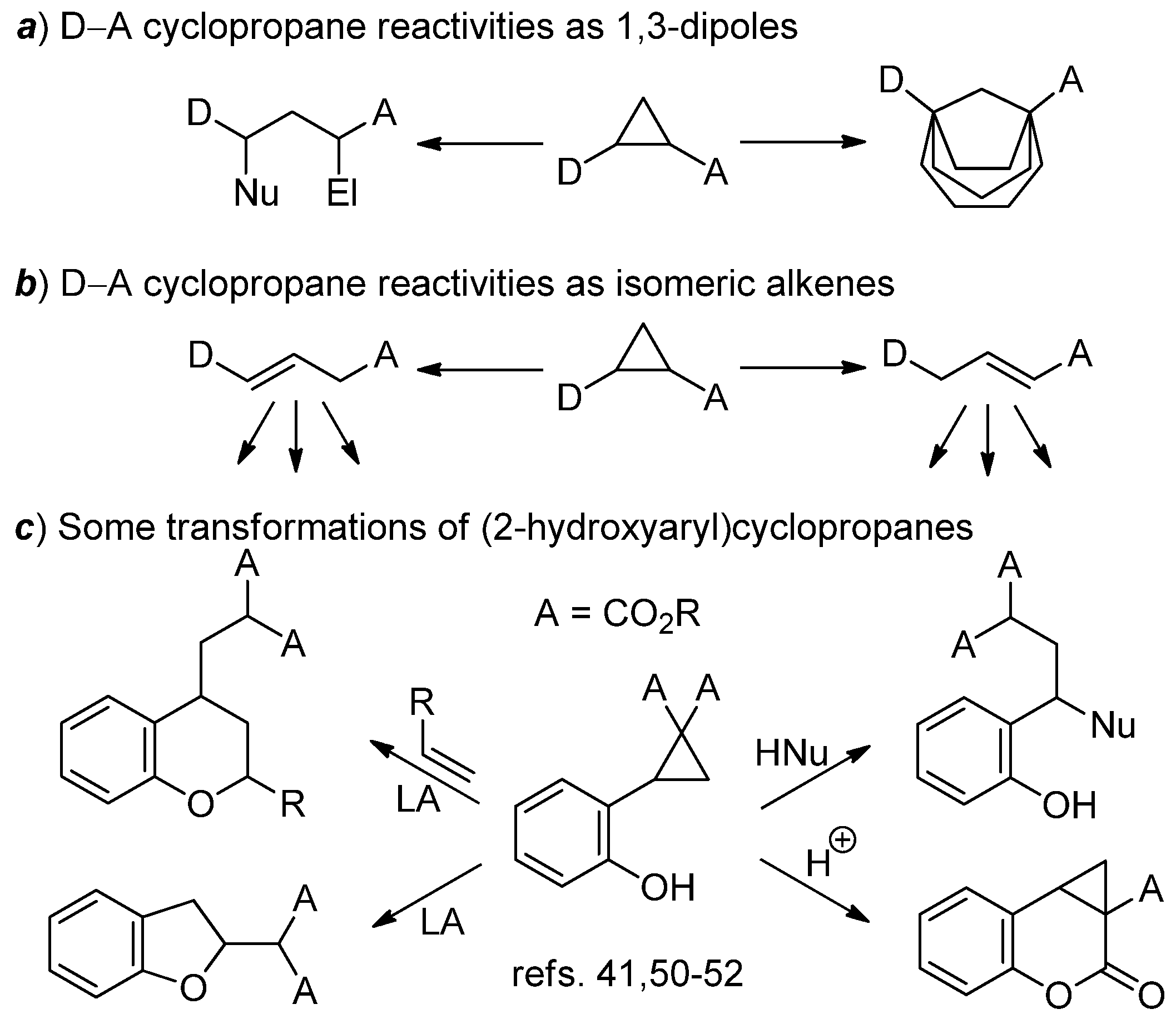
1. Introduction
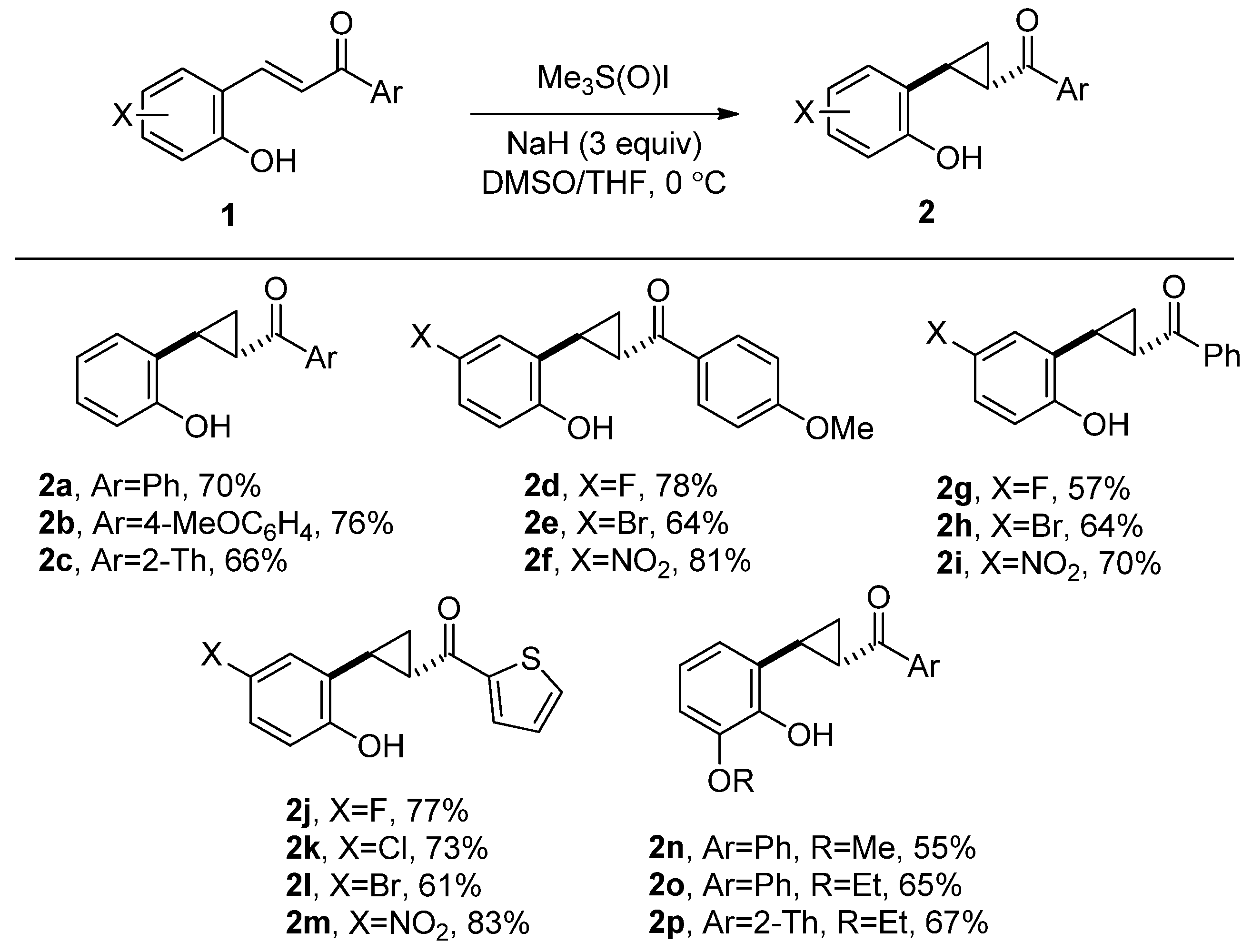
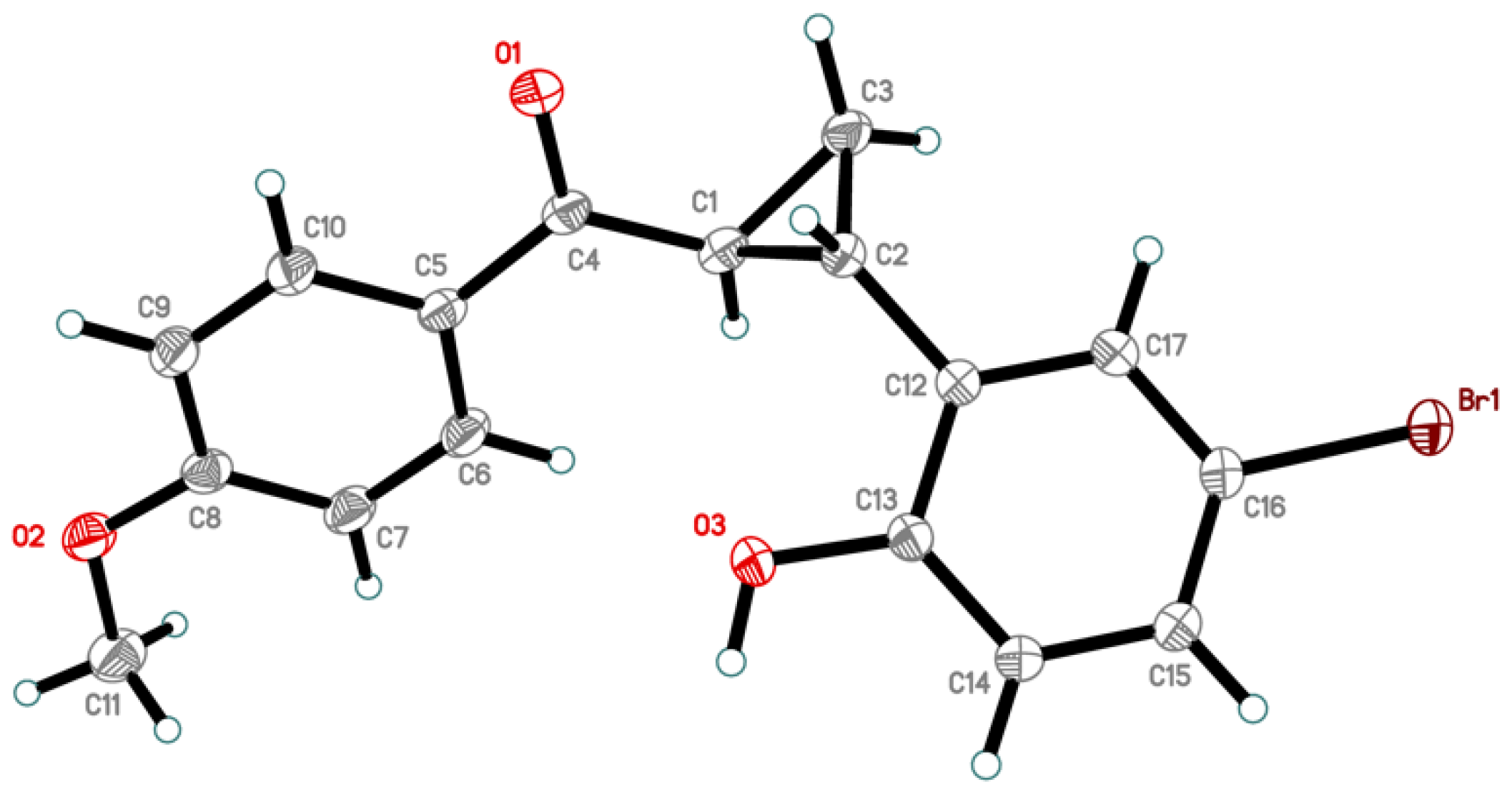
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Synthesis of 2-Hydroxychalcones 1
3.3. General Procedure for the Synthesis of Donor–Acceptor Cyclopropanes 2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reissig, H.-U.; Zimmer, R. Donor–Acceptor-Substituted Cyclopropane Derivatives and Their Application in Organic Synthesis. Chem. Rev. 2003, 103, 1151–1196. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.F.; Kaschel, J.; Werz, D.B. A New Golden Age for Donor–Acceptor Cyclopropanes. Angew. Chem. Int. Ed. 2014, 53, 5504–5523. [Google Scholar] [CrossRef] [PubMed]
- Cavitt, M.A.; Phun, L.H.; France, S. Intramolecular donor–acceptor cyclopropane ring-opening cyclizations. Chem. Soc. Rev. 2014, 43, 804–818. [Google Scholar] [CrossRef] [PubMed]
- Budynina, E.M.; Ivanov, K.L.; Sorokin, I.D.; Melnikov, M.Y. Ring Opening of Donor–Acceptor Cyclopropanes with N-Nucleophiles. Synthesis 2017, 49, 3035–3068. [Google Scholar] [CrossRef]
- Ivanova, O.A.; Trushkov, I.V. Donor–Acceptor Cyclopropanes in the Synthesis of Carbocycles. Chem. Rec. 2019, 19, 2189–2208. [Google Scholar] [CrossRef] [PubMed]
- Tomilov, Y.V.; Menchikov, L.G.; Novikov, R.A.; Ivanova, O.A.; Trushkov, I.V. Methods for the synthesis of donor–acceptor cyclopropanes. Russ. Chem. Rev. 2018, 87, 201–250. [Google Scholar] [CrossRef]
- Guin, A.; Rathod, T.; Gaykar, R.N.; Roy, T.; Biju, A.T. Lewis Acid Catalyzed Ring-Opening 1,3-Aminothiolation of Donor–Acceptor Cyclopropanes using Sulfenamides. Org. Lett. 2020, 22, 2276–2280. [Google Scholar] [CrossRef]
- Mondal, B.; Das, D.; Saha, J. Multicomponent, Tandem 1,3- and 1,4-Bisarylaltion of Donor–Acceptor Cyclopropanes and Cyclobutanes with Electron-Rich Arenes and Hypervalent Arylbismuth Reagents. Org. Lett. 2020, 22, 5115–5120. [Google Scholar] [CrossRef]
- Zhang, D.; Zhong, J.; Yin, L.; Chen, Y.; Man, J.; Zhang, Q.-F. Desymmetrization of 1-Symmetrical Donor–Acceptor (D–A) Cyclopropanes via Reactions with 1,3-Cyclodiones. J. Org. Chem. 2020, 85, 5778–5786. [Google Scholar] [CrossRef]
- Boichenko, M.A.; Andreev, I.A.; Chagarovskiy, A.O.; Levina, I.I.; Zhokhov, S.S.; Trushkov, I.V.; Ivanova, O.A. Ring Opening of Donor–Acceptor Cyclopropanes with Cyanide Ion and Its Surrogates. J. Org. Chem. 2020, 85, 1146–1157. [Google Scholar] [CrossRef]
- Sridhar, P.R.; Seshadri, K.; Reddy, G.M. Stereoselective synthesis of sugar fused β-disubstituted γ-butyro-lactones: C-spiro-glycosides from 1,2-cyclopropanecarboxylated sugars. Chem. Commun. 2012, 48, 756–758. [Google Scholar] [CrossRef] [PubMed]
- Cermola, F.; Di Gioia, L.; Garziano, M.L.; Iesce, M.R. Ring-opening reactions of cyclopropanes. Part 8. Nitrosation of donor-acceptor cyclopropanes. J. Chem. Res. 2005, 677–681. [Google Scholar] [CrossRef]
- Garve, L.K.B.; Barkawitz, P.; Jones, P.G.; Werz, D.B. Ring-Opening 1,3-Dichlorination of Donor–Acceptor Cyclopropanes by Iodobenzene Dichloride. Org. Lett. 2014, 16, 5804–5807. [Google Scholar] [CrossRef] [PubMed]
- Ieki, R.; Kani, Y.; Tsunoi, S.; Shibata, I. Transition-Metal-Free Coupling Reaction of Vinylcyclopropanes with Aldehydes Catalyzed by Tin Hydride. Chem. Eur. J. 2015, 21, 6295–6300. [Google Scholar] [CrossRef]
- Xu, C.; Wei, N.; Zhu, D.; Wang, M. Cyclopentene Synthesis by a Catalytic [3 + 2] Annulation of Donor–Acceptor Cyclopropanes with Polarized Alkenes. ChemistrySelect 2020, 5, 11399–11402. [Google Scholar] [CrossRef]
- Ahlburg, N.L.; Jones, P.G.; Werz, D.B. cis-Selective, Enantiospecific Addition of Donor–Acceptor Cyclopropanes to Activated Alkenes: An Iodination/Michael-Cyclization Cascade. Org. Lett. 2020, 22, 6404–6408. [Google Scholar] [CrossRef]
- Su, P.; Li, H.; Chen, W.; Luo, G.; Yang, G.; Chai, Z. Lewis Acid Catalyzed [3 + 2] Annulations of γ-Butyrolactam-Fused Donor–Acceptor Cyclopropanes with Aromatic Aldehydes and Aldimines. Eur. J. Org. Chem. 2020, 2020, 5380–5387. [Google Scholar] [CrossRef]
- Mloston, G.; Kowalczyk, M.; Augustin, A.U.; Jones, P.G.; Werz, D.B. Ferrocenyl-substituted tetrahydrothiophenes via formal [3 + 2]-cycloaddition reactions of ferrocenyl thioketones with donor-acceptor cyclopropanes. Beilstein J. Org. Chem. 2020, 16, 1288–1295. [Google Scholar] [CrossRef]
- Lücht, A.; Kreft, A.; Jones, P.G.; Werz, D.B. Ketenedithioacetals as Surrogates for the Formal Insertion of Ketenes into Donor–Acceptor Cyclopropanes. Eur. J. Org. Chem. 2020, 2020, 2560–2564. [Google Scholar] [CrossRef]
- Mikhaylov, A.A.; Kuleshov, A.V.; Solyev, P.N.; Korlyukov, A.A.; Dorovatovskii, P.V.; Mineev, K.S.; Baranov, M.S. Imidazol-5-one as an Acceptor in Donor–Acceptor Cyclopropanes: Cycloaddition with Aldehydes. Org. Lett. 2020, 22, 2740–2745. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Chand, H.R.; Golantsov, N.E.; Trushkov, I.V.; Voskressensky, L.G. Cyclopentene Assembly by Microwave-Assisted Domino Reaction of Donor–Acceptor Cyclopropanes with Ketals. Synlett 2020, 31, 295–299. [Google Scholar] [CrossRef]
- Xia, M.-M.; Li, F.-X.; Ma, Y.-P.; Song, L.-L.; Hou, Y.-N.; Shi, Z.-F.; Cao, X.P. Synthesis of Chiral Aza-[n.2.1] Skeletons by Separable Epimers of Intramolecular [3 + 2] Cross Cycloaddition of Cyclopropane 1,1-Diesters with Chiral Sulfinylimines. Adv. Synth. Catal. 2020, 362, 1112–1124. [Google Scholar] [CrossRef]
- Dhote, P.S.; Ramana, C.V. One-Pot Au(III)-/Lewis Acid Catalyzed Cycloisomerization of Nitroalkynes and [3 + 3]Cycloaddition with Donor–Acceptor Cyclopropanes. Org. Lett. 2019, 21, 6221–6224. [Google Scholar] [CrossRef] [PubMed]
- Petzold, M.; Jones, P.G.; Werz, D.B. (3 + 3)-Annulation of Carbonyl Ylides with Donor–Acceptor Cyclopropanes: Synergistic Dirhodium(II) and Lewis Acid Catalysis. Angew. Chem. Int. Ed. 2019, 58, 6225–6229. [Google Scholar] [CrossRef]
- Chagarovskiy, A.O.; Vasin, V.S.; Kuznetsov, V.V.; Ivanova, O.A.; Rybakov, V.B.; Shumsky, A.N.; Makhova, N.N.; Trushkov, I.V. (3 + 3)-Annulation of Donor–Acceptor Cyclopropanes with Diaziridines. Angew. Chem. Int. Ed. 2018, 57, 10338–10342. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, M.; Yang, G.; Chai, Z. Sc(OTf)3-Catalyzed Chemodivergent Annulations of γ-Butyrolactone-Fused Cyclopropanes with Anthranils. J. Org. Chem. 2020, 85, 430–440. [Google Scholar] [CrossRef]
- Augustin, A.U.; Merz, J.L.; Jones, P.G.; Mloston, G.; Werz, D.B. (4 + 3)-Cycloaddition of Donor–Acceptor Cyclopropanes with Thiochalcones: A Diastereoselective Access to Tetrahydrothiepines. Org. Lett. 2019, 21, 9405–9409. [Google Scholar] [CrossRef]
- Chagarovskiy, A.O.; Ivanova, O.A.; Budynina, E.M.; Kolychev, E.L.; Nechaev, M.S.; Trushkov, I.V.; Mel’nikov, M.Y. Reaction of donor-acceptor cyclopropanes with 1,3-diphenylisobenzofuran. Lewis acid effect on the reaction pathway. Russ. Chem. Bull. 2013, 62, 2407–2423. [Google Scholar] [CrossRef]
- Chagarovskiy, A.O.; Ivanova, O.A.; Rakhmankulov, E.R.; Budynina, E.M.; Trushkov, I.V.; Mel’nikov, M.Y. Lewis Acid-Catalyzed Isomerization of 2-Arylcyclopropane-1,1-dicarboxylates: A New Efficient Route to 2-Styrylmalonates. Adv. Synth. Catal. 2010, 352, 3179–3184. [Google Scholar] [CrossRef]
- Tsuruda, K.; Tokumoto, T.; Inoue, N.; Nakajima, M.; Nemoto, T. Synthesis of 7-Membered Ring Carbocycles via a Palladium-Catalyzed Intramolecular Allylic Alkylation–Isomerization–Cope Rearrangement Cascade. Eur. J. Org. Chem. 2018, 2018, 2836–2840. [Google Scholar] [CrossRef]
- Borisov, D.D.; Novikov, R.A.; Tomilov, Y.V. GaCl3-Mediated Reactions of Donor–Acceptor Cyclopropanes with Aromatic Aldehydes. Angew. Chem. Int. Ed. 2016, 55, 12233–12237. [Google Scholar] [CrossRef] [PubMed]
- Novikov, R.A.; Korolev, V.A.; Timofeev, V.P.; Tomilov, Y.V. New dimerization and cascade oligomerization reactions of dimethyl 2-phenylcyclopropan-1,1-dicarboxylate catalyzed by Lewis acids. Tetrahedron Lett. 2011, 52, 4996–4999. [Google Scholar] [CrossRef]
- Novikov, R.A.; Tarasova, A.V.; Tomilov, Y.V. GaGl3-Mediated Isomerization of Donor–Acceptor Cyclopropanes into (2-Arylalkylidene)malonates. Synlett 2016, 27, 1367–1370. [Google Scholar] [CrossRef]
- Ivanova, O.A.; Budynina, E.M.; Skvortsov, D.A.; Limoge, M.; Bakin, A.V.; Chagarovskiy, A.O.; Trushkov, I.V.; Mel’nikov, M.Y. A bioinspired route to indanes and cyclopentannulated hetarenes via (3+2)-cyclodimerization of donor-acceptor cyclopropanes. Chem. Commun. 2013, 49, 11482–11484. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Liu, J.; Yu, J.; Chen, L.; Zhang, C.; Wang, L. AlCl3-Promoted Formal [2 + 3]-Cycloaddition of 1,1-Cyclopropane Diesters with N-Benzylic Sulfonamides To Construct Highly Stereoselective Indane Derivatives. Org. Lett. 2014, 16, 1856–1859. [Google Scholar] [CrossRef]
- Ivanova, O.A.; Budynina, E.M.; Skvortsov, D.A.; Trushkov, I.V.; Mel’nikov, M.Y. Shortcut Approach to Cyclopenta[b]indoles by [3 + 2] Cyclodimerization of Indole-Derived Cyclopropanes. Synlett 2014, 25, 2289–2292. [Google Scholar] [CrossRef]
- Dousset, M.; Parrain, J.-L.; Chouraqui, G. Intriguing Elecgtrophilic Reactivity of Donor–Acceptor Cyclopropanes: Experimental and Theoretical Studies. Eur. J. Org. Chem. 2017, 2017, 5238–5245. [Google Scholar] [CrossRef]
- Verma, K.; Banerjee, P. Synthesis of Indenopyridine Derivatives via MgI2-Promoted [2 + 4] Cycloaddition Reaction of In-situ Generated 2-Styrylmalonate from Donor–Acceptor Cyclopropanes and Chalconimines. Adv. Synth. Catal. 2018, 360, 3687–3692. [Google Scholar] [CrossRef]
- Budynina, E.M.; Ivanov, K.L.; Chagarovskiy, A.O.; Rybakov, V.B.; Trushkov, I.V.; Mel’nikov, M.Y. From Umpolung to Alternation: Modified Reactivity of Donor–Acceptor Cyclopropanes Towards Nucleophiles in Reaction with Nitroalkanes. Chem. Eur. J. 2016, 22, 3692–3696. [Google Scholar] [CrossRef]
- Zotova, M.A.; Novikov, R.A.; Shulishov, E.V.; Tomilov, Y.V. GaGl3-Mediated “Inverted” Formal [3 + 2]-Cycloaddition of Donor–Acceptor Cyclopropanes to Allylic Systems. J. Org. Chem. 2018, 83, 8193–8207. [Google Scholar] [CrossRef]
- Ivanova, O.A.; Andronov, V.A.; Vasin, V.S.; Shumsky, A.N.; Rybakov, V.B.; Voskressensky, L.G.; Trushkov, I.V. Expanding the Reactivity of Donor–Acceptor Cyclopropanes: Synthesis of Benzannulated Five-Membered Heterocycles via Intramolecular Attack of a Pendant Nucleophilic Group. Org. Lett. 2018, 20, 7947–7952. [Google Scholar] [CrossRef] [PubMed]
- Novikov, R.A.; Borisov, D.D.; Tarasova, A.V.; Tkachev, Y.V.; Tomilov, Y.V. Three-Component Gallium(III)-Promoted Addition of Halide Anions and Acetylenes to Donor–Acceptor Cyclopropanes. Angew. Chem. Int. Ed. 2018, 57, 10293–10298. [Google Scholar] [CrossRef] [PubMed]
- Borisova, I.A.; Tarasova, A.V.; Potapov, K.V.; Novikov, R.A.; Tomilov, Y.V. Reactions of donor-acceptor cyclopropanes or benzylidenemalonate with benzyl azide by generating gallium trichloride 1,2-zwitterionic complexes. Russ. Chem. Bull. 2019, 68, 1504–1509. [Google Scholar] [CrossRef]
- Zotova, M.A.; Novikov, R.A.; Volodin, A.D.; Korlyukov, A.A.; Tkachev, Y.V.; Korolev, V.A.; Tomilov, Y.V. Four-Membered Cycle Formation Challenge: GaCl3-Promoted Formal [2 + 2]-Cycloaddition of Donor–Acceptor Cyclopropanes to Bicyclobutylidene. Eur. J. Org. Chem. 2019, 2019, 4207–4214. [Google Scholar] [CrossRef]
- Belaya, M.A.; Knyazev, D.A.; Novikov, R.A.; Tomilov, Y.V. “Diels-Alder reaction” in the ionic version: GaCl3-promoted formation of substituted cyclohexenes from donor-acceptor cyclopropanes and dienes. Tetrahedron Lett. 2020, 61, 151990. [Google Scholar] [CrossRef]
- Smith, R.J.; Nhu, D.; Clark, M.R.; Gai, S.; Lucas, N.T.; Hawkins, B.C. Synthesis of Chromones from 1,1-Diacylcyclopropanes: Toward the Synthesis of Bromophycoic Acid E. J. Org. Chem. 2017, 82, 5317–5327. [Google Scholar] [CrossRef] [PubMed]
- Dawande, S.G.; Harode, M.; Kalepu, J.; Katukojvala, S. Ag(I)-catalyzed intramolecular transannulation of enynone tethered donor–acceptor cyclopropanes: A new synthesis of 2,3-dihydronaphtho [1,2-b]furans. Chem. Commun. 2016, 52, 13699–13701. [Google Scholar] [CrossRef]
- Kumar, P.; Dey, R.; Banerjee, P. Exploitation of Cyclopropane Carbaldehydes to Prins Cyclization: Quick Access to (E)-Hexahydrooxonine and Octahydrocyclopenta[b]furan. Org. Lett. 2018, 20, 5163–5166. [Google Scholar] [CrossRef]
- Novikov, R.A.; Tarasova, A.V.; Denisov, D.A.; Borisov, D.D.; Korolev, V.A.; Timofeev, V.P.; Tomilov, Y.V. [4 + 2] Annulation of Donor–Acceptor Cyclopropanes with Acetylenes Using 1,2-Zwitterionic Reactivity. J. Org. Chem. 2017, 82, 2724–2739. [Google Scholar] [CrossRef]
- Ivanov, K.L.; Bezzubov, S.I.; Melnikov, M.Y.; Budynina, E.M. Donor-acceptor cyclopropanes as ortho-quinone methide equivalents in formal (4 + 2)-cycloaddition to alkenes. Org. Biomol. Chem. 2018, 16, 3897–3909. [Google Scholar] [CrossRef]
- Ivanova, O.A.; Andronov, V.A.; Levina, I.I.; Chagarovskiy, A.O.; Voskressensky, L.G.; Trushkov, I.V. Convenient Synthesis of Functionalized Cyclopropa[c]coumarin-1a-carboxylates. Molecules 2019, 24, 57. [Google Scholar] [CrossRef] [PubMed]
- Fuerst, D.E.; Stoltz, B.M.; Wood, J.L. Synthesis of C(3) Benzofuran-Derived Bisaryl Quaternary Centers: Approaches to Diazonamide, A. Org. Lett. 2000, 2, 3521–3523. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, L.-Q.; Chen, J.; Takaki, K.; Johnson, G.; Iben, L.; Mahle, C.D.; Ryan, E.; Xu, C. Design and synthesis of benzoxazole derivatives as novel melatoninergic ligands. Bioorg. Med. Chem. Lett. 2004, 14, 1197–1200. [Google Scholar] [CrossRef] [PubMed]
- Terauchi, T.; Takemura, A.; Doko, T.; Yoshida, Y.; Tanaka, T.; Sorimachi, K.; Naoe, Y.; Beuckmann, C.; Kazuta, Y. Cyclopropane Compound. U.S. Patent 2012/0095031A1, 19 April 2012. [Google Scholar]
- Banothu, J.; Basavoju, S.; Bavantula, R. Pyridinium Ylide Assisted Highly Stereoselective One-Pot Synthesis of trans-2-(4-Chlorobenzoyl)-3-aryl-spiro[cyclopropane-1,2′-inden]-1′,3′-diones and Their Antimocrobial and Nematicidal Activities. J. Heterocycl. Chem. 2015, 52, 853–860. [Google Scholar] [CrossRef]
- Bravo, P.; Fronza, G.; Ticozzi, C. Oxygen Heterocycles by Sulphur Ylide Annulation. The Reaction of Dimethylsulphoxonium Methylide on ortho-Hydroxybenzal Ketones: A Case of Annulation Leading to a Mixture of Unexpected Oxygen Heterocycles. Gazz. Chim. Ital. 1984, 114, 93–102. [Google Scholar]
- Murphy, W.S.; Wattanasin, S. Reactions of Aryl Cyclopropyl Ketones. A New Synthesis of Aryl Tetralones. J. Chem. Soc. Perkin Trans. 1 1981, 2920–2926. [Google Scholar] [CrossRef]
- Bravo, P.; Ticozzi, C. Oxygen Heterocycles by Sulphur Ylide Annulation: Reaction of o-Hydroxybenzalketones with Dimethyloxosulphonium Methylide. Heterocycles 1981, 16, 713–716. [Google Scholar] [CrossRef]
- Bravo, P.; Fronza, G.; Maggi, D.; Ticozzi, C. 2-(β-Hydroxy-β-methylsulfinylmethyl)-alkyl-2,3-dihydro-1-benzofurans by Addition of the Whole Molecules of Dimethyloxosulfonium Methylide to o-Hydroxybenzalketones. Tetrahedron Lett. 1977, 18, 1077–1078. [Google Scholar] [CrossRef]
- Donelly, J.A.; Fox, M.J.; Hoey, J.G. Cyclopropyl Epoxides. Reactions of Some αβ-Dibromoketones with Dimethyloxosulfoninum Methylide. J. Chem. Soc. Perkin Trans. 1 1979, 2629–2633. [Google Scholar] [CrossRef]
- Gil, A.; Pabon, A.; Galiano, S.; Burguete, A.; Perez-Silanes, S.; Deharo, E.; Monge, A.; Aldana, I. Synthesis, Biological Evaluation and Structure-Activity Relationships of New Quinoxaline Derivatives as Anti-Plasmodium falciparum Agents. Molecules 2014, 19, 2166–2180. [Google Scholar] [CrossRef]
- Paxton, R.J.; Taylor, R.J.K. Improved Dimethylsulfoxonim Methylide Cyclopropanation Procedures, Including a Tandem Oxidation Variant. Synlett 2007, 633–637. [Google Scholar] [CrossRef]
- Bastiansen, O.; Fritsch, F.N.; Hedberg, K. Least-Squares Refinement of Molecular Structures from Gaseous Electron-Diffraction Sector Microphotometer Data. III. Refinement of Cyclopropane. Acta Cryst. 1964, 17, 538–543. [Google Scholar] [CrossRef]
- Syam, S.; Abdelwahab, S.I.; Al-Mamary, M.A.; Mohan, S. Synthesis of Chalcones with Anticancer Activities. Molecules 2012, 17, 6179–6195. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Fan, L.; Ren, T.; Zheng, C.; Tao, Q.; Wu, A.; She, N. Synthesis of functionalized 2-aryl-4-(indol-3-yl)-4H-chromenes via iodine-catalyzed domino Michael addition–intramolecular cyclization reaction. Org. Biomol Chem. 2012, 10, 8877–8883. [Google Scholar] [CrossRef] [PubMed]
- Kadayat, T.M.; Kim, M.J.; Nam, T.; Park, P.-H.; Lee, E.-S. Thieny/Furanyl-hydroxyphenylpropenones as Inhibitors of LPS-induced ROS and NO Production in RAW 264.7 Marcophages, and Their Structure-Activity Relationship Study. Bull. Korean Chem. Soc. 2014, 35, 2481–2486. [Google Scholar] [CrossRef][Green Version]
- Lee, Y.T.; Jang, Y.-J.; Syu, S.; Chou, S.-C.; Lee, C.-J.; Lin, W. Preparation of functional benzofurans and indoles via chemoselective intramolecular Wittig reactions. Chem. Commun. 2012, 48, 8135–8137. [Google Scholar] [CrossRef]
- Saha, P.; Biswas, A.; Molleti, N.; Singh, V.K. Enantioselective Synthesis of Highly Substituted Chromans via the oxa-Michael–Michael Cascade Reaction with a Bifunctional Catalyst. J. Org. Chem. 2015, 80, 11115–11122. [Google Scholar] [CrossRef]
- Varano, F.; Catarzi, D.; Colotta, V.; Cecchi, L.; Filacchioni, G.; Galli, A.; Costagli, C. Structure-Activity Relationship Studies of Novel Pyrazolo[1,5-c][1,3]benzoxazines: Synthesis and Benzodiazepine Receptor Affinity. Arch. Pharm. 1996, 329, 529–534. [Google Scholar] [CrossRef]
- Kalogiros, C.; Hadjiarapoglu, L.P. Facile preparaton of bicyclo[2.2.2]octenone derivatives via Diels-Alder cycloadditions of in situ-generated masked o-benzoquinones. Tetrahedron 2011, 67, 3216–3225. [Google Scholar] [CrossRef]
- Tatsuzaki, J.; Bastow, K.F.; Nakagawa-Goto, K.; Nakamura, S.; Itokawa, H.; Lee, K.-H. Dehydrozingerone, Chalcone, and Isoeugenol Analogues as in Vitro Anticancer Agents. J. Nat. Prod. 2006, 69, 1445–1449. [Google Scholar] [CrossRef]
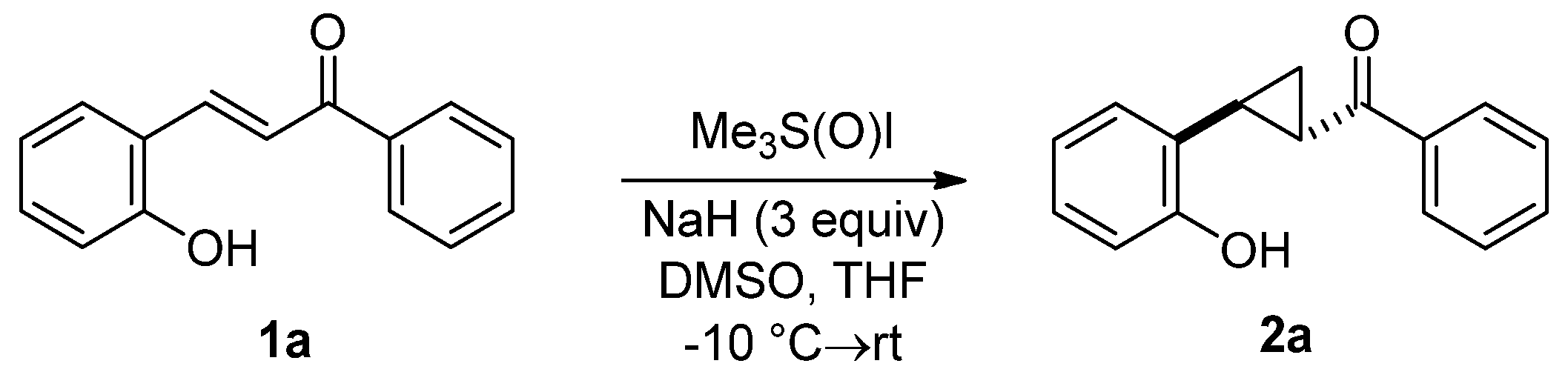
Sample Availability: Samples of the compounds are not available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fadeev, A.A.; Chagarovskiy, A.O.; Makarov, A.S.; Levina, I.I.; Ivanova, O.A.; Uchuskin, M.G.; Trushkov, I.V. Synthesis of (Het)aryl 2-(2-hydroxyaryl)cyclopropyl Ketones. Molecules 2020, 25, 5748. https://doi.org/10.3390/molecules25235748
Fadeev AA, Chagarovskiy AO, Makarov AS, Levina II, Ivanova OA, Uchuskin MG, Trushkov IV. Synthesis of (Het)aryl 2-(2-hydroxyaryl)cyclopropyl Ketones. Molecules. 2020; 25(23):5748. https://doi.org/10.3390/molecules25235748
Chicago/Turabian StyleFadeev, Alexander A., Alexey O. Chagarovskiy, Anton S. Makarov, Irina I. Levina, Olga A. Ivanova, Maxim G. Uchuskin, and Igor V. Trushkov. 2020. "Synthesis of (Het)aryl 2-(2-hydroxyaryl)cyclopropyl Ketones" Molecules 25, no. 23: 5748. https://doi.org/10.3390/molecules25235748
APA StyleFadeev, A. A., Chagarovskiy, A. O., Makarov, A. S., Levina, I. I., Ivanova, O. A., Uchuskin, M. G., & Trushkov, I. V. (2020). Synthesis of (Het)aryl 2-(2-hydroxyaryl)cyclopropyl Ketones. Molecules, 25(23), 5748. https://doi.org/10.3390/molecules25235748







