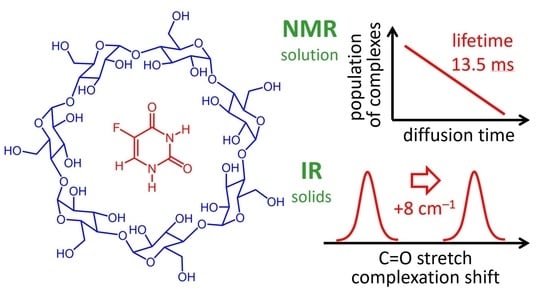
On Complex Formation between 5-Fluorouracil and β-Cyclodextrin in Solution and in the Solid State: IR Markers and Detection of Short-Lived Complexes by Diffusion NMR
Abstract
1. Introduction
- (a)
- NMR spectroscopy (standard 1D and rotating-frame nuclear Overhauser effect spectroscopy (ROESY) NMR, PFG NMR in aqueous solution at room temperature and neutral pH);
- (b)
- IR spectroscopy (ATR for the solid-state mixtures of 5-FU and β-CD prepared by physical mixing, kneading and co-precipitation);
- (c)
- Density Functional Theory (DFT) calculations (gas phase; structure optimization and theoretical IR spectra in the gas phase).
2. Results and Discussion
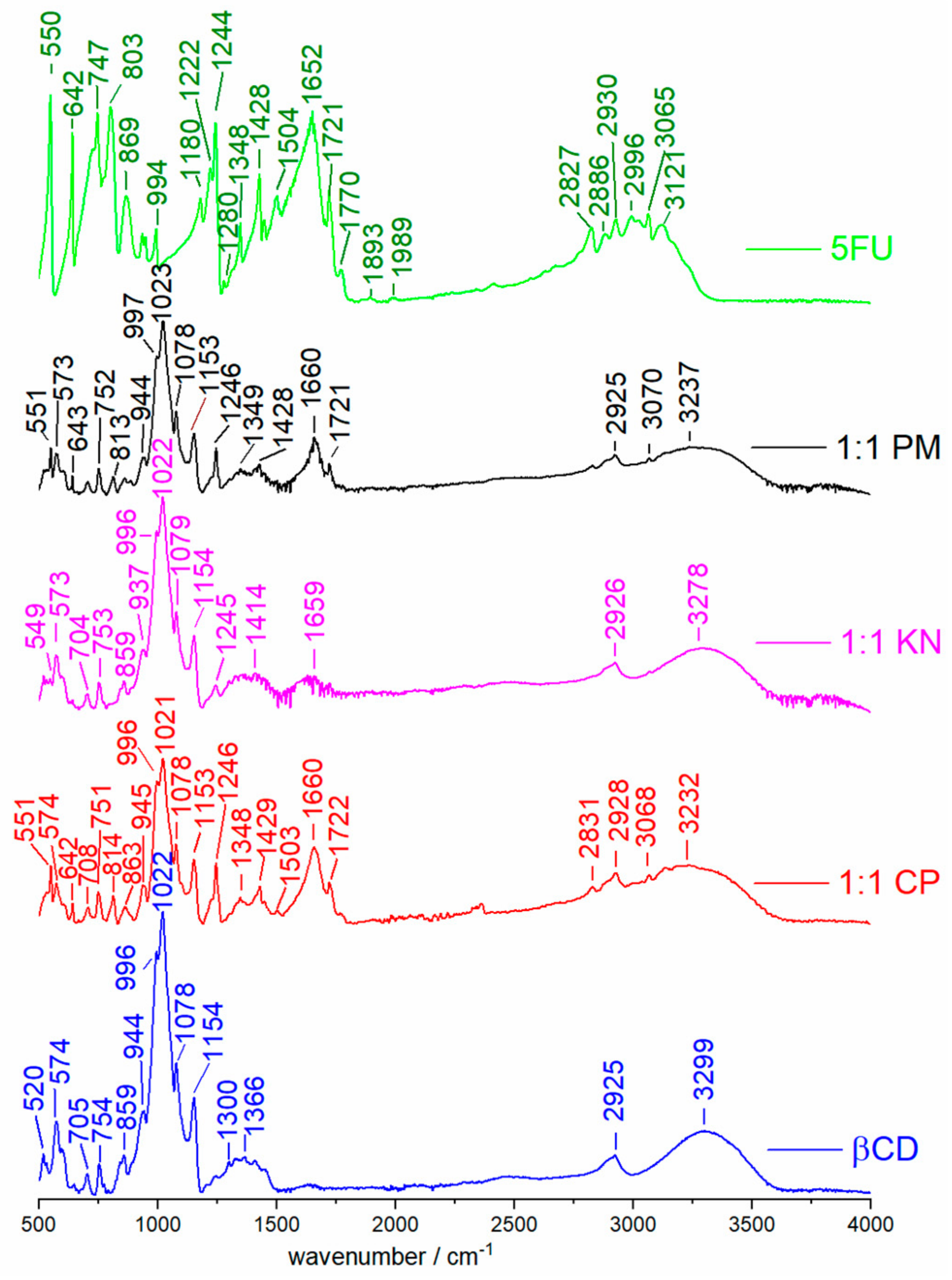
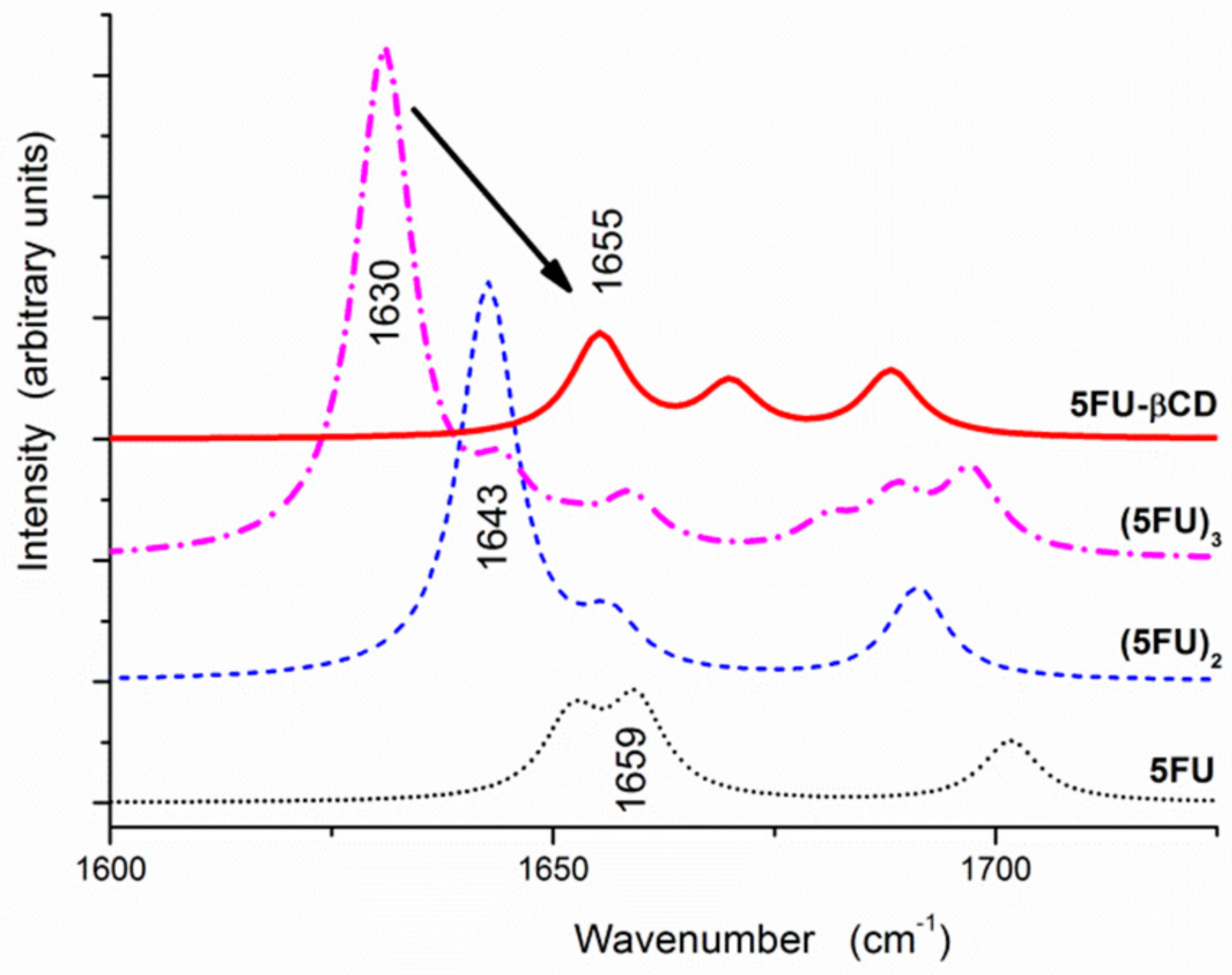
2.1. ATR Spectra
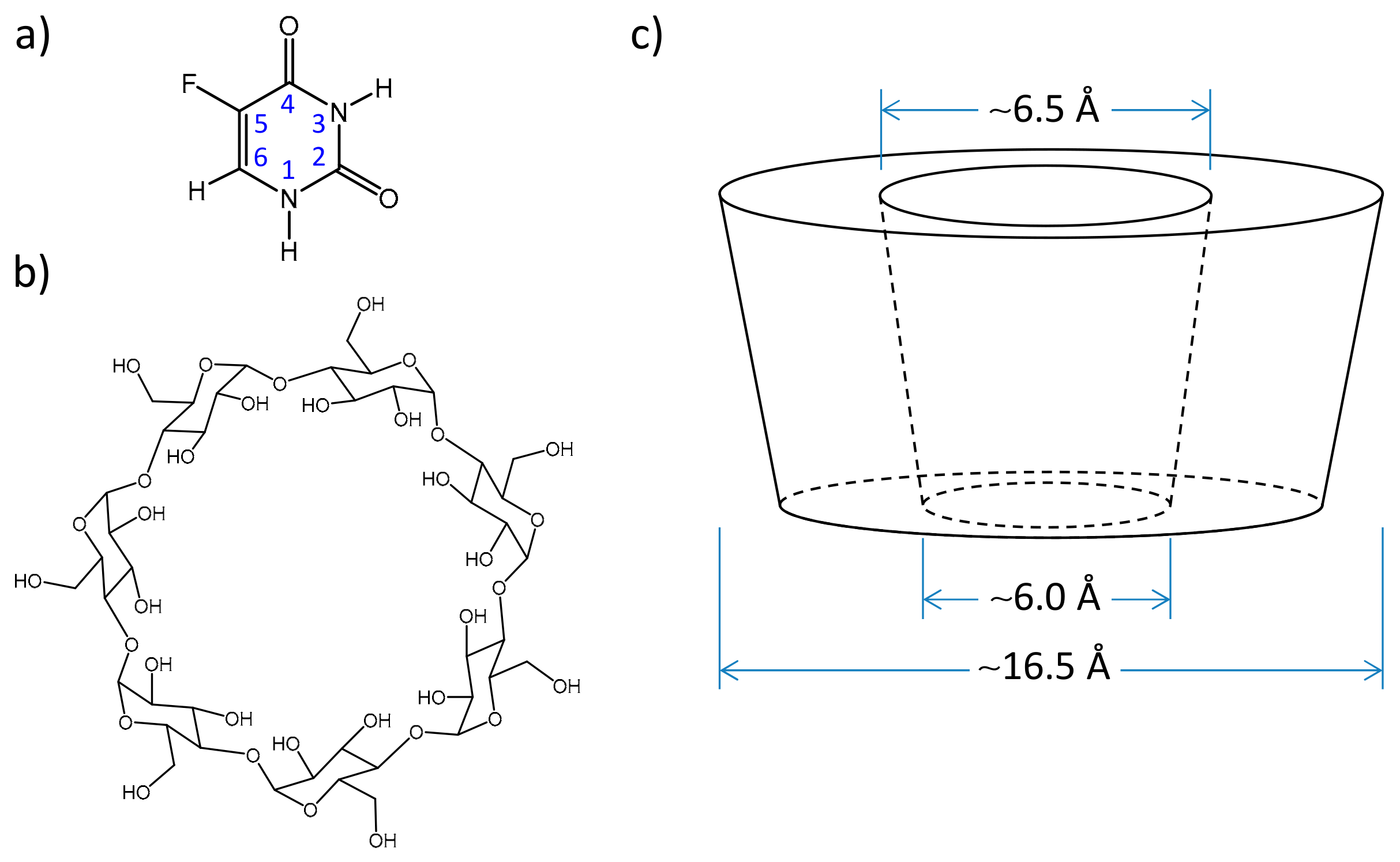
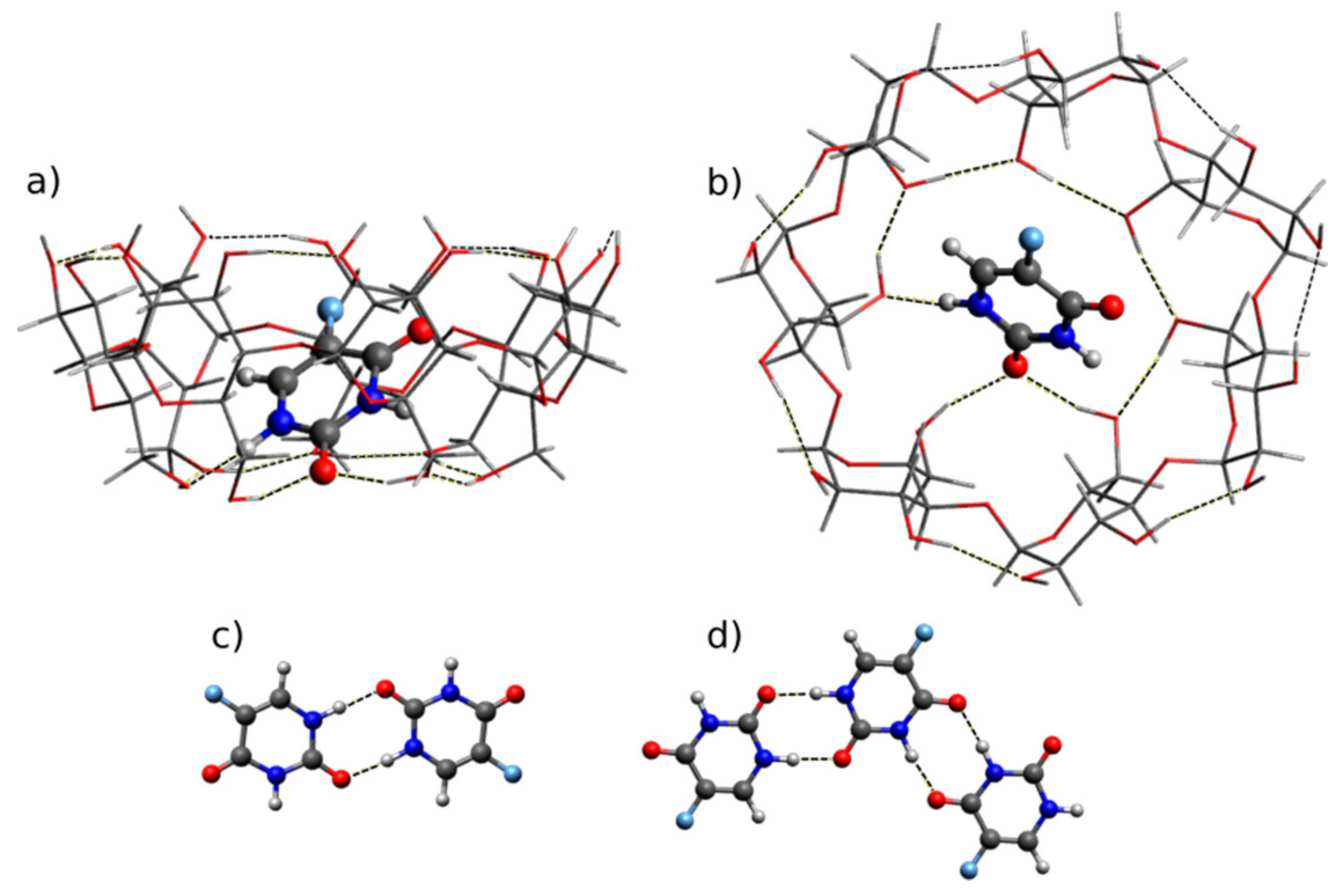
2.2. Quantum Chemical Calculations: Geometry
2.3. Quantum Chemical Calculations: Predicted IR Spectra
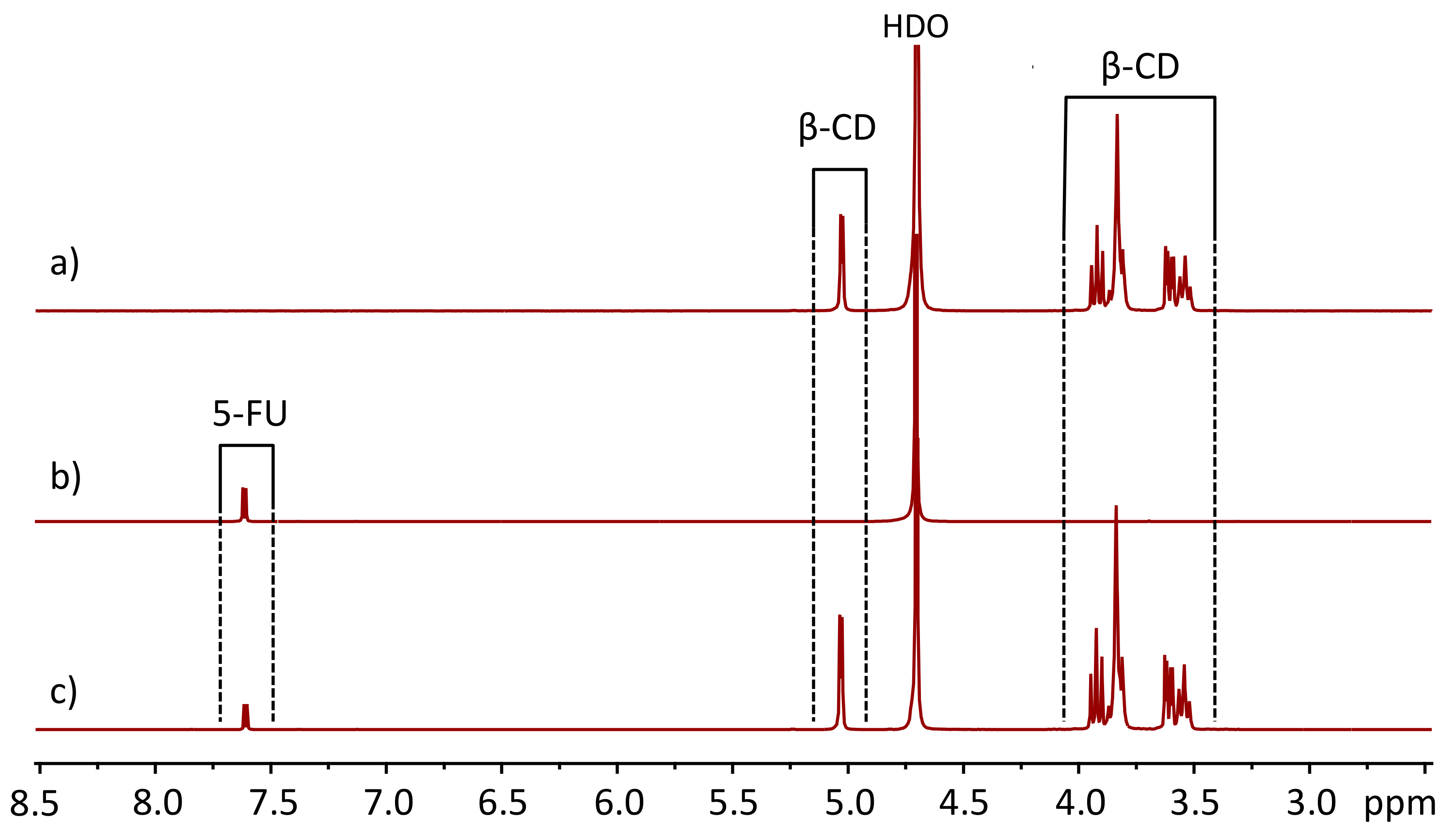
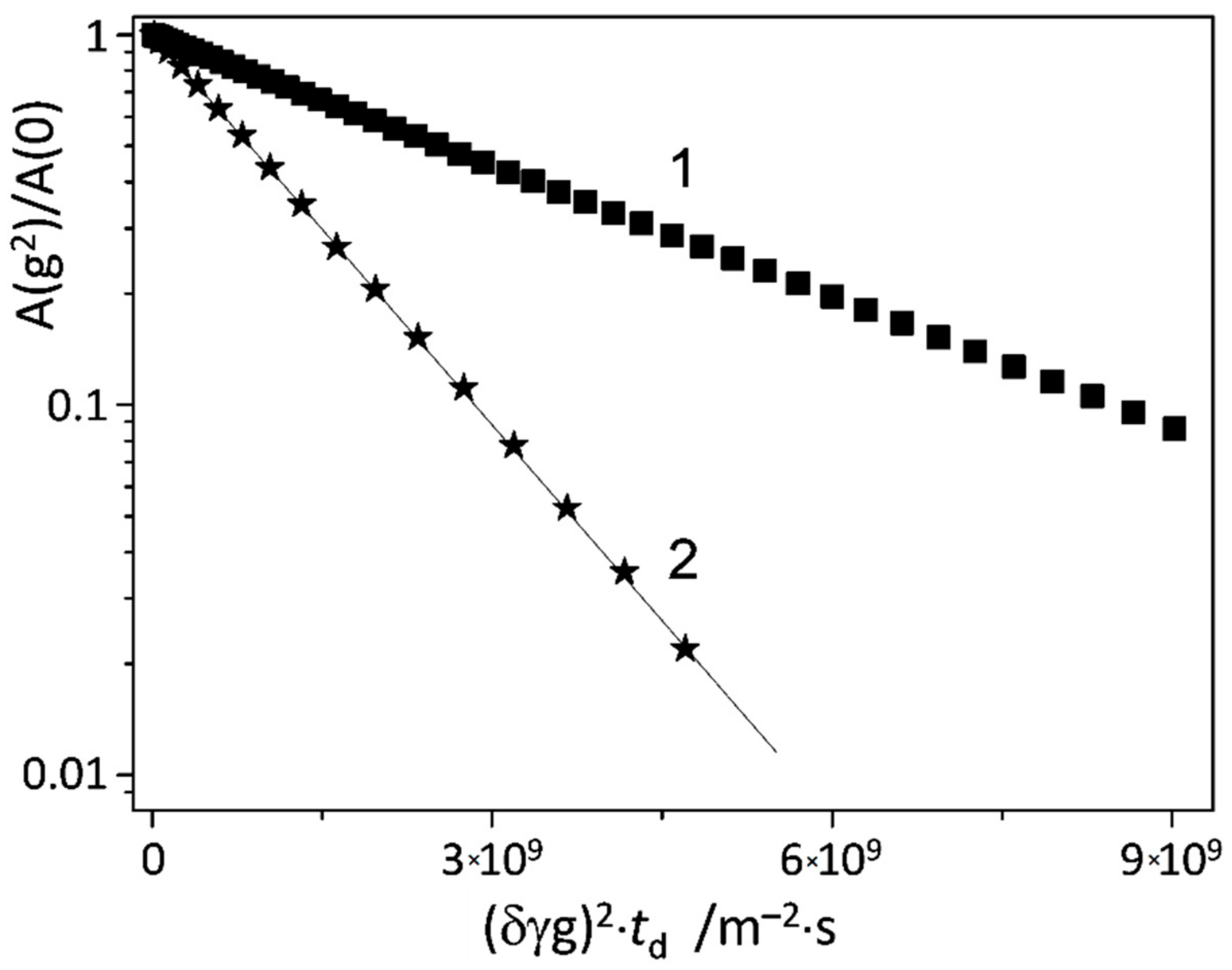
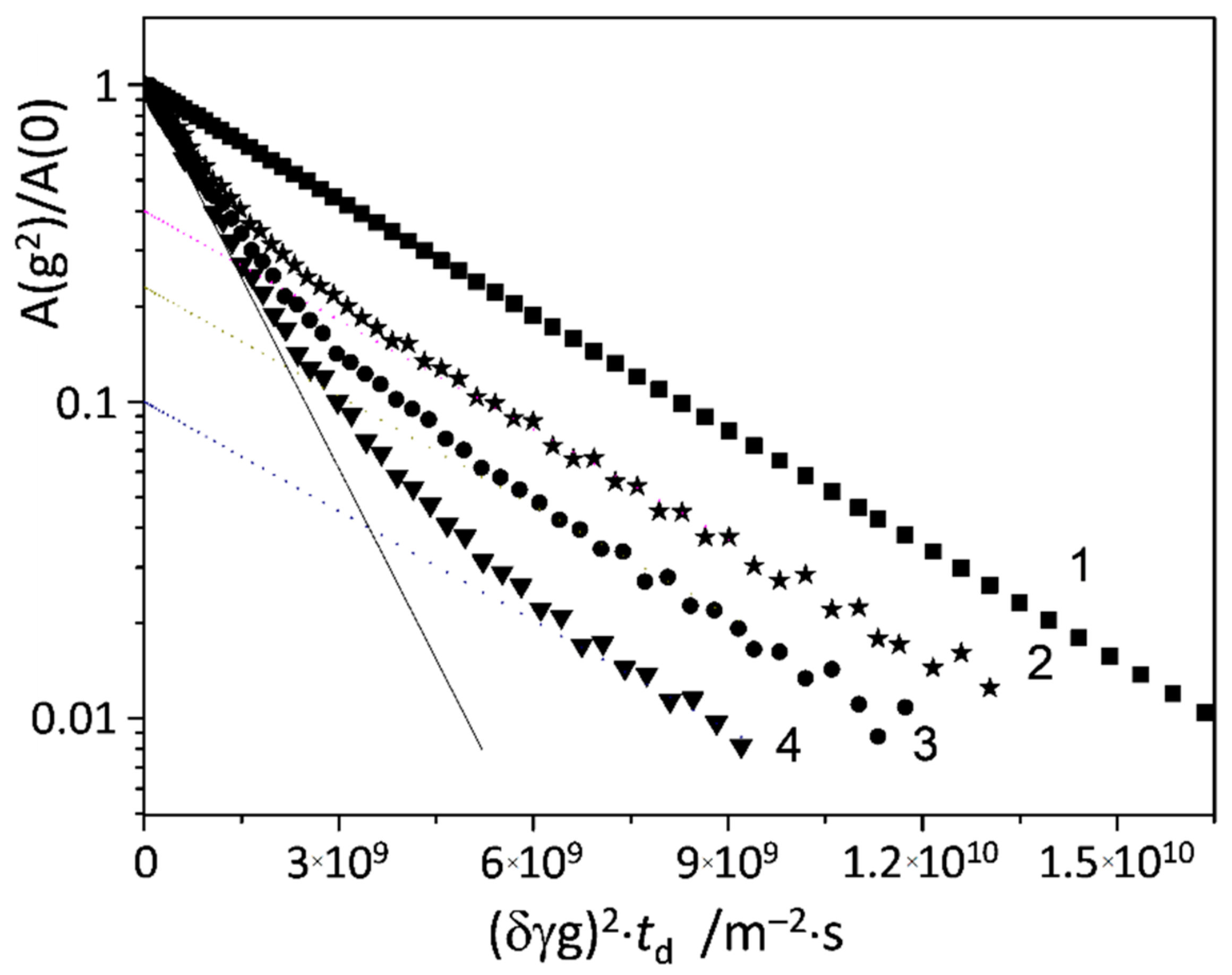
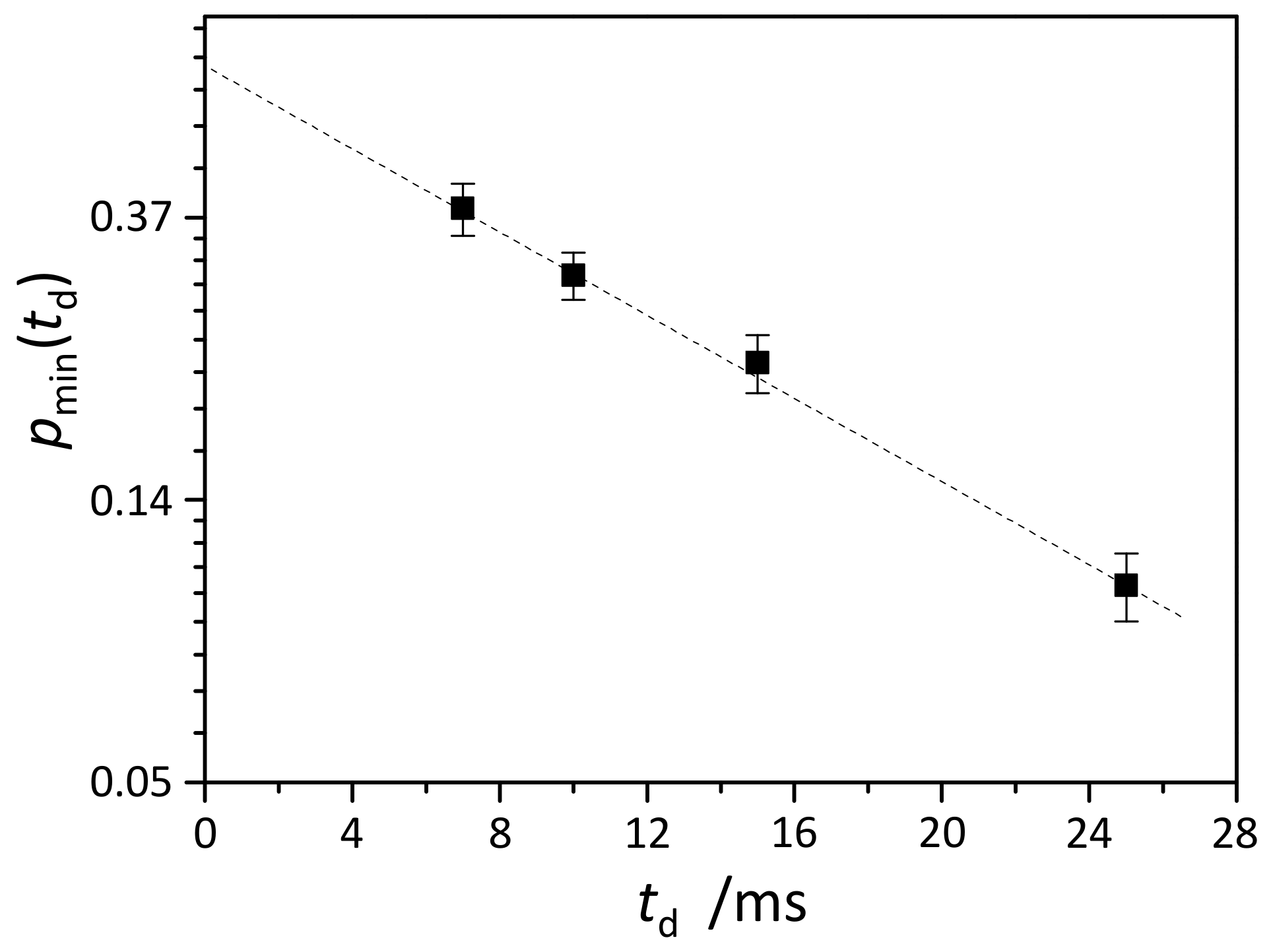
2.4. NMR Measurements
3. Experimental Methods
3.1. Sample Preparation for Solution-State (High-Resolution) NMR Experiments
3.2. Sample Preparation for Solid-State IR Experiments
3.3. Physical Mixture (PM)
3.4. Kneading Method (KN)
3.5. Co-precipitation Method (CP)
3.6. Attenuated Total Reflectance (ATR) Analysis
3.7. Computational Methods
3.8. 1D and 2D NMR experiments
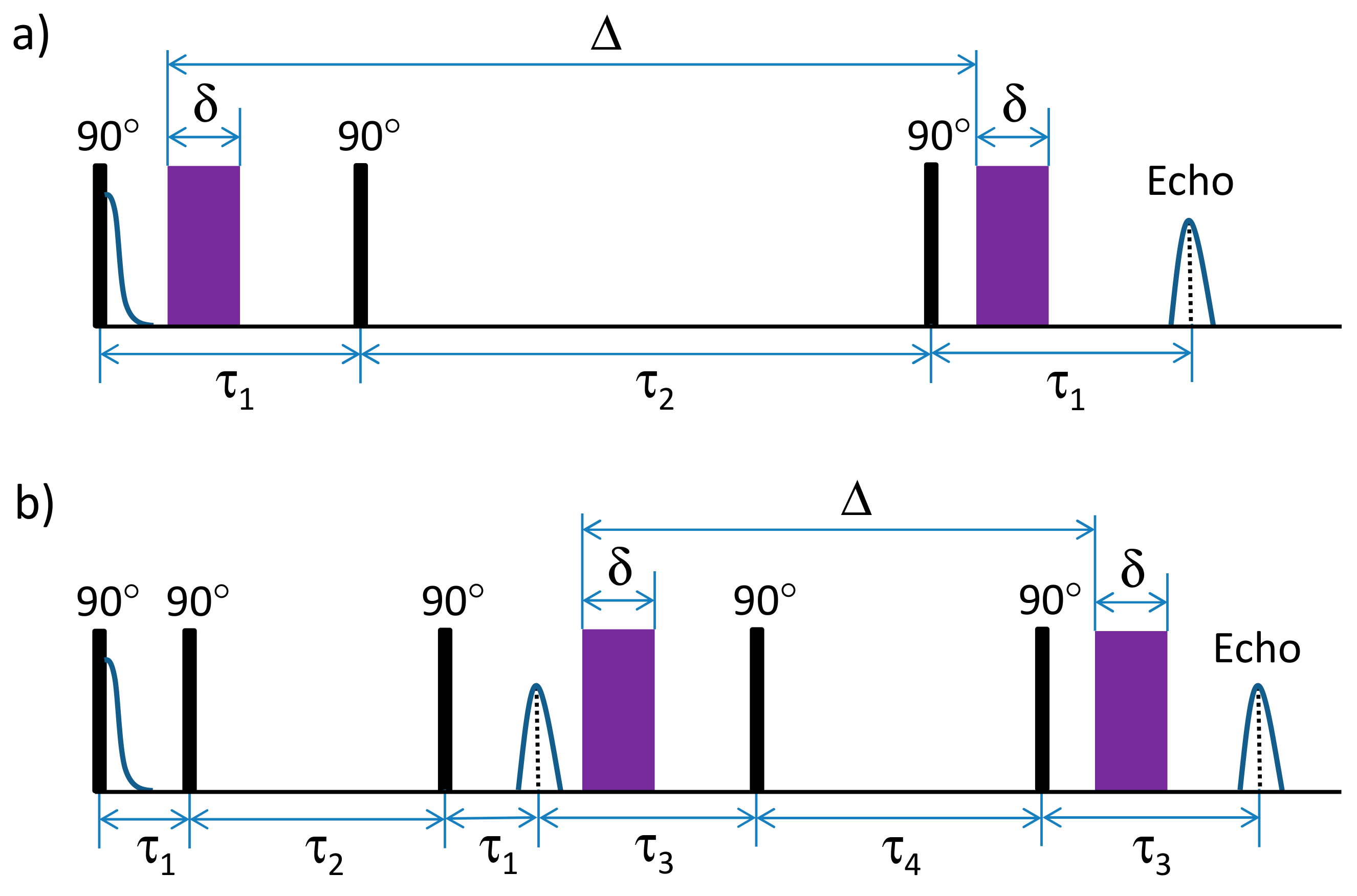
3.9. Diffusion NMR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Noordhuis, P.; Holwerda, U.; Van der Wilt, C.L.; Van Groeningen, C.J.; Smid, K.; Meijer, S.; Pinedo, H.M.; Peters, G.J. 5-Fluorouracil incorporation into RNA and DNA in relation to thymidylate synthase inhibition of human colorectal cancers. Ann. Oncol. 2004, 15, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- He, L.; Zhu, H.; Zhou, S.; Wu, T.; Wu, H.; Yang, H.; Mao, H.; SekharKathera, C.; Janardhan, A.; Edick, A.M.; et al. Wnt pathway is involved in 5-FU drug resistance of colorectal cancer cells. Exp. Mol. Med. 2018, 50, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sravanthi, V.; Mayure, V.K.; Finny, G.J.; Meher, C.P. Anti-cancer (Novel Agents): A review. Pharma Tutor 2014, 2, 119–153. [Google Scholar]
- Theilen, G.H. Veterinary Cancer Medicine Subsequent Edition; Madewell, B.R., Ed.; Lea and Febiger: Philadelphia, PH, USA, 1987. [Google Scholar]
- Wu, Y.; Deng, Z.; Wang, H.; Ma, W.; Zhou, C.; Zhang, S. Repeated cycles of 5-fluorouracil chemotherapy impaired anti-tumor functions of cytotoxic T cells in a CT26 tumor-bearing mouse model. BMC Immunol. 2016, 17, 29. [Google Scholar] [CrossRef] [PubMed]
- Stalzer, R.C.; Kiely, J.M.; Pease, G.L.; Brown, A.L., Jr. Effect of 5-fluorouracil on human hematopoiesis. A morphologic study. Cancer 1965, 18, 1071–1078. [Google Scholar] [CrossRef]
- Kojima, E.; Tsuboi, A. Effects of 5-fluorouracil on hematopoietic stem cells in normal and irradiated mice. J. Radiat. Res. 1992, 33, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Fraile, R.J.; Baker, L.H.; Buroker, T.R.; Horwitz, J.; Vaitkevicius, V.K. Pharmacokinetics of 5-fluorouracil administered orally, by rapid intravenous and by slow infusion. Cancer Res. 1980, 40, 2223–2228. [Google Scholar]
- Bruckner, H.W.; Motwani, B.T. Chemotherapy of advanced cancer of the colon and rectum. Semin. Oncol. 1991, 18, 443–461. [Google Scholar]
- Schilsky, R.L. Biochemical and clinical pharmacology of 5-fluorouracil. Oncology 1998, 12, 13–18. [Google Scholar]
- Milone, M.C. Pharmacogenomics Issues. In Therapeutic Drug Monitoring of Selected Anticancer Drugs; Dasgupta, A., Ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 291–321. [Google Scholar]
- Zhang, J.; Lan, C.Q.; Post, M.; Simard, B.; Deslandes, Y.; Hsieh, T.H. Design of nanoparticles as drug carriers for cancer therapy. Cancer Genom. Proteom. 2006, 3, 147–157. [Google Scholar]
- Yoshikawa, R.; Kusunoki, M.; Yanagi, H.; Noda, M.; Furuyama, J.-I.; Yamamura, T.; Tomoko, H.-T. Dual Antitumor Effects of 5-fluorouracil on the cell cycle in colorectal carcinoma cells: A novel target mechanism concept for pharmacokinetic modulating chemotherapy. Cancer Res. 2001, 61, 1029–1037. [Google Scholar] [PubMed]
- De Gramont, A.; Vignoud, J.; Tournigand, C.; Louvet, C.; André, T.; Varette, C.; Raymond, E.; Moreau, S.; Le Bail, N.; Krulik, M. Oxaliplatin with high-dose leucovorin and 5-fluorouracil 48-h continuous infusion in pretreated metastatic colorectal cancer. Eur. J. Cancer 1997, 33, 214–219. [Google Scholar] [CrossRef]
- Arkenau, H.T.; Bermann, A.; Rettig, K.; Strohmeyer, G.; Porschen, R. 5-Fluorouracil plus leucovorin is an effective adjuvant chemotherapy in curatively resected stage III colon cancer: Long-term follow-up results of the adjCCA-01 trial. Ann. Oncol. 2003, 14, 395–399. [Google Scholar] [CrossRef]
- Suzuki, R.; Kang, Y.A.; Li, X.; Roife, D.; Zhang, R.; Fleming, J.B. Genistein potentiates the antitumor effect of 5-Fluorouracil by inducing apoptosis and autophagy in human pancreatic cancer cells. Anticancer Res. 2014, 34, 4685–4692. [Google Scholar]
- Buur, A.; Bundgaard, H.; Falch, E. Prodrugs of 5-fluorouracil. IV. Hydrolysis kinetics, bioactivation and physicochemical properties of various N-acyloxymethyl derivatives of 5-fluorouracil. Int. J. Pharmaceut. 1985, 24, 43–60. [Google Scholar] [CrossRef]
- Lemaire, L.; Malet-Martino, M.C.; de Forni, M.; Martino, R.; Lasserre, B. Cardiotoxicity of commercial 5-fluorouracil vials stems from the alkaline hydrolysis of this drug. Brit. J. Cancer 1992, 66, 119–127. [Google Scholar] [CrossRef]
- Rzepiela, K.; Buczek, A.; Kupka, T.; Broda, M.A. Factors governing the chemical stability and NMR parameters of uracil tautomers and Its 5-halogen derivatives. Molecules 2020, 25, 3931. [Google Scholar] [CrossRef]
- Scanlan, M.J.; Hillier, I.H. An ab initio study of tautomerism of uracil, thymine, 5-fluorouracil, and cytosine. J. Am. Chem. Soc. 1984, 106, 3737–3745. [Google Scholar] [CrossRef]
- Rastogi, V.; Palafox, M. Vibrational spectra, tautomerism and thermodynamics of anticarcinogenic drug: 5-Fluorouracil. Spectrochim. Acta A 2011, 79, 970–977. [Google Scholar] [CrossRef]
- Markova, N.; Enchev, V.; Timtcheva, I. Oxo-hydroxy tautomerism of 5-fluorouracil: Water-assisted proton transfer. J. Phys. Chem. A 2005, 109, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Crampton, K.T.; Rathur, A.I.; Nei, Y.W.; Berden, G.; Oomens, J.; Rodgers, M.T. Protonation preferentially stabilizes minor tautomers of the halouracils: IRMPD action spectroscopy and theoretical studies. J. Am. Soc. Mass. Spectr. 2012, 23, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Jangir, D.K.; Charak, S.; Mehrotra, R.; Kundu, S. FTIR and circular dichroism spectroscopic study of interaction of 5-fluorouracil with DNA. J. Photochem. Photobiol. B Biol. 2011, 105, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Sharma, R.; Banerjee, U.C. Biotechnological applications of cyclodextrins. Biotechnol. Adv. 2002, 20, 341–359. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J. Pharm. Sci. 1996, 85, 1017–1025. [Google Scholar] [CrossRef]
- Steed, J.W.; Atwood, J.L. Supramolecular Chemistry; John Wiley and Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Chen, G.; Jiang, M. Cyclodextrin-based inclusion complexation bridging supramolecular chemistry and macromolecular self-assembly. Chem. Soc. Rev. 2011, 40, 2254–2266. [Google Scholar] [CrossRef]
- Van der Veen, B.A.; van Alebeek, G.J.; Uitdehaag, J.C.; Dijkstra, B.W.; Dijkhuizen, L. The three transglycosylation reactions catalyzed by cyclodextrin glycosyltransferase from Bacillus circulans (strain 251) proceed via different kinetic mechanisms. Eur. J. Biochem. 2000. [Google Scholar] [CrossRef]
- Connors, K.A. The stability of cyclodextrin complexes in solution. Chem. Rev. 1997, 97, 1325–1358. [Google Scholar] [CrossRef]
- Gratz, S.; Gamble, B.; Stalcup, A. Inclusion complexation: Liquid chromatography. In Encyclopedia of Separation Science; Academic Press: Cambridge, MA, USA, 2000; pp. 3079–3086. [Google Scholar]
- Buvári-Barcza, A.; Rohonczy, J.; Rozlosnik, N.; Gilányi, T.; Szabo, B.; Lovas, G.; Braun, T.; Samu, J.; Barcza, L. Aqueous solubilization of [60] fullerene via inclusion complex formation and the hydration of C60. J. Chem. Soc. Perkin Trans. 2 2001, 2, 191–196. [Google Scholar] [CrossRef]
- Saenger, W. Cyclodextrin inclusion compounds in research and industry. Angew. Chem. Int. Ed. Eng. 1980, 19, 344–362. [Google Scholar] [CrossRef]
- Pereva, S.; Nikolova, V.; Angelova, S.; Spassov, T.; Dudev, T. Water inside β-cyclodextrin cavity: Amount, stability and mechanism of binding. Beilstein J. Org. Chem. 2019, 15, 1592–1600. [Google Scholar] [CrossRef] [PubMed]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of cyclodextrins and drug/cyclodextrin complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef] [PubMed]
- Buczek, A.; Staś, M.; Hebenstreit, C.; Maller, C.; Broda, M.A.; Kupka, T.; Kelterer, A.-M. Interaction of 5-fluorouracil with β-cyclodextrin: A density functional theory study with dispersion correction. Int. J. Quantum Chem. 2020. [Google Scholar] [CrossRef]
- Szejtli, J. Past, present, and future of cyclodextrin research. Pure Appl. Chem. 2004, 76, 1825–1845. [Google Scholar] [CrossRef]
- Martin Del Valle, E.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Schmidt, B.V.K.J.; Barner-Kowollik, C. Dynamic macromolecular material design—The versatility of cyclodextrin-based host–guest chemistry. Angew. Chem. Int. Ed. Engl. 2017, 56, 8350–8369. [Google Scholar] [CrossRef]
- Wojcik, J.F.; Rohrbach, R.P. Small anion binding to cycloamylose. Equilibrium constants. J. Phys. Chem. 1975, 79, 2251–2253. [Google Scholar] [CrossRef]
- Cramer, F.; Henglein, F.M. Einschlußverbindungen der Cyclodextrine mit Gasen. Angew. Chem. 1956, 68, 649. [Google Scholar] [CrossRef]
- Dodziuk, H. (Ed.) Cyclodextrins and Their Complexes: Chemistry, Analytical Methods, Applications, 1st ed.; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Dsouza, R.N.; Pischel, U.; Nau, W.M. Fluorescent dyes and their supramolecular host/guest complexes with macrocycles in aqueous solution. Chem. Rev. 2011, 111, 7941–7980. [Google Scholar] [CrossRef]
- Crupi, V.; Ficarra, R.; Guardo, M.; Majolino, D.; Stancanelli, R.; Venuti, V. UV–vis and FTIR–ATR spectroscopic techniques to study the inclusion complexes of genistein with β-cyclodextrins. J. Pharm. Biomed. Anal. 2007, 44, 110–117. [Google Scholar] [CrossRef]
- Li, J.; Jiang, Q.; Deng, P.; Chen, Q.; Yu, M.; Shang, J.; Li, W. The formation of a host-guest inclusion complex system between β-cyclodextrin and baicalin and its dissolution characteristics. J. Pharm. Pharmacol. 2017, 69, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Uekama, K.; Hirayama, F.; Nasu, S.; Matsuo, N.; Irie, T. Determination of the stability constants for inclusion complexes of cyclodextrins with various drug molecules by high performance liquid chromatography. Chem. Pharm. Bull. 1978, 26, 3477–3484. [Google Scholar] [CrossRef][Green Version]
- Lucarini, M.; Luppi, B.; Pedulli, G.F.; Roberts, B.P. Dynamic aspects of cyclodextrin host–guest inclusion as studied by an EPR spin-probe technique. Chem. Eur. J. 1999, 5, 2048–2054. [Google Scholar] [CrossRef]
- Schneider, H.J.; Blatter, T.; Simova, S. Host guest chemistry. 26. NMR and fluorescence studies of cyclodextrin complexes with guest molecules containing both phenyl and naphthyl units. J. Am. Chem. Soc. 1991, 113, 1996–2000. [Google Scholar] [CrossRef]
- Alagona, G.; Ghio, C.; Monti, S. 5-fluorouracil dimers in aqueous solution: Molecular dynamics in water and continuum solvation. Int. J. Quantum Chem. 2002, 88, 133–146. [Google Scholar] [CrossRef]
- Di Donato, C.; Lavorgna, M.; Fattorusso, R.; Isernia, C.; Isidori, M.; Malgieri, G.; Piscitelli, C.; Russo, C.; Russo, L.; Iacovino, R. Alpha-and Beta-Cyclodextrin Inclusion Complexes with 5-Fluorouracil: Characterization and Cytotoxic Activity Evaluation. Molecules 2016, 21, 1644. [Google Scholar] [CrossRef]
- Schneider, H.J.; Hacket, F.; Rüdiger, V.; Ikeda, H. NMR studies of cyclodextrins and cyclodextrin complexes. Chem. Rev. 1998, 98, 1755–1785. [Google Scholar] [CrossRef]
- Cabaleiro-Lago, C.; Nilsson, M.; Söderman, O. Self-diffusion NMR studies of the host-guest interaction between beta-cyclodextrin and alkyltrimethylammonium bromide surfactants. Langmuir 2005, 21, 11637–11644. [Google Scholar] [CrossRef]
- Sun, D.-Z.; Li, L.; Qiu, X.-M.; Liu, F.; Yin, B.-L. Isothermal titration calorimetry and 1H NMR studies on host–guest interaction of paeonol and two of its isomers with β-cyclodextrin. Int. J. Pharm. 2006, 316, 7–13. [Google Scholar] [CrossRef]
- Gidwani, B.; Vyas, A. A Comprehensive review on cyclodextrin-based carriers for delivery of chemotherapeutic cytotoxic anticancer drugs. Biomed. Res. Int. 2015, 2015, 198268. [Google Scholar] [CrossRef]
- Kavitha, K.; Rao, A.; Nalini, C.N. An investigation on enhancement of solubility of 5 fluorouracil by applying complexation technique-characterization, dissolution and molecular-modeling studies. J. Appl. Pharm. Sci. 2013, 3, 162–166. [Google Scholar]
- Terekhova, I.V.; Kumeev, R.S.; Alper, G.A.; Agafonov, A.V. Thermodynamic characteristics of the formation of α- and β-cyclodextrin complexes with lumichrome, lumazine, and uracil in aqueous solution. Russ. J. Phys. Chem. A 2011, 85, 1844. [Google Scholar] [CrossRef]
- Neacsu, A.; Pincu, E.; Munteanu, G.; Munteanu, C.; Meltzer, V. Characterization and Comparison of the Solid State Inclusion Compounds of α-, β-Cyclodextrins and its 2-Hydroxypropyl Derivatives with Uracil and 5-fluorouracil. Rev. Chim. 2017, 68, 2756–2760. [Google Scholar] [CrossRef]
- Kulikov, O.V.; Terekhova, I.; Kumeev, R.; Al’per, G.A. Thermodynamic spectroscopic studies of interactions of alpha- and beta-cyclodextrins and 18-crown-6 ether with bases of nucleic acids. Russ. J. Phys. Chem. 2004, 78, 1488–1493. [Google Scholar]
- Sambasevam, K.; Mohamad, S.; Muhamad Sarih, N.; Ismail, N. Synthesis and Characterization of the Inclusion Complex of β-cyclodextrin and Azomethine. Int. J. Mol. Sci. 2013, 14, 3671–3682. [Google Scholar] [CrossRef]
- Figueiras, A.; Carvalho, R.A.; Ribeiro, L.; Torres-Labandeira, J.J.; Veiga, F.J.B. Solid-state characterization and dissolution profiles of the inclusion complexes of omeprazole with native and chemically modified β-cyclodextrin. Eur. J. Pharm. Biopharm. 2007, 67, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Rajendrakumar, K.; Madhusudan, S.; Pralhad, T. Cyclodextrin complexes of valdecoxib: Properties and anti-inflammatory activity in rat. Eur. J. Pharm. Biopharm. 2005, 60, 39–46. [Google Scholar] [CrossRef]
- Badr-Eldin, S.M.; Elkheshen, S.A.; Ghorab, M.M. Inclusion complexes of tadalafil with natural and chemically modified β-cyclodextrins. I: Preparation and in-vitro evaluation. Eur. J. Pharm. Biopharm. 2008, 70, 819–827. [Google Scholar] [CrossRef]
- Snor, W.; Liedl, E.; Weiss-Greiler, P.; Karpfen, A.; Viernstein, H.; Wolschann, P. On the structure of anhydrous β-cyclodextrin. Chem. Phys. Lett. 2007, 441, 159–162. [Google Scholar] [CrossRef]
- Hulme, A.T.; Price, S.L.; Tocher, D.A. A new polymorph of 5-fluorouracil found following computational crystal structure predictions. J. Am. Chem. Soc. 2005, 127, 1116–1117. [Google Scholar] [CrossRef]
- Andersson, M.P.; Uvdal, P. New scale factors for harmonic vibrational frequencies using the B3LYP density functional method with the triple-ζ basis set 6-311+G.(d,p). J. Phys. Chem. A 2005, 109, 2937–2941. [Google Scholar] [CrossRef] [PubMed]
- Pessine, F.B.T.; Calderini, A.; Alexandrino, G.L. Review: Cyclodextrin inclusion complexes probed by NMR techniques. In Magnetic Resonance Spectroscopy; Kim, D.E., Ed.; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar]
- Ali, S.; Muzaffar, S. Validating strategy of quantitative ROESY analysis for structure determination of cyclodextrin inclusion complexes. J. Mol. Struct. 2018, 1176. [Google Scholar] [CrossRef]
- Tanner, J.E. Use of the stimulated echo in NMR diffusion studies. J. Chem. Phys. 1970, 52, 2523–2526. [Google Scholar] [CrossRef]
- Maklakov, A.I.; Skirda, V.D.; Fatkullin, N.F. Self-diffusion in Polymer Solutions and Melts; Kazan University Press: Kazan, Russia, 1987. [Google Scholar]
- Maklakov, A.I.; Sevryugin, V.A.; Skirda, V.D.; Fatkullin, N.F. Self-diffusion of macromolecules in a polymer solution. Transl. Polym. Sci. USSR 1984, 26, 2502–2507. [Google Scholar]
- Skirda, V.D. Self-Diffusion in Polymer Systems. Ph.D. Thesis, Kazan State University, Kazan, Russia, 1992. [Google Scholar]
- Sevryugin, V.A.; Skirda, V.D.; Skirda, M.V. Exchange process in water solutions of saccharides. Russ. J. Phys. Chem. 1998, 72, 869–874. [Google Scholar]
- Sevryugin, V.A.; Zhuravleva, N.E.; Budanov, A.R.; Abine, S.A.; Zahainov, V.M. Boundary concentrations of areas of the diluted and concentrated electrolyte solutions. Environ. Radioecol. Appl. Ecol. 1998, 4, 221–224. [Google Scholar]
- Sevryugin, V.A.; Skirda, V.D.; Skirda, M.V. Obmennye processy v vodnyh rastvorah saharidov. Zhurnal Fiz. Khimii 1998, 72, 869–874. (In Russian) [Google Scholar]
- Maklakov, A.I.; Skirda, V.D.; Fatkullin, N.F. Self-difussion in polymer systems. In Encyclopedia of Fluid Mechanics; Gulf Publ. Co.: Houston, TX, USA, 1990; Volume 9, pp. 705–745. [Google Scholar]
- Valiullin, P.R.; Skirda, V.D.; Stapf, S.; Kimmich, R. Molecular exchange processes in partially filled porous glass as seen with NMR diffusometry. Phys. Rev. E 1997, 55, 2664–2671. [Google Scholar] [CrossRef]
- Angelova, S.; Nikolova, V.; Pereva, S.; Spassov, T.; Dudev, T. α-cyclodextrin: How effectively can its hydrophobic cavity be hydrated? J. Phys. Chem. B 2017, 121, 9260–9267. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct assymptotic-behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, S. A consistent and accurate ab initio parametrization of density functional dispersion correction (dft-d) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision, E.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Curtiss, L.A.; Frurip, D.J.; Blander, M. Studies of molecular association in H2O and D2O vapors by measurement of thermal conductivity. J. Chem. Phys. 1979, 71, 2703–2711. [Google Scholar] [CrossRef]
- Harris, R.K.; Becker, E.D.; Cabral de Menezes, S.M.; Goodfellow, R.; Grangers, P. NMR nomenclature. Nuclear spin properties and conventions for chemical shifts (IUPAC Recommendations 2001). Pure Appl. Chem. 2001, 73, 1795–1818. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are no more available. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melnikova, D.L.; Badrieva, Z.F.; Kostin, M.A.; Maller, C.; Stas, M.; Buczek, A.; Broda, M.A.; Kupka, T.; Kelterer, A.-M.; Tolstoy, P.M.; et al. On Complex Formation between 5-Fluorouracil and β-Cyclodextrin in Solution and in the Solid State: IR Markers and Detection of Short-Lived Complexes by Diffusion NMR. Molecules 2020, 25, 5706. https://doi.org/10.3390/molecules25235706
Melnikova DL, Badrieva ZF, Kostin MA, Maller C, Stas M, Buczek A, Broda MA, Kupka T, Kelterer A-M, Tolstoy PM, et al. On Complex Formation between 5-Fluorouracil and β-Cyclodextrin in Solution and in the Solid State: IR Markers and Detection of Short-Lived Complexes by Diffusion NMR. Molecules. 2020; 25(23):5706. https://doi.org/10.3390/molecules25235706
Chicago/Turabian StyleMelnikova, Daria L., Zilya F. Badrieva, Mikhail A. Kostin, Corina Maller, Monika Stas, Aneta Buczek, Malgorzata A. Broda, Teobald Kupka, Anne-Marie Kelterer, Peter M. Tolstoy, and et al. 2020. "On Complex Formation between 5-Fluorouracil and β-Cyclodextrin in Solution and in the Solid State: IR Markers and Detection of Short-Lived Complexes by Diffusion NMR" Molecules 25, no. 23: 5706. https://doi.org/10.3390/molecules25235706
APA StyleMelnikova, D. L., Badrieva, Z. F., Kostin, M. A., Maller, C., Stas, M., Buczek, A., Broda, M. A., Kupka, T., Kelterer, A.-M., Tolstoy, P. M., & Skirda, V. D. (2020). On Complex Formation between 5-Fluorouracil and β-Cyclodextrin in Solution and in the Solid State: IR Markers and Detection of Short-Lived Complexes by Diffusion NMR. Molecules, 25(23), 5706. https://doi.org/10.3390/molecules25235706