Antimicrobial Activity and Barrier Properties against UV Radiation of Alkaline and Enzymatically Treated Linen Woven Fabrics Coated with Inorganic Hybrid Material
Abstract
1. Introduction
2. Results and Discussion
2.1. Enzymatic Activity of Laccase
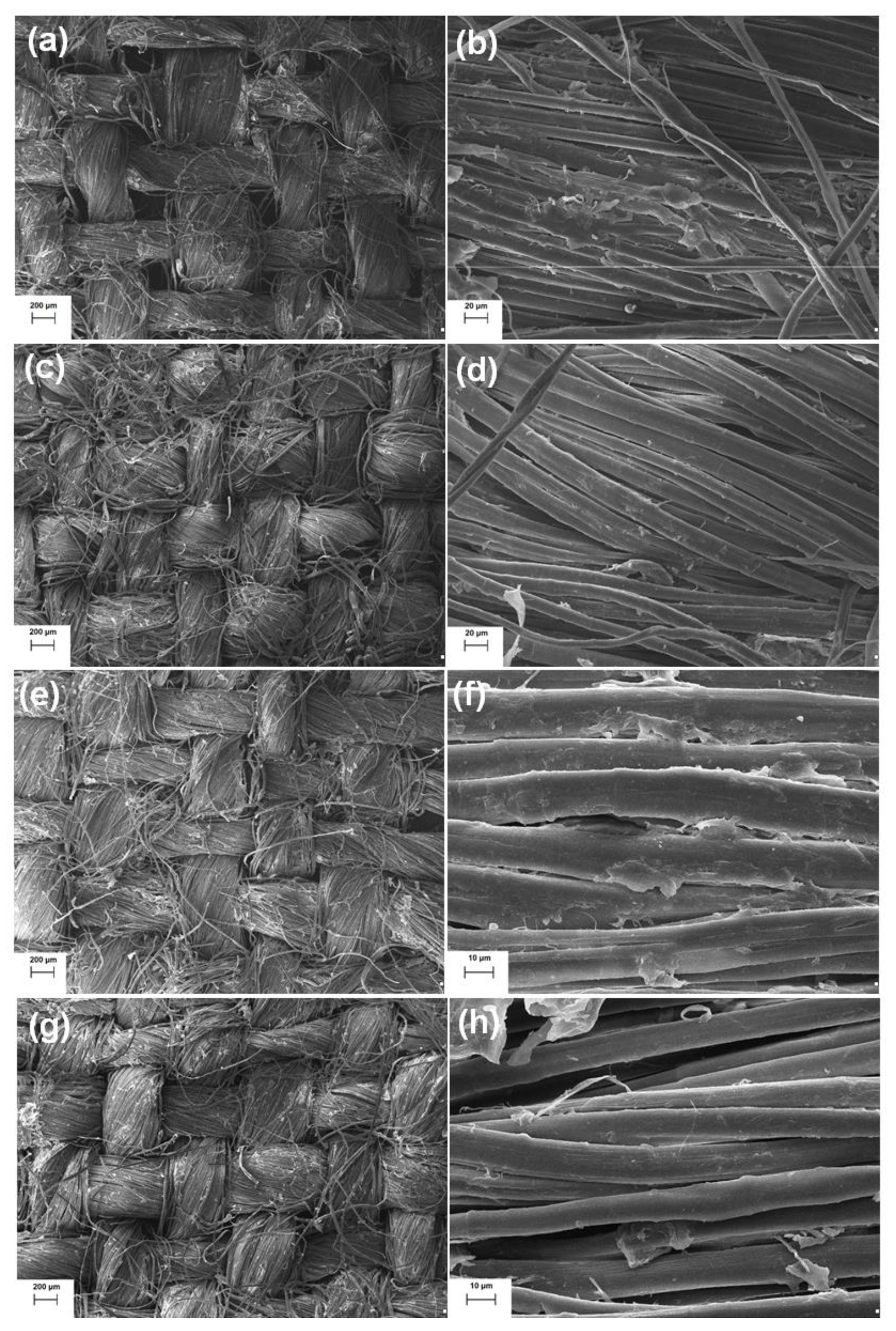
2.2. Morphological Analysis
2.3. Barrier Properties of Linen Woven Fabrics against UV Radiation
2.4. Antimicrobial Activity of Modified Linen Woven Fabrics
3. Materials and Methods
3.1. Materials
3.2. Production of Laccase and Its Enzymatic Activity
3.3. Synthesis of CuO-SiO2 Oxide System
3.4. Alkaline and Enzymatic Treatment of Linen Fabrics
3.5. Hybrid Inorganic Modification of Linen Woven Fabrics
3.6. Morphological Analysis
3.7. Barrier Properties of Linen Woven Fabrics against UV Radiation
3.8. Antimicrobial Activity of Linen Woven Fabrics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Akrman, J.; Přikryl, J. Application of benzotriazole reactive UV absorbers to cellulose and determining sun protection of treated fabric spectrophotometrically. J. Appl. Polym. Sci. 2008, 108, 334–341. [Google Scholar] [CrossRef]
- Kim, Y.K. Ultraviolet protection finishes for textiles. In Functional Finishes for Textiles; Elsevier Ltd.: New York, NY, USA, 2015; pp. 463–485. [Google Scholar]
- Shen, Y.; Zhu, J.; Chen, H.; Huang, D. Synthesis, characterization, and crystal structure of modified benzophenone UV-absorber containing reactive group. Res. Chem. Intermed. 2016, 42, 2909–2918. [Google Scholar] [CrossRef]
- Wong, W.Y.; Lam, J.K.C.; Kan, C.W.; Postle, R. Ultraviolet protection of weft-knitted fabrics. Text. Prog. 2016, 48, 1–54. [Google Scholar] [CrossRef]
- Rode, C.; Zieger, M.; Wyrwa, R.; Thein, S.; Wiegand, C.; Weiser, M.; Ludwig, A.; Wehner, D.; Hipler, U.-C. Antibacterial zinc oxide nanoparticle coating of polyester fabrics. J. Text. Sci. Technol. 2015, 1, 65–74. [Google Scholar] [CrossRef][Green Version]
- Madhu, A.; Chakraborty, J.N. Developments in application of enzymes for textile processing. J. Clean. Prod. 2017, 145, 114–133. [Google Scholar] [CrossRef]
- Hasan, H.H.; Nabi, F.; Mahmud, R. Benefits of enzymatic process in textile wet processing. J. Fibre Text. Res. 2003, 5, 16–19. [Google Scholar]
- Cavaco-Paulo, A.; Gubitz, G.M. Textile Processing with Enzymes; Woodhead Publishing Ltd & CRC Press LLC: New York, NY, USA, 2003. [Google Scholar]
- Jegannathan, K.R.; Nielsen, P.H. A literature review, Environmental assessment of enzyme use in indrustial production. J. Clean. Prod. 2013, 42, 228–240. [Google Scholar] [CrossRef]
- Sójka-Ledakowicz, J.; Lichawska-Olczyk, J.; Pyć, R. Integrated enzymatic pretreatment of cotton fabrics. J. Nat. Fibres 2006, 2, 199–207. [Google Scholar]
- Shrimali, K.; Dedhia, E. Enzymatic Finishing of Textiles. Int. J. Sci. Res. 2016, 5, 674–677. [Google Scholar]
- Patra, A.K.; Madhu, A. Studies on enzymatic pretreatment of linen. Indian J. Fibre Text. Res. 2010, 35, 337–341. [Google Scholar]
- Jawaid, M.; Abdul Khalil, H.P.S. Cellulosic/synthetic fibre reinforced polymer hybrid composites. A review. Carbohydr. Polym. 2011, 86, 1–18. [Google Scholar] [CrossRef]
- Vigneswaren, C.; Jayapriya, C. Effect on physical characteristics of jute fibers with cellulase and specific mixed enzyme systems. J. Text. Instit. 2010, 101, 506–513. [Google Scholar] [CrossRef]
- Sreenath, H.K.; Shah, A.B.; Yang, V.W.; Gharia, M.M.; Jeffries, T.W. Enzymatic Polishing of jute/cotton blended fabrics. J. Ferment. Bioeng 1996, 81, 18–20. [Google Scholar] [CrossRef]
- Strong, P.J.; Claus, H. Laccase: A Review of Its Past and Its Future in Bioremediation. Crit. Rev. Environ. Sci. Technol. 2011, 41, 373–434. [Google Scholar] [CrossRef]
- Sitarz, A.K.; Mikkelsen, J.D.; Meyer, A.S. Structure, functionality and tuning up of laccases for lignocellulose and other industrial applications. Crit. Rev. Biotechnol. 2016, 36, 70–86. [Google Scholar] [CrossRef]
- Michniewicz, A.; Ledakowicz, S.; Ullrich, R.; Hofrichter, M. Kinetics of the enzymatic decolorization of textile dyes by laccase from Cerrena unicolor. Dyes Pigment. 2008, 77, 295–302. [Google Scholar] [CrossRef]
- Kalia, S.; Thakur, K.; Celli, A.; Kiechel, M.A.; Schauer, C.L. Surface modification of plant fibers using environment friendly methods for their application in polymer composites, textile industry and antimicrobial activities: A review. J. Environ. Chem. Eng. 2013, 1, 97–112. [Google Scholar] [CrossRef]
- Polak, J.; Jarosz-Wilkolazka, A. Fungal laccases as green catalysts for dye synthesis. Proc. Biochem. 2012, 47, 1295–1307. [Google Scholar] [CrossRef]
- Sójka-Ledakowicz, J.; Lichawska-Olczyk, J.; Ledakowicz, S.; Michniewicz, A. Bio-scouring of linen fabrics with laccase complex from Cerrena unicolor. Fibres Text. East. Europ. 2007, 63, 86–89. [Google Scholar]
- Sójka-Ledakowicz, J.; Lewartowska, J.; Kudzin, M.; Leonowicz, M.; Jesionowski, T.; Siwińska-Stefańska, K.; Krysztafkiewicz, A. Functionalization of textile materials by aloxysilane-grafted titanium dioxide. J. Mater. Sci. 2009, 44, 3852–3860. [Google Scholar] [CrossRef]
- Sójka-Ledakowicz, J.; Lewartowska, J.; Kudzin, M.; Jesionowski, T.; Siwińska-Stefańska, K.; Krysztafkiewicz, A. Modification of textile material with nanostructured metal oxides. Fibres Text. East. Europ. 2008, 5, 112–116. [Google Scholar]
- Yu, J.Z.; Pang, J.; Zheng, C.; Zhou, T.; Zhang, J.; Zhou, H.; Wei, Q. Cotton fabric finished by PANI/TiO2 with multifunctions of conductivity, anti-ultraviolet and photocatalysis activity. Appl. Surf. Sci. 2019, 470, 84–90. [Google Scholar] [CrossRef]
- Nowacka, M.; Modrzejewska-Sikorska, A.; Chrzanowski, Ł.; Ambrożewicz, D.; Rozmanowski, T.; Myszka, K.; Czaczyk, K.; Bula, K.; Jesionowski, T. Electrokinetic and bioactive properties of CuO-SiO2 oxide composites. Bioelectrochem 2012, 87, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Trapalis, C.C.; Kokkoris, M.; Perdikakis, G.; Kordas, G. Study of antibacterial composite Cu/SiO2 thin coatings. J. Sol. Gel Sci. Technol. 2003, 26, 1213–1218. [Google Scholar] [CrossRef]
- Varghese, S.; ElFakhri, S.O.; Sheel, D.W.; Sheel, P.; Bolton, F.J.E.; Foste, H.A. Antimicrobial activity of novel nanostructured Cu-SiO2 coatings prepared by chemical vapour deposition against hospital related pathogens. AMB Express 2013, 3, 53–61. [Google Scholar] [CrossRef]
- Kalaivani, S.; Singh, R.K.; Ganesan, V.; Kannan, S. Effect of copper (Cu2+) inclusion on the bioactivity and antibacterial behavior of calcium silicate coatings on titanium metal. J. Mater. Chem. B 2014, 2, 846–858. [Google Scholar] [CrossRef]
- Ren, G.; Hu, D.; Cheng, E.W.C.; Vargas-Reus, M.A.; Reip, P.; Allaker, R.P. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int. J. Antimicrob. Agents 2009, 33, 587–590. [Google Scholar] [CrossRef]
- Gnanamani, A.; Jayaprakashvel, M.; Arulmani, M.; Sadulla, S. Effect of inducers and culturing processes on laccase synthesis in Phanerochaete chrysosporium NCIM 1197 and the constitutive expression of laccase isozymes. Enzym. Microb. Technol. 2006, 38, 1017–1021. [Google Scholar] [CrossRef]
- Technical Criteria No. KT 12/2015 Clothing Protecting against Ultraviolet Radiation (UV) and KT 13/2013 Roller Blinds/Covers Protecting against UV Radiation; Developed in Łukasiewicz—Institute of Leather Industry (Lodz, Poland) and Central Institute for Labour Protection—National Research Institute (Lodz, Poland) for Łukasiewicz—Textile Research Institute (Lodz, Poland) as Part of the Project ENVIROTEX POIG No. 01.03.01-00-006/08. 2015; unpublished. (In Polish)
- Janusz, G.; Rogalski, J.; Szczodrak, J. Increased production of laccase by Cerrena unicolor in submerged liquid cultures. World J. Microb. And Biotech. 2007, 23, 1459–1464. [Google Scholar] [CrossRef]
- Antecka, A.; Blatkiewicz, M.; Boruta, T.; Górak, A.; Ledakowicz, S. Comparison of downstream processing methods in purification of highly active laccase. Bioprocess. Biosyst Eng. 2019, 42, 1635–1645. [Google Scholar] [CrossRef]
- Eggert, C.; Temp, U.; Eriksson, K.E. The ligninolytic system of the white rot fungus Pycnoporus cinnabarinus: Purification and characterization of the laccase. Appl. Environ. Microbiol. 1996, 62, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Leonowicz, A.; Grzywnowicz, K. Quantitative estimation of laccase forms in some white-rot fungi using syringaldazine as a substrate. Enzyme Microb. Technol. 1981, 3, 55–58. [Google Scholar] [CrossRef]


| Sample Type | UPF | Transmittance (%) |
|---|---|---|
| Raw linen | 27 | TUVA = 4.52 TUVB = 3.9 Tav. = 4.22 |
| Linen/alkaline scouring | 39 | TUVA = 3.42 TUVB = 2.53 Tav. = 3.1 |
| Linen/2.5 U/g of laccase | 36 | TUVA = 3.83 TUVB = 2.89 Tav. = 3.62 |
| Linen/5.0 U/g of laccase | 36 | TUVA = 3.79 TUVB = 2.87 Tav. = 3.58 |
| Raw linen without pretreatment/5% wt. of CuO-SiO2 | 32 | TUVA = 3.94 TUVB = 3.20 Tav. = 3.81 |
| Raw linen without pretreatment/7% wt. of CuO-SiO2 | 29 | TUVA = 4.21 TUVB = 3.40 Tav. = 4.03 |
| Linen/alkaline scouring/5% wt. of CuO-SiO2 | 64 (>50) | TUVA = 1.81 TUVB = 1.41 Tav. = 1.61 |
| Linen/alkaline scouring/7% wt. of CuO-SiO2 | 72 (>50) | TUVA = 1.61 TUVB = 1.22 Tav. = 1.52 |
| Linen/2.5 U/g of laccase/5% wt. of CuO-SiO2 | 131 (>50) | TUVA = 0.34 TUVB = 0.20 Tav. = 0.31 |
| Linen/5.0 U/g of laccase/5% wt. of CuO-SiO2 | 110 (>50) | TUVA = 0.41 TUVB = 0.31 Tav. = 0.36 |
| Linen/2.5 U/g of laccase/7% wt. of CuO-SiO2 | 128 (>50) | TUVA = 0.86 TUVB = 0.75 Tav. = 0.83 |
| Linen/5.0 U/g of laccase/7% wt. of CuO-SiO2 | 99 (>50) | TUVA = 0.74 TUVB = 0.69 Tav. = 0.79 |
| Sample Type | Microbiological Activity against Escherchia coli (ATCC 11229) | Microbiological Activity against Staphylococcus aureus (ATCC 6538) | Microbiological Activity against Candida albicans (ATCC 10231) | |||
|---|---|---|---|---|---|---|
| A | L | A | L | A | L | |
| Raw linen | −0.2 | −5.0 | 0.0 | −4.1 | −1.4 | −3.8 |
| Linen/2.5 U/g of laccase/5% wt. of CuO-SiO2 | 4.5 | 0.9 | 3.0 | 0.1 | >5.7 | >2.0 |
| Linen/2.5 U/g of laccase/7% wt. of CuO-SiO2 | 6.3 | 1.5 | 4.0 | 0.2 | 5.5 | 1.8 |
| Linen/alkali-scouring/7% wt. of CuO-SiO2 | 6.3 | 1.2 | 4.0 | 0.5 | 5.3 | 1.6 |
Sample Availability: Samples of the compounds are not available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olczyk, J.; Sójka-Ledakowicz, J.; Walawska, A.; Antecka, A.; Siwińska-Ciesielczyk, K.; Zdarta, J.; Jesionowski, T. Antimicrobial Activity and Barrier Properties against UV Radiation of Alkaline and Enzymatically Treated Linen Woven Fabrics Coated with Inorganic Hybrid Material. Molecules 2020, 25, 5701. https://doi.org/10.3390/molecules25235701
Olczyk J, Sójka-Ledakowicz J, Walawska A, Antecka A, Siwińska-Ciesielczyk K, Zdarta J, Jesionowski T. Antimicrobial Activity and Barrier Properties against UV Radiation of Alkaline and Enzymatically Treated Linen Woven Fabrics Coated with Inorganic Hybrid Material. Molecules. 2020; 25(23):5701. https://doi.org/10.3390/molecules25235701
Chicago/Turabian StyleOlczyk, Joanna, Jadwiga Sójka-Ledakowicz, Anetta Walawska, Anna Antecka, Katarzyna Siwińska-Ciesielczyk, Jakub Zdarta, and Teofil Jesionowski. 2020. "Antimicrobial Activity and Barrier Properties against UV Radiation of Alkaline and Enzymatically Treated Linen Woven Fabrics Coated with Inorganic Hybrid Material" Molecules 25, no. 23: 5701. https://doi.org/10.3390/molecules25235701
APA StyleOlczyk, J., Sójka-Ledakowicz, J., Walawska, A., Antecka, A., Siwińska-Ciesielczyk, K., Zdarta, J., & Jesionowski, T. (2020). Antimicrobial Activity and Barrier Properties against UV Radiation of Alkaline and Enzymatically Treated Linen Woven Fabrics Coated with Inorganic Hybrid Material. Molecules, 25(23), 5701. https://doi.org/10.3390/molecules25235701









