Promising Lead Compounds in the Development of Potential Clinical Drug Candidate for Drug-Resistant Tuberculosis
Abstract
1. Introduction
- GenXpert MTB/RIF is an assay for nucleic acid amplification in sputum sample that analyses DNA and RIF resistance for the presence of mycobacterium tuberculosis. It is a very simple and reproducible procedure with 90% sensitivity and 99% accuracy. The GenXpert is an automated assay and requires no laboratory arrangements. Rifampicin resistance detection is also used for the prediction of MDR-tuberculosis with isoniazid resistance (in most of the cases) [14]. As an initial diagnostic examination, the WHO recently proposed GenXpert for patients with HIV supposed to have tuberculosis or for those who are at risk for rifampicin resistance and/or MDR tuberculosis. GenXpert (MTB/RIF) Ultra is theoretically facilitating, more precise, and sensitive bedside testing that can enhance tuberculosis detection in smear-negative patients, and similar assays are currently under development.
- Line probe assay (LPA), approved by WHO, is family of DNA strip-based tests for swift recognition of first- and second-line antitubercular agents drug resistance [31]. It can also be used for testing culture isolates along with direct testing of acid-fast bacilli as well as smear-positive and -negative sputum specimens. LPA can determine the frequently identified mutations in resistant strains [14,15].
2. Compounds with Promising Antimycobacterial Potentials
2.1. Classification of Antitubercular Drugs
- Group-A contains fluoroquinolones (high doses of levofloxacin, moxifloxacin, and gatifloxacin). Due to their bactericidal and sterilizing efficacy and strong safety profile, these are called vital products.
- Group-B contains injectable products like (streptomycin, kanamycin, amikacin, and capreomycin) that are incredibly bactericidal but have a lower safety rating than drugs in Group A.
- Ethionamide, cycloserine/terizidone, clofazimine, prothionamide, and linezolid are in Group-C. Given increasing proof of their effectiveness and tolerability, these medications are recommended as vital second-line medicines for multidrug-resistant tuberculosis.
- Group-D is classified into three subgroups: D1: large-dose isoniazid, ethambutol, and pyrazinamide; D2: delamanid and bedaquiline; and D3: para-aminosalicylic acid, meropenem, cilastatin-imipenem, clarithromycin, and clavulanate-amoxicillin.
2.2. Promising Novel Chemotherapeutics
2.2.1. Isoniazid Lead Derivatives
2.2.2. Coumarin Lead Derivatives
2.2.3. Griselimycin Lead Derivatives
2.3. Lead Antimicrobial Peptides
3. Conclusions
Funding
Conflicts of Interest
References
- World Health Organization (WHO). WHO Guidelines on Tuberculosis Infection Prevention and Control: 2019 Update; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Tiemersma, E.W.; van der Werf, M.J.; Borgdorff, M.W.; Williams, B.G.; Nagelkerke, N.J. Natural history of tuberculosis: Duration and fatality of untreated pulmonary tuberculosis in HIV negative patients: A systematic review. PLoS ONE 2011, 6, e17601. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Srivastva, G.; Kachhwaha, S.; Sharma, M.; Kothari, S. Antimycobacterial activity of Citrullus colocynthis (L.) Schrad. against drug sensitive and drug resistant Mycobacterium tuberculosis and MOTT clinical isolates. J. Ethnopharmacol. 2013, 149, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Tascon, R.; Soares, C.; Ragno, S.; Stavropoulos, E.; Hirst, E.; Colston, M. Mycobacterium tuberculosis-activated dendritic cells induce protective immunityin mice. Immunology 2000, 99, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Woodworth, J.; Sköld, M.; Behar, S.M. In vivo depletion of CD11c+ cells delays the CD4+ T cell response to Mycobacterium tuberculosis and exacerbates the outcome of infection. J. Immunol. 2005, 175, 3268–3272. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2019; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Harding, E. WHO global progress report on tuberculosis elimination. Lancet Respir. Med. 2020, 8, 19. [Google Scholar] [CrossRef]
- Dobbs, T.; Webb, R. Chemotherapy of Tuberculosis. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Shetye, G.S.; Franzblau, S.G.; Cho, S. New tuberculosis drug targets, their inhibitors, and potential therapeutic impact. Transl. Res. 2020, 220, 68–97. [Google Scholar] [CrossRef]
- Young, C.; Walzl, G.; Du Plessis, N. Therapeutic host-directed strategies to improve outcome in tuberculosis. Mucosal Immunol. 2020, 13, 190–204. [Google Scholar] [CrossRef]
- Mangtani, P.; Abubakar, I.; Ariti, C.; Beynon, R.; Pimpin, L.; Fine, P.E.; Rodrigues, L.C.; Smith, P.G.; Lipman, M.; Whiting, P.F. Protection by BCG vaccine against tuberculosis: A systematic review of randomized controlled trials. Clin. Infect. Dis. 2014, 58, 470–480. [Google Scholar] [CrossRef]
- Kaufmann, S.H.; Evans, T.G.; Hanekom, W.A. Tuberculosis vaccines: Time for a global strategy. Sci. Transl. Med. 2015, 7, fs8–fs276. [Google Scholar] [CrossRef][Green Version]
- Wayne, L.G.; Sohaskey, C.D. Nonreplicating persistence of Mycobacterium tuberculosis. Annu. Rev. Microbiol. 2001, 55, 139–163. [Google Scholar] [CrossRef] [PubMed]
- Rustad, T.R.; Harrell, M.I.; Liao, R.; Sherman, D.R. The enduring hypoxic response of Mycobacterium tuberculosis. PLoS ONE 2008, 3, e1502. [Google Scholar] [CrossRef] [PubMed]
- Cardona, P.; Ruiz-Manzano, J. On the nature of Mycobacterium tuberculosis-latent bacilli. Eur. Respir. J. 2004, 24, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Lata, M.; Sharma, D.; Deo, N.; Tiwari, P.K.; Bisht, D.; Venkatesan, K. Proteomic analysis of ofloxacin-mono resistant Mycobacterium tuberculosis isolates. J. Proteom. 2015, 127, 114–121. [Google Scholar] [CrossRef]
- Sharma, D.; Bisht, D. Secretory proteome analysis of streptomycin-resistant Mycobacterium tuberculosis clinical isolates. Slas Discov. Adv. Life Sci. R&D 2017, 22, 1229–1238. [Google Scholar]
- Sharma, D.; Bisht, D.M. tuberculosis hypothetical proteins and proteins of unknown function: Hope for exploring novel resistance mechanisms as well as future target of drug resistance. Front. Microbiol. 2017, 8, 465. [Google Scholar] [CrossRef]
- Sharma, D.; Bisht, D.; Khan, A.U. Potential alternative strategy against drug resistant tuberculosis: A proteomics prospect. Proteomes 2018, 6, 26. [Google Scholar] [CrossRef]
- Sharma, D.; Lata, M.; Singh, R.; Deo, N.; Venkatesan, K.; Bisht, D. Cytosolic proteome profiling of aminoglycosides resistant Mycobacterium tuberculosis clinical isolates using MALDI-TOF/MS. Front. Microbiol. 2016, 7, 1816. [Google Scholar] [CrossRef]
- Lee, M.; Lee, J.; Carroll, M.W.; Choi, H.; Min, S.; Song, T.; Via, L.E.; Goldfeder, L.C.; Kang, E.; Jin, B. Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N. Engl. J. Med. 2012, 367, 1508–1518. [Google Scholar] [CrossRef]
- Sotgiu, G.; Centis, R.; D’Ambrosio, L.; Spanevello, A.; Migliori, G.B. Linezolid to treat extensively drug-resistant TB: Retrospective data are confirmed by experimental evidence. Eur. Respir. J. 2013, 42, 288–290. [Google Scholar] [CrossRef]
- Dua, K.; Rapalli, V.K.; Shukla, S.D.; Singhvi, G.; Shastri, M.D.; Chellappan, D.K.; Satija, S.; Mehta, M.; Gulati, M.; Pinto, T.D.J.A. Multi-drug resistant Mycobacterium tuberculosis & oxidative stress complexity: Emerging need for novel drug delivery approaches. Biomed. Pharmacother. 2018, 107, 1218–1229. [Google Scholar] [PubMed]
- Alffenaar, J.; Van Der Laan, T.; Simons, S.; Van Der Werf, T.; Van De Kasteele, P.; De Neeling, H.; Van Soolingen, D. Susceptibility of clinical Mycobacterium tuberculosis isolates to a potentially less toxic derivate of linezolid, PNU-100480. Antimicrob. Agents Chemother. 2011, 55, 1287–1289. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, V.; Solapure, S.; Iyer, H.; Ghosh, A.; Sharma, S.; Kaur, P.; Deepthi, R.; Subbulakshmi, V.; Ramya, V.; Ramachandran, V. Bactericidal activity and mechanism of action of AZD5847, a novel oxazolidinone for treatment of tuberculosis. Antimicrob. Agents Chemother. 2014, 58, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Andries, K.; Villellas, C.; Coeck, N.; Thys, K.; Gevers, T.; Vranckx, L.; Lounis, N.; de Jong, B.C.; Koul, A. Acquired resistance of Mycobacterium tuberculosis to bedaquiline. PLoS ONE 2014, 9, e102135. [Google Scholar] [CrossRef]
- Zimenkov, D.V.; Nosova, E.Y.; Kulagina, E.V.; Antonova, O.V.; Arslanbaeva, L.R.; Isakova, A.I.; Krylova, L.Y.; Peretokina, I.V.; Makarova, M.V.; Safonova, S.G. Examination of bedaquiline-and linezolid-resistant Mycobacterium tuberculosis isolates from the Moscow region. J. Antimicrob. Chemother. 2017, 72, 1901–1906. [Google Scholar] [CrossRef]
- Veziris, N.; Bernard, C.; Guglielmetti, L.; Le Du, D.; Marigot-Outtandy, D.; Jaspard, M.; Caumes, E.; Lerat, I.; Rioux, C.; Yazdanpanah, Y. Rapid emergence of Mycobacterium tuberculosis bedaquiline resistance: Lessons to avoid repeating past errors. Eur. Respir. J. 2017, 49, 3. [Google Scholar] [CrossRef]
- Somoskovi, A.; Bruderer, V.; Hömke, R.; Bloemberg, G.V.; Böttger, E.C. A mutation associated with clofazimine and bedaquiline cross-resistance in MDR-TB following bedaquiline treatment. Eur. Respir. J. 2015, 45, 554–557. [Google Scholar] [CrossRef]
- Tadolini, M.; Lingtsang, R.D.; Tiberi, S.; Enwerem, M.; D’Ambrosio, L.; Sadutshang, T.D.; Centis, R.; Migliori, G.B. First case of extensively drug-resistant tuberculosis treated with both delamanid and bedaquiline. Eur. Respir. J. 2016, 48, 935–938. [Google Scholar] [CrossRef]
- Harausz, E.P.; Chervenak, K.A.; Good, C.E.; Jacobs, M.R.; Wallis, R.S.; Sanchez-Felix, M.; Boom, W.H. Activity of nitazoxanide and tizoxanide against Mycobacterium tuberculosis in vitro and in whole blood culture. Tuberculosis 2016, 98, 92–96. [Google Scholar] [CrossRef]
- Hancock, R.E.; Diamond, G. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 2000, 8, 402–410. [Google Scholar] [CrossRef]
- Ebenhan, T.; Gheysens, O.; Kruger, H.G.; Zeevaart, J.R.; Sathekge, M.M. Antimicrobial peptides: Their role as infection-selective tracers for molecular imaging. BioMed Res. Int. 2014, 2014, 867381. [Google Scholar] [CrossRef] [PubMed]
- Vilaplana, C.; Marzo, E.; Tapia, G.; Diaz, J.; Garcia, V.; Cardona, P.-J. Ibuprofen therapy resulted in significantly decreased tissue bacillary loads and increased survival in a new murine experimental model of active tuberculosis. J. Infect. Dis. 2013, 208, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Vilchèze, C.; Jacobs, J. William R, The mechanism of isoniazid killing: Clarity through the scope of genetics. Annu. Rev. Microbiol. 2007, 61, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.; Perdigao, J.; Ramos, J.; Couto, I.; Portugal, I.; Ritter, C.; Boettger, E.C.; Viveiros, M. High-level resistance to isoniazid and ethionamide in multidrug-resistant Mycobacterium tuberculosis of the Lisboa family is associated with inhA double mutations. J. Antimicrob. Chemother. 2013, 68, 1728–1732. [Google Scholar] [CrossRef]
- Petrini, B.; Hoffner, S. Drug-resistant and multidrug-resistant tubercle bacilli. Int. J. Antimicrob. Agents 1999, 13, 93–97. [Google Scholar] [CrossRef]
- Castelo-Branco, F.S.; de Lima, E.C.; de Oliveira Domingos, J.L.; Pinto, A.C.; Lourenço, M.C.S.; Gomes, K.M.; Costa-Lima, M.M.; Araujo-Lima, C.F.; Aiub, C.A.F.; Felzenszwalb, I. New hydrazides derivatives of isoniazid against Mycobacterium tuberculosis: Higher potency and lower hepatocytotoxicity. Eur. J. Med. Chem. 2018, 146, 529–540. [Google Scholar] [CrossRef]
- Loots, D.T. An altered Mycobacterium tuberculosis metabolome induced by katG mutations resulting in isoniazid resistance. Antimicrob. Agents Chemother. 2014, 58, 2144–2149. [Google Scholar] [CrossRef]
- Rajkhowa, S.; C Deka, R. DFT based QSAR/QSPR models in the development of novel anti-tuberculosis drugs targeting Mycobacterium tuberculosis. Curr. Pharm. Des. 2014, 20, 4455–4473. [Google Scholar] [CrossRef]
- Reddy, D.S.; Kongot, M.; Netalkar, S.P.; Kurjogi, M.M.; Kumar, R.; Avecilla, F.; Kumar, A. Synthesis and evaluation of novel coumarin-oxime ethers as potential anti-tubercular agents: Their DNA cleavage ability and BSA interaction study. Eur. J. Med. Chem. 2018, 150, 864–875. [Google Scholar] [CrossRef]
- Mangasuli, S.N.; Hosamani, K.M.; Devarajegowda, H.C.; Kurjogi, M.M.; Joshi, S.D. Synthesis of coumarin-theophylline hybrids as a new class of anti-tubercular and anti-microbial agents. Eur. J. Med. Chem. 2018, 146, 747–756. [Google Scholar] [CrossRef]
- Adeniji, A.A.; Knoll, K.E. Potential anti-TB investigational compounds and drugs with repurposing potential in TB therapy: A conspectus. Appl. Microbiol. Biotechnol. 2020, 104, 5633–5662. [Google Scholar] [CrossRef] [PubMed]
- Pires, C.T.; Scodro, R.B.; Cortez, D.A.; Brenzan, M.A.; Siqueira, V.L.; Caleffi-Ferracioli, K.R.; Vieira, L.C.; Monteiro, J.L.; Corrêa, A.G.; Cardoso, R.F. Structure–activity relationship of natural and synthetic coumarin derivatives against Mycobacterium tuberculosis. Future Med. Chem. 2020, 12, 1533–1546. [Google Scholar] [CrossRef] [PubMed]
- Kapp, E.; Visser, H.; Sampson, S.L.; Malan, S.F.; Streicher, E.M.; Foka, G.B.; Warner, D.F.; Omoruyi, S.I.; Enogieru, A.B.; Ekpo, O.E. Versatility of 7-substituted coumarin molecules as antimycobacterial agents, neuronal enzyme inhibitors and neuroprotective agents. Molecules 2017, 22, 1644. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Pfeiffer, B.; Altmann, K.-H. Recent developments in natural product-based drug discovery for tuberculosis. Drug Discov. Today 2017, 22, 585–591. [Google Scholar] [CrossRef]
- Kling, A.; Lukat, P.; Almeida, D.V.; Bauer, A.; Fontaine, E.; Sordello, S.; Zaburannyi, N.; Herrmann, J.; Wenzel, S.C.; König, C. Targeting DnaN for tuberculosis therapy using novel griselimycins. Science 2015, 348, 1106–1112. [Google Scholar] [CrossRef]
- Holzgrabe, U. New griselimycins for treatment of tuberculosis. Chem. Biol. 2015, 22, 981–982. [Google Scholar] [CrossRef]
- Lukat, P.; Katsuyama, Y.; Wenzel, S.; Binz, T.; König, C.; Blankenfeldt, W.; Brönstrup, M.; Müller, R. Biosynthesis of methyl-proline containing griselimycins, natural products with anti-tuberculosis activity. Chem. Sci. 2017, 8, 7521–7527. [Google Scholar] [CrossRef]
- Arranz-Trullén, J.; Lu, L.; Pulido, D.; Bhakta, S.; Boix, E. Host antimicrobial peptides: The promise of new treatment strategies against tuberculosis. Front. Immunol. 2017, 8, 1499. [Google Scholar] [CrossRef]
- Lee, I.-G.; Lee, S.J.; Chae, S.; Lee, K.-Y.; Kim, J.-H.; Lee, B.-J. Structural and functional studies of the Mycobacterium tuberculosis VapBC30 toxin-antitoxin system: Implications for the design of novel antimicrobial peptides. Nucl. Acids Res. 2015, 43, 7624–7637. [Google Scholar] [CrossRef]
- Gupta, S.; Winglee, K.; Gallo, R.; Bishai, W.R. Bacterial subversion of cAMP signalling inhibits cathelicidin expression, which is required for innate resistance to Mycobacterium tuberculosis. J. Pathol. 2017, 242, 52–61. [Google Scholar] [CrossRef]
- Rivas-Santiago, B.; Santiago, C.E.R.; Castañeda-Delgado, J.E.; León–Contreras, J.C.; Hancock, R.E.; Hernandez-Pando, R. Activity of LL-37, CRAMP and antimicrobial peptide-derived compounds E2, E6 and CP26 against Mycobacterium tuberculosis. Int. J. Antimicrob. Agents 2013, 41, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; P Higgins, M.; Whitehurst, J.; O Kisich, K.; I Voskuil, M.; S Hodges, R. Anti-tuberculosis activity of α-helical antimicrobial peptides: De novo designed L-and D-enantiomers versus L-and D-LL37. Protein Pept. Lett. 2011, 18, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Sosunov, V.; Mischenko, V.; Eruslanov, B.; Svetoch, E.; Shakina, Y.; Stern, N.; Majorov, K.; Sorokoumova, G.; Selishcheva, A.; Apt, A. Antimycobacterial activity of bacteriocins and their complexes with liposomes. J. Antimicrob. Chemother. 2007, 59, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.; Field, D.; O’Connor, P.M.; Cotter, P.D.; Coffey, A.; Hill, C.; O’Mahony, J. The gene encoded antimicrobial peptides, a template for the design of novel anti-mycobacterial drugs. Bioeng. Bugs 2010, 1, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.M.; Franco, D.P.; Vitorio, F.; Kummerle, A.E. Coumarin compounds in medicinal chemistry: Some important examples from the last years. Curr. Top. Med. Chem. 2018, 18, 124–148. [Google Scholar] [CrossRef]
- Neyts, J.; Clercq, E.D.; Singha, R.; Chang, Y.H.; Das, A.R.; Chakraborty, S.K.; Hong, S.C.; Tsay, S.-C.; Hsu, M.-H.; Hwu, J.R. Structure−activity relationship of new anti-Hepatitis C virus agents: Heterobicycle−coumarin conjugates. J. Med. Chem. 2009, 52, 1486–1490. [Google Scholar] [CrossRef]
- Tsay, S.-C.; Hwu, J.R.; Singha, R.; Huang, W.-C.; Chang, Y.H.; Hsu, M.-H.; Shieh, F.-k.; Lin, C.-C.; Hwang, K.C.; Horng, J.-C. Coumarins hinged directly on benzimidazoles and their ribofuranosides to inhibit hepatitis C virus. Eur. J. Med. Chem. 2013, 63, 290–298. [Google Scholar] [CrossRef]
- Manvar, A.; Bavishi, A.; Radadiya, A.; Patel, J.; Vora, V.; Dodia, N.; Rawal, K.; Shah, A. Diversity oriented design of various hydrazides and their in vitro evaluation against Mycobacterium tuberculosis H37Rv strains. Bioorganic Med. Chem. Lett. 2011, 21, 4728–4731. [Google Scholar] [CrossRef]
- Arshad, A.; Osman, H.; Bagley, M.C.; Lam, C.K.; Mohamad, S.; Zahariluddin, A.S.M. Synthesis and antimicrobial properties of some new thiazolyl coumarin derivatives. Eur. J. Med. Chem. 2011, 46, 3788–3794. [Google Scholar] [CrossRef]
- Farshori, N.N.; Banday, M.R.; Ahmad, A.; Khan, A.U.; Rauf, A. 7-Hydroxy-coumarin derivatives: Synthesis, characterization and preliminary antimicrobial activities. Med. Chem. Res. 2011, 20, 535–541. [Google Scholar] [CrossRef]
- López-Rojas, P.; Janeczko, M.; Kubiński, K.; Amesty, Á.; Masłyk, M.; Estévez-Braun, A. Synthesis and antimicrobial activity of 4-substituted 1, 2, 3-triazole-coumarin derivatives. Molecules 2018, 23, 199. [Google Scholar] [CrossRef] [PubMed]
- Kadhum, A.A.H.; Al-Amiery, A.A.; Musa, A.Y.; Mohamad, A.B. The antioxidant activity of new coumarin derivatives. Int. J. Mol. Sci. 2011, 12, 5747–5761. [Google Scholar] [CrossRef] [PubMed]
- Witaicenis, A.; Seito, L.N.; da Silveira Chagas, A.; de Almeida Junior, L.D.; Luchini, A.C.; Rodrigues-Orsi, P.; Cestari, S.H.; Di Stasi, L.C. Antioxidant and intestinal anti-inflammatory effects of plant-derived coumarin derivatives. Phytomedicine 2014, 21, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Manojkumar, P.; Ravi, T.; Subbuchettiar, G. Synthesis of coumarin heterocyclic derivatives with antioxidant activity and in vitro cytotoxic activity against tumour cells. Acta Pharm. 2009, 59, 159–170. [Google Scholar] [CrossRef]
- Sashidhara, K.V.; Kumar, A.; Chatterjee, M.; Rao, K.B.; Singh, S.; Verma, A.K.; Palit, G. Discovery and synthesis of novel 3-phenylcoumarin derivatives as antidepressant agents. Bioorganic Med. Chem. Lett. 2011, 21, 1937–1941. [Google Scholar] [CrossRef]
- Wang, S.-B.; Liu, H.; Li, G.-Y.; Li, J.; Li, X.-J.; Lei, K.; Wei, L.-C.; Quan, Z.-S.; Wang, X.-K.; Liu, R.-M. Coumarin and 3, 4-dihydroquinolinone derivatives: Synthesis, antidepressant activity, and molecular docking studies. Pharmacol. Rep. 2019, 71, 1244–1252. [Google Scholar] [CrossRef]
- Sashidhara, K.V.; Rao, K.B.; Singh, S.; Modukuri, R.K.; Teja, G.A.; Chandasana, H.; Shukla, S.; Bhatta, R.S. Synthesis and evaluation of new 3-phenylcoumarin derivatives as potential antidepressant agents. Bioorganic Med. Chem. Lett. 2014, 24, 4876–4880. [Google Scholar] [CrossRef]
- Kontogiorgis, C.A.; Hadjipavlou-Litina, D.J. Synthesis and antiinflammatory activity of coumarin derivatives. J. Med. Chem. 2005, 48, 6400–6408. [Google Scholar] [CrossRef]
- Fylaktakidou, K.C.; Hadjipavlou-Litina, D.J.; Litinas, K.E.; Nicolaides, D.N. Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activities. Curr. Pharm. Des. 2004, 10, 3813–3833. [Google Scholar] [CrossRef]
- Bansal, Y.; Sethi, P.; Bansal, G. Coumarin: A potential nucleus for anti-inflammatory molecules. Med. Chem. Res. 2013, 22, 3049–3060. [Google Scholar] [CrossRef]
- El-Haggar, R.; Al-Wabli, R.I. Anti-inflammatory screening and molecular modeling of some novel coumarin derivatives. Molecules 2015, 20, 5374–5391. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.K.; Kaur, N.; Bansal, Y.; Bansal, G. Novel coumarin–benzimidazole derivatives as antioxidants and safer anti-inflammatory agents. Acta Pharm. Sin. B 2014, 4, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Bi, J.; Su, W. Synthesis and Antitumor Activity of Novel Coumarin Derivatives via a Three-component Reaction in Water. Chin. J. Chem. 2013, 31, 507–514. [Google Scholar] [CrossRef]
- Qin, Q.-P.; Wang, Z.-F.; Huang, X.-L.; Tan, M.-X.; Zou, B.-Q.; Liang, H. Strong in vitro and vivo cytotoxicity of novel organoplatinum (II) complexes with quinoline-coumarin derivatives. Eur. J. Med. Chem. 2019, 184, 111751. [Google Scholar] [CrossRef]
- Musa, M.A.; Cooperwood, J.S.; Khan, M.O.F. A review of coumarin derivatives in pharmacotherapy of breast cancer. Curr. Med. Chem. 2008, 15, 2664–2679. [Google Scholar] [CrossRef]
- Bisi, A.; Cappadone, C.; Rampa, A.; Farruggia, G.; Sargenti, A.; Belluti, F.; Di Martino, R.M.; Malucelli, E.; Meluzzi, A.; Iotti, S. Coumarin derivatives as potential antitumor agents: Growth inhibition, apoptosis induction and multidrug resistance reverting activity. Eur. J. Med. Chem. 2017, 127, 577–585. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Z.; Zhou, M.; Wu, F.; Hou, X.; Luo, H.; Liu, H.; Han, X.; Yan, G.; Ding, Z. Synthesis and biological evaluation of 4-(1, 2, 3-triazol-1-yl) coumarin derivatives as potential antitumor agents. Bioorganic Med. Chem. Lett. 2014, 24, 799–807. [Google Scholar] [CrossRef]
- Alipour, M.; Khoobi, M.; Emami, S.; Fallah-Benakohal, S.; Ghasemi-Niri, S.F.; Abdollahi, M.; Foroumadi, A.; Shafiee, A. Antinociceptive properties of new coumarin derivatives bearing substituted 3, 4-dihydro-2H-benzothiazines. Daru J. Pharm. Sci. 2014, 22, 9. [Google Scholar] [CrossRef]
- Cheriyan, B.V., Sr.; Kadhirvelu, P., Sr.; Nadipelly, J., Jr.; Shanmugasundaram, J.; Sayeli, V., Sr.; Subramanian, V., Sr. Anti-nociceptive effect of 7-methoxy coumarin from Eupatorium Triplinerve vahl (Asteraceae). Pharmacogn. Mag. 2017, 13, 81. [Google Scholar]
- Park, S.-H.; Sim, Y.-B.; Kang, Y.-J.; Kim, S.-S.; Kim, C.-H.; Kim, S.-J.; Lim, S.-M.; Suh, H.-W. Antinociceptive profiles and mechanisms of orally administered coumarin in mice. Biol. Pharm. Bull. 2013, 36, 925–930. [Google Scholar] [CrossRef]
- Sanchez-Recillas, A.; Navarrete-Vázquez, G.; Hidalgo-Figueroa, S.; Rios, M.Y.; Ibarra-Barajas, M.; Estrada-Soto, S. Semisynthesis, ex vivo evaluation, and SAR studies of coumarin derivatives as potential antiasthmatic drugs. Eur. J. Med. Chem. 2014, 77, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Leal, L.K.A.M.; Silva, A.H.; de Barros Viana, G.S. Justicia pectoralis, a coumarin medicinal plant have potential for the development of antiasthmatic drugs? Rev. Bras. Farmacogn. 2017, 27, 794–802. [Google Scholar] [CrossRef]
- Wang, J.; Fu, Y.; Wei, Z.; He, X.; Shi, M.; Kou, J.; Zhou, E.; Liu, W.; Yang, Z.; Guo, C. Anti-asthmatic activity of osthole in an ovalbumin-induced asthma murine model. Respir. Physiol. Neurobiol. 2017, 239, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Piazzi, L.; Cavalli, A.; Colizzi, F.; Belluti, F.; Bartolini, M.; Mancini, F.; Recanatini, M.; Andrisano, V.; Rampa, A. Multi-target-directed coumarin derivatives: hAChE and BACE1 inhibitors as potential anti-Alzheimer compounds. Bioorganic Med. Chem. Lett. 2008, 18, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Shaik, J.B.; Palaka, B.K.; Penumala, M.; Kotapati, K.V.; Devineni, S.R.; Eadlapalli, S.; Darla, M.M.; Ampasala, D.R.; Vadde, R.; Amooru, G.D. Synthesis, pharmacological assessment, molecular modeling and in silico studies of fused tricyclic coumarin derivatives as a new family of multifunctional anti-Alzheimer agents. Eur. J. Med. Chem. 2016, 107, 219–232. [Google Scholar] [CrossRef]
- Patil, P.O.; Bari, S.B.; Firke, S.D.; Deshmukh, P.K.; Donda, S.T.; Patil, D.A. A comprehensive review on synthesis and designing aspects of coumarin derivatives as monoamine oxidase inhibitors for depression and Alzheimer’s disease. Bioorganic Med. Chem. 2013, 21, 2434–2450. [Google Scholar] [CrossRef]
- Ahmad, R.; Asad, M.; Siddiqui, Z.N. Evaluation of antipyretic and antinociceptive potential of new heterocyclic derivatives of 3-formyl-4-hydroxycoumarin in rats. Int. Res. J. Pharm. Appl. Sci. 2013, 3, 253–259. [Google Scholar]
- El-Sharkawy, K.A.; AlBratty, M.M.; Alhazmi, H.A. Synthesis of some novel pyrimidine, thiophene, coumarin, pyridine and pyrrole derivatives and their biological evaluation as analgesic, antipyretic and anti-inflammatory agents. Braz. J. Pharm. Sci. 2018, 54. [Google Scholar] [CrossRef]
- Sashidhara, K.V.; Kumar, A.; Kumar, M.; Sonkar, R.; Bhatia, G.; Khanna, A. Novel coumarin derivatives as potential antidyslipidemic agents. Bioorganic Med. Chem. Lett. 2010, 20, 4248–4251. [Google Scholar] [CrossRef]
- Sashidhara, K.V.; Kumar, A.; Kumar, M.; Srivastava, A.; Puri, A. Synthesis and antihyperlipidemic activity of novel coumarin bisindole derivatives. Bioorganic Med. Chem. Lett. 2010, 20, 6504–6507. [Google Scholar] [CrossRef]
- Asif, M. Pharmacologically potentials of different substituted coumarin derivatives. Chem. Int. 2015, 1, 1–11. [Google Scholar]
- Pari, L.; Rajarajeswari, N.; Saravanan, S.; Rathinam, A. Antihyperlipidemic effect of coumarin in experimental type 2 diabetic rats. Biomed. Prev. Nutr. 2014, 4, 171–176. [Google Scholar] [CrossRef]
- Keri, R.S.; Sasidhar, B.; Nagaraja, B.M.; Santos, M.A. Recent progress in the drug development of coumarin derivatives as potent antituberculosis agents. Eur. J. Med. Chem. 2015, 100, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhao, N.; Song, J.; Zhu, K.; Jiang, C.-s.; Shan, P.; Zhang, H. Design, synthesis and biological evaluation of novel coumarin-based hydroxamate derivatives as histone deacetylase (hdac) inhibitors with antitumor activities. Molecules 2019, 24, 2569. [Google Scholar] [CrossRef]
- Abdizadeh, T.; Kalani, M.R.; Abnous, K.; Tayarani-Najaran, Z.; Khashyarmanesh, B.Z.; Abdizadeh, R.; Ghodsi, R.; Hadizadeh, F. Design, synthesis and biological evaluation of novel coumarin-based benzamides as potent histone deacetylase inhibitors and anticancer agents. Eur. J. Med. Chem. 2017, 132, 42–62. [Google Scholar] [CrossRef]
- Niu, H.; Wang, W.; Li, J.; Lei, Y.; Zhao, Y.; Yang, W.; Zhao, C.; Lin, B.; Song, S.; Wang, S. A novel structural class of coumarin-chalcone fibrates as PPARα/γ agonists with potent antioxidant activities: Design, synthesis, biological evaluation and molecular docking studies. Eur. J. Med. Chem. 2017, 138, 212–220. [Google Scholar] [CrossRef]
- Terlain, B.; Thomas, J. Structure of griselimycin, polypeptide antibiotic extracted from streptomyces cultures. II. Structure of griselimycin. Bull. Soc. Chim. Fr. 1971, 6, 2357. [Google Scholar]
- Herrmann, J.; Rybniker, J.; Müller, R. Novel and revisited approaches in antituberculosis drug discovery. Curr. Opin. Biotechnol. 2017, 48, 94–101. [Google Scholar] [CrossRef]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Haney, E.F.; Vogel, H.J. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 2011, 29, 464–472. [Google Scholar] [CrossRef]
- Fjell, C.D.; Hiss, J.A.; Hancock, R.E.; Schneider, G. Designing antimicrobial peptides: Form follows function. Nat. Rev. Drug Discov. 2012, 11, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, A.; Pirri, G.; Nicoletto, S. Antimicrobial peptides: An overview of a promising class of therapeutics. Open Life Sci. 2007, 2, 1–33. [Google Scholar] [CrossRef]
- Bahar, A.A.; Ren, D. Antimicrobial peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef] [PubMed]
- Pushpanathan, M.; Gunasekaran, P.; Rajendhran, J. Antimicrobial peptides: Versatile biological properties. Int. J. Pept. 2013, 2013, 675391. [Google Scholar] [CrossRef] [PubMed]
- Jindal, M.; Le, C.; Mohd Yusof, M.; Sekaran, S. Net charge, hydrophobicity and specific amino acids contribute to the activity of antimicrobial peptides. J. Health Transl. Med. 2014, 17, 1–7. [Google Scholar]
- Abedinzadeh, M.; Gaeini, M.; Sardari, S. Natural antimicrobial peptides against Mycobacterium tuberculosis. J. Antimicrob. Chemother. 2015, 70, 1285–1289. [Google Scholar] [CrossRef]
- Kang, S.-J.; Kim, D.-H.; Mishig-Ochir, T.; Lee, B.-J. Antimicrobial peptides: Their physicochemical properties and therapeutic application. Arch. Pharmacal Res. 2012, 35, 409–413. [Google Scholar] [CrossRef]
- Linde, C.M.; Hoffner, S.E.; Refai, E.; Andersson, M. In vitro activity of PR-39, a proline-arginine-rich peptide, against susceptible and multi-drug-resistant Mycobacterium tuberculosis. J. Antimicrob. Chemother. 2001, 47, 575–580. [Google Scholar] [CrossRef]
- Hao, G.; Shi, Y.-H.; Tang, Y.-L.; Le, G.-W. The intracellular mechanism of action on Escherichia coli of BF2-A/C, two analogues of the antimicrobial peptide Buforin 2. J. Microbiol. 2013, 51, 200–206. [Google Scholar] [CrossRef]
- Fattorini, L.; Gennaro, R.; Zanetti, M.; Tan, D.; Brunori, L.; Giannoni, F.; Pardini, M.; Orefici, G. In vitro activity of protegrin-1 and beta-defensin-1, alone and in combination with isoniazid, against Mycobacterium tuberculosis. Peptides 2004, 25, 1075–1077. [Google Scholar] [CrossRef]
- Lan, Y.; Lam, J.T.; Siu, G.K.; Yam, W.C.; Mason, A.J.; Lam, J.K. Cationic amphipathic D-enantiomeric antimicrobial peptides with in vitro and ex vivo activity against drug-resistant Mycobacterium tuberculosis. Tuberculosis 2014, 94, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Santiago, B.; Hernandez-Pando, R.; Carranza, C.; Juarez, E.; Contreras, J.L.; Aguilar-Leon, D.; Torres, M.; Sada, E. Expression of cathelicidin LL-37 during Mycobacterium tuberculosis infection in human alveolar macrophages, monocytes, neutrophils, and epithelial cells. Infect. Immun. 2008, 76, 935–941. [Google Scholar] [CrossRef]
- Santos, J.C.; Silva-Gomes, S.; Silva, J.P.; Gama, M.; Rosa, G.; Gallo, R.L.; Appelberg, R. Endogenous cathelicidin production limits inflammation and protective immunity to Mycobacterium avium in mice. Immun. Inflamm. Dis. 2014, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, C.; Paul, M.; Xie, L.; Van Der Donk, W.A. Biosynthesis and mode of action of lantibiotics. Chem. Rev. 2005, 105, 633–684. [Google Scholar] [CrossRef] [PubMed]
- Guinane, C.; Cotter, P.; Hill, C.; Ross, R. Microbial solutions to microbial problems; lactococcal bacteriocins for the control of undesirable biota in food. J. Appl. Microbiol. 2005, 98, 1316–1325. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.; Draper, L.A.; O’Connor, P.M.; Coffey, A.; Hill, C.; Ross, R.P.; Cotter, P.D.; O’Mahony, J. Comparison of the activities of the lantibiotics nisin and lacticin 3147 against clinically significant mycobacteria. Int. J. Antimicrob. Agents 2010, 36, 132–136. [Google Scholar] [CrossRef]
- Yuk, J.-M.; Shin, D.-M.; Lee, H.-M.; Yang, C.-S.; Jin, H.S.; Kim, K.-K.; Lee, Z.-W.; Lee, S.-H.; Kim, J.-M.; Jo, E.-K. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe 2009, 6, 231–243. [Google Scholar] [CrossRef]
| Drug Class | Included Drugs |
|---|---|
| (A) Fluoroquinolones | Levofloxacin, gatifloxacin, moxifloxacin |
| (B) Second-line injectables | Streptomycin, kanamycin, amikacin, capreomycin |
| (C) Other core second-line drugs | Ethionamide, cycloserine/terizidone, prothionamide, linezolid, clofazimine |
| (D) Noncore, multidrug-resistant tubercular drugs | i. High dose—isoniazid, pyrazinamide, ethambutol ii. Delamanid and bedaquiline iii. Para-aminosalicylic acid, meropenem, cilastatin-imipenem, clarithromycin, clavulanate-amoxicillin |
| Drug Class | Lead Compounds | Molecular Targets/Mechanism of Action | MIC Range | References |
|---|---|---|---|---|
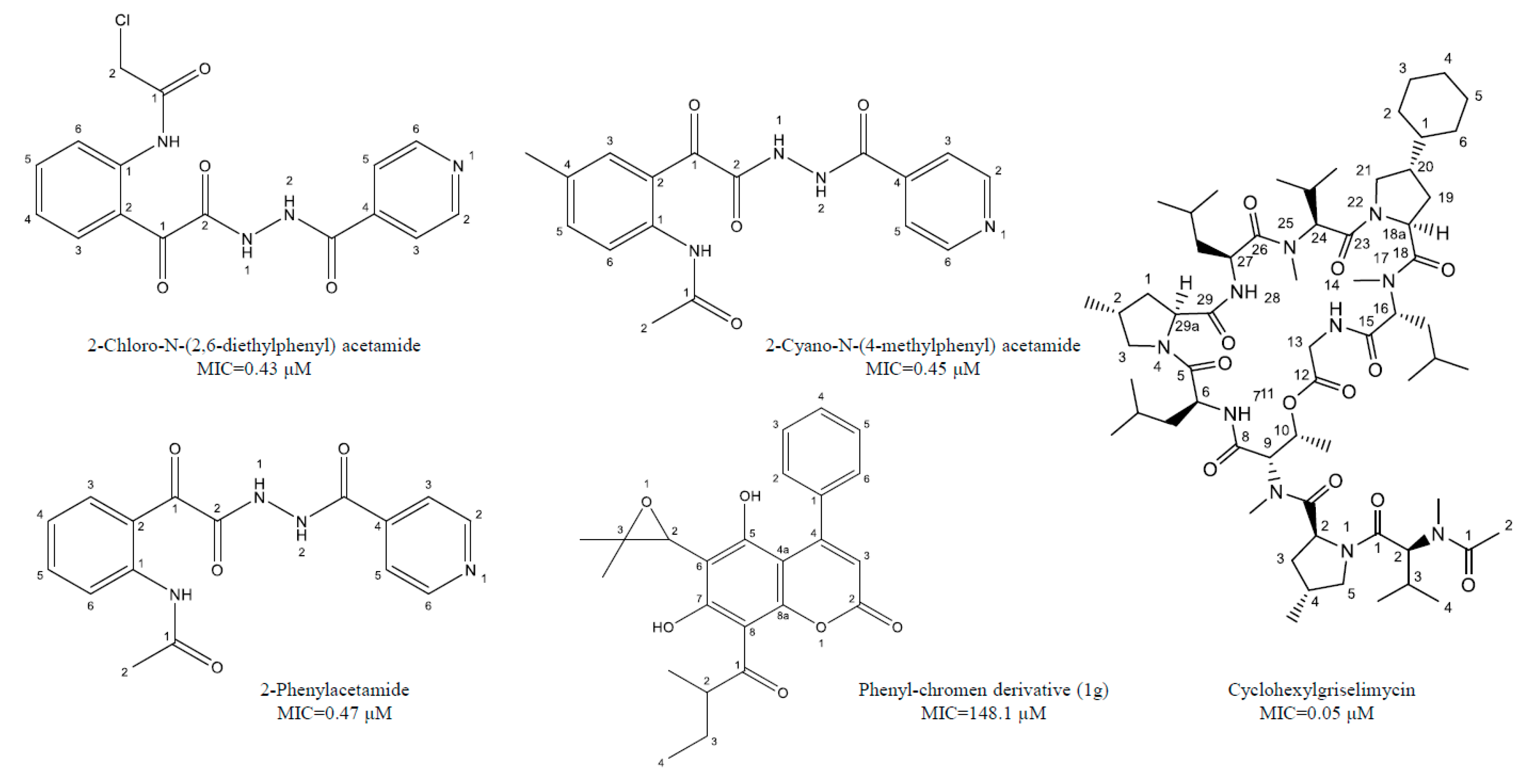
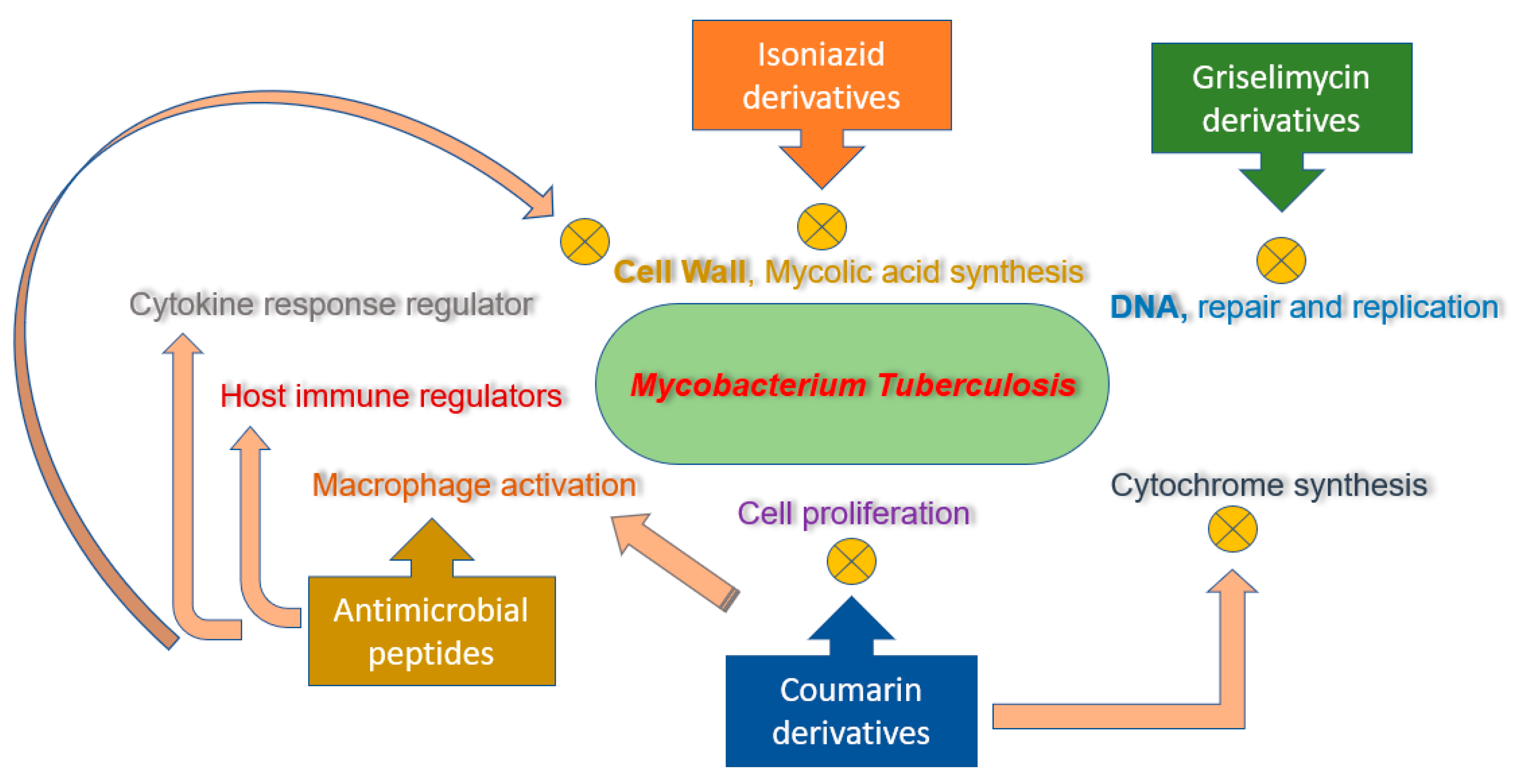
| Isoniazid derivatives | 2-Cyano-N-(4-methylphenyl) acetamide, 2-Chloro-N-(2,6-diethylphenyl) acetamide, 2-Phenylacetamide | Inhibition of mycolic acid synthesis and cell growth inhibitor | 0.43–0.47 μM | [38,39,40] |
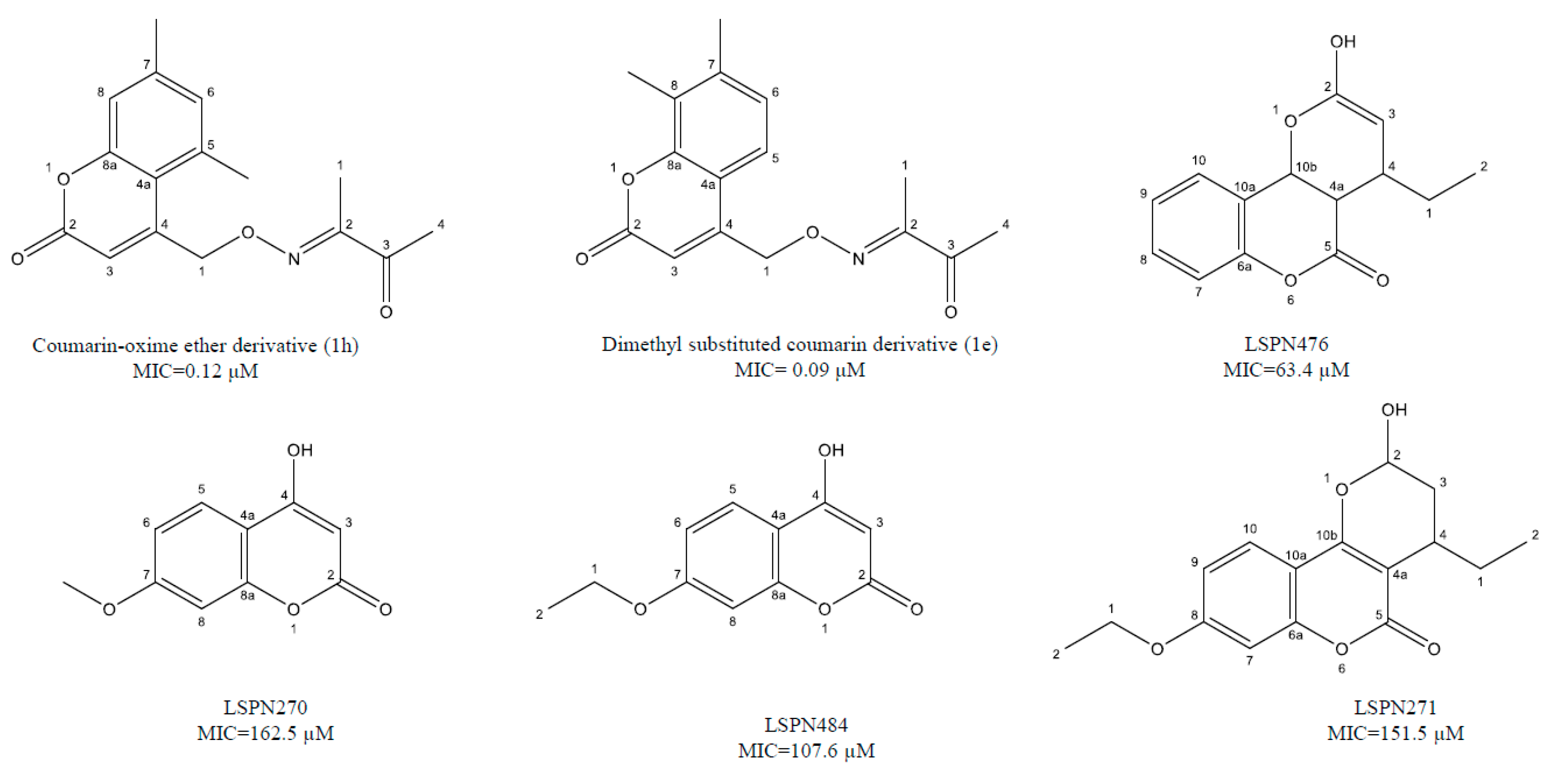
| Coumarin derivatives | 6-((3,3-dimethyloxiran-2-yl)-5,7-dihydroxy-8-(2-methylbutanoyl)-4-phenyl-2H-chromen-2-one (1g), Dimethyl substituted compound (1e), coumarin-oxime ether (1h), a coumarin-theophylline hybrid (3a), LSPN270, LSPN271, LSPN476, and LSPN484 | Cell proliferation inhibitors, cytochrome synthesis disruption, and macrophages activation | 0.12–148 μM | [41,42,43,44,45] |
| Griselimycin derivatives | Cyclohexyl griselimycin | Inhibits DNA repair and replication | 0.05–0.17 μM | [46,47,48,49] |
| Antimicrobial peptides | Bacteriocins (Bcn1–Bcn5), protegrin-1, nisin S, D-V13 K, cathelicidin LL37, D-LAK120-A, and D-LAK120-HP13 | Multifunctional host immune regulators, pro-inflammatory cytokine responses regulator, calcium influx, and apoptosis | 0.01–30 μM | [50,51,52,53,54,55,56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghamdi, S.; Rehman, S.U.; Shesha, N.T.; Faidah, H.; Khurram, M.; Rehman, S.U. Promising Lead Compounds in the Development of Potential Clinical Drug Candidate for Drug-Resistant Tuberculosis. Molecules 2020, 25, 5685. https://doi.org/10.3390/molecules25235685
Alghamdi S, Rehman SU, Shesha NT, Faidah H, Khurram M, Rehman SU. Promising Lead Compounds in the Development of Potential Clinical Drug Candidate for Drug-Resistant Tuberculosis. Molecules. 2020; 25(23):5685. https://doi.org/10.3390/molecules25235685
Chicago/Turabian StyleAlghamdi, Saad, Shaheed Ur Rehman, Nashwa Talaat Shesha, Hani Faidah, Muhammad Khurram, and Sabi Ur Rehman. 2020. "Promising Lead Compounds in the Development of Potential Clinical Drug Candidate for Drug-Resistant Tuberculosis" Molecules 25, no. 23: 5685. https://doi.org/10.3390/molecules25235685
APA StyleAlghamdi, S., Rehman, S. U., Shesha, N. T., Faidah, H., Khurram, M., & Rehman, S. U. (2020). Promising Lead Compounds in the Development of Potential Clinical Drug Candidate for Drug-Resistant Tuberculosis. Molecules, 25(23), 5685. https://doi.org/10.3390/molecules25235685







