Angular Regioselectivity in the Reactions of 2-Thioxopyrimidin-4-ones and Hydrazonoyl Chlorides: Synthesis of Novel Stereoisomeric Octahydro[1,2,4]triazolo[4,3-a]quinazolin-5-ones
Abstract
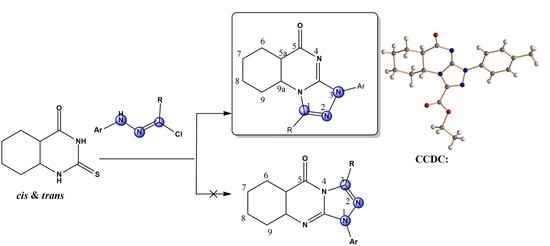
1. Introduction
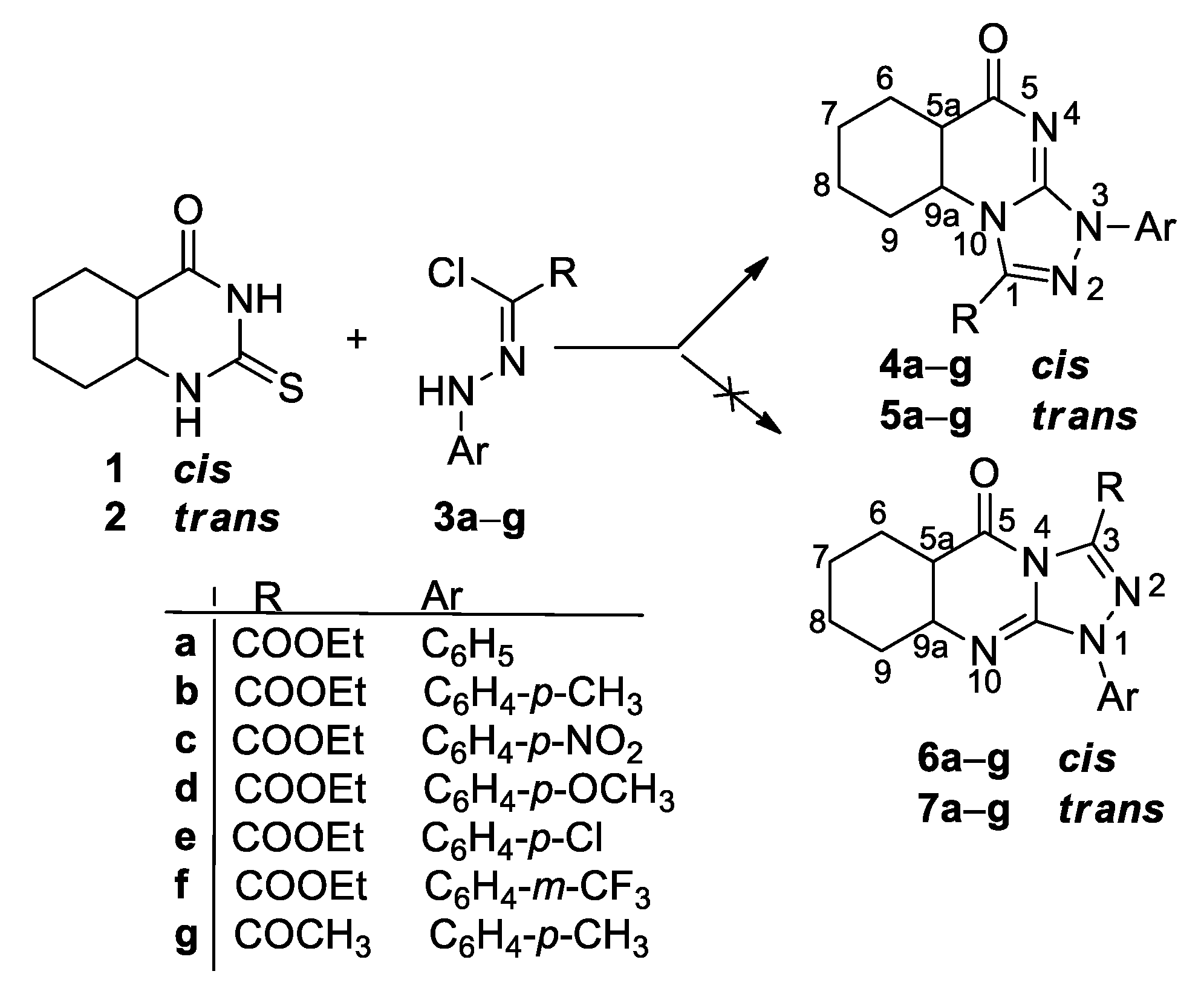
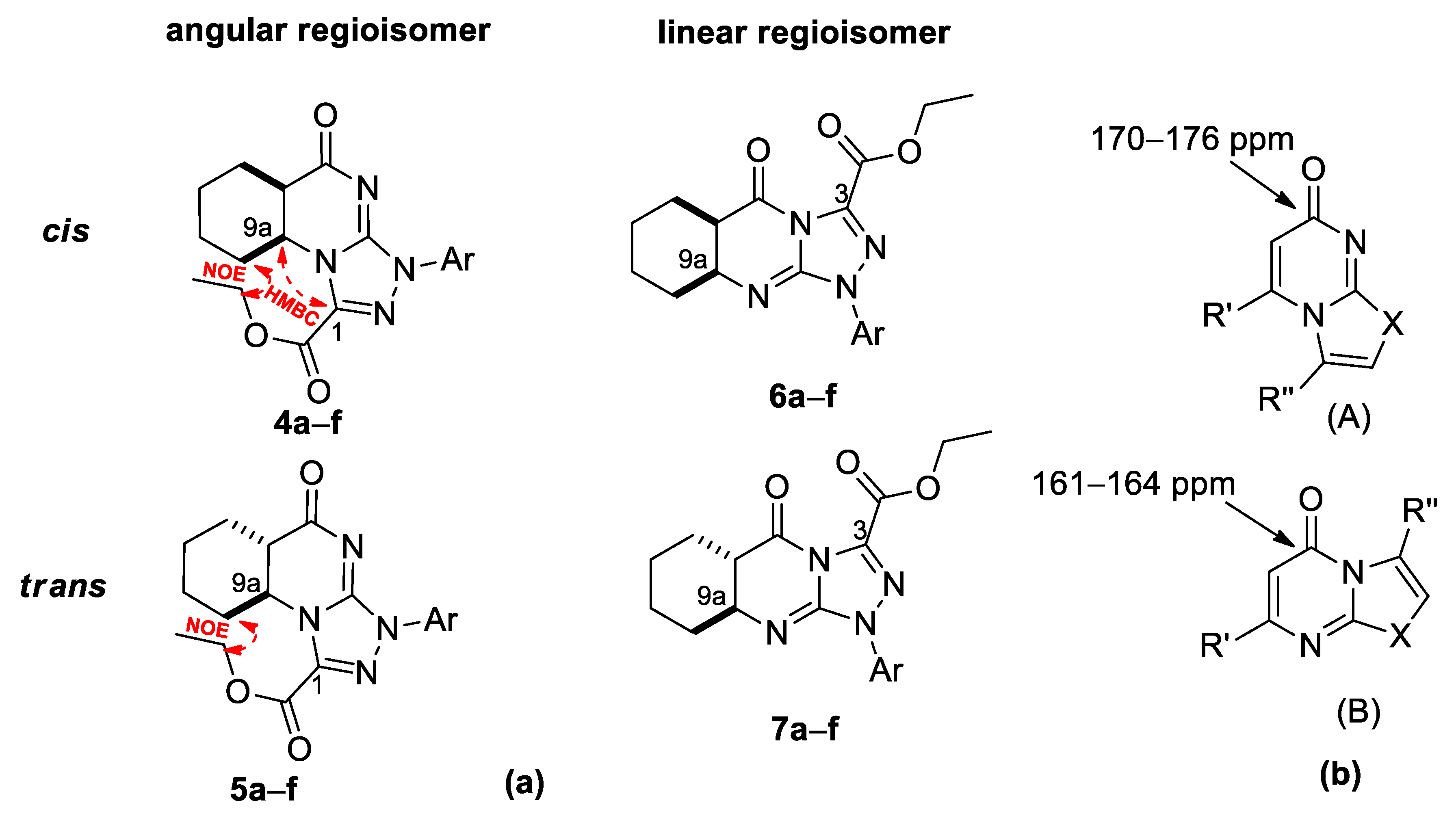
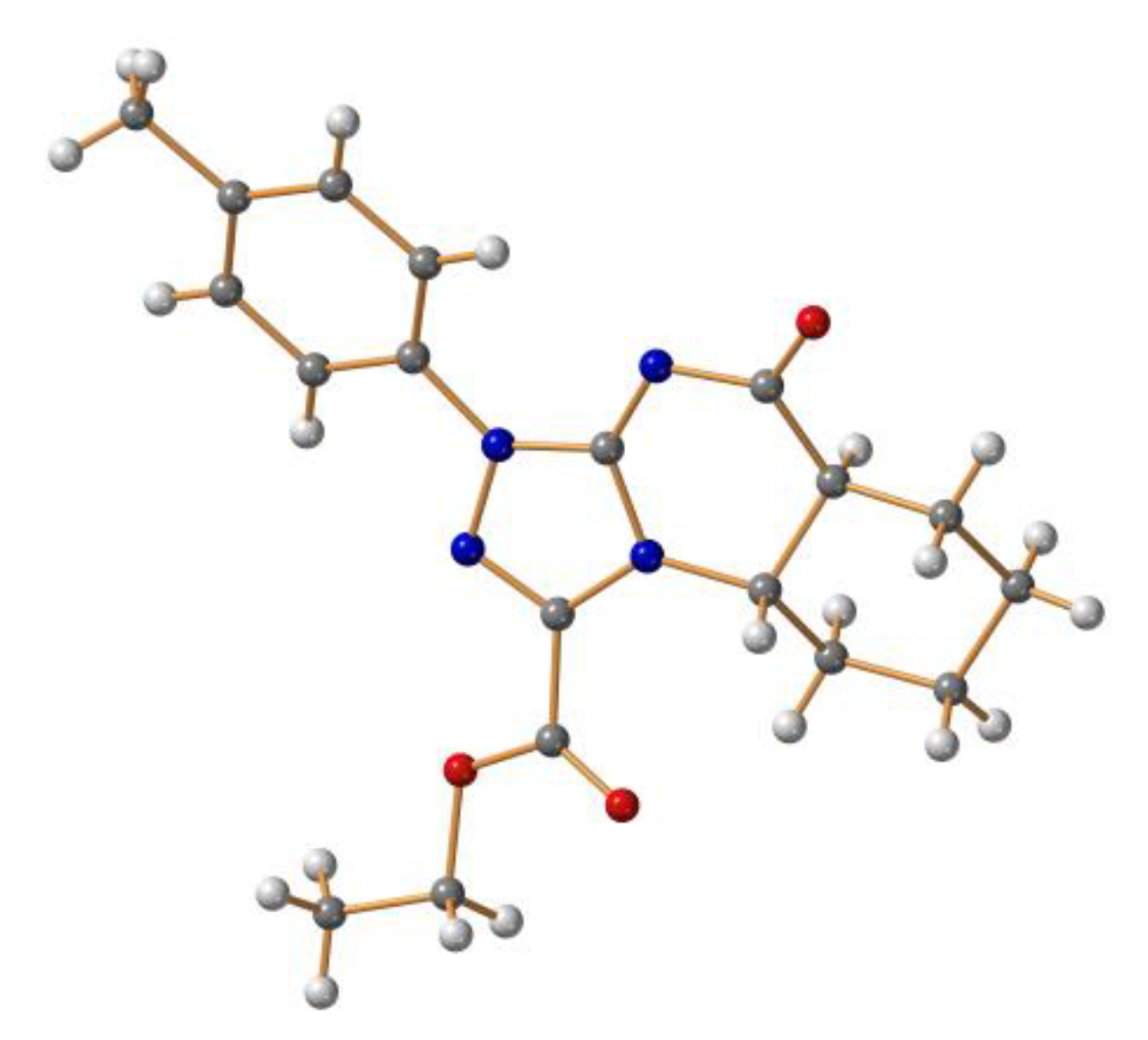
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. Synthesis of Cis- and Trans-[1,2,4]triazolo[4,3-a]quinazolin-5(3H)-one 4a–g and 5a–g
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fan, W.-Q.; Katritzky, A.R. 1,2,3-Triazoles. In Comprehensive Heterocycle Chemistry II; Katritzky, A.R., Rees, C.W., Scriven, E.F.V., Eds.; Pergamon Press: New York, NY, USA, 1996; Volume 4, pp. 1–126. [Google Scholar]
- Su, N.N.; Li, Y.; Yu, S.J.; Zhang, X.; Liu, X.H.; Zhao, W.G. Microwave-assisted synthesis of some novel 1,2,3-triazoles by click chemistry, and their biological activity. Res. Chem. Intermed. 2013, 39, 759–766. [Google Scholar] [CrossRef]
- Astakhov, A.V.; Chernyshev, V.M. Molecular structure of 3-amino[1,2,4]triazolo-[4,3-a] pyrimidin-5-one in various tautomeric forms: Investigation by DFT and QTAIM methods. Chem. Heterocycl. Compd. 2014, 50, 319–326. [Google Scholar] [CrossRef]
- Abdelhamid, A.O.; Gomha, S.M.; Abdelriheem, N.A.; Kandeel, S.M. Synthesis of New 3-Heteroarylindoles as Potential Anticancer Agents. Molecules 2016, 21, 929. [Google Scholar] [CrossRef]
- Gomha, S.M. A facile one-pot synthesis of 6,7,8,9-tetrahydrobenzo[4,5]thieno[2,3-d]-1,2,4-triazolo [4,5-a]pyrimidin-5-ones. Mon. Chem. 2009, 140, 213–220. [Google Scholar] [CrossRef]
- Fares, M.; Abou-Seri, S.M.; Abdel-Aziz, H.A.; Abbas, S.E.S.; Youssef, M.M.; Eladwy, R.A. Synthesis and antitumor activity of pyrido[2,3-d]pyrimidine and pyrido[2,3-d][1,2,4]triazolo[4,3-a]pyrimidine derivatives that induce apoptosis through G(1) cell-cycle arrest. Eur. J. Med. Chem. 2014, 83, 155–166. [Google Scholar] [CrossRef]
- Gomha, S.M.; Ahmed, S.A.; Abdelhamid, A.O. Synthesis and cytotoxicity evaluation of some novel thiazoles, thiadiazoles, and pyrido[2,3-d][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one incorporating triazole moiety. Molecules 2015, 20, 1357–1376. [Google Scholar] [CrossRef]
- Liu, X.H.; Sun, Z.H.; Yang, M.Y.; Tan, C.X.; Weng, J.Q.; Zhang, Y.G.; Ma, Y. Microwave assistant one pot synthesis, crystal structure, antifungal activities and 3D-QSAR of novel 1,2,4-triazolo[4,3-a]pyridines. Chem. Biol. Drug Des. 2014, 84, 342–347. [Google Scholar] [CrossRef]
- Gomha, S.M.; Badrey, M.G. Ecofriendly regioselective one-pot synthesis of chromeno[4,3-d][1,2,4]triazolo [4,3-a]pyrimidine. Eur. J. Chem. 2013, 4, 180–184. [Google Scholar] [CrossRef]
- Dieckmann, W.; Platz, L. Ueber eine neue Bildungsweise von Osotetrazonen. Ber. Dtsch. Chem. Ges. 1905, 38, 2986–2990. [Google Scholar] [CrossRef]
- Silvestri, R.; Cascio, M.G.; La Regina, G.; Piscitelli, F.; Lavecchia, A.; Brizzi, A.; Pasquini, S.; Botta, M.; Novellino, E.; Di Marzo, V.; et al. Synthesis, cannabinoid receptor affinity, and molecular modeling studies of substituted 1-aryl-5-(1H-pyrrol-1-yl)-1H-pyrazole-3-carboxamides. J. Med. Chem. 2008, 51, 1560–1576. [Google Scholar] [CrossRef]
- Liu, J.; Nie, M.; Wang, Y.; Hu, J.; Zhang, F.; Gao, Y.; Liu, Y.; Gong, P. Design, synthesis and structure-activity relationships of novel 4-phenoxyquinoline derivatives containing 1,2,4-triazolone moiety as c-Met kinase inhibitors. Eur. J. Med. Chem. 2016, 123, 431–446. [Google Scholar] [CrossRef]
- Abdelhamid, A.O.; Shawali, A.S.; Gomha, S.M.; El-Enany, W.M.A. Synthesis and antimicrobial evaluation of some novel thiazole, 1,3,4-thiadiazole and pyrido[2,3-d][1,2,4]triazolo[4,3-a]pyrimidine derivatives incorporating pyrazole moiety. Heterocycles 2015, 91, 2126–2142. [Google Scholar] [CrossRef]
- Riyadh, S.M. Enaminones as building blocks for the synthesis of substituted pyrazoles with antitumor and antimicrobial activities. Molecules 2011, 16, 1834–1853. [Google Scholar] [CrossRef]
- Said, A.I.; Palkó, M.; Haukka, M.; Fülöp, F. Retro Diels Alder Protocol for Regioselective Synthesis of Novel [1,2,4]triazolo[4,3-a]pyrimidin-7(1H)-ones. RSC Adv. 2020, 10, 33937–33943. [Google Scholar] [CrossRef]
- Said, A.I.; Haukka, M.; Fülöp, F. Microwave-Assisted Regioselective Synthesis of Variously Functionalized [1,2,4]triazolo[3,4-b]quinazolin-5(1H)-ones. Curr. Org. Chem. 2020, 24, 1892–1896. [Google Scholar] [CrossRef]
- Hassaneen, H.M.; Abdelhadi, H.A.; Abdallah, T.A. Novel synthesis of 1,2,4-triazolo[4,3-a]pyrimidin-5-one derivatives. Tetrahedron 2001, 57, 10133–10138. [Google Scholar] [CrossRef]
- Hassneen, H.M.; Abdallah, T.A. New Routes to Pyridino[2,3-d]pyrimidin-4-one and Pyridino[2,3-d] triazolino[4,5-a]pyrimidin-5-one Derivatives. Molecules 2003, 8, 333–341. [Google Scholar] [CrossRef]
- Abdel Hafez, N.A.; Farghaly, T.A.; Al-Omar, M.A.; Abdall, M.M. Synthesis of bioactive polyheterocyclic ring systems as 5α-reductase inhibitors. Eur. J. Med. Chem. 2010, 45, 4838–4844. [Google Scholar] [CrossRef]
- Abdallah, M.A.; Gomha, S.M.; Morad, M.A.; Elaasser, M.M. Synthesis of Pyridotriazolopyrimidines as Antitumor Agents. J. Heterocyclic Chem. 2017, 54, 1242–1251. [Google Scholar] [CrossRef]
- Abdallah, T.A.; Darwish, M.A.; Hassaneen, H.M. A Novel Synthesis of 1,2,4-Triazolopteridines. Molecules 2002, 7, 494–500. [Google Scholar] [CrossRef]
- Sohár, P.; Szöke-Molnár, Z.; Stájer, G.; Bernáth, G. Preparation and structure of cycloalkane-condensed [1,3]thiazino[3,2-a] pyrimidinones. Magn. Reson. Chem. 1989, 27, 959–963. [Google Scholar] [CrossRef]
- Elliott, A.J.; Callaghan, P.D.; Gibson, M.S.; Nemeth, S.T. Rearrangement of arylthiohydrazonates. Can. J. Chem. 1975, 53, 1484–1490. [Google Scholar] [CrossRef]
- Shawali, A.S.; Abbas, I.M.; Mahran, A.M. Facile Entries for Regioselective Synthesis of [1,2,4]Triazolo[4,3-a]pyrimidin-5(1H)-ones from 2-Thiouracil. JICS 2004, 1, 33–39. [Google Scholar] [CrossRef]
- Stajer, G.; Szabo, A.E.; Sohar, P. Synthesis and structure of norbornane/ene-fused thiouracils and thiazino[3,2-a]pyrimidinones. Heterocycles 1999, 51, 1849–1854. [Google Scholar] [CrossRef]
- Stájer, G.; Szabó, A.E.; Pintye, J.; Bernáth, G.; Sohár, P. Stereochemical Studies. Part 86. Saturated Heterocycles. Part 81 Preparation of New Thiouracils via Retrodiene Decomposition of Methylene-bridged Quinazolone Thiones. J. Chem. Soc. Perkin Trans. I 1985, 2483–2487. [Google Scholar] [CrossRef]
- Soliman, H.M.; Basuny, A.M.; Arafat, S.M. Utilization of Stearic acid Extracted from Olive Pomace for Production of Triazoles, Thiadiazoles and Thiadiazines Derivatives of Potential Biological Activities. J. Oleo. Sci. 2015, 64, 1019–1032. [Google Scholar] [CrossRef]
- Matiychuk, V.S.; Potopnyk, M.A.; Luboradzki, R.; Obushak, M.D. New Method for the Synthesis of 1-Aryl-1,2,4-triazole Derivatives. Synthesis 2011, 11, 1799–1813. [Google Scholar] [CrossRef]
- Rikagu Oxford Diffraction. CrysAlisPro; Agilent Technologies Inc.: Yarnton, UK, 2018. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Cryst. 2011, 44, 1281–1284. [Google Scholar] [CrossRef]
Sample Availability: Samples of all compounds are available from the authors. |

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Said, A.I.; Palkó, M.; Haukka, M.; Fülöp, F. Angular Regioselectivity in the Reactions of 2-Thioxopyrimidin-4-ones and Hydrazonoyl Chlorides: Synthesis of Novel Stereoisomeric Octahydro[1,2,4]triazolo[4,3-a]quinazolin-5-ones. Molecules 2020, 25, 5673. https://doi.org/10.3390/molecules25235673
Said AI, Palkó M, Haukka M, Fülöp F. Angular Regioselectivity in the Reactions of 2-Thioxopyrimidin-4-ones and Hydrazonoyl Chlorides: Synthesis of Novel Stereoisomeric Octahydro[1,2,4]triazolo[4,3-a]quinazolin-5-ones. Molecules. 2020; 25(23):5673. https://doi.org/10.3390/molecules25235673
Chicago/Turabian StyleSaid, Awad I., Márta Palkó, Matti Haukka, and Ferenc Fülöp. 2020. "Angular Regioselectivity in the Reactions of 2-Thioxopyrimidin-4-ones and Hydrazonoyl Chlorides: Synthesis of Novel Stereoisomeric Octahydro[1,2,4]triazolo[4,3-a]quinazolin-5-ones" Molecules 25, no. 23: 5673. https://doi.org/10.3390/molecules25235673
APA StyleSaid, A. I., Palkó, M., Haukka, M., & Fülöp, F. (2020). Angular Regioselectivity in the Reactions of 2-Thioxopyrimidin-4-ones and Hydrazonoyl Chlorides: Synthesis of Novel Stereoisomeric Octahydro[1,2,4]triazolo[4,3-a]quinazolin-5-ones. Molecules, 25(23), 5673. https://doi.org/10.3390/molecules25235673