Cardioprotective Effect of Tangeretin by Inhibiting PTEN/AKT/mTOR Axis in Experimental Sepsis-Induced Myocardial Dysfunction
Abstract
1. Introduction
2. Results
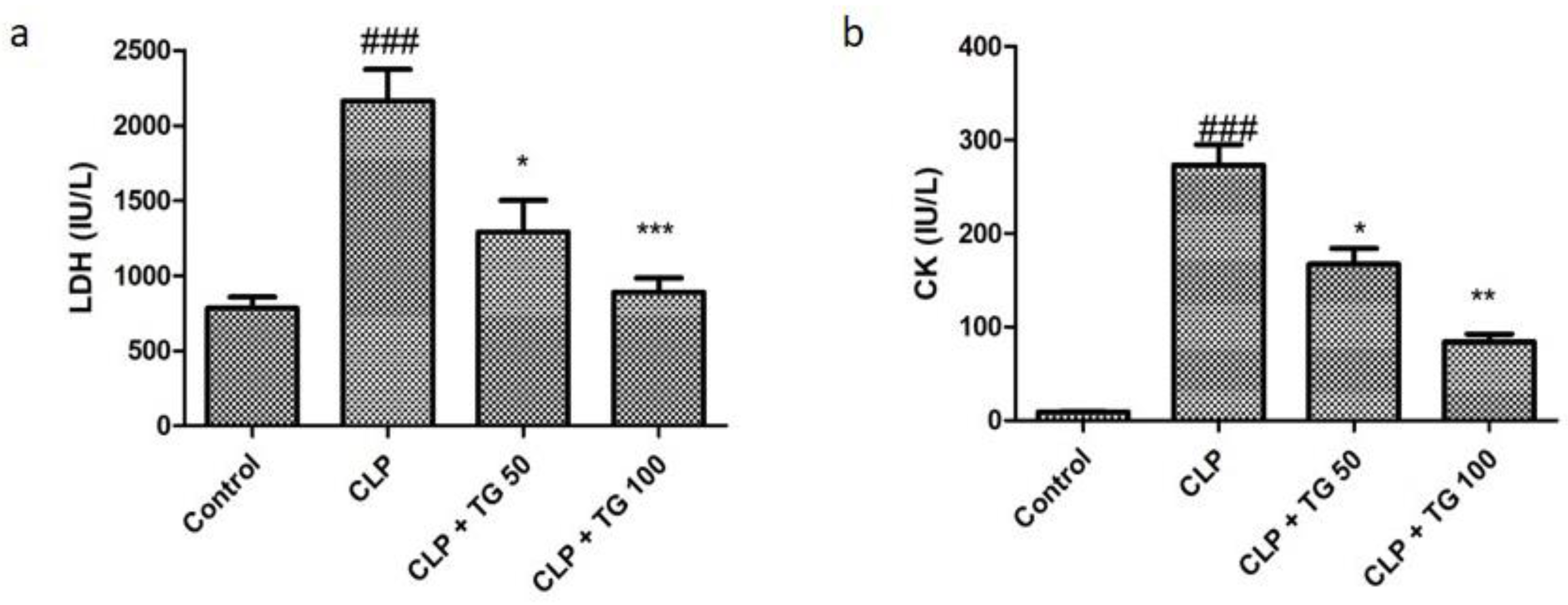
2.1. CLP Induced Sepsis and Sepsis Mediated Myocardial Dysfunction
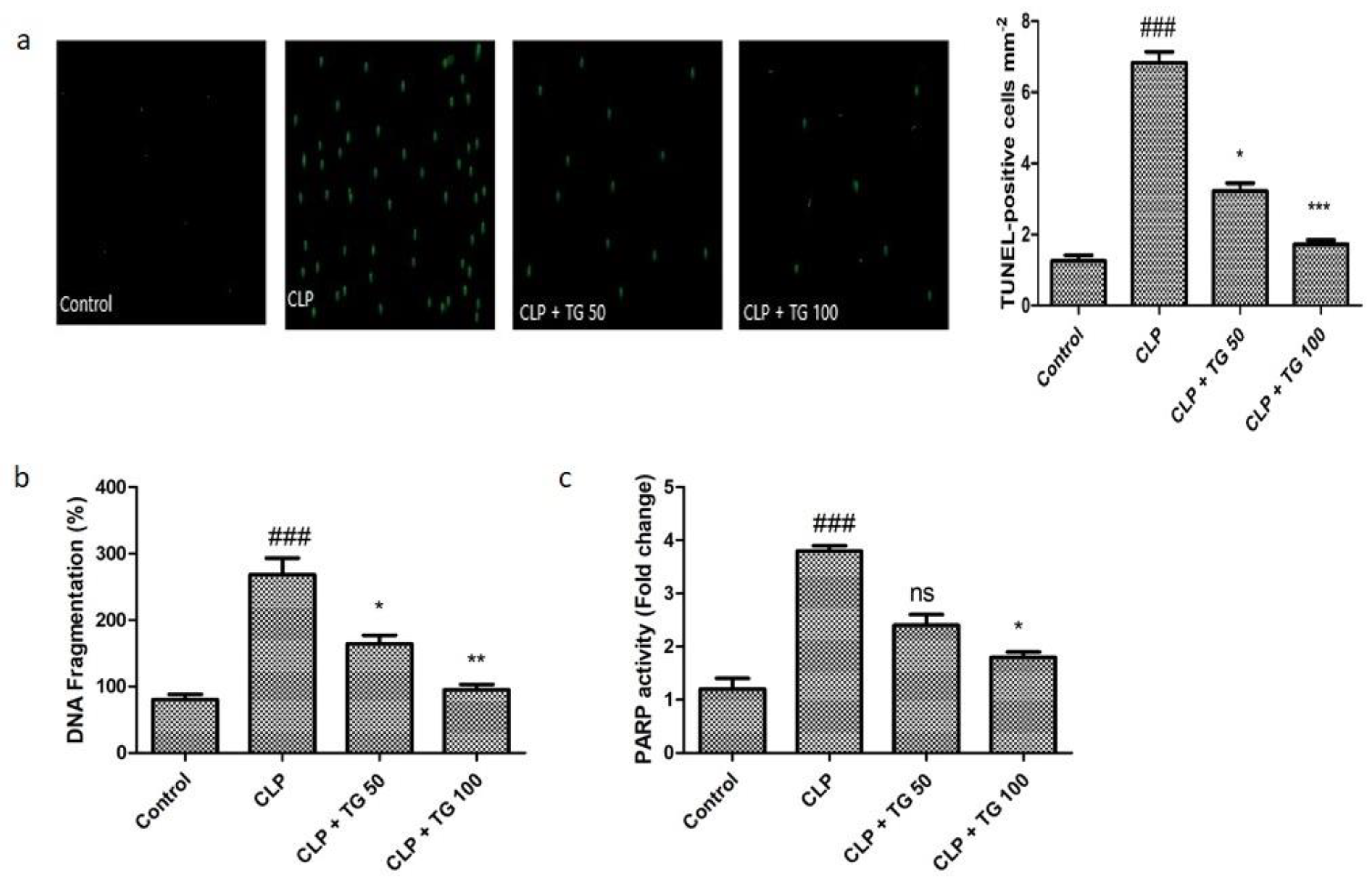
2.2. TUNEL Staining, Cell Death Markers, DNA Fragmentation, and PARP Activity
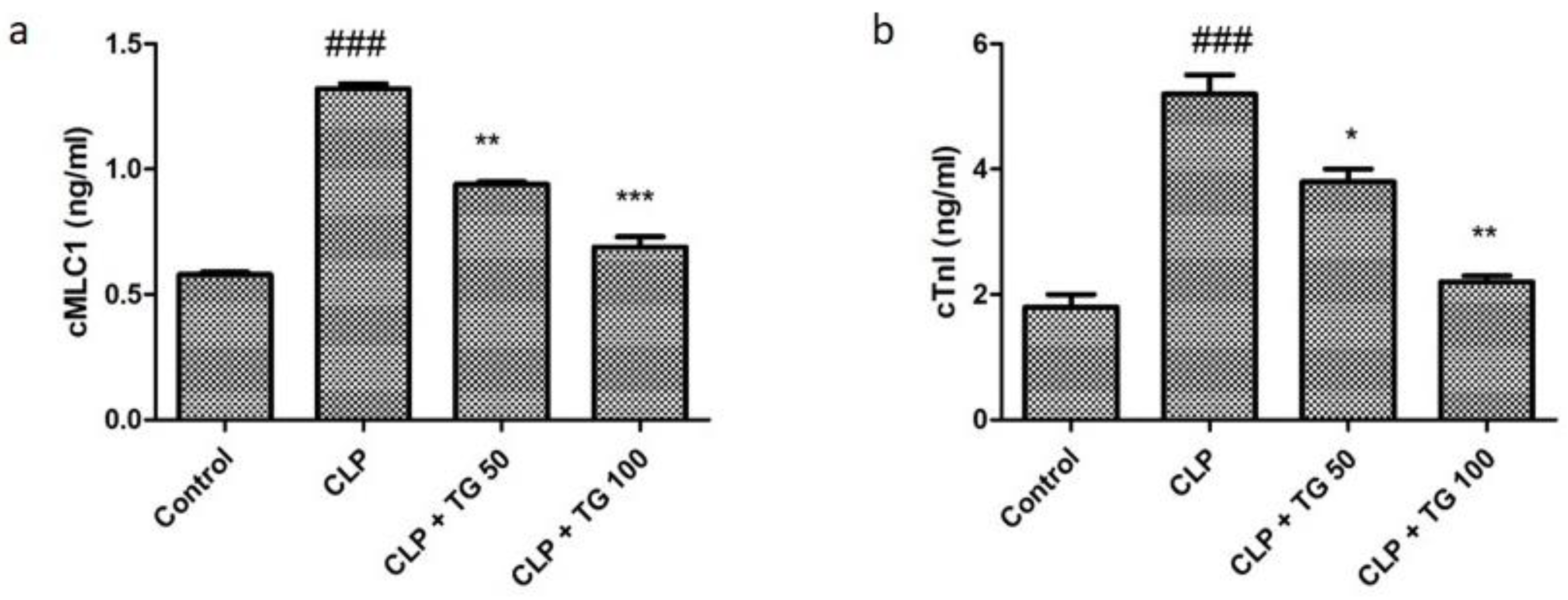
2.3. Analysis of Acute Myocardial Injury
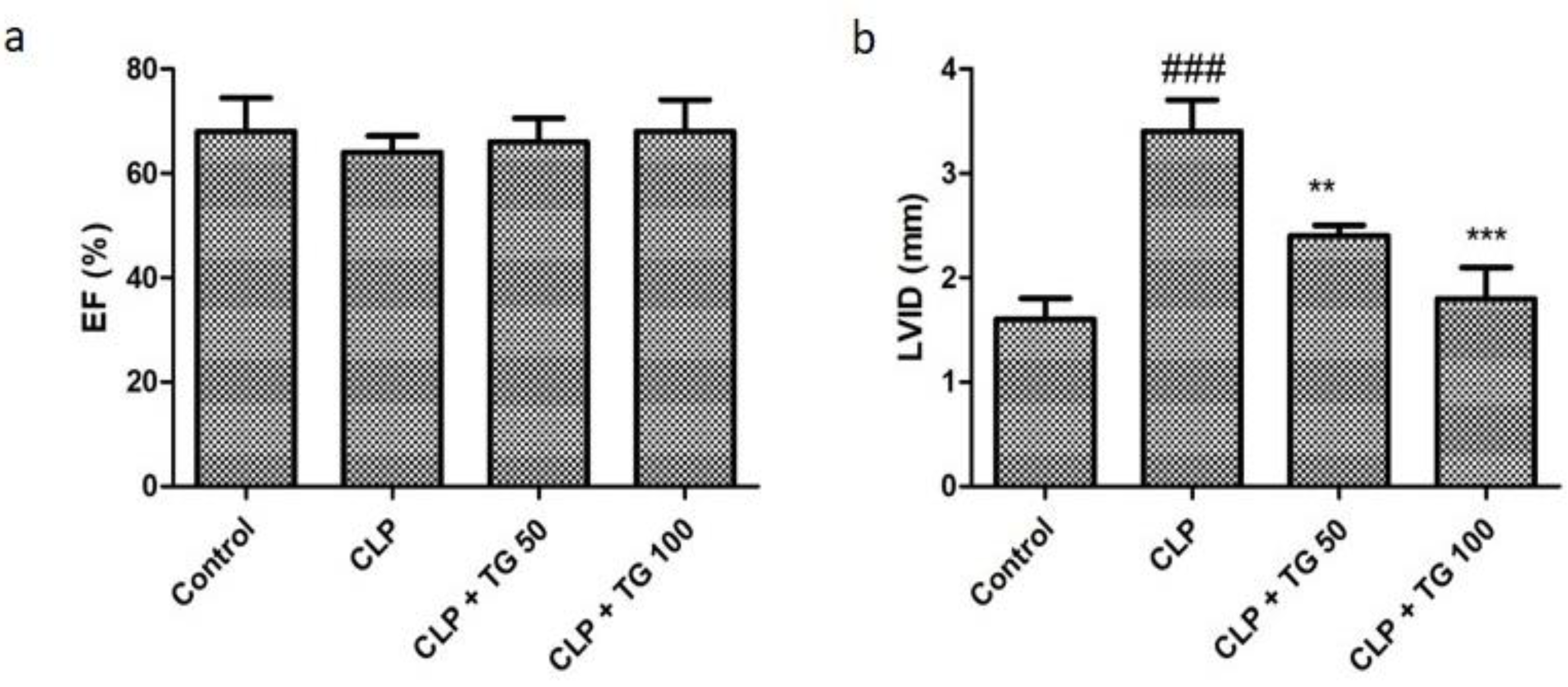
2.4. Echocardiography for Cardiac Functioning
2.5. Histopathology of Heart
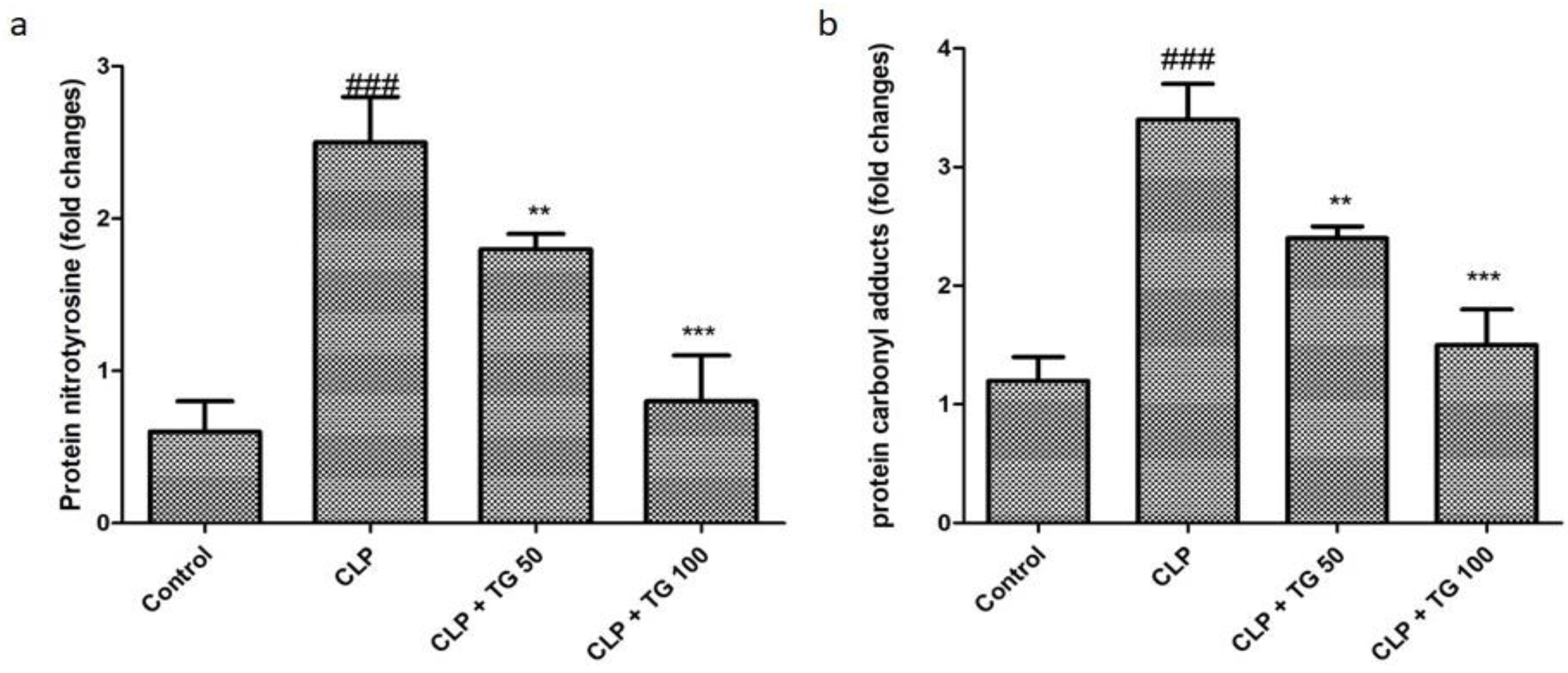
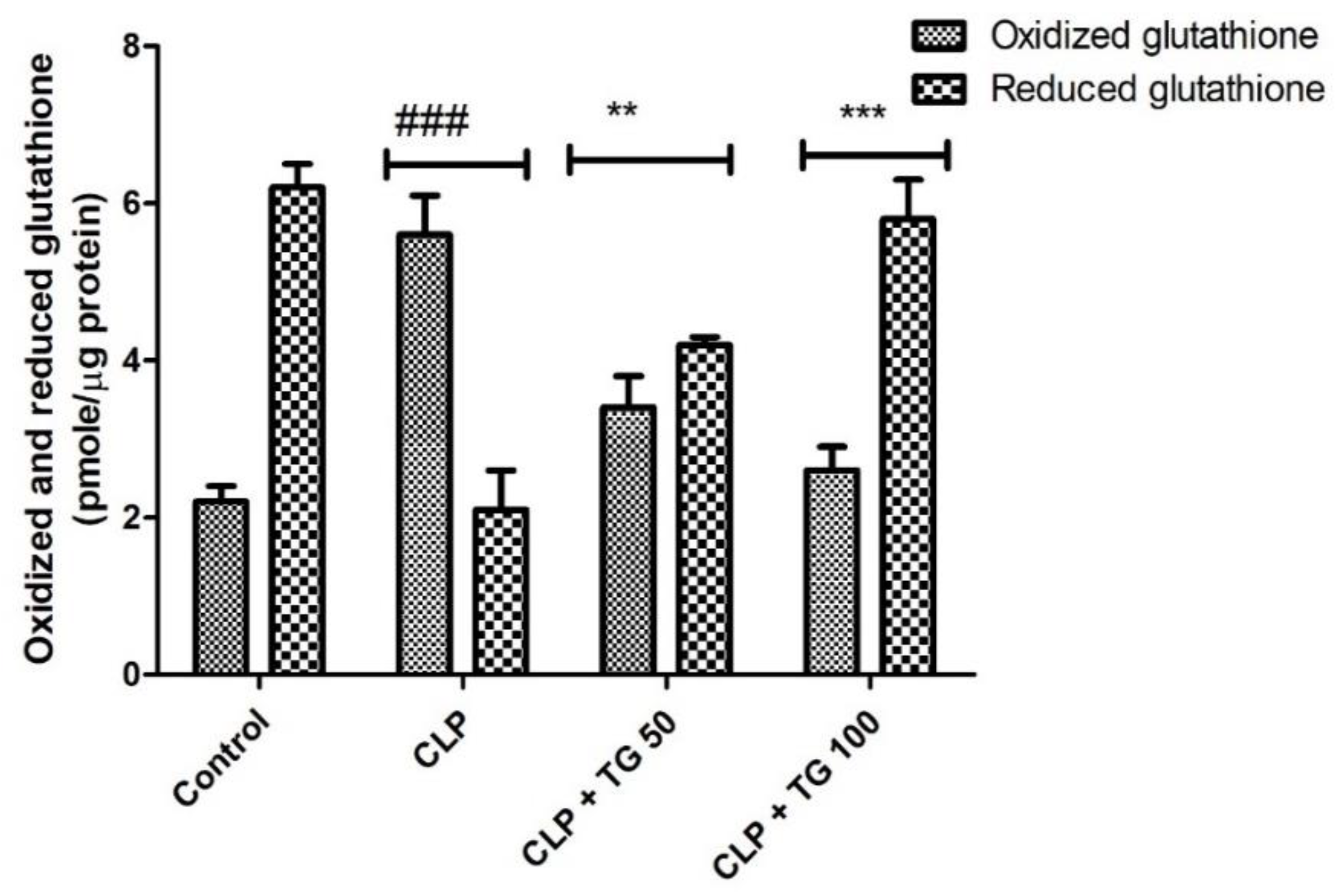
2.6. Estimation of Oxidative Markers
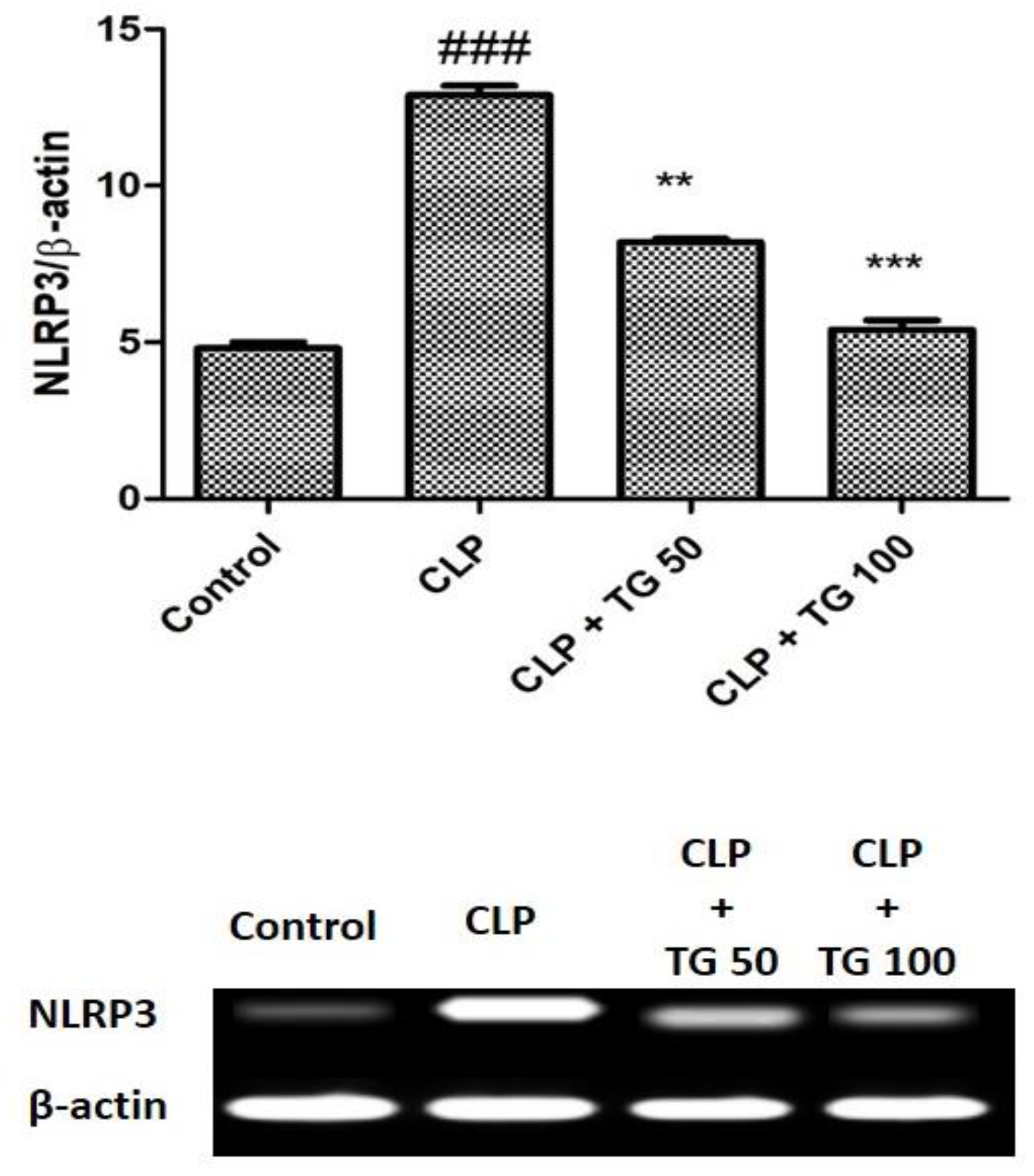
2.7. Analysis of NOD-Like Receptor Family, Pyrin Domain Containing 3 (NLRP3) Expression
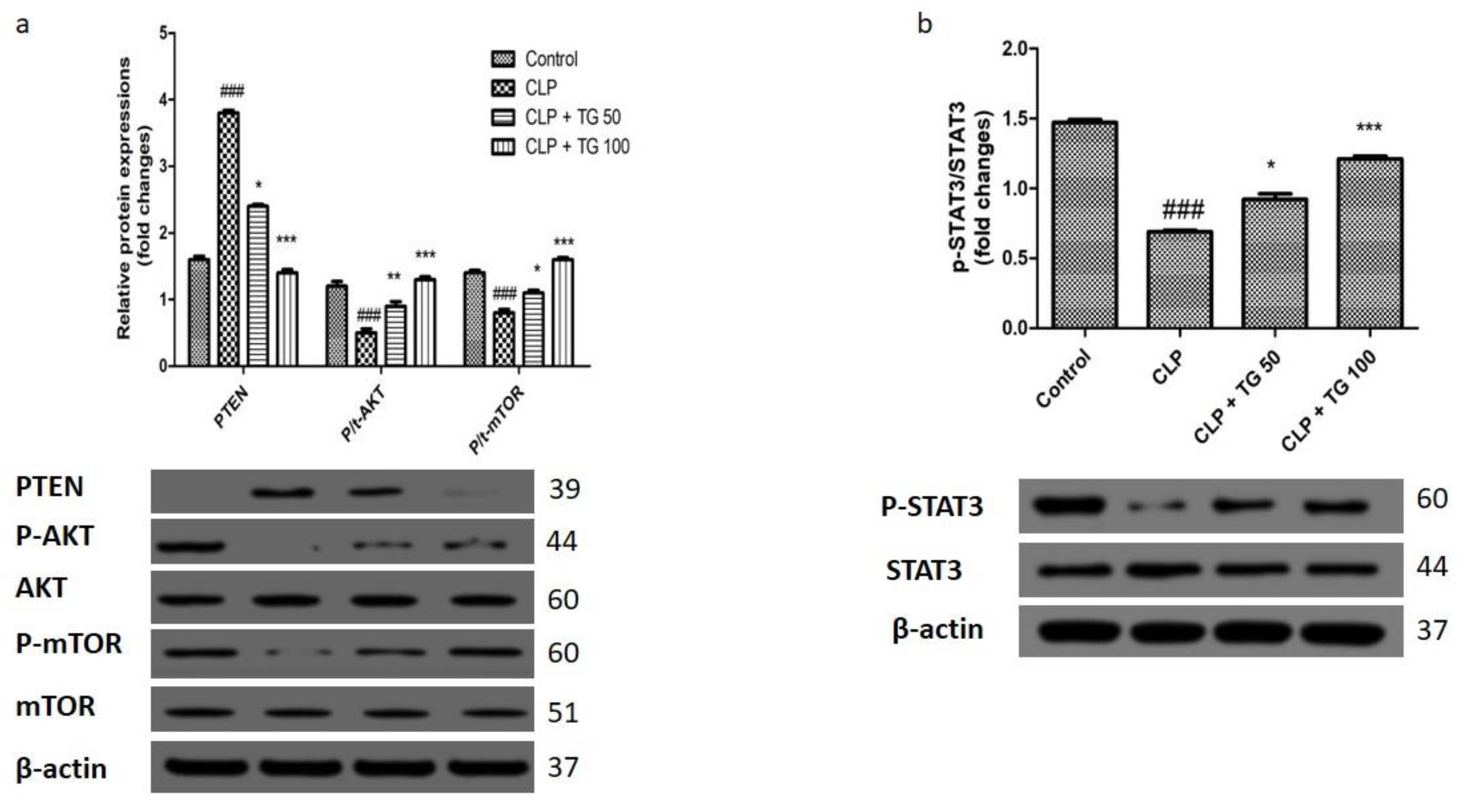
2.8. Analysis of Autophagy Markers Protein Light Chain 3 (LC)3, Cellular p62, PTEN/AKT/mTOR, and STAT3
3. Discussion
4. Materials and Methods
4.1. Materials Used
4.2. Animal Study
4.3. Tissue and Cardiac Injury Markers Analysis
4.4. Echocardiography
4.5. Histological Analysis
4.6. Cardiac TUNEL Staining
4.7. Cardiac Cell Death Markers
4.8. RT-PCR for NLRP3 Expression
4.9. Cardiac Glutathione Level
4.10. Cardiac Oxidative Stress Markers
4.11. Western Blot
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- An, R.; Zhao, L.; Xi, C.; Li, H.; Shen, G.; Liu, H.; Zhang, S.; Sun, L. Melatonin attenuates sepsis-induced cardiac dysfunction via a PI3K/Akt-dependent mechanism. Basic Res. Cardiol. 2016, 111, 8. [Google Scholar] [CrossRef]
- Ballard-Croft, C.; Maass, D.L.; Sikes, P.J.; Horton, J.W. Sepsis and burn complicated by sepsis alter cardiac transporter expression. Burns 2007, 33, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.W.; Li, Y.Z.; Liu, Z.Q.; Feng, S.L.; Zhou, H.; Liu, C.X.; Liu, L.; Xie, Y. Role of tangeretin as a potential bioavailability enhancer for silybin: Pharmacokinetic and pharmacological studies. Pharmacol. Res. 2018, 128, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Hagenlocher, Y.; Feilhauer, K.; Schäffer, M.; Bischoff, S.C.; Lorentz, A. Citrus peel polymethoxyflavones nobiletin and tangeretin suppress LPS- and IgE-mediated activation of human intestinal mast cells. Eur. J. Nutr. 2017, 56, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Y.; Lee, E.J.; Park, J.S.; Jang, S.E.; Kim, D.H.; Kim, H.S. Anti-Inflammatory and Antioxidant Mechanism of Tangeretin in Activated Microglia. J. Neuroimmune Pharmacol. 2016, 11, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.L.; Chang, W.S.; Lu, W.C.; Wei, G.J.; Wang, Y.; Ho, C.T.; Hwang, L.S. Pharmacokinetics, bioavailability, tissue distribution and excretion of tangeretin in rat. J. Food Drug Anal. 2018, 26, 849–857. [Google Scholar] [CrossRef]
- Zheng, J.; Shao, Y.; Jiang, Y.; Chen, F.; Liu, S.; Yu, N.; Zhang, D.; Liu, X.; Zou, L. Tangeretin inhibits hepatocellular carcinoma proliferation and migration by promoting autophagy-related BECLIN1. Cancer Manag. Res. 2019, 11, 5231–5242. [Google Scholar] [CrossRef]
- Liu, Z.J.; Liu, H.; Wu, C.; Xue, K. Effect of sepsis on the action potential and cardiac serotonin response in rats. Exp. Ther. Med. 2019, 18, 2207–2212. [Google Scholar] [CrossRef]
- Zhang, T.; Yin, Y.C.; Ji, X.; Zhang, B.; Wu, S.; Wu, X.Z.; Li, H.; Li, Y.D.; Ma, Y.L.; Wang, Y.; et al. AT1R knockdown confers cardioprotection against sepsis-induced myocardial injury by inhibiting the MAPK signaling pathway in rats. J. Cell. Biochem. 2020, 121, 25–42. [Google Scholar] [CrossRef]
- White, J.; Thomas, J.; Maass, D.L.; Horton, J.W. Cardiac effects of burn injury complicated by aspiration pneumonia-induced sepsis. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H47–H58. [Google Scholar] [CrossRef]
- Zhang, N.; Feng, H.; Liao, H.H.; Chen, S.; Yang, Z.; Deng, W.; Tang, Q.Z. Myricetin attenuated LPS induced cardiac injury in vivo and in vitro. Phytother. Res. 2018, 32, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zeng, Z.; Wu, C.; Liu, H. Tropisetron inhibits sepsis by repressing hyper-inflammation and regulating the cardiac action potential in rat models. Biomed. Pharmacother. 2019, 110, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Carlson, D.; Sun, Y.; Ma, L.; Wolf, S.E.; Minei, J.P.; Zang, Q.S. Mitochondrial ROS Induces Cardiac Inflammation via a Pathway through mtDNA Damage in a Pneumonia-Related Sepsis Model. PLoS ONE 2015, 10, e0139416. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, M.; Liu, L.; Tian, X.; Liang, Y. Protective effects of glucocorticoid on liver injury in a rat sepsis model. Exp. Ther. Med. 2019, 18, 3153–3160. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, H.; Chen, P. Sulfur dioxide attenuates sepsis-induced cardiac dysfunction via inhibition of NLRP3 inflammasome activation in rats. Nitric Oxide 2018, 81, 11–20. [Google Scholar] [CrossRef]
- Pai, M.H.; Wu, J.M.; Yang, P.J.; Lee, P.C.; Huang, C.C.; Yeh, S.L.; Lin, M.T. Antecedent Dietary Glutamine Supplementation Benefits Modulation of Liver Pyroptosis in Mice with Polymicrobial Sepsis. Nutrients 2020, 12, 1086. [Google Scholar] [CrossRef]
- Hoover, D.B.; Ozment, T.R.; Wondergem, R.; Li, C.; Williams, D.L. Impaired heart rate regulation and depression of cardiac chronotropic and dromotropic function in polymicrobial sepsis. Shock 2015, 43, 185–191. [Google Scholar] [CrossRef]
- Liu, L.; Jin, X.; Hu, C.F.; Li, R.; Zhou, Z.; Shen, C.X. Exosomes Derived from Mesenchymal Stem Cells Rescue Myocardial Ischaemia/Reperfusion Injury by Inducing Cardiomyocyte Autophagy Via AMPK and Akt Pathways. Cell. Physiol. Biochem. 2017, 43, 52–68. [Google Scholar] [CrossRef]
- Lee, K.H.; Cho, H.; Lee, S.; Woo, J.S.; Cho, B.H.; Kang, J.H.; Jeong, Y.M.; Cheng, X.W.; Kim, W. Enhanced-autophagy by exenatide mitigates doxorubicin-induced cardiotoxicity. Int. J. Cardiol. 2017, 232, 40–47. [Google Scholar] [CrossRef]
- Damy, T.; Kirsch, M.; Khouzami, L.; Caramelle, P.; Le Corvoisier, P.; Roudot-Thoraval, F.; Dubois-Randé, J.L.; Hittinger, L.; Pavoine, C.; Pecker, F. Glutathione deficiency in cardiac patients is related to the functional status and structural cardiac abnormalities. PLoS ONE 2009, 4, e4871. [Google Scholar] [CrossRef]
- Ho, E.; Karimi Galougahi, K.; Liu, C.C.; Bhindi, R.; Figtree, G.A. Biological markers of oxidative stress: Applications to cardiovascular research and practice. Redox Biol. 2013, 1, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Asif, A.H.; Nanjundan, P.; Basavarajappa, G.M.; SreeHarsha, N.; Chandur, U.; SRoopashree, T. Insulin Resistance Modulation with Lifestyle Modification-Proof-of-Concept Study in Rats. Indian J. Pharm. Educ. Res. 2020, 54. [Google Scholar] [CrossRef]


Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiroorkar, P.N.; Afzal, O.; Kazmi, I.; Al-Abbasi, F.A.; Altamimi, A.S.A.; Gubbiyappa, K.S.; Sreeharsha, N. Cardioprotective Effect of Tangeretin by Inhibiting PTEN/AKT/mTOR Axis in Experimental Sepsis-Induced Myocardial Dysfunction. Molecules 2020, 25, 5622. https://doi.org/10.3390/molecules25235622
Shiroorkar PN, Afzal O, Kazmi I, Al-Abbasi FA, Altamimi ASA, Gubbiyappa KS, Sreeharsha N. Cardioprotective Effect of Tangeretin by Inhibiting PTEN/AKT/mTOR Axis in Experimental Sepsis-Induced Myocardial Dysfunction. Molecules. 2020; 25(23):5622. https://doi.org/10.3390/molecules25235622
Chicago/Turabian StyleShiroorkar, Predeepkumar Narayanappa, Obaid Afzal, Imran Kazmi, Fahad A. Al-Abbasi, Abdulmalik Saleh Alfawaz Altamimi, Kumar Shiva Gubbiyappa, and Nagaraja Sreeharsha. 2020. "Cardioprotective Effect of Tangeretin by Inhibiting PTEN/AKT/mTOR Axis in Experimental Sepsis-Induced Myocardial Dysfunction" Molecules 25, no. 23: 5622. https://doi.org/10.3390/molecules25235622
APA StyleShiroorkar, P. N., Afzal, O., Kazmi, I., Al-Abbasi, F. A., Altamimi, A. S. A., Gubbiyappa, K. S., & Sreeharsha, N. (2020). Cardioprotective Effect of Tangeretin by Inhibiting PTEN/AKT/mTOR Axis in Experimental Sepsis-Induced Myocardial Dysfunction. Molecules, 25(23), 5622. https://doi.org/10.3390/molecules25235622






