Grapevine as a Rich Source of Polyphenolic Compounds
Abstract
1. Introduction
2. Extraction Techniques for Phenolic Compounds
2.1. Solid–Liquid Extraction
2.2. Ultrasound-Assisted Extraction
2.3. Microwave-Assisted Extraction
2.4. Enzyme-Assisted Extraction
3. Qualitative and Quantitative Analysis
4. Phenolic Compounds in Grapevines
4.1. Phenolic Acids
4.2. Stilbenes
4.3. Flavonoids
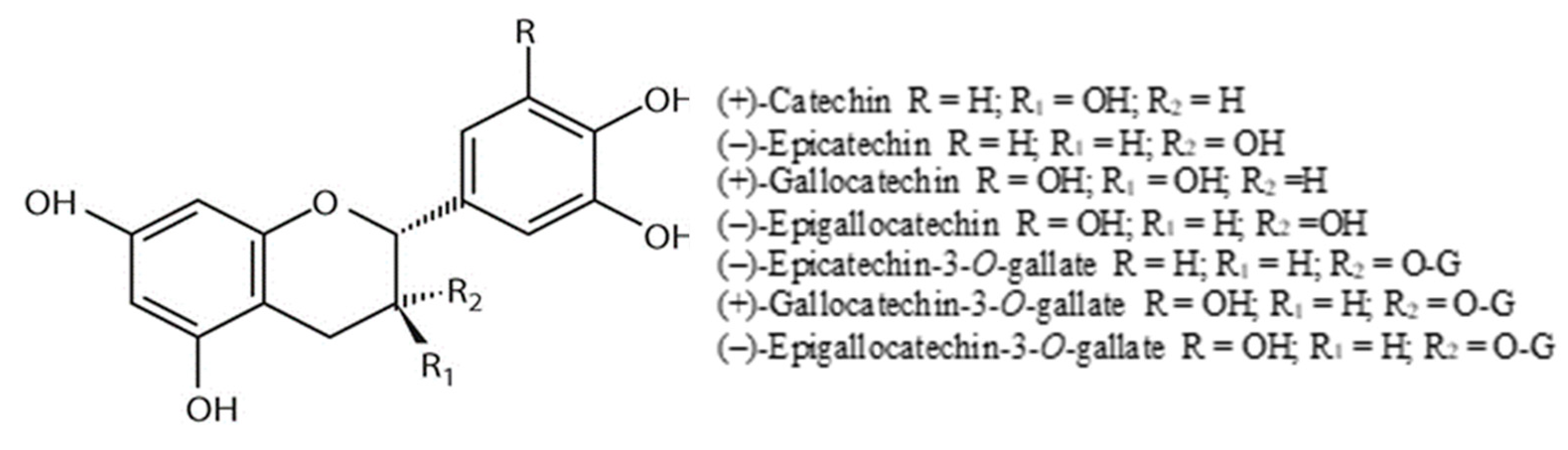
4.3.1. Anthocyanins
4.3.2. Flavonols
4.3.3. Flavan-3-ols
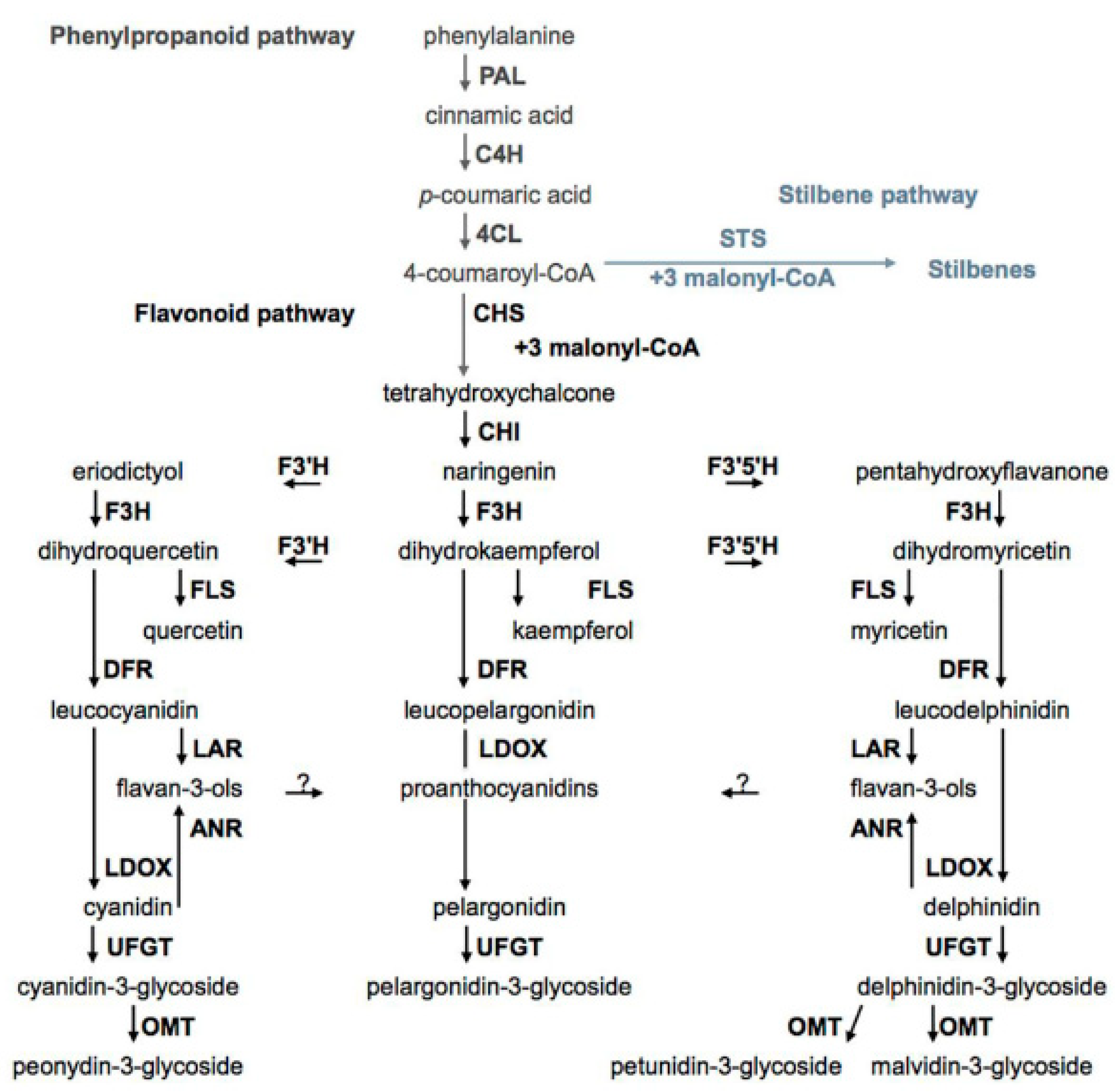
5. Biosynthesis of Phenolic Compounds
6. Grape Phenolics and Their Impact on Human Health
6.1. Antioxidant Activity
6.2. Anti-Inflammatory Activity
6.3. Cardiovascular Protection
6.4. Neuroprotective Activity
6.5. Anticancerogenic Activity
6.6. Antimicrobial Activity
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- OIV Statistical Report on World Vitiniculture 2018. Available online: http://www.oiv.int/public/medias/6371/oiv-statistical-report-on-world-vitiviniculture-2018.pdf (accessed on 10 May 2019).
- De Rosso, M.; Panighel, A.; Dalla Vedova, A.; Gardiman, M.; Flamini, R. Characterization of Non-Anthocyanic Flavonoids in Some Hybrid Red Grape Extracts Potentially Interesting for Industrial Uses. Molecules 2015, 20, 18095–18106. [Google Scholar] [CrossRef]
- De Rosso, M.; Tonidandel, L.; Larcher, R.; Nicolini, G.; Dalla Vedova, A.; De Marchi, F.; Gardiman, M.; Giust, M.; Flamini, R. Identification of new flavonols in hybrid grapes by combined liquid chromatography-mass spectrometry approaches. Food Chem. 2014, 163, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Kontić, J.K.; Jelušić, I.R.; Tomaz, I.; Preiner, D.; Marković, Z.; Stupić, D.; Andabaka, Ž.; Maletić, E. Polyphenolic Composition of the Berry Skin of Six Fungus-Resistant Red Grapevine Varieties. Int. J. Food Prop. 2015, 19, 1809–1824. [Google Scholar] [CrossRef]
- Ehrhardt, C.; Arapitsas, P.; Stefanini, M.; Flick, G.; Mattivi, F. Analysis of the phenolic composition of fungus-resistant grape varieties cultivated in Italy and Germany using UHPLC-MS/MS. J. Mass Spectrom. 2014, 49, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Myles, S.; Boyko, A.R.; Owens, C.L.; Brown, P.J.; Grassi, F.; Aradhya, M.K.; Prins, B.; Reynolds, A.; Chia, J.M.; Ware, D.; et al. Genetic structure and domestication history of the grape. P. Natl. Acad. Sci. USA 2011, 108, 3530–3535. [Google Scholar] [CrossRef] [PubMed]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouysegu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Ang. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Tomaz, I. Optimization of the Sample Preparation for Analysis of Polyphenolic Compounds in the Grape Skin by High Performance Liquid Chromatography. Ph.D. Thesis, University of Zagreb, Zagreb, 2016. [Google Scholar]
- Brossaud, F.; Cheynier, V.; Noble, A.C. Bitterness and astringency of grape and wine polyphenols. Aust. J. Grape Wine Res. 2001, 7, 33–39. [Google Scholar] [CrossRef]
- Gomez-Plaza, E.; Gil-Munoz, R.; Lopez-Roca, J.M.; Martinez-Cutillas, A.; Fernandez-Fernandez, J.I. Phenolic compounds and color stability of red wines: Effect of skin maceration time. Am. J. Enol. Vitic. 2001, 52, 266–270. [Google Scholar]
- Flamini, R.; Mattivi, F.; De Rosso, M.; Arapitsas, P.; Bavaresco, L. Advanced Knowledge of Three Important Classes of Grape Phenolics: Anthocyanins, Stilbenes and Flavonols. Int. J. Mol. Sci. 2013, 14, 19651–19669. [Google Scholar] [CrossRef]
- Renaud, S.; Delorgeril, M. Wine, alcohol, platelets, and the french paradox for coronary heart-disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Galinski, C.N.; Zwicker, J.I.; Kennedy, D.R. Revisiting the mechanistic basis of the French Paradox: Red wine inhibits the activity of protein disulfide isomerase in vitro. Thromb. Res. 2016, 137, 169–173. [Google Scholar] [CrossRef]
- Hu, X.T.; Wang, H.; Lv, X.H.; Chu, L.; Liu, Z.Y.; Wei, X.G.; Chen, Q.C.; Zhu, L.; Cui, W. Cardioprotective Effects of Tannic Acid on Isoproterenol-Induced Myocardial Injury in Rats: Further Insight into “French Paradox”. Phytother. Res. 2015, 29, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Franchini, M.; Favaloro, E.J.; Targher, G. Moderate Red Wine Consumption and Cardiovascular Disease Risk: Beyond the “French Paradox”. Semin. Thromb. Hemost. 2010, 36, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.Y.; Simonyi, A.; Sun, G.Y. The “French paradox” and beyond: Neuroprotective effects of polyphenols. Free Radic. Biol. Med. 2002, 32, 314–318. [Google Scholar] [CrossRef]
- Zern, T.L.; Fernandez, M.L. Cardioprotective effects of dietary polyphenols. J. Nutr. 2005, 135, 2291–2294. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lomillo, J.; Gonzalez-SanJose, M.L. Applications of Wine Pomace in the Food Industry: Approaches and Functions. Compr. Rev. Food. Sci. Food Saf. 2017, 16, 3–22. [Google Scholar] [CrossRef]
- Garrido, J.; Borges, F. Wine and grape polyphenols–A chemical perspective. Food Res. Int. 2013, 54, 1843–1858. [Google Scholar] [CrossRef]
- Lorrain, B.; Ky, I.; Pechamat, L.; Teissedre, P.L. Evolution of Analysis of Polyhenols from Grapes, Wines, and Extracts. Molecules 2013, 18, 1076–1100. [Google Scholar] [CrossRef]
- Tomaz, I.; Huzanić., N.; Preiner, D.; Stupić, D.; Andabaka, Ž; Maletić, E.; Karoglan Kontic, J.; Ašperger, D. Extraction Methods of Polyphenol from Grapes: Extractions of Grape Polyphenols. In Polyphenols in Plants, 2nd ed.; Watson, R.R., Ed.; Elsevier Inc.: London, UK, 2019; pp. 151–168. [Google Scholar]
- Castellarin, S.D.; Bavaresco, L.; Falginella, L.; Goncavles, M.I.V.Z.; Di Gaspero, G. Phenolics in Grape Berry and Key Antioxidants. In The Biochemistry of the Grape Berry; Geros, H., Chavez, M.M., Delrot, S., Eds.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2012. [Google Scholar]
- Tomaz, I.; Štambuk, P.; Andabaka, Ž.; Preiner, D.; Stupić, D.; Maletić, E.; Karoglan Kontić, J.; Ašperger, D. The Polyphenolic Profile of Grapes. In Grapes Polyphenolic Composition, Antioxidant Characteristics and Health Benefits; Thomas, S., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2017; pp. 1–70. [Google Scholar]
- Xia, E.Q.; Deng, G.F.; Guo, Y.J.; Li, H.B. Biological Activities of Polyphenols from Grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Giovinazzo, G.; Grieco, F. Functional Properties of Grape and Wine Polyphenols. Plant. Food Hum. Nutr. 2015, 70, 454–462. [Google Scholar] [CrossRef]
- Garcia-Martinez, D.J.; Funes, J.C.; Saborido, C.M.; Santos, C. Grape Polyphenols to Arrest in Vitro Proliferation of Human Leukemia Cells: A Systematic Review and Meta-analysis. Food Rev. Int. 2020, 1–18. [Google Scholar] [CrossRef]
- Pasinetti, G.M.; Wang, J.; Ho, L.; Zhao, W.; Dubner, L. Roles of resveratrol and other grape-derived polyphenols in Alzheimer’s disease prevention and treatment. Biochim. Biophy. Acta Mol. Basis Dis. 2015, 1852, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Pasinetti, G.M.; Ho, L. Role of grape seed polyphenols in Alzheimer’s disease neuropathology. Nutr. Diet. Suppl. 2010, 2, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Simon, J.E.; Wu, Q. A critical review on grape polyphenols for neuroprotection: Strategies to enhance bioefficacy. Crit. Rev. Food Sci. Nutr. 2020, 60, 597–625. [Google Scholar] [CrossRef]
- Caracci, F.; Harary, J.; Simkovic, S.; Pasinetti, G.M. Grape-Derived Polyphenols Ameliorate Stress-Induced Depression by Regulating Synaptic Plasticity. J. Agr. Food Chem. 2020, 68, 1808–1815. [Google Scholar] [CrossRef]
- Herman, F.; Westfall, S.; Brathwaite, J.; Pasinetti, G.M. Suppression of Presymptomatic Oxidative Stress and Inflammation in Neurodegeneration by Grape-Derived Polyphenols. Front. Pharmacol. 2018, 9, 20. [Google Scholar] [CrossRef]
- Haghighatdoost, F.; Gholami, A.; Hariri, M. Effect of grape polyphenols on selected inflammatory mediators: A systematic review and meta-analysis randomized clinical trials. EXCLI J. 2020, 19, 251–267. [Google Scholar] [CrossRef]
- Magrone, T.; Magrone, M.; Russo, M.A.; Jirillo, E. Recent Advances on the Anti-Inflammatory and Antioxidant Properties of Red Grape Polyphenols: In Vitro and In Vivo Studies. Antioxidants 2020, 9, 28. [Google Scholar] [CrossRef]
- Chuang, C.C.; McIntosh, M.K. Potential Mechanisms by Which Polyphenol-Rich Grapes Prevent Obesity-Mediated Inflammation and Metabolic Diseases. In Annual Review of Nutrition; Cousins, R.J., Bier, D.M., Bowman, B.A., Eds.; Annual Reviews: Palo Alto, CA, USA, 2011; Volume 31. [Google Scholar]
- Rasines-Perea, Z.; Teissedre, P.-L. Grape Polyphenols’ Effects in Human Cardiovascular Diseases and Diabetes. Molecules 2017, 22, 1–19. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Role of red grape polyphenols as antidiabetic agents. Integr. Med. Res. 2014, 3, 119–125. [Google Scholar] [CrossRef]
- Vinas, P.; Campillo, N. Gas Chromatography-Mass Spectrometry Analysis of Polyphenols in Foods; Elsevier Academic Press Inc.: San Diego, CA, USA, 2014; pp. 103–157. [Google Scholar] [CrossRef]
- Pinelo, M.; Del Fabbro, P.; Manzocco, L.; Nunez, M.J.; Nicoli, M.C. Optimization of continuous phenol extraction from Vitis vinifera byproducts. Food Chem. 2005, 92, 109–117. [Google Scholar] [CrossRef]
- Iacopini, P.; Baldi, M.; Storchi, P.; Sebastiani, L. Catechin, epicatechin, quercetin, rutin and resveratrol in red grape: Content, in vitro antioxidant activity and interactions. J. Food Compos. Anal. 2008, 21, 589–598. [Google Scholar] [CrossRef]
- Rusjan, D.; Korosec-Koruza, Z. A comparison of extraction methods for selected phenolic compounds from grape berry skins using liquid chromatography and spectrophotometry. Acta Chim. Slo. 2007, 54, 114–118. [Google Scholar]
- Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Extraction of Polyphenols From Aromatic and Medicinal Plants: An Overview of the Methods and the Effect of Extraction Parameters. In Polyphenols in Plants, 2nd ed.; Watson, R.R., Ed.; Academic Press: London, UK, 2019; pp. 243–259. [Google Scholar] [CrossRef]
- Pinelo, M.; Rubilar, M.; Jerez, M.; Sineiro, J.; Nunez, M.J. Effect of solvent, temperature, and solvent-to-solid ratio on the total phenolic content and antiradical activity of extracts from different components of grape pomace. J. Agric. Food Chem. 2005, 53, 2111–2117. [Google Scholar] [CrossRef]
- Chanioti, S.; Liadakis, G.; Tzia, C. Solid-Liquid Extraction. In Food Engineering Handbook: Food Process Engineering; CRC Press: Boca Raton, USA, 2014; pp. 253–286. [Google Scholar]
- Barba, F.J.; Zhu, Z.Z.; Koubaa, M.; Sant’Ana, A.S.; Orlien, V. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Chemat, F.; Zill e, H.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Rombaut, N.; Tixier, A.S.; Bily, A.; Chemat, F. Green extraction processes of natural products as tools for biorefinery. Biofuels Bioprod. Biorefin. 2014, 8, 530–544. [Google Scholar] [CrossRef]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. Trac-Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Vural, N.; Cavuldak, O.A.; Anli, R.E. Multi response optimisation of polyphenol extraction conditions from grape seeds by using ultrasound assisted extraction (UAE). Sep. Sci. Technol. 2018, 53, 1540–1551. [Google Scholar] [CrossRef]
- Mazza, K.E.L.; Santiago, M.; do Nascimento, L.S.M.; Godoy, R.L.O.; Souza, E.F.; Brigida, A.I.S.; Borguini, R.G.; Tonon, R.V. Syrah grape skin valorisation using ultrasound-assisted extraction: Phenolic compounds recovery, antioxidant capacity and phenolic profile. Int. J. Food Sci. Technol. 2019, 54, 641–650. [Google Scholar] [CrossRef]
- Alupului, A.; Calinescu, I.; Lavric, V. Microwave extraction of active principles from medicinal plants. Univ. Politeh. Buchar. Bull. Ser. B-Chem. Mater. Sci. 2012, 74, 129–142. [Google Scholar]
- Routray, W.; Orsat, V. Microwave-Assisted Extraction of Flavonoids: A Review. Food Bioproc. Technol. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- Liazid, A.; Guerrero, R.F.; Cantos, E.; Palma, M.; Barroso, C.G. Microwave assisted extraction of anthocyanins from grape skins. Food Chem. 2011, 124, 1238–1243. [Google Scholar] [CrossRef]
- Li, Y.; Fabiano-Tixier, A.S.; Vian, M.A.; Chemat, F. Solvent-free microwave extraction of bioactive compounds provides a tool for green analytical chemistry. Trac-Trends Anal. Chem. 2013, 47, 1–11. [Google Scholar] [CrossRef]
- Ortega-Regules, A.; Romero-Cascales, I.; Ros-Garcia, J.M.; Lopez-Roca, J.M.; Gomez-Plaza, E. A first approach towards the relationship between grape skin cell-wall composition and anthocyanin extractability. Anal. Chim. Acta 2006, 563, 26–32. [Google Scholar] [CrossRef]
- Pinelo, M.; Arnous, A.; Meyer, A.S. Upgrading of grape skins: Significance of plant cell-wall structural components and extraction techniques for phenol release. Trends Food Sci. Technol. 2006, 17, 579–590. [Google Scholar] [CrossRef]
- Meini, M.R.; Cabezudo, I.; Boschetti, C.E.; Romanini, D. Recovery of phenolic antioxidants from Syrah grape pomace through the optimization of an enzymatic extraction process. Food Chem. 2019, 283, 257–264. [Google Scholar] [CrossRef]
- Tomaz, I.; Maslov, L.; Stupic, D.; Preiner, D.; Asperger, D.; Kontic, J.K. Recovery of flavonoids from grape skins by enzyme-assisted extraction. Sep. Sci. Technol. 2016, 51, 255–268. [Google Scholar] [CrossRef]
- Gligor, O.; Mocan, A.; Moldovan, C.; Locatelli, M.; Crisan, G.; Ferreira, I. Enzyme-assisted extractions of polyphenols–A comprehensive review. Trends Food Sci. Technol. 2019, 88, 302–315. [Google Scholar] [CrossRef]
- Belur, P.D.; Mugeraya, G. Microbial production of tannase: State of the art. Res. J. Microbiol. 2011, 6, 25–40. [Google Scholar] [CrossRef]
- Benucci, I.; Segade, S.R.; Cerreti, M.; Giacosa, S.; Paissoni, M.A.; Liburdi, K.; Bautista-Ortin, A.B.; Gomez-Plaza, E.; Gerbi, V.; Esti, M.; et al. Application of enzyme preparations for extraction of berry skin phenolics in withered winegrapes. Food Chem. 2017, 237, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, V.; Stefova, M.; Chinnici, F. Determination of the polyphenol contents in Macedonian grapes and wines by standardized spectrophotometric methods. J. Serb. Chem. Soc. 2010, 75, 45–59. [Google Scholar] [CrossRef]
- Dobes, J.; Zitka, O.; Sochor, J.; Ruttkay-Nedecky, B.; Babula, P.; Beklova, M.; Kynicky, J.; Hubalek, J.; Klejdus, B.; Kizek, R.; et al. Electrochemical Tools for Determination of Phenolic Compounds in Plants. A Review. Int. J. Electrochem. Sci. 2013, 8, 4520–4542. [Google Scholar]
- Hoyos-Arbelaez, J.; Vazquez, M.; Contreras-Calderon, J. Electrochemical methods as a tool for determining the antioxidant capacity of food and beverages: A review. Food Chem. 2017, 221, 1371–1381. [Google Scholar] [CrossRef]
- Aguirre, M.J.; Chen, Y.Y.; Isaacs, M.; Matsuhiro, B.; Mendoza, L.; Torres, S. Electrochemical behaviour and antioxidant capacity of anthocyanins from Chilean red wine, grape and raspberry. Food Chem. 2010, 121, 44–48. [Google Scholar] [CrossRef]
- Ivanova, V.; Stefova, M.; Vojnoski, B.; Dornyei, A.; Mark, L.; Dimovska, V.; Stafilov, T.; Kilar, F. Identification of polyphenolic compounds in red and white grape varieties grown in R. Macedonia and changes of their content during ripening. Food Res. Int. 2011, 44, 2851–2860. [Google Scholar] [CrossRef]
- Jin, Z.M.; Bi, H.Q.; Liang, N.N.; Duan, C.Q. An Extraction Method for Obtaining the Maximum Non-Anthocyanin Phenolics from Grape Berry Skins. Anal. Let. 2010, 43, 776–785. [Google Scholar] [CrossRef]
- Ghassempour, A.; Heydari, R.; Talebpour, Z.; Fakhari, A.R.; Rassouli, A.; Davies, N.; Aboul-Enein, H.Y. Study of new extraction methods for separation of anthocyanins from red grape skins: Analysis by HPLC and LC-MS/MS. J. Liq. Chromatogr. Rel. Technol. 2008, 31, 2686–2703. [Google Scholar] [CrossRef]
- Novak, I.; Janeiro, P.; Seruga, M.; Oliveira-Brett, A.M. Ultrasound extracted flavonoids from four varieties of Portuguese red grape skins determined by reverse-phase high-performance liquid chromatography with electrochemical detection. Anal. Chim. Acta 2009, 648. [Google Scholar] [CrossRef]
- Liazid, A.; Barbero, G.F.; Palma, M.; Brigui, J.; Barroso, C.G. Rapid Determination of Simple Polyphenols in Grapes by LC Using a Monolithic Column. Chromatographia 2010, 72, 417–424. [Google Scholar] [CrossRef]
- Manns, D.C.; Mansfield, A.K. A core–shell column approach to a comprehensive high-performance liquid chromatography phenolic analysis of Vitis vinifera L. and interspecific hybrid grape juices, wines, and other matrices following either solid phase extraction or direct injection. J. Chromatogr. A 2012, 1251, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Mazzuca, P.; Ferranti, P.; Picariello, G.; Chianese, L.; Addeo, F. Mass spectrometry in the study of anthocyanins and their derivatives: Differentiation of Vitis vinifera and hybrid grapes by liquid chromatography electrospray ionization mass spectrometry and tandem mass spectrometry. J. Mass Spectrom. 2005, 40, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Delgado, M.A.; Malovana, S.; Perez, J.P.; Borges, T.; Montelongo, F.J.G. Separation of phenolic compounds by high-performance liquid chromatography with absorbance and fluorimetric detection. J. Chromatogr. A 2001, 912, 249–257. [Google Scholar] [CrossRef]
- Flamini, R. Recent application of Mass Spectrometry in the Study of Grape and Wine Polyphenols. ISRN Spectrosc. 2013, 2013, 1–45. [Google Scholar] [CrossRef]
- Adams, D.O. Phenolics and ripening in grape berries. Am. J. Enol. Vitic. 2006, 57, 249–256. [Google Scholar]
- Fontes, N.; Geros, H.; Delrot, S. Grape Berry Vacuole: A Complex and Heterogeneous Membrane System Specialized in the Accumulation of Solutes. Am. J. Enol. Vitic. 2011, 62, 270–278. [Google Scholar] [CrossRef]
- Downey, M.O.; Dokoozlian, N.K.; Krstic, M.P. Cultural practice and environmental impacts on the flavonoid composition of grapes and wine: A review of recent research. Am. J. Enol. Vitic. 2006, 57, 257–268. [Google Scholar]
- Nicoletti, I.; Bello, C.; De Rossi, A.; Corradini, D. Identification and quantification of phenolic compounds in grapes by HPLC-PDA-ESI-MS on a semimicro separation scale. J. Agric. Food Chem. 2008, 56, 8801–8808. [Google Scholar] [CrossRef]
- Burin, V.M.; Ferreira-Lima, N.E.; Panceri, C.P.; Bordignon-Luiz, M.T. Bioactive compounds and antioxidant activity of Vitis vinifera and Vitis labrusca grapes: Evaluation of different extraction methods. Microchem. J. 2014, 114, 155–163. [Google Scholar] [CrossRef]
- Tomaz, I.; Maslov, L. Simultaneous Determination of Phenolic Compounds in Different Matrices using Phenyl-Hexyl Stationary Phase. Food Anal. Met. 2016, 9, 401–410. [Google Scholar] [CrossRef]
- Pena-Neira, A.; Caceres, A.; Pastenes, C. Low molecular weight phenolic and anthocyanin composition of grape skins from cv. syrah (Vitis vinifera L.) in the maipo valley (Chile): Effect of clusters thinning and vineyard yield. Food Sci. Technol. Int. 2007, 13, 153–158. [Google Scholar] [CrossRef]
- Burns, J.; Gardner, P.T.; Matthews, D.; Duthie, G.G.; Lean, M.E.J.; Crozier, A. Extraction of phenolics and changes in antioxidant activity of red wines during vinification. J. Agric. Food Chem. 2001, 49, 5797–5808. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.C.; Owens, C.L.; Zhong, G.Y.; Cheng, L.L. Polyphenolic profiles detected in the ripe berries of Vitis vinifera germplasm. Food Chem. 2011, 129, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Hogan, S.; Zhang, L.; Li, J.R.; Zoecklein, B.; Zhou, K.Q. Antioxidant properties and bioactive components of Norton (Vitis aestivalis) and Cabernet Franc (Vitis vinifera) wine grapes. LWT 2009, 42, 1269–1274. [Google Scholar] [CrossRef]
- Pantelic, M.M.; Dabic Zagorac, D.; Davidovic, S.M.; Todic, S.R.; Beslic, Z.S.; Gasic, U.M.; Tesic, Z.L.; Natic, M.M. Identification and quantification of phenolic compounds in berry skin, pulp, and seeds in 13 grapevine varieties grown in Serbia. Food Chem. 2016, 211, 243–252. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R.T. Major flavonoids in grape seeds and skins: Antioxidant capacity of catechin, epicatechin, and gallic acid. J. Agric. Food Chem. 2004, 52, 255–260. [Google Scholar] [CrossRef]
- Butkhup, L.; Chowtivannakul, S.; Gaensakoo, R.; Prathepha, P.; Samappito, S. Study of the Phenolic Composition of Shiraz Red Grape Cultivar (Vitis vinifera L.) Cultivated in North-eastern Thailand and its Antioxidant and Antimicrobial Activity. S. Afr. J. Enol. Vitic. 2010, 31, 89–98. [Google Scholar] [CrossRef]
- Di Lecce, G.; Arranz, S.; Jauregui, O.; Tresserra-Rimbau, A.; Quifer-Rada, P.; Lamuela-Raventos, R.M. Phenolic profiling of the skin, pulp and seeds of Albarino grapes using hybrid quadrupole time-of-flight and triple-quadrupole mass spectrometry. Food Chem. 2014, 145, 874–882. [Google Scholar] [CrossRef]
- Pantelic, M.M.; Zagorac, D.C.D.; Ciric, I.Z.; Pergal, M.V.; Relic, D.J.; Todic, S.R.; Natic, M.M. Phenolic profiles, antioxidant activity and minerals in leaves of different grapevine varieties grown in Serbia. J. Food Compos. Anal. 2017, 62, 76–83. [Google Scholar] [CrossRef]
- Conde, C.; Silva, P.; Fontes, N.; Dias, A.C.P.; Tavares, R.M.; Sousa, M.J.; Agasse, A.; Delrot, S.; Geros, H. Biochemical changes throughout grape berry development and fruit and wine quality. Food Chem. 2007, 1, 1–22. [Google Scholar]
- Dopico-Garcia, M.S.; Fique, A.; Guerra, L.; Afonso, J.M.; Pereira, O.; Valentao, P.; Andrade, P.B.; Seabra, R.M. Principal components of phenolics to characterize red Vinho Verde grapes: Anthocyanins or non-coloured compounds? Talanta 2008, 75, 1190–1202. [Google Scholar] [CrossRef] [PubMed]
- Lingua, M.S.; Fabani, M.P.; Wunderlin, D.A.; Baroni, M.V. From grape to wine: Changes in phenolic composition and its influence on antioxidant activity. Food Chem. 2016, 208, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Samoticha, J.; Wojdylo, A.; Golis, T. Phenolic composition, physicochemical properties and antioxidant activity of interspecific hybrids of grapes growing in Poland. Food Chem. 2017, 215, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, M.; Travaglia, F.; Coisson, J.D.; Bordiga, M.; Arlorio, M. Phenolic composition of Nebbiolo grape (Vitis vinifera L.) from Piedmont: Characterization during ripening of grapes selected in different geographic areas and comparison with Uva Rara and Vespolina cv. Eur. Food Res. Technol. 2016, 242, 1057–1068. [Google Scholar] [CrossRef]
- Del-Castillo-Alonso, M.A.; Castagna, A.; Csepregi, K.; Hideg, E.; Jakab, G.; Jansen, M.A.K.; Jug, T.; Llorens, L.; Matai, A.; Martinez-Luscher, J.; et al. Environmental Factors Correlated with the Metabolite Profile of Vitis vinifera cv. Pinot Noir Berry Skins along a European Latitudinal Gradient. J. Agric. Food Chem. 2016, 64, 8722–8734. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Fernandes, F.; Pinto-Carnide, O.; Valentao, P.; Falco, V.; Martin, J.P.; Ortiz, J.M.; Arroyo-Garcia, R.; Andrade, P.B.; Castro, I. Identification of Vitis vinifera L. grape berry skin color mutants and polyphenolic profile. Food Chem. 2016, 194, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Lago-Vanzela, E.S.; Da-Silva, R.; Gomes, E.; Garcia-Romero, E.; Hermosin-Gutierrez, I. Phenolic Composition of the Brazilian Seedless Table Grape Varieties BRS Clara and BRS Morena. J. Agric. Food Chem. 2011, 59, 8314–8323. [Google Scholar] [CrossRef]
- Portu, J.; Lopez-Alfaro, I.; Gomez-Alonso, S.; Lopez, R.; Garde-Cerdan, T. Changes on grape phenolic composition induced by grapevine foliar applications of phenylalanine and urea. Food Chem. 2015, 180, 171–180. [Google Scholar] [CrossRef]
- Andjelkovic, M.; Radovanovic, B.; Radovanovic, A.; Andjelkovic, A.M. Changes in Polyphenolic Content and Antioxidant Activity of Grapes cv Vranac During Ripening. S. Afr. J. Enol. Vitic. 2013, 34, 147–155. [Google Scholar] [CrossRef]
- Rescic, J.; Mikulic-Petkovsek, M.; Rusjan, D. The impact of canopy managements on grape and wine composition of cv. ‘Istrian Malvasia’ (Vitis vinifera L.). J. Sci. Food Agric. 2016, 96, 4724–4735. [Google Scholar] [CrossRef]
- Singleton, V.L.; Trousdale, E. White Wine Phenolics–Varietal and Processing Differences as Shown by hplc. Am. J. Enol. Vitic. 1983, 34, 27–34. [Google Scholar]
- Valanciene, E.; Jonuskiene, I.; Syrpas, M.; Augustiniene, E.; Matulis, P.; Simonavicius, A.; Malys, N. Advances and Prospects of Phenolic Acids Production, Biorefinery and Analysis. Biomolecules 2020, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Bae, H. An Overview of Stress-Induced Resveratrol Synthesis in Grapes: Perspectives for Resveratrol-Enriched Grape Products. Molecules 2017, 22, 18. [Google Scholar] [CrossRef] [PubMed]
- Gatto, P.; Vrhovsek, U.; Muth, J.; Segala, C.; Romualdi, C.; Fontana, P.; Pruefer, D.; Stefanini, M.; Moser, C.; Mattivi, F.; et al. Ripening and Genotype Control Stilbene Accumulation in Healthy Grapes. J. Agric. Food Chem. 2008, 56, 11773–11785. [Google Scholar] [CrossRef]
- Adrian, M.; Jeandet, P.; Douillet-Breuil, A.C.; Tesson, L.; Bessis, R. Stilbene content of mature Vitis vinifera berries in response to UV-C elicitation. J. Agric. Food Chem. 2000, 48, 6103–6105. [Google Scholar] [CrossRef]
- Flamini, R.; De Rosso, M.; De Marchi, F.; Dalla Vedova, A.; Panighel, A.; Gardiman, M.; Maoz, I.; Bavaresco, L. An innovative approach to grape metabolomics: Stilbene profiling by suspect screening analysis. Metabolomics 2013, 9, 1243–1253. [Google Scholar] [CrossRef]
- Jeandet, P.; Bessis, R.; Sbaghi, M.; Meunier, P. Production of the phytoalexin resveratrol by grapes as a response to botrytis attack under natural conditions. J. Phytopathol. Phytopathol. Zeit. 1995, 143, 135–139. [Google Scholar] [CrossRef]
- Bavaresco, L.; Petegolli, D.; Cantù, E.; Fergoni, M.; Chiusa, G.; Trevisan, M. Elicitation and accumulation of stilbene phytoalexins in grapevine berries infected by Botrytis cinerea. Vitis 1997, 36, 77–83. [Google Scholar]
- Katalinic, V.; Mozina, S.S.; Skroza, D.; Generalic, I.; Abramovic, H.; Milos, M.; Ljubenkov, I.; Piskernik, S.; Pezo, I.; Terpinc, P.; et al. Polyphenolic profile, antioxidant properties and antimicrobial activity of grape skin extracts of 14 Vitis vinifera varieties grown in Dalmatia (Croatia). Food Chem. 2010, 119, 715–723. [Google Scholar] [CrossRef]
- Jin, Z.M.; He, J.J.; Bi, H.Q.; Cui, X.Y.; Duan, C.Q. Phenolic Compound Profiles in Berry Skins from Nine Red Wine Grape Cultivars in Northwest China. Molecules 2009, 14, 4922–4935. [Google Scholar] [CrossRef]
- Ali, K.; Maltese, F.; Choi, Y.H.; Verpoorte, R. Metabolomic constituents of grapevine and grape-derived products. Phytochem. Rev. 2010, 9, 357–378. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.; Eiras-Dias, J.; Castellarin, S.D.; Geros, H. Berry Phenolics of Grapevine under Challenging Environments. Int. J. Mol. Sci. 2013, 14, 18711–18739. [Google Scholar] [CrossRef] [PubMed]
- Terra, X.; Pallares, V.; Ardevol, A.; Blade, C.; Fernandez-Larrea, J.; Pujadas, G.; Salvado, J.; Arola, L.; Blay, M. Modulatory effect of grape-seed procyanidins on local and systemic inflammation in diet-induced obesity rats. J. Nutr. Biochem. 2011, 22, 380–387. [Google Scholar] [CrossRef] [PubMed]
- He, F.; He, J.J.; Pan, Q.H.; Duan, C.Q. Mass-spectrometry evidence confirming the presence of pelargonidin-3-O-glucoside in the berry skins of Cabernet Sauvignon and Pinot Noir (Vitis vinifera L.). Aust. J. Grape Wine Res. 2010, 16, 464–468. [Google Scholar] [CrossRef]
- He, F.; Mu, L.; Yan, G.L.; Liang, N.N.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Biosynthesis of Anthocyanins and Their Regulation in Colored Grapes. Molecules 2010, 15, 9057–9091. [Google Scholar] [CrossRef]
- De la Cruz, A.A.; Hilbert, G.; Riviere, C.; Mengin, V.; Ollat, N.; Bordenave, L.; Decroocq, S.; Delaunay, J.C.; Delrot, S.; Merillon, J.M.; et al. Anthocyanin identification and composition of wild Vitis spp. accessions by using LC-MS and LC-NMR. Anal. Chim. Acta 2012, 732, 145–152. [Google Scholar] [CrossRef]
- Janvary, L.; Hoffmann, T.; Pfeiffer, J.; Hausmann, L.; Topfer, R.; Fischer, T.C.; Schwab, W. A Double Mutation in the Anthocyanin 5-O-Glucosyltransferase Gene Disrupts Enzymatic Activity in Vitis vinifera L. J. Agric. Food Chem. 2009, 57, 3512–3518. [Google Scholar] [CrossRef]
- Xing, R.R.; Li, S.Y.; He, F.; Yang, Z.; Duan, C.Q.; Li, Z.; Wang, J.; Pan, Q.H. Mass spectrometric and enzymatic evidence confirm the existence of anthocyanidin 3,5-O-diglucosides in cabernet sauvignon (Vitis vinifera L.) grape berries. J. Agric. Food Chem. 2015, 63, 3251–3260. [Google Scholar] [CrossRef]
- Owens, C.L. Pigments in Grape. In Pigments in Fruits and Vegetables: Genomics and Dietetics; Chen, C., Ed.; Springer New York: New York, NY, USA, 2015; pp. 189–204. [Google Scholar]
- Castellarin, S.D.; Pfeiffer, A.; Sivilotti, P.; Degan, M.; Peterlunger, E.; Di Gaspero, G. Transcriptional regulation of anthocyanin biosynthesis in ripening fruits of grapevine under seasonal water deficit. Plant Cell Environ. 2007, 30, 1381–1399. [Google Scholar] [CrossRef]
- Mattivi, F.; Guzzon, R.; Vrhovsek, U.; Stefanini, M.; Velasco, R. Metabolite profiling of grape: Flavonols and anthocyanins. J. Agric. Food Chem. 2006, 54, 7692–7702. [Google Scholar] [CrossRef]
- Costa, E.; Cosme, F.; Jordao, A.M.; Mendes-Faia, A. Anthocyanin profile and antioxidant activity from 24 grape varieties cultivated in two portuguese wine regions. J. Int. Sci. Vign. Vin. 2014, 48, 51–62. [Google Scholar] [CrossRef]
- Guerrero, R.F.; Liazid, A.; Palma, M.; Puertas, B.; Gonzalez-Barrio, R.; Gil-Izquierdo, A.; Garcia-Barroso, C.; Cantos-Villar, E. Phenolic characterisation of red grapes autochthonous to Andalusia. Food Chem. 2009, 112, 949–955. [Google Scholar] [CrossRef]
- Cheng, G.; Zhou, S.H.; Liu, Y.; Yue, T.X.; Zhang, Z.W. Effect of bearing position on phenolics profiles in the skins of four cultivars of grapevine (Vitis vinifera L.). J. Hort. Sci. Biotechnol. 2015, 90, 356–363. [Google Scholar] [CrossRef]
- Mazza, G.; Fukumoto, L.; Delaquis, P.; Girard, B.; Ewert, B. Anthocyanins, phenolics, and color of Cabernet Franc, Merlot, and Pinot Noir wines from British Columbia. J. Agric. Food Chem. 1999, 47, 4009–4017. [Google Scholar] [CrossRef]
- Cacho, J.; Fernandez, P.; Ferreira, V.; Castells, J.E. Evolution of five anthocyanidin-3-glucosides in the skin of the Tempranillo, Moristel, and Garnacha grape varieties and influence of climatological variables. Am. J. Enol. Vitic. 1992, 43, 244–248. [Google Scholar]
- Hebrero, E.; Santosbuelga, C.; Rivasgonzalo, J.C. High-performance liquid chromatography diode array spectroscopy identification of anthocyanins of vitis-vinifera variety tempranillo. Am. J. Enol. Vitic. 1988, 39, 227–233. [Google Scholar]
- Mateus, N.; Proenca, S.; Machado, J.M.; De Freitas, V. Grape and wine polyphenolics composition of red Vitis vinifera varieties concerning vineyard altitude. Ciên. Tecnol. Alim. 2011, 3, 102–110. [Google Scholar]
- Makris, D.P.; Kallithraka, S.; Kefalas, P. Flavonols in grapes, grape products and wines: Burden, profile and influential parameters. J. Food Compos. Anal. 2006, 19, 396–404. [Google Scholar] [CrossRef]
- Hermosin-Gutierrez, I.; Castillo-Munoz, N.; Gomez-Alonso, S.; Garcia-Romero, E. Flavonol profiles for grape and wine authentication. Abstr. Pap. Am. Chem. Soc. 2010, 239, 1. [Google Scholar]
- Downey, M.O.; Harvey, J.S.; Robinson, S.P. Synthesis of flavonols and expression of flavonol synthase genes in the developing grape berries of Shiraz and Chardonnay (Vitis vinifera L.). Aust. J. Grape Wine Res. 2003, 9, 110–121. [Google Scholar] [CrossRef]
- Azuma, A.; Yakushiji, H.; Koshita, Y.; Kobayashi, S. Flavonoid biosynthesis-related genes in grape skin are differentially regulated by temperature and light conditions. Planta 2012, 236, 1067–1080. [Google Scholar] [CrossRef] [PubMed]
- Rustioni, L.; Bedgood, D.R.; Failla, O.; Prenzler, P.D.; Robards, K. Copigmentation and anti-copigmentation in grape extracts studied by spectrophotometry and post-column-reaction HPLC. Food Chem. 2012, 132, 2194–2201. [Google Scholar] [CrossRef]
- Castillo-Munoz, N.; Gomez-Alonso, S.; Garcia-Romero, E.; Hermosin-Gutierrez, I. Flavonol profiles of Vitis vinifera white grape cultivars. J. Food Compos. Anal. 2010, 23, 699–705. [Google Scholar] [CrossRef]
- Montealegre, R.R.; Peces, R.R.; Vozmediano, J.L.C.; Gascuena, J.M.; Romero, E.G. Phenolic compounds in skins and seeds of ten grape Vitis vinifera varieties grown in a warm climate. J. Food Compos. Anal. 2006, 19, 687–693. [Google Scholar] [CrossRef]
- Zerbib, M.; Mazauric, J.P.; Meudec, E.; Le Guerneve, C.; Lepak, A.; Nidetzky, B.; Cheynier, V.; Terrier, N.; Saucier, C. New flavanol O-glycosides in grape and wine. Food Chem. 2018, 266, 441–448. [Google Scholar] [CrossRef]
- Dixon, R.A.; Xie, D.Y.; Sharma, S.B. Proanthocyanidins–a final frontier in flavonoid research? New Phytol. 2005, 165, 9–28. [Google Scholar] [CrossRef]
- Fujita, A.; Soma, N.; Goto-Yamamoto, N.; Shindo, H.; Kakuta, T.; Koizumi, T.; Hashizume, K. Anthocyanidin reductase gene expression and accumulation of flavan-3-ols in grape berry. Am. J. Enol. Vitic. 2005, 56, 336–342. [Google Scholar]
- De Freitas, V.A.P.; Glories, Y. Concentration and compositional changes of procyanidins in grape seeds and skin of white Vitis vinifera varieties. J. Sci. Food Agric. 1999, 79, 1601–1606. [Google Scholar] [CrossRef]
- de Freitas, V.A.P.; Glories, Y.; Monique, A. Developmental changes of procyanidins in grapes of red Vitis vinifera varieties and their composition in respective wines. Am. J. Enol. Vitic. 2000, 51, 397–403. [Google Scholar]
- Borbalan, A.M.A.; Zorro, L.; Guillen, D.A.; Barroso, C.G. Study of the polyphenol content of red and white grape varieties by liquid chromatography-mass spectrometry and its relationship to antioxidant power. J. Chromatogr. A 2003, 1012, 31–38. [Google Scholar] [CrossRef]
- Mattivi, F.; Vrhovsek, U.; Masuero, D.; Trainotti, D. Differences in the amount and structure of extractable skin and seed tannins amongst red grape varieties. Aust. J. Grape Wine Res. 2009, 15, 27–35. [Google Scholar] [CrossRef]
- Monages, M.; Nunez, V.; Bartolome, B.; Gomez-Cordoves, C. Anthocyanin-derived pigments in Graciano, Tempranillo, and Cabernet Sauvignon wines produced in Spain. Am. J. Enol. Vitic. 2003, 54, 163–169. [Google Scholar]
- Chira, K.; Schmauch, G.; Saucier, C.; Fabre, S.; Teissedre, P.L. Grape Variety Effect on Proanthocyanidin Composition and Sensory Perception of Skin and Seed Tannin Extracts from Bordeaux Wine Grapes (Cabernet Sauvignon and Merlot) for Two Consecutive Vintages (2006 and 2007). J. Agric. Food Chem. 2009, 57, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Mateus, N.; Marques, S.; Goncalves, A.C.; Machado, J.M.; De Freitas, V. Proanthocyanidin composition of red Vitis vinifera varieties from the Douro valley during ripening: Influence of cultivation altitude. Am. J. Enol. Vitic. 2001, 52, 115–121. [Google Scholar]
- Peleg, H.; Gacon, K.; Schlich, P.; Noble, A.C. Bitterness and astringency of flavan-3-ol monomers, dimers and trimers. J. Sci. Food Agric. 1999, 79, 1123–1128. [Google Scholar] [CrossRef]
- Pastor, R.F.; Restani, P.; Di Lorenzo, C.; Orgiu, F.; Teissedre, P.L.; Stockley, C.; Ruf, J.C.; Quini, C.I.; Tejedor, N.G.; Gargantini, R.; et al. Resveratrol, human health and winemaking perspectives. Crit. Rev. Food Sci. Nutr. 2019, 59, 1237–1255. [Google Scholar] [CrossRef]
- Lima, G.P.P.; Vianello, F.; Correa, C.R.; Campos, R.A.S.; Borguini, M.G. Polyphenols in Fruits and Vegetables and Its Effect on Human Health. Food Nutr. Sci. 2014, 5, 1065–1082. [Google Scholar] [CrossRef]
- Leifert, W.R.; Abeywardena, M.Y. Cardioprotective actions of grape polyphenols. Nutr. Res. 2008, 28, 729–737. [Google Scholar] [CrossRef]
- Castilla, P.; Echarri, R.; Davalos, A.; Cerrato, F.; Ortega, H.; Teruel, J.L.; Lucas, M.F.; Gomez-Coronado, D.; Ortuno, J.; Lasuncion, M.A. Concentrated red grape juice exerts antioxidant, hypolipidemic, and antiinflammatory effects in both hemodialysis patients and healthy subjects. Am. J. Clin. Nutr. 2006, 84, 252–262. [Google Scholar] [CrossRef]
- Leong, S.Y.; Burritt, D.J.; Oey, I. Evaluation of the anthocyanin release and health-promoting properties of Pinot Noir grape juices after pulsed electric fields. Food Chem. 2016, 196, 833–841. [Google Scholar] [CrossRef]
- Wang, S.; Mateos, R.; Goya, L.; Amigo-Benavent, M.; Sarria, B.; Bravo, L. A phenolic extract from grape by-products and its main hydroxybenzoic acids protect Caco-2 cells against pro-oxidant induced toxicity. Food Chem. Toxicol. 2016, 88, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Lingua, M.S.; Theumer, M.G.; Kruzynski, P.; Wunderlin, D.A.; Baroni, M.V. Bioaccessibility of polyphenols and antioxidant properties of the white grape by simulated digestion and Caco-2 cell assays: Comparative study with its winemaking product. Food Res. Int. 2019, 122, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, P.; Kalariya, H.M.; Poulev, A.; Ribnicky, D.M.; Jaja-Chimedza, A.; Roopchand, D.E.; Raskin, I. Grape polyphenols reduce gut-localized reactive oxygen species associated with the development of metabolic syndrome in mice. PLoS ONE 2018, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Garbetta, A.; Nicassio, L.; D’Antuono, I.; Cardinali, A.; Linsalata, V.; Attolico, G.; Minervini, F. Influence of in vitro digestion process on polyphenolic profile of skin grape (cv. Italia) and on antioxidant activity in basal or stressed conditions of human intestinal cell line (HT-29). Food Res. Int. 2018, 106, 878–884. [Google Scholar] [CrossRef]
- Cristea, E.; Sturza, R.; Jauregi, P.; Niculaua, M.; Ghendov-Mosanu, A.; Patras, A. Influence of pH and ionic strength on the color parameters and antioxidant properties of an ethanolic red grape marc extract. J. Food Biochem. 2019, 43, 9. [Google Scholar] [CrossRef]
- Messina, C.M.; Manuguerra, S.; Catalano, G.; Arena, R.; Cocchi, M.; Morghese, M.; Montenegro, L.; Santulli, A. Green biotechnology for valorisation of residual biomasses in nutraceutic sector: Characterization and extraction of bioactive compounds from grape pomace and evaluation of the protective effects in vitro. Nat. Prod. Res. 2019, 6, 1–6. [Google Scholar] [CrossRef]
- Maia, M.; Ferreira, A.E.N.; Laureano, G.; Marques, A.P.; Torres, V.M.; Silva, A.B.; Matos, A.R.; Cordeiro, C.; Figueiredo, A.; Silva, M.S. Vitis vinifera ‘Pinot noir’ leaves as a source of bioactive nutraceutical compounds. Food Funct. 2019, 10, 3822–3827. [Google Scholar] [CrossRef]
- Georgiev, V.; Ananga, A.; Tsolova, V. Recent Advances and Uses of Grape Flavonoids as Nutraceuticals. Nutrients 2014, 6, 391–415. [Google Scholar] [CrossRef]
- Li, W.G.; Zhang, X.Y.; Wu, Y.J.; Tian, X. Anti-inflammatory effect and mechanism of proanthocyanidins from grape seeds. Acta Pharmacol. Sin. 2001, 22, 1117–1120. [Google Scholar]
- Dvorakova, M.; Landa, P. Anti-inflammatory activity of natural stilbenoids: A review. Pharmacol. Res. 2017, 124, 126–145. [Google Scholar] [CrossRef]
- Martin, A.R.; Villegas, I.; La Casa, C.; de la Lastra, C.A. Resveratrol, a polyphenol found in grapes, suppresses oxidative damage and stimulates apoptosis during early colonic inflammation in rats. Biochem. Pharmacol. 2004, 67, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Csaki, C.; Nebrich, S.; Mobasheri, A. Resveratrol suppresses interleukin-1β-induced inflammatory signaling and apoptosis in human articular chondrocytes: Potential for use as a novel nutraceutical for the treatment of osteoarthritis. Biochem. Pharmacol. 2008, 76, 1426–1439. [Google Scholar] [CrossRef] [PubMed]
- Colica, C.; Milanovic, M.; Milic, N.; Aiello, V.; De Lorenzo, A.; Abenavoli, L. A Systematic Review on Natural Antioxidant Properties of Resveratrol. Nat. Prod. Commun. 2018, 13, 1195–1203. [Google Scholar] [CrossRef]
- Hansen, A.S.; Marckmann, P.; Dragsted, L.O.; Nielsen, I.L.F.; Nielsen, S.E.; Gronbaek, M. Effect of red wine and red grape extract on blood lipids, haemostatic factors, and other risk factors for cardiovascular disease. Eur. J. Clin. Nutr. 2005, 59, 449–455. [Google Scholar] [CrossRef] [PubMed]
- de Lange, D.W.; Scholman, W.L.G.; Kraaijenhagen, R.J.; Akkerman, J.W.N.; van de Wiel, A. Alcohol and polyphenolic grape extract inhibit platelet adhesion in flowing blood. Eur. J. Clin. Investig. 2004, 34, 818–824. [Google Scholar] [CrossRef]
- Draijer, R.; de Graaf, Y.; Slettenaar, M.; de Groot, E.; Wright, C.I. Consumption of a Polyphenol-Rich Grape-Wine Extract Lowers Ambulatory Blood Pressure in Mildly Hypertensive Subjects. Nutrients 2015, 7, 3138–3153. [Google Scholar] [CrossRef]
- Chaves, A.A.; Joshi, M.S.; Coyle, C.M.; Brady, J.E.; Dech, S.J.; Schanbacher, B.L.; Baliga, R.; Basuray, A.; Bauer, J.A. Vasoprotective endothelial effects of a standardized grape product in humans. Vasc. Pharmacol. 2009, 50, 20–26. [Google Scholar] [CrossRef]
- Chacar, S.; Hajal, J.; Saliba, Y.; Bois, P.; Louka, N.; Maroun, R.G.; Faivre, J.F.; Fares, N. Long-term intake of phenolic compounds attenuates age-related cardiac remodeling. Aging Cell 2019, 18, 13. [Google Scholar] [CrossRef]
- Poti, F.; Santi, D.; Spaggiari, G.; Zimetti, F.; Zanotti, I. Polyphenol Health Effects on Cardiovascular and Neurodegenerative Disorders: A Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 26. [Google Scholar] [CrossRef]
- Vauzour, D.; Vafeiadou, K.; Rodriguez-Mateos, A.; Rendeiro, C.; Spencer, J.P.E. The neuroprotective potential of flavonoids: A multiplicity of effects. Gen. Nutr. 2008, 3, 115–126. [Google Scholar] [CrossRef]
- Ben Youssef, S.; Brisson, G.; Doucet-Beaupre, H.; Castonguay, A.-M.; Gora, C.; Amri, M.; Levesque, M. Neuroprotective benefits of grape seed and skin extract in a mouse model of Parkinson’s disease. Nutr. Neurosci. 2019, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.S.; Smith, C. Ageing-Associated Oxidative Stress and Inflammation Are Alleviated by Products from Grapes. Oxid. Med. Cell. Longev. 2016, 2016, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.G. Molecular mechanism of the chemopreventive effect of resveratrol. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2003, 523, 145–150. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Wu, J.M. Suppression of cell proliferation and gene expression by combinatorial synergy of EGCG, resveratrol and γ-tocotrienol in estrogen receptor-positive MCF-7 breast cancer cells. Int. J. Oncol. 2008, 33, 851–859. [Google Scholar] [CrossRef]
- Gatouillat, G.; Balasse, E.; Joseph-Pietras, D.; Morjani, H.; Madoulet, C. Resveratrol Induces Cell-Cycle Disruption and Apoptosis in Chemoresistant B16 Melanoma. J. Cell. Biochem. 2010, 110, 893–902. [Google Scholar] [CrossRef]
- Nivelle, L.; Hubert, J.; Courot, E.; Jeandet, P.; Aziz, A.; Nuzillard, J.M.; Renault, J.H.; Clement, C.; Martiny, L.; Delmas, D.; et al. Anti-Cancer Activity of Resveratrol and Derivatives Produced by Grapevine Cell Suspensions in a 14 L Stirred Bioreactor. Molecules 2017, 22, 14. [Google Scholar] [CrossRef]
- Grimes, K.L.; Stuart, C.M.; McCarthy, J.J.; Kaur, B.; Cantu, E.J.; Forester, S.C. Enhancing the Cancer Cell Growth Inhibitory Effects of Table Grape Anthocyanins. J. Food Sci. 2018, 83, 2369–2374. [Google Scholar] [CrossRef]
- Sorrenti, V.; Vanella, L.; Acquaviva, R.; Cardile, V.; Giofre, S.; Di Giacomo, C. Cyanidin induces apoptosis and differentiation in prostate cancer cells. Int. J. Oncol. 2015, 47, 1303–1310. [Google Scholar] [CrossRef]
- Kumar, A.; D’Silva, M.; Dholakia, K.; Levenson, A.S. In Vitro Anticancer Properties of Table Grape Powder Extract (GPE) in Prostate Cancer. Nutrients 2018, 10, 12. [Google Scholar] [CrossRef]
- Signorelli, P.; Fabiani, C.; Brizzolari, A.; Paroni, R.; Casas, J.; Fabrias, G.; Rossi, D.; Ghidoni, R.; Caretti, A. Natural Grape Extracts Regulate Colon Cancer Cells Malignancy. Nutr. Cancer 2015, 67, 494–503. [Google Scholar] [CrossRef]
- Nirmala, J.G.; Celsia, S.E.; Swaminathan, A.; Narendhirakannan, R.T.; Chatterjee, S. Cytotoxicity and apoptotic cell death induced by Vitis vinifera peel and seed extracts in A431 skin cancer cells. Cytotechnology 2018, 70, 537–554. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Longo, C.; Gerardi, C.; Trosko, J.E. Pro-Apoptotic Effect of Grape Seed Extract on MCF-7 Involves Transient Increase of Gap Junction Intercellular Communication and Cx43 Up-Regulation: A Mechanism of Chemoprevention. Int. J. Mol. Sci. 2019, 20, 3244. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agent. 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Matias, A.A.; Serra, A.T.; Silva, A.C.; Perdigao, R.; Ferreira, T.B.; Marcelino, I.; Silva, S.; Coelho, A.V.; Alves, P.M.; Duarte, C.M.M. Portuguese winemaking residues as a potential source of natural anti-adenoviral agents. Internat. J. Food Sci. Nutrit. 2010, 61, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Baert, L.; Zhang, D.; Xia, M.; Zhong, W.; Van Coillie, E.; Jiang, X.; Uyttendaele, M. Effect of Grape Seed Extract on Human Norovirus GII. 4 and Murine Norovirus 1 in Viral Suspensions, on Stainless Steel Discs, and in Lettuce Wash Water. Appl. Environment. Microbiol. 2012, 78, 7572–7578. [Google Scholar] [CrossRef]
- Hwang, D.; Lim, Y.H. Resveratrol controls Escherichia coli growth by inhibiting the AcrAB-TolC efflux pump. Fems Microbiol. Lett. 2019, 366. [Google Scholar] [CrossRef]
- Hwang, D.; Lim, Y.H. Resveratrol antibacterial activity against Escherichia coli is mediated by Z-ring formation inhibition via suppression of FtsZ expression. Sci. Rep. 2015, 5, 10029. [Google Scholar] [CrossRef]
- Lee, W.; Lee, D.G. Resveratrol induces membrane and DNA disruption via pro-oxidant activity against Salmonella typhimurium. Biochem. Bioph. Res. Commun. 2017, 489, 228–234. [Google Scholar] [CrossRef]
- Jung, H.J.; Hwang, I.A.; Sung, W.S.; Kang, H.; Kang, B.S.; Seu, Y.B.; Lee, D.G. Fungicidal effect of resveratrol on human infectious fungi. Arch. Pharmacal. Res. 2005, 28, 557–560. [Google Scholar] [CrossRef]
- Yeni, F.; Yavas, S.; Alpas, H.; Soyer, Y. Most Common Foodborne Pathogens and Mycotoxins on Fresh Produce: A Review of Recent Outbreaks. Crit. Rev. Food Sci. Nutr. 2016, 56, 1532–1544. [Google Scholar] [CrossRef] [PubMed]
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadi, M.; Chorianopoulos, N.G.; Nychas, G.-J.E.; Haroutounian, S.A. Antilisterial Activities of Polyphenol-Rich Extracts of Grapes and Vinification Byproducts. J. Agric. Food Chem. 2009, 57, 457–463. [Google Scholar] [CrossRef]
- Silvan, J.M.; Mingo, E.; Hidalgo, M.; de Pascual-Teresa, S.; Carrascosa, A.V.; Martinez-Rodriguez, A.J. Antibacterial activity of a grape seed extract and its fractions against Campylobacter spp. Food Cont. 2013, 29, 25–31. [Google Scholar] [CrossRef]
- Xu, C.M.; Yagiz, Y.; Hsu, W.Y.; Simonne, A.; Lu, J.; Marshall, M.R. Antioxidant, Antibacterial, and Antibiofilm Properties of Polyphenols from Muscadine Grape (Vitis rotundifolia Michx.) Pomace against Selected Foodborne Pathogens. J. Agric. Food Chem. 2014, 62, 6640–6649. [Google Scholar] [CrossRef]
- Xu, C.M.; Yagiz, Y.; Zhao, L.; Simonne, A.; Lu, J.; Marshall, M.R. Fruit quality, nutraceutical and antimicrobial properties of 58 muscadine grape varieties (Vitis rotundifolia Michx.) grown in United States. Food Chem. 2017, 215, 149–156. [Google Scholar] [CrossRef]
- Al-Habib, A.; Al-Saleh, E.; Safer, A.M.; Afzal, M. Bactericidal effect of grape seed extract on methicillin-resistant Staphylococcus aureus (MRSA). J. Toxicol. Sci. 2010, 35, 357–364. [Google Scholar] [CrossRef]
- Sheng, L.; Olsen, S.A.; Hu, J.; Yue, W.; Means, W.J.; Zhu, M.J. Inhibitory effects of grape seed extract on growth, quorum sensing, and virulence factors of CDC “top-six” non-O157 Shiga toxin producing E. coli. Int. J. Food Microbiol. 2016, 229, 24–32. [Google Scholar] [CrossRef]
- Zhu, M.J.; Olsen, S.A.; Sheng, L.; Xue, Y.; Yue, W. Antimicrobial efficacy of grape seed extract against Escherichia coli O157:H7 growth, motility and Shiga toxin production. Food Cont. 2015, 51, 177–182. [Google Scholar] [CrossRef]
- Adamez, J.D.; Samino, E.G.; Sanchez, E.V.; Gonzalez-Gomez, D. In vitro estimation of the antibacterial activity and antioxidant capacity of aqueous extracts from grape-seeds (Vitis vinifera L.). Food Cont. 2012, 24, 136–141. [Google Scholar] [CrossRef]
- Sanhueza, L.; Tello, M.; Vivanco, M.; Mendoza, L.; Wilkens, M. Relation between Antibacterial Activity against Food Transmitted Pathogens and Total Phenolic Compounds in Grape Pomace Extracts from Cabernet Sauvignon and Syrah Varieties. Adv. Microbiol. 2014, 4, 225–232. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Selvi, T.; Sakariah, K.K. Antibacterial and antioxidant activities of grape (Vitis vinifera) seed extracts. Food Res. Int. 2003, 36, 117–122. [Google Scholar] [CrossRef]
- Brown, J.C.; Huang, G.H.; Haley-Zitlin, V.; Jiang, X.P. Antibacterial Effects of Grape Extracts on Helicobacter pylori. Appl. Environ. Microbiol. 2009, 75, 848–852. [Google Scholar] [CrossRef]
- Martini, S.; D’Addario, C.; Braconi, D.; Bernardini, G.; Salvini, L.; Bonechi, C.; Figura, N.; Santucci, A.; Rossi, C. Antibacterial Activity of Grape Extracts on cagA-Positive and -Negative Helicobacter pylori Clinical Isolates. J. Chemother. 2009, 21, 507–513. [Google Scholar] [CrossRef]
- Furiga, A.; Lonvaud-Funel, A.; Badet, C. In vitro study of antioxidant capacity and antibacterial activity on oral anaerobes of a grape seed extract. Food Chem. 2009, 113, 1037–1040. [Google Scholar] [CrossRef]
- Pflieger, A.; Teguo, P.W.; Papastamoulis, Y.; Chaignepain, S.; Subra, F.; Munir, S.; Delelis, O.; Lesbats, P.; Calmels, C.; Andreola, M.-L.; et al. Natural Stilbenoids Isolated from Grapevine Exhibiting Inhibitory Effects against HIV-1 Integrase and Eukaryote MOS1 Transposase In Vitro Activities. PLoS ONE 2013, 8, e81184. [Google Scholar] [CrossRef]
- Yu, B.; Jiang, Y.; Zhang, B.; Yang, H.; Ma, T. Resveratrol dimer trans-epsilon-viniferin prevents rotaviral diarrhea in mice by inhibition of the intestinal calcium-activated chloride channel. Pharmacol. Res. 2018, 129, 453–461. [Google Scholar] [CrossRef]
- Lee, S.; Yoon, K.D.; Lee, M.; Cho, Y.; Choi, G.; Jang, H.; Kim, B.; Jung, D.-H.; Oh, J.-G.; Kim, G.-W.; et al. Identification of a resveratrol tetramer as a potent inhibitor of hepatitis C virus helicase. Br. J. Pharmacol. 2016, 173, 191–211. [Google Scholar] [CrossRef]
- Lee, S.; Mailar, K.; Kim, M.I.; Park, M.; Kim, J.; Min, D.-H.; Heo, T.-H.; Bae, S.K.; Choi, W.; Lee, C. Plant-Derived Purification, Chemical Synthesis, and In Vitro/In Vivo Evaluation of a Resveratrol Dimer, Viniferin, as an HCV Replication Inhibitor. Viruses 2019, 11, 890. [Google Scholar] [CrossRef]
- Chisholm, C.; Lopez, L. Cutaneous Infections Caused by Herpesviridae: A Review. Arch. Pathol. Lab. Med. 2011, 135, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Evers, D.L.; Wang, X.; Huong, S.M.; Huang, D.Y.; Huang, E.S. 3,4′,5-trihydroxy-trans-stilbene (resveratrol) inhibits human cytomegalovirus replication and virus-induced cellular signaling. Antivir. Res. 2004, 63, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Faith, S.A.; Sweet, T.J.; Bailey, E.; Booth, T.; Docherty, J.J. Resveratrol suppresses nuclear factor-kappa B in herpes simplex virus infected cells. Antivir. Res. 2006, 72, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Docherty, J.J.; Sweet, T.J.; Bailey, E.; Faith, S.A.; Booth, T. Resveratrol inhibition of varicella-zoster virus replication in vitro. Antivir. Res. 2006, 72, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Palamara, A.T.; Nencioni, L.; Aquilano, K.; De Chiara, G.; Hernandez, L.; Cozzolino, F.; Ciriolo, M.R.; Garaci, E. Inhibition of influenza A virus replication by resveratrol. J. Inf. Dis. 2005, 191, 1719–1729. [Google Scholar] [CrossRef] [PubMed]
- Hartenian, E.; Nandakumar, D.; Lari, A.; Ly, M.; Tucker, J.M.; Glaunsinger, B.A. The molecular virology of coronaviruses. J. Biol. Chem. 2020, 295, 12910–12934. [Google Scholar] [CrossRef]
- Naqvi, A.A.T.; Fatima, K.; Mohammad, T.; Fatima, U.; Singh, I.K.; Singh, A.; Atif, S.M.; Hariprasad, G.; Hasan, G.M.; Hassan, M.I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochim. Biophy. Acta Mol. Basis Dis. 2020, 1866. [Google Scholar] [CrossRef]
- Muchtaridi, M.; Fauzi, M.; Ikram, N.K.K.; Gazzali, A.M.; Wahab, H.A. Natural Flavonoids as Potential Angiotensin-Converting Enzyme 2 Inhibitors for Anti-SARS-CoV-2. Molecules 2020, 25, 3980. [Google Scholar] [CrossRef]
- Joshi, R.S.; Jagdale, S.S.; Bansode, S.B.; Shankar, S.S.; Tellis, M.B.; Pandya, V.K.; Chugh, A.; Giri, A.P.; Kulkarni, M.J. Discovery of potential multi-target-directed ligands by targeting host-specific SARS-CoV-2 structurally conserved main protease. J. Biomol. Struct. Dyn. 2020, 1–16. [Google Scholar] [CrossRef]
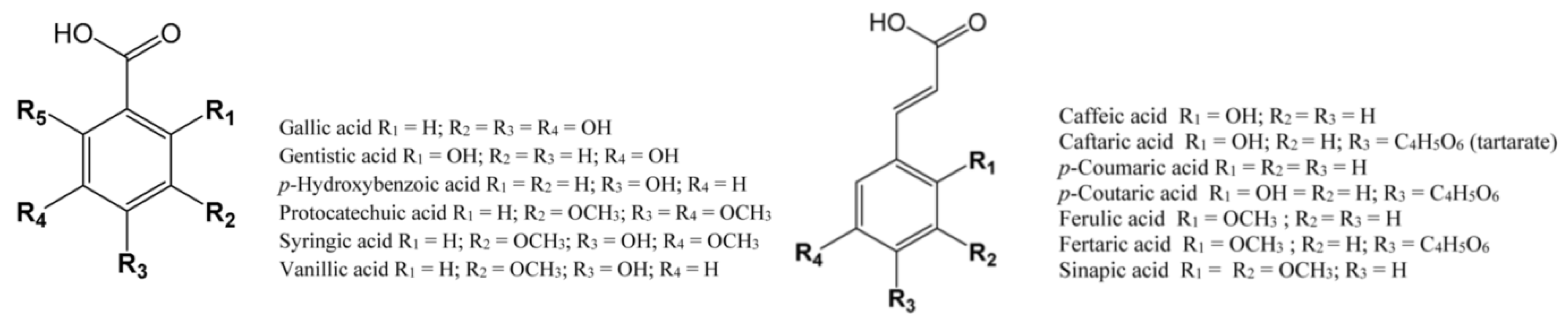
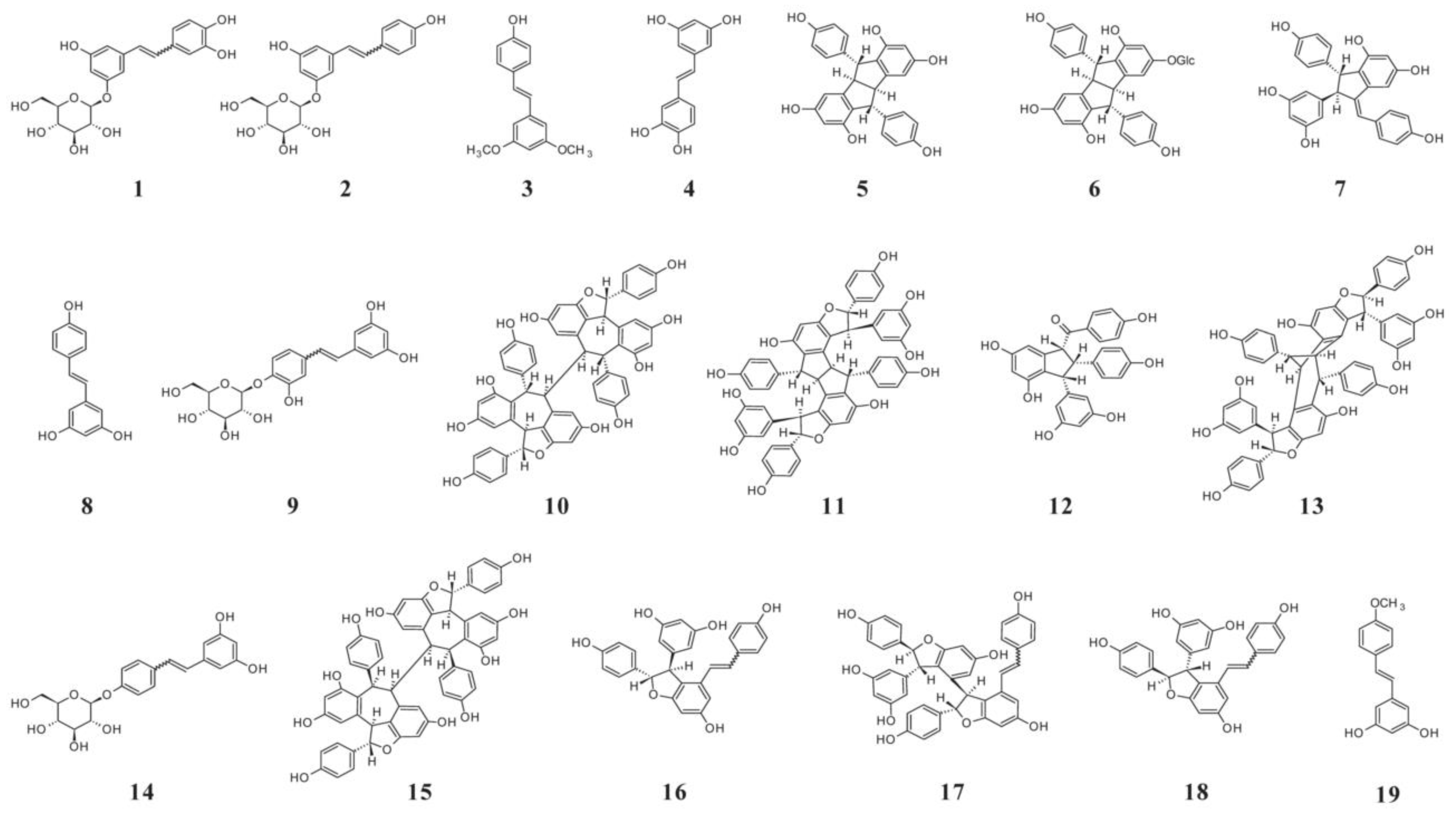
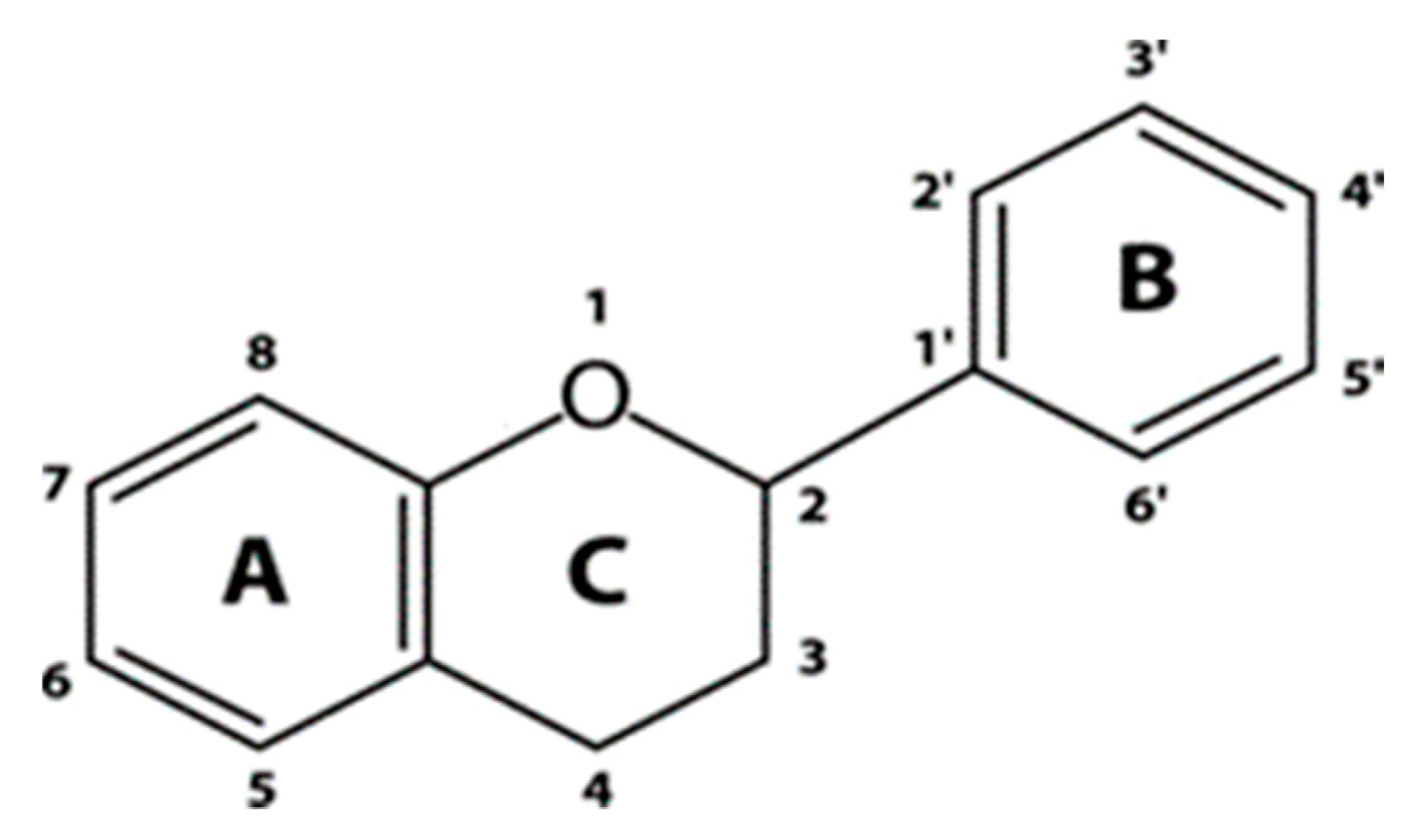

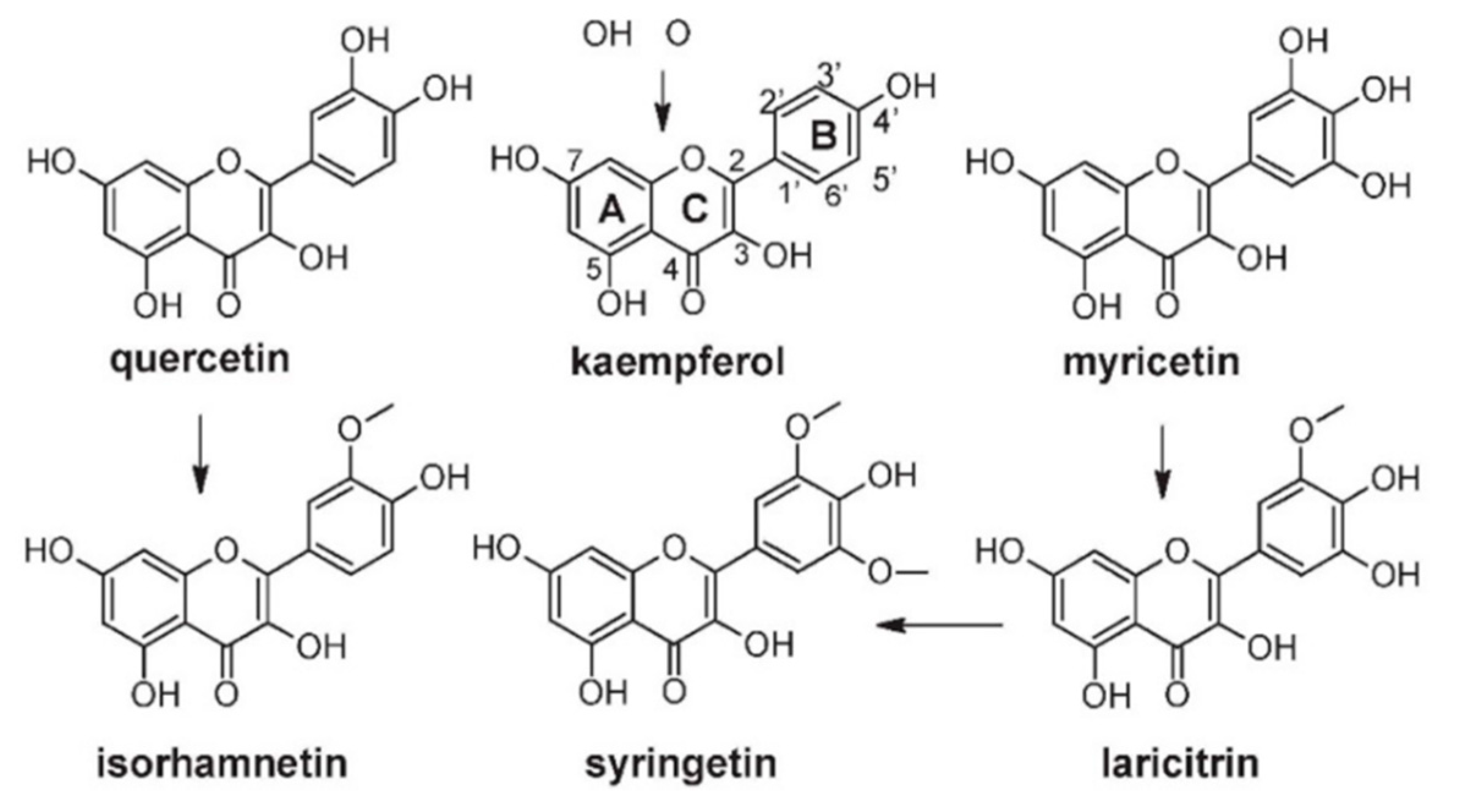
| Cultivar (Country of Origin *) | Gallic Acid | Protocatechuic Acid | p-Hydroxybenzoic Acid | Gentisic Acid | Vanillic Acid | Syringic Acid | Analysis Method ** | Ref. |
|---|---|---|---|---|---|---|---|---|
| Grapes–Red Cultivars | ||||||||
| Alphonse Lavallee (FRA) | 0 m | 0 m | n.a. *** | n.a. | n.a. | n.a. | 1 | [77] |
| Azal Tinto (POR) | 3.4 m | n.a. | n.a. | n.a. | n.a. | 2.5 m | 2 | [78] |
| Borracal (ESP) | 4.6 m | n.a. | n.a. | n.a. | n.a. | 13 m | 3 | [78] |
| Cabernet Sauvignon (FRA) | 11.93 a | n.a. | n.a. | n.a. | n.a. | n.a. | 4 | [79] |
| 21.5–27.7 d | n.a. | n.a. | n.a. | n.a. | n.a. | 4 | [80] | |
| 3.66 i | 0.44 i | 1.60 i | 6.74 i | n.a. | n.a. | 5 | [81] | |
| n.a. | n.a. | n.a. | n.a. | 0.003 l | n.a. | 6 | [82] | |
| 82.6 o | 15.4 o | n.a. | n.a. | 108.5 o | 120.9 o | 3 | [83] | |
| Cabernet Franc (FRA) | 16.7–16.9 f | n.a. | n.a. | n.a. | 2.2–2.5 f | n.a. | 4 | [83] |
| 8.76 i | 0.43 i | 0.44 i | 0 i | n.a. | n.a. | 5 | [84] | |
| Dornfelder (GER) | n.a. | n.a. | n.a. | n.a. | 0.011 l | n.a. | 6 | [82] |
| Espadeiro (POR) | 5.1 m | n.a. | n.a. | n.a. | n.a. | 0 m | 2 | [78] |
| Merlot (FRA) | 19.3–40.0 d | n.a. | n.a. | n.a. | n.a. | n.a. | 4 | [81] |
| 3.66 i | 0.48 i | 0 i | 0 i | n.a. | n.a. | 5 | [84] | |
| 3 j | n.a. | n.a. | n.a. | n.a. | n.a. | 4 | [85] | |
| 125.6 o | 51.7 o | n.a. | n.a. | 197.9 o | 121.8 o | 3 | [78] | |
| 66.6 m | 328.7 m | n.a. | n.a. | n.a. | n.a. | 1 | [77] | |
| Negroamaro (ITA) | 7.3 m | 42.0 m | n.a. | n.a. | n.a. | n.a. | 1 | [77] |
| Pedral (POR) | 2.2 m | n.a. | n.a. | n.a. | n.a. | 0 m | 2 | [78] |
| Pinot Noir (FRA) | 38.4 g | n.a. | n.a. | n.a. | n.a. | 32.8 g | 4 | [84] |
| 2.42 i | 0.40 i | 1.42 i | 5.64 i | n.a. | n.a. | 5 | [84] | |
| n.a. | n.a. | n.a. | n.a. | 0.014 l | n.a. | 6 | [82] | |
| Pinot Gris (FRA) | 16.1 g | n.a. | n.a. | n.a. | n.a. | 20.2 g | 4 | [84] |
| 2.34 i | 0.54 i | 0 i | 5.90 i | n.a. | n.a. | 5 | [84] | |
| Plavac Mali (CRO) | n.a. | n.a. | n.a. | n.a. | 0.047 l | n.a. | 6 | [82] |
| Primitivo (CRO) | 0 m | 13.4 m | n.a. | n.a. | n.a. | n.a. | 1 | [77] |
| Prokupac (MNE) | 3.90 i | 0.52 i | 0 i | 7.15 i | n.a. | n.a. | 5 | [84] |
| Sangiovese (ITA) | 4.80 i | 0.44 i | 0.30 i | 10.16 i | n.a. | n.a. | 5 | [84] |
| Syrah (FRA) | 0.23–0.33 c | n.a. | n.a. | n.a. | n.a. | n.a. | 4 | [80] |
| 5.85 i | 0.44 i | 0.77 i | 8.74 i | n.a. | n.a. | 5 | [84] | |
| 16.45 k | n.a. | n.a. | n.a. | n.a. | n.a. | 4 | [86] | |
| Touriga (POR) | n.a. | n.a. | n.a. | n.a. | 0.015 l | n.a. | 6 | [82] |
| Tempranillo (ESP) | 0.40 b | n.a. | n.a. | n.a. | n.a. | n.a. | 2 | [84] |
| Vinhao (POR) | 2.7 m | n.a. | n.a. | n.a. | n.a. | 8.9 m | 2 | [78] |
| Vranac (SRB) | 0.41 h | n.a. | n.a. | n.a. | n.a. | n.a. | 4 | [84] |
| Grapes–White Cultivars | ||||||||
| Albarino (ESP) | 1.19 n | n.a. | 2.32 n | n.a. | n.a. | n.a. | 7 | [87] |
| Chardonnay (FRA) | 2.78 i | 0.49 i | 0 i | 5.87 i | n.a. | n.a. | 5 | [84] |
| 5 j | n.a. | n.a. | n.a. | n.a. | n.a. | 4 | [85] | |
| n.a. | n.a. | n.a. | n.a. | 0.017 l | n.a. | 6 | [82] | |
| 29.4 o | 1.51 o | n.a. | n.a. | 249.8 o | 42.7 o | 3 | [78] | |
| Malvasia Fina (POR) | 5.7 g | n.a. | n.a. | n.a. | n.a. | 5.5 g | 4 | [84] |
| Pinot Blanc (FRA) | 18.3 g | n.a. | n.a. | n.a. | n.a. | 17.2 g | 4 | [84] |
| Riesling (GER) | 2.44 i | 0.35 i | 0 i | 0 i | n.a. | n.a. | 5 | [84] |
| Sauvignon blanc (FRA) | 4.25 i | 0.55 i | 0 i | 1.47 i | n.a. | n.a. | 5 | [84] |
| n.a. | n.a. | n.a. | n.a. | 0.030 l | n.a. | 6 | [82] | |
| 16.8 o | 2.10 o | n.a. | n.a. | 264.7 o | 95.2 o | 3 | [78] | |
| Verdelho (POR) | 4.6 m | n.a. | n.a. | n.a. | n.a. | 0 m | 3 | [78] |
| Vermentino (ITA) | 11.9 o | 0 o | n.a. | n.a. | 74.9 o | 55.3 o | 3 | [78] |
| Viognier (FRA) | 10.2 o | 0 o | n.a. | n.a. | 153.9 o | 33.6 o | 3 | [78] |
| Welschriesling (NN) | 4.57 i | 0.44 i | 0 i | 0 i | n.a. | n.a. | 5 | [84] |
| Leaves | ||||||||
| Cabernet franc (FRA) | 4.72 r | 1.78 r | 33.9 r | 2.75 r | n.a. | n.a. | 8 | [88] |
| Cabernet Sauvignon (FRA) | 4.49 r | 3.47 r | 72.2 r | 3.76 r | n.a. | n.a. | ||
| Chardonnay (FRA) | 4.69 r | 5.90 r | 31.1 r | 2.70 r | n.a. | n.a. | ||
| Merlot (FRA) | 4.61 r | 1.25 r | 29.6 r | 2.71 r | n.a. | n.a. | ||
| Pinot Noir (FRA) | 4.38 r | 3.24 r | 57.6 r | 4.38 r | n.a. | n.a. | ||
| Prokupac (SRB) | 4.67 r | 4.95 r | 15.8 r | 2.75 r | n.a. | n.a. | ||
| Riesling (GER) | 4.13 r | 2.74 r | 54.6 r | 0.597 r | n.a. | n.a. | ||
| Sauvignon blanc (FRA | 5.35 r | 4.05 r | 110 r | 2.75 r | n.a. | n.a. | ||
| Syrah (FRA) | 3.58 r | 2.55 r | 0 r | 4.07 r | n.a. | n.a. | ||
| Vranac (MNE) | 4.78 r | 3.11 r | 93.4 r | 0.986 r | n.a. | n.a. | ||
| Welschriesling (NN) | 4.12 r | 3.08 r | 35.9 r | 2.73 r | n.a. | n.a. | ||
| Cultivar (Country of Origin *) | Caftaric Acid | Caffeic Acid | trans-Coutaric Acid | trans-Coumaric Acid | Fertaric Acid | Ferulic Acid | Analysis Method ** | Ref. |
|---|---|---|---|---|---|---|---|---|
| Grapes–Red Cultivars | ||||||||
| Aglianico (ITA) | 320.4 d | n.a. ** | n.a. | n.a. | n.a. | n.a. | 1 | [77] |
| Alphonse Lavallee (FRA) | 645.0 d | n.a. | n.a. | n.a. | n.a. | n.a. | 1 | [77] |
| Azal Tinto (POR) | 11 d | n.a. | 5.5 d | n.a. | n.a. | n.a. | 2 | [90] |
| Borracal (ESP) | 13 d | n.a. | 3.5 d | n.a. | n.a. | n.a. | 2 | [90] |
| Brancelho (POR) | 2.6 d | n.a. | 0.73 d | n.a. | n.a. | n.a. | 2 | [90] |
| Cabernet Sauvignon (FRA) | n.a. | 0.59 b | n.a. | 1.14 b | n.a. | 1.59 b | 3 | [84] |
| 127.55 d | Trace | 84.86 d | n.a. | 3.62 d | n.a. | 4 | [91] | |
| 162.98 f | 50.57 f | n.a. | 12.76 f | n.a. | 0 f | 5 | [79] | |
| 54.9 l | n.a. | 2.3 l | 0 l | n.a. | n.a. | 6 | [92] | |
| 0 n | 901.2 n | n.a. | 367.5 n | n.a. | 412.3 n | 7 | [78] | |
| Cabernet Franc (FRA) | n.a. | 0.50 b | n.a. | 0 b | n.a. | 10.33 b | 3 | [84] |
| 0.1–2.6 i | n.a. | n.a. | 2.8–4.2 i | n.a. | 0.1–0.3 i | 5 | [83] | |
| Cesanese (ITA) | 28.8 d | n.a. | n.a. | n.a. | n.a. | n.a. | 1 | [77] |
| Docal (POR) | 3.9 d | n.a. | 0.75 d | n.a. | n.a. | n.a. | 2 | [90] |
| Espadeiro (POR) | 44 d | n.a. | 8.9 d | n.a. | n.a. | n.a. | 2 | [90] |
| Malvasia Nera (GRE) | 171.9 d | n.a. | n.a. | n.a. | n.a. | n.a. | 1 | [77] |
| Merlot (FRA) | n.a. | 0.53 b | n.a. | 0 b | n.a. | 2.20 b | 3 | [84] |
| 100.23 d | Trace | 39.00 d | n.a. | 2.72 d | n.a. | 4 | [91] | |
| 0 n | 1363.6 n | n.a. | 336.7 n | n.a. | 515.1 n | 7 | [78] | |
| 746.3 d | n.a. | n.a. | n.a. | n.a. | n.a. | 1 | [77] | |
| Nebbiolo (ITA) | 0.05–0.08 f | n.a. | 0.05–0.53 f | 0.02–0.06 f | n.a. | n.a. | 1 | [93] |
| Negroamaro (ITA) | 8.5 d | n.a. | n.a. | n.a. | n.a. | n.a. | 1 | [77] |
| Pedral (POR) | 17 d | n.a. | 3.3 d | n.a. | n.a. | n.a. | 2 | [90] |
| Pinot Noir (FRA) | n.a. | 0.54 b | n.a. | 0 b | n.a. | 2.12 b | 3 | [84] |
| 144–6195 e | n.a. * | 14.8–1016 e | n.a. | 1.8–5.9 e | n.a. | 8 | [94] | |
| 53.9 k | n.a. | n.a. | n.a. | n.a. | n.a. | 5 | [95] | |
| Primitivo (CRO) | 1.89 d | n.a. | n.a. | n.a. | n.a. | n.a. | 1 | [77] |
| Sangiovese (ITA) | n.a. | 0.84 b | n.a. | 5.59 b | n.a. | 6.62 b | 3 | [84] |
| Susumaniello (ITA) | 171.7 d | n.a. | n.a. | n.a. | n.a. | n.a. | 1 | [77] |
| Syrah (FRA) | n.a. | 0.67 b | n.a. | 4.39 b | n.a. | 6.98 b | 3 | [84] |
| n.a. | 1.58 g | n.a. | 0 b | n.a. | 4.20 g | 5 | [86] | |
| 154.73 d | Trace | 136.05 d | n.a. | 2.62 d | n.a. | 9 | [96] | |
| Tempranillo (ESP) | 0.8 f | n.a. | 0.9 f | n.a. | 0.65 f | n.a. | 2 | [97] |
| Uva di Troia (ITA) | 93.3 d | n.a. | n.a. | n.a. | n.a. | n.a. | 1 | [77] |
| Vinhao (POR) | 11 d | n.a. | 5.3 d | n.a. | n.a. | n.a. | 2 | [90] |
| Vranac (MNE) | n.a. * | 0.085 a | 0.011 a | 0.009 a | n.a. | n.a. | 5 | [98] |
| Grapes–White Cultivars | ||||||||
| Albarino (ESP) | 4.04 m | n.a. | 0.27 m | 1.96 m | 1.68 m | n.a. | 9 | [87] |
| Chardonnay (FRA) | n.a. | 0.58 b | n.a. | 0.72 b | n.a. | 13.95 b | 3 | [84] |
| 1918 n | 1880.2 n | n.a. | 271.6 n | n.a. | 195.3 n | 7 | [78] | |
| Istrian Malvasia (CRO) | 0.17–0.62 f | n.a. | n.a. | n.a. | n.a. | n.a. | 10 | [99] |
| Moscato (NN) | 48.4 d | n.a. | n.a. | n.a. | n.a. | n.a. | 1 | [77] |
| Riesling (GER) | n.a. | 0.65 b | n.a. | 0 b | n.a. | 11.83 b | 3 | [84] |
| Sauvignon blanc (FRA) | n.a. | 0.49 b | n.a. | 0 b | n.a. | 10.94 b | 3 | [84] |
| 1430 n | 2498.4 n | n.a. | 275.1 n | n.a. | 136.4 n | 7 | [78] | |
| Semillon (FRA) | 33.1 f | n.a. | 7.3 f | n.a. | n.a. | n.a. | 5 | [100] |
| Viognier (FRA) | 1075.2 n | 2285.1 n | n.a. | 301.7 n | n.a. | 140.7 n | 7 | [78] |
| Vermentino (ITA) | 1076.7 n | 1661.8 n | n.a. | 270.2 n | n.a. | 201.6 n | 7 | [78] |
| Verdelho (POR) | 3.2 d | n.a. | 1.3 d | n.a. | n.a. | n.a. | 2 | [90] |
| Welschriesling (NN) | n.a. | 0.63 b | n.a. | 0.22 b | n.a. | 8.95 b | 3 | [84] |
| Leaves | ||||||||
| Cabernet Franc (FRA) | n.a. | 5.36 o | n.a. | 4.69 o | n.a. | 39.0 o | 11 | [88] |
| Cabernet Sauvignon (FRA) | n.a. | 5.21 o | n.a. | 0.772 o | n.a. | 22.4 o | ||
| Chardonnay (FRA) | n.a. | 5.03 o | n.a. | 3.96 o | n.a. | 27.0 o | ||
| Merlot (FRA) | n.a. | 2.72 o | n.a. | 2.84 o | n.a. | 18.3 o | ||
| Riesling (GER) | n.a. | 4.18 o | n.a. | 1.29 o | n.a. | 19.1 o | ||
| Sangiovese (ITA) | n.a. | 8.95 o | n.a. | 1.15 o | n.a. | 21.5 o | ||
| Sauvignon blanc (FRA) | n.a. | 4.10 o | n.a. | 1.10 o | n.a. | 25.5 o | ||
| Syrah (FRA) | n.a. | 4.96 o | n.a. | 1.91 o | n.a. | 18.2 o | ||
| Vranac (MNE) | n.a. | 4.58 o | n.a. | 1.10 o | n.a. | 25.5 o | ||
| Welschriesling (NN) | n.a. | 3.20 o | n.a. | 3.94 o | n.a. | 7.1 o | ||
| Cultivar (Country of Origin **) | trans-Resveratrol | cis-Resveratrol | trans-Piceid | cis-Piceid | Analysis Method * | Ref. |
|---|---|---|---|---|---|---|
| Grapes–Red Cultivars | ||||||
| Aglianico (ITA) | 61.1 d | n.a. *** | 75.7 d | n.a. | 1 | [77] |
| Alphonse Lavalleen (FRA) | 40.0 d | n.a. | 24.1 d | n.a. | 1 | [77] |
| Babić (CRO) | 0.44 a | 0.42 a | 0.18 a | n.a. | 2 | [108] |
| Brancelho (POR) | n.a. | n.a. | 2.8 m | n.a. | 3 | [90] |
| Borracal (ESP) | n.a. | n.a. | 14 m | n.a. | 3 | [90] |
| Cabernet Sauvignon (FRA) | 0 e | n.a. | 69.77 e | n.a. | 2 | [79] |
| 9.61 i | n.a. | n.a. | n.a. | 4 | [84] | |
| 56.4 n | n.a. | n.a. | n.a. | 5 | [78] | |
| 0.47 h | n.a. | n.a. | n.a. | 6 | [91] | |
| n.a. | n.a. | 5.2–6.9 h | 0 h | 7 | [109] | |
| 25.5 j | n.a. | n.a. | n.a. | 2 | [39] | |
| Gamay (FRA) | n.a. | n.a. | 6.2–17.8 h | 0 h | 7 | [109] |
| Merlot (FRA) | 5.80 i | n.a. | n.a. | n.a. | 4 | [84] |
| 1.02 a | 0.36 a | 0.31 a | n.a. | 2 | [108] | |
| 54.1 n | n.a. | n.a. | n.a. | 5 | [78] | |
| 9.2 d | n.a. | 26.3 d | n.a. | 1 | [77] | |
| 6.99 h | n.a. | n.a. | n.a. | 6 | [91] | |
| n.a. | n.a. | 25.6–28.1 h | 0–13.1 h | 7 | [109] | |
| 10.5 j | n.a. | n.a. | n.a. | 2 | [39] | |
| Negroamaro (ITA) | 3.6 d | n.a. | 4.14 d | n.a. | 1 | [77] |
| Pedral (POR) | n.a. | n.a. | 38 m | n.a. | 3 | [90] |
| Pinot Noir (FRA) | 6.4–123 g | n.a. | 2.2–805 g | n.a. | 8 | [94] |
| 5.80 i | n.a. | n.a. | n.a. | 4 | [84] | |
| n.a. | n.a. | 5.5 b | n.a. | 2 | [95] | |
| Pinot Gris (FRA) | n.a. | n.a. | 2.1 b | n.a. | 2 | [95] |
| Plavina (CRO) | 0.30 a | 0.07 a | 0.17 a | n.a. | 2 | [108] |
| Primitivo (CRO) | 13.9 d | n.a. | 30.7 d | n.a. | 1 | [77] |
| 1136.40 k | n.a. | 2332.10 k | 1776.20 k | 9 | [105] | |
| Prokupac (SRB) | 13.42 i | n.a. | n.a. | n.a. | 4 | [84] |
| Raboso Piave (ITA) | 1134.80 k | n.a. | 395.30 k | 1476.80 k | 9 | [105] |
| Syrah (FRA) | 3.6 d | n.a. | n.a. | n.a. | 2 | [86] |
| 0.08 h | n.a. | n.a. | n.a. | 6 | [91] | |
| Tempranillo (ESP) | 0.12 e | 0.10 e | 0.65 e | 0.40 e | 3 | [97] |
| Trnjak (CRO) | 0.41 a | 1.74 a | 0.11 a | n.a. | 2 | [108] |
| Vinhao (POR) | n.a. | n.a. | 163 m | n.a. | 3 | [90] |
| Vranac (MNE) | 0.78 a | 0.93 a | 0 a | n.a. | 2 | [108] |
| Verdelho (POR) | n.a. | n.a. | 32 m | n.a. | 3 | [90] |
| Albarino (ESP) | 1.43 l | n.a. | 6.93 l | n.a. | 10 | [87] |
| Chardonnay (FRA) | 29.4 n | n.a. | n.a. | n.a. | 5 | [78] |
| Istrian Malvasia (CRO) | n.a. | n.a. | 0.01 f | n.a. | 11 | [99] |
| Kujundžuša (CRO) | 0.27 a | 0.84 a | 1.11 a | n.a. | 2 | [108] |
| Zlatarica (CRO) | 0.10 a | 0.26 a | 0.10 a | n.a. | 2 | [108] |
| Maraština (CRO) | 0.17 a | 0.29 a | 0.65 a | n.a. | 2 | [108] |
| Debit (CRO) | 0.72 a | 0.26 a | 0.29 a | n.a. | 2 | [108] |
| Cultivar (Country of Origin *) | 3-O-Glucosides | 3-O-Acetylglucosides | 3-O-Coumaroylglucosides | 3-O-Caffeoylglucosides | Analysis Method ** | Ref. | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dp 1 | Cy 2 | Pt 3 | Pn 4 | Mv 5 | Dp | Cy | Pt | Pn | Mv | Dp | Cy | Pt | Pn | Mv | Pn | Mv | |||
| Aglianico (ITA) | 594.7 g | 52.1 g | 596.5 g | 553.6 g | 3348.0 g | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 5 | [77] |
| Alfrocheiro (POR) | 0.03 k | 0 k | 0.16 k | 0.14 k | 2.90 k | n.a. | 0 k | 0 k | 0.04 k | 0.18 k | n.a. | n.a. | 0.06 k | 0.04 k | 1.46 k | n.a. | n.a. | 1 | [121] |
| Alphonse Lavallee (FRA) | 219.8 g | 46.4 g | 241.1 g | 52.5 g | 1851.1 g | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 5 | [77] |
| Alvarlhão (POR) | 0.08 k | 0.24 k | 0.12 k | 1.04 k | 0.99 k | n.a. | 0.01 k | 0.01 k | 0.06 k | 0.05 k | n.a. | n.a. | 0.01 k | 0.12 k | 0.12 k | n.a. | n.a. | 1 | [121] |
| Borracal (ESP) | 481 b | 55 b | 513 b | 259 b | 1469 b | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 42 b | 26 b | 122 b | n.a. | n.a. | 7 | [90] |
| Cabernet Sauvignon (FRA) | 7124 c | 653 c | 2860 c | 560 c | 9119 c | n.a. *** | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 1 | [79] |
| 1.59 g | 0.79 g | 7.40 g | 16.70 g | 328.86 g | 1.28 g | 0.17 g | 11.62 g | 32.08 g | 539.01 g | 0.37 g | n.a. | 2.52 g | 23.02 g | 71.08 g | n.a. | 0.16 g | 2 | [91] | |
| 2057 g | 43 g | 1558 g | 847 g | 6438 g | 502 g | 134 g | 408 g | 179 g | 1828 g | 376 g | 77 g | 315 g | 328 g | 2380 g | 39 g | 86 g | 3 | [109] | |
| 46 h | Tr. h | 51 h | 43 h | 665 h | 21 h | n.a. | 26 h | 29 h | 425 h | n.a. | Tr. h | 21 h | n.a. | 220 h | n.a. | 62 h | 1 | [122] | |
| 6540 g | 686 g | 1622 g | 1835 g | 9354 g | 687 g | 178 g | 542 g | 441 g | 4513 g | 178 g | 53 g | 130 g | 261 g | 1346 g | 43.63 g | 0 g | 4 | [117] | |
| 1206.86 g | 147.38 g | 719.03 g | 809.19 g | 5708.98 g | 384.02 g | 124.70 g | 342.41 g | 672.4 g | 4675.9 g | 124.26 g | n.a. | n.a. | 388.12 g | 1959.9 g | n.a. | n.a. | 3 | [123] | |
| 115.7 f | 53.8 f | 73.1 f | 106.2 f | 188.3 f | 31.5 f | 14.0 f | 23.0 f | 35.2 f | 55.4 f | 8.3 f | 5.1 f | 5.5 f | 17.4 f | 23.0 f | 1.6 f | n.a. | 1 | [124] | |
| Cabernet Franc (FRA) | 0.95 k | 0.04 k | 1.03 k | 0.17 k | 4.10 k | n.a. | 0.01 k | 0.02 k | 0.20 k | 0.27 k | n.a. | n.a. | 0.22 k | 0.02 k | 1.52 k | n.a. | n.a. | 1 | [121] |
| Carignan Noir (FRA) | 0.34 k | 0.02 k | 0.57 k | 0.56 k | 5.60 k | n.a. | 0 k | 0 k | 0.04 k | 0.53 k | n.a. | n.a. | 0.16 k | 0.35 k | 3.44 k | n.a. | n.a. | 1 | [121] |
| Gamay (FRA) | 200 g | 57 g | 259 g | 534 g | 3847 g | 0 g | 0 g | 0 g | 0 g | 131 g | 0 g | 0 g | 0 g | 37 g | 189 g | 0 g | 0 g | 3 | [109] |
| 0.02 k | 0 k | 0.08 k | 0.12 k | 1.77 k | n.a. | 0 k | 0 k | 0.04 k | 0.23 k | n.a. | n.a. | 0.02 k | 0.11 k | 1.61 k | n.a. | n.a. | 1 | [121] | |
| Gewürztraminer (NN) | 0.21 k | 0.01 k | 0.44 k | 0.26 k | 4.92 k | n.a. | 0.03 k | 0.39 k | 0.07 k | 1.54 k | n.a. | n.a. | 0.39 k | 0.07 k | 0.88 k | n.a. | n.a. | 1 | [121] |
| Grenache (ESP) | n.a. | 42.9 c | 98.8 c | 156 c | 355 c | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 1 | [125] |
| 0.25 k | 0.04 k | 0.14 k | 0.18 k | 1.44 k | n.a. | 0 k | 0 k | 0 k | 0.01 k | n.a. | n.a. | 0.05 k | 0.05 k | 0.17 k | n.a. | n.a. | 1 | [121] | |
| Merlot (FRA) | 6.91 g | 1.78 g | 25.05 g | 58.89 g | 251.54 g | 3.39 g | 0.65 g | 19.92 g | 47.79 g | 258.20 g | 3.57 g | n.a. | 7.04 g | 23.02 g | 49.58 g | n.a. | 0.47 g | 2 | [91] |
| 2059 g | 363 g | 1470 g | 295 g | 6835 g | 450 g | 133 g | 350 g | 286 g | 1813 g | 201 g | 254 g | 169 g | 218 g | 1202 g | 0 g | 0 g | 3 | [109] | |
| 974.5 g | 25.93 g | 534.4 g | 580.2 g | 12180 g | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 5 | [77] | |
| 1544.63 g | 310.21 g | 1252.03 g | 1357.51 g | 8027.00 g | 495.00 g | 161.14 g | 340.34 g | 168.16 g | 4092.97 g | 228.73 g | n.a. | n.a. | 369.91 g | 2415.44 g | n.a. | n.a. | 3 | [123] | |
| 250.6 f | 87.5 f | 125.6 f | 74.2 f | 203.0 f | 46.5 f | 15.4 f | 29.2 f | 12.7 f | 43.6 f | 16.6 f | 9.5 f | 9.4 f | 8.0 f | 20.6 f | 2.1 f | n.a. | 1 | [124] | |
| Moristel (ESP) | n.a. | 12.1 c | 56.8 c | 33.7 c | 265 c | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 1 | [125] |
| Negroamaro (ITA) | 112.8 g | 50.6 g | 203.6 g | 110.2 g | 662.6 g | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 5 | [77] |
| Pinot Noir (FRA) | 2.9 b | 4.4 b | 6.4 b | 34.9 b | 61.5 b | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 6 | [94] |
| 2373 g | 1325 g | 1249 g | 1528 g | 6019 g | 337 g | 90.2 g | 274 g | 288 g | 901 g | 0 g | 0 g | 142 g | 88 g | 597 g | 0 g | 0 g | 3 | [109] | |
| 105.6 f | 24.1 f | 104.5 f | 114.2 f | 378.5 f | 0 f | 0 f | 0 f | 0 f | 0 f | 0 f | 0 f | 0 f | 0 f | 0 f | 0 f | n.a. | 1 | [124] | |
| Primitivo (CRO) | 44.7 g | 14.7 g | 168.4 g | 207.7 g | 1883.0 g | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 5 | [77] |
| Syrah (FRA) | 3.30 g | 0.70 g | 24.08 g | 48.42 g | 308.45 g | 1.86 g | 0.18 g | 17.68 g | 72.53 g | 816.78 g | 8 g | n.a. | 17.20 g | 63.86 g | 251.70 g | n.a. | 2.46 g | 2 | [91] |
| 1660 g | 111 g | 987 g | 679 g | 3574 g | 164 g | 0 g | 231 g | 142 g | 1303 g | 0 g | 0 g | 0 g | 288 g | 1629 g | 0 g | 0 g | 3 | [109] | |
| Tempranillo (ESP) | n.a. | 17.5 c | 76.6 c | 21.8 c | 239 c | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 1 | [125] |
| 275.68 f | 107.81 f | 204.33 f | 207.57 f | 608.36 f | 7.02 f | 1.09 f | 4.86 f | 2.38 f | 20.07 f | 36.73 f | 16.21 f | 29.68 f | 24.66 f | 205.25 f | 0.34 f | 2.54 f | 1 | [126] | |
| 256.94 c | 84.28 c | 164.80 c | 115.37 c | 342.44 c | 4.49 c | 0.83 c | 2.85 c | 0.20 c | 5.82 c | 24.37 c | 7.99 c | 15.49 c | 11.85 c | 42.06 c | n.a. | 0.48 c | 7 | [97] | |
| 107 h | 15 h | 91 h | 30 h | 292 h | 10 h | n.a. | 15 h | Tr. h | 49 h | n.a. | Tr. h | 56 h | n.a. | 220 h | n.a. | Tr. h | 1 | [122] | |
| Tinta Barroca (POR) | 0.21 k | 0.06 k | 0.41 k | 0.57 k | 4.93 k | n.a. | 0 k | 0 k | 0.07 k | 0.26 k | n.a. | n.a. | 0.11 k | 0.13 k | 1.94 k | n.a. | n.a. | 1 | [121] |
| Tinto Cão (POR) | 0.27 k | 0.01 k | 0.39 k | 0.13 k | 2.65 k | n.a. | 0 k | 0.01 k | 0.16 k | 0.37 k | n.a. | n.a. | 0.25 k | 0.05 k | 2.57 k | n.a. | n.a. | 1 | [121] |
| Touriga Francesca (POR) | 0.05 i | 0.01 i | 0.08 i | 0.02 i | 1.02 i | 0 i | 0.01 i | 0.06 i | 0.31 i | 0.11 i | 0 i | 0.01 i | 0.11 i | 0.08 i | 1.25 i | 0.01 i | 0.10 i | 1 | [127] |
| Touriga Nacional (POR) | 691.2 a | 0.73 a | 1761 a | 542 a | 2232 a | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 8 | [68] |
| 0.48 i | 0.07 i | 0.47 i | 0.52 i | 2.51 i | 0.05 i | 0.01 i | 0.07 i | 0.09 i | 0.52 i | 0.01 i | 0 i | 0.13 i | 0.22 i | 0.85 i | 0.01 i | 0.12 i | 1 | [127] | |
| Verdelho tinto (POR) | 318 b | 28 b | 320 b | 145 b | 1486 b | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 56 b | 32 b | 260 b | n.a. | n.a. | 7 | [90] |
| Vinhao (POR) | 3653 b | 409 b | 2054 b | 623 b | 4736 b | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 106 b | 29 b | 233 b | n.a. | n.a. | 7 | [90] |
| 745 g | 243 g | 735 g | 706 g | 3579 g | 45 g | 0 g | 44 g | 103 g | 172 g | 0 g | 0 g | 102 g | 114 g | 754 g | 0 g | 0 g | 3 | [109] | |
| Vranac (MNE) | 3.28 d | 0.98 d | 3.60 d | 2.41 d | 7.09 d | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 1 | [98] |
| Cultivar (Country of Origin *) | Myricetin | Quercetin | Kaempferol | Isorhamnetin | Syringentin | Laricitrin | Analysis Method ** | Ref. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glc 1 | Glr 1 | Gal 3 | Glc | Glr | Rut 4 | Gal | Glc | Glr | Gal | Glc | Glr | Glc | Glc | |||
| Grapes–Red Cultivars | ||||||||||||||||
| Alphonse Lavallee (FRA) | n.a. *** | n.a. | n.a. | 4.1 d | n.a. | 0 d | n.a. | 4.3 d | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 1 | [77] |
| Aglianico (ITA) | n.a. | n.a. | n.a. | 6.7 d | n.a. | 6.1 d | n.a. | 20.9 d | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 1 | [77] |
| Azal Tinto (POR) | 21 d | n.a. | n.a. | 27 d | n.a. | n.a. | n.a. | 2.9 d | n.a. | n.a. | 26 d | n.a. | 21 d | 15 d | 2 | [90] |
| Babić (CRO) | n.a. | n.a. | n.a. | 1.18 c | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 2 | [108] |
| Borracal (POR) | 32 d | n.a. | n.a. | 40 d | n.a. | n.a. | n.a. | 4.4 d | n.a. | n.a. | 22 d | n.a. | 26 d | 11 d | 2 | [90] |
| Brancelho (POR) | 8.4 d | n.a. | n.a. | 30 d | n.a. | n.a. | n.a. | 5.1 d | n.a. | n.a. | 21 d | n.a. | 15 d | 8.0 d | 2 | [90] |
| Cabernet Sauvignon (FRA) | 279.87 d | n.a. | n.a. | 754.37 d | n.a. | 35.36 d | n.a. | n.a. | n.a. | n.a. | 73.91 d | n.a. | n.a. | n.a. | 2 | [79] |
| 198.92 d | 3.96 d | n.a. | n.a. | 46.79 d | n.a. | n.a. | 0.52 d | n.a. | n.a. | 34.46 d | n.a. | 29.80 d | 5.56 d | 3 | [91] | |
| 95.4 d | n.a. | n.a. | 76.7 d | 61.3 d | n.a. | 16.7 d | 71.4 d | n.a. | 10.5 d | 91.7 d | 0 d | 136.3 d | 22.9 d | 4 | [109] | |
| 22 c | 10 c | n.a. | 48 c | 59 c | n.a. | n.a. | 13 c | n.a. | n.a. | 28 c | n.a. | n.a. | n.a. | 2 | [134] | |
| 248.86 d | n.a. | n.a. | 297.33 d | 159.52 d | n.a. | 59.68 d | 197.36 d | n.a. | 39.51 d | 261.25 d | 0 d | 140.88 d | 115.61 d | 5 | [114] | |
| 168.08 d | n.a. | 417.60 d | 23.55 d | 120.88 d | 150.90 d | 46.50 d | 0 d | n.a. | 148.10 d | n.a. | n.a. | 109.38 d | 30.15 d | 4 | [123] | |
| Cesanese (ITA) | n.a. | n.a. | n.a. | 2.87 d | n.a. | 54.7 d | n.a. | 40.7 d | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 1 | [77] |
| Docal (POR) | 41 d | n.a. | n.a. | 25 d | n.a. | n.a. | n.a. | 3.4 d | n.a. | n.a. | 15 d | n.a. | 27 d | 20 d | 6 | [90] |
| Espadeiro (POR) | 33 d | n.a. | n.a. | 93 d | n.a. | n.a. | n.a. | 23 d | n.a. | n.a. | 48 d | n.a. | 38 d | 45 d | 6 | [90] |
| Gamay (FRA) | 0 d | n.a. | n.a. | 10.3 d | 30.7 d | n.a. | 0 d | 0 d | n.a. | 12.4 d | 46.7 d | 0 d | 46.1 d | 9.5 d | 4 | [109] |
| Gewürtztramminer (NN) | 0 c | 0 c | n.a. | 24 c | 17 c | n.a. | n.a. | 6.7 c | n.a. | n.a. | 2.3 c | n.a. | n.a. | n.a. | 2 | [134] |
| Malvasia Nera (ITA) | n.a. | n.a. | n.a. | 9.0 d | n.a. | 0 d | n.a. | 31.2 d | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 1 | [77] |
| Merlot (FRA) | n.a. | n.a. | n.a. | 1.65 c | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 2 | [108] |
| 75.92 d | 1.57 d | n.a. | n.a. | 35.18 d | n.a. | n.a. | 0.20 d | n.a. | n.a. | 11.41 d | n.a. | 7.16 d | 2.21 d | 3 | [91] | |
| n.a. | n.a. | n.a. | 45.0 d | n.a. | 30.2 d | n.a. | 97.7 d | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 1 | [77] | |
| 150 d | n.a. | n.a. | 67.4 d | 58.9 d | n.a. | 0 d | 65.9 d | n.a. | 0 d | 134.8 d | 0 d | 82.2 d | 28.3 d | 4 | [109] | |
| 142.22 d | n.a. | 286.82 d | 150.00 d | 34.80 d | 144.54 d | 39.52 d | 0 d | n.a. | 32.47 d | n.a. | n.a. | 103.46 d | 29.24 d | 4 | [123] | |
| 13 c | 5.8 c | n.a. | 31 c | 43 c | n.a. | n.a. | 8 c | n.a. | n.a. | 17 c | n.a. | n.a. | n.a. | 2 | [134] | |
| Negroamaro (ITA) | n.a. | n.a. | n.a. | 1.03 d | n.a. | 1.39 d | n.a. | 32.0 d | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 1 | [77] |
| Padeiro de Basto (POR) | 28 d | n.a. | n.a. | 15 d | n.a. | n.a. | n.a. | 23 d | n.a. | n.a. | 17 d | n.a. | 61 d | 22 d | 6 | [90] |
| Pedral (POR) | 11 d | n.a. | n.a. | 13 d | n.a. | n.a. | n.a. | 3.3 d | n.a. | n.a. | 4.7 d | n.a. | 15 d | 6.2 d | 6 | [90] |
| Pinot Noir (FRA) | 20.3 i | n.a. | n.a. | 67.2 i | n.a. | 30.5 i | 33.8 i | 25.8 i | n.a. | n.a. | 13.0 i | n.a. | 8.6 i | n.a. | 1 | [95] |
| 1066 b | 391 b | n.a. | 105 b | 2726 b | 272 b | 240 b | 177 b | n.a. | n.a. | 319 b | n.a. | 171 b | n.a. | 7 | [94] | |
| 88.1 d | n.a. | n.a. | 28.5 d | 31.7 d | n.a. | 0 d | 0 d | n.a. | 0 d | 0 d | 0 d | 35.5 d | 16.9 d | 4 | [109] | |
| Plavina (CRO) | n.a. | n.a. | n.a. | 0.79 c | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 2 | [108] |
| Primitivo (CRO) | 38.1 d | n.a. | n.a. | 11.5 d | 53.5 d | n.a. | 0 d | 27.7 d | n.a. | 0 d | 0 d | 0 d | 166.3 d | 0 d | 2 | [108] |
| n.a. | n.a. | n.a. | 6.3 d | n.a. | 6.2 d | n.a. | 33.9 d | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 1 | [77] | |
| Susumaniello (ITA) | n.a. | n.a. | n.a. | 7.6 d | n.a. | 10.6 d | n.a. | 22.0 d | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 1 | [77] |
| Syrah (FRA) | 163.70 f | n.a. | 15.04 f | 675.34 f | n.a. | n.a. | 93.8 f | 116.05 f | 4.83 f | 293.67 f | 181.44 f | n.a. | n.a. | n.a. | 2 | [80] |
| 209.61 d | 1.06 d | n.a. | n.a. | 10.95 d | n.a. | n.a. | 0.14 d | n.a. | n.a. | 50.24 d | n.a. | 25.38 d | 4.32 d | 3 | [91] | |
| 181.8 d | n.a. | n.a. | 358.6 d | 155.7 d | n.a. | 56.2 d | 118.3 d | n.a. | 34.3 d | 265.6 d | 60.8 d | 107.9 d | 35 d | 4 | [109] | |
| 21 c | 7.3 c | n.a. | 55 c | 35 c | n.a. | n.a. | 18 c | n.a. | n.a. | 48 c | n.a. | n.a. | n.a. | 2 | [134] | |
| 260.90 d | n.a. | n.a. | 1411.45 d | 210.96 d | n.a. | 213.86 d | 378.97 d | n.a. | 91.68 d | 937.34 d | 255.79 d | 305.59 d | 175.53 d | 5 | [66] | |
| Tempranillo (SPA) | 1.59 e | 18.76 e | 0.72 e | 6.20 e | 4.60 e | 0.39 e | 0.57 e | 0.77 e | 0.16 e | 0.18 e | 0.40 e | 0.11 e | 0.71 e | 2.30 e | 4 | [97] |
| Trnjak (CRO) | n.a. | n.a. | n.a. | 1.16 c | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 2 | [108] |
| Uva di Troia (ITA) | n.a. | n.a. | n.a. | 15.0 d | n.a. | 41.4 d | n.a. | 45.8 d | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 1 | [77] |
| Vinhao (POR) | 163 d | n.a. | n.a. | 13 d | n.a. | n.a. | n.a. | 1.6 d | n.a. | n.a. | 7.6 d | n.a. | 26 d | 21 d | 6 | [90] |
| Verdelho tinto (POR) | 56 d | n.a. | n.a. | 58 d | n.a. | n.a. | n.a. | 11 d | n.a. | n.a. | 13 d | n.a. | 31 d | 22 d | 6 | [90] |
| Vranac (MNE) | 0.11 h | n.a. | n.a. | 0.32 h | n.a. | 0.44 h | n.a. | 0.09 h | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 2 | [98] |
| n.a. | n.a. | n.a. | 1.73 c | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 2 | [108] | |
| Grapes–White Cultivars | ||||||||||||||||
| Albarino (POR) | n.a. | n.a. | n.a. | 12.43 j | 0.98 j | 0.42 j | n.a. | 8.43 j | 3.21 j | n.a. | n.a. | n.a. | n.a. | n.a. | 8 | [87] |
| Chardonnay (FRA) | 0 c | 0 c | n.a. | 17 c | 25 c | n.a. | n.a. | 8.4 c | n.a. | n.a. | 0.48 c | n.a. | n.a. | n.a. | 2 | [134] |
| 0 d | n.a. | n.a. | 587.49 d | 174.60 d | n.a. | 124.55 d | 277.05 d | n.a. | 0 d | 0 d | 0 d | 0 d | 60.25 d | 5 | [66] | |
| 0 d | n.a. | 0 d | 161.66 d | 67.34 d | 78.85 d | 6.64 d | 15.20 d | n.a. | 44.90 d | n.a. | n.a. | 0 d | 0 d | 4 | [123] | |
| Debit (CRO) | n.a. | n.a. | n.a. | 0.49 c | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 2 | [108] |
| Istrian Malvasia (CRO) | n.a. | n.a. | n.a. | 178.6 g | 12.3 g | 6.7 g | 52.3 g | 62.2 g | 1.1 g | 12.7 g | n.a. | n.a. | n.a. | n.a. | 9 | [99] |
| Italian Riesling (NN) | 0 d | n.a. | n.a. | 74.29 d | 128.63 d | n.a. | 13.75 d | 36.46 d | n.a. | 0 d | 0 d | 0 d | 0 d | 0 d | 5 | [66] |
| 0 d | n.a. | 0 d | 35.29 d | 106.27 d | 164.56 d | 72.56 d | 5.96 d | n.a. | 38.89 d | n.a. | n.a. | 0 d | 0 d | 4 | [123] | |
| Kujundžuša (CRO) | n.a. | n.a. | n.a. | 2.55 c | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 2 | [108] |
| Maraština (CRO) | n.a. | n.a. | n.a. | 0.21 c | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 2 | [108] |
| Moscato (ITA) | n.a. | n.a. | n.a. | 136.5 d | n.a. | 25.2 d | n.a. | 101.0 d | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 1 | [77] |
| Rabo de Ovelha (POR) | 40 d | n.a. | n.a. | 26 d | n.a. | n.a. | n.a. | 31 d | n.a. | n.a. | 15 d | n.a. | 28 d | 19 d | 6 | [90] |
| Riesling (GER) | 0 c | 0 c | n.a. | 22 c | 30 c | n.a. | n.a. | 2.9 c | n.a. | n.a. | Tr. c | n.a. | n.a. | n.a. | 2 | [134] |
| Sauvignon Blanc (FRA) | 0 c | 0 c | n.a. | 8.9 c | 12 c | n.a. | n.a. | 2.0 c | n.a. | n.a. | 0.78 c | n.a. | n.a. | n.a. | 2 | [134] |
| Zlatarica (CRO) | n.a. | n.a. | n.a. | 0.58 c | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 2 | [108] |
| Cultivar | Catechin | Epicatechin | Galocatechin | Epigallocatechin | Epicatechin Gallate | Epigallocatechin Gallate | Procyanidins | Analysis Method | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B1 | B2 | B3 | B4 | |||||||||
| Grapes–Red Cultivars | ||||||||||||
| Aglianico (ITA) | 3214.9 a | 1890.1 a | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 1 | [77] |
| Alphonse Lavallee (FRA) | 331.20 a | 32.4 a | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 1 | [99] |
| Babić (CRO) | 2.6 c | 0 c | n.a. | n.a. | 0.45 c | n.a. | 1.53 c | 1.19 c | n.a. | n.a. | 2 | [108] |
| Cabernet Franc (FRA) | 0 n | 0 n | 3.81 n | 2.19 n | n.a. | 0 n | n.a. | n.a. | n.a. | n.a. | 3 | [84] |
| Cabernet Sauvignon (FRA) | 102.4 b | 28.79 b | n.a. | 82.71 b | n.a. | n.a. | 39.17 b | 23.83 b | n.a. | n.a. | 2 | [79] |
| 5.9 n | 3.01 n | 4.13 n | 1.95 n | n.a. | 0 n | n.a. | n.a. | n.a. | n.a. | 3 | [84] | |
| 73.5 r | 792.4 r | n.a. * | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 4 | [78] | |
| 28.37 a | 57.45 a | n.a. | n.a. | 6.81 a | n.a. | n.a. | n.a. | n.a. | n.a. | 5 | [91] | |
| 23.5 a | 0 a | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 6 | [109] | |
| 4.4 c | 0.4 c | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 2 | [122] | |
| 17 k | 6.2 k | n.a. | n.a. | n.a. | n.a. | 12 k | 0.99 k | 27 k | n.a. | 2 | [134] | |
| 0.31 h | 0.1 h | n.a. | n.a. | 0.08 h | n.a. | 0.11 | 0.84 h | 0.25 h | 0 h | 2 | [138] | |
| 118.42 f | 32.38 f | n.a. | n.a. | n.a. | 1.96 f | n.a. | n.a. | n.a. | n.a. | 12 | [140] | |
| 2.6 l | 0.9 l | 1.8 l | 1.5 l | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 7 | [141] | |
| 0.206 m | 0.171 m | n.a. | n.a. | 0 m | n.a. | 0 m | 0 m | 0 m | 0 m | 8 | [142] | |
| 0.212 h | 0.119 h | n.a. | n.a. | 0 h | n.a. | 0.005 h | 0 h | 0.04 h | 0 h | 9 | [143] | |
| Carmenere (FRA) | 3.5 l | 1.6 l | 2.8 l | 2.5 l | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 7 | [141] |
| Cesanese (ITA) | 178.8 a | 8.9 a | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 1 | [77] |
| Gamay (FRA) | 19.2 a | 0 a | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 6 | [109] |
| Gewurztraminer (NN) | 19 k | 8.3 k | n.a. | n.a. | n.a. | n.a. | 21 k | 48 k | n.a. | n.a. | 2 | [134] |
| Graciano (SPA) | 0.104 m | 0.235 m | n.a. | n.a. | 0 m | n.a. | 0 m | 0 m | 0 m | 0 m | 8 | [142] |
| Jean Tinto (FRA) | 3.2 c | 0.6 c | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 2 | [122] |
| Malvasia Nera (ITA) | 966.0 a | 734.7 a | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 1 | [77] |
| Merlot (FRA) | 7.47 n | 0 n | 4.62 n | 2.24 n | n.a. | 0 n | n.a. | n.a. | n.a. | n.a. | 3 | [84] |
| 16 o | 13 o | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 2 | [85] | |
| 74.2 r | 389.2 r | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 4 | [78] | |
| 601.0 a | 980.7 a | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 1 | [77] | |
| 20.85 a | 68.09 a | n.a. | n.a. | 75.3 a | n.a. | n.a. | n.a. | n.a. | n.a. | 5 | [91] | |
| 3.12 c | 0 c | n.a. | n.a. | 6.54 c | n.a. | 2.74 c | 0 c | n.a. | n.a. | 2 | [108] | |
| 13.1 a | 37.4 a | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 6 | [109] | |
| 25 k | 13 k | n.a. | n.a. | n.a. | n.a. | 21 k | 2.2 k | 35 k | n.a. | 2 | [134] | |
| 0.57 h | 0.16 h | n.a. | n.a. | 0.23 h | n.a. | 0.08 | 0.92 h | 0.02 h | 0 h | 2 | [138] | |
| 5.5 l | 1.9 l | 2.1 l | 2.2 l | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 7 | [141] | |
| 0.61 h | 0.072 h | n.a. | n.a. | 0 h | n.a. | 0.015 h | 0 h | 0.019 h | 0 h | 9 | [143] | |
| Negroamaro (ITA) | 118.2 a | 27.8 a | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 1 | [77] |
| Palomino Negro (SPA) | 2.7 c | 0.1 c | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 2 | [122] |
| Pedro Ximenez (SPA) | 22.4 f | 8.95 f | n.a. | n.a. | n.a. | 1.13 f | n.a. | n.a. | n.a. | n.a. | 12 | [140] |
| Pinot Noir (FRA) | 0 n | 0 n | 2.91 n | 2.42 n | n.a. | 0 n | n.a. | n.a. | n.a. | n.a. | 3 | [84] |
| 355 d | 7.2 d | n.a. | n.a. | n.a. | n.a. | 633 d | n.a. | n.a. | n.a. | 10 | [94] | |
| 0 a | 0 a | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 6 | [109] | |
| 2.4 l | 0.7 l | n.a. * | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 7 | [141] | |
| Pinot Gris (FRA) | 0 n | 0 n | 3.09 n | 2.09 n | n.a. | 2.07 n | n.a. | n.a. | n.a. | n.a. | 3 | [78] |
| Plavina (CRO) | 2.48 c | 0 c | n.a. | n.a. | 0.7 c | n.a. | 1.81 c | 0 c | n.a. | n.a. | 2 | [108] |
| Primitivo (CRO) | 307.1 a | 49.8 a | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 1 | [77] |
| 0 a | 0 a | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 6 | [109] | |
| Sangiovese (ITA) | 3.93 n | 0 n | 3.08 n | 2.03 n | n.a. | 0 n | n.a. | n.a. | n.a. | n.a. | 3 | [84] |
| Syrah (FRA) | 5.42 n | 3.02 n | 0 n | 1.9 n | n.a. | 0 n | n.a. | n.a. | n.a. | n.a. | 3 | [84] |
| 28.7 a | 74.8 a | n.a. | n.a. | 25.39 a | n.a. | n.a. | n.a. | n.a. | n.a. | 5 | [91] | |
| 34.5 a | 0 a | n.a. * | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 6 | [109] | |
| 8.5 k | 6.9 k | n.a. | n.a. | n.a. | n.a. | 8.4 k | 0.75 k | 16 k | n.a. | 2 | [134] | |
| 42.61 f | 28.64 f | n.a. | n.a. | n.a. | 1.12 f | n.a. | n.a. | n.a. | n.a. | 12 | [140] | |
| 6.4 l | 2 l | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 7 | [141] | |
| Susumaniello (ITA) | 147.1 a | 73.5 a | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 1 | [77] |
| Tempranillo (SPA) | 165 b | 35 b | n.a. | 1.2 b | 9.5 b | n.a. | 14 b | 18 b | n.a. | n.a. | 12 | [97] |
| 6.1 c | 1.5 c | n.a. * | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 2 | [122] | |
| 22.87 f | 12.05 f | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 12 | [140] | |
| 0.61 m | 0.079 m | n.a. | n.a. | 0 m | n.a. | 0 m | 0 m | 0 m | 0 m | 8 | [142] | |
| Tintilla de Rota (ITA) | 1.5 c | 0.3 c | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 2 | [122] |
| Touriga Nacional (POR) | 1.1 i | tr i | n.a. | n.a. | n.a. | 0 i | 14.2 i | 0 i | 0 i | 0 i | 2 | [144] |
| Touriga Francesca (POR) | 0.5 i | 0.5 i | n.a. | n.a. | n.a. | 0 i | 5.9 i | 0.7 i | n.a. | n.a. | 2 | [144] |
| Trnjak (CRO) | 2.05 c | 0 c | n.a. | n.a. | 1.47 c | n.a. | 18.13 c | 2.54 c | n.a. | n.a. | 2 | [108] |
| Uva di Troia (ITA) | 127.6 a | 62.5 a | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 1 | [77] |
| Vranac (MNE) | 0 h | 0.018 h | n.a. | n.a. | n.a. | 0 h | n.a. | 0.027 h | n.a. | n.a. | 2 | [98] |
| 2.05 c | 0 c | n.a. | n.a. | 2.83 c | n.a. | 2.51 c | 2.74 c | n.a. | n.a. | 2 | [108] | |
| Grapes–White Cultivars | ||||||||||||
| Albarino (POR) | 11.45 s | 0.23 s | n.a. | 2.09 s | n.a. | 0 s | 0 s | 8.65 s | 0 s | 8.04 s | 11 | [87] |
| Chardonnay (FRA) | 0 n | 2.95 n | 0 n | 2.25 n | n.a. | 2.51 n | n.a. | n.a. | n.a. | n.a. | 3 | [84] |
| 63 o | 44 o | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 2 | [85] | |
| 147.9 r | 87.5 r | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 4 | [78] | |
| 23 k | 5.8 k | n.a. | n.a. | n.a. | n.a. | 37 k | 23 k | n.a. | n.a. | 2 | [134] | |
| Debit (CRO) | 2.1 c | 2.22 c | n.a. | n.a. | 0.63 c | n.a. | 10.89 c | 4.27 c | n.a. | n.a. | 2 | [108] |
| Istrian Malvasia (CRO) | 8.5 g | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 7 | [99] |
| Italian Riesling (NN) | 6.6 n | 0 n | 0 n | 1.98 n | n.a. | 2.1 n | n.a. | n.a. | n.a. | n.a. | 3 | [84] |
| Kujundžuša (CRO) | 2.93 c | 2.08 c | n.a. | n.a. | 1.66 c | n.a. | 6.75 c | 1.95 c | n.a. | n.a. | 2 | [108] |
| Maraština (CRO) | 3.36 c | 1.42 c | n.a. | n.a. | 0.64 c | n.a. | 6.36 c | 2.8 c | n.a. | n.a. | 2 | [108] |
| Moscatel (ITA) | 16 k | 2.6 k | n.a. | n.a. | n.a. | n.a. | 23 k | 21 k | n.a. | n.a. | 2 | [134] |
| 45.33 f | 9.39 f | n.a. | n.a. | n.a. | 1.86 f | n.a. | n.a. | n.a. | n.a. | 12 | [140] | |
| Palomino Fino (SPA) | 5.99 f | 1.4 f | n.a. | n.a. | n.a. | 0.6 f | n.a. | n.a. | n.a. | n.a. | 12 | [140] |
| Petra (SRB) | 3.27 n | 3.56 n | 2.65 n | 0 n | n.a. | 2.15 n | n.a. | n.a. | n.a. | n.a. | 3 | [84] |
| Prokupac (SRB) | 0 n | 0 n | 0 n | 1.98 n | n.a. | 0 n | n.a. | n.a. | n.a. | n.a. | 3 | [84] |
| Riesling (GER) | 0 n | 0 n | 0 n | 2.01 n | n.a. | 2.13 n | n.a. | n.a. | n.a. | n.a. | 3 | [84] |
| 14 k | tr k | n.a. | n.a. | n.a. | n.a. | 29 k | 12 k | n.a. | n.a. | 2 | [134] | |
| Sauvignon Blanc (FRA) | 0 n | 0 n | 0 n | 1.98 n | n.a. | 1.95 n | n.a. | n.a. | n.a. | n.a. | 3 | [84] |
| 102.3 r | 210.7 r | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 4 | [78] | |
| 9.5 k | 3.4 k | n.a. | n.a. | n.a. | n.a. | 25 k | 16 k | n.a. | n.a. | 2 | [134] | |
| Semillon (FRA) | 214 b | 313 b | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 2 | [100] |
| 35.2 h | 1.6 h | n.a. | n.a. | 0.05 h | n.a. | 0.4 h | 0 h | 0.2 h | 0 h | 2 | [138] | |
| Ugni Blanc (FRA) | 222 h | 3 h | n.a. | n.a. | 0.07 h | n.a. | 1.1 h | 0.2 h | 0.2 h | 0.04 h | 2 | [138] |
| Viognier (FRA) | 112.7 r | 105.1 r | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 4 | [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šikuten, I.; Štambuk, P.; Andabaka, Ž.; Tomaz, I.; Marković, Z.; Stupić, D.; Maletić, E.; Kontić, J.K.; Preiner, D. Grapevine as a Rich Source of Polyphenolic Compounds. Molecules 2020, 25, 5604. https://doi.org/10.3390/molecules25235604
Šikuten I, Štambuk P, Andabaka Ž, Tomaz I, Marković Z, Stupić D, Maletić E, Kontić JK, Preiner D. Grapevine as a Rich Source of Polyphenolic Compounds. Molecules. 2020; 25(23):5604. https://doi.org/10.3390/molecules25235604
Chicago/Turabian StyleŠikuten, Iva, Petra Štambuk, Željko Andabaka, Ivana Tomaz, Zvjezdana Marković, Domagoj Stupić, Edi Maletić, Jasminka Karoglan Kontić, and Darko Preiner. 2020. "Grapevine as a Rich Source of Polyphenolic Compounds" Molecules 25, no. 23: 5604. https://doi.org/10.3390/molecules25235604
APA StyleŠikuten, I., Štambuk, P., Andabaka, Ž., Tomaz, I., Marković, Z., Stupić, D., Maletić, E., Kontić, J. K., & Preiner, D. (2020). Grapevine as a Rich Source of Polyphenolic Compounds. Molecules, 25(23), 5604. https://doi.org/10.3390/molecules25235604








