Novel Potential Application of Chitosan Oligosaccharide for Attenuation of Renal Cyst Growth in the Treatment of Polycystic Kidney Disease
Abstract
1. Introduction
2. Results
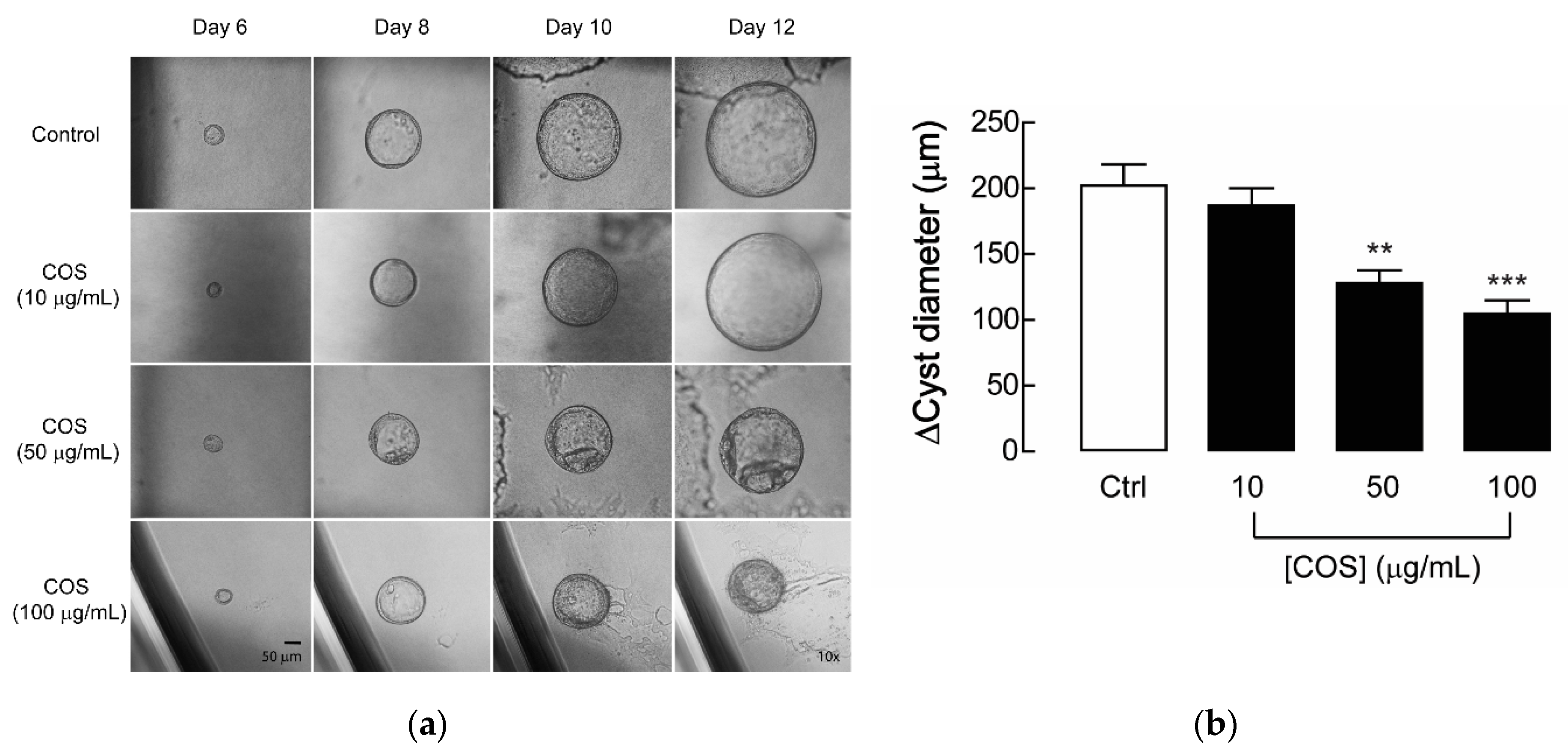
2.1. Effect of COS on Renal Cyst Progression
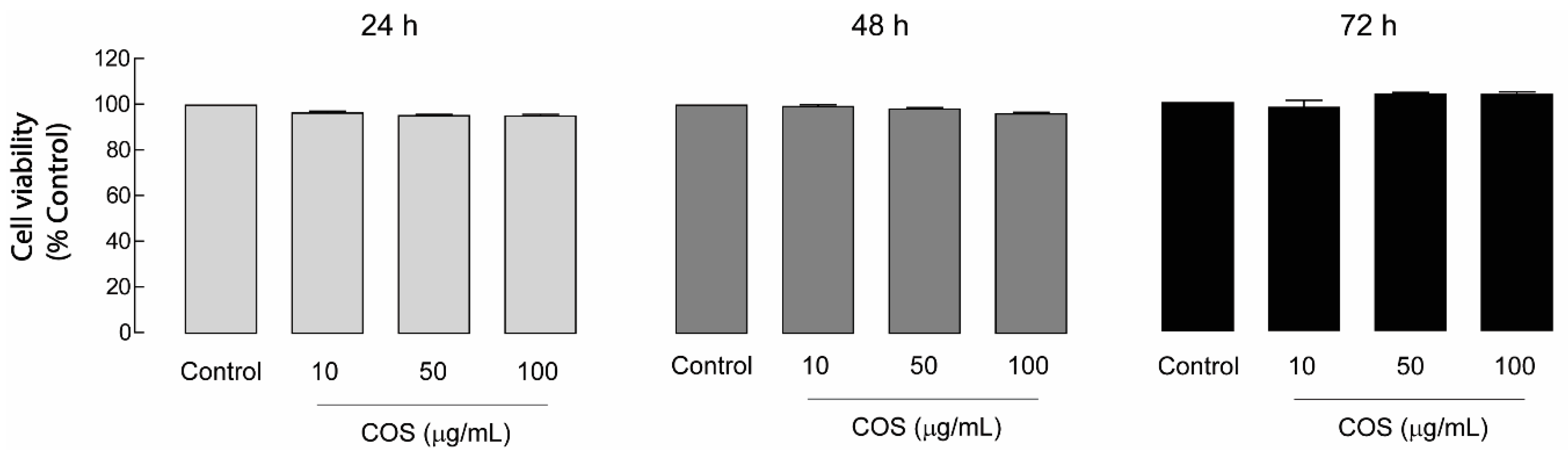
2.2. Non-Cytotoxic Effect of COS in MDCK Cells
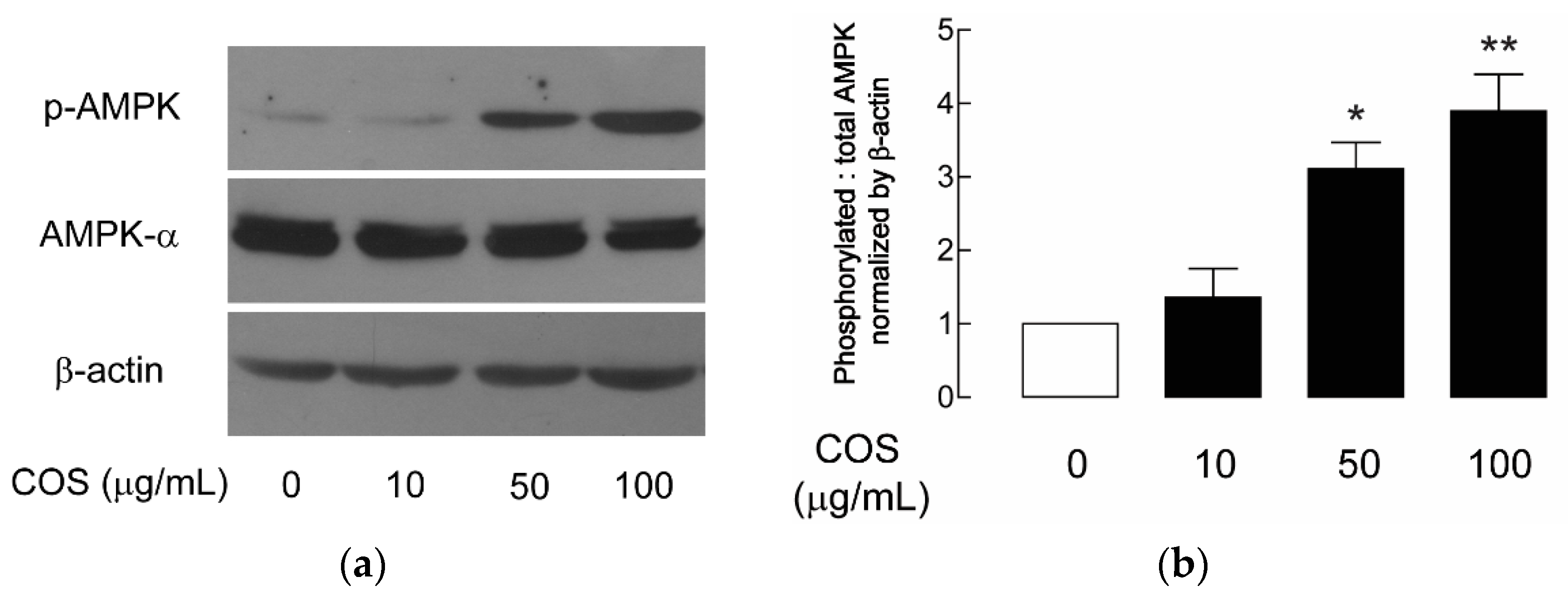
2.3. AMPK Activation by COS in MDCK Cells
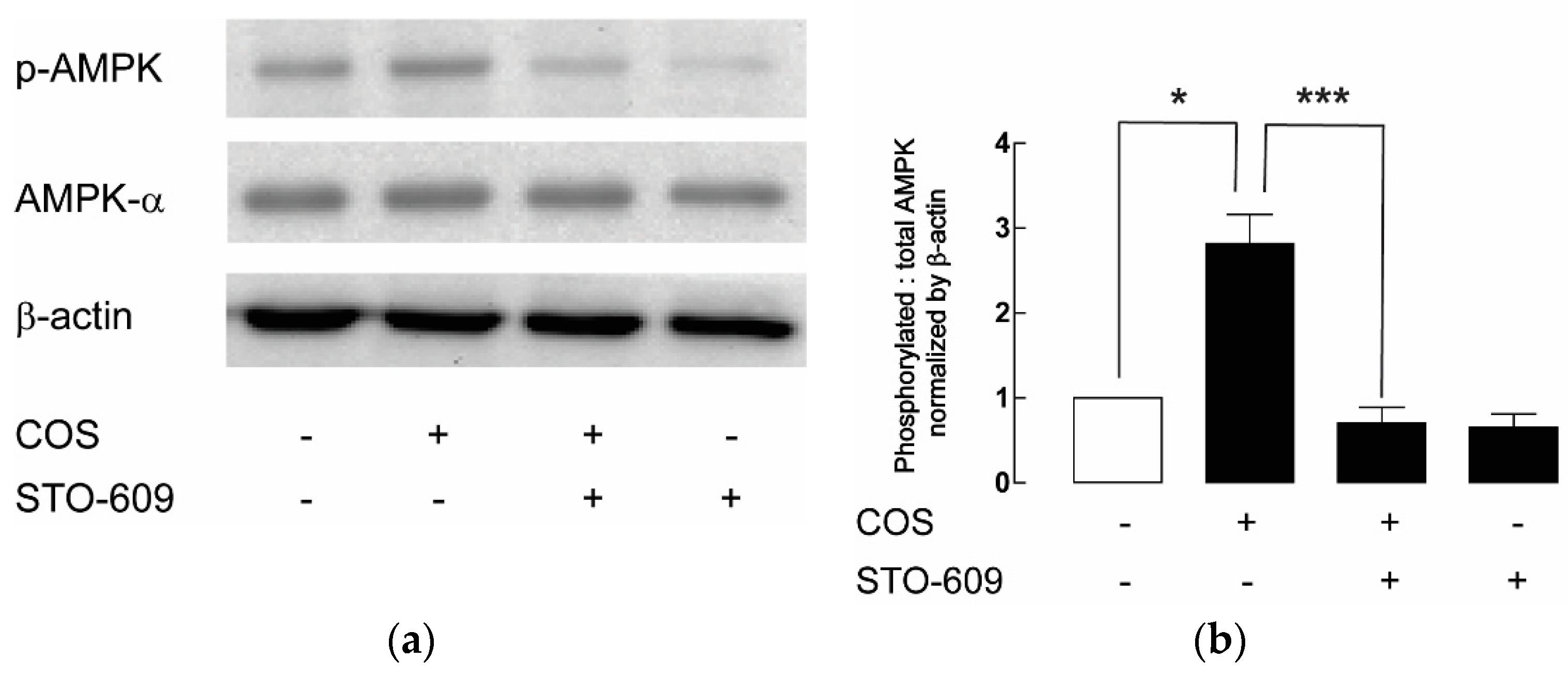
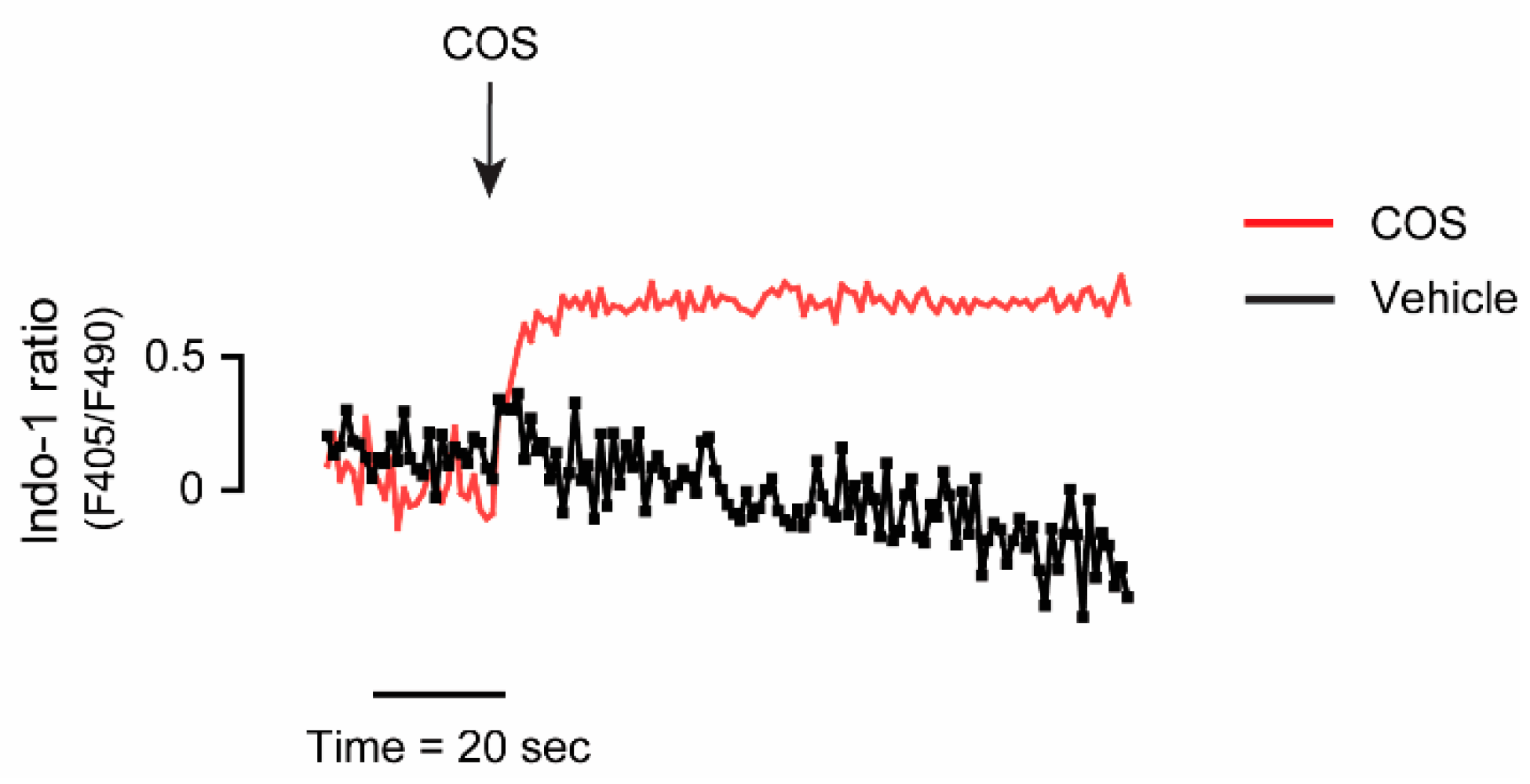
2.4. Upstream Target of COS-Induced AMPK Activation
3. Discussion
4. Materials and Methods
4.1. Chemical Reagents and Antibodies
4.2. Preparation of Chitosan Oligosaccharide
4.3. Cell Culture
4.4. MDCK Cyst Model
4.5. Cell Viability Assay
4.6. Immunoblotting
4.7. Intracellular Calcium Measurement
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Willey, C.J.; Blais, J.D.; Hall, A.K.; Krasa, H.B.; Makin, A.J.; Czerwiec, F.S. Prevalence of autosomal dominant polycystic kidney disease in the European Union. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2017, 32, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, C.; Guay-Woodford, L.M.; Harris, P.C.; Horie, S.; Peters, D.J.M.; Torres, V.E. Polycystic kidney disease. Nat. Rev. Dis. Primers 2018, 4, 50. [Google Scholar] [CrossRef] [PubMed]
- Lanktree, M.B.; Haghighi, A.; Guiard, E.; Iliuta, I.A.; Song, X.; Harris, P.C.; Paterson, A.D.; Pei, Y. Prevalence Estimates of Polycystic Kidney and Liver Disease by Population Sequencing. J. Am. Soc. Nephrol. JASN 2018, 29, 2593–2600. [Google Scholar] [CrossRef]
- Cornec-Le, G.E.; Torres, V.E.; Harris, P.C. Genetic Complexity of Autosomal Dominant Polycystic Kidney and Liver Diseases. J. Am. Soc. Nephrol. JASN 2018, 29, 13–23. [Google Scholar] [CrossRef]
- Mekahli, D.; Parys, J.B.; Bultynck, G.; Missiaen, L.; De Smedt, H. Polycystins and cellular Ca2+ signaling. Cell. Mol. Life Sci. CMLS 2013, 70, 2697–2712. [Google Scholar] [CrossRef]
- Douguet, D.; Patel, A.; Honoré, E. Structure and function of polycystins: Insights into polycystic kidney disease. Nat. Rev. Nephrol. 2019, 15, 412–422. [Google Scholar] [CrossRef]
- Mochizuki, T.; Makabe, S.; Aoyama, Y.; Kataoka, H.; Nitta, K. New Insights into Cystic Kidney Diseases. Contrib. Nephrol. 2018, 195, 31–41. [Google Scholar] [CrossRef]
- Mochizuki, T.; Tsuchiya, K.; Nitta, K. Autosomal dominant polycystic kidney disease: Recent advances in pathogenesis and potential therapies. Clin. Exp. Nephrol. 2013, 17, 317–326. [Google Scholar] [CrossRef]
- Kalluri, H.V.; Hardinger, K.L. Current state of renal transplant immunosuppression: Present and future. World J. Transplant. 2012, 2, 51–68. [Google Scholar] [CrossRef]
- Wallace, D.P. Cyclic AMP-mediated cyst expansion. Biochim. Biophys. Acta 2011, 1812, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.E.; Harris, P.C. Strategies targeting cAMP signaling in the treatment of polycystic kidney disease. J. Am. Soc. Nephrol. JASN 2014, 25, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Chapin, H.C.; Caplan, M.J. The cell biology of polycystic kidney disease. J. Cell Biol. 2010, 191, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Ibraghimov-Beskrovnaya, O.; Natoli, T.A. mTOR signaling in polycystic kidney disease. Trends Mol. Med. 2011, 17, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Antignac, C.; Calvet, J.P.; Germino, G.G.; Grantham, J.J.; Guay-Woodford, L.M.; Harris, P.C.; Hildebrandt, F.; Peters, D.J.; Somlo, S.; Torres, V.E.; et al. The Future of Polycystic Kidney Disease Research—As Seen By the 12 Kaplan Awardees. J. Am. Soc. Nephrol. JASN 2015, 26, 2081–2095. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Carling, D. AMP-activated protein kinase: The current landscape for drug development. Nat. Rev. Drug Discov. 2019, 18, 527–551. [Google Scholar] [CrossRef]
- Glosse, P.; Föller, M. AMP-Activated Protein Kinase (AMPK)-Dependent Regulation of Renal Transport. Int. J. Mol. Sci. 2018, 19, 3481. [Google Scholar] [CrossRef]
- Rajani, R.; Pastor-Soler, N.M.; Hallows, K.R. Role of AMP-activated protein kinase in kidney tubular transport, metabolism, and disease. Curr. Opin. Nephrol. Hypertens. 2017, 26, 375–383. [Google Scholar] [CrossRef]
- Rowe, I.; Chiaravalli, M.; Mannella, V.; Ulisse, V.; Quilici, G.; Pema, M.; Song, X.W.; Xu, H.; Mari, S.; Qian, F.; et al. Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat. Med. 2013, 19, 488–493. [Google Scholar] [CrossRef]
- Takiar, V.; Nishio, S.; Seo-Mayer, P.; King, J.D., Jr.; Li, H.; Zhang, L.; Karihaloo, A.; Hallows, K.R.; Somlo, S.; Caplan, M.J. Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 2462–2467. [Google Scholar] [CrossRef] [PubMed]
- Muanprasat, C.; Chatsudthipong, V. Chitosan oligosaccharide: Biological activities and potential therapeutic applications. Pharmacol. Ther. 2017, 170, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Thadathil, N.; Velappan, S.P. Recent developments in chitosanase research and its biotechnological applications: A review. Food Chem. 2014, 150, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Phil, L.; Sohail, M.; Hasnat, M.; Baig, M.; Ihsan, A.U.; Shumzaid, M.; Kakar, M.U.; Mehmood Khan, T.; Akabar, M.D.; et al. Chitosan oligosaccharide (COS): An overview. Int. J. Biol. Macromol. 2019, 129, 827–843. [Google Scholar] [CrossRef] [PubMed]
- Phil, L.; Naveed, M.; Mohammad, I.S.; Bo, L.; Bin, D. Chitooligosaccharide: An evaluation of physicochemical and biological properties with the proposition for determination of thermal degradation products. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 102, 438–451. [Google Scholar] [CrossRef]
- Sanna, V.; Pala, N.; Sechi, M. Targeted therapy using nanotechnology: Focus on cancer. Int. J. Nanomed. 2014, 9, 467–483. [Google Scholar] [CrossRef]
- Aied, A.; Greiser, U.; Pandit, A.; Wang, W. Polymer gene delivery: Overcoming the obstacles. Drug Discov. Today 2013, 18, 1090–1098. [Google Scholar] [CrossRef]
- Dong, W.; Han, B.; Feng, Y.; Song, F.; Chang, J.; Jiang, H.; Tang, Y.; Liu, W. Pharmacokinetics and biodegradation mechanisms of a versatile carboxymethyl derivative of chitosan in rats: In vivo and in vitro evaluation. Biomacromolecules 2010, 11, 1527–1533. [Google Scholar] [CrossRef]
- Dong, W.; Han, B.; Shao, K.; Yang, Z.; Peng, Y.; Yang, Y.; Liu, W. Effects of molecular weights on the absorption, distribution and urinary excretion of intraperitoneally administrated carboxymethyl chitosan in rats. J. Mater. Sci. Mater. Med. 2012, 23, 2945–2952. [Google Scholar] [CrossRef]
- Yoon, S.P.; Han, M.S.; Kim, J.W.; Chang, I.Y.; Kim, H.L.; Chung, J.H.; Shin, B.C. Protective effects of chitosan oligosaccharide on paraquat-induced nephrotoxicity in rats. Food Chem. Toxicol. 2011, 49, 1828–1833. [Google Scholar] [CrossRef]
- Jing, S.B.; Li, L.; Ji, D.; Takiguchi, Y.; Yamaguchi, T. Effect of chitosan on renal function in patients with chronic renal failure. J. Pharm. Pharmacol. 1997, 49, 721–723. [Google Scholar] [CrossRef] [PubMed]
- Muanprasat, C.; Wongkrasant, P.; Satitsri, S.; Moonwiriyakit, A.; Pongkorpsakol, P.; Mattaveewong, T.; Pichyangkura, R.; Chatsudthipong, V. Activation of AMPK by chitosan oligosaccharide in intestinal epithelial cells: Mechanism of action and potential applications in intestinal disorders. Biochem. Pharmacol. 2015, 96, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Pathomthongtaweechai, N.; Soodvilai, S.; Chatsudthipong, V.; Muanprasat, C. Pranlukast inhibits renal epithelial cyst progression via activation of AMP-activated protein kinase. Eur. J. Pharmacol. 2014, 724, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Yuajit, C.; Homvisasevongsa, S.; Chatsudthipong, L.; Soodvilai, S.; Muanprasat, C.; Chatsudthipong, V. Steviol reduces MDCK Cyst formation and growth by inhibiting CFTR channel activity and promoting proteasome-mediated CFTR degradation. PLoS ONE 2013, 8, e58871. [Google Scholar] [CrossRef]
- Yuajit, C.; Muanprasat, C.; Homvisasevongsa, S.; Chatsudthipong, V. Steviol stabilizes polycystin 1 expression and promotes lysosomal degradation of CFTR and β-catenin proteins in renal epithelial cells. Biomed. Pharmacother. 2017, 94, 820–826. [Google Scholar] [CrossRef]
- Gao, J.; Zhou, H.; Lei, T.; Zhou, L.; Li, W.; Li, X.; Yang, B. Curcumin inhibits renal cyst formation and enlargement in vitro by regulating intracellular signaling pathways. Eur. J. Pharmacol. 2011, 654, 92–99. [Google Scholar] [CrossRef]
- Yang, B.; Sonawane, N.D.; Zhao, D.; Somlo, S.; Verkman, A.S. Small-molecule CFTR inhibitors slow cyst growth in polycystic kidney disease. J. Am. Soc. Nephrol. JASN 2008, 19, 1300–1310. [Google Scholar] [CrossRef]
- Dasgupta, B.; Seibel, W. Compound C/Dorsomorphin: Its Use and Misuse as an AMPK Inhibitor. Methods Mol. Biol. 2018, 1732, 195–202. [Google Scholar] [CrossRef]
- Kunanusornchai, W.; Witoonpanich, B.; Tawonsawatruk, T.; Pichyangkura, R.; Chatsudthipong, V.; Muanprasat, C. Chitosan oligosaccharide suppresses synovial inflammation via AMPK activation: An in vitro and in vivo study. Pharmacol. Res. 2016, 113, 458–467. [Google Scholar] [CrossRef]
- Yousef, M.; Pichyangkura, R.; Soodvilai, S.; Chatsudthipong, V.; Muanprasat, C. Chitosan oligosaccharide as potential therapy of inflammatory bowel disease: Therapeutic efficacy and possible mechanisms of action. Pharmacol. Res. 2012, 66, 66–79. [Google Scholar] [CrossRef]
- Gattone, V.H., 2nd; Chen, N.X.; Sinders, R.M.; Seifert, M.F.; Duan, D.; Martin, D.; Henley, C.; Moe, S.M. Calcimimetic inhibits late-stage cyst growth in ADPKD. J. Am. Soc. Nephrol. JASN 2009, 20, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Harris, P.C.; Somlo, S.; Batlle, D.; Torres, V.E. Effect of calcium-sensing receptor activation in models of autosomal recessive or dominant polycystic kidney disease. Nephrol. Dial. Transplant. 2009, 24, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, D.; Brown, E.M. Physiology and pathophysiology of the calcium-sensing receptor in the kidney. Am. J. Physiol. Ren. Physiol. 2010, 298, F485–F499. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, D.; Valenti, G. Localization and function of the renal calcium-sensing receptor. Nat. Rev. Nephrol. 2016, 12, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Toka, H.R.; Pollak, M.R.; Houillier, P. Calcium Sensing in the Renal Tubule. Physiology 2015, 30, 317–326. [Google Scholar] [CrossRef]
- Zheng, B.; Cantley, L.C. Regulation of epithelial tight junction assembly and disassembly by AMP-activated protein kinase. Proc. Natl. Acad. Sci. USA 2007, 104, 819–822. [Google Scholar] [CrossRef]
- Jouret, F.; Wu, J.; Hull, M.; Rajendran, V.; Mayr, B.; Schöfl, C.; Geibel, J.; Caplan, M.J. Activation of the Ca2+-sensing receptor induces deposition of tight junction components to the epithelial cell plasma membrane. J. Cell Sci. 2013, 126, 5132–5142. [Google Scholar] [CrossRef]
- Rowart, P.; Wu, J.; Caplan, M.J.; Jouret, F. Implications of AMPK in the Formation of Epithelial Tight Junctions. Int. J. Mol. Sci. 2018, 19, 2040. [Google Scholar] [CrossRef]
- Zhang, J.; Han, J.; Zhang, X.; Jiang, J.; Xu, M.; Zhang, D.; Han, J. Polymeric nanoparticles based on chitooligosaccharide as drug carriers for co-delivery of all-trans-retinoic acid and paclitaxel. Carbohydr. Polym. 2015, 129, 25–34. [Google Scholar] [CrossRef]
- Zhou, P.; Sun, X.; Zhang, Z. Kidney-targeted drug delivery systems. Acta Pharm. Sin. B 2014, 4, 37–42. [Google Scholar] [CrossRef]
- Quiñones, J.P.; Peniche, H.; Peniche, C. Chitosan Based Self-Assembled Nanoparticles in Drug Delivery. Polymers 2018, 10, 235. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Gong, T.; Sun, X.; Tang, J.Z.; Zhang, Z. Chitosan oligomers as drug carriers for renal delivery of zidovudine. Carbohydr. Polym. 2012, 87, 2284–2290. [Google Scholar] [CrossRef]
- Sørbotten, A.; Horn, S.J.; Eijsink, V.G.; Vårum, K.M. Degradation of chitosans with chitinase B from Serratia marcescens. Production of chito-oligosaccharides and insight into enzyme processivity. FEBS J. 2005, 272, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Kudan, S.; Pichyangkura, R. Purification and characterization of thermostable chitinase from Bacillus Licheniformis SK-1. Appl. Biochem. Biotechnol. 2009, 157, 23–35. [Google Scholar] [CrossRef]
- Liang, S.; Sun, Y.; Dai, X. A Review of the Preparation, Analysis and Biological Functions of Chitooligosaccharide. Int. J. Mol. Sci. 2018, 19, 2197. [Google Scholar] [CrossRef]
- Suphasiriroj, W.; Yotnuengnit, P.; Surarit, R.; Pichyangkura, R. The fundamental parameters of chitosan in polymer scaffolds affecting osteoblasts (MC3T3-E1). J. Mater. Sci. Mater. Med. 2009, 20, 309–320. [Google Scholar] [CrossRef]
Sample Availability: Samples of the chitosan oligosaccharide (COS) are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pathomthongtaweechai, N.; Soodvilai, S.; Pichyangkura, R.; Muanprasat, C. Novel Potential Application of Chitosan Oligosaccharide for Attenuation of Renal Cyst Growth in the Treatment of Polycystic Kidney Disease. Molecules 2020, 25, 5589. https://doi.org/10.3390/molecules25235589
Pathomthongtaweechai N, Soodvilai S, Pichyangkura R, Muanprasat C. Novel Potential Application of Chitosan Oligosaccharide for Attenuation of Renal Cyst Growth in the Treatment of Polycystic Kidney Disease. Molecules. 2020; 25(23):5589. https://doi.org/10.3390/molecules25235589
Chicago/Turabian StylePathomthongtaweechai, Nutthapoom, Sunhapas Soodvilai, Rath Pichyangkura, and Chatchai Muanprasat. 2020. "Novel Potential Application of Chitosan Oligosaccharide for Attenuation of Renal Cyst Growth in the Treatment of Polycystic Kidney Disease" Molecules 25, no. 23: 5589. https://doi.org/10.3390/molecules25235589
APA StylePathomthongtaweechai, N., Soodvilai, S., Pichyangkura, R., & Muanprasat, C. (2020). Novel Potential Application of Chitosan Oligosaccharide for Attenuation of Renal Cyst Growth in the Treatment of Polycystic Kidney Disease. Molecules, 25(23), 5589. https://doi.org/10.3390/molecules25235589





