Alkaloids: Therapeutic Potential against Human Coronaviruses
Abstract
1. Introduction
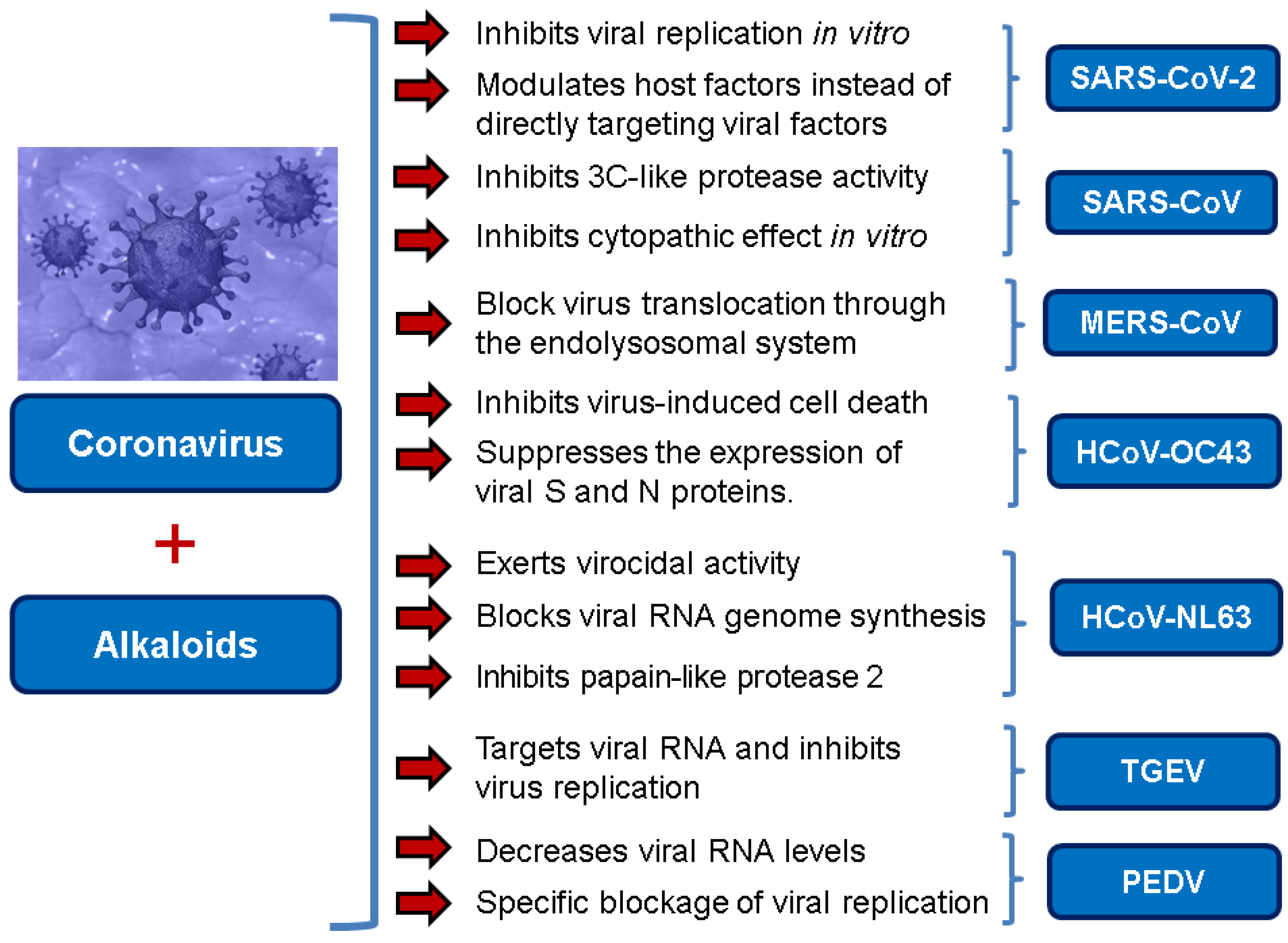
2. Results
Molecular Docking Study
3. Conclusions
4. Methodology
Molecular Docking
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 229-E | Human coronavirus-229E |
| 3CLpro | 3C-like protease |
| BCoV-L9 | Bovine coronavirus strain L9 |
| CEP | Cepharanthine |
| CoVs | Coronaviruses |
| COVID-19 | Coronavirus disease 2019 |
| CPE | Cytopathic effect |
| DV | Dengue virus |
| EV | Ebolavirus |
| EV-71 | Enterovirus 71 |
| FAN | Fangchinoline |
| HBV | Hepatitis B virus |
| HCMV | Human cytomegalovirus |
| HCV | Hepatitis C virus |
| HECoV-4408 | Human enteric coronavirus-4408 |
| HEV1 | Human echovirus 1 |
| HHT | Homoharringtonine |
| HIV | Human immunodeficiency virus |
| HKU1 | Human coronavirus-HKU1 |
| HSV1 | Human T-lymphotropic virus type |
| HTLV-1 | Herpes simplex virus type 1 |
| JEV | Japanese encephalitis virus |
| MERS-CoV | Middle east respiratory syndrome-coronavirus |
| MHV | Mouse hepatitis virus |
| NDV | Newcastle disease virus |
| NL63 | Human coronavirus-NL63 |
| OC43 | Human coronavirus-OC43 |
| PEDV | Porcine epidemic diarrhoea coronavirus |
| PLpro | Papain-like protease |
| RVFV | Rift Valley fever virus |
| SARS-CoV | Severe acute respiratory syndrome-coronavirus |
| SARS-CoV-2 | Severe acute respiratory syndrome-coronavirus-2 |
| S-RBD | Spike protein receptor-binding domain |
| TET | Bis-benzylisoquinoline alkaloids tetrandrine |
| TGEV | Transmissible gastroenteritis coronavirus |
| VSV | Vesicular stomatitis virus |
| VZV | Varicella-zoster virus |
| ZV | Zika virus |
References
- Sanders, J.M.; Monogue, M.L.; Jodlowski, T.Z.; Cutrell, J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): A review. JAMA 2020, 323, 1824–1836. [Google Scholar] [CrossRef] [PubMed]
- Fuzimoto, A.D.; Isidoro, C. The antiviral and the coronavirus-host protein pathways inhibiting properties of herbs and natural compounds-Additional weapons in the fight against the COVID-19 pandemic? J. Tradit. Complement. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Choy, K.-T.; Wong, A.Y.-L.; Kaewpreedee, P.; Sia, S.-F.; Chen, D.; Hui, K.P.Y.; Chu, D.K.W.; Chan, M.C.W.; Cheung, P.P.-H.; Huang, X. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir. Res. 2020, 104786. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, S.; Sato, M.; Tsunematsu, Y.; Watanabe, K. Evaluation of Biosynthetic Pathway and Engineered Biosynthesis of Alkaloids. Molecules 2016, 21, 1078. [Google Scholar] [CrossRef]
- Aniszewski, T. Alkaloids: Chemistry, Biology, Ecology, and Applications; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 0444594620. [Google Scholar]
- Martin, N.J.; Ferreiro, S.F.; Barbault, F.; Nicolas, M.; Lecellier, G.; Paetz, C.; Gaysinski, M.; Alonso, E.; Thomas, O.P.; Botana, L.M.; et al. Indole alkaloids from the Marquesan plant Rauvolfia nukuhivensis and their effects on ion channels. Phytochemistry 2015, 109, 84–95. [Google Scholar] [CrossRef]
- Chen, Q.-B.; Gao, J.; Zou, G.-A.; Xin, X.-L.; Aisa, H.A. Piperidine Alkaloids with Diverse Skeletons from Anacyclus pyrethrum. J. Nat. Prod. 2018, 81, 1474–1482. [Google Scholar] [CrossRef]
- Yu, X.; Gao, X.; Zhu, Z.; Cao, Y.; Zhang, Q.; Tu, P.; Chai, X. Alkaloids from the Tribe Bocconieae (Papaveraceae): A Chemical and Biological Review. Molecules 2014, 19, 13042–13060. [Google Scholar] [CrossRef]
- Arato Ferreira, P.H.; dos Santos, D.A.P.; da Silva, M.F.D.G.; Vieira, P.C.; King-Diaz, B.; Lotina-Hennsen, B.; Veiga, T.A.M. Acridone Alkaloids from Swinglea glutinosa (Rutaceae) and Their Effects on Photosynthesis. Chem. Biodivers. 2016, 13, 100–106. [Google Scholar] [CrossRef]
- Heinig, U.; Aharoni, A. Analysis of Steroidal Alkaloids and Saponins in Solanaceae Plant Extracts Using UPLC-qTOF Mass Spectrometry; Humana Press: New York, NY, USA, 2014; pp. 171–185. [Google Scholar]
- Oliveira, S.L.; da Silva, M.S.; Tavares, J.F.; Sena-Filho, J.G.; Lucena, H.F.S.; Romero, M.A.V.; Barbosa-Filho, J.M. Tropane Alkaloids from Erythroxylum Genus: Distribution and Compilation of 13C-NMR Spectral Data. Chem. Biodivers. 2010, 7, 302–326. [Google Scholar] [CrossRef]
- He, L.-J.; Liu, J.-S.; Luo, D.; Zheng, Y.-R.; Zhang, Y.-B.; Wang, G.-C.; Li, Y.-L. Quinolizidine alkaloids from Sophora tonkinensis and their anti-inflammatory activities. Fitoterapia 2019, 139, 104391. [Google Scholar] [CrossRef]
- Brook, K.; Bennett, J.; Desai, S.P. The Chemical History of Morphine: An 8000-year Journey, from Resin to de-novo Synthesis. J. Anesth. Hist. 2017, 3, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Cushnie, B.; Lamb, A.J. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int. J. Antimicrob. Agents 2014, 44, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Mubarak, M.S.; Amin, S. Antifungal Potential of Alkaloids As An Emerging Therapeutic Target. Curr. Drug Targets 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.L.; Nidorf, S.M. Colchicine: An Affordable Anti-Inflammatory Agent for Atherosclerosis. Curr. Opin. Lipidol. 2018, 29, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Manayi, A.; Nabavi, S.M.; Setzer, W.N.; Jafari, S. Piperine as a Potential Anti-cancer Agent: A Review on Preclinical Studies. Curr. Med. Chem. 2019, 25, 4918–4928. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, M.; Liu, H.; Wei, K.; He, M.; Li, X.; Hu, D.; Yang, S.; Zheng, Y. Antiviral activity of aconite alkaloids from Aconitum carmichaelii Debx. Nat. Prod. Res. 2019, 33, 1486–1490. [Google Scholar] [CrossRef]
- Hung, T.-C.; Jassey, A.; Liu, C.-H.; Lin, C.-J.; Lin, C.-C.; Wong, S.H.; Wang, J.Y.; Yen, M.-H.; Lin, L.-T. Berberine inhibits hepatitis C virus entry by targeting the viral E2 glycoprotein. Phytomedicine 2019, 53, 62–69. [Google Scholar] [CrossRef]
- Luganini, A.; Mercorelli, B.; Messa, L.; Palù, G.; Gribaudo, G.; Loregian, A. The isoquinoline alkaloid berberine inhibits human cytomegalovirus replication by interfering with the viral Immediate Early-2 (IE2) protein transactivating activity. Antivir. Res. 2019, 164, 52–60. [Google Scholar] [CrossRef]
- Varghese, F.S.; Thaa, B.; Amrun, S.N.; Simarmata, D.; Rausalu, K.; Nyman, T.A.; Merits, A.; McInerney, G.M.; Ng, L.F.P.; Ahola, T. The Antiviral Alkaloid Berberine Reduces Chikungunya Virus-Induced Mitogen-Activated Protein Kinase Signaling. J. Virol. 2016, 90, 9743–9757. [Google Scholar] [CrossRef]
- Diosa-Toro, M.; Troost, B.; van de Pol, D.; Heberle, A.M.; Urcuqui-Inchima, S.; Thedieck, K.; Smit, J.M. Tomatidine, a novel antiviral compound towards dengue virus. Antivir. Res. 2019, 161, 90–99. [Google Scholar] [CrossRef]
- McMahon, J.B.; Currens, M.J.; Gulakowski, R.J.; Buckheit, R.W.; Lackman-Smith, C.; Hallock, Y.F.; Boyd, M.R. Michellamine B, a novel plant alkaloid, inhibits human immunodeficiency virus-induced cell killing by at least two distinct mechanisms. Antimicrob. Agents Chemother. 1995, 39, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.-P.; Wang, Q.-W.; Su, Y.; Gu, L.-M.; Deng, H.-X.; Chen, X.-X.; Li, W.-Z.; Li, K.-S. Oxymatrine Inhibits Influenza A Virus Replication and Inflammation via TLR4, p38 MAPK and NF-κB Pathways. Int. J. Mol. Sci. 2018, 19, 965. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.-J.; Lu, J.-W.; Huang, Y.-L.; Lai, Z.-Z. Palmatine inhibits Zika virus infection by disrupting virus binding, entry, and stability. Biochem. Biophys. Res. Commun. 2019, 518, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.-T.; Karimi, A.; Rafieian-Kopaei, M.; Fotouhi, F. In vitro antiviral effects of Peganum harmala seed extract and its total alkaloids against Influenza virus. Microb. Pathog. 2017, 110, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Nawawi, A.; Nakamura, N.; Meselhy, M.R.; Hattori, M.; Kurokawa, M.; Shiraki, K.; Kashiwaba, N.; Ono, M. In vivo Antiviral Activity of Stephania cepharantha against Herpes Simplex Virus Type-1. Phyther. Res. 2001, 15, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zheng, T.-T.; Li, X.; Liang, Y.; Wang, L.-J.; Huang, Y.-C.; Xiao, H.-T. Plant-Derived Alkaloids: The Promising Disease-Modifying Agents for Inflammatory Bowel Disease. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Thawabteh, A.; Juma, S.; Bader, M.; Karaman, D.; Scrano, L.; Bufo, S.A.; Karaman, R. The Biological Activity of Natural Alkaloids against Herbivores, Cancerous Cells and Pathogens. Toxins 2019, 11, 656. [Google Scholar] [CrossRef]
- Li, S.; Chen, C.; Zhang, H.; Guo, H.; Wang, H.; Wang, L.; Zhang, X.; Hua, S.; Yu, J.; Xiao, P. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antivir. Res. 2005, 67, 18–23. [Google Scholar] [CrossRef]
- Kim, D.; Min, J.; Jang, M.; Lee, J.; Shin, Y.; Park, C.; Song, J.; Kim, H.; Kim, S.; Jin, Y.-H.; et al. Natural Bis-Benzylisoquinoline Alkaloids-Tetrandrine, Fangchinoline, and Cepharanthine, Inhibit Human Coronavirus OC43 Infection of MRC-5 Human Lung Cells. Biomolecules 2019, 9, 696. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; O’Brien, S.; Cortes, J. Homoharringtonine/Omacetaxine Mepesuccinate: The Long and Winding Road to Food and Drug Administration Approval. Clin. Lymphoma Myeloma Leuk. 2013, 13, 530–533. [Google Scholar] [CrossRef]
- Lü, S.; Wang, J. Homoharringtonine and omacetaxine for myeloid hematological malignancies. J. Hematol. Oncol. 2014, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-E.; Song, Y.-J. Anti-varicella-zoster virus activity of cephalotaxine esters in vitro. J. Microbiol. 2019, 57, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.; Serrano, M.; Efferth, T.; Alvarez, M.; Marin, J. Effect of Cantharidin, Cephalotaxine and Homoharringtonine on “in vitro” Models of Hepatitis B Virus (HBV) and Bovine Viral Diarrhoea Virus (BVDV) Replication. Planta Med. 2007, 73, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Andersen, P.I.; Krpina, K.; Ianevski, A.; Shtaida, N.; Jo, E.; Yang, J.; Koit, S.; Tenson, T.; Hukkanen, V.; Anthonsen, M.W.; et al. Novel Antiviral Activities of Obatoclax, Emetine, Niclosamide, Brequinar, and Homoharringtonine. Viruses 2019, 11, 964. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.-J.; Wang, Z.-H.; Meng, W.; Li, C.-C.; Hu, Y.-X.; Zhou, L.; Wang, X.-J. The Natural Compound Homoharringtonine Presents Broad Antiviral Activity In Vitro and In Vivo. Viruses 2018, 10, 601. [Google Scholar] [CrossRef]
- Cao, J.; Forrest, J.C.; Zhang, X. A screen of the NIH Clinical Collection small molecule library identifies potential anti-coronavirus drugs. Antivir. Res. 2015, 114, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Yang, P.; Zhou, Q. Multiple biological functions and pharmacological effects of lycorine. Sci. China Chem. 2013, 56, 1382–1391. [Google Scholar] [CrossRef]
- Saltan Çitoğlu, G.; Bahadır Acıkara, Ö.; Sever Yılmaz, B.; Özbek, H. Evaluation of analgesic, anti-inflammatory and hepatoprotective effects of lycorine from Sternbergia fisheriana (Herbert) Rupr. Fitoterapia 2012, 83, 81–87. [Google Scholar] [CrossRef]
- Wang, P.; Li, L.-F.; Wang, Q.-Y.; Shang, L.-Q.; Shi, P.-Y.; Yin, Z. Anti-Dengue-Virus Activity and Structure-Activity Relationship Studies of Lycorine Derivatives. ChemMedChem 2014, 9, 1522–1533. [Google Scholar] [CrossRef]
- Chen, H.; Lao, Z.; Xu, J.; Li, Z.; Long, H.; Li, D.; Lin, L.; Liu, X.; Yu, L.; Liu, W.; et al. Antiviral activity of lycorine against Zika virus in vivo and in vitro. Virology 2020, 546, 88–97. [Google Scholar] [CrossRef]
- Oluyemisi, O.O.; Oriabure, A.E.; Adekunle, A.J.; Ramsay, K.S.T.; Shyyaula, S.; Choudhary, M.I. Bioassay-guided isolation of Poliovirus-inhibiting constituents from Zephyranthes candida. Pharm. Biol. 2015, 53, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Cai, J.; Cheng, J.; Jing, C.; Yin, J.; Jiang, J.; Peng, Z.; Hao, X. Design, Synthesis and Structure-Activity Relationship Optimization of Lycorine Derivatives for HCV Inhibition. Sci. Rep. 2015, 5, 14972. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, Y.; Xu, Y.; Ma, C.; Qin, C.; Zhang, L. Lycorine reduces mortality of human enterovirus 71-infected mice by inhibiting virus replication. Virol. J. 2011, 8, 483. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, T.; Yang, Y.; Yu, L.; Pan, X.; Li, Y. Lycorine Derivative LY-55 Inhibits EV71 and CVA16 Replication Through Downregulating Autophagy. Front. Cell. Infect. Microbiol. 2019, 9. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, J.H.; Zhang, X.L.; Lao, G.J.; Su, G.M.; Wang, L.; Li, Y.L.; Ye, W.C.; He, J. Tandem mass tag-based quantitative proteomic analysis of lycorine treatment in highly pathogenic avian influenza H5N1 virus infection. PeerJ 2019, 7, e7697. [Google Scholar] [CrossRef]
- Renard-Nozaki, J.; Kim, T.; Imakura, Y.; Kihara, M.; Kobayashi, S. Effect of alkaloids isolated from Amaryllidaceae on herpes simplex virus. Res. Virol. 1989, 140, 115–128. [Google Scholar] [CrossRef]
- Gabrielsen, B.; Monath, T.P.; Huggins, J.W.; Kefauver, D.F.; Pettit, G.R.; Groszek, G.; Hollingshead, M.; Kirsi, J.J.; Shannon, W.M.; Schubert, E.M.; et al. Antiviral (RNA) Activity of Selected Amaryllidaceae Isoquinoline Constituents and Synthesis of Related Substances. J. Nat. Prod. 1992, 55, 1569–1581. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Zhang, Q.-Y.; Li, X.-D.; Xiong, J.; Xiao, S.-Q.; Wang, Z.; Zhang, Z.-R.; Deng, C.-L.; Yang, X.-L.; Wei, H.-P.; et al. Gemcitabine, lycorine and oxysophoridine inhibit novel coronavirus (SARS-CoV-2) in cell culture. Emerg. Microbes Infect. 2020, 9, 1170–1173. [Google Scholar] [CrossRef]
- He, J.; Qi, W.-B.; Wang, L.; Tian, J.; Jiao, P.-R.; Liu, G.-Q.; Ye, W.-C.; Liao, M. Amaryllidaceae alkaloids inhibit nuclear-to-cytoplasmic export of ribonucleoprotein (RNP) complex of highly pathogenic avian influenza virus H5N1. Influenza Other Respi. Viruses 2013, 7, 922–931. [Google Scholar] [CrossRef]
- Rui, C.; Yuxiang, L.; Ning, J.; Ningtian, M.; Qingluan, Z.; Yinju, H.; Ru, Z.; Lin, M.; Tao, S.; Jianqiang, Y. Anti-apoptotic and Neuroprotective Effects of Oxysophoridine on Cerebral Ischemia Both In Vivo and In Vitro. Planta Med. 2013, 79, 916–923. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Li, Y.-X.; Zhao, P.; Wang, H.-B.; Zhou, R.; Hao, Y.-J.; Wang, J.; Wang, S.-J.; Du, J.; Ma, L.; et al. Anti-inflammation Effects of Oxysophoridine on Cerebral Ischemia–Reperfusion Injury in Mice. Inflammation 2015, 38, 2259–2268. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Zhang, Y.; Long, W.; Liu, P. Oxysophoridine suppresses the growth of hepatocellular carcinoma in mice: In Vivo and cDNA microarray studies. Chin. J. Integr. Med. 2012, 18, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Gao, J.; Jia, Y.; Yan, L.; Yu, J.; Jiang, Y. Oxysophoridine through intrathecal injection induces antinociception and increases the expression of the GABAAα1 receptor in the spinal cord of mice. Planta Med. 2012, 78, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Chen, L.; Liu, Y.; Peng, T. Oxysophoridine rescues spinal cord injury via anti-inflammatory, anti-oxidative stress and anti-apoptosis effects. Mol. Med. Rep. 2017. [Google Scholar] [CrossRef]
- Wan, Z.; Lu, Y.; Liao, Q.; Wu, Y.; Chen, X. Fangchinoline Inhibits Human Immunodeficiency Virus Type 1 Replication by Interfering with gp160 Proteolytic Processing. PLoS ONE 2012, 7, e39225. [Google Scholar] [CrossRef]
- Matsuda, K.; Hattori, S.; Komizu, Y.; Kariya, R.; Ueoka, R.; Okada, S. Cepharanthine inhibited HIV-1 cell–cell transmission and cell-free infection via modification of cell membrane fluidity. Bioorg. Med. Chem. Lett. 2014, 24, 2115–2117. [Google Scholar] [CrossRef]
- Toyama, M.; Hamasaki, T.; UTO, T.; Aoyama, H.; Okamoto, M.; Hashmoto, Y.; Baba, M. Synergistic inhibition of HTLV-1-infected cell proliferation by combination of cepharanthine and a tetramethylnaphthalene derivative. Anticancer Res. 2012, 32, 2639–2645. [Google Scholar]
- Liou, J.-T.; Chen, Z.-Y.; Ho, L.-J.; Yang, S.-P.; Chang, D.-M.; Liang, C.-C.; Lai, J.-H. Differential effects of triptolide and tetrandrine on activation of COX-2, NF-κB, and AP-1 and virus production in dengue virus-infected human lung cells. Eur. J. Pharmacol. 2008, 589, 288–298. [Google Scholar] [CrossRef]
- Sakurai, Y.; Kolokoltsov, A.A.; Chen, C.-C.; Tidwell, M.W.; Bauta, W.E.; Klugbauer, N.; Grimm, C.; Wahl-Schott, C.; Biel, M.; Davey, R.A. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science 2015, 347, 995–998. [Google Scholar] [CrossRef]
- Hu, S.; Dutt, J.; Zhao, T.; Foster, C.S. Tetrandrine potently inhibits herpes simplex virus type-1-induced keratitis in BALB/c mice. Ocul. Immunol. Inflamm. 1997, 5, 173–180. [Google Scholar] [CrossRef]
- Zhou, Y.-B.; Wang, Y.-F.; Zhang, Y.; Zheng, L.-Y.; Yang, X.-A.; Wang, N.; Jiang, J.-H.; Ma, F.; Yin, D.-T.; Sun, C.-Y.; et al. In vitro activity of cepharanthine hydrochloride against clinical wild-type and lamivudine-resistant hepatitis B virus isolates. Eur. J. Pharmacol. 2012, 683, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Gunaratne, G.S.; Yang, Y.; Li, F.; Walseth, T.F.; Marchant, J.S. NAADP-dependent Ca2+ signaling regulates Middle East respiratory syndrome-coronavirus pseudovirus translocation through the endolysosomal system. Cell Calcium 2018, 75, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Raina, V.; Raina, S. The responsiveness of leukocyte adenyl cyclase to tylophorine in asthmatic subjects. Biochem. Biophys. Res. Commun. 1980, 94, 1074–1077. [Google Scholar] [CrossRef]
- You, X.; Pan, M.; Gao, W.; Shiah, H.-S.; Tao, J.; Zhang, D.; Koumpouras, F.; Wang, S.; Zhao, H.; Madri, J.A.; et al. Effects of a novel tylophorine analog on collagen-induced arthritis through inhibition of the innate immune response. Arthritis Rheum. 2006, 54, 877–886. [Google Scholar] [CrossRef]
- Yang, C.-W.; Chuang, T.-H.; Wu, P.-L.; Huang, W.-H.; Lee, S.-J. Anti-inflammatory effects of 7-methoxycryptopleurine and structure–activity relations of phenanthroindolizidines and phenanthroquinolizidines. Biochem. Biophys. Res. Commun. 2007, 354, 942–948. [Google Scholar] [CrossRef]
- Wang, Y.; Lee, S.; Ha, Y.; Lam, W.; Chen, S.-R.; Dutschman, G.E.; Gullen, E.A.; Grill, S.P.; Cheng, Y.; Fürstner, A.; et al. Tylophorine Analogs Allosterically Regulates Heat Shock Cognate Protein 70 And Inhibits Hepatitis C Virus Replication. Sci. Rep. 2017, 7, 10037. [Google Scholar] [CrossRef]
- Pham, L.V.; Ngo, H.T.T.; Lim, Y.-S.; Hwang, S.B. Hepatitis C virus non-structural 5B protein interacts with cyclin A2 and regulates viral propagation. J. Hepatol. 2012, 57, 960–966. [Google Scholar] [CrossRef]
- Lee, Y.-Z.; Yang, C.-W.; Hsu, H.-Y.; Qiu, Y.-Q.; Yeh, T.-K.; Chang, H.-Y.; Chao, Y.-S.; Lee, S.-J. Synthesis and Biological Evaluation of Tylophorine-Derived Dibenzoquinolines as Orally Active Agents: Exploration of the Role of Tylophorine E Ring on Biological Activity. J. Med. Chem. 2012, 55, 10363–10377. [Google Scholar] [CrossRef]
- Yang, C.-W.; Lee, Y.-Z.; Kang, I.-J.; Barnard, D.L.; Jan, J.-T.; Lin, D.; Huang, C.-W.; Yeh, T.-K.; Chao, Y.-S.; Lee, S.-J. Identification of phenanthroindolizines and phenanthroquinolizidines as novel potent anti-coronaviral agents for porcine enteropathogenic coronavirus transmissible gastroenteritis virus and human severe acute respiratory syndrome coronavirus. Antivir. Res. 2010, 88, 160–168. [Google Scholar] [CrossRef]
- Yang, C.-W.; Lee, Y.-Z.; Hsu, H.-Y.; Shih, C.; Chao, Y.-S.; Chang, H.-Y.; Lee, S.-J. Targeting Coronaviral Replication and Cellular JAK2 Mediated Dominant NF-κB Activation for Comprehensive and Ultimate Inhibition of Coronaviral Activity. Sci. Rep. 2017, 7, 4105. [Google Scholar] [CrossRef]
- Qin, G.; Xu, R. Recent advances on bioactive natural products from Chinese medicinal plants. Med. Res. Rev. 1998, 18, 375–382. [Google Scholar] [CrossRef]
- Chang, S.-J.; Chang, Y.-C.; Lu, K.-Z.; Tsou, Y.-Y.; Lin, C.-W. Antiviral Activity of Isatis indigotica Extract and Its Derived Indirubin against Japanese Encephalitis Virus. Evid. Based Complement. Altern. Med. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Ho, Y.-L.; Chang, Y.-S. Studies on the antinociceptive, anti-inflammatory and antipyretic effects of Isatis indigotica root. Phytomedicine 2002, 9, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-W.; Tsai, F.-J.; Tsai, C.-H.; Lai, C.-C.; Wan, L.; Ho, T.-Y.; Hsieh, C.-C.; Chao, P.-D.L. Anti-SARS coronavirus 3C-like protease effects of Isatis indigotica root and plant-derived phenolic compounds. Antivir. Res. 2005, 68, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.; Fielding, B.; Gamieldien, J. Potential Broad Spectrum Inhibitors of the Coronavirus 3CLpro: A Virtual Screening and Structure-Based Drug Design Study. Viruses 2015, 7, 6642–6660. [Google Scholar] [CrossRef] [PubMed]
- Shahni, R.; Handique, P.J. Antibacterial properties of leaf extracts of Strobilanthes cusia (Nees) Kuntze, a rare ethno-medicinal plant of Manipur, India. Int. J. PharmTech Res. 2013, 5, 1281–1285. [Google Scholar]
- Tanaka, T.; Ikeda, T.; Kaku, M.; Zhu, X.-H.; Okawa, M.; Yokomizo, K.; Uyeda, M.; Nohara, T. A New Lignan Glycoside and Phenylethanoid Glycosides from Strobilanthes cusia BREMEK. Chem. Pharm. Bull. 2004, 52, 1242–1245. [Google Scholar] [CrossRef]
- Gu, W.; Wang, W.; Li, X.; Zhang, Y.; Wang, L.; Yuan, C.; Huang, L.; Hao, X. A novel isocoumarin with anti-influenza virus activity from Strobilanthes cusia. Fitoterapia 2015, 107, 60–62. [Google Scholar] [CrossRef]
- Lee, C.-L.; Wang, C.-M.; Hu, H.-C.; Yen, H.-R.; Song, Y.-C.; Yu, S.-J.; Chen, C.-J.; Li, W.-C.; Wu, Y.-C. Indole alkaloids indigodoles A–C from aerial parts of Strobilanthes cusia in the traditional Chinese medicine Qing Dai have anti-IL-17 properties. Phytochemistry 2019, 162, 39–46. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Lee, C.-L.; Yen, H.-R.; Chang, Y.-S.; Lin, Y.-P.; Huang, S.-H.; Lin, C.-W. Antiviral Action of Tryptanthrin Isolated from Strobilanthes cusia Leaf against Human Coronavirus NL63. Biomolecules 2020, 10, 366. [Google Scholar] [CrossRef]
- Choudhary, S.; Malik, Y.S.; Tomar, S. Identification of SARS-CoV-2 cell entry inhibitors by drug repurposing using in silico structure-based virtual screening approach. Front. Immunol. 2020, 11, 1664. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Ye, G.; Shi, K.; Wan, Y.S.; Aihara, H.; Li, F. Structure of 2019-nCoV chimeric receptor-binding domain complexed with its receptor human ACE2. Worldw. Protein Data Bank 2020. [Google Scholar] [CrossRef]
- Brooks, B.R.; Bruccoleri, R.E.; Olafson, B.D.; States, D.J.; Swaminathan, S.A.; Karplus, M. CHARMM: A program for macromolecular energy, minimization, and dynamics calculations. J. Comput. Chem. 1983, 4, 187–217. [Google Scholar] [CrossRef]
- Chandrashekharappa, S.; Venugopala, K.N.; Tratrat, C.; Mahomoodally, F.M.; Aldhubiab, B.E.; Haroun, M.; Venugopala, R.; Mohan, M.K.; Kulkarni, R.S.; Attimarad, M.V. Efficient synthesis and characterization of novel indolizines: Exploration of in vitro COX-2 inhibitory activity and molecular modelling studies. New J. Chem. 2018, 42, 4893–4901. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Y.; Guo, M.; Xie, J.; Jiang, X. A Virtual Screening Method for Inhibitory Peptides of Angiotensin I–Converting Enzyme. J. Food Sci. 2014, 79, C1635–C1642. [Google Scholar] [CrossRef]

| Alkaloid | Coronavirus | Main Finding | Reference |
|---|---|---|---|
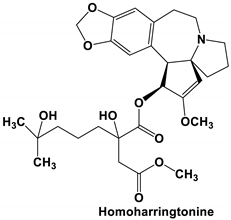
| Homoharringtonine (HHT) | SARS-CoV-2 | EC50 2.10 μM (reduction in viral copy number) EC50 2.55 μM (reduction in infectious virus) | [3] |
| MHV, BCoV-L9 and HECoV-4408 | Inhibits viral replication IC50 11 nM | [38] | |
| PEDV | IC50 0.112 μM in Vero E6 cells Decreases viral RNA levels in vivo in piglets Specific blockage of viral replication | [37] | |
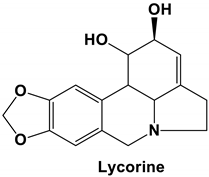
| Lycorine | SARS-CoV | IC50 15.7 nM | [30] |
| SARS-CoV-2 | Anti-CoV activity likely due to the lycorine modulating host factors instead of directly targeting viral factors | [50] | |
| Oxysophoridine | SARS-CoV-2 | EC50 0.18 μM and CC50 > 40 μM | [50] |
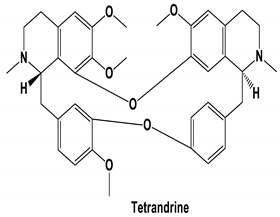
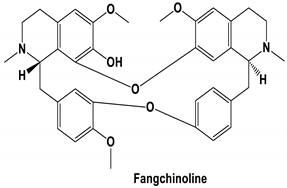
| Tetrandrine, Fangchinoline, and Cepharanthine | MERS-CoV HCoV-OC43 | Block MERS-pseudovirus translocation through the endolysosomal system Inhibited HCoV-OC43-induced cell death in the early stage of infection and reduced virus replication by suppressing the expression of viral S and N proteins. | [64] [31] |
| Tylophorine and Tylophorine analogs | SARS-CoV, MHV, and TGEV SARS-CoV, MERS-CoV, and TGEV | Anti-CoV replication activity; blocks virus-induced apoptosis and subsequent cytopathic effect in cells in vitro EC50 values for the natural and synthetic tylophorine compounds 8 to 1468 nM and 5 to 340 nM in ST and Vero 76 cells, respectively Targets viral RNA, thereby inhibiting TGEV replication Acts jointly with JAK family inhibitor for comprehensive anti-CoV | [71] [72] |
| Indigo | SARS-CoV | Inhibits the cleavage activities of the 3CLpro IC50 values for cell-free and cell-based assays of 300 μM and 752 μM, respectively | [76] |
| Tryptanthrin and Indigodole B | HCoV-NL63 | Reduces viral yield: tryptanthrin (IC50 1.52 μM); indigodole B (2.60 μM) Virucidal activity: tryptanthrin (IC50 = 0.06 μM); indigodole B (IC50 = 2.09 μM) Tryptanthrin blocks viral RNA genome synthesis and the activity of the papain-like protease 2 | [82] |
| Alkaloid | Type of Virus/Cell Lines | Concentration/Dose | Antiviral Effect | Reference |
|---|---|---|---|---|
| Homoharringtonine | VZV/HFF cells HBV/HepG2 2.2.15 cells Echovirus 1/RPE cells VSV/HEK293T cells HSV1/Vero cells | 10 ng/mL 0.03 μM 2 μM 0.12 μM 50 nM 139 nM | Down-regulation of VZV lytic gene transcripts Induces a 50% inhibition in HBsAg release Induces a 50% inhibition in HBV-DNA release Inhibit echovirus replication Inhibits the late stage of vesicular stomatitis virus replication Inhibits 50% of HSV1 replication | [34] [35] [36] [37] |
| Tylophorine | HCV | 0.06 μM | Reduced replication of the HCV through inhibition of Cyclin A2 | [69] |
| Fangchinoline | HIV1/MT-4, PM1, and human embryonic kidney cell line 293T cells | 0.8 to 1.7 μM | Inhibits HIV1 replication by interfering with gp160 proteolytic processing | [57] |
| Lycorine | DV-2/A549 cells ZV/Vero, Huh7, and A549 cells RD cells HCV/Huh 7.5 cells Avian influenza H5N1 virus/GD178 and MDCK cells EV-71 H/Vero cells Coxsackievirus A16/Vero cells | 0.8 μM 0.22 to 0.39 μM 0.058 μg/mL 0.316 μM 0.52 μM 2.04 μM 3.2 μM | Inhibits 50% of envelope protein production Inhibits 50% of ZV protein and envelope biosynthesis Inhibits 50% of virus replication Reduces HCV replication by 50% through suppresses the expression of Hsc70 Reduces the expression of viral proteins Inhibits 50% of virus replication Inhibits 50% of virus replication | [41] [42] [43] [44] [47] [46] |
| Indigo | JEV/BHK-21 cells | 37.5 μg/ml | Inhibits 50% of virus replication | [74] |
| Tetrandrine | DV/A549 cells Ebolavirus/human macrophages HSV/BALB/c mice | 1–10 μM 8 μM 15 mg/kg (i.p.) | Inhibited the DNA binding activity of NF-κB induced by DV and suppressed viral production Inhibited the infection of human macrophages by Ebolavirus Inhibited keratitis induced by HSV-I | [60] [61] [62] |
| Cepharanthine | HIV1/Molt-4 T cell line | 5–20 μg/mL | Inhibited the entry of the virus by reducing the fluidity of the plasma membrane | [58] |
| Cepharanthine hydrochloride | HBV/HepG2 cells | 2.14 μM 31.89 μM | Inhibited the virus replication Inhibited HBeAg production | [63] |
| Alkaloids | 2D Diagram | Libdock Score (Kcal/mol) | Key Amino Acids |
|---|---|---|---|
 |  | 109.11 | TYR 453 PRO 491 GLN 493 SER 494 SER 494 |
 | 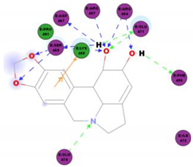 | 86.92 | ARG 454 PHE 456 SER 469 Glu 471 Gln 474 |
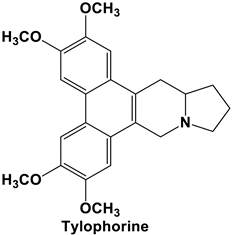 |  | 89.77 | TYR 351 TYR 453 GLN 493 SER 494 |
 | 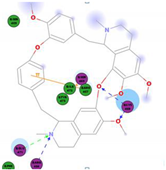 | 72.96 | ARG 454 LYS 458 LYS 458 GLU 471 |
 |  | 92.66 | ARG 454 LYS 458 LYS 458 SER 469 GLN 474 |
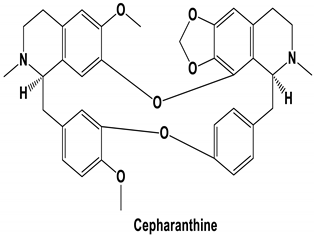 | 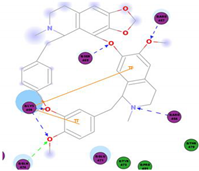 | 106.74 | ARG 454 ARG 457 LYS 458 LYS 458 SER 469 GLN 474 |
 |  | 76.08 | SER 469 SER 469 GLU 471 GLN 474 |
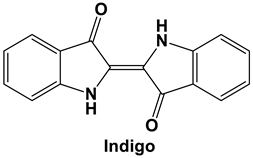 | 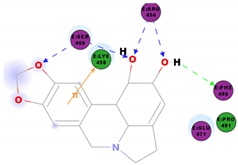 | 64.26 | PHE 347 ASN 448 ASN 450 |
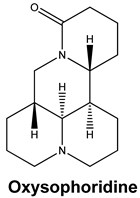 | 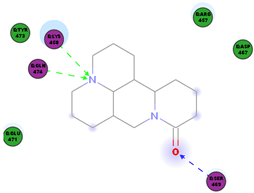 | 79.62 | LYS 458 SER 469 GLN 474 |
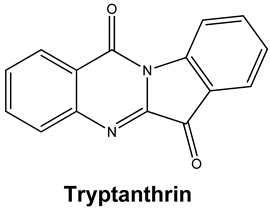 |  | 76.46 | ARG 454 LYS 458 SER 469 |
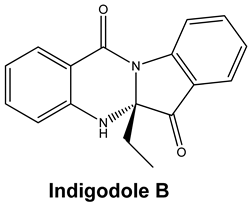 |  | 74.59 | ARG 454 ASP 467 SER 469 |
Sample Availability: Samples of the compounds are not available from the authors. Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fielding, B.C.; da Silva Maia Bezerra Filho, C.; Ismail, N.S.M.; Sousa, D.P.d. Alkaloids: Therapeutic Potential against Human Coronaviruses. Molecules 2020, 25, 5496. https://doi.org/10.3390/molecules25235496
Fielding BC, da Silva Maia Bezerra Filho C, Ismail NSM, Sousa DPd. Alkaloids: Therapeutic Potential against Human Coronaviruses. Molecules. 2020; 25(23):5496. https://doi.org/10.3390/molecules25235496
Chicago/Turabian StyleFielding, Burtram C., Carlos da Silva Maia Bezerra Filho, Nasser S. M. Ismail, and Damião Pergentino de Sousa. 2020. "Alkaloids: Therapeutic Potential against Human Coronaviruses" Molecules 25, no. 23: 5496. https://doi.org/10.3390/molecules25235496
APA StyleFielding, B. C., da Silva Maia Bezerra Filho, C., Ismail, N. S. M., & Sousa, D. P. d. (2020). Alkaloids: Therapeutic Potential against Human Coronaviruses. Molecules, 25(23), 5496. https://doi.org/10.3390/molecules25235496








