Effect of Vitamin B Complex Treatment on Macrophages to Schwann Cells Association during Neuroinflammation after Peripheral Nerve Injury
Abstract
1. Introduction
2. Results
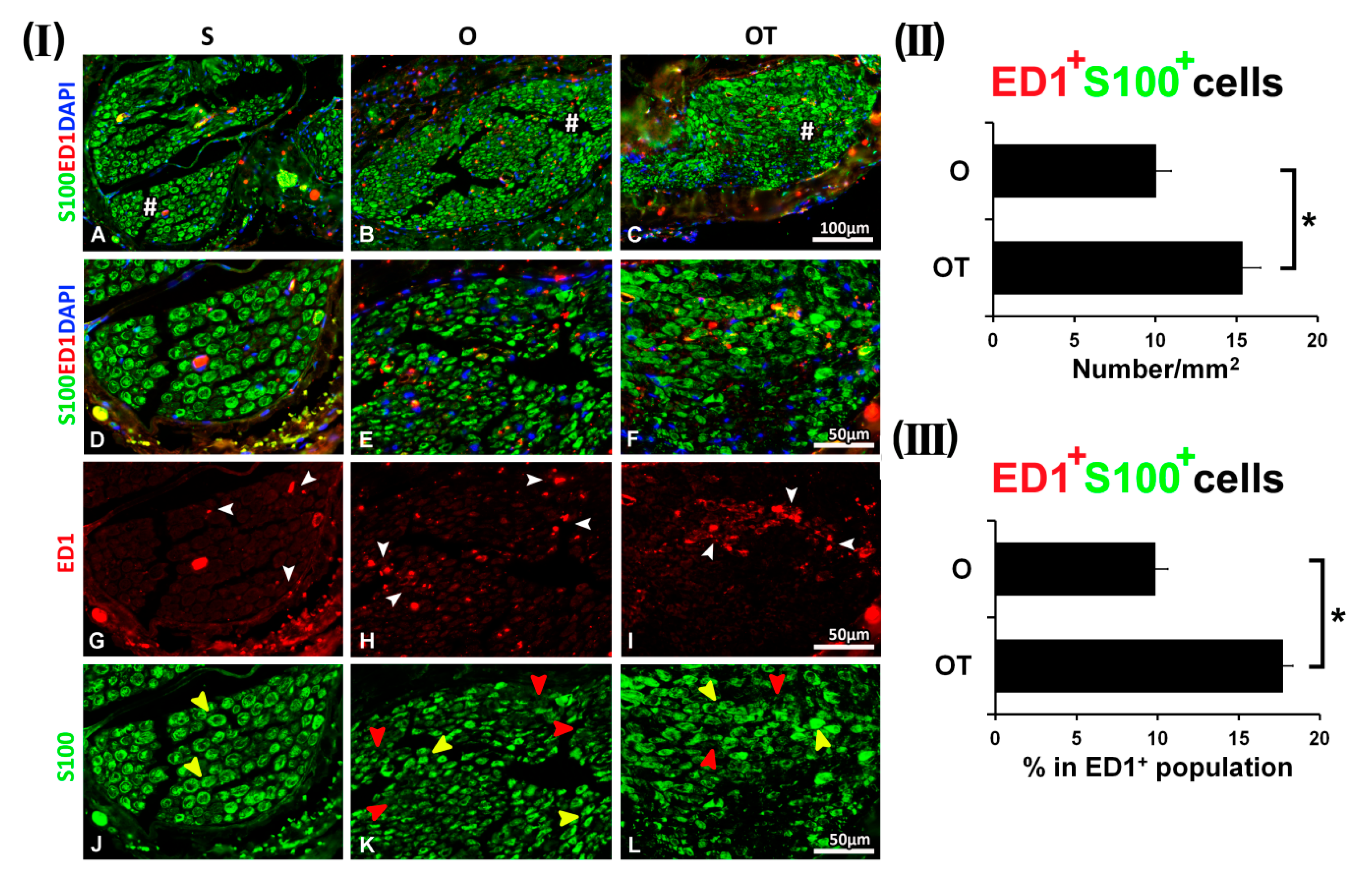
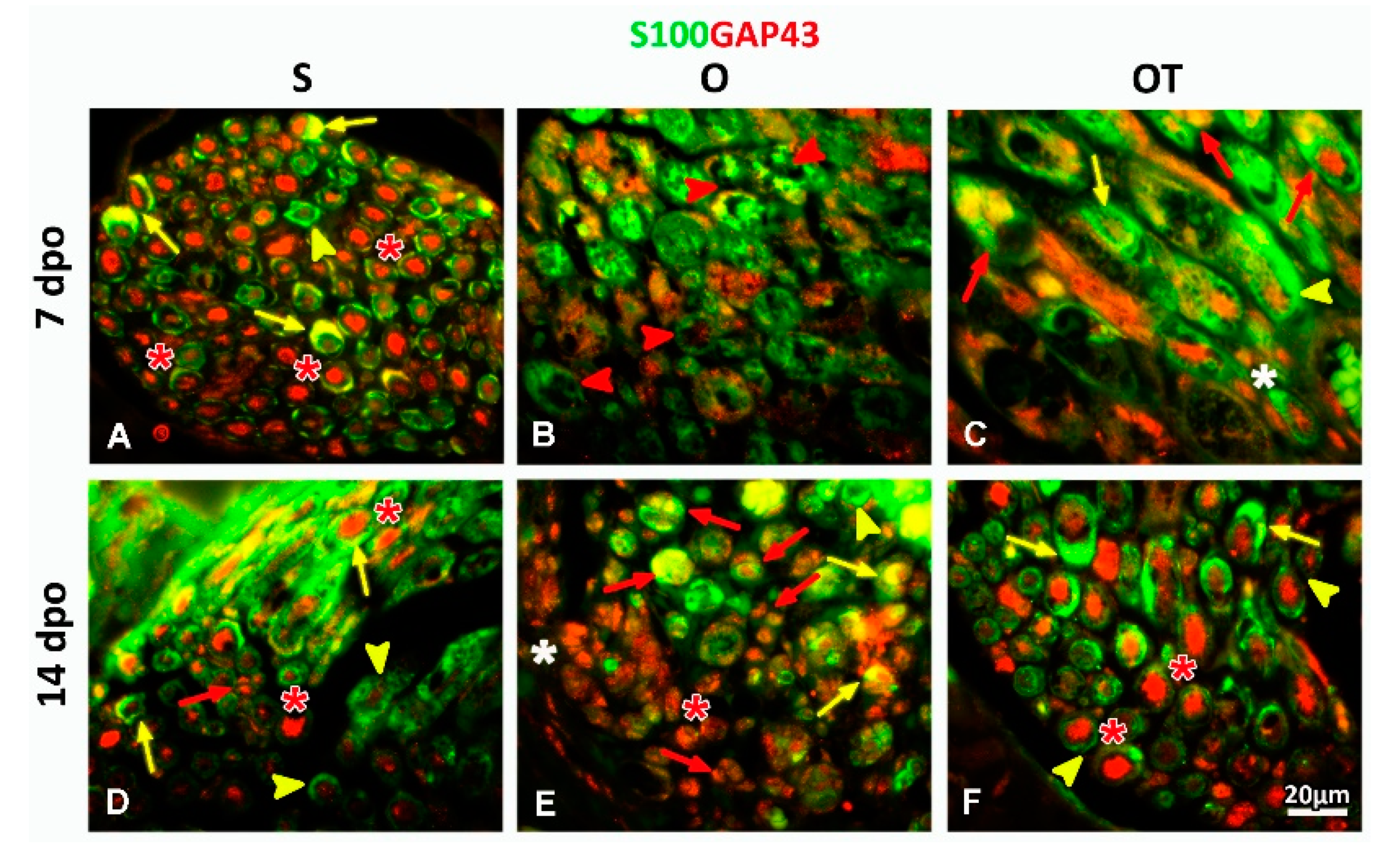
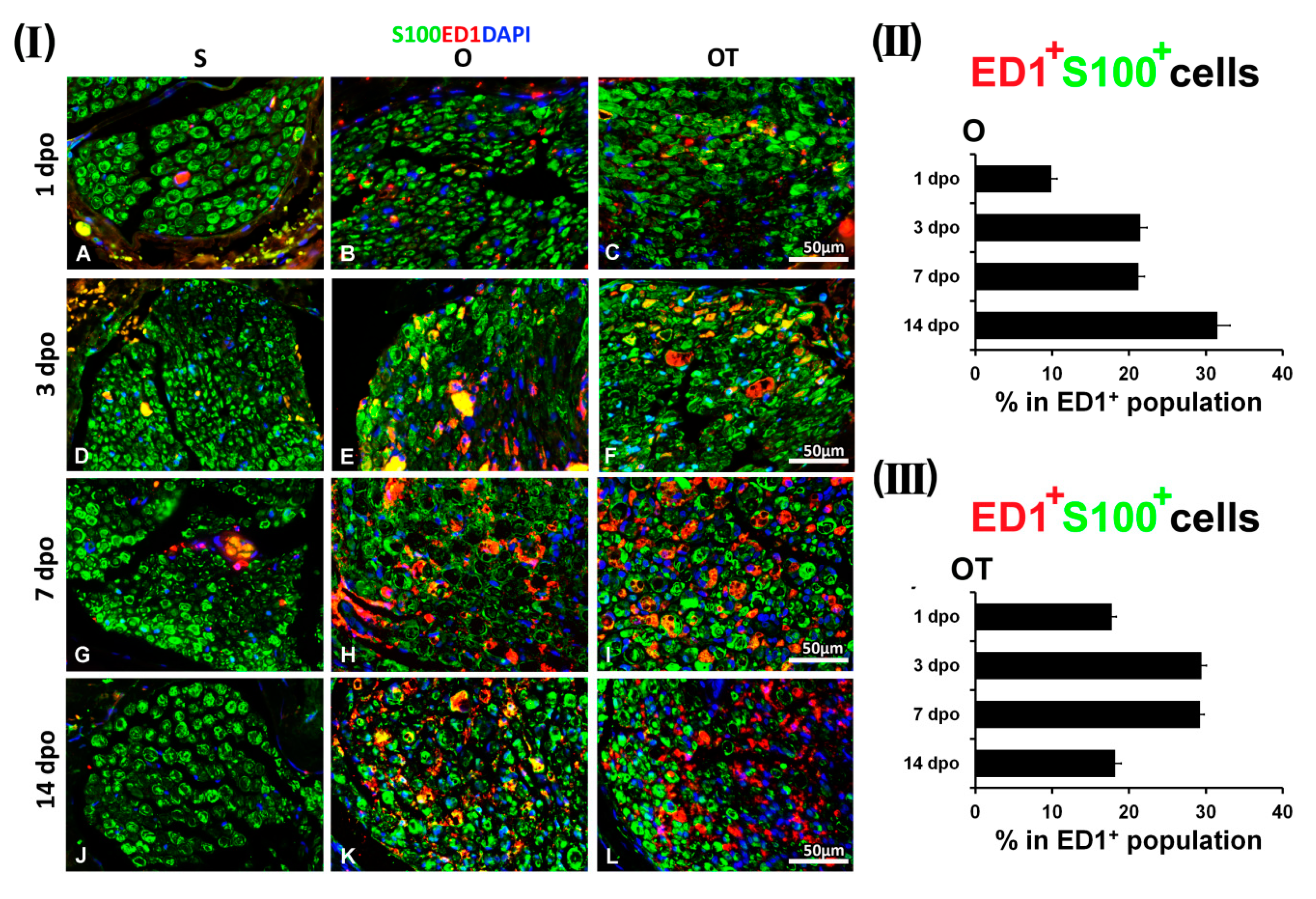
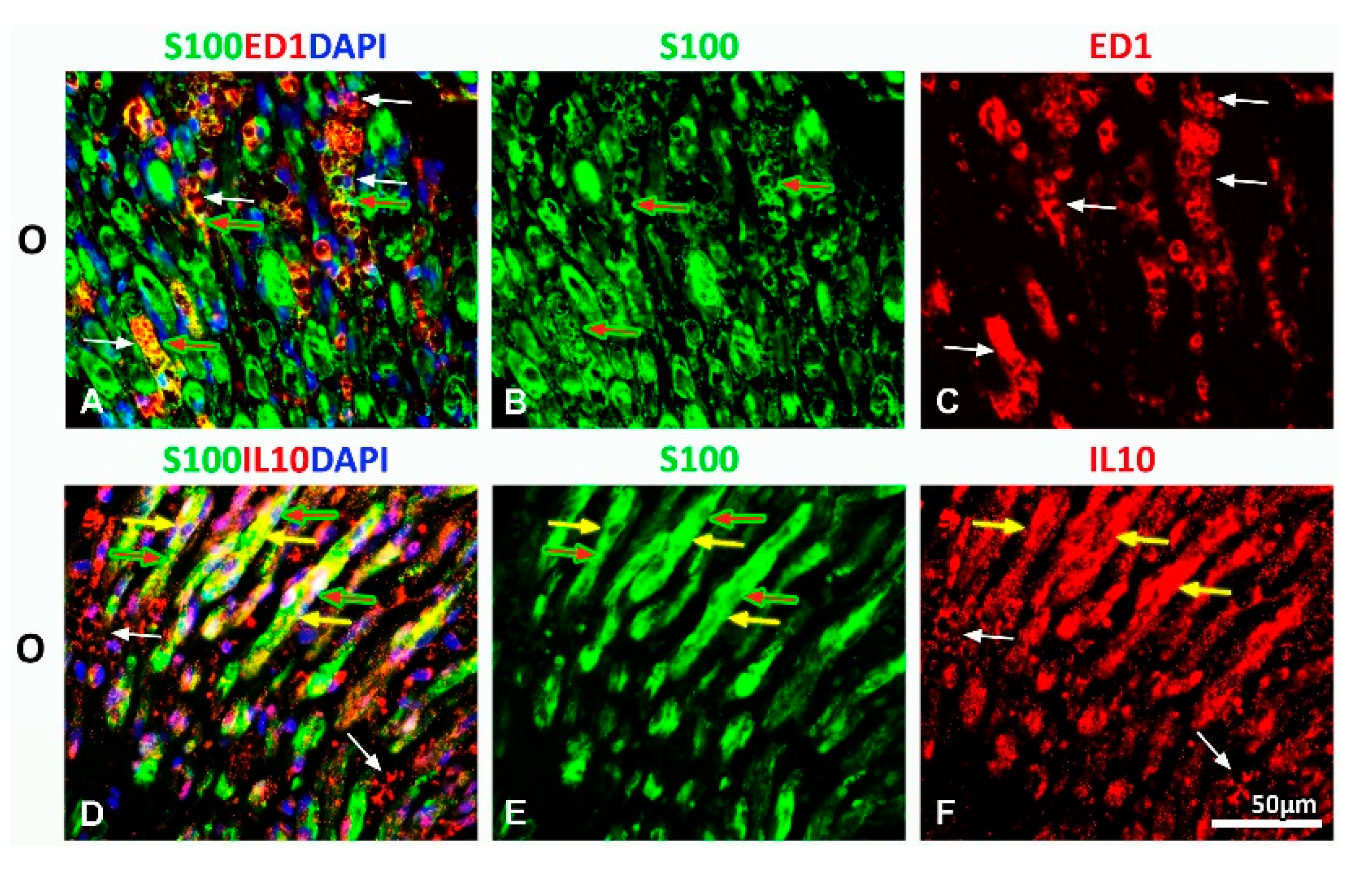
2.1. Treatment with Vitamin B Complex Altered Macrophages/Schwann Cells Interaction during the Recovery Period after PNI
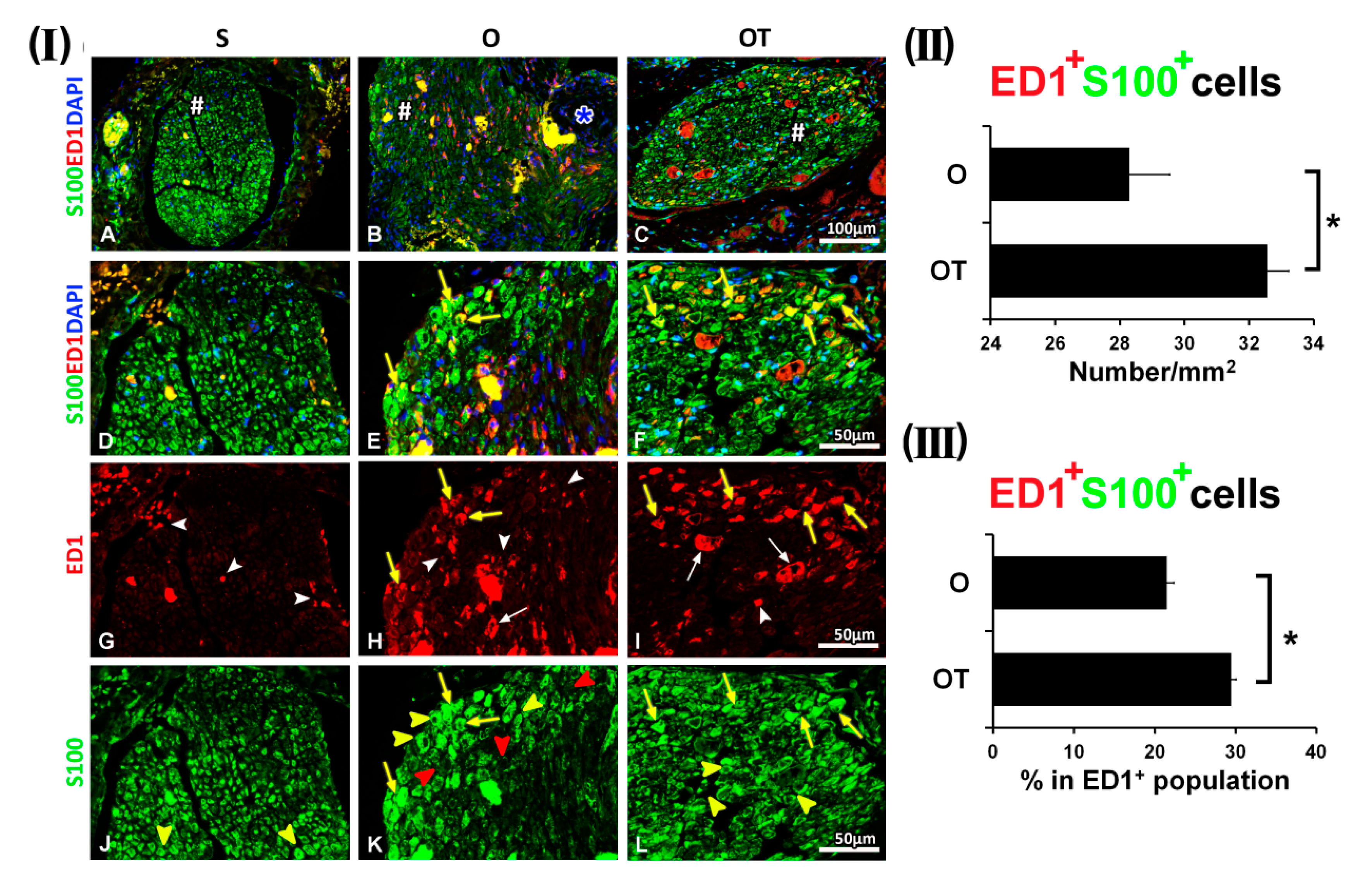
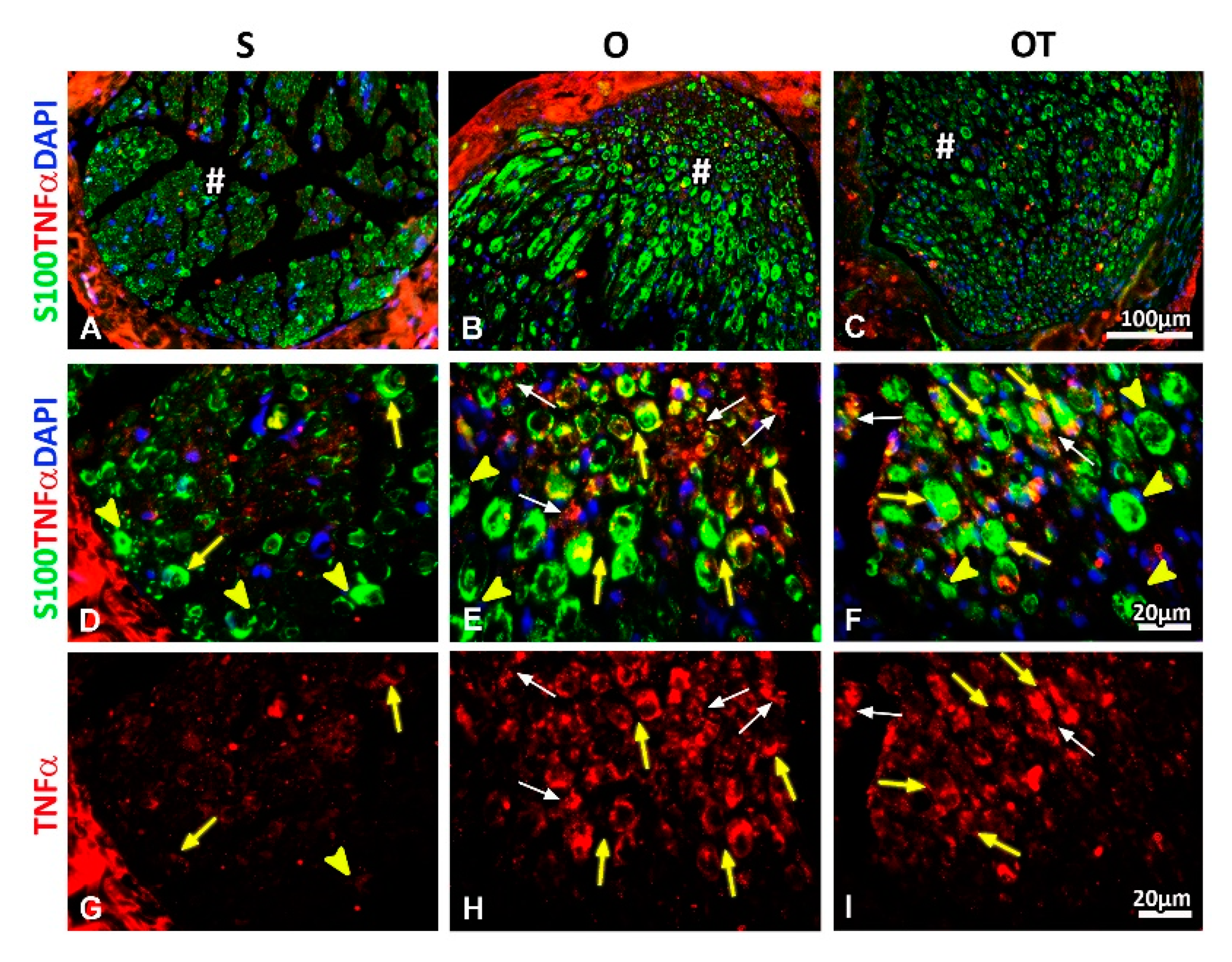
2.2. Administration of the Vitamin B Complex Reduced the Expression of Proinflammatory Cytokine TNF-α in SCs after the PNI
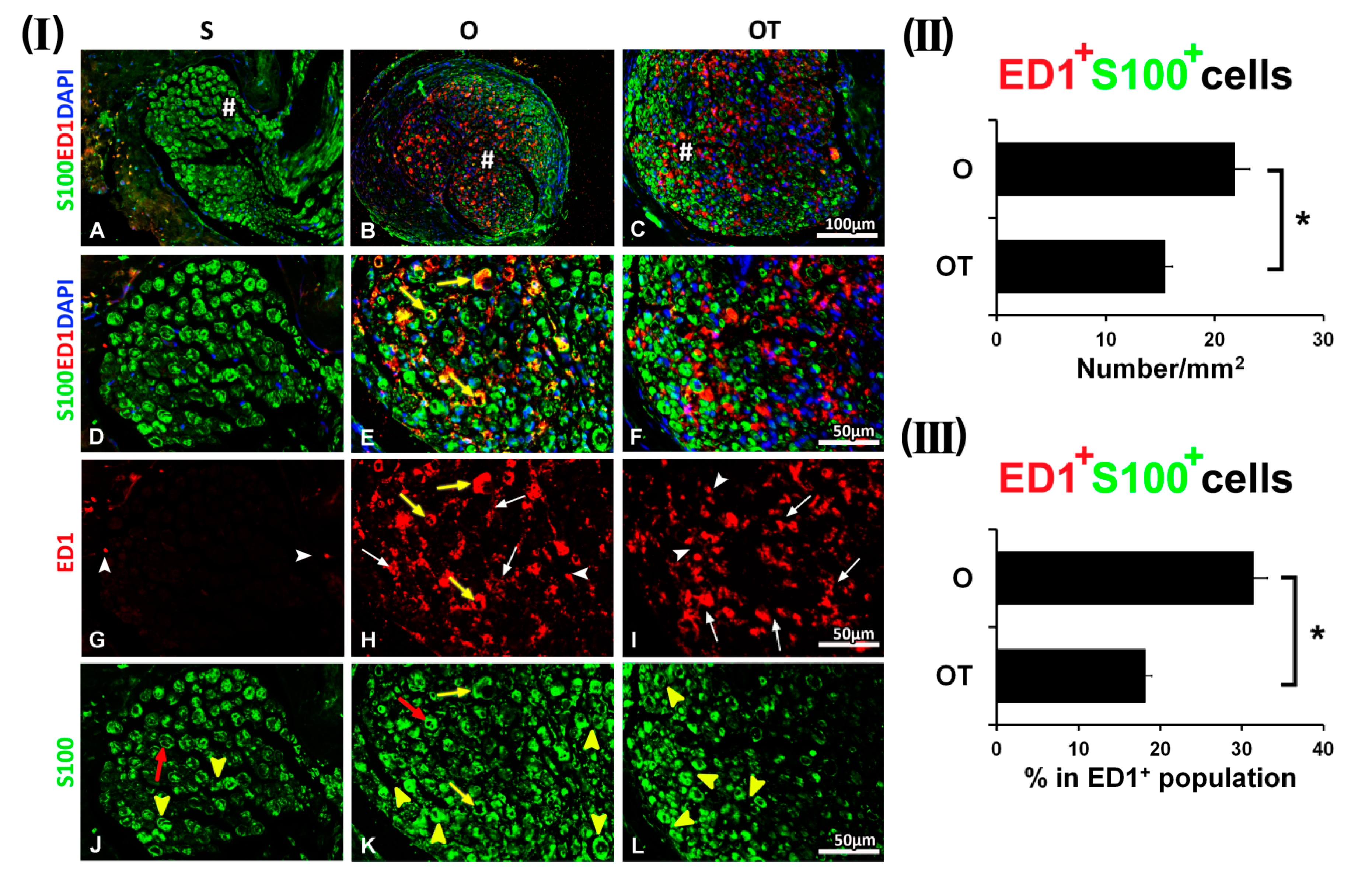
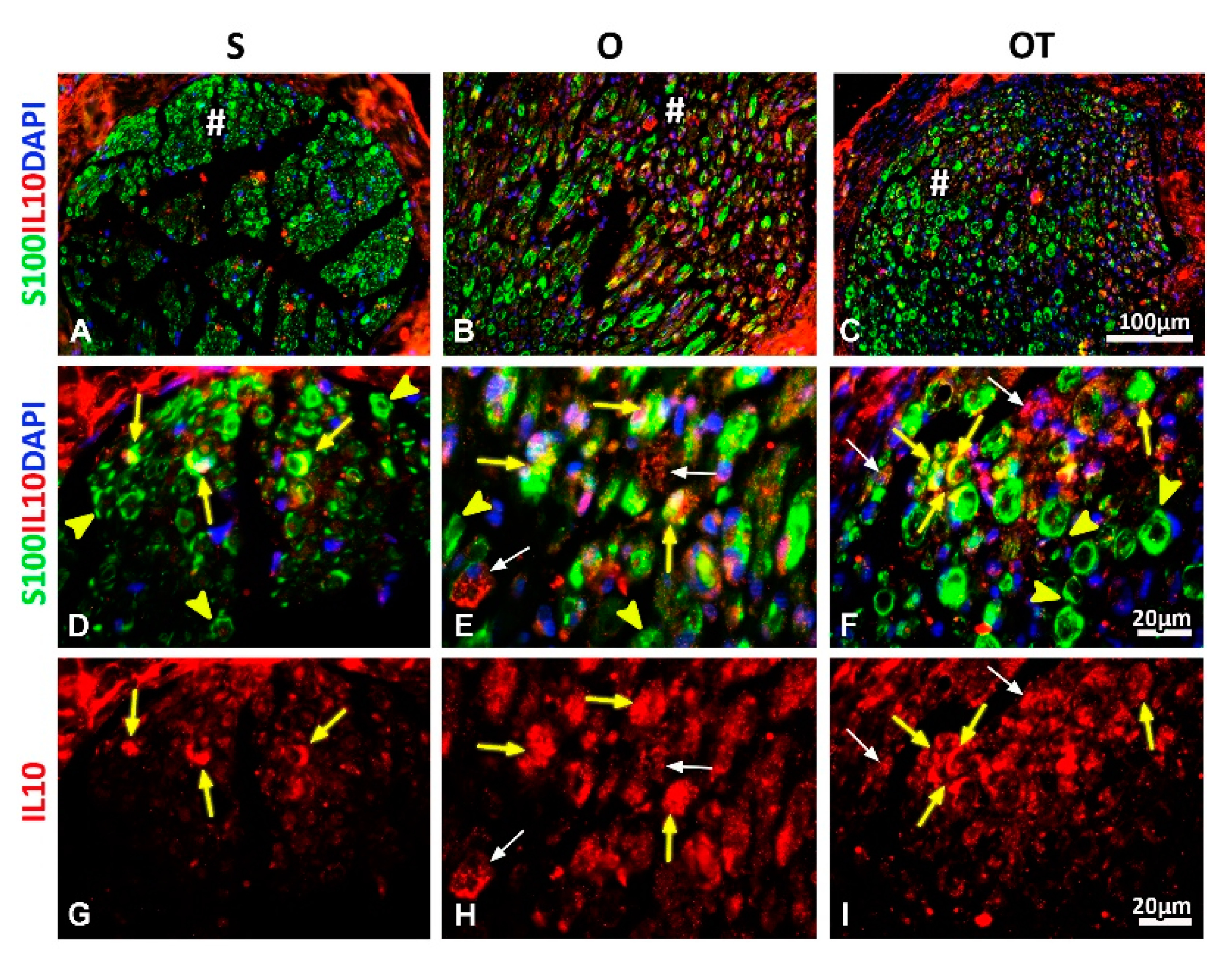
2.3. Effect of PNI and the Vitamin B Complex Treatment on the Expression of Anti-Inflammatory Cytokine IL-10 in SCs
3. Discussion
4. Materials and Methods
4.1. Ethical Approval and Consent to Participate
4.2. Femoral Nerve Injury Rat Model
4.3. Protocol for Vitamin B Complex Treatment
4.4. Femoral Nerve Processing Procedure for Immunofluorescence Staining
4.5. Procedure of Immunofluorescence Staining and Digital Image Processing
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ciardelli, G.; Chiono, V. Materials for peripheral nerve regeneration. Macromol. Biosci. 2006, 6, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Bigbee, J.W.; Yoshino, J.E.; DeVries, G.H. Morphological and proliferative responses of cultured Schwann cells following rapid phagocytosis of a myelin-enriched fraction. J. Neurocytol. 1987, 16, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Stoll, G.; Griffin, J.; Li, C.Y.; Trapp, B. Wallerian degeneration in the peripheral nervous system: Participation of both Schwann cells and macrophages in myelin degradation. J. Neurocytol. 1989, 18, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Hirata, K.; Kawabuchi, M. Myelin phagocytosis by macrophages and nonmacrophages during Wallerian degeneration. Microsc. Res. Tech. 2002, 57, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Tofaris, G.K.; Patterson, P.H.; Jessen, K.R.; Mirsky, R. Denervated Schwann cells attract macrophages by secretion of leukemia inhibitory factor (LIF) and monocyte chemoattractant protein-1 in a process regulated by interleukin-6 and LIF. J. Neurosci. 2002, 22, 6696–6703. [Google Scholar] [CrossRef] [PubMed]
- Fleur, M.L.; Underwood, J.L.; Rappolee, D.A.; Werb, Z. Basement membrane and repair of injury to peripheral nerve: Defining a potential role for macrophages, matrix metalloproteinases, and tissue inhibitor of metalloproteinases-1. J. Exp. Med. 1996, 184, 2311–2326. [Google Scholar] [CrossRef]
- Barrette, B.; Hébert, M.-A.; Filali, M.; Lafortune, K.; Vallieres, N.; Gowing, G.; Julien, J.-P.; Lacroix, S. Requirement of myeloid cells for axon regeneration. J. Neurosci. 2008, 28, 9363–9376. [Google Scholar] [CrossRef]
- Stratton, J.A.; Shah, P.T. Macrophage polarization in nerve injury: Do Schwann cells play a role? Neural Regen. Res. 2016, 11, 53. [Google Scholar] [CrossRef]
- Brown, H.C. Macrophages and the Nervous System. Ph.D. Thesis, University of Oxford, Oxford, UK, 1996. [Google Scholar]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef]
- Kolter, J.; Kierdorf, K.; Henneke, P. Origin and differentiation of nerve-associated macrophages. J. Immunol. 2020, 204, 271–279. [Google Scholar] [CrossRef]
- Pan, B.; Shi, Z.-J.; Yan, J.-Y.; Li, J.-H.; Feng, S.-Q. Long non-coding RNA NONMMUG014387 promotes Schwann cell proliferation after peripheral nerve injury. Neural Regen. Res. 2017, 12, 2084. [Google Scholar] [PubMed]
- Lutz, A.B.; Barres, B.A. Contrasting the glial response to axon injury in the central and peripheral nervous systems. Dev. Cell 2014, 28, 7–17. [Google Scholar]
- Scholz, T.; Krichevsky, A.; Sumarto, A.; Jaffurs, D.; Wirth, G.A.; Paydar, K.; Evans, G.R. Peripheral nerve injuries: An international survey of current treatments and future perspectives. J. Reconstr. Microsurg. 2009, 25, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.-C.; Yang, D.-Y.; Ho, S.-P.; Sheu, M.-L.; Chen, C.-J.; Hwang, S.-M.; Chang, M.-H.; Cheng, F.-C. Escalated regeneration in sciatic nerve crush injury by the combined therapy of human amniotic fluid mesenchymal stem cells and fermented soybean extracts, Natto. J. Biomed. Sci. 2009, 16, 75. [Google Scholar] [CrossRef] [PubMed]
- Wiberg, M.; Terenghi, G. Will it be possible to produce peripheral nerves? Surg. Technol. Int. 2003, 11, 303–310. [Google Scholar] [PubMed]
- Fernández-Villa, D.; Jiménez Gómez-Lavín, M.; Abradelo, C.; San Román, J.; Rojo, L. Tissue engineering therapies based on folic acid and other vitamin B derivatives. Functional mechanisms and current applications in regenerative medicine. Int. J. Mol. Sci. 2018, 19, 4068. [Google Scholar] [CrossRef] [PubMed]
- Altun, I.; Kurutaş, E.B. Vitamin B complex and vitamin B12 levels after peripheral nerve injury. Neural Regen. Res. 2016, 11, 842. [Google Scholar] [CrossRef]
- Nedeljković, P.; Zmijanjac, D.; Drašković-Pavlović, B.; Vasiljevska, M.; Vučević, D.; Božić, B.; Bumbaširević, M. Vitamin B complex treatment improves motor nerve regeneration and recovery of muscle function in a rodent model of peripheral nerve injury. Arch. Biol. Sci. 2017, 69, 361–368. [Google Scholar] [CrossRef]
- Ehmedah, A.; Nedeljkovic, P.; Dacic, S.; Repac, J.; Draskovic Pavlovic, B.; Vucevic, D.; Pekovic, S.; Bozic Nedeljkovic, B. Vitamin B Complex Treatment Attenuates Local Inflammation after Peripheral Nerve Injury. Molecules 2019, 24, 4615. [Google Scholar] [CrossRef]
- Liao, W.-C.; Wang, Y.-J.; Huang, M.-C.; Tseng, G.-F. Methylcobalamin facilitates collateral sprouting of donor axons and innervation of recipient muscle in end-to-side neurorrhaphy in rats. PLoS ONE 2013, 8, e76302. [Google Scholar] [CrossRef]
- Wang, Z.-B.; Gan, Q.; Rupert, R.L.; Zeng, Y.-M.; Song, X.-J. Thiamine, pyridoxine, cyanocobalamin and their combination inhibit thermal, but not mechanical hyperalgesia in rats with primary sensory neuron injury. Pain 2005, 114, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Fujii, A.; Matsumoto, H.; Yamamoto, H. Effect of vitamin B complex on neurotransmission and neurite outgrowth. Gen. Pharmacol. Vasc. Syst. 1996, 27, 995–1000. [Google Scholar] [CrossRef]
- Shearman, J.D.; Franks, A.J. S-100 protein in Schwann cells of the developing human peripheral nerve. Cell Tissue Res. 1987, 249, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Mata, M.; Alessi, D.; Fink, D.J. S100 is preferentially distributed in myelin-forming Schwann cells. J. Neurocytol. 1990, 19, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jin, Y.-Q.; Chen, L.; Wang, Y.; Yang, X.; Cheng, J.; Wu, W.; Qi, Z.; Shen, Z. Specific marker expression and cell state of Schwann cells during culture in vitro. PLoS ONE 2015, 10, e0123278. [Google Scholar] [CrossRef]
- Donnelly, C.J.; Park, M.; Spillane, M.; Yoo, S.; Pacheco, A.; Gomes, C.; Vuppalanchi, D.; McDonald, M.; Kim, H.H.; Merianda, T.T. Axonally synthesized β-actin and GAP-43 proteins support distinct modes of axonal growth. J. Neurosci. 2013, 33, 3311–3322. [Google Scholar] [CrossRef]
- Curtis, R.; Stewart, H.; Hall, S.M.; Wilkin, G.P.; Mirsky, R.; Jessen, K.R. GAP-43 is expressed by nonmyelin-forming Schwann cells of the peripheral nervous system. J. Cell Biol. 1992, 116, 1455–1464. [Google Scholar] [CrossRef]
- Sensenbrenner, M.; Lucas, M.; Deloulme, J.-C. Expression of two neuronal markers, growth-associated protein 43 and neuron-specific enolase, in rat glial cells. J. Mol. Med. 1997, 75, 653–663. [Google Scholar] [CrossRef]
- Irintchev, A. Potentials and limitations of peripheral nerve injury models in rodents with particular reference to the femoral nerve. Ann. Anat. Anat. Anz. 2011, 193, 276–285. [Google Scholar] [CrossRef]
- Rotshenker, S. Wallerian degeneration: The innate-immune response to traumatic nerve injury. J. Neuroinflamm. 2011, 8, 109. [Google Scholar] [CrossRef]
- Lubińska, L. Early course of Wallerian degeneration in myelinated fibres of the rat phrenic nerve. Brain Res. 1977, 130, 47–63. [Google Scholar] [CrossRef]
- Luttges, M.W.; Kelly, P.T.; Gerren, R.A. Degenerative changes in mouse sciatic nerves: Electrophoretic and electrophysiologic characterizations. Exp. Neurol. 1976, 50, 706–733. [Google Scholar] [CrossRef]
- Tsao, J.W.; George, E.B.; Griffin, J.W. Temperature modulation reveals three distinct stages of Wallerian degeneration. J. Neurosci. 1999, 19, 4718–4726. [Google Scholar] [CrossRef] [PubMed]
- Stratton, J.A.; Holmes, A.; Rosin, N.L.; Sinha, S.; Vohra, M.; Burma, N.E.; Trang, T.; Midha, R.; Biernaskie, J. Macrophages regulate Schwann cell maturation after nerve injury. Cell Rep. 2018, 24, 2561–2572.e6. [Google Scholar] [CrossRef]
- Jessen, K.; Mirsky, R. The repair Schwann cell and its function in regenerating nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef]
- Griffin, J.W.; Thompson, W.J. Biology and pathology of nonmyelinating Schwann cells. Glia 2008, 56, 1518–1531. [Google Scholar] [CrossRef]
- Armati, P.J.; Mathey, E.K. An update on Schwann cell biology—immunomodulation, neural regulation and other surprises. J. Neurol. Sci. 2013, 333, 68–72. [Google Scholar] [CrossRef]
- Al-saaeed, S.M.; Al-khalisy, M.H. The Regenerative Role of Vitamins B1, B6, B12 in Treatment of Peripheral Neuropathy. Int. J. Sci. Res. 2017, 6, 2411–2415. [Google Scholar]
- Nishimoto, S.; Tanaka, H.; Okamoto, M.; Okada, K.; Murase, T.; Yoshikawa, H. Methylcobalamin promotes the differentiation of Schwann cells and remyelination in lysophosphatidylcholine-induced demyelination of the rat sciatic nerve. Front. Cell. Neurosci. 2015, 9, 298. [Google Scholar] [CrossRef]
- Kang, W.-B.; Chen, Y.-J.; Lu, D.-Y.; Yan, J.-Z. Folic acid contributes to peripheral nerve injury repair by promoting Schwann cell proliferation, migration, and secretion of nerve growth factor. Neural Regen. Res. 2019, 14, 132. [Google Scholar]
- Taskinen, H.; Olsson, T.; Bucht, A.; Khademi, M.; Svelander, L.; Röyttä, M. Peripheral nerve injury induces endoneurial expression of IFN-γ, IL-10 and TNF-α mRNA. J. Neuroimmunol. 2000, 102, 17–25. [Google Scholar] [CrossRef]
- Okamoto, K.; Martin, D.P.; Schmelzer, J.D.; Mitsui, Y.; Low, P.A. Pro-and anti-inflammatory cytokine gene expression in rat sciatic nerve chronic constriction injury model of neuropathic pain. Exp. Neurol. 2001, 169, 386–391. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Kleinschnitz, C.; Zelenka, M.; Brinkhoff, J.; Stoll, G.; Sommer, C. Wallerian degeneration after crush or chronic constriction injury of rodent sciatic nerve is associated with a depletion of endoneurial interleukin-10 protein. Exp. Neurol. 2004, 188, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Sawada, T.; Sano, M.; Omura, T.; Omura, K.; Hasegawa, T.; Funahashi, S.; Nagano, A. Spatiotemporal quantification of tumor necrosis factor-alpha and interleukin-10 after crush injury in rat sciatic nerve utilizing immunohistochemistry. Neurosci. Lett. 2007, 417, 55–60. [Google Scholar] [CrossRef]
- Vidal, P.M.; Lemmens, E.; Dooley, D.; Hendrix, S. The role of “anti-inflammatory” cytokines in axon regeneration. Cytokine Growth Factor Rev. 2013, 24, 1–12. [Google Scholar] [CrossRef]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef]
- Wang, N.; Liang, H.; Zen, K. Molecular mechanisms that influence the macrophage M1–M2 polarization balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef]
- Chen, P.; Piao, X.; Bonaldo, P. Role of macrophages in Wallerian degeneration and axonal regeneration after peripheral nerve injury. Acta Neuropathol. 2015, 130, 605–618. [Google Scholar] [CrossRef]
- Rőszer, T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediat. Inflamm. 2015, 2015, 816460. [Google Scholar] [CrossRef]
- Dubový, P.; Klusáková, I.; Hradilová Svíženská, I. Inflammatory profiling of Schwann cells in contact with growing axons distal to nerve injury. Biomed Res. Int. 2014, 2014. [Google Scholar] [CrossRef][Green Version]
- Menorca, R.M.; Fussell, T.S.; Elfar, J.C. Peripheral nerve trauma: Mechanisms of injury and recovery. Hand Clin. 2013, 29, 317. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. It is commercial product Beviplex (Beviplex®, Galenika a.d. Belgrade, Serbia) and it is written in the Section 4.3. Protocol for Vitamin B Complex Treatment. |

| Antibodies | Dilution | Company |
|---|---|---|
| Mouse monoclonal anti-CD68 (Clone ED1) | 1:100 | Abcam, Cambridge, MA, USA |
| Goat monoclonal anti-TNF-α | 1:100 | Santa Cruz Biotechnology, CA, USA |
| Goat monoclonal anti-IL-10 | 1:100 | Santa Cruz Biotechnology, CA, USA |
| Mouse monoclonal anti-S100 | 1:200 | Chemicon International, CA, USA |
| Rabbit monoclonal anti-S100 | 1:200 | Bio-Rad Laboratories, CA, USA |
| Rabbit monoclonal anti-GAP43 | 1:200 | Millipore, Darmstadt, Germany |
| Donkey anti-goat IgG (Alexa Fluor 555) | 1:200 | Invitrogen, Carlsbad, CA, USA |
| Donkey anti-rabbit IgG (Alexa Fluor 488) | 1:200 | Invitrogen, Carlsbad, CA, USA |
| Donkey anti-rabbit IgG (Alexa Flour 555) | 1:200 | Invitrogen, Carlsbad, CA, USA |
| Donkey anti-mouse IgG (Alexa Fluor 488) | 1:200 | Invitrogen, Carlsbad, CA, USA |
| Donkey anti-mouse IgG (Alexa Fluor 555) | 1:200 | Invitrogen, Carlsbad, CA, USA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehmedah, A.; Nedeljkovic, P.; Dacic, S.; Repac, J.; Draskovic-Pavlovic, B.; Vučević, D.; Pekovic, S.; Nedeljkovic, B.B. Effect of Vitamin B Complex Treatment on Macrophages to Schwann Cells Association during Neuroinflammation after Peripheral Nerve Injury. Molecules 2020, 25, 5426. https://doi.org/10.3390/molecules25225426
Ehmedah A, Nedeljkovic P, Dacic S, Repac J, Draskovic-Pavlovic B, Vučević D, Pekovic S, Nedeljkovic BB. Effect of Vitamin B Complex Treatment on Macrophages to Schwann Cells Association during Neuroinflammation after Peripheral Nerve Injury. Molecules. 2020; 25(22):5426. https://doi.org/10.3390/molecules25225426
Chicago/Turabian StyleEhmedah, Adil, Predrag Nedeljkovic, Sanja Dacic, Jelena Repac, Biljana Draskovic-Pavlovic, Dragana Vučević, Sanja Pekovic, and Biljana Bozic Nedeljkovic. 2020. "Effect of Vitamin B Complex Treatment on Macrophages to Schwann Cells Association during Neuroinflammation after Peripheral Nerve Injury" Molecules 25, no. 22: 5426. https://doi.org/10.3390/molecules25225426
APA StyleEhmedah, A., Nedeljkovic, P., Dacic, S., Repac, J., Draskovic-Pavlovic, B., Vučević, D., Pekovic, S., & Nedeljkovic, B. B. (2020). Effect of Vitamin B Complex Treatment on Macrophages to Schwann Cells Association during Neuroinflammation after Peripheral Nerve Injury. Molecules, 25(22), 5426. https://doi.org/10.3390/molecules25225426






