Design and Synthesis of New Benzo[d]oxazole-Based Derivatives and Their Neuroprotective Effects on β-Amyloid-Induced PC12 Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Effects of Compounds on Cell Viability of Aβ25-35-Induced PC12 Cells and Selection of Active Compounds
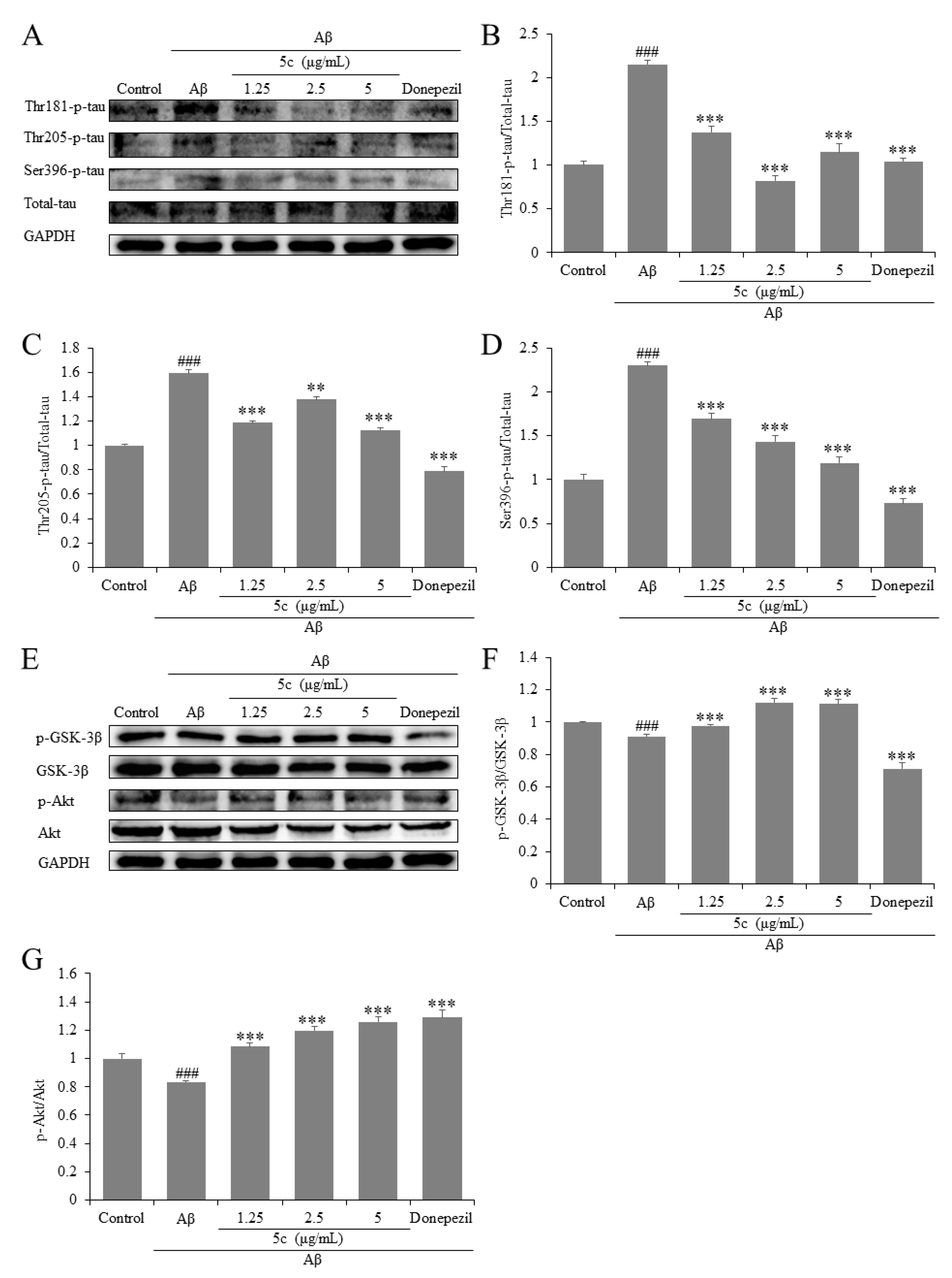
2.3. Compound 5c Reduced Tau Phosphorylation, Akt and GSK-3β Activation in Aβ25-35-Induced PC12 Cells
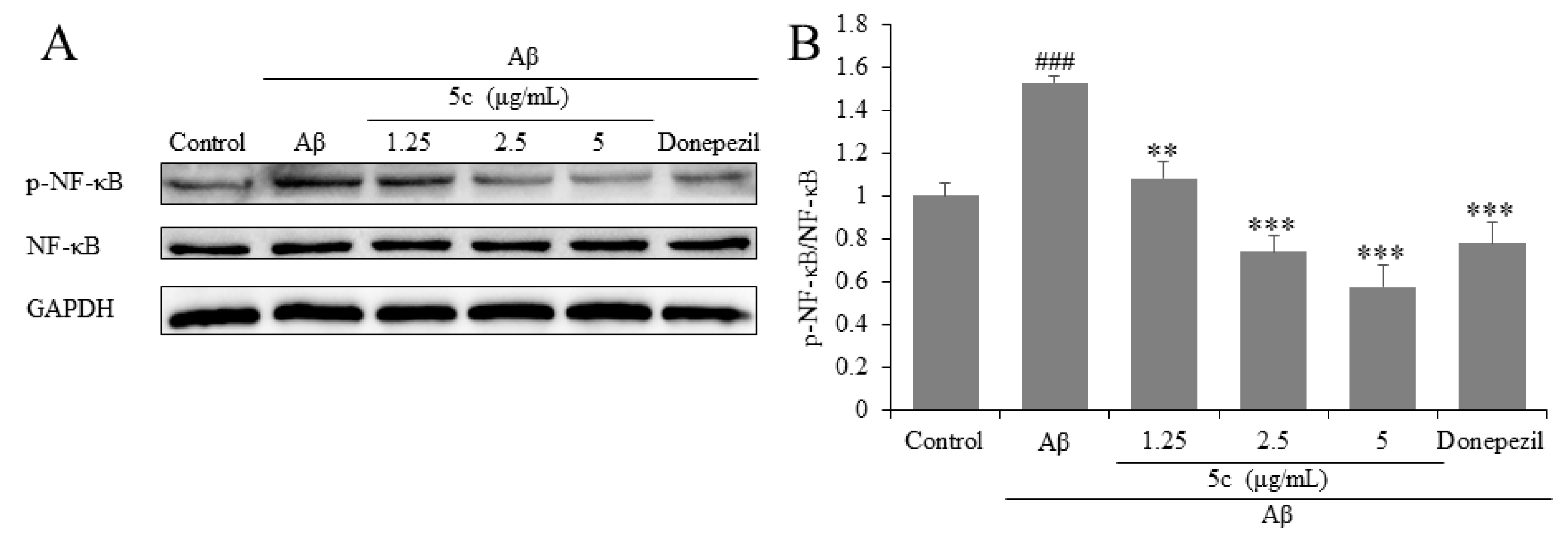
2.4. Compound 5c Inhibited Expression of NF-κB in Aβ25-35-Induced PC12 Cells
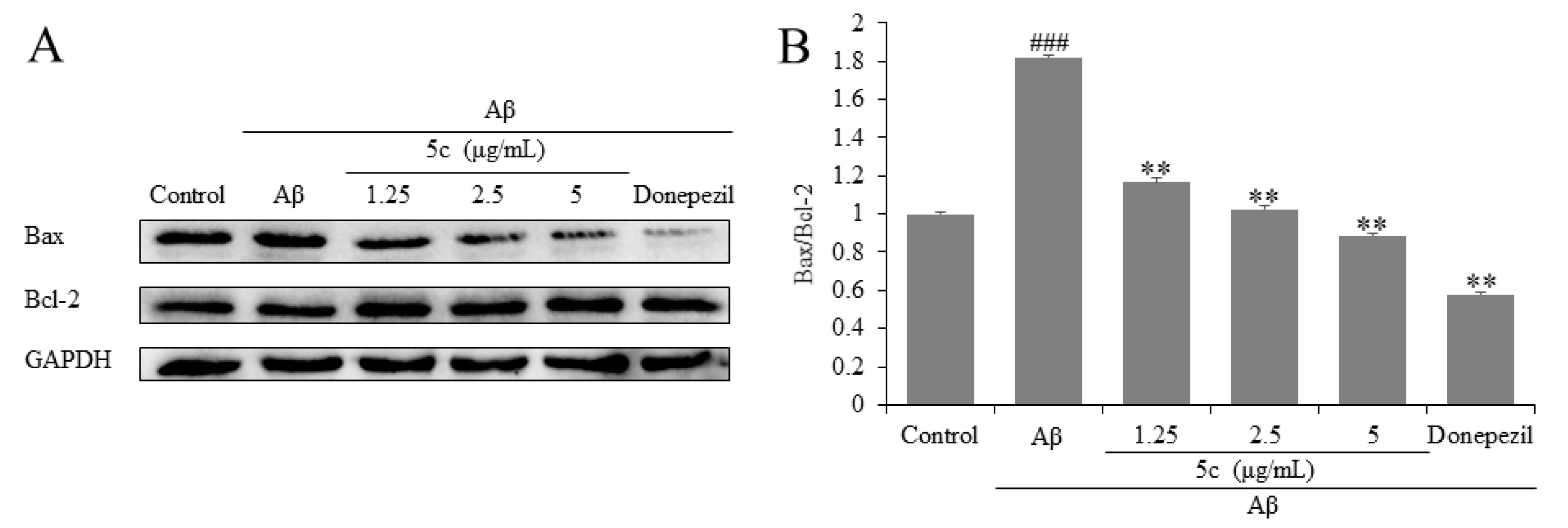
2.5. Compound 5c Inhibited Expression of Bax and Bcl-2 in Aβ25-35-Induced PC12 Cells
2.6. Compound 5c Inhibited Expression of BACE1 in Aβ25-35-Induced PC12 Cells
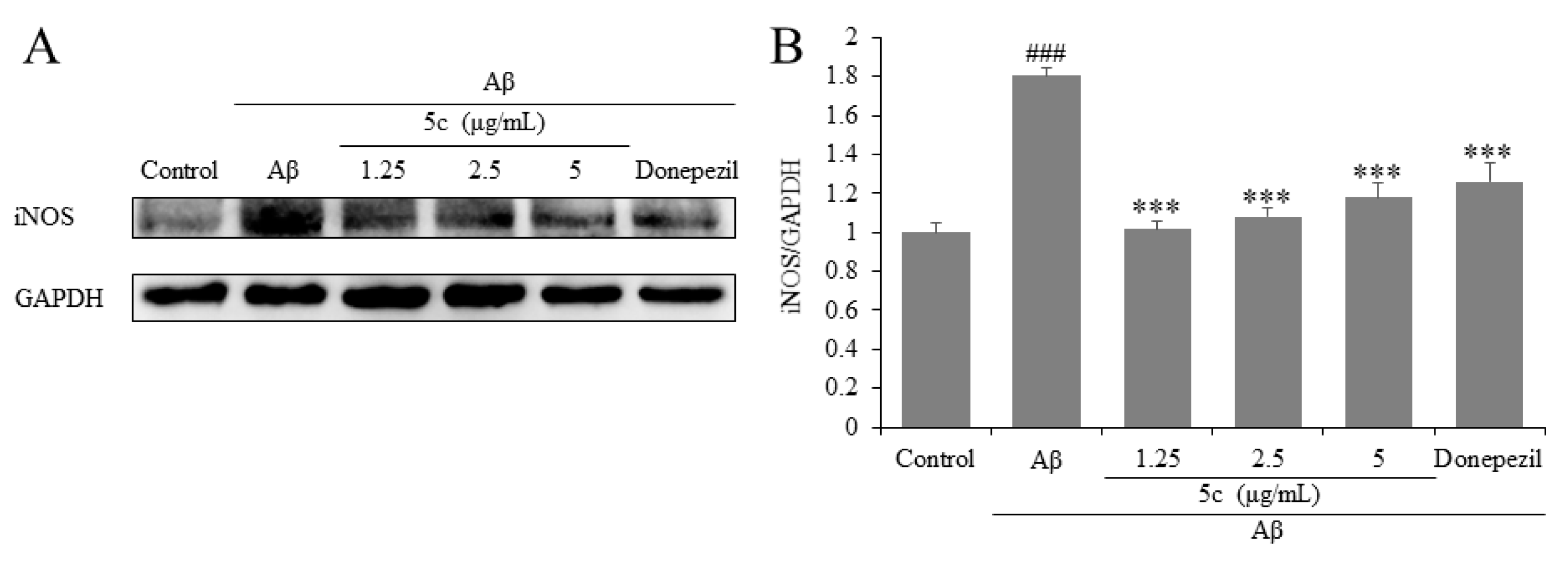
2.7. Compound 5c Inhibited Inflammation-Related Factors iNOS of Aβ25-35-Induced PC12 Cells
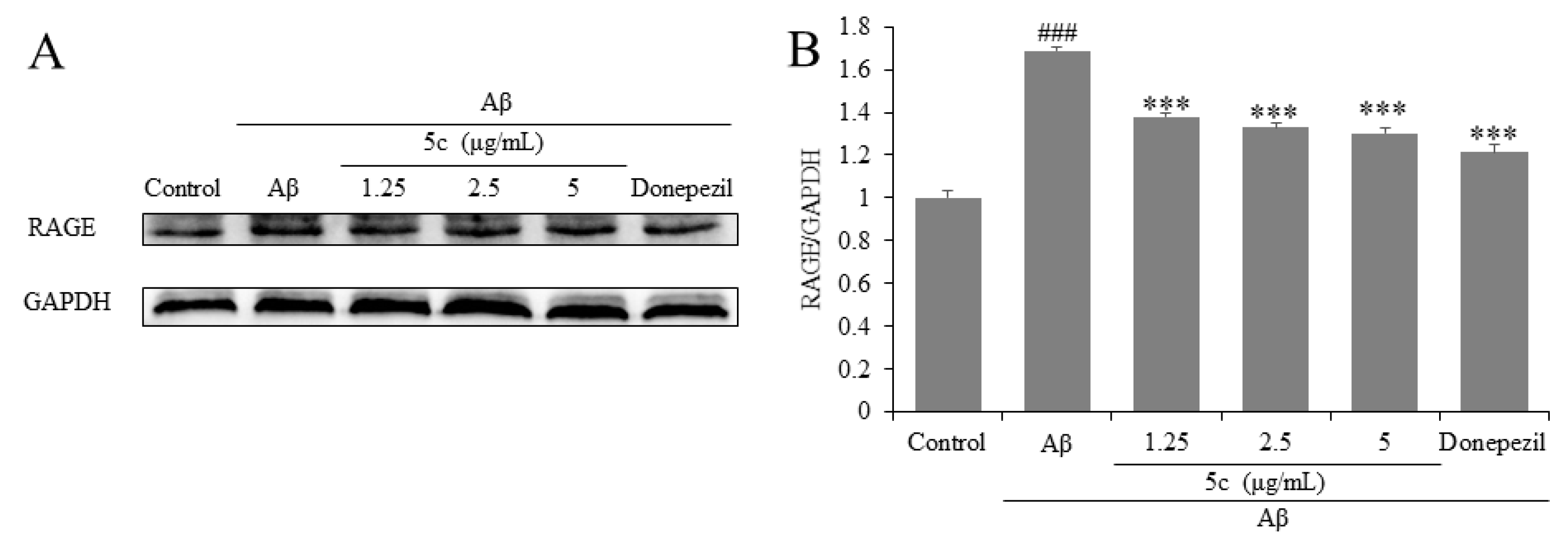
2.8. Compound 5c Inhibited Expression of RAGE of Aβ25-35-Induced PC12 Cells
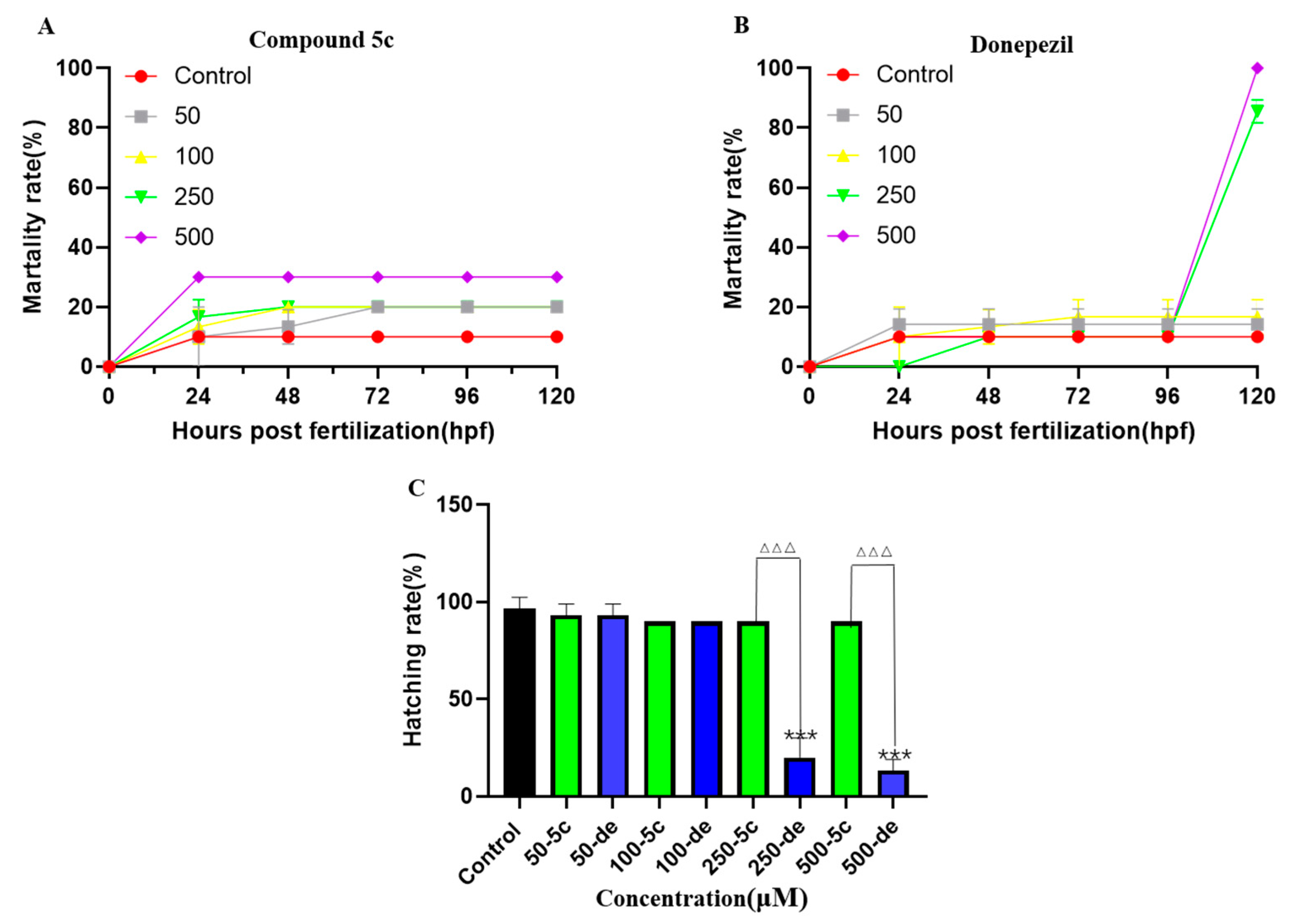
2.9. Effects of Compound 5c and Donepezil on Zebrafish Mortality and Hatching Rate
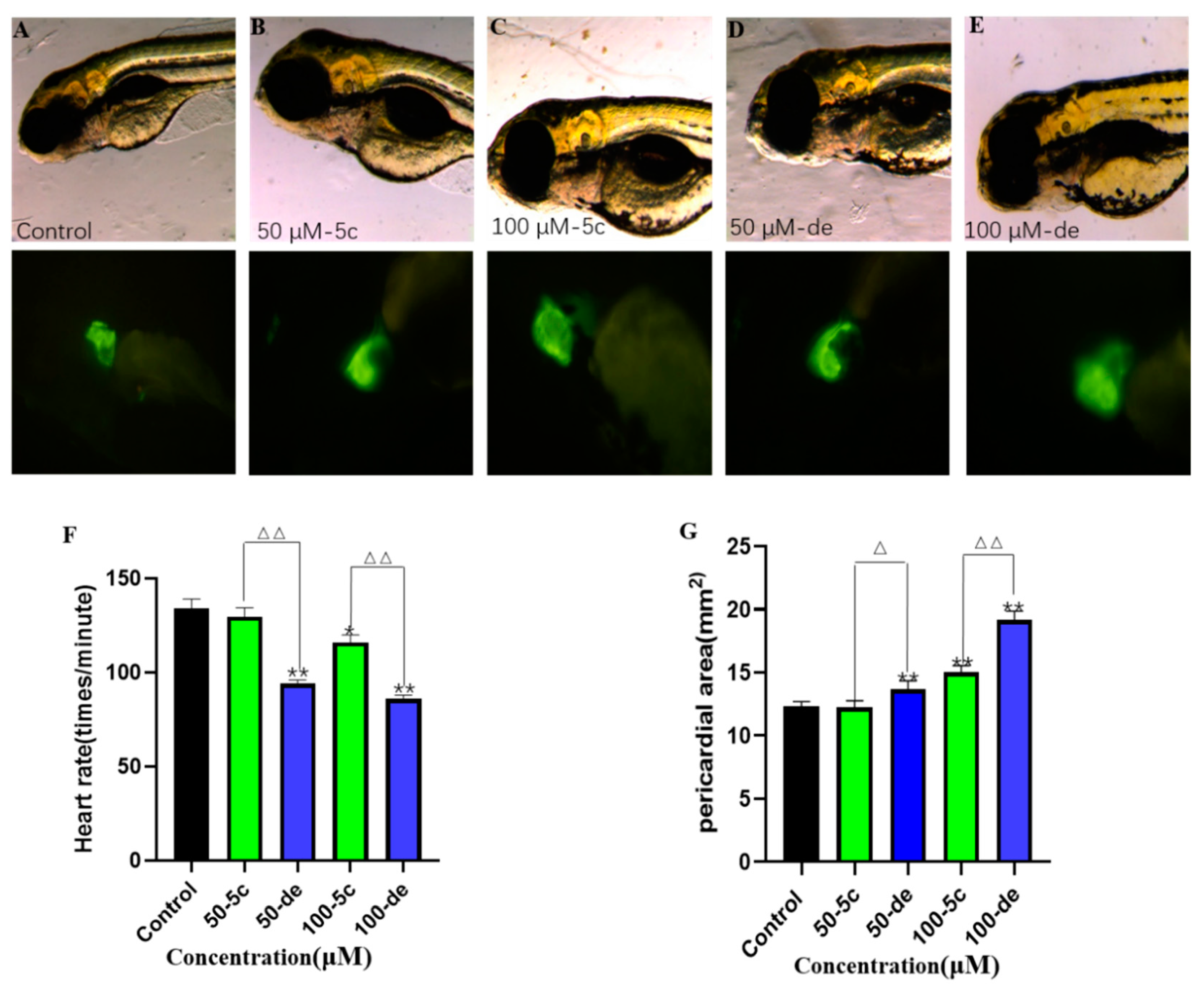
2.10. Effects of Compound 5c and Donepezil on Heart Rate
2.11. Effects of Compound 5c and Donepezil on the Pericardial Cavity Area
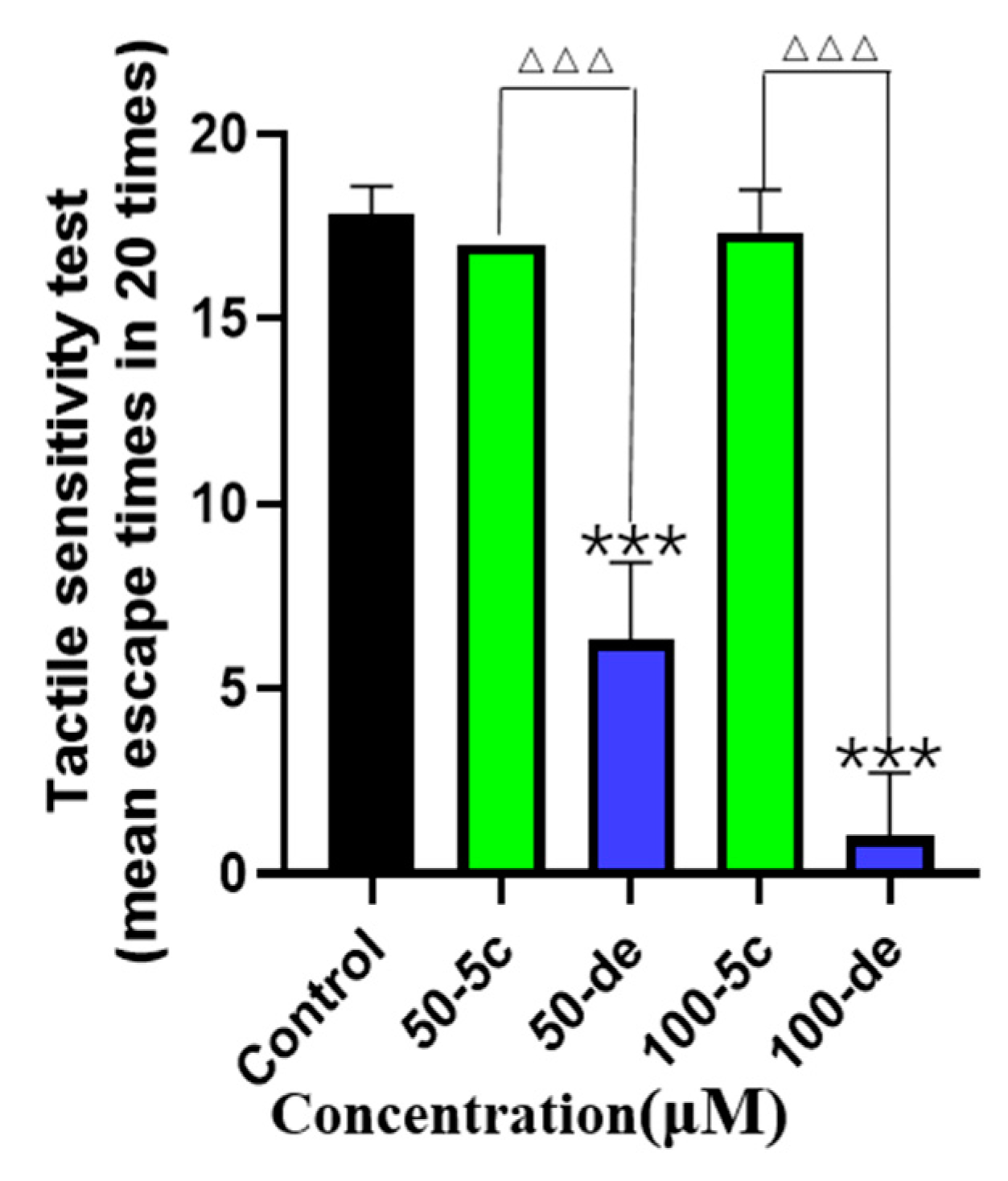
2.12. Effects of Compound 5c and Donepezil on Tactile Sensitivity
3. Experimental Section
3.1. Chemistry
3.2. General Procedure for the Preparation of Compound 2
3.3. General Procedure for the Synthesis of Compounds 4a–4v
3.4. General Procedure for the Synthesis of Compounds 5a–5v
3.5. Preparation of Aβ25-35
3.6. Cell Culture and Treatment
3.7. Cell Viability
3.8. Western Blotting Analysis
3.9. Zebrafish
3.10. Mortality
3.11. Measuring Area of Pericardial Cavity
3.12. Measuring Tactile Sensitivity
3.13. Statistical Analysis
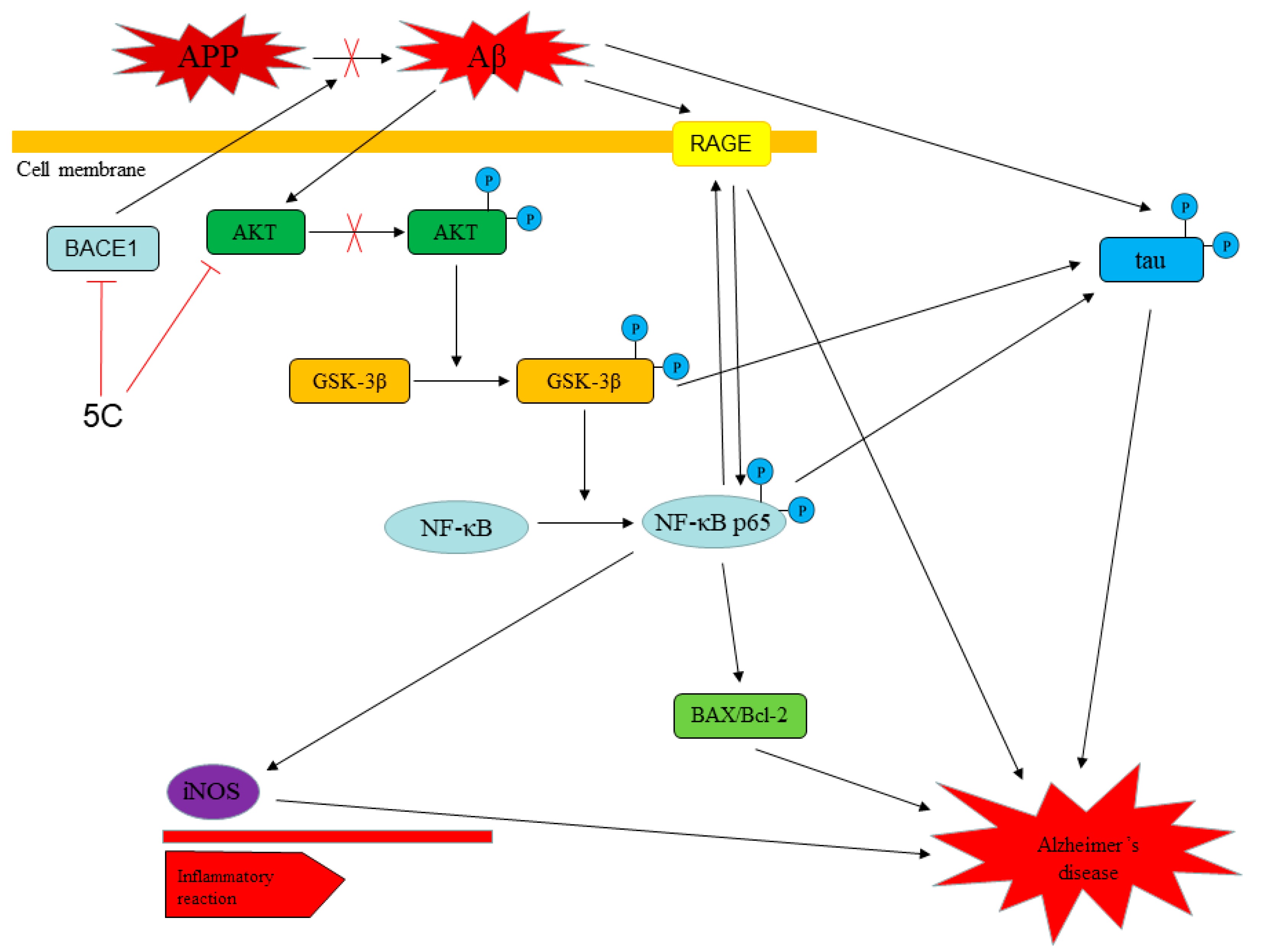
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Conti, P.; Dallanoce, C.; De Amici, M.; De Micheli, C.; Klotz, K.N. Synthesis of new delta 2-isoxazoline derivatives and their pharmacological characterization as beta-adrenergic receptor antagonists. Bioorg. Med. Chem. 1998, 6, 401–408. [Google Scholar] [CrossRef]
- Yatam, S.; Jadav, S.S.; Gundla, R.; Gundla, K.P.; Reddy, G.M.; Ahsan, M.J.; Chimakurthy, J. Design, synthesis and biological evaluation of 2-(((5-aryl-1,2,4-oxadiazol-3-yl)methyl)thio)benzo[d]oxazoles: New anti-inflammatory and antioxidant agents. Chemistryselect 2018, 3, 10305–10310. [Google Scholar] [CrossRef]
- Gaikar, R.B.; Gadhave, A.G.; Karale, B.K. Synthesis and antimicrobial screening of 2-(1-(2,4- difluorophenyl)-1W-pyrazol-4-yl) benzo[d]oxazole. Indian. J. Heterocy Chem. 2011, 20, 221–224. [Google Scholar]
- Wei, C.X.; Wu, D.; Sun, Z.G.; Chai, K.Y.; Quan, Z.S. Synthesis of 6-(3-substituted-4H-1,2,4-triazol-4-yl)-2-phenylbenzo[d]oxazoles as potential anticonvulsant agents. Med. Chem. Res. 2009, 19, 925–935. [Google Scholar] [CrossRef]
- Li, Y.; Geng, J.; Liu, Y.; Yu, S.; Zhao, G. Thiadiazole-a promising structure in medicinal chemistry. ChemMedChem 2013, 8, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Pouramiri, B.; Moghimi, S.; Mahdavi, M.; Nadri, H.; Moradi, A.; Tavakolinejad-Kermani, E.; Firoozpour, L.; Asadipour, A.; Foroumadi, A. Synthesis and anticholinesterase activity of new substituted benzo[d]oxazole-based derivatives. Chem. Biol. Drug Des. 2017, 89, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Gurjar, A.S.; Andrisano, V.; Simone, A.D.; Velingkar, V.S. Design, synthesis, in silico and in vitro screening of 1,2,4-thiadiazole analogues as non-peptide inhibitors of beta-secretase. Bioorg. Chem. 2014, 57, 90–98. [Google Scholar] [CrossRef]
- Najafi, Z.; Saeedi, M.; Mahdavi, M.; Sabourian, R.; Khanavi, M.; Tehrani, M.B.; Moghadam, F.H.; Edraki, N.; Karimpor-Razkenari, E.; Sharifzadeh, M.; et al. Design and synthesis of novel anti-Alzheimer’s agents: Acridine-chromenone and quinoline-chromenone hybrids. Bioorg. Chem. 2016, 67, 84–94. [Google Scholar] [CrossRef]
- Brusnikina, M.; Silyukov, O.; Chislov, M.; Volkova, T.; Proshin, A.; Mazur, A.; Tolstoy, P.; Terekhova, I. Effect of cyclodextrin complexation on solubility of novel anti-Alzheimer 1,2,4-thiadiazole derivative. J. Mol. Liq. 2017, 130, 443–450. [Google Scholar] [CrossRef]
- Wortmann, M. Dementia: A global health priority-highlights from an ADI and World Health Organization report. Alzheimers Res. Ther. 2012, 4, 40. [Google Scholar] [CrossRef]
- Gao, Y.; Tan, L.; Yu, J.T.; Tan, L. Tau in Alzheimer’s Disease: Mechanisms and Therapeutic Strategies. Curr. Alzheimer Res. 2018, 15, 283–300. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.W.; Liu, W.W.; Wu, Y.T.; Wu, Q.; Lu, H.Y.; Xu, Z.H.; Gao, H.Y.; Zhao, Q.C. Notopterygium incisum extract (NRE) rescues cognitive deficits in APP/PS1 Alzhneimer’s disease mice by attenuating amyloid-beta, tau, and neuroinflammation pathology. J. Ethnopharmacol. 2020, 249, 112433. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhou, F.; Wang, K.X.; Zhou, Y.Z.; Du, G.H.; Qin, X.M. Baicalein protects PC12 cells from Aβ25-35-induced cytotoxicity via inhibition of apoptosis and metabolic disorders. Life Sci. 2020, 248, 117471. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Jin, J.; Liu, Q.; Ma, D.; Li, J.; Zhang, Y.; Liu, Y. Optimization of Degradation Conditions with PRG, a Polysaccharide from Phellinus ribis, by RSM and the Neuroprotective Activity in PC12 Cells Damaged by Aβ25-35. Molecules 2019, 24, 3010. [Google Scholar] [CrossRef]
- Bowroju, S.K.; Mainali, N.; Ayyadevara, S.; Penthala, N.R.; Krishnamachari, S.; Kakraba, S.; Reis, R.J.S.; Crooks, P.A. Design and Synthesis of Novel Hybrid 8-Hydroxy Quinoline-Indole Derivatives as Inhibitors of Aβ Self-Aggregation and Metal Chelation-Induced Aβ Aggregation. Molecules 2020, 25, 3610. [Google Scholar] [CrossRef]
- Leroy, K.; Yilmaz, Z.; Brion, J.P. Increased level of active GSK-3beta in Alzheimer’s disease and accumulation in argyrophilic grains and in neurones at different stages of neurofibrillary degeneration. Neuropathol. Appl. Neurobiol. 2007, 33, 43–55. [Google Scholar] [CrossRef]
- Ly, P.T.; Wu, Y.; Zou, H.; Wang, R.; Zhou, W.; Kinoshita, A.; Zhang, M.; Yang, Y.; Cai, F.; Woodgett, J.; et al. Inhibition of GSK3beta-mediated BACE1 expression reduces Alzheimer-associated phenotypes. J. Clin. Investig. 2013, 123, 224–235. [Google Scholar] [CrossRef]
- Boissiere, F.; Hunot, S.; Faucheux, B.; Duyckaerts, C.; Hauw, J.J.; Agid, Y.; Hirsch, E.C. Nuclear translocation of NF-kappaB in cholinergic neurons of patients with Alzheimer’s disease. Neuroreport 1997, 8, 2849–2852. [Google Scholar] [CrossRef]
- Chen, S.; Jia, J. Tenuifolin Attenuates Amyloid-β42-Induced Neuroinflammation in Microglia through the NF-κB Signaling Pathway. J. Alzheimers Dis. 2020, 76, 195–205. [Google Scholar] [CrossRef]
- Avrahami, L.; Licht-Murava, A.; Eisenstein, M.; Eldar-Finkelman, H. GSK-3 inhibition: Achieving moderate efficacy with high selectivity. Biochim. Biophys. Acta 2013, 1834, 1410–1414. [Google Scholar] [CrossRef]
- McCorkell, K.A.; May, M.J. NEMO-binding domain peptide inhibition of inflammatory signal-induced NF-κB activation in vivo. Methods Mol. Biol. 2015, 1280, 505–525. [Google Scholar] [PubMed]
- Zhan, D.; Guo, L.; Zheng, L. Inhibition of the receptor for advanced glycation promotes proliferation and repair of human periodontal ligament fibroblasts in response to high glucose via the NF-κB signaling pathway. Arch. Oral Biol. 2018, 87, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Kiang, J.G.; Smith, J.T.; Cannon, G.; Anderson, M.N.; Ho, C.; Zhai, M.; Cui, W.; Xiao, M. Ghrelin, a novel therapy, corrects cytokine and NF-κB-AKT-MAPK network and mitigates intestinal injury induced by combined radiation and skin-wound trauma. Cell Biosci. 2020, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Zhou, W.; Liu, S.; Deng, Y.; Cai, F.; Tone, M.; Tone, Y.; Tong, Y.; Song, W. Increased NF-kappaB signalling up-regulates BACE1 expression and its therapeutic potential in Alzheimer’s disease. Int. J. Neuropsychopharmacol. 2012, 15, 77–90. [Google Scholar] [CrossRef]
- Tian, J.; Shi, J.; Zhang, L.; Yin, J.; Hu, Q.; Xu, Y.; Sheng, S.; Wang, P.; Ren, Y.; Wang, R.; et al. GEPT extract reduces Abeta deposition by regulating the balance between production and degradation of Abeta in APPV717I transgenic mice. Curr. Alzheimer Res. 2009, 6, 118–131. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, R.; Lin, Y.; Shi, P. Inhibition of NF-κB might enhance the protective role of roflupram on SH-SY5Y cells under amyloid β stimulation via PI3K/AKT/mTOR signaling pathway. Int. J. Neurosci. 2020, 7, 1–11. [Google Scholar] [CrossRef]
- Porquet, D.; Casadesús, G.; Bayod, S.; Vicente, A.; Canudas, A.M.; Vilaplana, J.; Pelegrí, C.; Sanfeliu, C.; Camins, A.; Pallàs, M.; et al. Dietary resveratrol prevents Alzheimer’s markers and increases life span in SAMP8. Age 2013, 35, 1851–1865. [Google Scholar] [CrossRef]
- Beurel, E.; Grieco, S.F.; Jope, R.S. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmacol. Ther. 2015, 148, 114–131. [Google Scholar] [CrossRef]
- Noh, M.Y.; Koh, S.H.; Kim, S.M.; Maurice, T.; Ku, S.K.; Kim, S.H. Neuroprotective effects of donepezil against Aβ42-induced neuronal toxicity are mediated through not only enhancing PP2A activity but also regulating GSK-3β and nAChRs activity. J. Neurochem. 2013, 127, 562–574. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Ding, J.; Liu, N.; Peng, S.; Wang, J.; Wang, F.; Zhang, Y. Targeting Akt by SC66 triggers GSK-3β mediated apoptosis in colon cancer therapy. Cancer Cell Int. 2019, 19, 124. [Google Scholar] [CrossRef]
- Jovanović, A. Cardioprotective signalling: Past, present and future. Eur. J. Pharmacol. 2018, 833, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Tanno, M.; Kuno, A.; Ishikawa, S.; Miki, T.; Kouzu, H.; Yano, T.; Murase, H.; Tobisawa, T.; Ogasawara, M.; Horio, Y.; et al. Translocation of glycogen synthase kinase-3β (GSK-3β), a trigger of permeability transition, is kinase activity-dependent and mediated by interaction with voltage-dependent anion channel 2 (VDAC2). J. Biol. Chem. 2014, 289, 29285–29296. [Google Scholar] [CrossRef] [PubMed]
- Vélez, D.E.; Mestre-Cordero, V.E.; Hermann, R.; Perego, J.; Harriet, S.; Fernandez-Pazos, M.L.M.; Mourglia, J.; Marina-Prendes, M.G. Rosuvastatin protects isolated hearts against ischemia-reperfusion injury: Role of Akt-GSK-3β, metabolic environment, and mitochondrial permeability transition pore. J. Physiol. Biochem. 2020, 76, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.M.; Josephson, L.; Pilozzi, A.R.; Huang, X. A novel dual fluorochrome near-infrared imaging probe for potential alzheimer’s enzyme biomarkers-bace1 and cathepsin D. Molecules 2020, 25, 274. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zeng, Y.; Wu, A.; Yu, C.; Tang, Y.; Wang, X.; Xiong, R.; Chen, H.; Wu, J.; Qin, D. Lychee Seed Fraction Inhibits Aβ1-42-Induced Neuroinflammation in BV-2 Cells via NF-κB Signaling Pathway. Front. Pharmacol. 2018, 9, 380. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Yang, S.; Lee, C.; Park, E.K.; Kim, K.M.; Ku, S.K.; Bae, J.S. Aloin reduces inflammatory gene iNOS via inhibition activity and p-STAT-1 and NF-κB. Food Chem. Toxicol. 2019, 126, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, X.; Niu, M.; Zhang, X.; Wang, J.; Zhou, C.; Xie, A. RAGE Silencing Ameliorates Neuroinflammation by Inhibition of p38-NF-κB Signaling Pathway in Mouse Model of Parkinson’s Disease. Front. Neurosci. 2020, 14, 353. [Google Scholar] [CrossRef]
- Chen, F.; Ghosh, A.; Hu, M.; Long, Y.; Sun, H.; Kong, L.; Hong, H.; Tang, S. RAGE-NF-κB-PPARγ Signaling is Involved in AGEs-Induced Upregulation of Amyloid-β Influx Transport in an In Vitro BBB Model. Neurotox. Res. 2018, 33, 284–299. [Google Scholar] [CrossRef]
- Järemo, P.; Jejcic, A.; Jelic, V.; Shahnaz, T.; Oweling, M.; Winblad, B.; Behbahani, H. Erythrocyte Amyloid Beta Peptide Isoform Distributions in Alzheimer and Mild Cognitive Impairment. Curr. Alzheimer Res. 2019, 16, 1050–1054. [Google Scholar] [CrossRef]
- Fernando, W.M.A.D.B.; Martins, I.J.; Morici, M.; Bharadwaj, P.; Rainey-Smith, S.R.; Lim, W.L.F.; Martins, R.N. Sodium Butyrate Reduces Brain Amyloid-β Levels and Improves Cognitive Memory Performance in an Alzheimer’s Disease Transgenic Mouse Model at an Early Disease Stage. J. Alzheimers Dis. 2020, 74, 91–99. [Google Scholar] [CrossRef]
- Shi, X.L.; Yan, N.; Cui, Y.J.; Liu, Z.P. A Unique GSK-3β inhibitor B10 Has a Direct Effect on Aβ, Targets Tau and Metal Dyshomeostasis, and Promotes Neuronal Neurite Outgrowth. Cells 2020, 9, 649. [Google Scholar] [CrossRef] [PubMed]
- Jęśko, H.; Lukiw, W.J.; Wilkaniec, A.; Cieślik, M.; Gąssowska-Dobrowolska, M.; Murawska, E.; Hilgier, W.; Adamczyk, A. Altered Expression of Urea Cycle Enzymes in Amyloid-β Protein Precursor Overexpressing PC12 Cells and in Sporadic Alzheimer’s Disease Brain. J. Alzheimers Dis. 2018, 62, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.J.; Barrett, T. Gliosis Precedes Amyloid-β Deposition and Pathological Tau Accumulation in the Neuronal Cell Cycle Re-Entry Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. Rep. 2020, 4, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganathan, B.; Suryanarayanan, V.; Sathya, S.; Narenkumar, M.; Singh, S.K.; Ruckmani, K.; Pandima-Devi, K. Anti-amyloidogenic and anti-apoptotic effect of α-bisabolol against Aβ induced neurotoxicity in PC12 cells. Eur. J. Med. Chem. 2018, 143, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yuan, B.; Chu, Q.; Wang, C.; Bi, H. Protective roles of isoastilbin against Alzheimer’s disease via Nrf2-mediated antioxidation and anti-apoptosis. Int. J. Mol. Med. 2019, 43, 1406–1416. [Google Scholar] [CrossRef]
- Chen, N.; Wang, J.; He, Y.; Xu, Y.; Zhang, Y.; Gong, Q.; Yu, C.; Gao, J. Trilobatin Protects Against Aβ25-35-Induced Hippocampal HT22 Cells Apoptosis Through Mediating ROS/p38/Caspase 3-Dependent Pathway. Front. Pharmacol. 2020, 11, 584. [Google Scholar] [CrossRef]
- Cheng, W.; Chen, W.; Wang, P.; Chu, J. Asiatic acid protects differentiated PC12 cells from Aβ25-35-induced apoptosis and tau hyperphosphorylation via regulating PI3K/Akt/GSK-3β signaling. Life Sci. 2018, 208, 96–101. [Google Scholar] [CrossRef]
- Chinchalongporn, V.; Shukla, M.; Govitrapong, P. Melatonin ameliorates Aβ42-induced alteration of βAPP processing secretases via the melatonin receptor through the Pin1/GSK3β/NF-κB pathway in SH-SY5Y cells. J. Pineal Res. 2018, 64, 1. [Google Scholar] [CrossRef]
- Parajuli, P.; Joshee, N.; Chinni, S.R.; Rimando, A.M.; Mittal, S.; Sethi, S.; Yadav, A.K. Delayed growth of glioma by scutellaria flavonoids involve inhibition of akt, gsk-3 and nf-κb signaling. J. Neuro-Oncol. 2011, 101, 15–24. [Google Scholar] [CrossRef]
- Wang, L.; Cao, J.; Shi, Z.; Fan, W.; Liu, H.; Deng, J. Experimental study on the neurotoxic effect of beta-amyloid on the cytoskeleton of PC12 cells. Int. J. Mol. Med. 2018, 41, 2764–2770. [Google Scholar]
- Duronio, V. The life of a cell: Apoptosis regulation by the PI3K/PKB pathway. Biochem. J. 2008, 415, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Xiong, R.; Wu, A.G.; Yu, C.L.; Zhao, Y.; Qiu, W.Q.; Wang, X.L.; Teng, J.F.; Liu, J.; Chen, H.X.; et al. Polyphenols Derived from Lychee Seed Suppress Aβ (1-42)-Induced Neuroinflammation. Int. J. Mol. Sci. 2018, 19, 2109. [Google Scholar] [CrossRef]
- Shi, Z.; Chen, T.; Yao, Q.B.; Zheng, L.; Zhang, Z.; Wang, J.Z.; Hu, Z.M.; Cui, H.M.; Han, Y.W.; Han, X.H.; et al. The circular RNA ci RS-7 promotes APP and BACE1 degradation in an NF-κB-dependent manner. FEBS J. 2017, 284, 1096–1109. [Google Scholar] [CrossRef] [PubMed]
- Park, R.; Kook, S.Y.; Park, J.C.; Mook-Jung, I. Aβ1-42 reduces P-glycoprotein in the blood-brain barrier through RAGE-NF-κB signaling. Cell Death Dis. 2014, 5, e1299. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.M.; Yan, S.D.; Stern, D.; Saraiva, M.J. Interaction of the receptor for advanced glycation end products (rage) with transthyretin triggers nuclear transcription factor kb (Nf-κb) activation. Lab. Investig. 2000, 80, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Schmidt, A.M. Characterization and functional analysis of the promoter of RAGE, the receptor for advanced glycation end products. J. Biol. Chem. 1997, 272, 16498–16506. [Google Scholar] [CrossRef]
- Liao, Y.; Qi, X.L.; Cao, Y.; Yu, W.F.; Ravid, R.; Winblad, B.; Pei, J.J.; Guan, Z.Z. Elevations in the Levels of NF-κB and Inflammatory Chemotactic Factors in the Brains with Alzheimer’s Disease-One Mechanism May Involve α3 Nicotinic Acetylcholine Receptor. Curr. Alzheimer Res. 2016, 13, 1290–1301. [Google Scholar] [CrossRef]
- Zhou, J.; Gu, X.; Fan, X.; Zhou, Y.; Wang, H.; Si, N.; Yang, J.; Bian, B.; Zhao, H. Anti-inflammatory and Regulatory Effects of Huanglian Jiedu Decoction on Lipid Homeostasis and the TLR4/MyD88 Signaling Pathway in LPS-Induced Zebrafish. Front. Physiol. 2019, 10, 1241. [Google Scholar] [CrossRef]
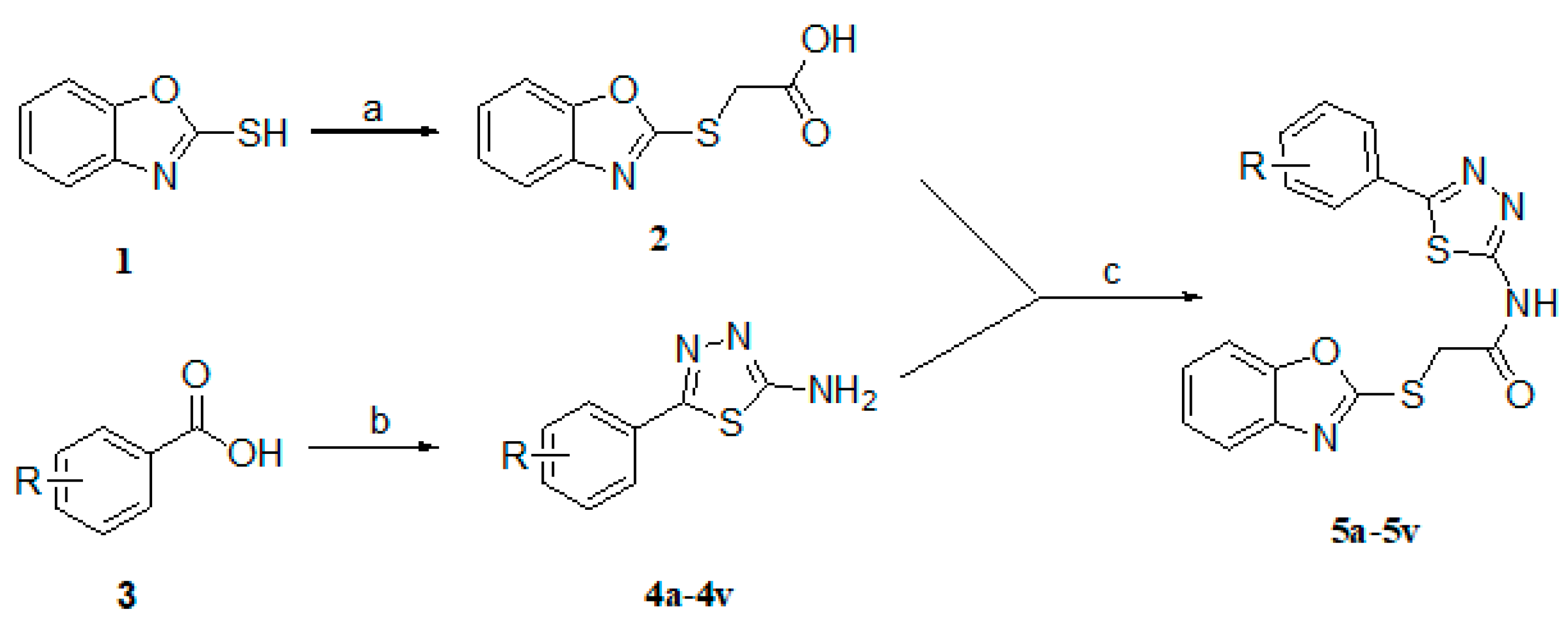


| Compound | R | Compound | R | Compound | R |
|---|---|---|---|---|---|
| 5a | H | 5i | 4-Br | 5q | 2-OCH3 |
| 5b | 2-Cl | 5j | 2-CF3 | 5r | 3-OCH3 |
| 5c | 3-Cl | 5k | 3-CF3 | 5s | 4-OCH3 |
| 5d | 4-Cl | 5l | 4-CF3 | 5t | 2,5-F2 |
| 5e | 2-F | 5m | 2-CH3 | 5u | 2-Cl-5-F |
| 5f | 3-F | 5n | 3-CH3 | 5v | 3,5-(CH3)2 |
| 5g | 4-F | 5o | 4-CH3 | ||
| 5h | 2-Br | 5p | 3,4,5-(OCH3)3 |
Sample Availability: All samples of the compounds are available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Bian, M.; Ma, Q.-Q.; Zhang, Z.; Du, H.-H.; Wei, C.-X. Design and Synthesis of New Benzo[d]oxazole-Based Derivatives and Their Neuroprotective Effects on β-Amyloid-Induced PC12 Cells. Molecules 2020, 25, 5391. https://doi.org/10.3390/molecules25225391
Liu Z, Bian M, Ma Q-Q, Zhang Z, Du H-H, Wei C-X. Design and Synthesis of New Benzo[d]oxazole-Based Derivatives and Their Neuroprotective Effects on β-Amyloid-Induced PC12 Cells. Molecules. 2020; 25(22):5391. https://doi.org/10.3390/molecules25225391
Chicago/Turabian StyleLiu, Zheng, Ming Bian, Qian-Qian Ma, Zhuo Zhang, Huan-Huan Du, and Cheng-Xi Wei. 2020. "Design and Synthesis of New Benzo[d]oxazole-Based Derivatives and Their Neuroprotective Effects on β-Amyloid-Induced PC12 Cells" Molecules 25, no. 22: 5391. https://doi.org/10.3390/molecules25225391
APA StyleLiu, Z., Bian, M., Ma, Q.-Q., Zhang, Z., Du, H.-H., & Wei, C.-X. (2020). Design and Synthesis of New Benzo[d]oxazole-Based Derivatives and Their Neuroprotective Effects on β-Amyloid-Induced PC12 Cells. Molecules, 25(22), 5391. https://doi.org/10.3390/molecules25225391




