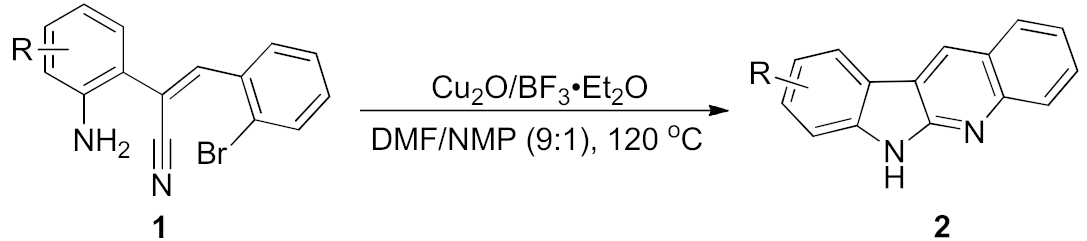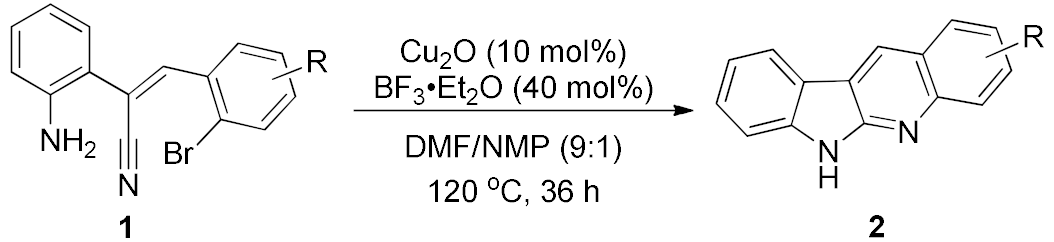Abstract
A synthetic approach to quinindoline derivatives by the Cu-catalyzed dual cyclization has been developed. This catalytic reaction is a practical method for the systematic synthesis of quinindoline core structure, which contains a limited-step synthetic strategy and can tolerant a wide variety of substituents. In addition, the mechanistic study reveals that the reaction initiates from a Lewis acid accelerated addition of aniline to nitrile and provides the indole substructure, and then the subsequent Cu-catalyzed C-N coupling reaction furnishes the quinoline subunit and affords the quinindoline structure.
1. Introduction
Quinindoline, the tetra-fused N-heterocycle, is an important structure, particularly in the field of medical and biological chemistry, which contains the common bioactive subunits quinoline and indole in its skeleton. The most useful and representative natural alkaloids containing the simplest substituent on the quinindoline core structure are norneocryptolepine (without any substituent on the core) and neocryptolepine (with a solely N-methyl group) [1,2,3] (Figure 1). These two important alkaloids have attracted considerable attention from chemists because of their versatile bioactivities, including antimalaria, antitumor, antibacterial properties [4,5,6]. They are the important components of traditional herbal medicine in the West and Central Africa [7], and are also the model structures for the design of pharmaceutical compounds. Therefore, many related studies, for example, their structure–activity relationship, strategies for the synthetic approaches, and spectroscopy have been developed in recent decades [8].
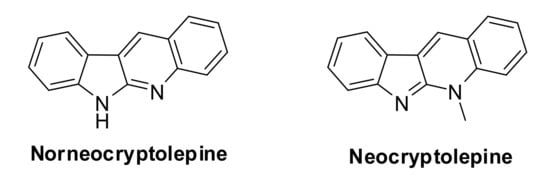
Figure 1.
Natural alkaloids with quinindoline core structure.
Because of the complicity in the structure of tetra-fused rings, the developed methods for the synthesis of quinindolines cannot satisfy the comprehensiveness of synthesis on the structures, and thus so many different approaches appeared based on this. The typical approaching routes mainly relied on three pathways. The first one is extension of two aromatic rings from an indole substrate, which is also the most commonly involved strategy [9,10,11,12,13]. The second route is the ring expansion of a quinoline derivative. This is the oldest strategy to afford the quinindolines, and reported as the first method to synthesize the neocryptolepine [14,15,16]. The dual annulation of an N-alkynylaryl-N-aryl carbodiimide through diradical pathway is the third common strategy, but not as often recorded as other two routes [17,18,19]. Despite these three dominant pathways, there have been several modern strategies reported [11,12,13,20,21,22]. In this respect, our recent work in the synthesis of norneocryptolepine derivatives through a Pd-catalysis can systematically offer the unprotected quinindolines various substituents on the benzene moieties (Scheme 1) [22]. This work and our experience in the construction of heterocycles [23,24,25,26,27,28,29,30] encouraged us to continuously study in this field. In addition, during the investigation of optimized conditions, we found the possibility to proceed with the dual cyclization under a Cu/Lewis acid catalytic system. This discovery offered an alternative protocol in a lower cost catalytic system and also the potential for industrial applications. Herein, we report a Cu/Lewis acid catalyzed dual cyclization for the synthesis of quinindolines.
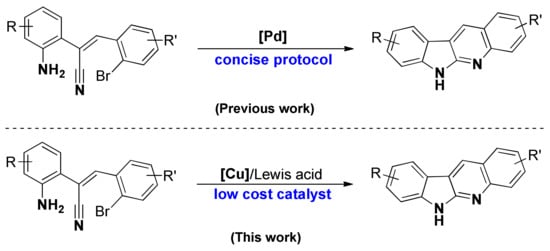
Scheme 1.
Palladium and copper-catalyzed dual cyclization to synthesize quinindolines.
2. Results and Discussion
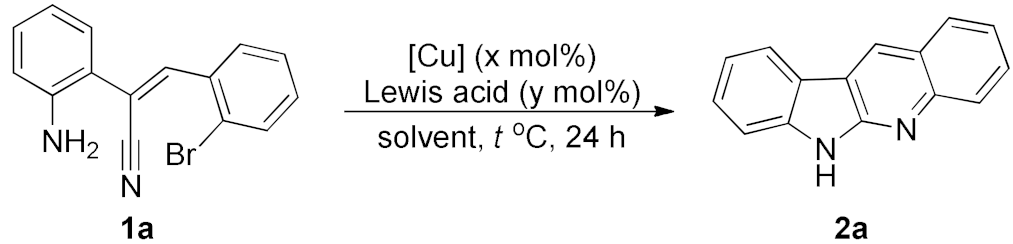
Our study was initiated from the inadvertently discovered condition of copper catalysis, although it did not show satisfactory performance (Table 1, entry 1). We then investigated various factors of the conditions for this copper catalysis, and the results are summarized in Table 1 (also see Supplementary Materials in detail). We first examined different solvents and found that because the high reaction temperature is required, only the polar solvents with high boiling points can advance the reaction effectively (entries 2, 3). The cosolvent system is helpful for this reaction (entries 4–6), and the best yield was observed in a 9:1 ratio of DMF/NMP. Increase or decrease in the temperature reduced the reaction yields (entries 7, 8). We also test different kinds of ligand; however, they did not promote the reaction yields at all. When we introduced the Lewis acid BF3·OEt2 into the reaction, the reaction yield was significantly improved (entry 9). We further reduced the loading of Cu2O and increase the amount of BF3·OEt2, and found that the yield was further increased under 36 h reaction time (entry 10). Other copper sources cannot perform as good as the Cu2O (entry 11). The Lewis acid is crucial, and we found that only the boron acids can improve the reaction yield, other Lewis acids such as AlCl3, TiCl4 and FeCl3 are not able to provide the desired product in good yield (entries 12–14). Moreover, in the absence of copper source, the reaction can still proceed in a bad yield (entry 15). We then tried to reduce the loading amount of copper sources by fine-tuning the combination of every factor, but did not well succeed (see Supplementary Materials for the detail). The moderate or better reaction yields required at least 20 mol% of copper sources (10 mol% Cu2O).

Table 1.
Optimization of reaction conditions a.
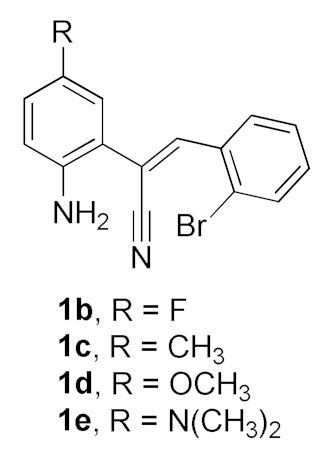
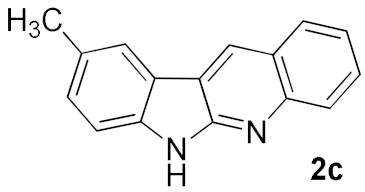
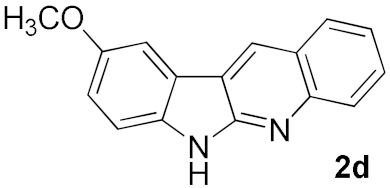
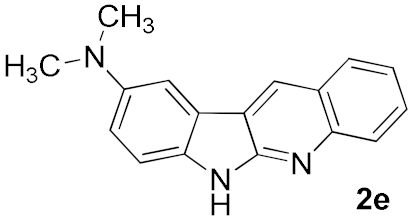
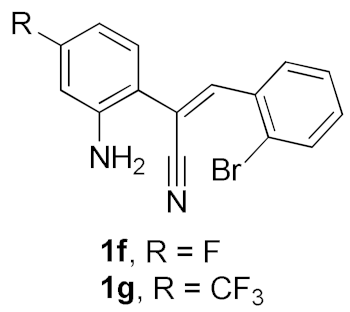
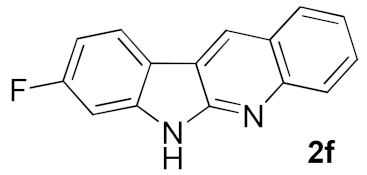
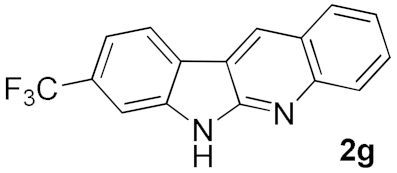
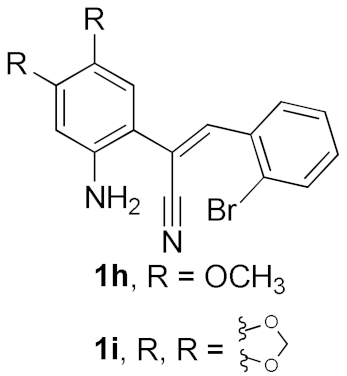
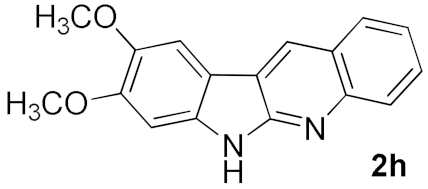
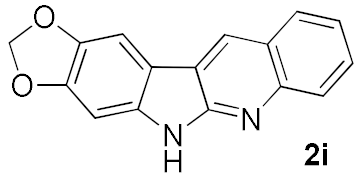
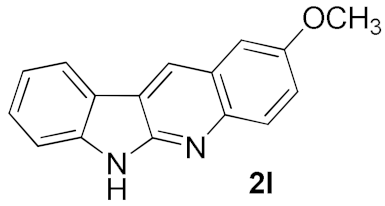
After studying the factors of the reaction conditions, we then investigated the reaction scope by testing various substituents to understand the capacity of this copper catalysis. We selected two conditions (entries 9, 10) as our standard conditions since we found that different structures fit different conditions. The reactivity for the substituent on the aniline moiety (left ring, Table 2) was surveyed first. It was found that an electron-donating group on the aniline moiety can generally provide higher yields of the desired products than those with an electron-withdrawing group on it. In addition, for the substrates with an electron-donating group, the condition A (10 mol% of Cu2O and 40 mol% of BF3·OEt2) can provide higher yields of the desired products than the condition B (20 mol% of Cu2O and 20 mol% of BF3·OEt2). On the contrary to the electron-donating substituents, the substrates with an electron-withdrawing group can offer higher yields under the condition B. Thus, for a substituent para to the amino group (entries 1–3), the desired product 2b can be afforded in 41% yield under condition B; and the products 2c, 2d and 2e can be obtained in 68%, 76% and 45% yields, respectively, by using condition A. The substrates with a substituent meta to the amino group (1f and 1g) can also advance the reaction smoothly and afford the corresponding products 2f and 2g in moderate yields with condition B. The disubstituted products 2h and 2i can be obtained in 71% and 56% yields, respectively, with condition A.

Table 2.
Reaction scope a.
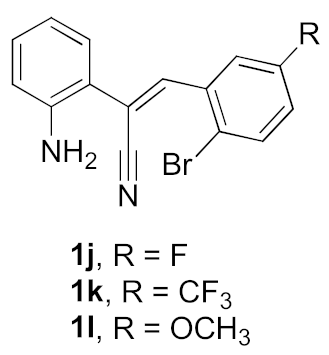
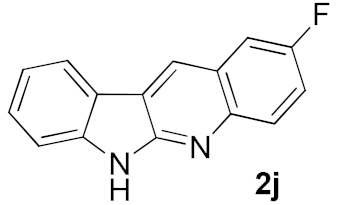
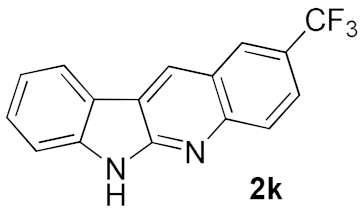
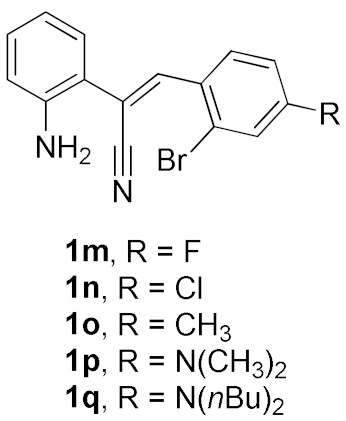
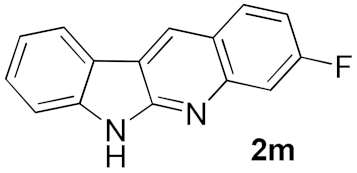
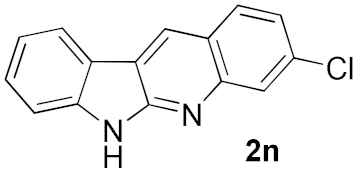
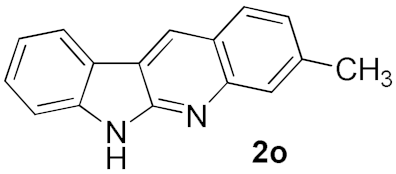
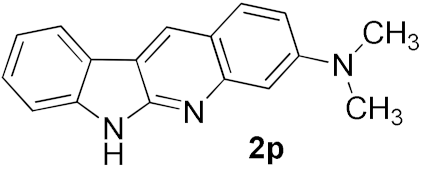
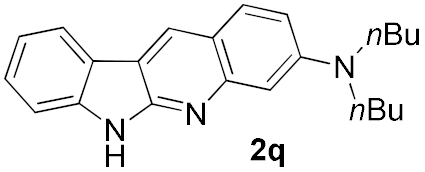
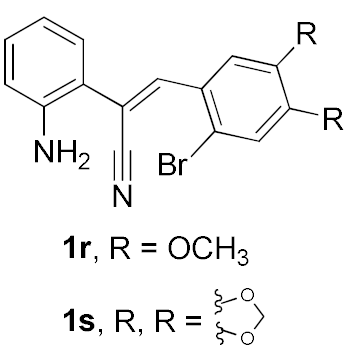
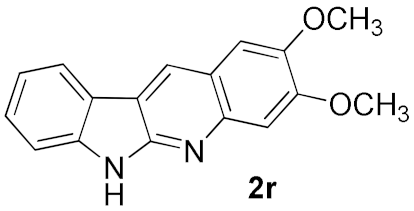
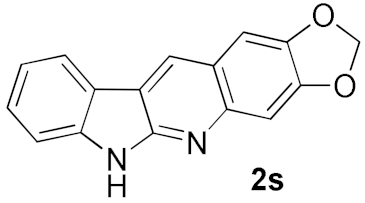
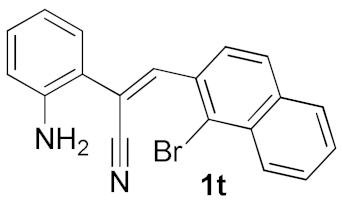
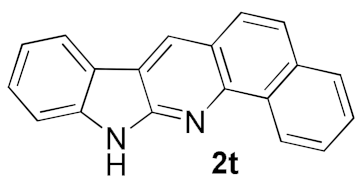
We further surveyed the substrates with a substituent on the ortho-bromostyryl moiety. As indicated in Table 3, the products were consistently obtained in higher yields with condition A. For the substrates with a substituent para to the bromide (1j, 1k and 1l), the corresponding products 2j, 2k and 2l can be afforded in moderate-to-good yields. The distribution of yields reveals that the electronic property on the styryl moiety also significantly affects the reaction yields. Substrates with a substituent meta to the bromide advance the reaction smoothly as well. Thus, the meta-fluoro and chloro groups resulted in similar yields of the desired products (2m and 2n), while the meta-methyl substituted product 2o was generated in a slightly lower yield. The amino substituted products 2p and 2q were obtained in much lower yields comparing with other meta-substituted products. The disubstituted products 2r and 2s can be also generated in moderate yields; the yields of the desired products are 62% and 57%, respectively. Moreover, the naphthyl group is able to be tolerated to give the product 2t in 49% yield.

Table 3.
Reaction scope a.
From the results of this and our previous works, we can find that the reactivity of substrates is very different between conditions of the Pd- and the Cu-catalysis. Therefore, we tried to figure out the reaction pathway of this Cu-catalytic dual cyclization. We set up some control experiments to carefully study the nature of this reaction (Scheme 2). We first monitored the relationship between the reaction time and the reaction behavior. When the reaction time was increased, the recovered amount of substrate 1a decreased, and the yield of product 2a increased. A supposed intermediate 3a was detected in low yields. We then removed the copper source and checked the difference of the reaction, and the results were unexpected. The supposed intermediate 3a was always detected, and the detected amount of 3a was steady around 20% for the reaction time greater than 16 h. In addition, a trace amount of the desired product 2a was detected, which was probably caused by the intramolecular SNAr reaction. We further investigated the variety of the amount of 3a by solely using Cu2O as the catalyst. It was found that the substrate is fully consumed in a much longer reaction time compared with the standard condition, and the crude 1H NMR spectrum is messier. Some unidentified compounds appear at 18 h reaction time. The amount of supposed intermediate 3a was generally kept in a low yield for different reaction times. The formation of 3a implied that the cyclization pathway is likely similar to the reaction with the condition of BF3·OEt2 only. The results of control experiments can be briefly concluded as the following. First, the supposed intermediate 3a was detected in every control experiment; therefore, it is highly possible that 3a is the key intermediate in the copper catalysis. Second, the amount of 3a is higher while the BF3·OEt2 is introduced into the reaction. This phenomena is obvious when the BF3·OEt2 is solely introduced without any copper source. Third, during the reaction, the amount of 3a is steady, and would not change as the product formed. This is probably because the subsequent step is faster than the formation of 3a.
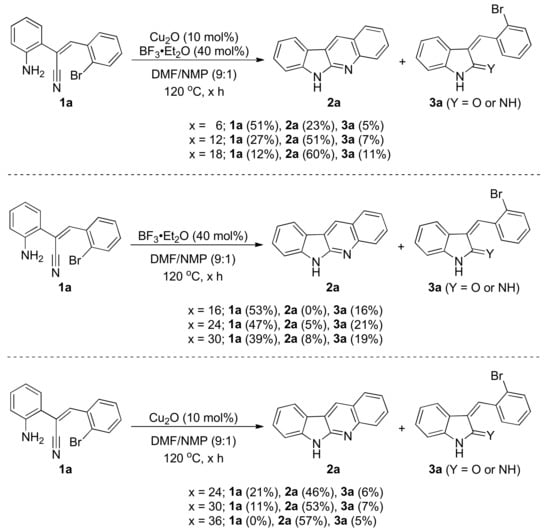
Scheme 2.
Control experiments a; a the yields were estimated by GC-MS.
Based on the above results and the previous report [31], a tentative reaction pathway can be proposed as below (Scheme 3). The reaction is likely to be initiated by the coordination of substrate with BF3, which accelerates the cyclization to form the intermediate A. Transmetalation of the boron species A generates the copper complex B, which facilitates the intramolecular addition to form the complex C. Aromatization and release of [Cu]Br affords the desired product 2a. The [Cu]Br can react with proton to regenerate the [Cu]+.
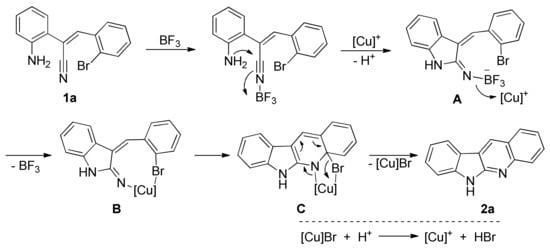
Scheme 3.
Proposed reaction mechanism.
3. Materials and Methods
All reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA), Alfa-Aesar (Haverhill, MA, USA), TCI (Tokyo, Japan) and Fisher-Acros (Loughborough, UK), and were used without further purification unless otherwise noted. All manipulations of oxygen- and moisture-sensitive materials were conducted with a standard Schlenk technique or in the glove box. Flash column chromatography was performed using silica gel (230–400 mesh). Analytical thin layer chromatography (TLC) was performed on 60 F254 (0.25 mm) plates and visualization was accomplished with UV light (254 and 354 nm) and/or an aqueous alkaline KMnO4 solution followed by heating. Proton and carbon nuclear magnetic resonance spectra (1H NMR and 13C NMR) were recorded on Bruker 300 or Bruker 600 spectrometer with Me4Si or solvent resonance as the internal standard (1H NMR, Me4Si at 0 ppm, CDCl3 at 7.26 ppm, d6-DMSO at 2.49 ppm; 13C NMR, Me4Si at 0 ppm, CDCl3 at 77.0 ppm, d6-DMSO at 39.7 ppm). 1H NMR data are reported as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, quint = quintet, sext = sextet, sept = septet, br = broad, m = multiplet), coupling constants (Hz), and integration. IR spectral data were recorded on a Bruker TENSOR 37 spectrometer (Bruker, Billerica, MA, USA). Melting points (mp) were determined using a SRS OptiMelt MPA100 (Stanford Research Systems, Sunnyvale, CA, USA). GC-MS data were obtained from the HP 5890 Series II GC/HP 5972 GC MASS Spectrometer System. High resolution mass spectral data were obtained from MAT-95XL HRMS by using EI method.
4. Conclusions
In conclusion, we have developed a catalytic dual cyclization to approach the quinindolines by using the copper and boron species as the catalytic system. This catalytic cyclization could proceed for most substrates and provide the desired products in moderate-to-good yields with tolerance of various substituents. Study of the reaction mechanism via the control experiments indicates that the reaction goes through the formation of an oxindole-related intermediate, and the copper species can facilitate the intramolecular SNAr reaction. Moreover, this reaction protocol with low cost catalysts may represent a practical synthesis with potential in industrial applications.
Supplementary Materials
The following are available online, experimental procedures for the synthesis of substrates (1) and products (2). Table S1–S3: optimization study.
Author Contributions
Experiments, H.-K.W., Y.-L.C., and G.P.; resources, H.-L.W.; methodologies and paper writing, J.-C.H.; paper editing and correction, H.-L.S. All authors have read and agreed to the published version of the manuscript.
Funding
We are grateful for the financial support of this work from the Ministry of Science and Technology of Republic of China (MOST 108-2113-M-032-001) and the postdoctoral fellowship for Gangaram Pallikonda (MOST 107-2811-M-032-002).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Subbaraju, G.V.; Kavitha, J.; Rajasekhar, D.; Jimenez, J.I. Jusbetonin, the First Indolo[3,2-b]quinoline Alkaloid Glycoside, from Justicia betonica. J. Nat. Prod. 2004, 67, 461–462. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, M.H.M.; Schiff, P.L., Jr.; Tackie, A.N.; Phoebe, C.H., Jr.; Martin, G.E. Two new indoloquinoline alkaloids from cryptolepis sanguinolenta: Cryptosanguinolentine and cryptotackieine. J. Heterocycl. Chem. 1996, 33, 239–243. [Google Scholar] [CrossRef]
- Cimanga, K.; De Bruyne, T.; Pieters, L.; Claeys, M.; Vlietinck, A. New alkaloids from Cryptolepis sanguinolenta. Tetrahedron Lett. 1996, 37, 1703–1706. [Google Scholar] [CrossRef]
- Jonckers, T.H.M.; van Miert, S.; Cimanga, K.; Bailly, C.; Colson, P.; De Pauw-Gillet, M.-C.; van der Heuvel, H.; Claeys, M.; Lemière, F.; Esmans, E.L.; et al. Synthesis, Cytotoxicity, and Antiplasmodial and Antitrypanosomal Activity of New Neocryptolepine Derivatives. J. Med. Chem. 2002, 45, 3497–3508. [Google Scholar] [CrossRef] [PubMed]
- Sidoryk, K.; Świtalska, M.; Jaromin, A.; Cmoch, P.; Bujak, I.; Kaczmarska, M.; Wietrzyk, J.; Dominguez, E.G.; Żarnowski, R.; Andes, D.R.; et al. The synthesis of indolo[2,3-b]quinoline derivatives with a guanidine group: Highly selective cytotoxic agents. Eur. J. Med. Chem. 2015, 105, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Godlewska, J.; Luniewski, W.; Zagrodzki, B.; Kaczmarek, Ł.; Bielawska-Pohl, A.; Dus, D.; Wietrzyk, J.; Opolski, A.; Siwko, M.; Jaromin, A.; et al. Biological Evaluation of ω-(Dialkylamino)alkyl Derivatives of 6H-indolo[2,3-b]quinoline-Novel Cytotoxic DNA Topoisomerase II Inhibitors. Anticancer Res. 2005, 25, 2857–2868. [Google Scholar]
- Lavrado, J.; Moreira, R.; Paulo, A. Indoloquinolines as scaffolds for drug discovery. Curr. Med. Chem. 2010, 17, 2348–2370. [Google Scholar] [CrossRef]
- Bracca, A.B.J.; Heredia, D.A.; Larghi, E.L.; Kaufman, T.S. Neocryptolepine (Cryprotackieine), a Unique Bioactive Natural Product: Isolation, Synthesis, and Profile of Its Biological Activity. Eur. J. Org. Chem. 2014, 36, 7979–8003. [Google Scholar] [CrossRef]
- Sundaram, G.S.M.; Venkatesh, C.; Syam Kumar, U.K.; Ila, H.; Junjappa, H. A Concise Formal Synthesis of Alkaloid Cryptotackiene and Substituted 6H-Indolo[2,3-b]quinolines. J. Org. Chem. 2004, 69, 5760–5762. [Google Scholar] [CrossRef]
- Parvatkar, P.T.; Parameswaran, P.S.; Tilve, S.G. An Expeditious I2-Catalyzed Entry into 6H-Indolo[2,3-b]quinoline System of Cryptotackieine. J. Org. Chem. 2009, 74, 8369–8372. [Google Scholar] [CrossRef]
- Yu, S.; Li, Y.; Zhou, X.; Wang, H.; Kong, L.; Li, X. Access to Structurally Diverse Quinoline-Fused Heterocycles via Rhodium(III)-Catalyzed C–C/C–N Coupling of Bifunctional Substrates. Org. Lett. 2016, 18, 2812–2815. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wang, B. Tandem Rh(III)-Catalyzed C–H Amination/Annulation Reactions: Synthesis of Indoloquinoline Derivatives in Water. Org. Lett. 2016, 18, 2820–2823. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Liu, M.; Ye, Y.; Yin, G. Synthesis of 6-Substituted 6H-Indolo[2,3-b]quinolines from Isoindigos. Org. Lett. 2017, 19, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Holt, S.J.; Petrow, V. Carbazoles, carbolines, and related compounds. Part III. Quinindoline derivatives. J. Chem. Soc. 1948, 1948, 922–924. [Google Scholar] [CrossRef]
- Kaczmarek, Ł.; Balicki, R.; Nantka-Namirski, P.; Peczyńska-Czoch, W.; Mordarski, M. Cancerostatics, VI. Synthesis and antineoplastic properties of some benzo-iso-alpha-carbolines. Arch. Pharm. 1988, 321, 463–467. [Google Scholar] [CrossRef]
- Timári, G.; Soós, T.; Hajós, G. A convenient synthesis of two new indoloquinoline alkaloids. Synlett 1997, 1067–1068. [Google Scholar] [CrossRef]
- Schmittel, M.; Steffen, J.-P.; Engels, B.; Lennartz, C.; Hanrath, M. Two Novel Thermal Biradical Cyclizations in Theory and Experiment: New Synthetic Routes to 6H-Indolo[2,3-b]quinolines and 2-Aminoquinolines from Enyne-Carbodiimides. Angew. Chem. Int. Ed. 1998, 37, 2371–2373. [Google Scholar] [CrossRef]
- Schmittel, M.; Rodríguez, D.; Steffen, J.-P. A Highly Efficient Triplet Analogue of a Thermal Biradical Cyclization-The Photochemical C2–C6 Cyclization of Enyne-Heteroallenes. Angew. Chem. Int. Ed. 2000, 39, 2152–2155. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, C.; Zhang, H.-R.; Wang, K.K. Synthesis of 6H-Indolo[2,3-b][1,6]naphthyridines and Related Compounds as the 5-Aza Analogues of Ellipticine Alkaloids. J. Org. Chem. 2000, 65, 7977–7983. [Google Scholar] [CrossRef]
- Nallapati, S.B.; Prasad, B.; Sreenivas, B.Y.; Sunke, R.; Poornachandra, Y.; Kumar, C.G.; Sridhar, B.; Shivashankar, S.; Mukkanti, K.; Pal, M. Apparent Carbon Monoxide Insertion via Double Isocyanide Incorporation during Palladium-Catalyzed Construction of Indoloquinoline Ring in a Single Pot: Synthesis of New Cytotoxic Agents. Adv. Synth. Catal. 2016, 358, 3387–3393. [Google Scholar] [CrossRef]
- Yan, Z.; Wan, C.; Wan, J.; Wang, Z. An efficient iron-promoted synthesis of 6H-indolo [2,3-b]quinolines and neocryptolepine derivatives. Org. Biomol. Chem. 2016, 14, 4405–4408. [Google Scholar] [CrossRef] [PubMed]
- Yeh, L.-H.; Wang, H.-K.; Pallikonda, G.; Ciou, Y.-L.; Hsieh, J.-C. Palladium-Catalyzed Dual Annulation: A Method for the Synthesis of Norneocryptolepine. Org. Lett. 2019, 21, 1730–1734. [Google Scholar] [CrossRef] [PubMed]
- Thorat, V.H.; Hsieh, J.-C.; Cheng, C.-H. Transition-Metal-Free Tandem Cyclization/N-Arylation Reaction: A Method to Access Biaryl Sultam Derivatives via a Diradical Pathway. Org. Lett. 2020, 22, 6623–6627. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.-C.; Su, H.-L. Synthesis of N-Heterocycles via the Transition-Metal-Catalyzed Tandem Addition/Cyclization of a Nitrile. Synthesis 2020, 52, 819–833. [Google Scholar] [CrossRef]
- Chen, M.-H.; Hsieh, J.-C.; Lee, Y.-H.; Cheng, C.-H. Controlled Synthesis of Enantioselective 1-Aminoindenes via Cobalt-Catalyzed [3+2] Annulation Reaction. ACS Catal. 2018, 8, 9364–9369. [Google Scholar] [CrossRef]
- Jhang, Y.-Y.; Fan-Chiang, T.-T.; Huang, J.-M.; Hsieh, J.-C. Copper-Catalyzed Annulation: A Method for the Systematic Synthesis of Phenanthridinium Bromide. Org. Lett. 2016, 18, 1154–1157. [Google Scholar] [CrossRef]
- Fan-Chiang, T.-T.; Wang, H.-K.; Hsieh, J.-C. Synthesis of Phenanthridine Skeletal Amaryllidaceae Alkaloids. Tetrahedron 2016, 72, 5640–5645. [Google Scholar] [CrossRef]
- Chen, W.-L.; Chen, C.-Y.; Chen, Y.-F.; Hsieh, J.-C. Hydride-Induced Anionic Cyclization: An Efficient Method for the Synthesis of 6-H-Phenanthridines via a Transition-Metal-Free Process. Org. Lett. 2015, 17, 1613–1616. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Hsieh, J.-C. Synthesis of Polysubstituted Phenanthridines via Ligand-Free Copper-Catalyzed Annulation. Org. Lett. 2014, 16, 4642–4645. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Wu, Y.-S.; Jhan, Y.-H.; Hsieh, J.-C. An Efficient Synthesis of (NH)-Phenanthridinones via Ligand-Free Copper-Catalyzed Annulation. Org. Chem. Front. 2014, 1, 253–257. [Google Scholar] [CrossRef]
- Yu, X.; Gao, L.; Jia, L.; Yamamoto, Y.; Bao, M. Synthesis of Quinazolin-4(3H)-ones via the Reaction of 2-Halobenzamides with Nitriles. J. Org. Chem. 2018, 83, 10352–10358. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1a–1t and 2a–2t are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).