Implementing Curcumin in Translational Oncology Research
Abstract
1. Introduction
2. Curcumin-Pleiotropic Molecule in a Setting of Personalized Medicine
3. Low Bioavailability: How to Understand the Mechanism of Action?
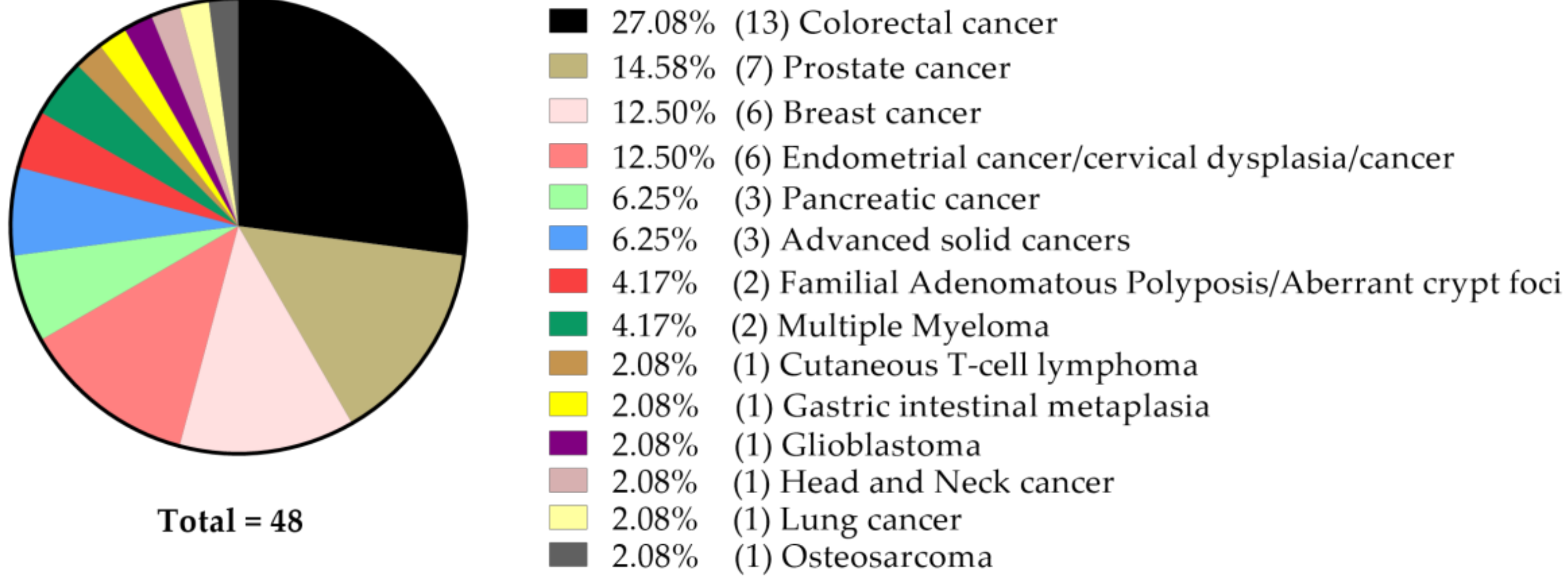
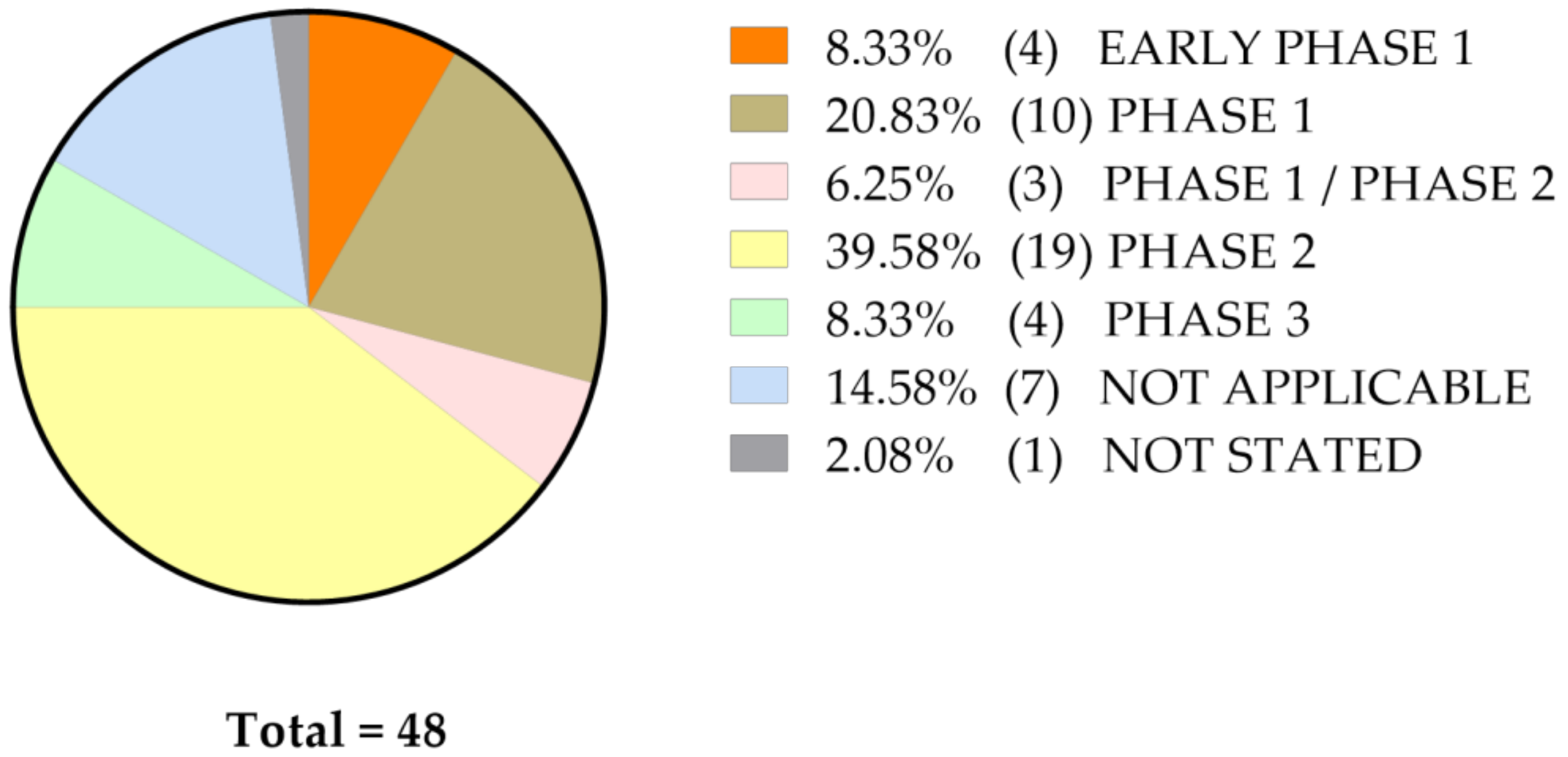
4. Current Status of Curcumin-Related Oncological Clinical Studies Listed at ClinicalTrials.Gov
The Status of Clinical Studies with Curcumin Only (n = 48)
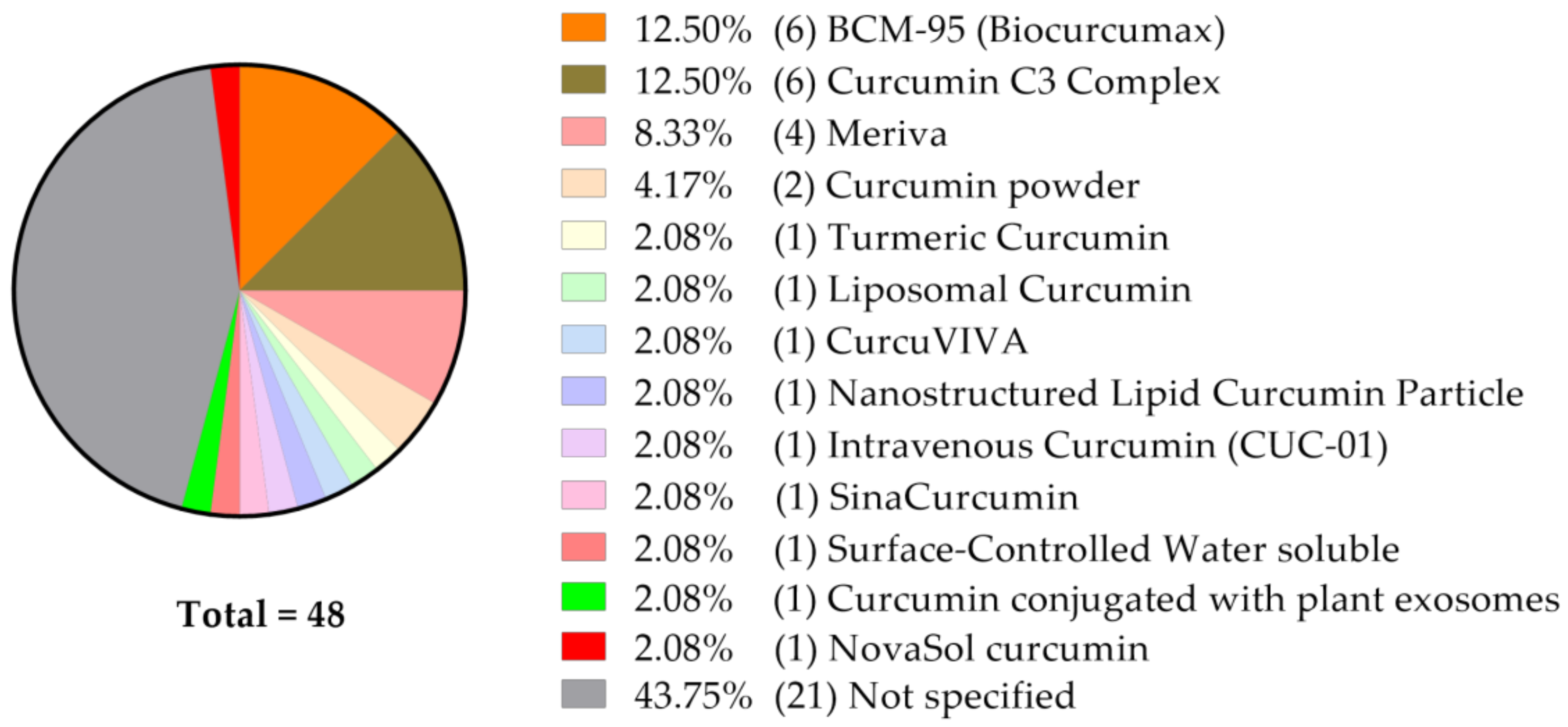
5. The Effect of Curcumin Formulations Targeted at Improving its Bioavailability
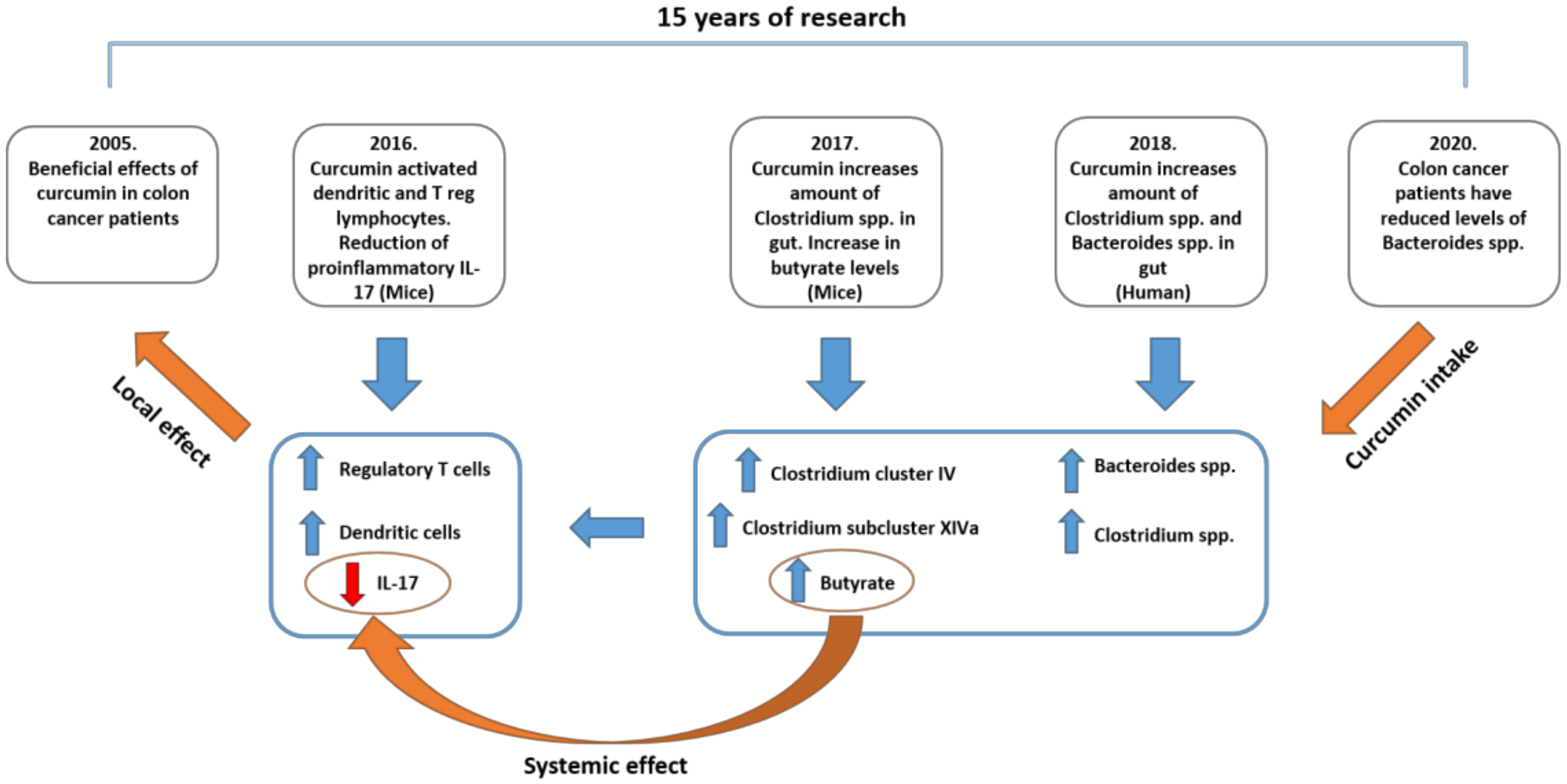
6. Microbiota and Curcumin: Acting Locally Benefiting Systemically?
7. Curcumin in Targeting Metabolic Liabilities in Cancer
Curcumin-Mediated Suppression of NNMT and Other Metabolic Enzymes in Circumventing Therapy Resistance
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Farber, S.; Diamond, L.K. Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid. N. Engl. J.Med. 1948, 238, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Rixe, O.; Ortuzar, W.; Alvarez, M.; Parker, R.; Reed, E.; Paull, K.; Fojo, T. Oxaliplatin, tetraplatin, cisplatin, and carboplatin: Spectrum of activity in drug-resistant cell lines and in the cell lines of the National Cancer Institute’s Anticancer Drug Screen panel. Biochem. Pharmacol. 1996, 52, 1855–1865. [Google Scholar] [CrossRef]
- Weiss, R.B.; Christian, M.C. New cisplatin analogues in development. A review. Drugs 1993, 46, 360–377. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.C.; Sorger, P.K. Combination Cancer Therapy Can Confer Benefit via Patient-to-Patient Variability without Drug Additivity or Synergy. Cell 2017, 171, 1678–1691. [Google Scholar] [CrossRef] [PubMed]
- Gajria, D.; Chandarlapaty, S. HER2-amplified breast cancer: Mechanisms of trastuzumab resistance and novel targeted therapies. Expert Rev. Anticanc. 2011, 11, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.; Pusztai, L.; Swanton, C. Cancer heterogeneity: Implications for targeted therapeutics. Br. J. Cancer 2013, 108, 479–485. [Google Scholar] [CrossRef]
- Yuan, M.; Huang, L.L.; Chen, J.H.; Wu, J.; Xu, Q. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal. Transduct Target. Ther. 2019, 4, 61. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Moazzen, S.; van der Sloot, K.J.W.; Bock, G.H.; Alizadeh, B.Z. Systematic review and meta-analysis of diet quality and colorectal cancer risk: Is the evidence of sufficient quality to develop recommendations? Crit Rev. Food Sci. Nutr. 2020, 2, 1–10. [Google Scholar] [CrossRef]
- Mentella, M.C.; Scaldaferri, F.; Ricci, C.; Gasbarrini, A.; Miggiano, G.A.D. Cancer and Mediterranean Diet: A Review. Nutrients 2019, 11, 2059. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.L.; Huang, T.S.; Lin, J.K. Curcumin, an antioxidant and anti-tumor promoter, induces apoptosis in human leukemia cells. Biochim. Biophys Acta 1996, 1317, 95–100. [Google Scholar] [CrossRef]
- Abegg, D.; Frei, R.; Cerato, L.; Prasad Hari, D.; Wang, C.; Waser, J.; Adibekian, A. Proteome-Wide Profiling of Targets of Cysteine reactive Small Molecules by Using Ethynyl Benziodoxolone Reagents. Angew Chem. Int. Ed. Engl. 2015, 54, 10852–10857. [Google Scholar] [CrossRef]
- Mary, C.P.V.; Vijayakumar, S.; Shankar, R. Metal chelating ability and antioxidant properties of Curcumin-metal complexes-A DFT approach. J. Mol. Graph. Model. 2018, 79, 1–14. [Google Scholar] [CrossRef]
- Paciello, F.; Fetoni, A.R.; Mezzogori, D.; Rolesi, R.; Di Pino, A.; Paludetti, G.; Grassi, C.; Troiani, D. The dual role of curcumin and ferulic acid in counteracting chemoresistance and cisplatin-induced ototoxicity. Sci. Rep. 2020, 10, 1063. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Morón, E.; Calderón-Montaño, J.M.; Salvador, J.; Robles, A.; López-Lázaro, M. The dark side of curcumin. Int. J. Cancer 2010, 126, 1771–1775. [Google Scholar] [CrossRef]
- Tiekou Lorinczova, H.; Fitzsimons, O.; Mursaleen, L.; Renshaw, D.; Begum, G.; Zariwala, M.G. Co-Administration of Iron and a Bioavailable Curcumin Supplement Increases Serum BDNF Levels in Healthy Adults. Antioxidants 2020, 9, 645. [Google Scholar] [CrossRef]
- Ammon, H.P.; Wahl, M.A. Pharmacology of Curcuma longa. Planta Med. 1991, 57, 1–7. [Google Scholar] [CrossRef]
- Tayyem, R.F.; Heath, D.D.; Al-Delaimy, W.K.; Rock, C.L. Curcumin content of turmeric and curry powders. Nutr. Cancer 2006, 55, 126–131. [Google Scholar] [CrossRef]
- Thangavel, K.D.K. Determination of curcumin, starch and moisture content in turmeric by Fourier transform near infrared spectroscopy (FT-NIR). Eng. Agr. Environ. Food 2019, 12, 264–269. [Google Scholar] [CrossRef]
- Goel, A.; Kunnumakkara, A.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 5, 787–809. [Google Scholar] [CrossRef]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef]
- Wahlstrom, B.; Blennow, G. A study on the fate of curcumin in the rat. Acta Pharmacol. Toxicol. (Copenh) 1978, 43, 86–92. [Google Scholar] [CrossRef]
- Holder, G.M.; Plummer, J.L.; Ryan, A.J. The metabolism and excretion of curcumin (1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) in the rat. Xenobiotica 1978, 8, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Irving, G.R.; Howells, L.M.; Sale, S.; Kralj-Hans, I.; Atkin, W.S.; Clark, S.K.; Britton, R.G.; Jones, D.J.; Scott, E.N.; Berry, D.P.; et al. Prolonged biologically active colonic tissue levels of curcumin achieved after oral administration--a clinical pilot study including assessment of patient acceptability. Cancer Prev. Res. 2013, 6, 119–128. [Google Scholar] [CrossRef]
- Scazzocchio, B.; Minghetti, L.; D’Archivio, M. Interaction between Gut Microbiota and Curcumin: A New Key of Understanding for the Health Effects of Curcumin. Nutrients 2020, 12, 2499. [Google Scholar] [CrossRef]
- Salvucci, E. The human-microbiome superorganism and its modulation to restore health. Int. J. Food Sci. Nutr. 2019, 70, 781–795. [Google Scholar] [CrossRef]
- Oppenheimer, A. Turmeric (curcumin) in biliary diseases. Lancet 1937, 229, 619–621. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef]
- Jiao, Y.; Wilkinson, J.t.; Di, X.; Wang, W.; Hatcher, H.; Kock, N.D.; D’Agostino, R., Jr.; Knovich, M.A.; Torti, F.M.; Torti, S.V. Curcumin, a cancer chemopreventive and chemotherapeutic agent, is a biologically active iron chelator. Blood 2009, 113, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Devassy, J.G.; Nwachukwu, I.D.; Jones, P.J. Curcumin and cancer: Barriers to obtaining a health claim. Nutr. Rev. 2015, 73, 155–165. [Google Scholar] [CrossRef]
- DeVito, N.J.; Bacon, S.; Goldacre, B. Compliance with legal requirement to report clinical trial results on ClinicalTrials.gov: A cohort study. Lancet 2020, 395, 361–369. [Google Scholar] [CrossRef]
- Greil, R.; Greil-Ressler, S.; Weiss, L.; Schonlieb, C.; Magnes, T.; Radl, B.; Bolger, G.T.; Vcelar, B.; Sordillo, P.P. A phase 1 dose-escalation study on the safety, tolerability and activity of liposomal curcumin (Lipocurc™) in patients with locally advanced or metastatic cancer. Cancer Chemother. Pharmacol. 2018, 82, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Pires, N.; Gota, V.; Gulia, A.; Hingorani, L.; Agarwal, M.; Puri, A. Safety and pharmacokinetics of Withaferin-A in advanced stage high grade osteosarcoma: A phase I trial. J. Ayurveda Integr. Med. 2020, 11, 68–72. [Google Scholar] [CrossRef]
- Saadipoor, A.; Razzaghdoust, A.; Simforoosh, N.; Mahdavi, A.; Bakhshandeh, M.; Moghadam, M.; Abdollahi, H.; Mofid, B. Randomized, double-blind, placebo-controlled phase II trial of nanocurcumin in prostate cancer patients undergoing radiotherapy. Phytother. Res. 2019, 33, 370–378. [Google Scholar] [CrossRef]
- Cruz-Correa, M.; Hylind, L.M.; Marrero, J.H.; Zahurak, M.L.; Murray-Stewart, T.; Casero, R.A., Jr.; Montgomery, E.A.; Iacobuzio-Donahue, C.; Brosens, L.A.; Offerhaus, G.J.; et al. Efficacy and Safety of Curcumin in Treatment of Intestinal Adenomas in Patients With Familial Adenomatous Polyposis. Gastroenterology 2018, 155, 668–673. [Google Scholar] [CrossRef]
- Lao, C.D.; Ruffin, M.T.t.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Carroll, R.E.; Benya, R.V.; Turgeon, D.K.; Vareed, S.; Neuman, M.; Rodriguez, L.; Kakarala, M.; Carpenter, P.M.; McLaren, C.; Meyskens, F.L., Jr.; et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev. Res. (Phila) 2011, 4, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Latimer, B.; Ekshyyan, O.; Nathan, N.; Moore-Medlin, T.; Rong, X.; Ma, X.; Khandelwal, A.; Christy, H.T.; Abreo, F.; McClure, G.; et al. Enhanced Systemic Bioavailability of Curcumin Through Transmucosal Administration of a Novel Microgranular Formulation. Anticancer Res. 2015, 35, 6411–6418. [Google Scholar]
- Howells, L.M.; Iwuji, C.O.O.; Irving, G.R.B.; Barber, S.; Walter, H.; Sidat, Z.; Griffin-Teall, N.; Singh, R.; Foreman, N.; Patel, S.R.; et al. Curcumin Combined with FOLFOX Chemotherapy Is Safe and Tolerable in Patients with Metastatic Colorectal Cancer in a Randomized Phase IIa Trial. J. Nutr. 2019, 149, 1133–1139. [Google Scholar] [CrossRef]
- James, M.I.; Iwuji, C.; Irving, G.; Karmokar, A.; Higgins, J.A.; Griffin-Teal, N.; Thomas, A.; Greaves, P.; Cai, H.; Patel, S.R.; et al. Curcumin inhibits cancer stem cell phenotypes in ex vivo models of colorectal liver metastases, and is clinically safe and tolerable in combination with FOLFOX chemotherapy. Cancer Lett. 2015, 364, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Irving, G.R.; Iwuji, C.O.; Morgan, B.; Berry, D.P.; Steward, W.P.; Thomas, A.; Brown, K.; Howells, L.M. Combining curcumin (C3-complex, Sabinsa) with standard care FOLFOX chemotherapy in patients with inoperable colorectal cancer (CUFOX): Study protocol for a randomised control trial. Trials 2015, 16, 110. [Google Scholar] [CrossRef]
- Choi, Y.H.; Han, D.H.; Kim, S.W.; Kim, M.J.; Sung, H.H.; Jeon, H.G.; Jeong, B.C.; Seo, S.I.; Jeon, S.S.; Lee, H.M.; et al. A randomized, double-blind, placebo-controlled trial to evaluate the role of curcumin in prostate cancer patients with intermittent androgen deprivation. Prostate 2019, 79, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Saghatelyan, T.; Tananyan, A.; Janoyan, N.; Tadevosyan, A.; Petrosyan, H.; Hovhannisyan, A.; Hayrapetyan, L.; Arustamyan, M.; Arnhold, J.; Rotmann, A.R.; et al. Efficacy and safety of curcumin in combination with paclitaxel in patients with advanced, metastatic breast cancer: A comparative, randomized, double-blind, placebo-controlled clinical trial. Phytomedicine 2020, 70, 153218. [Google Scholar] [CrossRef]
- Hejazi, J.; Rastmanesh, R.; Taleban, F.A.; Molana, S.H.; Hejazi, E.; Ehtejab, G.; Hara, N. Effect of Curcumin Supplementation During Radiotherapy on Oxidative Status of Patients with Prostate Cancer: A Double Blinded, Randomized, Placebo-Controlled Study. Nutr. Cancer 2016, 68, 77–85. [Google Scholar] [CrossRef]
- Dützmann, S.; Schiborr, C.; Kocher, A.; Pilatus, U.; Hattingen, E.; Weissenberger, J.; Geßler, F.; Quick-Weller, J.; Franz, K.; Seifert, V.; et al. Intratumoral Concentrations and Effects of Orally Administered Micellar Curcuminoids in Glioblastoma Patients. Nutr. Cancer 2016, 68, 943–948. [Google Scholar] [CrossRef]
- Tuyaerts, S.; Rombauts, K.; Everaert, T.; Van Nuffel, A.M.T.; Amant, F. A Phase 2 Study to Assess the Immunomodulatory Capacity of a Lecithin-based Delivery System of Curcumin in Endometrial Cancer. Front. Nutr. 2018, 5, 138. [Google Scholar] [CrossRef]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef]
- Antony, B.; Merina, B.; Iyer, V.S.; Judy, N.; Lennertz, K.; Joyal, S. A Pilot Cross-Over Study to Evaluate Human Oral Bioavailability of BCM-95CG (Biocurcumax), A Novel Bioenhanced Preparation of Curcumin. Indian J. Pharm Sci. 2008, 70, 445–449. [Google Scholar] [CrossRef]
- Garcea, G.; Jones, D.J.; Singh, R.; Dennison, A.R.; Farmer, P.B.; Sharma, R.A.; Steward, W.P.; Gescher, A.J.; Berry, D.P. Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br. J. Cancer 2004, 90, 1011–1015. [Google Scholar] [CrossRef] [PubMed]
- Dahmke, I.N.; Backes, C.; Rudzitis-Auth, J.; Laschke, M.W.; Leidinger, P.; Menger, M.D.; Meese, E.; Mahlknecht, U. Curcumin intake affects miRNA signature in murine melanoma with mmu-miR-205-5p most significantly altered. PLoS ONE 2013, 8, e81122. [Google Scholar] [CrossRef]
- Cuomo, J.; Appendino, G.; Dern, A.S.; Schneider, E.; McKinnon, T.P.; Brown, M.J.; Togni, S.; Dixon, B.M. Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation. J. Nat. Prod. 2011, 74, 664–669. [Google Scholar] [CrossRef]
- Gota, V.S.; Maru, G.B.; Soni, T.G.; Gandhi, T.R.; Kochar, N.; Agarwal, M.G. Safety and pharmacokinetics of a solid lipid curcumin particle formulation in osteosarcoma patients and healthy volunteers. J. Agric. Food Chem. 2010, 58, 2095–2099. [Google Scholar] [CrossRef]
- Sasaki, H.; Sunagawa, Y.; Takahashi, K.; Imaizumi, A.; Fukuda, H.; Hashimoto, T.; Wada, H.; Katanasaka, Y.; Kakeya, H.; Fujita, M.; et al. Innovative preparation of curcumin for improved oral bioavailability. Biol. Pharm. Bull. 2011, 34, 660–665. [Google Scholar] [CrossRef]
- Mizumoto, A.; Ohashi, S.; Kamada, M.; Saito, T.; Nakai, Y.; Baba, K.; Hirohashi, K.; Mitani, Y.; Kikuchi, O.; Matsubara, J.; et al. Combination treatment with highly bioavailable curcumin and NQO1 inhibitor exhibits potent antitumor effects on esophageal squamous cell carcinoma. J. Gastroenterol. 2019, 54, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, R. Bioavailable curcumin formulations: A review of pharmacokinetic studies in healthy volunteers. J. Integr. Med. 2018, 16, 367–374. [Google Scholar] [CrossRef]
- Jamwal, R. Corrigendum to “Bioavailable curcumin formulations: A review of pharmacokinetic studies in healthy volunteers” [J. Integr. Med. 16 (2018) 367–374]. J. Integr. Med. 2019, 17, 310. [Google Scholar] [CrossRef]
- Troselj, K.G.; Kujundzic, R.N. Curcumin in combined cancer therapy. Curr. Pharm.Des. 2014, 20, 6682–6696. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Kraehe, P.; Lueders, C.; Shayan, P.; Goel, A.; Shakibaei, M. Curcumin suppresses crosstalk between colon cancer stem cells and stromal fibroblasts in the tumor microenvironment: Potential role of EMT. PLoS ONE 2014, 9, e107514. [Google Scholar] [CrossRef]
- Toden, S.; Okugawa, Y.; Buhrmann, C.; Nattamai, D.; Anguiano, E.; Baldwin, N.; Shakibaei, M.; Boland, C.R.; Goel, A. Novel Evidence for Curcumin and Boswellic Acid-Induced Chemoprevention through Regulation of miR-34a and miR-27a in Colorectal Cancer. Cancer Prev. Res. (Phila) 2015, 8, 431–443. [Google Scholar] [CrossRef]
- Yoshida, K.; Toden, S.; Ravindranathan, P.; Han, H.; Goel, A. Curcumin sensitizes pancreatic cancer cells to gemcitabine by attenuating PRC2 subunit EZH2, and the lncRNA PVT1 expression. Carcinogenesis 2017, 38, 1036–1046. [Google Scholar] [CrossRef]
- Toden, S.; Okugawa, Y.; Jascur, T.; Wodarz, D.; Komarova, N.L.; Buhrmann, C.; Shakibaei, M.; Boland, C.R.; Goel, A. Curcumin mediates chemosensitization to 5-fluorouracil through miRNA-induced suppression of epithelial-to-mesenchymal transition in chemoresistant colorectal cancer. Carcinogenesis 2015, 36, 355–367. [Google Scholar] [CrossRef]
- Kuriakose, M.A.; Ramdas, K.; Dey, B.; Iyer, S.; Rajan, G.; Elango, K.K.; Suresh, A.; Ravindran, D.; Kumar, R.R.; Prathiba, R.; et al. A Randomized Double-Blind Placebo-Controlled Phase IIB Trial of Curcumin in Oral Leukoplakia. Cancer Prev. Res. (Phila) 2016, 9, 683–691. [Google Scholar] [CrossRef]
- Purbadi, S.; Rustamadji, P.; Prijanti, A.R.; Sekarutami, S.M.; Sutrisna, B.; Suyatna, F.D.; Andrijono. Biocurcumin as Radiosensitiser for Cervical Cancer Study (BRACES): A Double-Blind Randomised Placebo-Controlled Trial. Evid. Based Complement. Alternat. Med. 2020, 2020, 1986793. [Google Scholar] [CrossRef] [PubMed]
- Arun, P.; Sagayaraj, A.; Azeem Mohiyuddin, S.M.; Santosh, D. Role of turmeric extract in minimising mucositis in patients receiving radiotherapy for head and neck squamous cell cancer: A randomised, placebo-controlled trial. J. Laryngol. Otol. 2020, 7, 1–6. [Google Scholar] [CrossRef]
- Shakibaei, M.; Buhrmann, C.; Kraehe, P.; Shayan, P.; Lueders, C.; Goel, A. Curcumin chemosensitizes 5-fluorouracil resistant MMR-deficient human colon cancer cells in high density cultures. PLoS ONE 2014, 9, e85397. [Google Scholar] [CrossRef]
- Du, B.; Shim, J.S. Targeting Epithelial-Mesenchymal Transition (EMT) to Overcome Drug Resistance in Cancer. Molecules 2016, 21, 965. [Google Scholar] [CrossRef]
- Shakibaei, M.; Kraehe, P.; Popper, B.; Shayan, P.; Goel, A.; Buhrmann, C. Curcumin potentiates antitumor activity of 5-fluorouracil in a 3D alginate tumor microenvironment of colorectal cancer. BMC Cancer 2015, 15, 250. [Google Scholar] [CrossRef]
- Siddappa, G.; Kulsum, S.; Ravindra, D.R.; Kumar, V.V.; Raju, N.; Raghavan, N.; Sudheendra, H.V.; Sharma, A.; Sunny, S.P.; Jacob, T.; et al. Curcumin and metformin-mediated chemoprevention of oral cancer is associated with inhibition of cancer stem cells. Mol. Carcinog. 2017, 56, 2446–2460. [Google Scholar] [CrossRef]
- Ravindranathan, P.; Pasham, D.; Balaji, U.; Cardenas, J.; Gu, J.; Toden, S.; Goel, A. A combination of curcumin and oligomeric proanthocyanidins offer superior anti-tumorigenic properties in colorectal cancer. Sci. Rep. 2018, 8, 13869. [Google Scholar] [CrossRef] [PubMed]
- Aoki, H.; Takada, Y.; Kondo, S.; Sawaya, R.; Aggarwal, B.B.; Kondo, Y. Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: Role of Akt and extracellular signal-regulated kinase signaling pathways. Mol. Pharmacol. 2007, 72, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Sung, B.; Kunnumakkara, A.B.; Sethi, G.; Anand, P.; Guha, S.; Aggarwal, B.B. Curcumin circumvents chemoresistance in vitro and potentiates the effect of thalidomide and bortezomib against human multiple myeloma in nude mice model. Mol. Cancer Ther. 2009, 8, 959–970. [Google Scholar] [CrossRef]
- Kim, T.; Davis, J.; Zhang, A.J.; He, X.; Mathews, S.T. Curcumin activates AMPK and suppresses gluconeogenic gene expression in hepatoma cells. Biochem. Biophys. Res. Commun. 2009, 388, 377–382. [Google Scholar] [CrossRef]
- Clark, C.A.; McEachern, M.D.; Shah, S.H.; Rong, Y.; Rong, X.; Smelley, C.L.; Caldito, G.C.; Abreo, F.W.; Nathan, C.O. Curcumin inhibits carcinogen and nicotine-induced Mammalian target of rapamycin pathway activation in head and neck squamous cell carcinoma. Cancer Prev. Res. (Phila) 2010, 3, 1586–1595. [Google Scholar] [CrossRef]
- Kakarala, M.; Brenner, D.E.; Korkaya, H.; Cheng, C.; Tazi, K.; Ginestier, C.; Liu, S.; Dontu, G.; Wicha, M.S. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res. Treat. 2010, 122, 777–785. [Google Scholar] [CrossRef]
- Tu, S.P.; Jin, H.; Shi, J.D.; Zhu, L.M.; Suo, Y.; Lu, G.; Liu, A.; Wang, T.C.; Yang, C.S. Curcumin induces the differentiation of myeloid-derived suppressor cells and inhibits their interaction with cancer cells and related tumor growth. Cancer Prev. Res. (Phila) 2012, 5, 205–215. [Google Scholar] [CrossRef]
- Alexandrow, M.G.; Song, L.J.; Altiok, S.; Gray, J.; Haura, E.B.; Kumar, N.B. Curcumin: A novel Stat3 pathway inhibitor for chemoprevention of lung cancer. Eur. J. Cancer Prev. 2012, 21, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Sonavane, K.; Phillips, J.; Ekshyyan, O.; Moore-Medlin, T.; Roberts Gill, J.; Rong, X.; Lakshmaiah, R.R.; Abreo, F.; Boudreaux, D.; Clifford, J.L.; et al. Topical curcumin-based cream is equivalent to dietary curcumin in a skin cancer model. J. Skin Cancer 2012, 2012, 147863. [Google Scholar] [CrossRef]
- Khandelwal, A.R.; Rong, X.; Moore-Medlin, T.; Ekshyyan, O.; Abreo, F.; Gu, X.; Nathan, C.A. Photopreventive Effect and Mechanism of AZD4547 and Curcumin C3 Complex on UVB-Induced Epidermal Hyperplasia. Cancer Prev. Res. (Phila) 2016, 9, 296–304. [Google Scholar] [CrossRef]
- Colacino, J.A.; McDermott, S.P.; Sartor, M.A.; Wicha, M.S.; Rozek, L.S. Transcriptomic profiling of curcumin-treated human breast stem cells identifies a role for stearoyl-coa desaturase in breast cancer prevention. Breast Cancer Res. Treat. 2016, 158, 29–41. [Google Scholar] [CrossRef]
- Messner, D.J.; Robinson, T.; Kowdley, K.V. Curcumin and Turmeric Modulate the Tumor-Promoting Effects of Iron In Vitro. Nutr. Cancer 2017, 69, 481–489. [Google Scholar] [CrossRef]
- Khandelwal, A.R.; Moore-Medlin, T.; Ekshyyan, O.; Gu, X.; Abreo, F.; Nathan, C.O. Local and systemic Curcumin C3 complex inhibits 4NQO-induced oral tumorigenesis via modulating FGF-2/FGFR-2 activation. Am. J. Cancer Res. 2018, 8, 2538–2547. [Google Scholar]
- Di Meo, F.; Filosa, S.; Madonna, M.; Giello, G.; Di Pardo, A.; Maglione, V.; Baldi, A.; Crispi, S. Curcumin C3 complex(R)/Bioperine(R) has antineoplastic activity in mesothelioma: An in vitro and in vivo analysis. J. Exp. Clin. Cancer Res. 2019, 38, 360. [Google Scholar] [CrossRef]
- Lin, Y.G.; Kunnumakkara, A.B.; Nair, A.; Merritt, W.M.; Han, L.Y.; Armaiz-Pena, G.N.; Kamat, A.A.; Spannuth, W.A.; Gershenson, D.M.; Lutgendorf, S.K.; et al. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-kappaB pathway. Clin. Cancer Res. 2007, 13, 3423–3430. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Diagaradjane, P.; Guha, S.; Deorukhkar, A.; Shentu, S.; Aggarwal, B.B.; Krishnan, S. Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor-kappaB-regulated gene products. Clin. Cancer Res. 2008, 14, 2128–2136. [Google Scholar] [CrossRef]
- Siddiqui, R.A.; Hassan, S.; Harvey, K.A.; Rasool, T.; Das, T.; Mukerji, P.; DeMichele, S. Attenuation of proteolysis and muscle wasting by curcumin c3 complex in MAC16 colon tumour-bearing mice. Br. J. Nutr. 2009, 102, 967–975. [Google Scholar] [CrossRef]
- Phillips, J.M.; Clark, C.; Herman-Ferdinandez, L.; Moore-Medlin, T.; Rong, X.; Gill, J.R.; Clifford, J.L.; Abreo, F.; Nathan, C.O. Curcumin inhibits skin squamous cell carcinoma tumor growth in vivo. Otolaryngol. Head Neck Surg. 2011, 145, 58–63. [Google Scholar] [CrossRef]
- Phillips, J.; Moore-Medlin, T.; Sonavane, K.; Ekshyyan, O.; McLarty, J.; Nathan, C.A. Curcumin inhibits UV radiation-induced skin cancer in SKH-1 mice. Otolaryngol. Head Neck Surg. 2013, 148, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wu, R.; Gaspar, J.M.; Sargsyan, D.; Su, Z.Y.; Zhang, C.; Gao, L.; Cheng, D.; Li, W.; Wang, C.; et al. DNA methylome and transcriptome alterations and cancer prevention by curcumin in colitis-accelerated colon cancer in mice. Carcinogenesis 2018, 39, 669–680. [Google Scholar] [CrossRef]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef]
- Garcea, G.; Berry, D.P.; Jones, D.J.; Singh, R.; Dennison, A.R.; Farmer, P.B.; Sharma, R.A.; Steward, W.P.; Gescher, A.J. Consumption of the putative chemopreventive agent curcumin by cancer patients: Assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 120–125. [Google Scholar]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef]
- Golombick, T.; Diamond, T.H.; Badmaev, V.; Manoharan, A.; Ramakrishna, R. The potential role of curcumin in patients with monoclonal gammopathy of undefined significance--its effect on paraproteinemia and the urinary N-telopeptide of type I collagen bone turnover marker. Clin. Cancer Res. 2009, 15, 5917–5922. [Google Scholar] [CrossRef] [PubMed]
- Rai, B.; Kaur, J.; Jacobs, R.; Singh, J. Possible action mechanism for curcumin in pre-cancerous lesions based on serum and salivary markers of oxidative stress. J. Oral. Sci. 2010, 52, 251–256. [Google Scholar] [CrossRef]
- Golombick, T.; Diamond, T.H.; Manoharan, A.; Ramakrishna, R. Monoclonal gammopathy of undetermined significance, smoldering multiple myeloma, and curcumin: A randomized, double-blind placebo-controlled cross-over 4g study and an open-label 8g extension study. Am. J. Hematol. 2012, 87, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Guha, S.; Krishnan, S.; Diagaradjane, P.; Gelovani, J.; Aggarwal, B.B. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007, 67, 3853–3861. [Google Scholar] [CrossRef] [PubMed]
- Epelbaum, R.; Schaffer, M.; Vizel, B.; Badmaev, V.; Bar-Sela, G. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr. Cancer 2010, 62, 1137–1141. [Google Scholar] [CrossRef]
- Kanai, M.; Yoshimura, K.; Asada, M.; Imaizumi, A.; Suzuki, C.; Matsumoto, S.; Nishimura, T.; Mori, Y.; Masui, T.; Kawaguchi, Y.; et al. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother. Pharmacol. 2011, 68, 157–164. [Google Scholar] [CrossRef]
- Ibrahim, A.; El-Meligy, A.; Fetaih, H.; Dessouki, A.; Stoica, G.; Barhoumi, R. Effect of curcumin and Meriva on the lung metastasis of murine mammary gland adenocarcinoma. In Vivo 2010, 24, 401–408. [Google Scholar]
- Chandra, D.; Jahangir, A.; Cornelis, F.; Rombauts, K.; Meheus, L.; Jorcyk, C.L.; Gravekamp, C. Cryoablation and Meriva have strong therapeutic effect on triple-negative breast cancer. Oncoimmunology 2016, 5, e1049802. [Google Scholar] [CrossRef]
- Belcaro, G.; Hosoi, M.; Pellegrini, L.; Appendino, G.; Ippolito, E.; Ricci, A.; Ledda, A.; Dugall, M.; Cesarone, M.R.; Maione, C.; et al. A controlled study of a lecithinized delivery system of curcumin (Meriva®) to alleviate the adverse effects of cancer treatment. Phytother. Res. 2014, 28, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Howells, L.M.; Sale, S.; Sriramareddy, S.N.; Irving, G.R.; Jones, D.J.; Ottley, C.J.; Pearson, D.G.; Mann, C.D.; Manson, M.M.; Berry, D.P.; et al. Curcumin ameliorates oxaliplatin-induced chemoresistance in HCT116 colorectal cancer cells in vitro and in vivo. Int. J. Cancer 2011, 129, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Ledda, A.; Belcaro, G.; Dugall, M.; Luzzi, R.; Scoccianti, M.; Togni, S.; Appendino, G.; Ciammaichella, G. Meriva®, a lecithinized curcumin delivery system, in the control of benign prostatic hyperplasia: A pilot, product evaluation registry study. Panminerva Med. 2012, 54, 17–22. [Google Scholar]
- Panahi, Y.; Saadat, A.; Beiraghdar, F.; Sahebkar, A. Adjuvant therapy with bioavailability-boosted curcuminoids suppresses systemic inflammation and improves quality of life in patients with solid tumors: A randomized double-blind placebo-controlled trial. Phytother. Res. 2014, 28, 1461–1467. [Google Scholar] [CrossRef]
- Pastorelli, D.; Fabricio, A.S.C.; Giovanis, P.; D’Ippolito, S.; Fiduccia, P.; Soldà, C.; Buda, A.; Sperti, C.; Bardini, R.; Da Dalt, G.; et al. Phytosome complex of curcumin as complementary therapy of advanced pancreatic cancer improves safety and efficacy of gemcitabine: Results of a prospective phase II trial. Pharmacol. Res. 2018, 132, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.P.; Mukerjee, A.; Gdowski, A.; Helson, L.; Bouchard, A.; Majeed, M.; Vishwanatha, J.K. Curcumin-ER Prolonged Subcutaneous Delivery for the Treatment of Non- Small Cell Lung Cancer. J. Biomed. Nanotechnol. 2016, 12, 679–688. [Google Scholar] [CrossRef]
- Withers, S.S.; York, D.; Johnson, E.; Al-Nadaf, S.; Skorupski, K.A.; Rodriguez, C.O., Jr.; Burton, J.H.; Guerrero, T.; Sein, K.; Wittenburg, L.; et al. In vitro and in vivo activity of liposome-encapsulated curcumin for naturally occurring canine cancers. Vet. Comp. Oncol. 2018, 16, 571–579. [Google Scholar] [CrossRef]
- Bolger, G.T.; Licollari, A.; Tan, A.; Greil, R.; Vcelar, B.; Greil-Ressler, S.; Weiss, L.; Schönlieb, C.; Magnes, T.; Radl, B.; et al. Pharmacokinetics of liposomal curcumin (Lipocurc™) infusion: Effect of co-medication in cancer patients and comparison with healthy individuals. Cancer Chemother. Pharmacol. 2019, 83, 265–275. [Google Scholar] [CrossRef]
- Maiti, P.; Al-Gharaibeh, A.; Kolli, N.; Dunbar, G.L. Solid Lipid Curcumin Particles Induce More DNA Fragmentation and Cell Death in Cultured Human Glioblastoma Cells than Does Natural Curcumin. Oxid. Med. Cell Longev. 2017, 2017, 9656719. [Google Scholar] [CrossRef]
- Hazarey, V.K.; Sakrikar, A.R.; Ganvir, S.M. Efficacy of curcumin in the treatment for oral submucous fibrosis-A randomized clinical trial. J. Oral. Maxillofac. Pathol. 2015, 19, 145–152. [Google Scholar] [CrossRef]
- Milano, F.; Mari, L.; van de Luijtgaarden, W.; Parikh, K.; Calpe, S.; Krishnadath, K.K. Nano-curcumin inhibits proliferation of esophageal adenocarcinoma cells and enhances the T cell mediated immune response. Front. Oncol. 2013, 3, 137. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Ho, J.N.; Kook, H.R.; Lee, S.; Oh, J.J.; Hong, S.K.; Lee, S.E.; Byun, S.S. Theracurmin® efficiently inhibits the growth of human prostate and bladder cancer cells via induction of apoptotic cell death and cell cycle arrest. Oncol. Rep. 2016, 35, 1463–1472. [Google Scholar] [CrossRef]
- Ide, H.; Lu, Y.; Noguchi, T.; Muto, S.; Okada, H.; Kawato, S.; Horie, S. Modulation of AKR1C2 by curcumin decreases testosterone production in prostate cancer. Cancer Sci. 2018, 109, 1230–1238. [Google Scholar] [CrossRef]
- Adachi, S.; Hamoya, T.; Fujii, G.; Narita, T.; Komiya, M.; Miyamoto, S.; Kurokawa, Y.; Takahashi, M.; Takayama, T.; Ishikawa, H.; et al. Theracurmin inhibits intestinal polyp development in Apc-mutant mice by inhibiting inflammation-related factors. Cancer Sci. 2020, 111, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- De Velasco, M.A.; Lu, Y.; Kura, Y.; China, T.; Inoue, Y.; Nakayama, A.; Okada, H.; Horie, S.; Uemura, H.; Ide, H. Chemopreventive effects of nanoparticle curcumin in a mouse model of Pten-deficient prostate cancer. Hum. Cell 2020, 33, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Izumi, Y.; Yamamoto, J.; Nomori, H. Coadministration of erlotinib and curcumin augmentatively reduces cell viability in lung cancer cells. Phytother. Res. 2014, 28, 728–735. [Google Scholar] [CrossRef]
- Kanai, M.; Otsuka, Y.; Otsuka, K.; Sato, M.; Nishimura, T.; Mori, Y.; Kawaguchi, M.; Hatano, E.; Kodama, Y.; Matsumoto, S.; et al. A phase I study investigating the safety and pharmacokinetics of highly bioavailable curcumin (Theracurmin) in cancer patients. Cancer Chemother. Pharmacol. 2013, 71, 1521–1530. [Google Scholar] [CrossRef]
- Feng, W.; Wang, H.; Zhang, P.; Gao, C.; Tao, J.; Ge, Z.; Zhu, D.; Bi, Y. Modulation of gut microbiota contributes to curcumin-mediated attenuation of hepatic steatosis in rats. Biochim. Biophys Acta. Gen. Subj. 2017, 1861, 1801–1812. [Google Scholar] [CrossRef]
- Lou, Y.; Zheng, J.; Hu, H.; Lee, J.; Zeng, S. Application of ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry to identify curcumin metabolites produced by human intestinal bacteria. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 985, 38–47. [Google Scholar] [CrossRef]
- Nagpal, R.; Wang, S.; Solberg Woods, L.C.; Seshie, O.; Chung, S.T.; Shively, C.A.; Register, T.C.; Craft, S.; McClain, D.A.; Yadav, H. Comparative Microbiome Signatures and Short-Chain Fatty Acids in Mouse, Rat, Non-human Primate, and Human Feces. Front. Microbiol. 2018, 9, 2897. [Google Scholar] [CrossRef]
- Peterson, C.T.; Vaughn, A.R.; Sharma, V.; Chopra, D.; Mills, P.J.; Peterson, S.N.; Sivamani, R.K. Effects of Turmeric and Curcumin Dietary Supplementation on Human Gut Microbiota: A Double-Blind, Randomized, Placebo-Controlled Pilot Study. J. Evid. Based Integr. Med. 2018, 23, 2515690X18790725. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Liu, L.; Ji, H.F. Regulative effects of curcumin spice administration on gut microbiota and its pharmacological implications. Food Nutr. Res. 2017, 61, 1361780. [Google Scholar] [CrossRef]
- Ohno, M.; Nishida, A.; Sugitani, Y.; Nishino, K.; Inatomi, O.; Sugimoto, M.; Kawahara, M.; Andoh, A. Nanoparticle curcumin ameliorates experimental colitis via modulation of gut microbiota and induction of regulatory T cells. PLoS ONE 2017, 12, e0185999. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Kaiko, G.E.; Ryu, S.H.; Koues, O.I.; Collins, P.L.; Solnica-Krezel, L.; Pearce, E.J.; Pearce, E.L.; Oltz, E.M.; Stappenbeck, T.S. The Colonic Crypt Protects Stem Cells from Microbiota-Derived Metabolites. Cell 2016, 165, 1708–1720. [Google Scholar] [CrossRef]
- Sánchez-Alcoholado, L.; Ordóñez, R.; Otero, A.; Plaza-Andrade, I.; Laborda-Illanes, A.; Medina, J.A.; Ramos-Molina, B.; Gómez-Millán, J.; Queipo-Ortuño, M.I. Gut Microbiota-Mediated Inflammation and Gut Permeability in Patients with Obesity and Colorectal Cancer. Int. J. Mol. Sci. 2020, 21, 6782. [Google Scholar] [CrossRef]
- Kujundžić, R.N.; Stepanić, V.; Milković, L.; Gašparović, A.; Tomljanović, M.; Trošelj, K.G. Curcumin and its Potential for Systemic Targeting of Inflamm-Aging and Metabolic Reprogramming in Cancer. Int. J. Mol. Sci. 2019, 20, 1180. [Google Scholar] [CrossRef]
- Zhao, H.M.; Xu, R.; Huang, X.Y.; Cheng, S.M.; Huang, M.F.; Yue, H.Y.; Wang, X.; Zou, Y.; Lu, A.P.; Liu, D.Y. Curcumin improves regulatory T cells in gut-associated lymphoid tissue of colitis mice. World J. Gastroenterol. 2016, 22, 5374–5383. [Google Scholar] [CrossRef]
- Larussa, T.; Gervasi, S.; Liparoti, R.; Suraci, E.; Marasco, R.; Imeneo, M.; Luzza, F. Downregulation of Interleukin- (IL-) 17 through Enhanced Indoleamine 2,3-Dioxygenase (IDO) Induction by Curcumin: A Potential Mechanism of Tolerance towards Helicobacter pylori. J. Immunol. Res. 2018, 2018, 3739593. [Google Scholar] [CrossRef] [PubMed]
- Madhur, M.S.; Lob, H.E.; McCann, L.A.; Iwakura, Y.; Blinder, Y.; Guzik, T.J.; Harrison, D.G. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 2010, 55, 500–507. [Google Scholar] [CrossRef]
- Vitiello, G.A.; Miller, G. Targeting the interleukin-17 immune axis for cancer immunotherapy. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef]
- Sethi, V.; Kurtom, S.; Tarique, M.; Lavania, S.; Malchiodi, Z.; Hellmund, L.; Zhang, L.; Sharma, U.; Giri, B.; Garg, B.; et al. Gut Microbiota Promotes Tumor Growth in Mice by Modulating Immune Response. Gastroenterology 2018, 155, 33–37. [Google Scholar] [CrossRef]
- Serrano-Gomez, S.J.; Maziveyi, M.; Alahari, S.K. Regulation of epithelial-mesenchymal transition through epigenetic and post-translational modifications. Mol. Cancer 2016, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.H.; Donaher, J.L.; Murphy, D.A.; Chau, S.; Yang, J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell 2012, 22, 725–736. [Google Scholar] [CrossRef]
- Wei, Q.; Qian, Y.; Yu, J.; Wong, C.C. Metabolic rewiring in the promotion of cancer metastasis: Mechanisms and therapeutic implications. Oncogene 2020, 39, 6139–6156. [Google Scholar] [CrossRef]
- Bae, M.K.; Kim, S.H.; Jeong, J.W.; Lee, Y.M.; Kim, H.S.; Kim, S.R.; Yun, I.; Bae, S.K.; Kim, K.W. Curcumin inhibits hypoxia-induced angiogenesis via down-regulation of HIF-1. Oncol. Rep. 2006, 15, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Majeed, M.; Sahebkar, A. Curcumin: A potent agent to reverse epithelial-to-mesenchymal transition. Cell Oncol. (Dordr) 2019, 42, 405–421. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.; Liang, J.; Li, W.; Zhu, Y.; Zhang, M.; Wang, C.; Hou, J. Deregulation of Hexokinase II Is Associated with Glycolysis, Autophagy, and the Epithelial-Mesenchymal Transition in Tongue Squamous Cell Carcinoma under Hypoxia. Biomed. Res. Int. 2018, 2018, 8480762. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhang, L.F.; Zhang, H.W.; Hu, S.; Lu, M.H.; Liang, S.; Li, B.; Li, Y.; Li, D.; Wang, E.D.; et al. A novel miR-155/miR-143 cascade controls glycolysis by regulating hexokinase 2 in breast cancer cells. EMBO J. 2012, 31, 1985–1998. [Google Scholar] [CrossRef]
- Geng, C.; Li, J.; Ding, F.; Wu, G.; Yang, Q.; Sun, Y.; Zhang, Z.; Dong, T.; Tian, X. Curcumin suppresses 4-hydroxytamoxifen resistance in breast cancer cells by targeting SLUG/Hexokinase 2 pathway. Biochem. Biophys Res. Commun. 2016, 473, 147–153. [Google Scholar] [CrossRef]
- Wang, K.; Fan, H.; Chen, Q.; Ma, G.; Zhu, M.; Zhang, X.; Zhang, Y.; Yu, J. Curcumin inhibits aerobic glycolysis and induces mitochondrial-mediated apoptosis through hexokinase II in human colorectal cancer cells in vitro. Anticancer Drugs 2015, 26, 15–24. [Google Scholar] [CrossRef]
- Yang, P.; Li, Z.; Fu, R.; Wu, H.; Li, Z. Pyruvate kinase M2 facilitates colon cancer cell migration via the modulation of STAT3 signalling. Cell Signal. 2014, 26, 1853–1862. [Google Scholar] [CrossRef]
- Hamabe, A.; Konno, M.; Tanuma, N.; Shima, H.; Tsunekuni, K.; Kawamoto, K.; Nishida, N.; Koseki, J.; Mimori, K.; Gotoh, N.; et al. Role of pyruvate kinase M2 in transcriptional regulation leading to epithelial-mesenchymal transition. Proc. Natl. Acad. Sci. USA 2014, 111, 15526–15531. [Google Scholar] [CrossRef]
- Siddiqui, F.A.; Prakasam, G.; Chattopadhyay, S.; Rehman, A.U.; Padder, R.A.; Ansari, M.A.; Irshad, R.; Mangalhara, K.; Bamezai, R.N.K.; Husain, M.; et al. Curcumin decreases Warburg effect in cancer cells by down-regulating pyruvate kinase M2 via mTOR-HIF1alpha inhibition. Sci. Rep. 2018, 8, 8323. [Google Scholar] [CrossRef]
- Mojzeš, A.; Tomljanović, M.; Milković, L.; Kujundžić, R.N.; Gašparović, A.; Trošelj, K.G. Cell-Type Specific Metabolic Response of Cancer Cells to Curcumin. Int. J. Mol. Sci. 2020, 21, 1661. [Google Scholar] [CrossRef]
- Lee, A.Y.; Fan, C.C.; Chen, Y.A.; Cheng, C.W.; Sung, Y.J.; Hsu, C.P.; Kao, T.Y. Curcumin Inhibits Invasiveness and Epithelial-Mesenchymal Transition in Oral Squamous Cell Carcinoma Through Reducing Matrix Metalloproteinase 2, 9 and Modulating p53-E-Cadherin Pathway. Integr. Cancer Ther. 2015, 14, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, H.; Xu, C.; Song, L.; Huang, L.; Lai, Y.; Wang, Y.; Chen, H.; Gu, D.; Ren, L.; et al. Curcumin inhibits tumor epithelialmesenchymal transition by downregulating the Wnt signaling pathway and upregulating NKD2 expression in colon cancer cells. Oncol. Rep. 2016, 35, 2615–2623. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mazumdar, M.; Chakraborty, S.; Manna, A.; Saha, S.; Khan, P.; Bhattacharjee, P.; Guha, D.; Adhikary, A.; Mukhjerjee, S.; et al. Curcumin inhibits breast cancer stem cell migration by amplifying the E-cadherin/beta-catenin negative feedback loop. Stem. Cell Res. Ther. 2014, 5, 116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cheng, X.; Gao, Y.; Zhang, C.; Bao, J.; Guan, H.; Yu, H.; Lu, R.; Xu, Q.; Sun, Y. Curcumin inhibits metastasis in human papillary thyroid carcinoma BCPAP cells via down-regulation of the TGF-beta/Smad2/3 signaling pathway. Exp. Cell Res. 2016, 341, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Ali, S.; Ahmad, A.; Azmi, A.S.; Li, Y.; Banerjee, S.; Kong, D.; Sethi, S.; Aboukameel, A.; Padhye, S.B.; et al. Hypoxia-induced aggressiveness of pancreatic cancer cells is due to increased expression of VEGF, IL-6 and miR-21, which can be attenuated by CDF treatment. PLoS ONE 2012, 7, e50165. [Google Scholar] [CrossRef]
- Yang, M.H.; Wu, M.Z.; Chiou, S.H.; Chen, P.M.; Chang, S.Y.; Liu, C.J.; Teng, S.C.; Wu, K.J. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat. Cell Biol. 2008, 10, 295–305. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, X.; Peng, Y.; Wu, M.; Zhang, P.; Xie, R.; Wu, Y.; Yan, Q.; Liu, S.; Wang, J. HIF-1alpha Promotes Epithelial-Mesenchymal Transition and Metastasis through Direct Regulation of ZEB1 in Colorectal Cancer. PLoS ONE 2015, 10, e0129603. [Google Scholar] [CrossRef]
- Yu, X.; Ma, R.; Wu, Y.; Zhai, Y.; Li, S. Reciprocal Regulation of Metabolic Reprogramming and Epigenetic Modifications in Cancer. Front. Genet. 2018, 9, 394. [Google Scholar] [CrossRef]
- Kanska, J.; Aspuria, P.P.; Taylor-Harding, B.; Spurka, L.; Funari, V.; Orsulic, S.; Karlan, B.Y.; Wiedemeyer, W.R. Glucose deprivation elicits phenotypic plasticity via ZEB1-mediated expression of NNMT. Oncotarget 2017, 8, 26200–26220. [Google Scholar] [CrossRef]
- Ulanovskaya, O.A.; Zuhl, A.M.; Cravatt, B.F. NNMT promotes epigenetic remodeling in cancer by creating a metabolic methylation sink. Nat. Chem. Biol. 2013, 9, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, D.B.; Waring, R.H.; Parsons, R.B.; Barlow, D.J.; Williams, A.C. Nicotinamide N-Methyltransferase: Genomic Connection to Disease. Int. J. Tryptophan Res. 2020, 13, 1178646920919770. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, L.; Wang, W.; Ma, S.; Liu, H.; Zang, X.; Zhang, Y.; Guan, F. Downregulation of nicotinamide N-methyltransferase inhibits migration and epithelial-mesenchymal transition of esophageal squamous cell carcinoma via Wnt/β-catenin pathway. Mol. Cell Biochem. 2019, 460, 93–103. [Google Scholar] [CrossRef]
- Hahn, Y.I.; Kim, S.J.; Choi, B.Y.; Cho, K.C.; Bandu, R.; Kim, K.P.; Kim, D.H.; Kim, W.; Park, J.S.; Han, B.W.; et al. Curcumin interacts directly with the Cysteine 259 residue of STAT3 and induces apoptosis in H-Ras transformed human mammary epithelial cells. Sci. Rep. 2018, 8, 6409. [Google Scholar] [CrossRef]
- Li, S.; Qiao, L.; Yang, Z.; He, C. Prognostic Value of Nicotinamide N-Methyltransferase Expression in Patients With Solid Tumors: A Systematic Review and Meta-Analysis. Front. Physiol. 2018, 9, 1407. [Google Scholar] [CrossRef]
- Eckert, M.A.; Coscia, F.; Chryplewicz, A.; Chang, J.W.; Hernandez, K.M.; Pan, S.; Tienda, S.M.; Nahotko, D.A.; Li, G.; Blaženović, I.; et al. Proteomics reveals NNMT as a master metabolic regulator of cancer-associated fibroblasts. Nature 2019, 569, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Tomida, M.; Ohtake, H.; Yokota, T.; Kobayashi, Y.; Kurosumi, M. Stat3 up-regulates expression of nicotinamide N-methyltransferase in human cancer cells. J. Cancer Res. Clin. Oncol. 2008, 134, 551–559. [Google Scholar] [CrossRef]
- Pissios, P. Nicotinamide N-Methyltransferase: More Than a Vitamin B3 Clearance Enzyme. Trends Endocrinol. Metab. 2017, 28, 340–353. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, D.; Wang, W.; Zhang, L.; Liu, H.; Ma, S.; Guo, W.; Yao, M.; Zhang, K.; Li, W.; et al. Nicotinamide N-methyltransferase decreases 5-fluorouracil sensitivity in human esophageal squamous cell carcinoma through metabolic reprogramming and promoting the Warburg effect. Mol. Carcinog 2020, 59, 940–954. [Google Scholar] [CrossRef]
- Li, W.C.; Huang, C.H.; Hsieh, Y.T.; Chen, T.Y.; Cheng, L.H.; Chen, C.Y.; Liu, C.J.; Chen, H.M.; Huang, C.L.; Lo, J.F.; et al. Regulatory Role of Hexokinase 2 in Modulating Head and Neck Tumorigenesis. Front. Oncol. 2020, 10, 176. [Google Scholar] [CrossRef]
- Wu, X.; Xia, J.; Zhang, J.; Zhu, Y.; Wu, Y.; Guo, J.; Chen, S.; Lei, Q.; Meng, B.; Kuang, C.; et al. Phosphoglycerate dehydrogenase promotes proliferation and bortezomib resistance through increasing reduced glutathione synthesis in multiple myeloma. Br. J. Haematol. 2020, 190, 52–66. [Google Scholar] [CrossRef]
- Ye, J.; Fan, J.; Venneti, S.; Wan, Y.W.; Pawel, B.R.; Zhang, J.; Finley, L.W.; Lu, C.; Lindsten, T.; Cross, J.R.; et al. Serine catabolism regulates mitochondrial redox control during hypoxia. Cancer Discov. 2014, 4, 1406–1417. [Google Scholar] [CrossRef]
- Woo, C.C.; Chen, W.C.; Teo, X.Q.; Radda, G.K.; Lee, P.T. Downregulating serine hydroxymethyltransferase 2 (SHMT2) suppresses tumorigenesis in human hepatocellular carcinoma. Oncotarget 2016, 7, 53005–53017. [Google Scholar] [CrossRef]
- Angelo, L.S.; Maxwell, D.S.; Wu, J.Y.; Sun, D.; Hawke, D.H.; McCutcheon, I.E.; Slopis, J.M.; Peng, Z.; Bornmann, W.G.; Kurzrock, R. Binding partners for curcumin in human schwannoma cells: Biologic implications. Bioorg. Med. Chem. 2013, 21, 932–939. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, Q.; Li, Y.; Tang, H.; Liu, W.; Yang, X. Doxorubicin and curcumin co-delivery by lipid nanoparticles for enhanced treatment of diethylnitrosamine-induced hepatocellular carcinoma in mice. Eur. J. Pharm. Biopharm. 2015, 93, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Pignanelli, C.; Ma, D.; Noel, M.; Ropat, J.; Mansour, F.; Curran, C.; Pupulin, S.; Larocque, K.; Wu, J.; Liang, G.; et al. Selective Targeting of Cancer Cells by Oxidative Vulnerabilities with Novel Curcumin Analogs. Sci. Rep. 2017, 7, 1105. [Google Scholar] [CrossRef]
- Edwards, R.L.; Luis, P.B.; Varuzza, P.V.; Joseph, A.I.; Presley, S.H.; Chaturvedi, R.; Schneider, C. The anti-inflammatory activity of curcumin is mediated by its oxidative metabolites. J. Biol. Chem. 2017, 292, 21243–21252. [Google Scholar] [CrossRef] [PubMed]
- Bahadori, F.; Demiray, M. A Realistic View on “The Essential Medicinal Chemistry of Curcumin”. ACS Med. Chem. Lett. 2017, 8, 893–896. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; DeBerardinis, R.J. Mechanisms and Implications of Metabolic Heterogeneity in Cancer. Cell Metab. 2019, 30, 434–446. [Google Scholar] [CrossRef]
- Ward, P.S.; Thompson, C.B. Metabolic reprogramming: A cancer hallmark even warburg did not anticipate. Cancer Cell 2012, 21, 297–308. [Google Scholar] [CrossRef]

| BCM-95® (Biocurcumax™) | ||
|---|---|---|
| MONO | ||
| Cell Culture Models | Animal Models | Clinical Studies |
| Suppression of cross-talk between HCT-116 colon cancer stem cells and MRC-5 fibroblasts in the 3D co-culture model [59]. Cytotoxic effect on HCT116 and SW480 colon cancer cell lines; promotion of cell-cycle arrest and apoptosis, strong upregulation of hsa-miR 34a and enhanced expression of F-Box and WD Repeat Containing 7 (FBXW7) [60]. A strong effect on cellular viability and colony formation ability of pancreatic cell lines (BxPC3-G and Panc1), already as 5 μM [61]. | HCT116-5-FUR (5-fluorouracil resistant) xenografts in nude mice (50 mg BCM-95®/kg daily i.p. for 40 days): attenuation of tumor growth [62]. HCT116 xenografts in mice (25 mg BCM-95®/kg daily i.p.; 20 days): enhanced suppression of tumor growth; decrease of miR-27a; increase of miR-34a [60]. The BxPC3-G xenografts in athymic mice (100 mg BCM-95®/kg daily i.p.; 28 days): suppression of tumor growth (p < 0.05) downregulation of plasmocytoma variant translocation 1 (PVT1), Myc and multidrug resistance-1 (MDR1) [61]. | A double-blinded, randomized, placebo-controlled study (n = 40) involving prostate cancer patients being treated with radiotherapy. Giving 3 g of BCM-95® starting 1 week before the initiation of radiotherapy and continuing until its completion: no significant effect on treatment outcome, but recordable effect on oxidative status during and after radiotherapy, presented as increased total plasma antioxidative activity and decreased activity of plasma superoxide dismutase (SOD) [45]. A randomized double-blind placebo-controlled phase II B trial, (n = 223) involving 111 patients with oral leukoplakia + 121 patients in the placebo group. Giving BCM-95® (3.6 g/day) for six months was well-tolerated and demonstrated significant (p = 0.02) and durable clinical response for 6 months [63]. Double-blind placebo randomized-controlled trial (n = 121) involving patients with cervical cancer being treated with radiotherapy. Giving 1 g of BCM-95® orally, three times daily for 9 weeks: there was no evidence of improved clinical response to radiation treatment [64]. A randomized, blind placebo-controlled trial (n = 61); patients receiving radiotherapy for head and neck squamous cell cancer and receiving three daily doses of 500 mg BCM-95® CG capsules; total daily dose 1.5 g until radiotherapy completion: decreased incidence and severity of radiation-induced mucositis, p < 0.001 [65]. Adverse Events (AE): A total of 61 (27.4%) subjects experienced at least one AE during the study period, 26 (23.4%) in curcumin arm, and 35 (31.3%) in placebo arm. Four subjects from curcumin arm and 1 from placebo arm withdrew from the study due to Adverse Events (AEs)/Serious Adverse Events (SAEs). Moderate/severe AEs were recorded in four patients in the curcumin group: anemia, skin/subcutaneous tissue disorders and hypertension. The Independent Data and Safety Monitoring Board did not identify any safety concerns, as there was no statistically significant difference in major AEs between the curcumin and placebo arms [63]. The most common adverse events were gastrointestinal nausea/vomiting (30% curcumin vs. 24.5% placebo), stomatitis (20% curcumin vs. 25% placebo), diarrhea (3.33% curcumin vs. 0% placebo), increased liver enzymes: aspartate aminotransferase, AST (5% curcumin vs. 3.3% placebo) and alanine aminotransferase, ALT (5% curcumin vs. 0% placebo) and increased creatinine (5% curcumin vs. 1.6% placebo). However, no statistically significant difference between treated and placebo group was found [64] |
| COMBINED | ||
| Cell Culture Models | Animal Models | |
| Chemosensitization to 5-FU (5-fluorouracil) of DNA mismatch repair (MMR)-deficient and MMR-proficient HCT116 colon cancer cell line through targeting the cancer stem cells subpopulation and the inhibition of β-catenin and NF-κB signaling pathways [66]. Moderate chemosensitization of 5-FU resistant colon cancer cell lines, HCT116R and SW480R, through suppressive micro-RNA (miR-34a, -200c, -141, -429) mediated inhibition of epithelial-to-mesenchymal transition (EMT). In 5-FU resistant cell lines, the combined treatment with BCM-95® increased the rate of apoptosis (10 µM 5-FU, 10 µM curcumin) with respect to 5-FU alone, through upregulated p21, and altered cell cycle through downregulated cyclin D1 (CycD1) and c-MYC [62,67]. Chemosensitization of MMR-deficient and MMR-proficient HCT116 colon cancer cell line to 5-FU in a 3D alginate mimicking tumor microenvironment [68]. Chemosensitization of pancreatic cancer cells (BxPC3-G and Panc1) to gemcitabine by attenuating Polycomb Repressive Complex 2 (PRC2) subunit Enhancer of Zeste 2 (EZH2), and the long non-coding RNA Plasmacytoma Variant Translocation 1 (PVT1) expression [61]. Primary culture of oral cavity cancer cells: decrease of CD44+ population treated with BCM-95® (p = 0.0001), and combination of curcumin and metformin (45.7%, p = 0.0001). The treatment also inhibited the migratory and self-renewal properties of cancer stem cells (CSC) [69]. Combination of oligomeric proanthocyanidins (OPC) and BCM-95®: strong anti-tumorigenic effect shown in six colorectal cancer cell lines. Decrease of tumor organoid formation and growth of patient-derived colorectal cancer cell. Combined treatment, as contrasted with individual treatments, strongly influenced Hedgehog signaling pathway and pathways related to insulin and peroxisome proliferator-activated receptors PPAR [70]. | HCT116-5-FUR xenografts in nude mice: 20 mg/kg every 2 days of 5-FU; 50 mg/kg daily BCM-95® i.p. for 40 days; attenuation of tumor growth, indicating significance for re-sensitization against 5-FU [62]. The BxPC3-G xenografts in athymic mice (100 mg BCM-95®/kg daily i.p.; 25 mg gemcitabine/kg body weight once every 4 days for 28 days): significant decrease of tumor weight (p < 0.01) [61]. 4-nitro quinoline-1-oxide (4NQO) induced murine oral carcinogenesis model (drugs in drinking water: BCM-95® 14.3 μg/mL; metformin: 5 mg/mL for 8 weeks): the combination regimen decreased tumor volume (p = 0.0431) and expression of NF-κB and pS6. Improved overall survival with downregulation of the number of cancer stem cells [69]. Athymic mice with subcutaneous xenografs of HCT116 cells: the combination of OPCs and curcumin (both agents at 100 mg/kg) was more potent in decreasing tumor growth than the individual agents (p = 0.0006 for tumor volume, p =0.000214 for tumor weight) [70]. | |
| Curcumin C3 Complex® | ||
|---|---|---|
| MONO | ||
| Cell Culture Models | Animal Models | Clinical Studies |
| The treatment with 40 μM C3 Complex® for 72 h induced autophagy, but not apoptosis in glioma U87-MG and U373-MG cell lines. The mode of action was highly dependent on the status of AKT [71]. Decreased viability of several human multiple myeloma cell lines [72]. Activation of 5-AMP (Adenosine 5′-Monophosphate)-Activated Protein Kinase (AMPK) and suppression of gluconeogenic gene expression in hepatoma cell lines of rat (H4IIE) and human origin Hep3B [73]. Inhibition of carcinogen and nicotine-induced Mammalian Target of Rapamycin (mTOR) pathway activation, cell proliferation, migration and invasion in several head and neck squamous cell carcinoma (HNSCC) cell lines [74]. Inhibition of breast stem cells self-renewal and Wnt signaling. Significant reduction of Aldehyde Dehydrogenase 1 Family Member A1 (ALDH-1A1) expressing cells (from 7.3% to 1.5%) achieved during 72 h with 10 μM C3 Complex® [75]. Induction of differentiation of myeloid-derived suppressor cells (MDSCs) and inhibition of their interaction with MKN-45 gastric cancer cells with consequential decrease of the growth advantage of cancer cells acquired from the interaction with MDSCs [76]. Antiproliferative effect through suppression of Signal Transducer and Activator of Transcription 3 (STAT3) activation in non-tumor-derived, immortalized human bronchial epithelial cells (AALE) and human lung adenocarcinoma cell line H441 [77]. A strong inhibitory effect on the aggressive skin cancer cell line SRB12-p9 presented on the second day of treatment at 20 μM C3 Complex® through an inhibitory effect on pAKT, pS6, phosphorylated Eukaryotic Translation Initiation Factor 4E-Binding Protein 1 (p-4EBP1), pSTAT3 and phosphorylated Extracellular Signal-Regulated Kinases 1 and 2 (pERK1/2) [78]. The treatment (20 µM C3 Complex® or vehicle −0.1% dimethyl sulfoxide (DMSO); control) for 48 h induced change of expression of several miRNAs tested. A strong increase of miR-205-5p was recorded in murine melanoma B78H1 cell line and human melanoma SK-MEL-28 cell line, but not in lymph node metastasis human MeWo cell line [51]. Inhibition of UV-B/FGF-2/mTOR–induced proliferation, progression and colony formation of murine epithelial JB6 cells [79]. Already 5 μM C3 Complex® inhibits formation of primary mammosphere (breast cancer cell lines and breast cells isolated from voluntary mammoplasty patients). A strong decrease of transcriptional activity of genes associated with breast stemness was observed [80]. Protection of rat liver epithelial cell line T51B against iron-related neoplastic cell transformation but only when applied in high concentration [81]. Reduction of tumorigenic properties of mesothelioma cell lines (MSTO-221H, NCI-H2452, Ist-Mes-2) by impairing cellular self-renewal ability and decrease of proliferation/migration but only when combined with Bioperine* [83]. | Several ovarian carcinoma cell lines xenografts in nude mice; C3 Complex®: oral gavage of 500 mg/kg for maximally 6 days, starting one week after inoculation. C3 Complex® monotherapy: significant decrease of tumor weight (55%; p = 0.01) in the HeyA8 docetaxel-sensitive model and reduction in tumor burden in the HeyA8 docetaxel-resistant model, when compared with controls (47%; p = 0.05) [84]. Xenografts of U87-MG glioma cell line in nude mice: C3 Complex® (100 mg/kg in DMSO in PBS) was given intratumorally every 24 h for 7 days. After 16 days, the size of the tumors in curcumin-treated animals were significantly smaller than in control animals (3.5 ± 2.8-fold versus 12.5 ± 5.9-fold; p < 0.05) and was associated with strong induction of autophagy [71]. Sensitization of human colorectal HCT116 cancer xenografts in mice (C3 Complex® 1 g/kg, once daily orally, one week after implantation of cancer cells) to γ-radiation by targeting NF-κB and NF-κB-regulated genes [85]. MAC16 colon tumor-bearing mice (C3 Complex® 250 mg/kg, daily, 21 days): prevention and/or reversion of cachexia through the inhibition of chymotrypsin-like proteasome 20S activity [86]. SCC40 xenografts in Balb/c nu/nu mice: a) treatment (daily oral gavage of 5, 10, or 15 mg in corn oil for 24 days: day 0: the day when tumors reached 40 mm3); b) chemoprevention: C3 Complex® application for 4 days (same dosage and way of application as under “a“), prior to grafting. Highly effective in both suppressing tumor growth and initiation, the activity is associated with modulation of various signaling pathways, including mTOR’s downstream target, ribosomal protein pS6 [74]. Dose-dependent inhibition of skin squamous cell carcinoma (SSCC) growth (p = 0.0012) in mice pretreated with 5 or 15 mg of C3 Complex®, three days prior to SSCC cells injection in each flank, and gavaged daily for 24 days. Inhibition of S6 phosphorylation, suggesting inhibition of the mTOR pathway [87]. Human gastric (MKN-45) cancer xenograft model and a mouse colon cancer (CT26) allograft model: treatment with C3 Complex® in the diet (2% C3 Complex® diet for 4 weeks) or by i.p. injection (50 mg/kg for 3 weeks). Significant inhibition of tumor growth and decreased percentage of MDSCs in the spleen, blood and tumor tissues, associated with reduced interleukin 6 (Il-6) level in both serum and tumor tissues [76]. Lung adenocarcinoma in mice (50mg/2.5ml/kg daily 3 or 9 days i.p.): reduction of pStat3 and the proliferative markers CycD1 and Minichromosome Maintenance Complex Component 2 (Mcm2) in murine lung tissues [77]. Murine squamous cell skin carcinoma model: topical formulation (15 mg/100 μL cream) was as effective as oral C3 Complex® (15 mg daily) in suppressing tumor growth when applied for 3 days prior to xenograft injection and continuing for another 29 days [78]. Inhibition of UV-B radiation-induced skin cancer in mice (p =0.01) receiving identical formulations and dosages as described [78]. No significant difference in average number of tumors per mouse whether receiving C3 Complex® orally or topically [88]. Murine amelanotic melanoma (B78H1) cells injected in the flank of C57BL/6 mice (4% C3 Complex® diet two weeks prior to injection of tumor cells until termination of the experiment): a markedly decreased tumor volume in curcumin-treated animals at day 28. The miRNA expression signature in tumors was substantially altered, with mmu-miR-205-5p being over 100 times increasingly expressed in treated vs. control tumors [51]. Photopreventive effect with respect to UVB-induced epidermal hyperplasia in mice (C3 Complex®: 15 mg/kg for 5 days a week for 2 weeks) [79]. Inhibition of proliferation of tongue squamous cell carcinoma OSC19 exposed to Fibroblast Growth Factor-2 (FGF2) [82]. Reduced number of oral lesions in mice exposed to 4NQO when applied locally and systemically (C3 Complex®: 15 mg daily for 4 weeks, both treatments) [82]. Azoxymethane-dextran sulfate sodium (AOM-DSS) induced colitis-associated colon cancer in mice fed a C3 Complex® diet 2% (wt/wt) from 5 weeks of age until the end of the 12th week. The decrease in tumor incidence and tumor multiplicity by C3 Complex® was statistically significant. C3 Complex® administration reversed the gene expression patterns modified by AOM + DSS, especially for genes in anti-inflammatory and anti-oxidative pathways clusters through changes of DNA methylation [89]. Mesothelioma xenograft tumor model in mice (40 mg/kg of C3 Complex® i.p. daily for 4 weeks): delayed tumor growth by reducing angiogenesis and increasing apoptosis in combination with Bioperine [83]. | A phase I clinical trial (n = 15) involving patients with advanced colorectal cancer for which no additional conventional therapies were available: curcumin and its metabolites were detected in plasma and urine in patients taking 3.6 g of C3 Complex® daily for 4 months. These patients had a decrease of an inducible prostaglandin E2 (PGE2) in blood samples (indicative of biological activity and systemic pharmacological properties), irrespective of curcumin’s measurable presence/absence in plasma [90]. Pilot trial (n = 12; patients with advanced colorectal cancer presented with hepatic metastases; 0.45–3.6 g of C3 Complex® daily, 1 week prior to surgery): low nanomolar levels of curcumin and its conjugates were found in the peripheral or portal circulation. Only the trace levels of metabolic reduction products were detected in normal liver tissue from one patient receiving 3.6 g of C3 Complex® daily. Levels of malondialdehyde (MDA)-DNA adduct, which reflect oxidative DNA changes, were not decreased in post-treated normal and malignant liver tissue when compared to pretreatment samples [50]. Twelve patients with confirmed colorectal carcinoma. Giving C3 Complex® capsules of 3.6, 1.8, or 0.45 g daily for 7 days pre-surgery: measurable amount of curcumin conjugates in colon tissue (both normal and cancer) after oral intake of a daily dose of 3.6 g C3 Complex®, but lack of quantifiable curcumin in plasma [91]. Nonrandomized, open-label, phase II trial (n = 25; patients with advanced pancreatic cancer; 8 g C3 Complex® orally/daily until disease progression, with restaging every 2 months): high tolerability to daily intake of 8 g; despite limited absorption, stable level of conjugated curcumin in plasma was achieved on the day 3. Some improvements were described in four study participants. [92]. The single-blind, cross-over pilot study: Patients suffering from monoclonal gammopathy of undefined significance (MGUS, n = 26; oral intake, 4 g daily for 6 months): decrease of excessive paraprotein load (≥20 g/L) in 5/10 patients observed. In addition, 27% of patients on C3 Complex® had a > 25% decrease in urinary N-telopeptide of type I collagen [93]. A clinical study (n = 100 participants; 25 control subjects and 75 patients with several diagnoses related to oral cavity (leukoplakia, submucous fibrosis, oral lichen planus); daily intake 1 g for maximally 218 days): increased level of vitamins C and E in saliva, decreased level of markers of oxidative stress malondialdehyde and 8-Hydroxy-2′-Deoxyguanosine (8-OHdG). The size of lesions significantly decreased [94]. Phase IIa clinical trial including forty-one patients (smokers) who completed the trial which explored potential influence of curcumin on ACF (daily doses: 2 and 4 g for 22 and 19 patients respectively, for 30 days). There was a significant decrease of rectal ACFs (17.8 ± 2.0, baseline, vs. 11.1 ± 2.8, postintervention; p < 0.005) in patients who were taking a higher daily dose (4 g). The decrease of PGE2 and 5-hydroxyeicosatetraenoic acid (5-HETE) was not recorded [38]. Randomized, double-blind placebo-controlled cross-over study including 36 patients suffering from MGUS or smoldering multiple myeloma (SMM): one group received 4 g C3 Complex® and the other 4 g placebo, crossing over at 3 months. At completion of the 4 g arm (completed by 28 patients), patients entered an open-label, 8 g dose extension study for 3 months (completed by 18 patients). Based on numerous parameters measured, C3 Complex® was shown to have a potential to benefit some but not all patients with MGUS or SMM [95]. A clinical pilot study (n =28; colorectal cancer patients undergoing colorectal endoscopy or surgical resection; daily intake of 5 × 470 mg orally for 2 weeks): curcuminoides and their metabolites were detectable in plasma of only 4 patients and urine samples of all patients, as well as in the colon mucosa of few (not all) patients who underwent colorectal endoscopy or surgical resection [24]. Clinical study (8 healthy volunteers and 15 head and neck squamous cell carcinoma (HNSCC) patients with newly diagnosed malignant tumors of the oral cavity, oropharynx, hypopharynx or larynx): self-administration of mouth dissolving microgranular formulation of C3 Complex® for 10 min; 3–4 weeks regimen of 2 × 4 g daily. Only FGF-2 was significantly decreased in post-treatment tumor samples in 7 out of 11 patients when compared to evaluable matched pre-treatment tumor samples (p =0.0261). The decrease of FGF-2, Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) and IL-17 in serum was significant [39]. Adverse Events: Two types of gastrointestinal AEs were reported by patients, which were probably related to curcumin consumption. Diarrhea occurred in two patients receiving 0.45 g and 3.6 g of C3 Complex® daily. One patient consuming 0.45 g C3 Complex® daily and one patient consuming 3.6 g C3 Complex® daily developed diarrhea (grades 1 and 2, respectively). Nausea occurred in one patient consuming 0.9 g C3 Complex® daily (toxicity grade 2). There was a rise in serum alkaline phosphatase in 4 patients (grade 1 and 2 toxicity) and increase of serum lactate dehydrogenase to >150% of pretreatment values in three patients [90]. Only one participant reported abdominal pain, bloating, nausea and diarrhea [24]. Gastrointestinal disturbances (diarrhea and distension, gastroesophageal reflux) were present in 25 participants (61%). The grade of toxicity was 1 and 2 [38]. |
| COMBINED | ||
| Cell Culture Models | Animal Models | Clinical Studies |
| Enhancement of the effect of docetaxel in HeyA8 andSKOV3ip1 ovarian carcinoma cell lines and inhibition of Tumor Necrosis Factor-Alpha (TNF-α)-mediated NF-κB activation [84]. Inhibits proliferation and potentiates the apoptotic effects of gemcitabine. Strong inhibition of constitutive NF-κB activation in four pancreatic cancer-derived cell lines (BxPC-3, MIA PaCa-2, Panc-1, Mpanc-96) [96]. C3 Complex® potentiated thalidomide- and bortezomib-induced apoptosis (25% to 85% and 10% to 75%, respectively) of multiple myeloma cells and amplified inhibitory effect of bortezomib and thalidomide on NF-κB activation [72]. | Several ovarian carcinoma cell lines xenografts in nude mice. C3 Complex®: oral gavage of 500 mg/kg for maximum 6 days, starting one week after inoculation. The combination of C3 Complex® and docetaxel (35 μg) had the greatest efficacy in reduction of tumor burden in SKOV3ip1 and HeyA8 models (96%, p < 0.001 and 77%, p = 0.002, respectively). The same combination reduced the tumor mass by 66% beyond docetaxel monotherapy (p = 0.01) [84]. Orthotopic model of pancreatic cancer (athymic nu/nu mice injected with MiaPaca) combination of C3 Complex®: 1 g/kg, once daily p.o., gemcitabine: 25 mg/kg, twice weekly by i.p. injection and gemcitabine + C3 Complex® for 4 weeks) potentiates antitumor activity of gemcitabine through suppression of proliferation, angiogenesis and inhibition of NFκB-regulated genes [96]. Human multiple myeloma U266 model xenograft in mice: combination of C3 Complex® (1 g/kg, orally, daily) and bortezomib (0.25 mg/kg, 100 μL, weekly) for up to 20 days: potentiates the effect of bortezomib recorded as decreased tumor volume (control vs. curcumin + bortezomib p < 0.001; bortezomib vs. curcumin + bortezomib p < 0.001) [72]. Spheroids obtained from human colorectal cancer samples were exposed to 5 µM curcumin, 2 µM oxaliplatin + 5 µM 5-FU and 5 µM C3 Complex® + 2 µM oxaliplatin + 5 µM 5-FU for 2 weeks. The triple combination significantly downregulated expression of pluripotent stem cell markers OCT3-4, alpha-fetoprotein (AFP) and Forkhead Box Protein A2 (FOXA2) at 24 h, and Nanog, Orthodenticle Homeobox 2 (OTX2) and Vascular Endothelial Growth Factor Receptor 2 (VEGFR2) at 72 h. Enhancement of anti-proliferative and pro-apoptotic effects in a portion of patient-derived explants was observed, associated with reduced expression of stem cell-associated markers by the addition of curcumin to oxaliplatin/5-FU [41]. | An open-labeled Phase II trial for 17 patients with previously untreated locally advanced or metastatic adenocarcinoma of the pancreas. Combined treatment, gemcitabine (1000 mg/m2 intravenously weekly) with C3 Complex®: (2 × 4 g daily), for 1 week to 12 months (median 2.5 months). The effect was reported for 11 patients: partial response in one patient during 7 months, stable disease from 2–12 months in four patients, tumor progression in six patients [97]. A phase I/II study of gemcitabine-based chemotherapy plus C3 Complex® for patients with gemcitabine-resistant pancreatic cancer (n = 21; 8 g oral C3 Complex®: daily in combination with gemcitabine-based chemotherapy): combination therapy with gemcitabine-based chemotherapy is safe and feasible [98]. The phase I dose escalation study (n = 12) of patients with colorectal liver metastases: 500 mg (1 capsule) of oral curcumin C3 Complex®: daily, 7 days prior to the scheduled chemotherapy (up to 2 g daily): safe and tolerable adjunct to folinic acid/5-fluorouracil/oxaliplatin chemotherapy (FOLFOX); by treatment end, 81.8% patients had no concerns regarding side-effects from C3 Complex® [41]. Randomized phase IIa trial to assess safety, efficacy, quality of life, neurotoxicity, curcuminoids and C-X-C motif chemokine ligand 1 (CXCL1) in patients with metastatic colorectal cancer receiving FOLFOX compared with FOLFOX + 2 g oral C3 Complex®/d (CUFOX): combination of C3 Complex®: and FOLFOX chemotherapy is safe and tolerable. There was no significant difference between arms for quality of life or neurotoxicity. Curcumin glucuronide was detectable at concentrations >1.00 pmol/mL in 15 of 18 patients receiving CUFOX. Curcumin did not significantly alter CXCL1 over time [40]. Adverse Events: Kanai et al. observed neutropenia (38%; grades 3–4) and fatigue (10%; grades 3–4), which were not attributed to C3 Complex®, but to the gemcitabine-based chemotherapy or disease progression [98]. There is a possibility that some side effects developed in patients receiving C3 Complex® and FOLFOX were attributable to curcumin: diarrhea was the most frequently reported event (in 66.7% of patients) [41]. The most common AE reported as possibly or probably related to C3 Complex® was diarrhea, nausea, oral mucositis and constipation [40]. Gastrointestinal toxicity manifested as abdominal fullness and pain in 7 patients. In 5/7 patients the adverse event was intractable (Grade 3) and resulted in cessation of C3 Complex® administration. In one of these patients, the duodenal peptic ulcer exacerbated. Two patients experienced Grade 2 abdominal pain. In these patients, the daily dose was reduced and continued on a reduced dose of 4 g/day of curcumin, after 2 and 6 weeks of full treatment, respectively. Mild hematological toxicity was observed in four patients (neutropenia grade 2 in two patients; 2 thrombocytopenia of grade 1 and 2). It was concluded that a daily dose of 8 g of C3 Complex® may be above the maximum tolerated dose when combined with gemcitabine [97]. |
| Meriva® | ||
|---|---|---|
| MONO | ||
| Animal Models | Clinical Studies | |
| Xenograft study of a mammary gland tumor cell line ENU1564 in athymic nude mice (n = 18): Meriva® group was fed daily with Tekled 2019 containing 6% Meriva®. Decreased number of metastatic foci in the lung (p < 0.041) in comparison to the control and curcumin groups. Reduced expression of matrix proteinase 9 (MMP-9), but not Vascular Endothelial Growth Factor (VEGF), was shown in Meriva® fed animals’ tumors [99]. BALB/c mice injected with triple-negative breast cancer cell line 4T1 (only 3 animals per group): Cryoablation plus Meriva® (but not Meriva® alone) strongly reduced tumor growth and the number of lung metastases. Overall survival was improved in animals treated with combination of Meriva® and cryoablation. When 10 mg of Meriva® (every 3 days for 14 days starting when tumors are 1–1.5 cm in diameter) was given, there was a decrease in Il-6 production and number of metastatic foci [100]. | A controlled study that included 160 cancer patients (terminal patients excluded) with various solid tumors: one tablet containing 0.5 g Meriva® was given three times daily between the 4th and 16th weeks from surgery, for at least 60 consecutive days. The observational framework: 4 months, starting from the day after their first cycle of chemotherapy or radiotherapy. Semi-quantitative assessment of side effects: statistically significantly improved (p < 0.05) plasma oxidative status between Meriva® group and control group [101]. | |
| COMBINED | ||
| Cell Culture Models | Animal Models | Clinical Studies |
| In combination with oxaliplatin decreases proliferative capacity of oxaliplatin-resistantHCT116 colorectal cell line, irrespective of their Tumor Protein P53 (TP53) status [102]. | Xenograft colorectal cancer (HCT116) in nude mice: 1.13% Meriva® mixed with a standard diet for 21 days. The decrease of tumor volume was 53%, 35% and 16% (Meriva® + oxaliplatin vs. Meriva® vs. oxaliplatin). No significant difference in DNA platinating ability but significantly increased cleaved-caspase-3-positive cells by 4.4-fold (p <0.05) when Meriva® + oxaliplatin were applied [102]. | A pilot, product evaluation registry study (n = 33; patients with benign prostate hyperplasia; 2 × 0.5 g of Meriva®/day for at least 24 weeks + “BSM” (Best Standard Management) vs. the BSM group (n = 28)): the addition to the standard treatment contributed to the reduction of signs and symptoms of the disease without causing any significant additional side effect. Statistically significant decrease of PSA, p < 0.025 [103]. A randomized, double-blind, placebo-controlled trial (n = 80; patients with solid tumors receiving a conventional chemotherapy; Meriva®: 180 mg/day for 8 weeks). Suppression of some (TNF-α, calcitonin gene-related peptide (CGRP), monocyte chemoattractant protein 1 (MCP-1): p < 0.001), but not all (IL-8) measured systemic inflammation markers. An improvement of quality of life was reported [104]. Single-center, single-arm prospective phase II trial (n = 52; advanced pancreatic cancer; gemcitabine 10 mg/m2/min infused over 100 min on days 1, 8, 15 and Meriva® 2 g/day continuously, each cycle repeated every 28 days). There is no strong evidence on improved safety and efficacy of gemcitabine when combined with Meriva® in patients suffering from advanced pancreatic cancer [105]. Adverse Events: Mild gastrointestinal side effects (not specified) occurred in 8 patients [104]. |
| Lipocurc™ | ||
|---|---|---|
| Cell Culture Models | Animal Models | Clinical Studies |
| Significantly higher activity when compared to curcumin: reduction in the IC50 in A549 lung cancer cells [106]. Cell-type-specific dose-dependent response to Lipocurc™ and curcumin was recorded as significantly higher in human osteosarcoma cell lines than in canine cell lines originating from various cancers. Parameters explored: cellular viability, migration and formation of tubes [107]. | Xenograft tumor growth of A549 non-small cell lung cancer cells in athymic nude mice (20 mg/kg s.c. twice a week for seven weeks). Regression of tumor volume was significant and was associated with a strong decrease of NFκB-p65, Ki-67 and Annexin A2, as recorded by immunohistochemistry [106]. Naturally occurring various canine lung cancers (initially included 11 dogs; Lipocurc™ infusion administered four times per week, 10 mg/kg): the study reports mixed results. Four out of six dogs experienced stable disease, and no radiographic responses were detected [107]. | Phase I, single-center, open-label study in patients with advanced metastatic tumors (n = 32): maximum tolerated dose in patients with metastatic tumors was determined (300 mg/m2 over 6 h) and recommended as starting dose for testing Lipocurc™ in clinical cancer trials. The study is not conclusive with respect to the primary disease [33]. Cancer patients: Analysis of the impact of co-medication on infusion rate normalized plasma levels and comparison of the plasma levels between cancer patients and healthy individuals: either co-medications or health status, or both, can impact the pharmacokinetics of Lipocurc™ infusion in cancer patients [108]. Adverse Events: Among143 AEs recorded in 30 patients (93.8%), only 11 AEs (in 9 patients) were considered definitely or probably related to the treatment itself. These included facial edema, anemia, hemolysis, mild gastrointestinal symptoms and a few others. It was difficult to make a clear distinction between symptoms and complications associated with the advanced primary disease and the treatment itself [33]. |
| LongVida® | |
|---|---|
| Cell Culture Models | Clinical Studies |
| Effective in human and mice glioblastoma cell lines, where it was shown to have significantly stronger response than with natural curcumin. Parameters tested: cellular viability, DNA fragmentation and apoptosis.A statistically significant increase of P53 and decrease of c-Myc [109]. | Safety and pharmacokinetics study (n = 11; patients with osteosarcoma; doses of 2, 3 and 4 g): in both healthy individuals and osteosarcoma patients, high interindividual variability in pharmacokinetics and nonlinear dose dependency was observed; overall, good tolerability was noted in both groups [53]. A randomized clinical trial (n = 30; oral submucous fibrosis patients): the total daily dose of 2 g was given for 3 months duration and follow-up was done for 6 months: promising in the treatment of oral submucous fibrosis with respect to tested parameters [110]. Adverse Events: Pigmentation of the tongue and teeth [110]. |
| Theracurmin™ | ||
|---|---|---|
| MONO | ||
| Cell Culture Models | Animal Models | Clinical Studies |
| Inhibition of proliferation and increased apoptosis of three different esophageal cancer cell lines (50 µM for 48 h). Modulation of the cytokine profile of activated T cells toward a profile that negatively affects tumor cell growth and migration (reduced secretion of TNF-α, and four interleukins: IL-8, IL-6, IL-10 and IL-1β) [111]. Efficiently reduces viability and inhibits the growth of human prostate and bladder cancer cell lines via induction of apoptotic cell death and cell cycle arrest [112]. Inhibition of cell proliferation and induction of apoptosis of human prostate cancer cell lines LNCaP and 22Rv1 in a dose-dependent manner. Strong influence on the proteins involved in steroidogenesis (decrease of Cytochrome P450 Family 11 Subfamily A Member 1 (CYP11A1) and Hydroxy-Delta-5-Steroid Dehydrogenase, 3 Beta- and Steroid Delta-Isomerase 2 (HSD3B2), increase of Cytochrome P450 Family 17 Subfamily A Member 1 (CYP17A1)), supporting the decrease of testosterone production [113]. Induction of cytotoxic and antiproliferative effects in several cell lines originating from esophageal squamous cell carcinoma (ESCC). Theracurmin™associated with increase of ROS and activation of the NRF2–NMRAL2P–NQO1 (Nuclear Factor (Erythroid-Derived 2)-Like 2-NMRA Like Redox Sensor 2-NAD(P)H Quinone Dehydrogenase 1) axis [55]. Decreased activity of NF-κB and its targets in two colon cancer cell lines (HCT116 and DLD1) [114]. Reduced viability of castration-naïve and castration-resistant prostate cancer-derived cell lines with conditional Pten (Phosphatase and Tensin Homologue) knock-out in a time- and dose-dependent fashion [115]. | Prostate tissues from the transgenic adenocarcinoma of the mouse prostate (TRAMP) model were obtained after 1-month oral administration of 200 mg/kg/day Theracurmin™. Decreased testosterone levels in the prostate tissues were observed (p < 0.01), but there was no influence on the serum testosterone level [113]. Hairless SCID male mice subcutaneously injected with 1.5 × 106 TE-11R cells, randomly assigned to three groups and fed with control diet, curcumin or Theracurmin™ supplemented diet from day 0 to day 70. Theracurmin™ group had greater reduction of tumor mass (43%) compared to curcumin group (11%) on day 70. Patient-derived ESCC xenografted tumors in C57BL/6 male mice who were drinking Theracurmin™-containing water from day 21 to day 70; 5000 ppm. A strong suppression of tumor growth, especially when combined with the NQO1 inhibitor [55]. Treatment with 500 ppm Theracurmin™ for 8 weeks inhibits development of polyps which were <0.5 mm or 0.5 < 0.9 mm in diameter (p < 0.05 vs. control group), in Apc mutant mice. However, there was no significant decrease of the total number of polyps. Inhibition of proliferation (proliferating cell nuclear antigen; PCNA) and inflammation-related (Mcp-1 and Il-6 mRNA) factors [114]. Mouse model of Pten-deficient prostate cancer: sixteen weeks of dietary supplementation of 76 mg/kg/day or 380 mg/kg/day nanoparticle Theracurmin™ did not influence tumor burden, but mice fed with high-dose Theracurmin™ had lower cancer cell proliferation rates at 12- and 16-, but not at the 20-week time [115]. | |
| COMBINED | ||
| Cell Culture Models | Animal Models | Clinical Studies |
| Combination with Erlotinib augmentatively reduces cell viability in four human lung cancer cell lines (p < 0.001; H1299, H460, A549 and PC9), partly through IkappaB expression [116]. Knockdown of NQO1 in TE-11R esophageal carcinoma cell line increased susceptibility to Theracurmin™. Conversely, overexpression of NQO1 in TE-11R cells resulted in a decrease of 8-OHdG-indicated oxidative damage after THC treatment and was associated with resistance to THC treatment [55]. | Human lung adenocarcinoma (PC9) tumors in mice: animals were receivingTheracurmin™(100 mg/kg by oral gavage, every 2 days for 2 weeks), administratedwith or without Erlotinib.The combined treatment significantly attenuated the tumor weight (p < 0.01) [116]. Patient-derived xenograft (PDX) esophageal squamous cell carcinomas in hairless SCID male mice. Animals were given either pure water or Theracurmin™-containing water (5000 ppm) from day 21 to day 70. The combination of THC and NQO1 inhibitor resulted in a significant inhibition of PDX tumor growth compared with that after control or monotherapy (inhibition at day 70: NQO1 inhibitor 1.9%, THC 40.7%, THC plus NQO1 inhibitor 72.6%) [55]. | Phase I study (n = 16; pancreatic or biliary tract cancer patients who failed standard chemotherapy): water solution of Theracurmin™ containing 200 mg of THC as a starting dose which swas safely escalated to 400 mg, orally administered every day with standard gemcitabine-based chemotherapy. A repetitive, systemic exposure to high concentrations of curcumin achieved by Theracurmin™ did not increase the incidence of adverse events in cancer patients receiving gemcitabine-based chemotherapy. The changes of NF-κB activity (in PBMCs), IL-6 and TNF-α (in plasma) were not demonstrated [117]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trošelj, K.G.; Samaržija, I.; Tomljanović, M.; Kujundžić, R.N.; Đaković, N.; Mojzeš, A. Implementing Curcumin in Translational Oncology Research. Molecules 2020, 25, 5240. https://doi.org/10.3390/molecules25225240
Trošelj KG, Samaržija I, Tomljanović M, Kujundžić RN, Đaković N, Mojzeš A. Implementing Curcumin in Translational Oncology Research. Molecules. 2020; 25(22):5240. https://doi.org/10.3390/molecules25225240
Chicago/Turabian StyleTrošelj, Koraljka Gall, Ivana Samaržija, Marko Tomljanović, Renata Novak Kujundžić, Nikola Đaković, and Anamarija Mojzeš. 2020. "Implementing Curcumin in Translational Oncology Research" Molecules 25, no. 22: 5240. https://doi.org/10.3390/molecules25225240
APA StyleTrošelj, K. G., Samaržija, I., Tomljanović, M., Kujundžić, R. N., Đaković, N., & Mojzeš, A. (2020). Implementing Curcumin in Translational Oncology Research. Molecules, 25(22), 5240. https://doi.org/10.3390/molecules25225240







