Carbohydrate Hydrolase-Inhibitory Activity of Juice-Based Phenolic Extracts in Correlation to Their Anthocyanin/Copigment Profile
Abstract
:1. Introduction
2. Results and Discussion
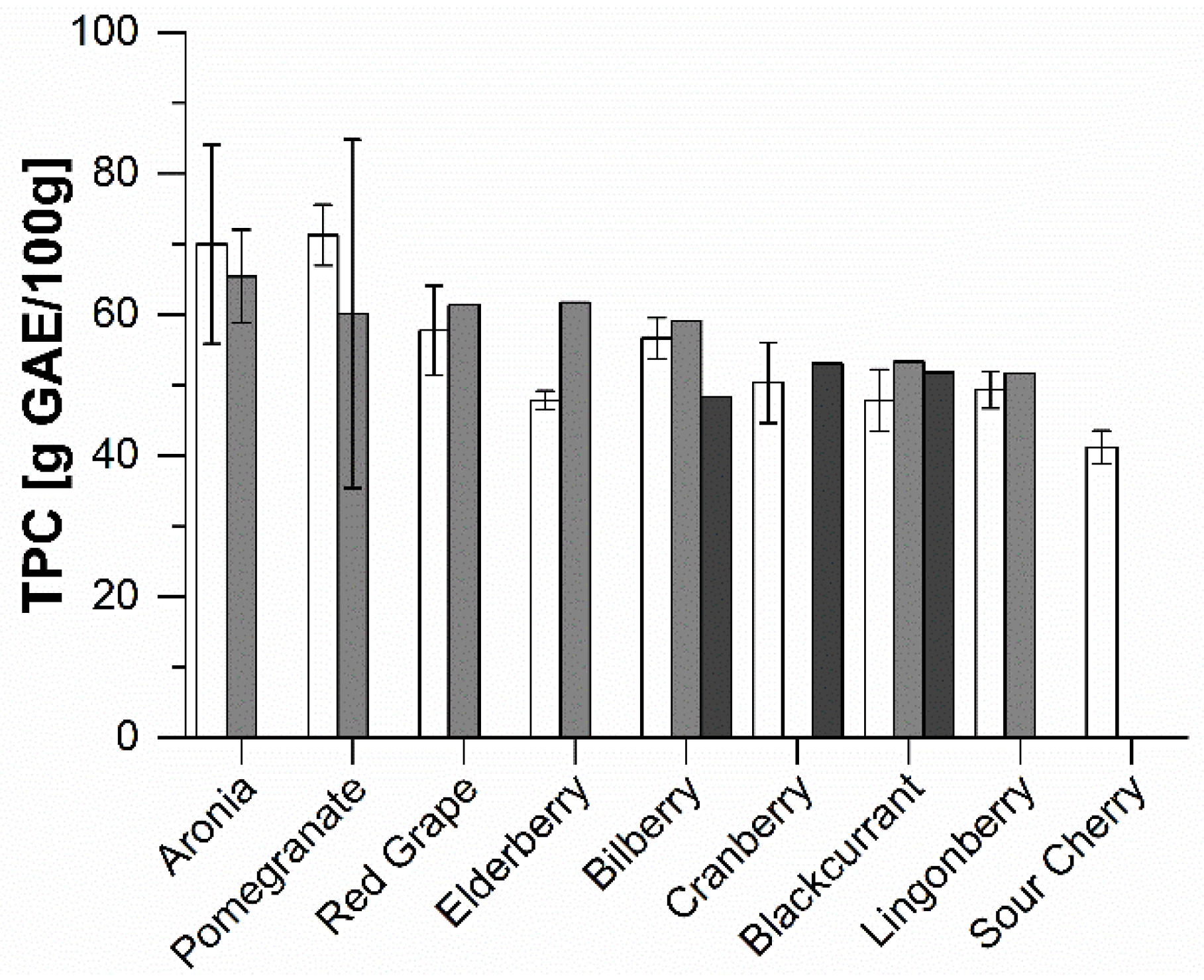
2.1. Phenolic Composition of the Extracts
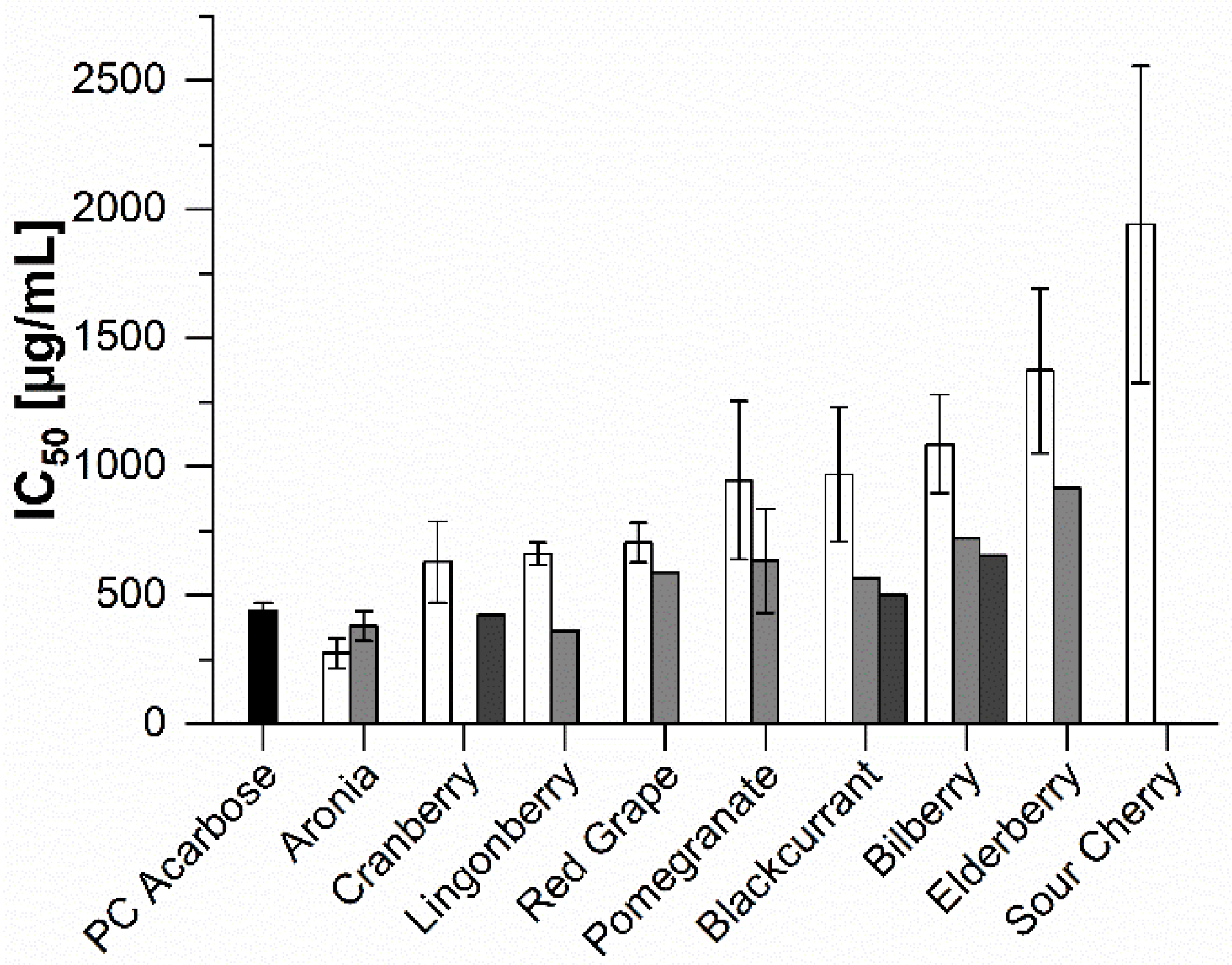
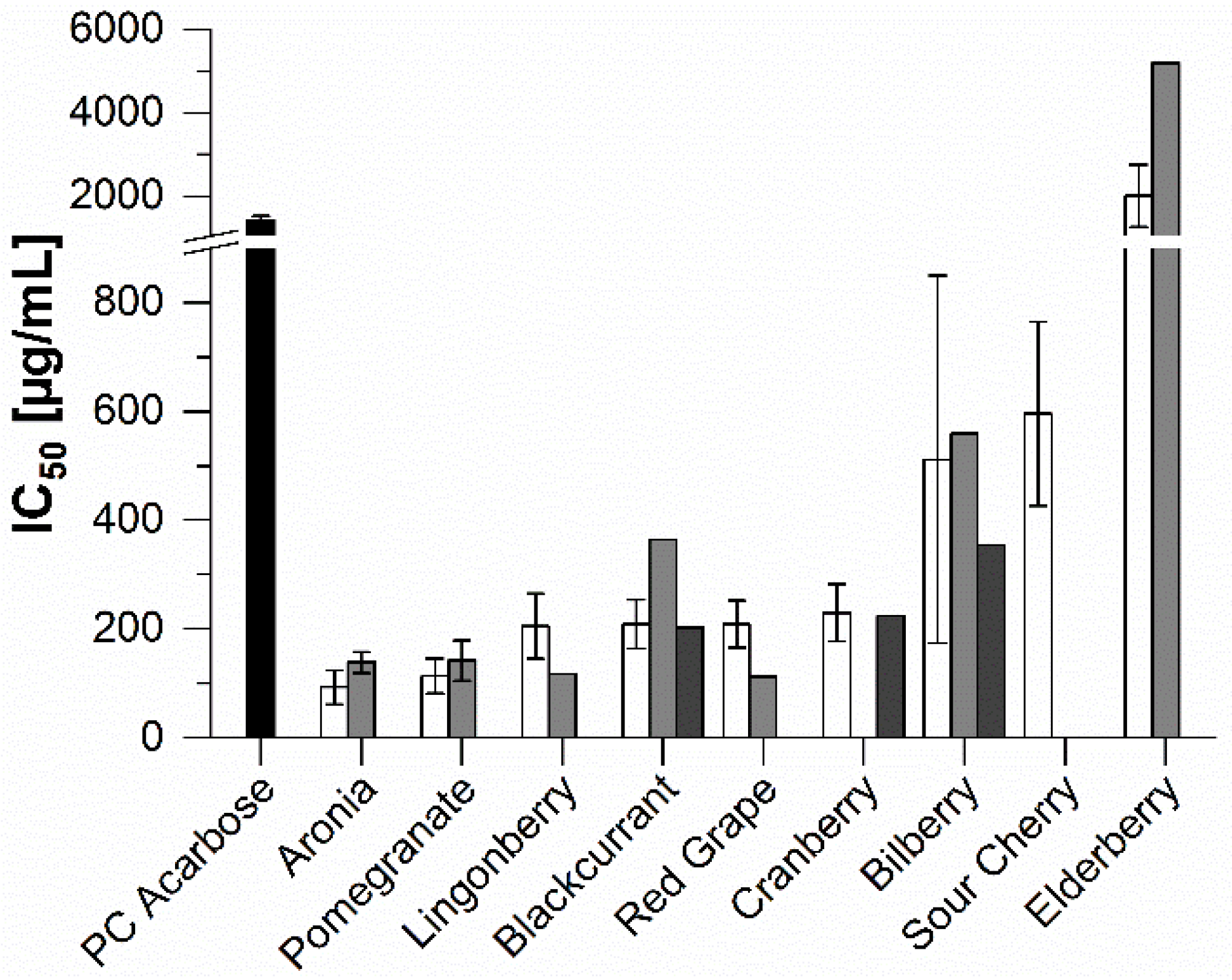
2.2. In Vitro Inhibition Study
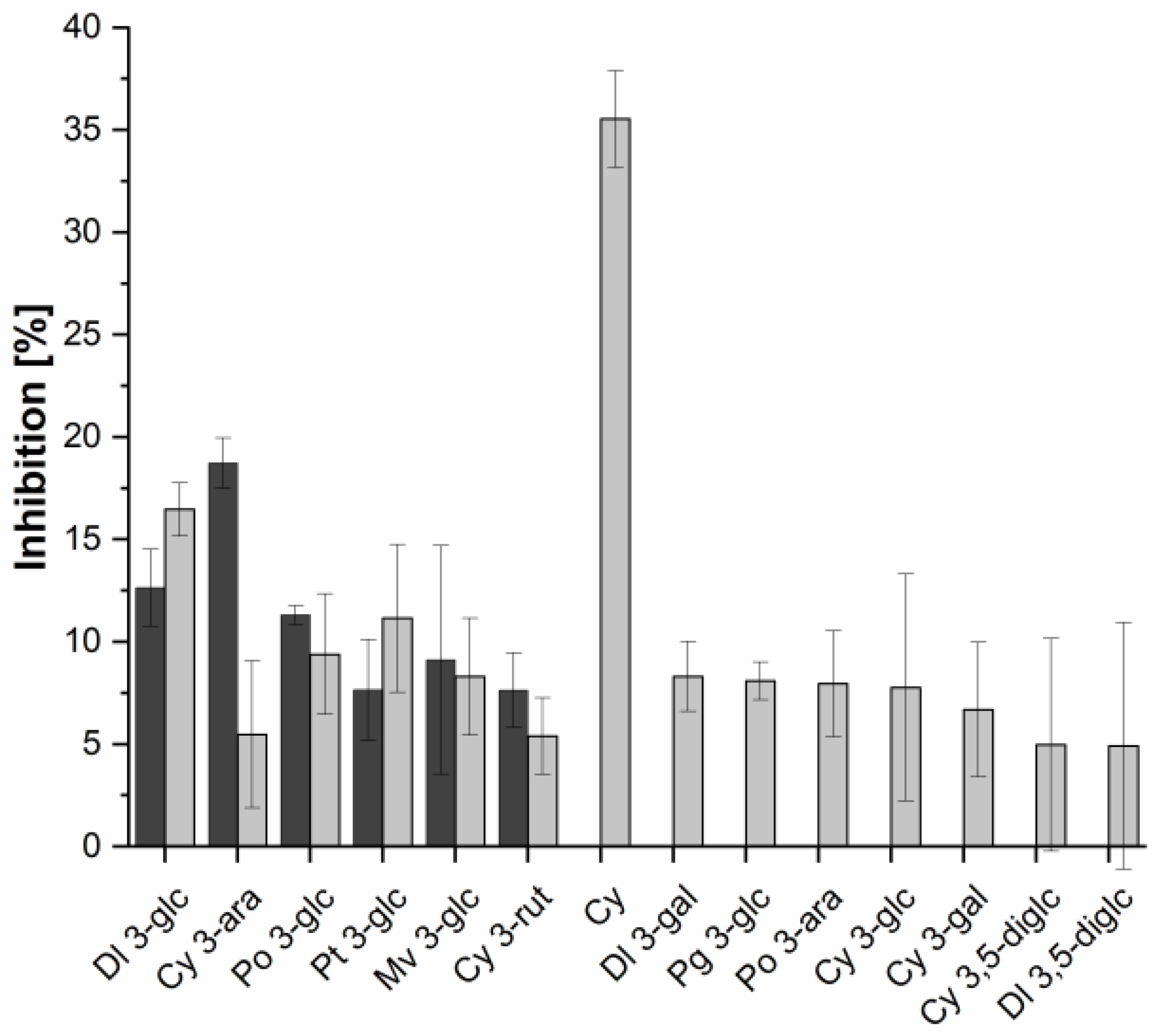
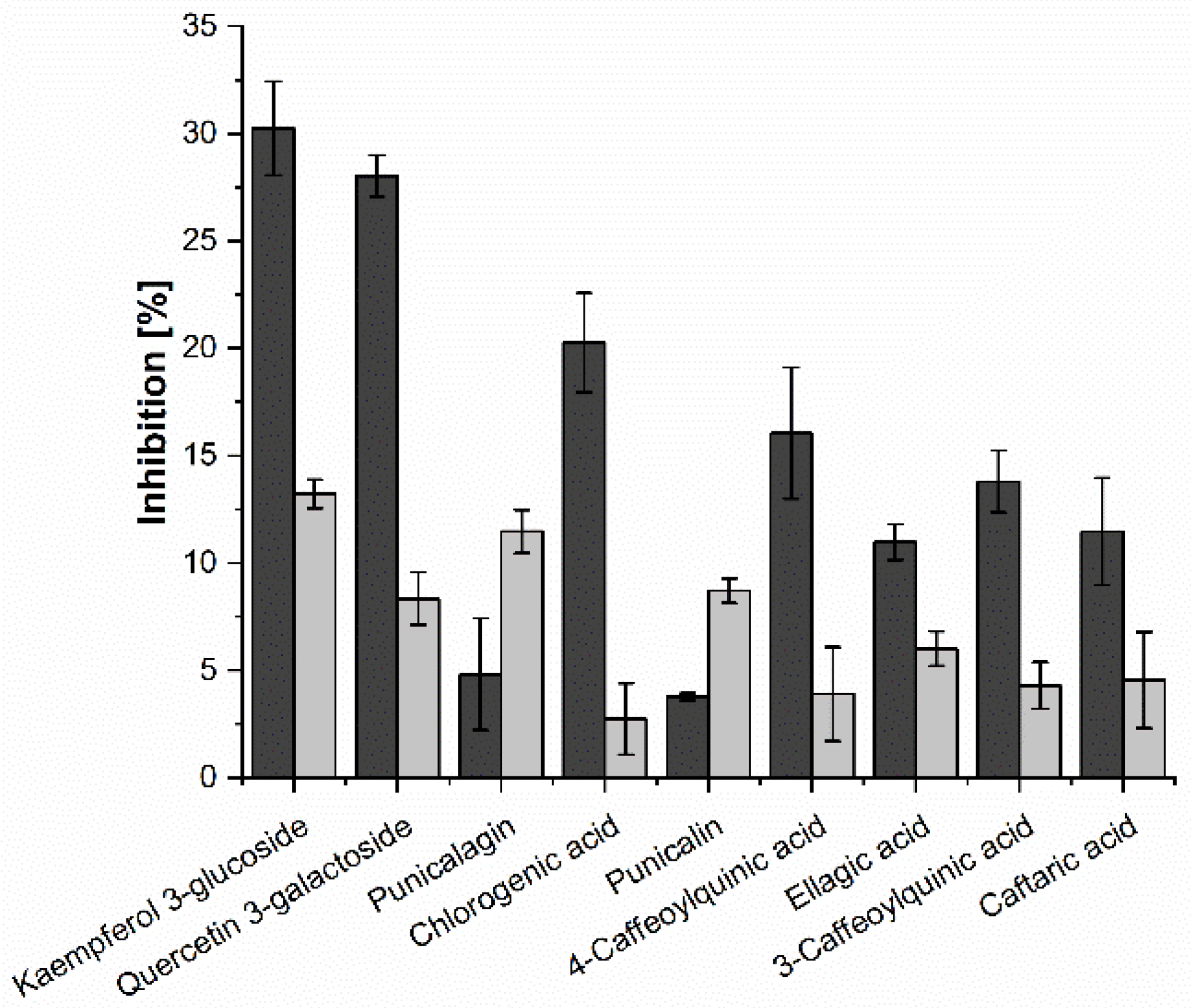
2.3. Investigation of Single Anthocyanins and Copigments
3. Materials and Methods
3.1. Chemicals
3.2. Materials and Preparation of Extracts
3.3. HPLC/DAD/ESI-MSn Analysis
3.4. Folin-Ciocalteu Assay
3.5. In Vitro α-Amylase Inhibition Study
3.6. In Vitro α-Glucosidase Inhibition Study
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GAE | gallic acid equivalents |
| IC50 | half maximal inhibitory concentration |
| JC | juice concentrate |
| NFC | juice not from concentrate |
| PC | positive control |
| TPC | total phenolic content |
References
- Ghosh, D.; Konishi, T. Anthocyanins and anthocyanin-rich extracts: Role in diabetes and eye function. Asia Pac. J. Clin. Nutr. 2007, 16, 200–208. [Google Scholar] [PubMed]
- Trouillas, P.; Sancho-García, J.C.; Freitas, V.d.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and modulating color by copigmentation: Insights from theory and experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fröhling, B.; Patz, C.; Dietrich, H.; Will, F. Anthocyane, Gesamtphenole und antioxidative Kapazität in kommerziellen roten Traubensäften, schwarzen Johannisbeer-und Sauerkirschnektaren. Flüssiges Obst. 2012, 7, 260–264. [Google Scholar]
- Joseph, S.V.; Edirisinghe, I.; Burton-Freeman, B.M. Berries: Anti-inflammatory effects in humans. J. Agric. Food Chem. 2014, 62, 3886–3903. [Google Scholar] [CrossRef]
- Watzl, B.; Briviba, K.; Rechkemmer, G. Anthocyane. Ernährungs Umsch. 2002, 49, 148–150. [Google Scholar]
- Oliveira, H.; Fernandes, A.; Brás, N.F.; Mateus, N.; Freitas, V.D.; Fernandes, I. Anthocyanins as antidiabetic agents—in vitro and in silico approaches of preventive and therapeutic effects. Molecules 2020, 25, 3813. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019; ISBN 978-2-930229-87-4. [Google Scholar]
- Adisakwattana, S.; Ruengsamran, T.; Kampa, P.; Sompong, W. In vitro inhibitory effects of plant-based foods and their combinations on intestinal α-glucosidase and pancreatic α-amylase. BMC Complement. Altern. Med. 2012, 12, 110. [Google Scholar] [CrossRef] [Green Version]
- Kam, A.; Li, K.M.; Razmovski-Naumovski, V.; Nammi, S.; Shi, J.; Chan, K.; Li, G.Q. A comparative study on the inhibitory effects of different parts and chemical constituents of pomegranate on α-amylase and α-glucosidase. Phytother. Res. 2013, 27, 1614–1620. [Google Scholar] [CrossRef]
- Ueno, H.; Tsuchimochi, W.; Wang, H.-W.; Yamashita, E.; Tsubouchi, C.; Nagamine, K.; Sakoda, H.; Nakazato, M. Effects of miglitol, acarbose, and sitagliptin on plasma insulin and gut peptides in type 2 diabetes mellitus: A crossover study. Diabetes Ther. 2015, 6, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Lo Piparo, E.; Scheib, H.; Frei, N.; Williamson, G.; Grigorov, M.; Chou, C.J. Flavonoids for controlling starch digestion: Structural requirements for inhibiting human alpha-amylase. J. Med. Chem. 2008, 51, 3555–3561. [Google Scholar] [CrossRef] [PubMed]
- Yuan, E.; Liu, B.; Wei, Q.; Yang, J.; Chen, L.; Li, Q. Structure activity relationships of flavonoids as potent α-amylase inhibitors. Nat. Prod. Commun. 2014, 9, 1934578X1400900829. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.-G.; Wu, S.-F.; Zhang, Q.-F.; Yin, Z.-P.; Zhang, L. α-Glucosidase inhibitory effect of anthocyanins from Cinnamomum camphora fruit: Inhibition kinetics and mechanistic insights through in vitro and in silico studies. Int. J. Biol. Macromol. 2020, 143, 696–703. [Google Scholar] [CrossRef]
- Cheplick, S.; Kwon, Y.-I.; Bhowmik, P.; Shetty, K. Clonal variation in raspberry fruit phenolics and revelance for diabetes and hypertension management. J. Food Biochem. 2007, 31, 656–679. [Google Scholar] [CrossRef]
- Ostberg-Potthoff, J.J.; Berger, K.; Richling, E.; Winterhalter, P. Activity-guided fractionation of red fruit extracts for the identification of compounds influencing glucose metabolism. Nutrients 2019, 11, 1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oszmiański, J.; Lachowicz, S. Effect of the production of dried fruits and juice from chokeberry (Aronia melanocarpa L.) on the content and antioxidative activity of bioactive compounds. Molecules 2016, 21, 1098. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD-ESI/MS(n). Food Chem. 2011, 127, 807–821. [Google Scholar] [CrossRef]
- Kammerer, D.; Claus, A.; Carle, R.; Schieber, A. Polyphenol screening of pomace from red and white grape varieties (Vitis vinifera L.) by HPLC-DAD-MS/MS. J. Agric. Food Chem. 2004, 52, 4360–4367. [Google Scholar] [CrossRef] [PubMed]
- Podsędek, A.; Majewska, I.; Redzynia, M.; Sosnowska, D.; Koziołkiewicz, M. In vitro inhibitory effect on digestive enzymes and antioxidant potential of commonly consumed fruits. J. Agric. Food Chem. 2014, 62, 4610–4617. [Google Scholar] [CrossRef]
- Sales, P.M.; Souza, P.M.; Simeoni, L.A.; Silveira, D. α-Amylase inhibitors: A review of raw material and isolated compounds from plant source. J. Pharm. Pharm. Sci. 2012, 15, 141–183. [Google Scholar] [CrossRef] [Green Version]
- Heber, D. Pomegranate Ellagitannins. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie IFF, W.-G.S., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011; pp. 201–210. ISBN 9781439807163. [Google Scholar]
- Li, Y.Q.; Zhou, F.C.; Gao, F.; Bian, J.S.; Shan, F. Comparative evaluation of quercetin, isoquercetin and rutin as inhibitors of alpha-glucosidase. J. Agric. Food Chem. 2009, 57, 11463–11468. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, A.I.; Díaz-Sánchez, Á.G.; La Rosa, L.A.d.; Bustos-Jaimes, I.; Alvarez-Parrilla, E. Inhibition of α-amylase by flavonoids: Structure activity relationship (SAR). Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 206, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Gowd, V.; Jia, Z.; Chen, W. Anthocyanins as promising molecules and dietary bioactive components against diabete—A review of recent advances. Trends Food Sci. Technol. 2017, 68, 1–13. [Google Scholar] [CrossRef]
- Kaeswurm, J.A.H.; Claasen, B.; Fischer, M.-P.; Buchweitz, M. Interaction of structurally diverse phenolic compounds with porcine pancreatic α-amylase. J. Agric. Food Chem. 2019, 67, 11108–11118. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, Y.; Zhou, W. In vitro and in silico studies of the inhibition activity of anthocyanins against porcine pancreatic α-amylase. J. Funct. Foods 2016, 21, 50–57. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144. [Google Scholar]
- Ashok Kumar, B.S.; Lakshman, K.; Nandeesh, R.; Arun Kumar, P.A.; Manoj, B.; Kumar, V.; Sheshadri Shekar, D. In vitro alpha-amylase inhibition and in vivo antioxidant potential of Amaranthus spinosus in alloxan-induced oxidative stress in diabetic rats. Saudi J. Biol. Sci. 2011, 18, 1–5. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |
| Compound | Mode [M + H]+/− | Ion m/z | Fragments m/z | Red Fruit | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ar | Bi | Bc | Cb | Eb | Lb | Pg | Rg | Sc | ||||
| Anthocyanin | ||||||||||||
| Cyanidin 3-arabinoside | + | 419 | 287 | × | × | × | × | |||||
| Cyanidin 3,5-diglucoside | + | 611 | 449/287 | × | ||||||||
| Cyanidin 3-galactoside | + | 449 | 287 | × | × | × | × | |||||
| Cyanidin 3-glucoside | + | 449 | 287 | × | × | × | × | × | × | × | ||
| Cyanidin 3-(2G-glucosyl-rutinoside) | + | 757 | 677/287 | × | ||||||||
| Cyanidin 3-rutinoside | + | 595 | 287 | × | × | |||||||
| Cyanidin 3-sambubioside | + | 581 | 287 | × | ||||||||
| Delphinidin 3,5-diglucoside | + | 627 | 303 | × | ||||||||
| Delphinidin 3-galactoside | + | 465 | 303 | × | ||||||||
| Delphinidin 3-glucoside | + | 465 | 303 | × | × | × | × | |||||
| Delphinidin 3-rutinoside | + | 611 | 303 | × | ||||||||
| Malvidin 3-glucoside | + | 493 | 331 | × | × | |||||||
| Myricetin 3-glucoside | - | 479 | 316 | × | ||||||||
| Peonidin 3-arabinoside | + | 433 | 301 | × | ||||||||
| Peonidin 3-galactoside | + | 463 | 301 | × | ||||||||
| Peonidin 3-glucoside | + | 463 | 301 | × | × | × | ||||||
| Petunidin 3-glucoside | + | 479 | 317 | × | × | |||||||
| Copigment | ||||||||||||
| Caftaric acid | - | 311 | 179 | × | ||||||||
| 3-Caffeoylquinic acid | - | 353 | 191/179/135 | × | × | × | ||||||
| 4-Caffeoylquinic acid | - | 353 | 191/179/173 | × | × | × | ||||||
| Chlorogenic acid | - | 353 | 191/179/161 | × | × | × | × | × | × | |||
| Coumaroyl iridoid | - | 535 | 371 | × | ||||||||
| Ellagic acid | - | 301 | 229 | × | ||||||||
| Kaempferol 3-glucoside | - | 447 | 285 | × | ||||||||
| Punicalagin | - | 1083 | 601 | × | ||||||||
| Punicalin | - | 781 | 601 | × | ||||||||
| Quercetin 3-galactoside | - | 463 | 301 | × | × | × | × | |||||
| Quercetin-3-glucoronide | - | 477 | 301 | × | ||||||||
| Quercetin 3-rhamnoside | - | 447 | 301 | × | ||||||||
| Quercetin 3-rutinoside | - | 609 | 301 | × | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berger, K.; Ostberg-Potthoff, J.J.; Bakuradze, T.; Winterhalter, P.; Richling, E. Carbohydrate Hydrolase-Inhibitory Activity of Juice-Based Phenolic Extracts in Correlation to Their Anthocyanin/Copigment Profile. Molecules 2020, 25, 5224. https://doi.org/10.3390/molecules25225224
Berger K, Ostberg-Potthoff JJ, Bakuradze T, Winterhalter P, Richling E. Carbohydrate Hydrolase-Inhibitory Activity of Juice-Based Phenolic Extracts in Correlation to Their Anthocyanin/Copigment Profile. Molecules. 2020; 25(22):5224. https://doi.org/10.3390/molecules25225224
Chicago/Turabian StyleBerger, Kirsten, Johanna Josefine Ostberg-Potthoff, Tamara Bakuradze, Peter Winterhalter, and Elke Richling. 2020. "Carbohydrate Hydrolase-Inhibitory Activity of Juice-Based Phenolic Extracts in Correlation to Their Anthocyanin/Copigment Profile" Molecules 25, no. 22: 5224. https://doi.org/10.3390/molecules25225224






