Optimization and Characterization of Mesoporous Sulfonated Carbon Catalyst and Its Application in Modeling and Optimization of Acetin Production
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of the Sulfonated Catalyst
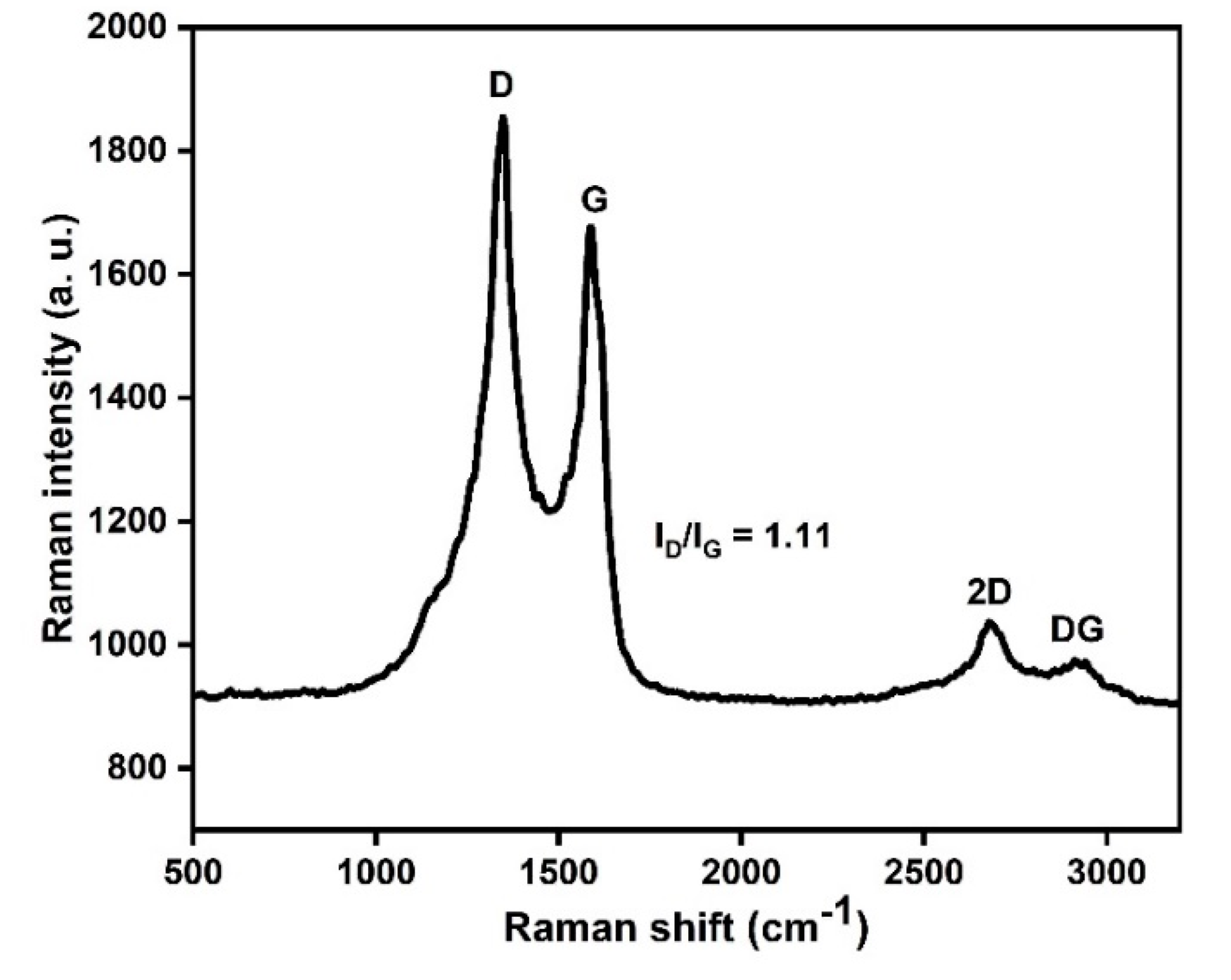
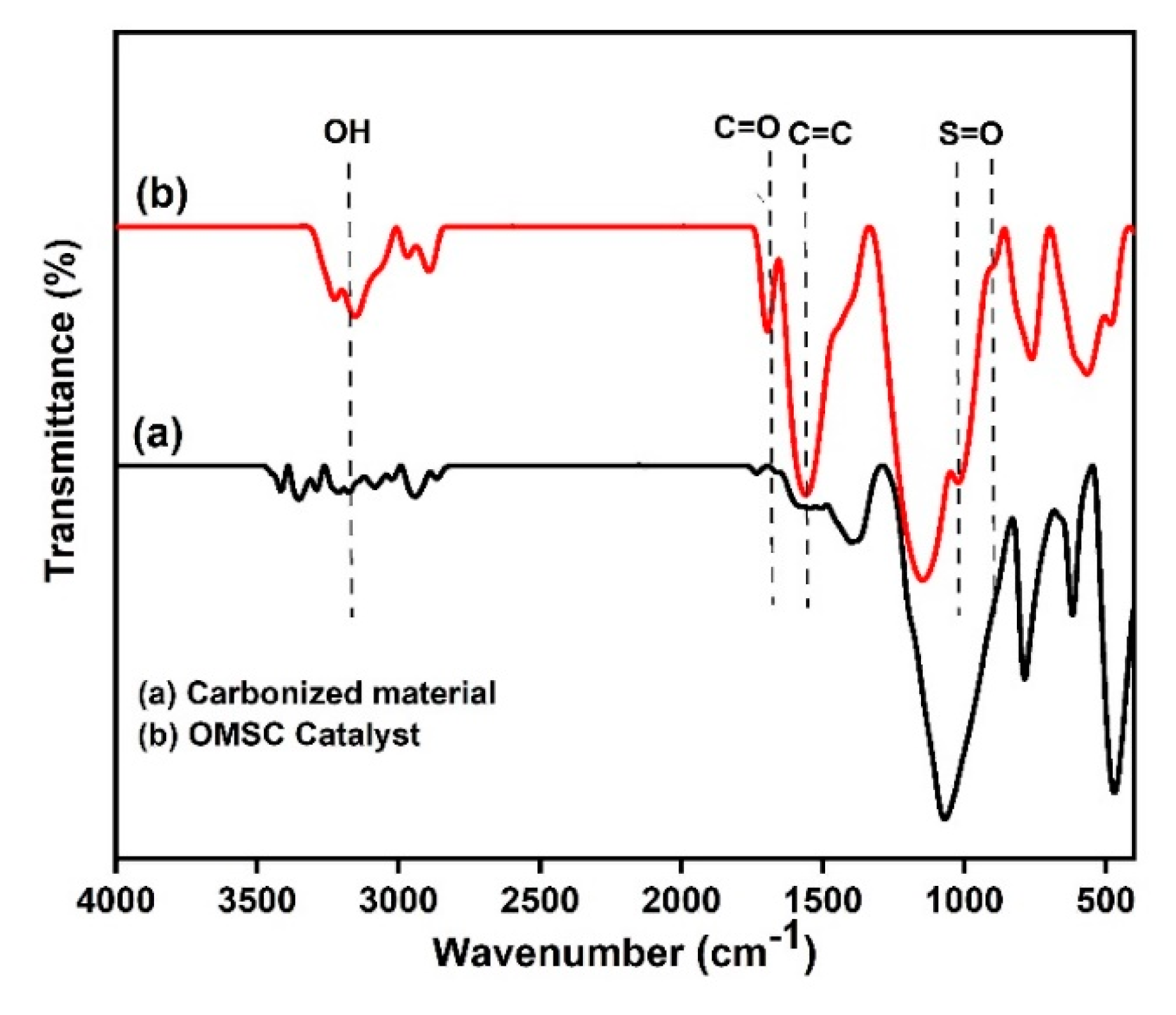
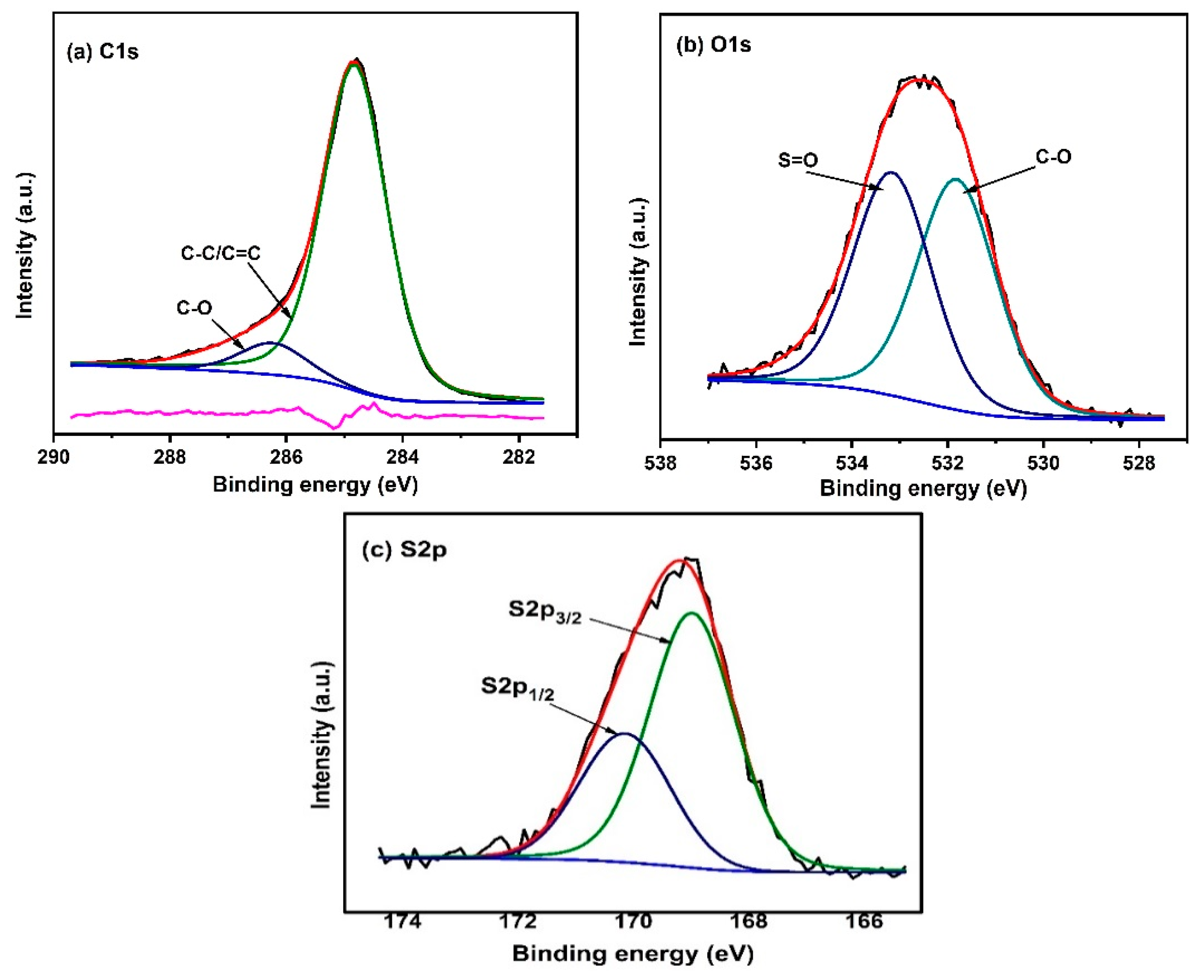
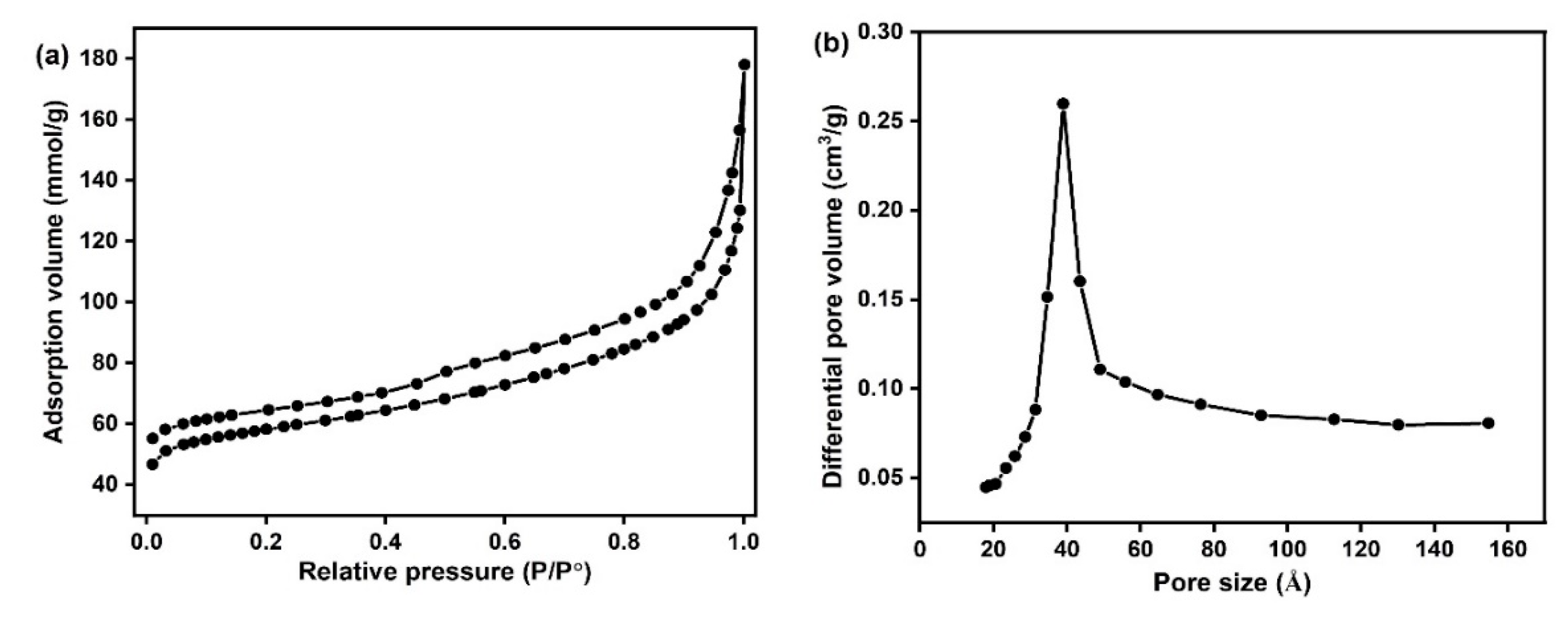
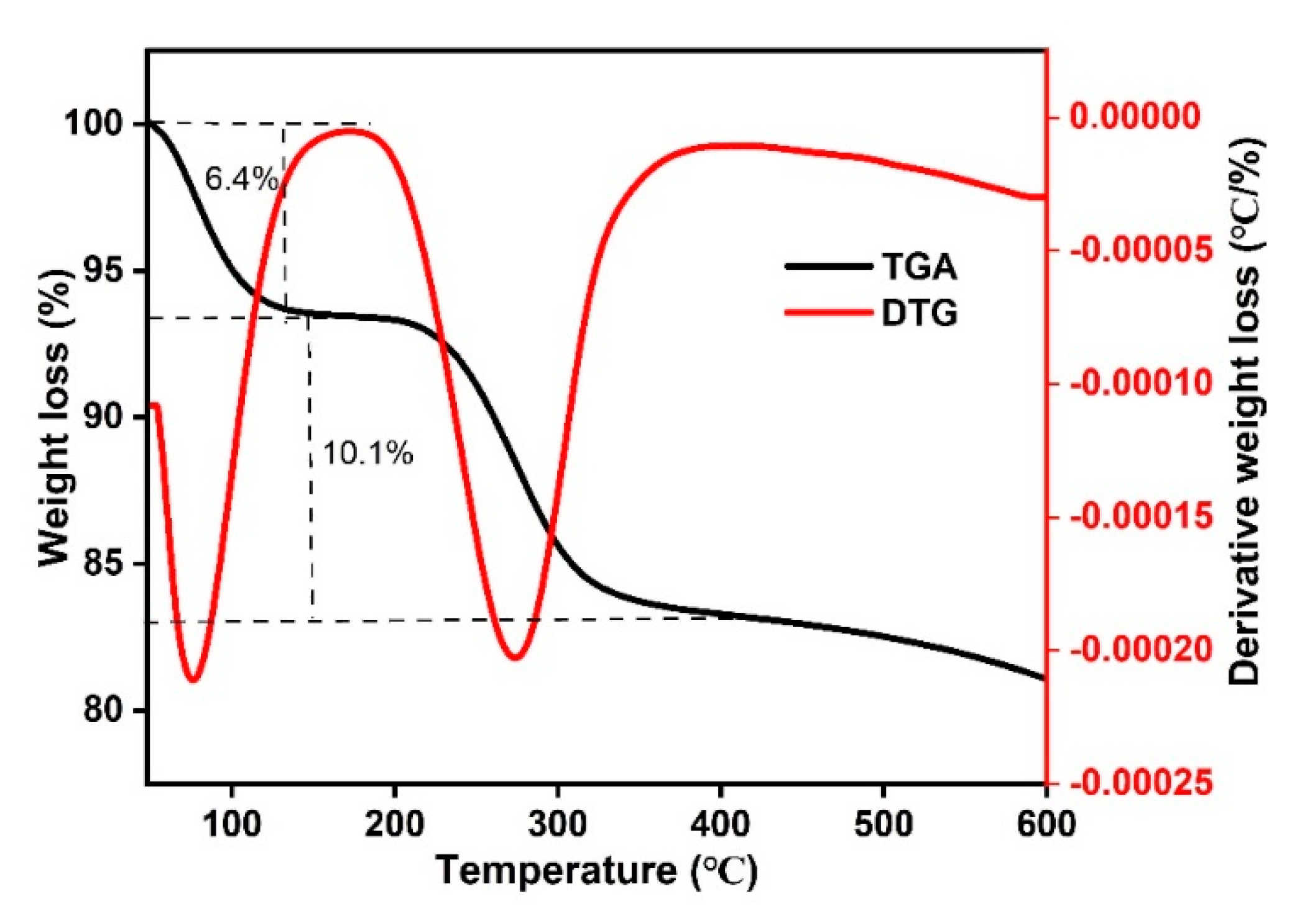
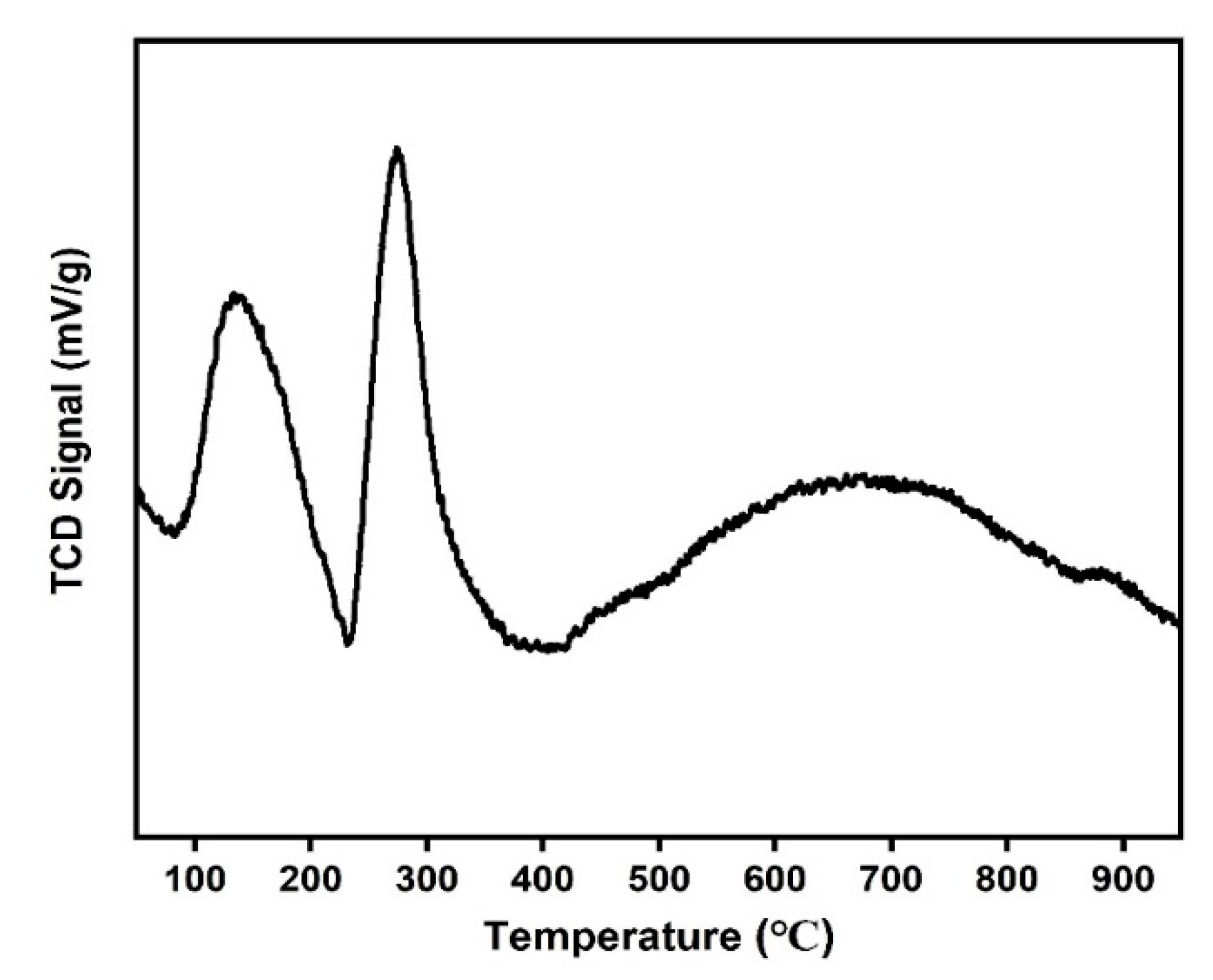
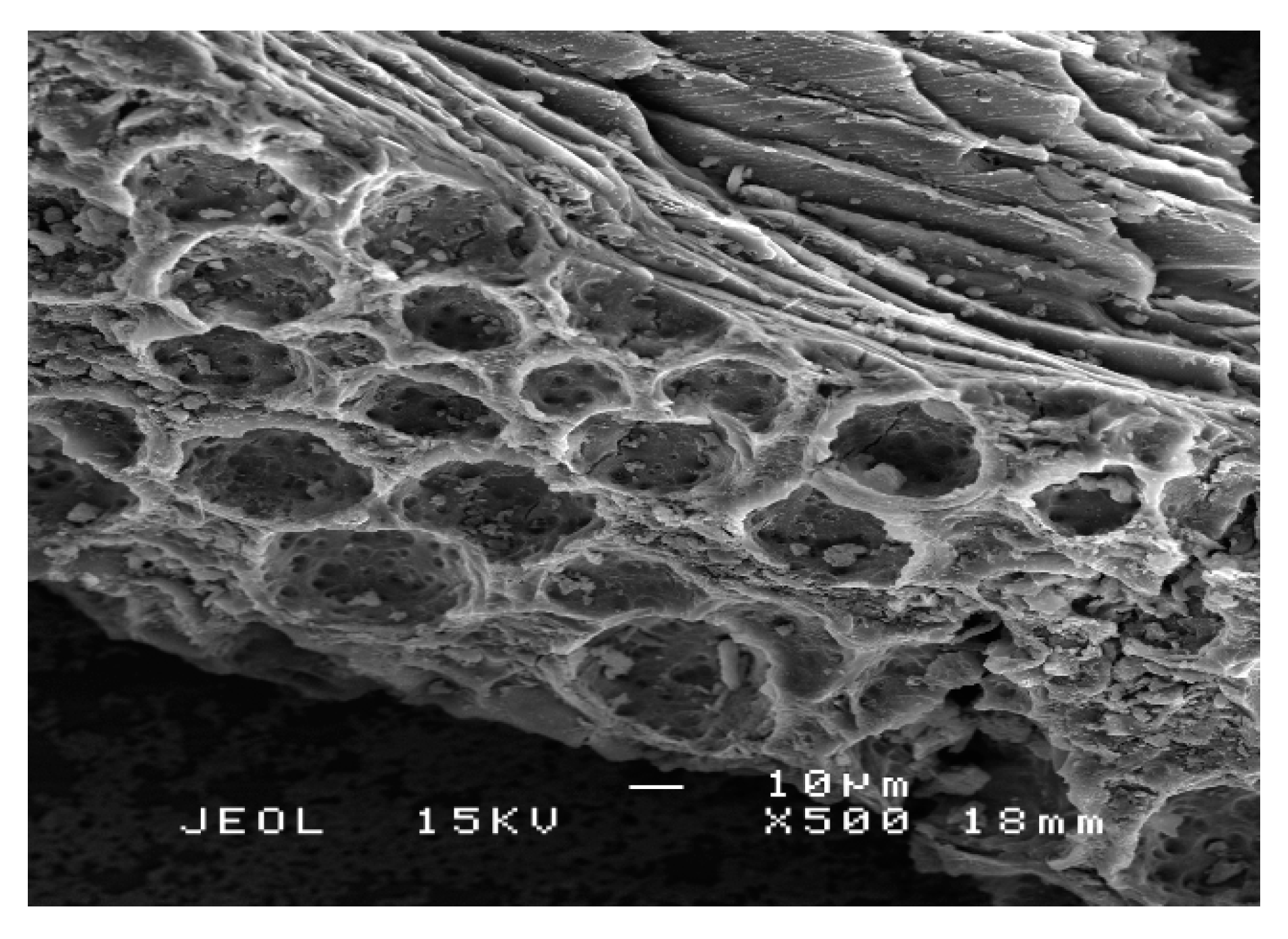
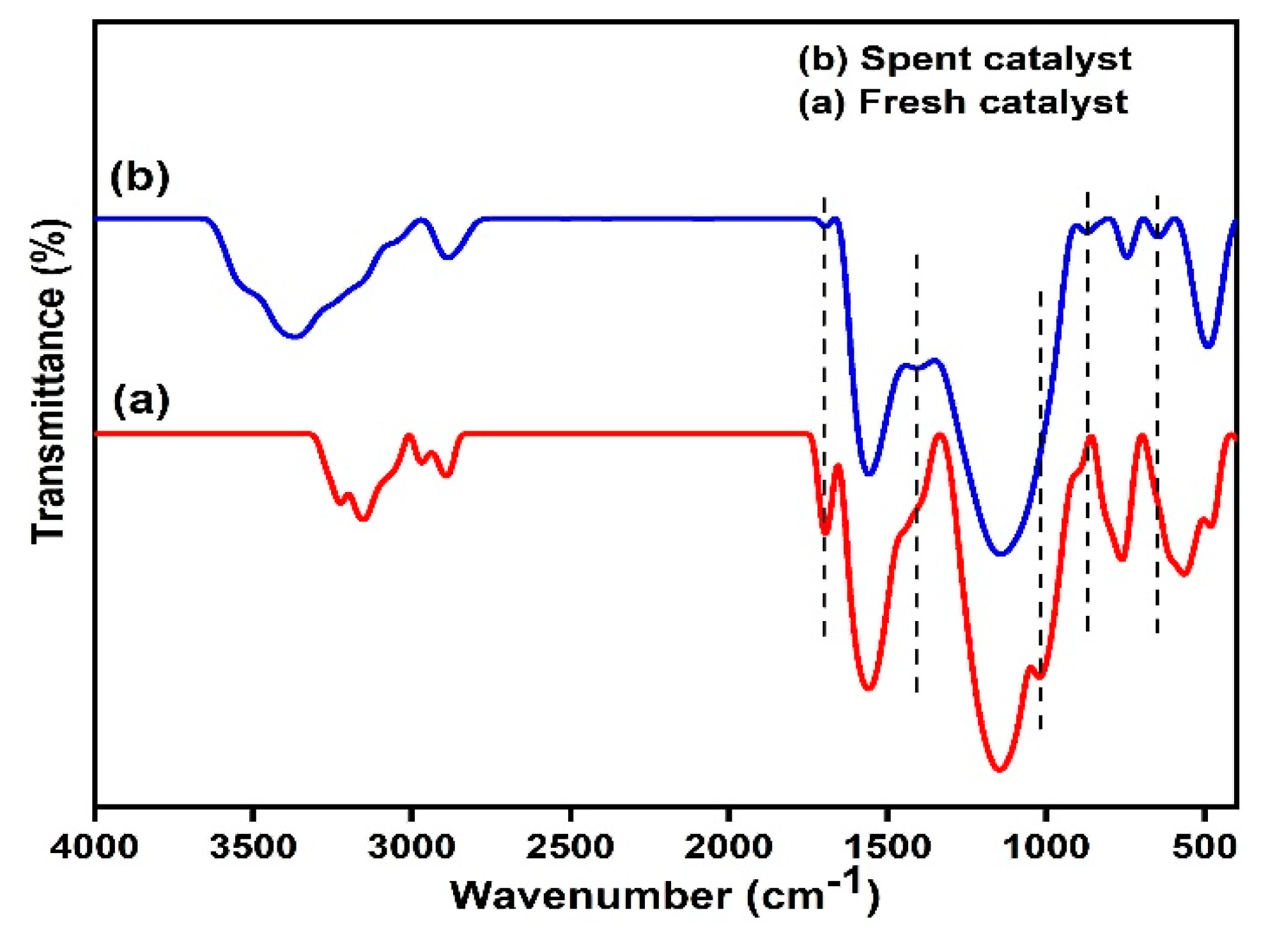
2.2. Catalyst Characterization
Structural and Surface Characteristics of the OMSC Catalyst
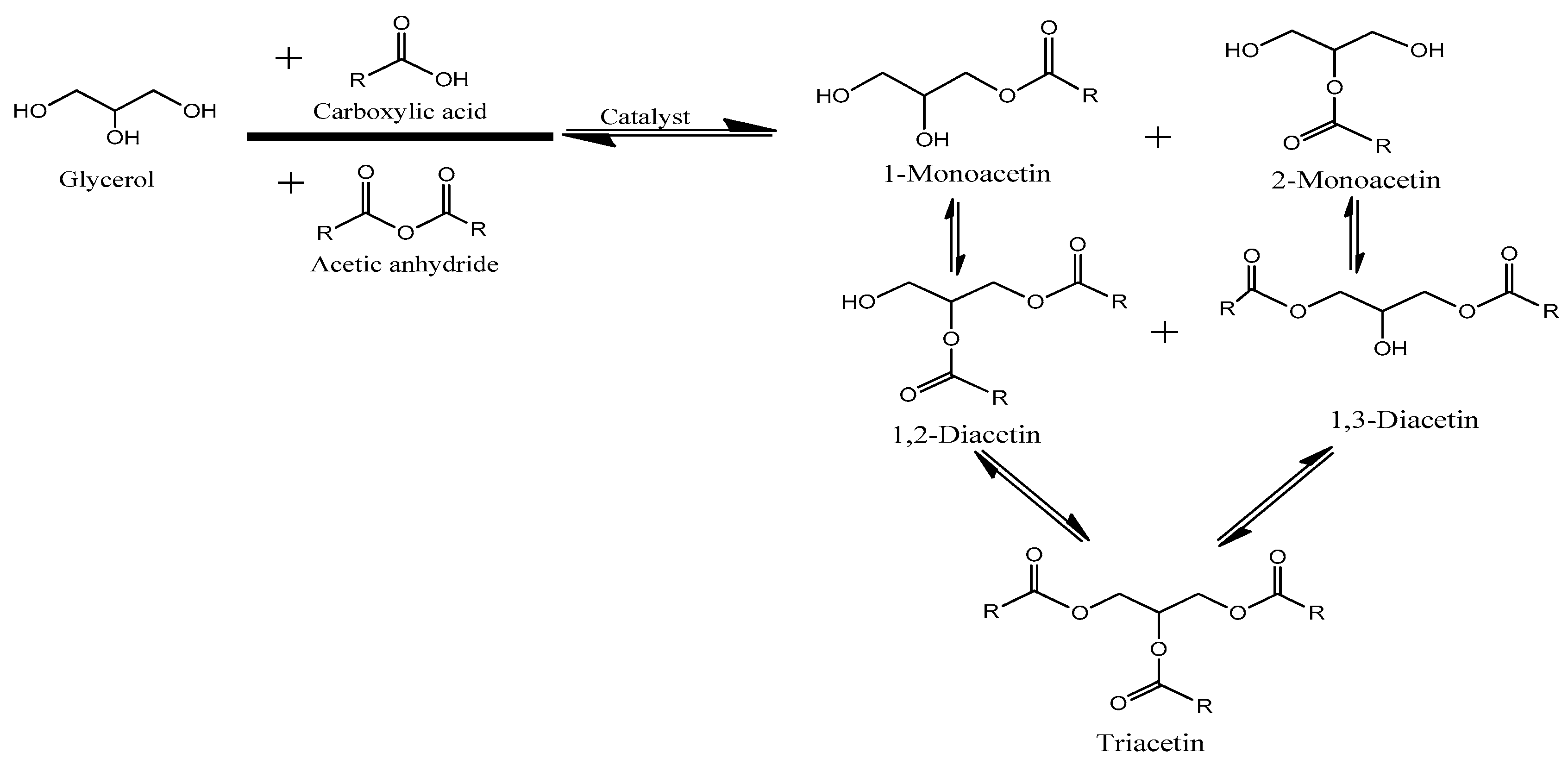
2.3. Modeling and Optimization of the Acetin Production
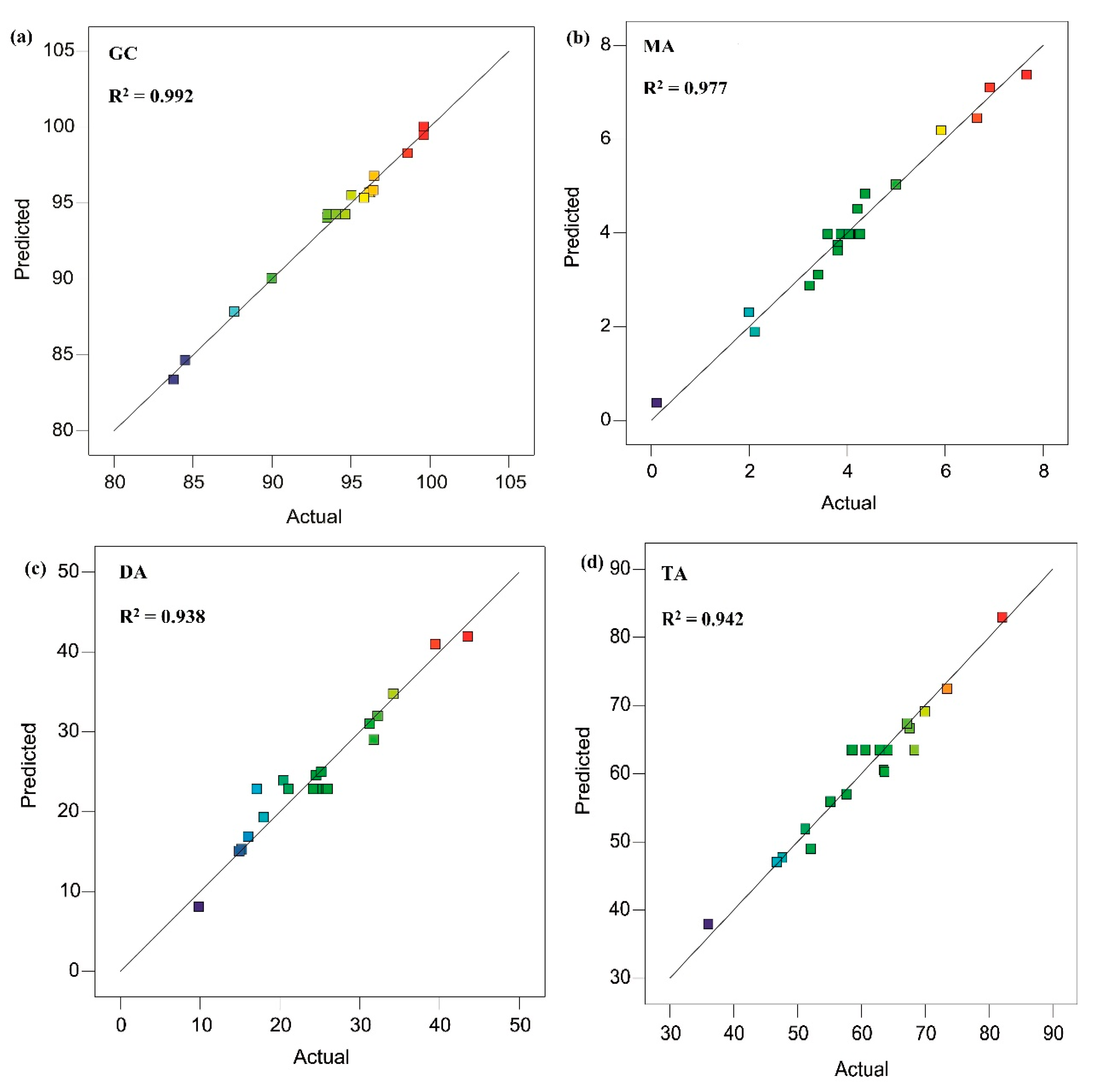
2.3.1. Analysis of Variance (ANOVA), Regression Model, and Model Fitting for GC
2.3.2. Analysis of Variance (ANOVA), Regression Model, and Model Fitting for MA Selectivity
2.3.3. Analysis of Variance (ANOVA), Regression Model, and Model Fitting for DA Selectivity
2.3.4. Analysis of Variance (ANOVA), Regression Model, and Model Fitting for TA Selectivity
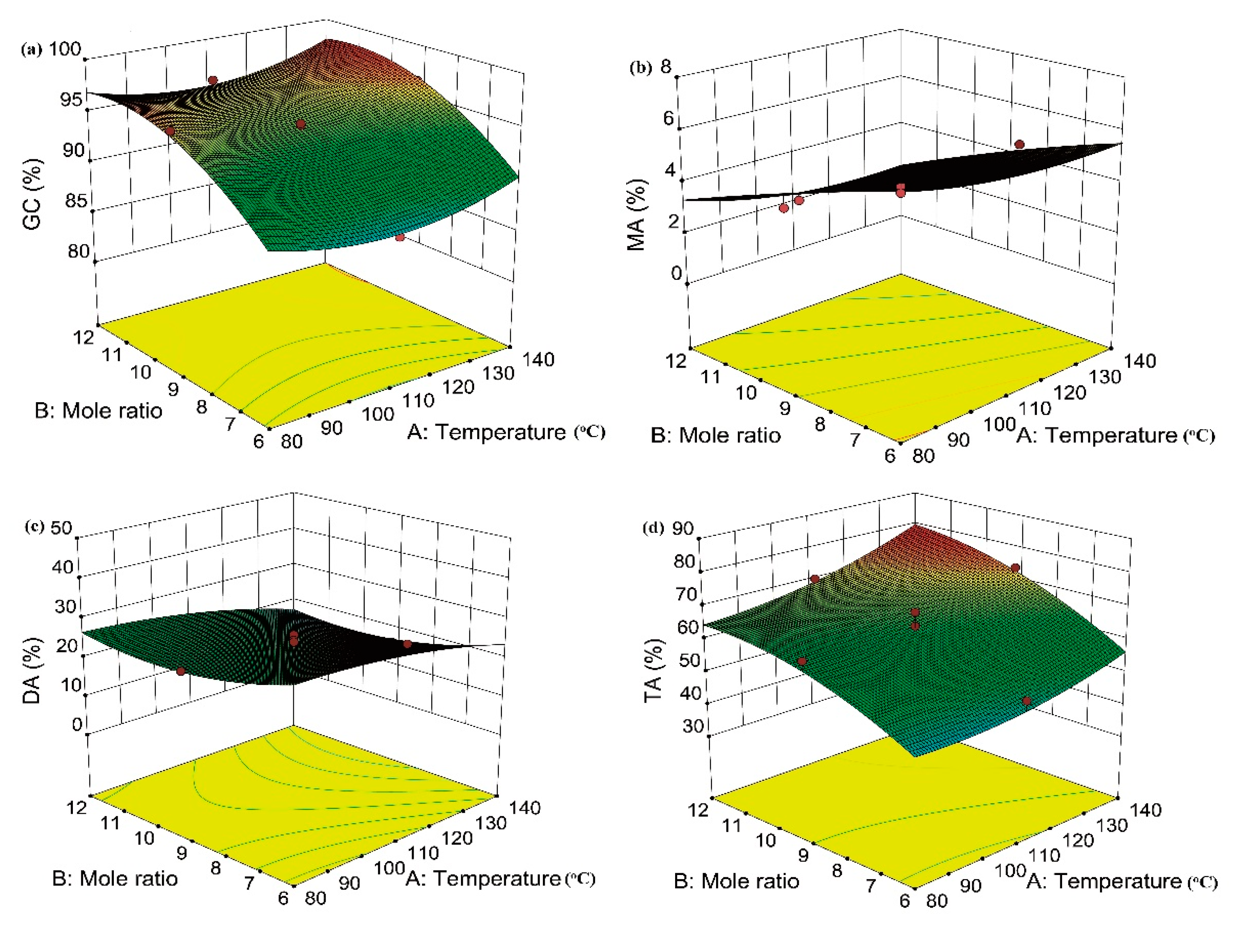
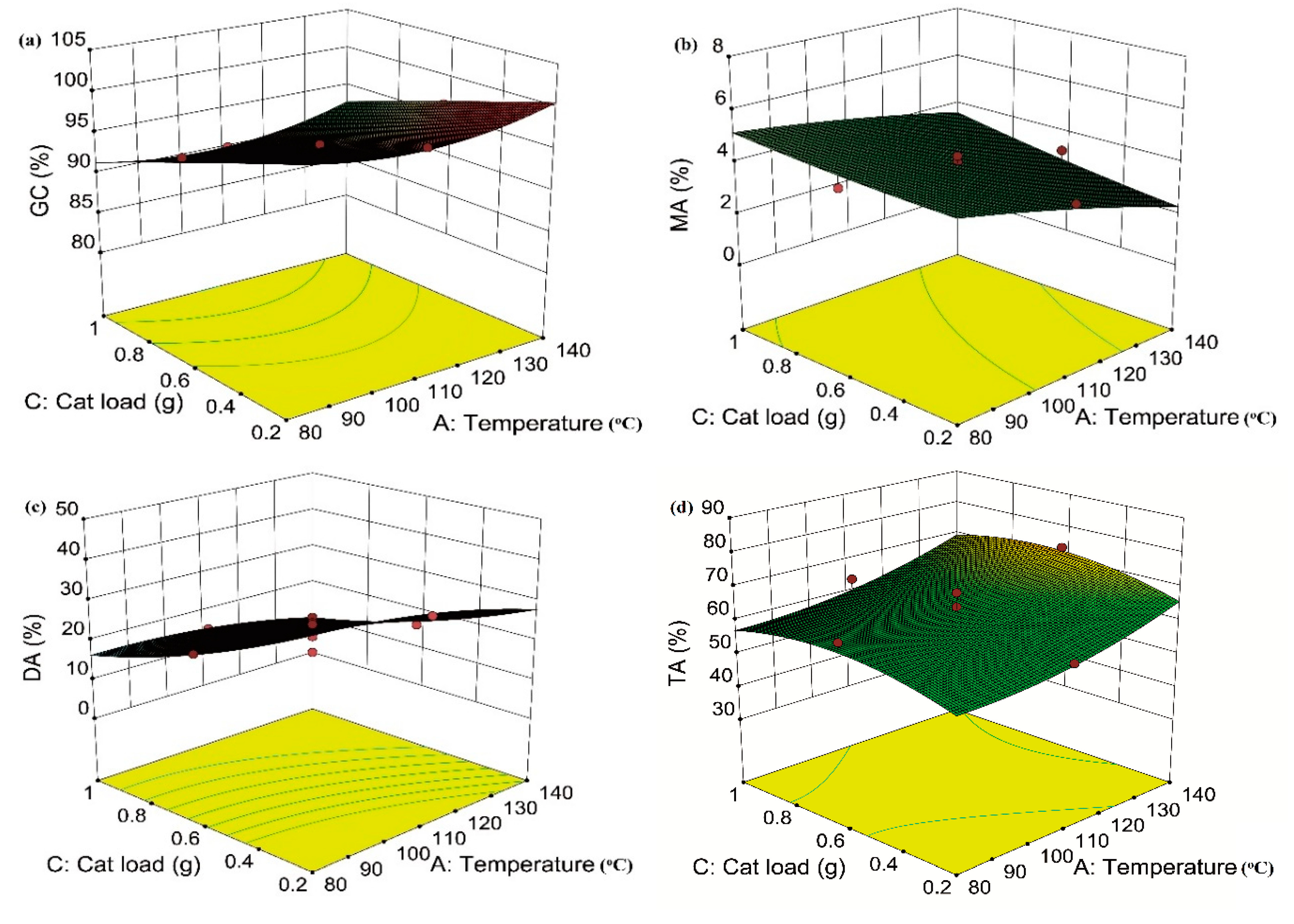
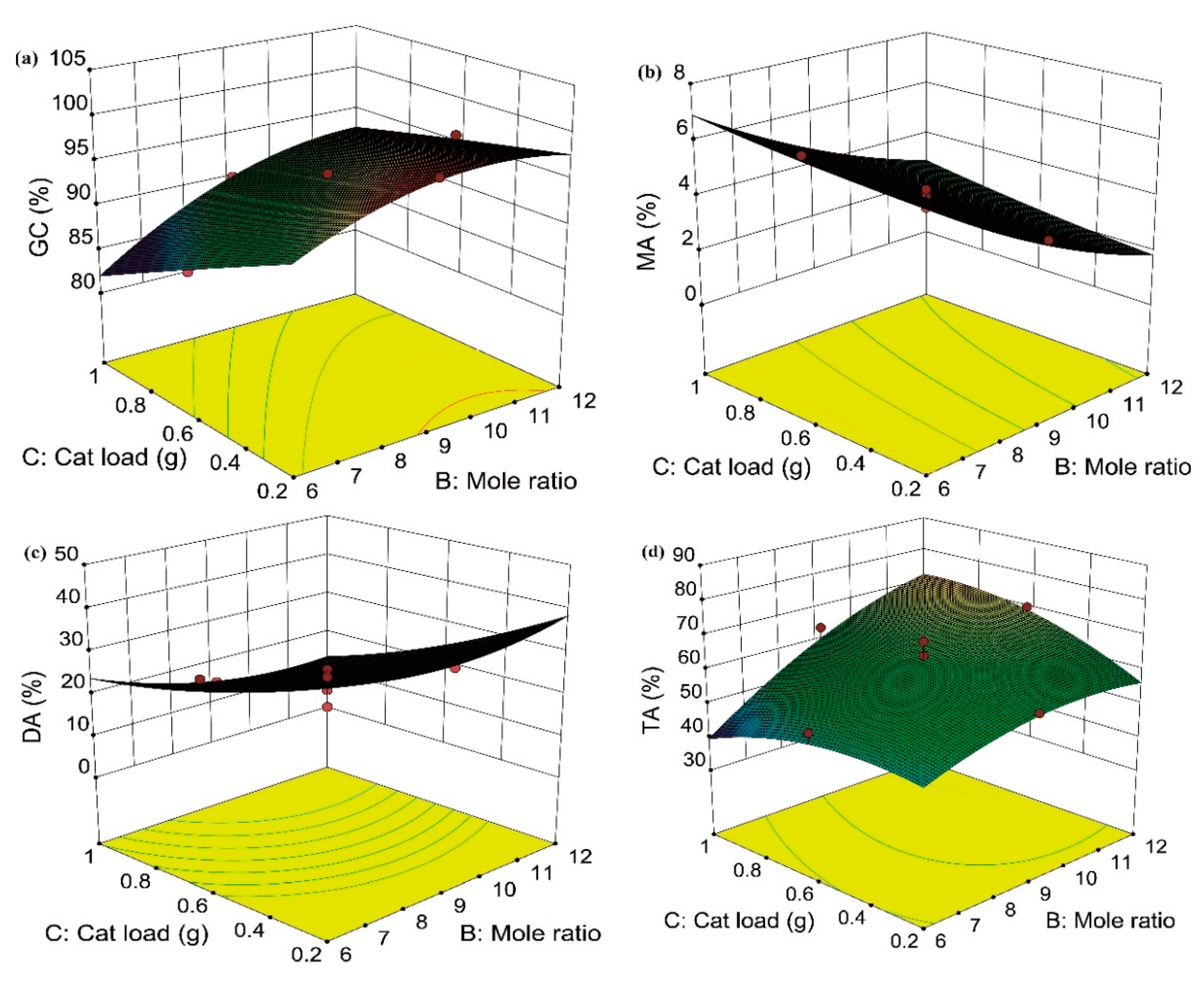
2.4. Effect of Variables and Their Interaction on GC and Selectivity to MA, DA, and TA
2.5. Optimization
2.6. Catalyst Reusability
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Mesoporous Sulfonated Carbon Catalyst
3.3. Characterization of the Catalyst
3.4. Catalytic Activity
3.5. Response Surface Methodology (RSM)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Knothe, G.; Razon, L.F. Biodiesel fuels. Prog. Energy Combust. Sci. 2017, 58, 36–59. [Google Scholar] [CrossRef]
- Mardhiah, H.H.; Ong, H.C.; Masjuki, H.H.; Lim, S.; Lee, H.V. A review on latest developments and future prospects of heterogeneous catalyst in biodiesel production from non-edible oils. Renew. Sustain. Energy Rev. 2017, 67, 1225–1236. [Google Scholar] [CrossRef]
- Keogh, J.; Tiwari, M.S.; Manyar, H. Esterification of Glycerol with Acetic Acid Using Nitrogen-Based Brønsted-Acidic Ionic Liquids. Ind. Eng. Chem. Res. 2019, 58, 17235–17243. [Google Scholar] [CrossRef]
- Nanda, M.R.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Poirier, M.; Xu, C.C. Thermodynamic and kinetic studies of a catalytic process to convert glycerol into solketal as an oxygenated fuel additive. Fuel 2014, 117, 470–477. [Google Scholar] [CrossRef]
- Neto, A.S.B.; Oliveira, A.C.; Filho, J.M.; Amadeo, N.; Dieuzeide, M.L.; de Sousa, F.F.; Oliveira, A.C. Characterizations of nanostructured nickel aluminates as catalysts for conversion of glycerol: Influence of the preparation methods. Adv. Powder Technol. 2017, 28, 131–138. [Google Scholar] [CrossRef]
- Okoye, P.U.; Abdullah, A.Z.; Hameed, B.H. A review on recent developments and progress in the kinetics and deactivation of catalytic acetylation of glycerol—A byproduct of biodiesel. Renew. Sustain. Energy Rev. 2017, 74, 387–401. [Google Scholar] [CrossRef]
- Liu, B.; Gao, F. Navigating Glycerol Conversion Roadmap and Heterogeneous Catalyst Selection Aided by Density Functional Theory: A Review. Catalysts 2018, 8, 44. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Li, X.; Gao, X. Esterification of glycerol and acetic acid in a pilot-scale reactive distillation column: Experimental investigation, model validation, and process analysis. J. Taiwan Inst. Chem. Eng. 2018, 89, 56–66. [Google Scholar] [CrossRef]
- Kim, I.; Kim, J.; Lee, D. A comparative study on catalytic properties of solid acid catalysts for glycerol acetylation at low temperatures. Appl. Catal. B Environ. 2014, 148, 295–303. [Google Scholar] [CrossRef]
- Rastegari, H.; Ghaziaskar, H.S.; Yalpani, M. Valorization of biodiesel derived glycerol to acetins by continuous esterification in acetic acid: Focusing on high selectivity to diacetin and triacetin with no byproducts. Ind. Eng. Chem. Res. 2015, 54, 3279–3284. [Google Scholar] [CrossRef]
- Arun, P.; Pudi, S.M.; Biswas, P. Acetylation of Glycerol over Sulfated Alumina: Reaction Parameter Study and Optimization Using Response Surface Methodology. Energy Fuels 2016, 30, 584–593. [Google Scholar]
- Caballero, K.V.; Guerrero-Amaya, H.; Baldovino-Medrano, V.G. Revisiting glycerol esterification with acetic acid over Amberlyst-35 via statistically designed experiments: Overcoming transport limitations. Chem. Eng. Sci. 2019, 207, 91–104. [Google Scholar] [CrossRef]
- Magar, S.; Mohanraj, G.T.; Jana, S.K.; Rode, C.V. Synthesis and characterization of supported heteropoly acid: Efficient solid acid catalyst for glycerol esterification to produce biofuel additives. Inorg. Nano Met. Chem. 2020, 50, 1157–1165. [Google Scholar] [CrossRef]
- Dewajani, H.; Zamrudy, W.; Saroso, H.; Paramarta, S.; Mulya, W. Conversion of Crude Glycerol from by-Product Biodiesel into Bio-additive of Fuel through Acetylation Reaction based on Modified Zeolite Catalyst. Alchemy 2019, 7, 46. [Google Scholar] [CrossRef]
- Liao, X.; Zhu, Y.; Wang, S.; Li, Y. Producing triacetylglycerol with glycerol by two steps: Esteri fi cation and acetylation. Fuel Process. Technol. 2009, 90, 988–993. [Google Scholar] [CrossRef]
- Chandrakala, U.; Prasad, R.B.N.; Prabhavathi Devi, B.L.A. Glycerol valorization as biofuel additives by employing a carbon-based solid acid catalyst derived from glycerol. Ind. Eng. Chem. Res. 2014, 53, 16164–16169. [Google Scholar] [CrossRef]
- García-Martín, J.F.; Alés-Álvarez, F.J.; Torres-García, M.; Feng, C.H.; Álvarez-Mateos, P. Production of oxygenated fuel additives from residual glycerine using biocatalysts obtained from heavy-metal-contaminated Jatropha Curcas L. roots. Energies 2019, 12, 740. [Google Scholar] [CrossRef]
- Okoye, P.U.; Abdullah, A.Z.; Hameed, B.H. Synthesis of oxygenated fuel additives via glycerol esterification with acetic acid over bio-derived carbon catalyst. Fuel 2017, 209, 538–544. [Google Scholar] [CrossRef]
- Rezayat, M.; Ghaziaskar, H.S. Continuous synthesis of glycerol acetates in supercritical carbon dioxide using Amberlyst 15®. Green Chem. 2009, 11, 710–771. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Sun, Y.; Jian, R.; Jian, P.; Wang, D. Selective synthesis of triacetin from glycerol catalyzed by HZSM-5/MCM-41 micro/mesoporous molecular sieve. Chinese J. Chem. Eng. 2019, 59, 1073–1078. [Google Scholar] [CrossRef]
- Nda-Umar, U.I.; Ramli, I.; Muhamad, E.N.; Taufiq-Yap, Y.H.; Azri, N. Synthesis and characterization of sulfonated carbon catalysts derived from biomass waste and its evaluation in glycerol acetylation. Biomass Convers. Biorefinery 2020. [Google Scholar] [CrossRef]
- Goscianska, J.; Malaika, A. A facile post-synthetic modification of ordered mesoporous carbon to get efficient catalysts for the formation of acetins. Catal. Today 2020, 357, 84–93. [Google Scholar] [CrossRef]
- Kakasaheb, Y.N.; Prashant, S.N.; Vijay, V.B. Synthesis of Oxygenated Fuel Additives via Acetylation of Bio-Glycerol over H2SO4 Modified Montmorillonite K10 Catalyst. 2018. Available online: https://www.semanticscholar.org/paper/Synthesis-of-Oxygenated-Fuel-Additives-via-of-over-Bokade/27865ce2d7f8fc5748d4474f52f02ce682ceda27 (accessed on 1 October 2020).
- Marwan, M.; Indarti, E.; Darmadi, D.; Rinaldi, W.; Hamzah, D.; Rinaldi, T. Production of triacetin by microwave assisted esterification of glycerol using activated natural zeolite. Bull. Chem. React. Eng. Catal. 2019, 14, 672–677. [Google Scholar] [CrossRef]
- Bureros, G.M.A.; Tanjay, A.A.; Cuizon, D.E.S.; Go, A.W.; Cabatingan, L.K.; Agapay, R.C.; Ju, Y.H. Cacao shell-derived solid acid catalyst for esterification of oleic acid with methanol. Renew. Energy 2019, 138, 489–501. [Google Scholar] [CrossRef]
- Yu, H.; Niu, S.; Lu, C.; Li, J.; Yang, Y. Preparation and esterification performance of sulfonated coal-based heterogeneous acid catalyst for methyl oleate production. Energy Convers. Manag. 2016, 126, 488–496. [Google Scholar] [CrossRef]
- Zhou, Y.; Niu, S.; Li, J. Activity of the carbon-based heterogeneous acid catalyst derived from bamboo in esterification of oleic acid with ethanol. Energy Convers. Manag. 2016, 114, 188–196. [Google Scholar] [CrossRef]
- Rastegari, H.; Ghaziaskar, H.S. From glycerol as the by-product of biodiesel production to value-added monoacetin by continuous and selective esterification in acetic acid. J. Ind. Eng. Chem. 2015, 21, 856–861. [Google Scholar] [CrossRef]
- Tan, Y.H.; Abdullah, M.O.; Nolasco-Hipolito, C.; Ahmad Zauzi, N.S. Application of RSM and Taguchi methods for optimizing the transesterification of waste cooking oil catalyzed by solid ostrich and chicken-eggshell derived CaO. Renew. Energy 2017, 114, 437–447. [Google Scholar] [CrossRef]
- Ma, H.; Li, J.; Liu, W.; Cheng, B.; Cao, X.; Mao, J.; Zhu, S. Hydrothermal preparation and characterization of novel corncob-derived solid acid catalysts. J. Agric. Food Chem. 2014, 62, 5345–5353. [Google Scholar] [CrossRef]
- Ning, Y.; Niu, S. Preparation and catalytic performance in esterification of a bamboo-based heterogeneous acid catalyst with microwave assistance. Energy Convers. Manag. 2017, 153, 446–454. [Google Scholar] [CrossRef]
- Tao, M.L.; Guan, H.Y.; Wang, X.H.; Liu, Y.C.; Louh, R.F. Fabrication of sulfonated carbon catalyst from biomass waste and its use for glycerol esterification. Fuel Process. Technol. 2015, 138, 355–360. [Google Scholar] [CrossRef]
- Ezebor, F.; Khairuddean, M.; Abdullah, A.Z.; Boey, P.L. Oil palm trunk and sugarcane bagasse derived solid acid catalysts for rapid esterification of fatty acids and moisture-assisted transesterification of oils under pseudo-infinite methanol. Bioresour. Technol. 2014, 157, 254–262. [Google Scholar] [CrossRef]
- Konwar, L.J.; Mäki-Arvela, P.; Begum, P.; Kumar, N.; Jyoti, T.A.; Mikkola, J.; Deka, C.R.; Deka, D. Shape selectivity and acidity effects in glycerol acetylation with acetic anhydride: Selective synthesis of triacetin over Y-zeolite and sulfonated mesoporous carbons. J. Catal. 2015, 329, 237–247. [Google Scholar] [CrossRef]
- Lee, D. Preparation of a sulfonated carbonaceous material from lignosulfonate and its usefulness as an esterification catalyst. Molecules 2013, 18, 8168–8180. [Google Scholar] [CrossRef]
- Bastos, R.R.C.; da Luz Corrêa, A.P.; da Luz, P.T.S.; da Rocha Filho, G.N.; Zamian, J.R.; da Conceição, L.R.V. Optimization of biodiesel production using sulfonated carbon-based catalyst from an amazon agro-industrial waste. Energy Convers. Manag. 2020, 205, 112457. [Google Scholar] [CrossRef]
- Wang, W.; Lu, P.; Tang, H.; Ma, Y.; Yang, X. Zanthoxylum bungeanum seed oil based carbon solid acid catalyst for the production of biodiesel. New J. Chem. 2017, 41, 9256–9261. [Google Scholar] [CrossRef]
- Nakhate, A.V.; Yadav, G.D. Synthesis and Characterization of Sulfonated Carbon-Based Graphene Oxide Monolith by Solvothermal Carbonization for Esterification and Unsymmetrical Ether Formation. ACS Sustain. Chem. Eng. 2016, 4, 1963–1973. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, J.; An, S.; Zhang, Q.; Guo, Y.; Song, D.; Shang, Q. Selective esterification of glycerol with acetic acid or lauric acid over rod-like carbon-based sulfonic acid functionalized ionic liquids. Fuel 2017, 207, 136–145. [Google Scholar] [CrossRef]
- Rebelo, S.L.H.; Guedes, A.; Szefczyk, M.E.; Pereira, A.M.; Araújo, J.P.; Freire, C. Progress in the Raman spectra analysis of covalently functionalized multiwalled carbon nanotubes: Unraveling disorder in graphitic materials. Phys. Chem. Chem. Phys. 2016, 18, 12784–12796. [Google Scholar] [CrossRef]
- Xie, J.; Han, Q.; Feng, B.; Liu, Z. Preparation of amphiphilic mesoporous carbon-based solid acid from krft lignin activated by phosphoric acid and its catalytic performance for hydration of α-pinene. BioResources 2019, 14, 4284–4303. [Google Scholar]
- Bai, Y.Y.; Xiao, L.P.; Sun, R.C. Efficient hydrolyzation of cellulose in ionic liquid by novel sulfonated biomass-based catalysts. Cellulose 2014, 21, 2327–2336. [Google Scholar] [CrossRef]
- Nagasundaram, N.; Kokila, M.; Sivaguru, P.; Santhosh, R.; Lalitha, A. SO3H@carbon powder derived from waste orange peel: An efficient, nano-sized greener catalyst for the synthesis of dihydropyrano[2,3-c]pyrazole derivatives. Adv. Powder Technol. 2020, 31, 1516–1528. [Google Scholar] [CrossRef]
- Lesiak, B.; Jiricek, P.; Bieloshapka, I. Chemical and structural properties of Pd nanoparticle-decorated graphene—Electron spectroscopic methods and QUASES. Appl. Surf. Sci. 2017, 404, 300–309. [Google Scholar] [CrossRef]
- Dalla Costa, B.O.; Decolatti, H.P.; Legnoverde, M.S.; Querini, C.A. Influence of acidic properties of different solid acid catalysts for glycerol acetylation. Catal. Today 2017, 289, 222–230. [Google Scholar] [CrossRef]
- Santos, E.M.; Teixeira, A.P.D.C.; Da Silva, F.G.; Cibaka, T.E.; Araújo, M.H.; Oliveira, W.X.C.; Medeiros, F.; Brasil, A.N. New heterogeneous catalyst for the esterification of fatty acid produced by surface aromatization/sulfonation of oilseed cake. Fuel 2015, 150, 408–414. [Google Scholar] [CrossRef]
- Figueiredo, J.L. Functionalization of porous carbons for catalytic applications. J. Mater. Chem. A 2013, 1, 9351. [Google Scholar] [CrossRef]
- Laohapornchaiphan, J.; Smith, C.B.; Smith, S.M. One-step Preparation of Carbon-based Solid Acid Catalyst from Water Hyacinth Leaves for Esterification of Oleic Acid and Dehydration of Xylose. Chem. Asian J. 2017, 12, 3178–3186. [Google Scholar] [CrossRef]
- Konwar, L.J.; Mäki-Arvela, P.; Salminen, E.; Kumar, N.; Thakur, A.J.; Mikkola, J.P.; Deka, D. Towards carbon efficient biorefining: Multifunctional mesoporous solid acids obtained from biodiesel production wastes for biomass conversion. Appl. Catal. B Environ. 2015, 176, 20–35. [Google Scholar] [CrossRef]
- Okoye, P.U.; Hameed, B.H. Review on recent progress in catalytic carboxylation and acetylation of glycerol as a byproduct of biodiesel production. Renew. Sustain. Energy Rev. 2016, 53, 558–574. [Google Scholar] [CrossRef]
- Zeng, D.; Zhang, Q.; Chen, S.; Liu, S.; Wang, G. Synthesis porous carbon-based solid acid from rice husk for esterification of fatty acids. Microporous Mesoporous Mater. 2016, 219, 54–58. [Google Scholar] [CrossRef]
- Shen, Z.; Yu, X.; Chen, J. Production of 5-hydroxymethylfurfural from fructose catalyzed by sulfonated bamboo-derived carbon prepared by simultaneous carbonization and sulfonation. BioResources 2016, 11, 3094–3109. [Google Scholar] [CrossRef]
- Nayebzadeh, H.; Saghatoleslami, N.; Tabasizadeh, M. Optimization of the activity of KOH/calcium aluminate nanocatalyst for biodiesel production using response surface methodology. J. Taiwan Inst. Chem. Eng. 2016, 68, 379–386. [Google Scholar] [CrossRef]
- Zamzuri, N.H.; Mat, R.; Amin, N.A.S.; Talebian-Kiakalaieh, A. Hydrogen production from catalytic steam reforming of glycerol over various supported nickel catalysts. J. Hydrog. 2016, 124, 1–12. [Google Scholar] [CrossRef]
- Kulkarni, R.M.; Britto, P.J.; Narula, A.; Saqline, S.; Anand, D.; Bhagyalakshmi, C.; Herle, R.N. Kinetic studies on the synthesis of fuel additives from glycerol using CeO2-ZrO2 metal oxide catalyst. Biofuel Res. J. 2020, 7, 1100–1108. [Google Scholar] [CrossRef]
- Kong, P.S.; Pérès, Y.; Wan Daud, W.M.A.; Cognet, P.; Aroua, M.K. Esterification of glycerol with oleic acid over hydrophobic zirconia-silica acid catalyst and commercial acid catalyst: Optimization and influence of catalyst acidity. Front. Chem. 2019, 7, 205. [Google Scholar] [CrossRef] [PubMed]
- Lathiya, D.R.; Bhatt, D.V.; Maheria, K.C. Synthesis of sulfonated carbon catalyst from waste orange peel for cost effective biodiesel production. Bioresour. Technol. Rep. 2018, 2, 69–76. [Google Scholar] [CrossRef]
- Ngaosuwan, K.; Goodwin, J.G.; Prasertdham, P. A green sulfonated carbon-based catalyst derived from coffee residue for esterification. Renew. Energy 2016, 86, 262–269. [Google Scholar] [CrossRef]
- Jiménez Toro, M.J.; Dou, X.; Ajewole, I.; Wang, J.; Chong, K.; Ai, N.; Zeng, G.; Chen, T. Preparation and Optimization of Macroalgae-Derived Solid Acid Catalysts. Waste Biomass Valorization 2017, 10, 805–816. [Google Scholar] [CrossRef]
- Konwar, L.J.; Mäki-Arvela, P.; Kumar, N.; Mikkola, J.P.; Sarma, A.K.; Deka, D. Selective esterification of fatty acids with glycerol to monoglycerides over –SO3H functionalized carbon catalysts. React. Kinet. Mech. Catal. 2016, 119, 121–138. [Google Scholar] [CrossRef]
Sample Availability: Samples are not available from the authors. |
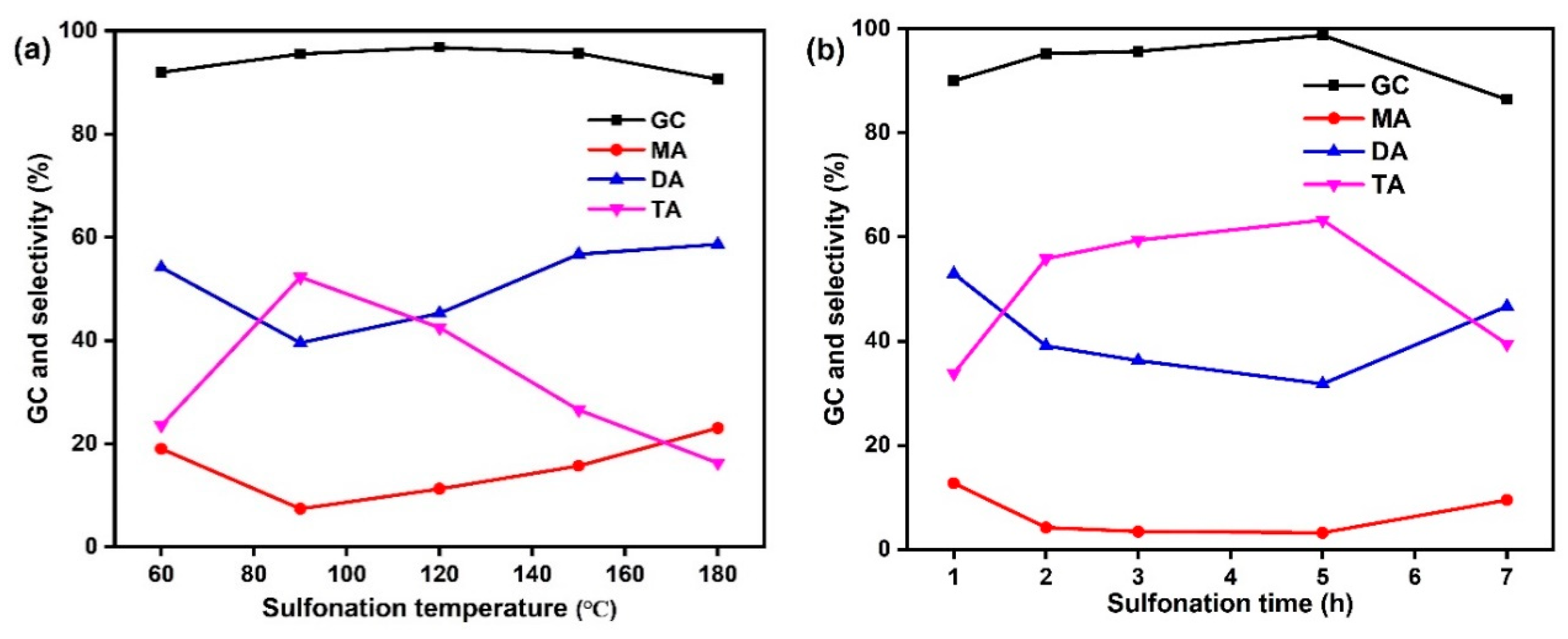



| Sulfonation Temperature (°C) | S a (%) | SAD b (mmol/g) | SAD c (mmol/g) | TAD d (mmol/g) | Sulfonation time (h) | S a (%) | SAD b (mmol/g) | SAD c (mmol/g) | TAD d (mmol/g) |
|---|---|---|---|---|---|---|---|---|---|
| 60 | 3.06 | 0.96 | 1.33 | 5.00 | 1 | 1.93 | 0.60 | 1.20 | 5.10 |
| 90 | 3.72 | 1.16 | 2.57 | 7.10 | 2 | 2.32 | 0.72 | 1.47 | 5.25 |
| 120 | 3.24 | 1.01 | 2.23 | 6.00 | 3 | 3.09 | 0.96 | 1.70 | 5.40 |
| 150 | 3.12 | 0.97 | 1.90 | 6.50 | 5 | 3.27 | 1.02 | 1.80 | 5.45 |
| 180 | 2.79 | 0.87 | 1.67 | 5.80 | 7 | 1.96 | 0.61 | 1.27 | 5.35 |
| Sample | Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (Å) | Acid Site Density a (µmol/g) |
|---|---|---|---|---|
| Carbonized | 484 | 0.327 | 27.09 | ND |
| OMSC Catalyst | 217 | 0.164 | 47.04 | 9568.3 |
| Run | Conditions of Reaction | Predicted Value (%) | Desirability | Experimental Value (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | MR | CL | GC | MA | DA | TA | GC | MA | DA | TA | ||
| 1. | 126 | 10.4 | 0.45 | 97.52 | 2.26 | 23.96 | 69.21 | 1.000 * | 96.97 | 4.87 | 27.76 | 66.49 |
| 2. | 80 | 12 | 0.2 | 99.47 | 3.11 | 41.95 | 51.93 | 1.000 | 96.87 | 5.68 | 50.03 | 43.07 |
| 3. | 110 | 9 | 0.6 | 94.27 | 3.98 | 22.85 | 63.51 | 1.000 | 97.46 | 5.55 | 34.84 | 58.41 |
| 4. | 110 | 9 | 0.2 | 98.29 | 3.62 | 37.74 | 57.02 | 1.000 | 97.19 | 5.24 | 39.67 | 54.23 |
| Sample | S a (%) | SAD b (mmol/g) | SAD c (mmol/g) | TAD d (mmol/g) |
|---|---|---|---|---|
| Fresh catalyst | 3.72 | 1.16 | 1.57 | 7.10 |
| Spent catalyst | 0.95 | 0.30 | 0.33 | 1.69 |
| Levels | ||||
|---|---|---|---|---|
| Factor | Unit | Code | Minimum | Maximum |
| −1 | 1 | |||
| Temperature | °C | A | 80 | 140 |
| Mole ratio | - | B | 6 | 12 |
| Catalyst load | g | C | 0.2 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nda-Umar, U.I.; Ramli, I.; Muhamad, E.N.; Azri, N.; Taufiq-Yap, Y.H. Optimization and Characterization of Mesoporous Sulfonated Carbon Catalyst and Its Application in Modeling and Optimization of Acetin Production. Molecules 2020, 25, 5221. https://doi.org/10.3390/molecules25225221
Nda-Umar UI, Ramli I, Muhamad EN, Azri N, Taufiq-Yap YH. Optimization and Characterization of Mesoporous Sulfonated Carbon Catalyst and Its Application in Modeling and Optimization of Acetin Production. Molecules. 2020; 25(22):5221. https://doi.org/10.3390/molecules25225221
Chicago/Turabian StyleNda-Umar, Usman Idris, Irmawati Ramli, Ernee Noryana Muhamad, Norsahida Azri, and Yun Hin Taufiq-Yap. 2020. "Optimization and Characterization of Mesoporous Sulfonated Carbon Catalyst and Its Application in Modeling and Optimization of Acetin Production" Molecules 25, no. 22: 5221. https://doi.org/10.3390/molecules25225221
APA StyleNda-Umar, U. I., Ramli, I., Muhamad, E. N., Azri, N., & Taufiq-Yap, Y. H. (2020). Optimization and Characterization of Mesoporous Sulfonated Carbon Catalyst and Its Application in Modeling and Optimization of Acetin Production. Molecules, 25(22), 5221. https://doi.org/10.3390/molecules25225221






