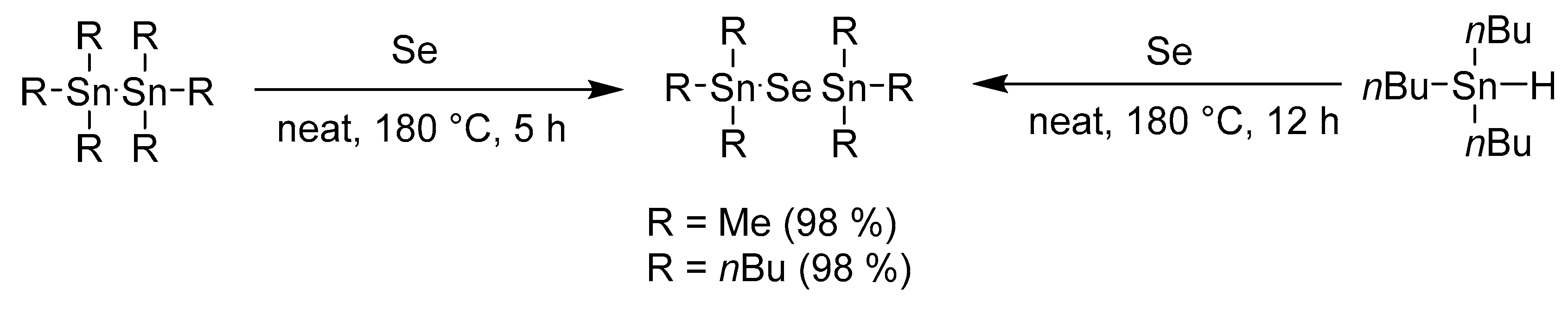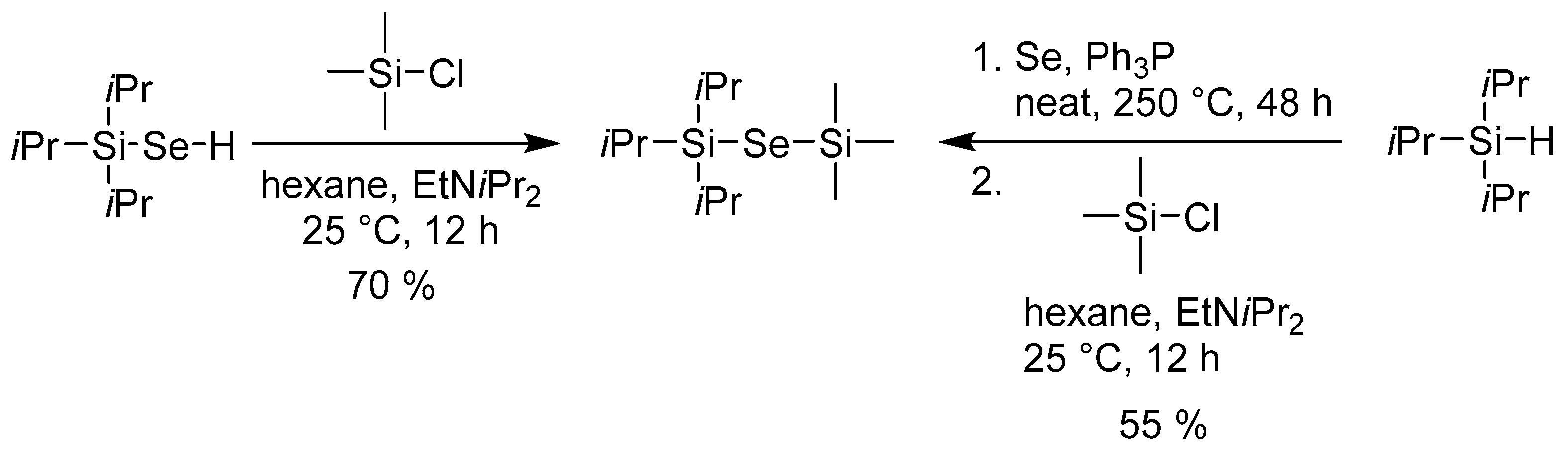Abstract
The current portfolio of organoselenium compounds applicable as volatile precursors for atomic layer deposition can be denoted as very limited. Hence, we report herein facile and cost-effective preparation of two bis(trialkylstannyl)selenides as well as one selenole and three bis(trialkylsilyl)selenides. Their syntheses have been optimized to: (i) use readily available and inexpensive starting materials, (ii) involve operationally simple methodology (heating in a pressure vessel), (iii) use a minimum amount of additives and catalysts, and (iv) either exclude additional purification or involve only simple distillation. The chemical structure of prepared Se derivatives was confirmed by multinuclear NMR and GC/MS. Their fundamental thermal properties were investigated by differential scanning calorimetry (DSC) and TGA methods that revealed thermal stability within the range of 160–300 °C.
1. Introduction
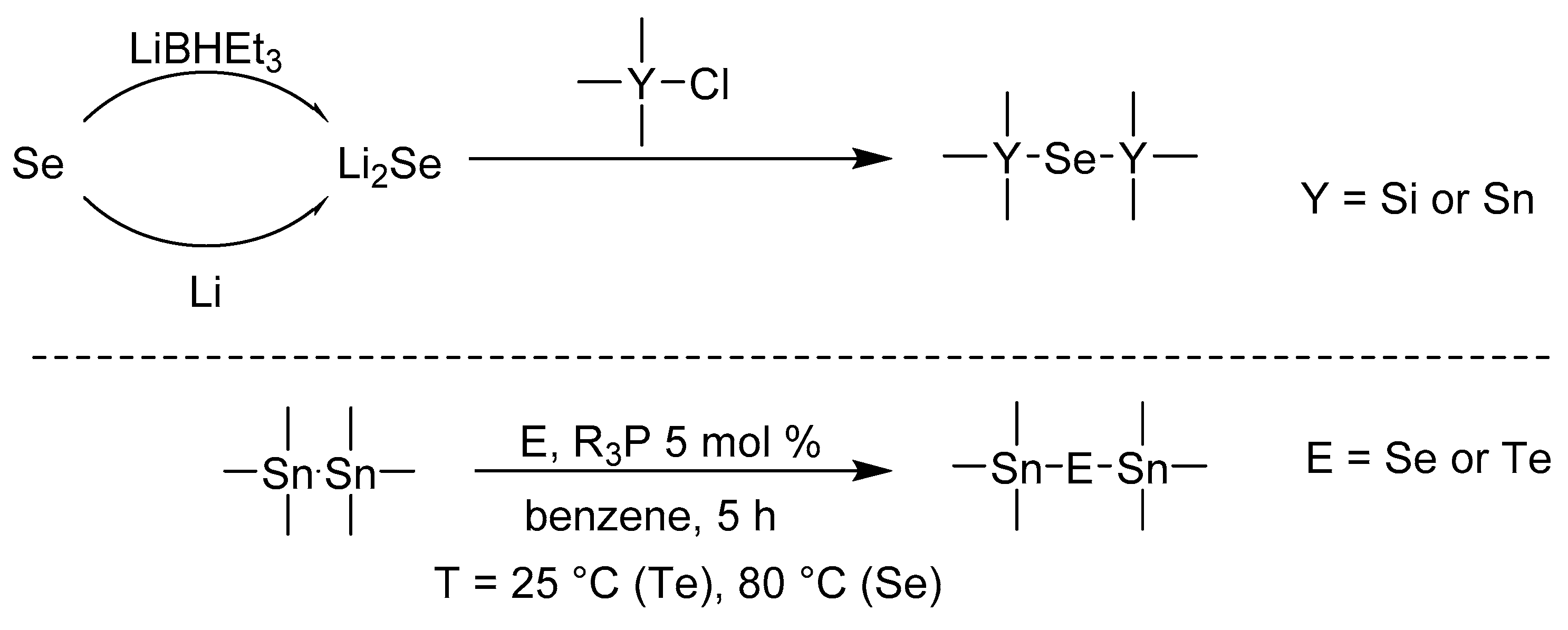
In addition to its indisputable essential and biological importance, selenium and its organo derivatives have also found important applications in materials science. For instance, chalcogenide forms metal selenides that belong to a group of TMDs (Transition Metal Dichalcogenides) with highly exploitable potential in many fields. Thin layers of metal sulfides, selenides, and tellurides have turned out to be very active in electrocatalysis; this topic was recently summarized by Lei [1]. Sb2Se3 is another example that showed interesting performance in optoelectronics [2]. Vanadium [3] or tungsten selenides [4] are well-known semiconducting materials for electronic technologies such as spintronics, oscillators, or memory storage devices. The successful application of the aforementioned selenides relies on their supramolecular arrangement, which is mostly affected by the synthetic method and deposition technique used. In this respect, Atomic Layer Deposition (ALD) is a burgeoning coating technology, which allows the manufacture of various thin films. The self-limiting nature of ALD provides a high compactness of nanolayer with exceptional coverage [5]. This holds true even for differently shaped substrates such as nanotubes [6], while ALD also allows a selective deposition over the particular substrate surface area [7]. ALD is a cyclic process composed of a few basic steps. The first one involves the reaction of the ALD precursor with free surface functional groups of the substrate in the reaction chamber. Subsequent evacuation of the chamber ensures the removal of precursor excess and possible by-products. After insertion of a second precursor, which is the third step, an atomic layer is deposited. The number of these cycles defines the thickness of the manufactured thin film, while the selection of the precursors allows the design of its composition. For instance, using water or oxygen leads to oxides, H2S to sulfides etc. However, the situation with metal precursors is complicated because such precursors need to be transported in a gas phase. Hence, the molecule used must be volatile enough and possess a good trade-off between thermal stability and chemical reactivity. In fact, there are only a few ALD precursors that deposit metal selenides. The previously used Et2Se (Et2Se2), which usually does not provide desired results, and toxic H2Se were mostly replaced with bis(trialkylsilyl)selenides [8]. In 2019, selenium dimethyldithiocarbamate, which reacts with tris(dimethylamino)antimony to form a Sb2Se3 nanolayer, was introduced by Sarkar et al. [9]. We have recently reported on a series of cyclic-silylselenides; in particular, the six-membered derivative bearing two selenium atoms provided very promising results during the depositing of MoSe2 by ALD [10]. Our further synthetic work [11] has identified bis(trialkylstanyl)selenides, structurally related to bis(trialkylsilyl)selenides, as an ALD selenium precursor with lowered air sensitivity. Indeed, (Me3Sn)2Se turned out to be a useful selenium source for MoSe2 nanolayers; however, the previous work lacked in-depth optimization of the synthesis. Easy and cost-effective preparation of the given organoselenium compound is another important aspect that must be considered when designing a new ALD precursor. Scheme 1 shows two reactions that in our opinion possess potential to be used for the preparation of selenium ALD precursors.
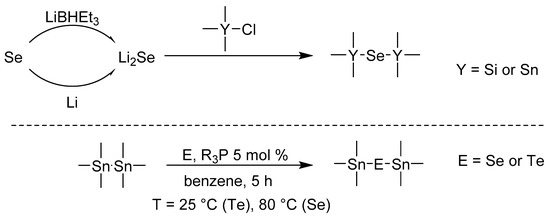
Scheme 1.
Known synthetic approaches towards (R3Y)2Se and (Me3Sn)2E compounds.
The most straightforward and efficient synthesis towards compounds of general formula (R3Y)2Se involves the in situ preparation of Li2Se, which subsequently reacts with trialkylsilyl chloride. Depending on the R-alkyl groups, the obtained yields vary from 43 to 90% [11,12,13,14,15,16,17]. Li2E is usually generated by the reaction of elemental selenium with either lithium or superhydride. Although the first approach is time-consuming and provides generally lower yields, the latter is fast but uses expensive LiBHEt3. Another interesting synthesis was reported by Tanaka et al. [18]. They used catalytic R3P oxidation by elemental Se or Te affording R3P = E. These agents are capable of inserting chalcogen into Sn–Sn (and Pb–Pb) bond (Scheme 1). Depending on the used catalyst, the yields reported for (Me3Sn)2Se were up to 46% (using Ph3P).
2. Materials and Methods
2.1. Synthesis of Selenium Precursors
All reactions were performed under argon using the Schlenk technique. The used solvents were water- and oxygen-free. Organic selenides are generally toxic; thus, all used glassware was treated with sodium hypochlorite after experiments. The reactions were carried out in Ace pressure tubes (Merck, Kenilworth, NJ, USA). A standard oil bath and a magnetic stirrer were used to accomplish the heating and stirring of the reaction mixtures. 1H, 13C, 29Si, and 77Se NMR spectra were recorded with Bruker AVANCE 400 and Bruker AscendTM 500 instruments equipped with a cryo-probe at 25 °C. Chemical shifts are reported in ppm relative to the signal of Me4Si. The MS spectra were measured with a GC/EI–MS configuration including gas chromatography Agilent Technologies 6890N (HP–5MS, 30 m column, I.D. 0.25 mm, film 0.25 µm) equipped with a mass detector Network MS detector 5973 (EI 70 eV, range 33–550 Da).
2.1.1. Bis(Trimethylstannyl)Selenide—(Me3Sn)2Se
Me6Sn2 (5.0 g; 15.26 mmol) and fine selenium powder (1.4 g; 17.73 mmol) were placed into a pressure vessel and heated to 180 °C for 5 h. After cooling to room temperature, the selenium excess was filtered off to give 6.1 g (98%) of (Me3Sn)2Se as a yellow liquid.
1H–NMR (500 MHz, 25 °C, CDCl3): δ = 0.51 (s, 18H, CH3) ppm. 13C–NMR APT (125 MHz, 25 °C, CDCl3): δ = −1.86 ppm. 119Sn–NMR (186 MHz, 25 °C, CDCl3): δ = 50.06 ppm. 77Se–NMR (95 MHz, 25 °C, CDCl3): δ = −550.76 ppm. EI–MS: m/z = 408 (10, M+), 391 (40), 165 (100), 135.
2.1.2. Bis(Tributylstannyl)Selenide—(Bu3Sn)2Se
Bu6Sn2 (5.0 g; 4.35 mL; 8.62 mmol) and fine selenium powder (0.82 g, 10.34 mmol) were placed into the pressure vessel and heated to 180 °C for 12 h. After cooling to room temperature, the selenium excess was filtered off to give 5.6 g (98%) of (Bu3Sn)2Se as a yellow oil.
Bu3SnH (3.7 g; 3.42 mL; 12.71 mmol) and selenium powder (0.6 g; 7.6 mmol) were placed into a pressure vessel and heated to 180 °C for 12 h. After cooling to room temperature, the selenium excess was filtered off to give 4 g (98%) of (Bu3Sn)2Se as a yellow oil.
1H–NMR (500 MHz, 25 °C, CDCl3): δ = 0.91 (t, J = 7.5 Hz, 18H, CH3), 1.12 (t, J = 8.2 Hz, 12H, CH2), 1.30–1.38 (m, 12H, CH2), 1.53–1.60 (m, 12H, CH2) ppm. 13C–NMR APT (125 MHz, 25 °C, CDCl3): δ = 13.61, 15.44, 27.08, 29.00 ppm. 119Sn–NMR (186 MHz, 25 °C, CDCl3): δ = 57.14 ppm. 77Se–NMR (95 MHz, 25 °C, CDCl3): δ = −651.72 ppm. EI–MS: m/z = 545 (20), 317, 291 (90), 235 (50), 179 (100).
2.1.3. Triisopropylsilylselenole—iPr3Si–SeH
iPr3Si–H (3.86 g; 5.0 mL; 24.4 mmol), Se powder (2.3 g; 29.29 mmol), and Ph3P (50 mg; 0.19 mmol) were placed into a pressure vessel and the reaction mixture was heated to 250 °C for 48 h. After cooling to room temperature, the selenium excess was filtered off. The residue was purified by vacuum distillation at 80–85 °C (5 torr) to give 4.68 g (81%) of iPr3Si–SeH as an orange liquid.
1H–NMR (400 MHz, 25 °C, C6D6): δ = −2.81 (s, 1H, SeH), 0.98–1.04 (m, 21H, CH, CH3) ppm. 13C–NMR APT (100 MHz, 25 °C, C6D6): δ = 13.96, 18.72 ppm. 29Si–NMR (99 MHz, 25 °C, C6D6): δ = 32.22 ppm. 77Se–NMR (95 MHz, 25 °C, C6D6, gated): δ = −420.7 (d, J = 47.17 Hz) ppm. EI–MS: m/z = 238 (20, M+) 195 (100), 153 (60), 125 (65), 59 (15).
2.1.4. Bis(Dimethylphenylsilyl)Selenide—PhMe2Si–Se–SiMe2Ph
Selenium powder (0.5 g; 6.33 mmol), PhMe2Si–H (2.6 g; 3.0 mL; 19 mmol), and Ph3P (10 mg) were placed into a pressure vessel and the reaction mixture was heated to 250 °C for 48 h. Vacuum distillation of the crude product at 160–165 °C (2 torr) gave 1.0 g (43%) of a colorless oil.
1H–NMR (400 MHz, 25 °C, C6D6): δ = 0.45 (s, 12H, CH3), 7.14–7.16 (m, 6, Ph), 7.50–7.52 (m, 4H, Ph) ppm. 13C–NMR APT (100 MHz, 25 °C, C6D6): δ = 127.93, 129.66, 133.94, 138.44 ppm. 29Si–NMR (99 MHz, 25 °C, C6D6): δ = 5.44 ppm. 77Se–NMR (95 MHz, 25 °C, C6D6): δ = −341.15 ppm. EI–MS: m/z = 350 (10, M+), 257 (10), 197 (20), 135 (100).
2.1.5. Bis(Triethylsilyl)Selenide—Et3Si–Se–SiEt3
Selenium powder (0.5 g; 6.33 mmol), Et3Si–H (1.5 g; 2.0 mL; 12.66 mmol), Ph3Si–H (30 mg), and Ph3P (10 mg) were placed into a pressure vessel and the reaction mixture was heated to 250 °C for 48 h. After cooling to room temperature, the mixture was filtered and purified by vacuum distillation at 120–125 °C (3 torr) to give 0.7 g (35%) of an orange liquid.
1H–NMR (500 MHz, 25 °C, CDCl3): δ = 0.81 (q, J = 8.0 Hz, 12H, CH2), 1.01 (t, J = 8.0 Hz, 18H, CH3) ppm. 13C–NMR APT (125 MHz, 25 °C, CDCl3): δ = 7.70, 8.16 ppm. 29Si–NMR (99 MHz, 25 °C, CDCl3): δ = 23.41 ppm. 77Se–NMR (95 MHz, 25°C, CDCl3): δ = −485.70 ppm. EI–MS: m/z = 310 (20, M+), 281 (100), 253 (60), 115 (70), 87, 59.
2.1.6. Triisopropylsilyl–Trimethylsilylselenide—iPr3Si–Se–SiMe3
iPr3Si–H (3.86 g; 5.0 mL; 24.4 mmol), Se powder (2.3 g; 29.29 mmol), and Ph3P (50 mg; 0.19 mmol) were placed into a pressure vessel and the reaction mixture was heated to 250 °C for 48 h. After cooling to room temperature, hexane (30 mL) and EtNiPr2 (3.15 g; 4.26 mL, 24.4 mmol) were added. The mixture was cooled to 0 °C and Me3SiCl (2.64 g; 3.58 mL; 24.4 mmol) was added dropwise. After stirring for 12 h at 25 °C, the mixture was filtered, and hexane was evaporated in vacuo. The residue was purified by vacuum distillation at 100–105 °C (2 torr) to give 3.6 g (50%) of a yellow liquid.
Hexane (30 mL), iPr3Si–SeH (1.22 g; 5.14 mmol) and EtNiPr2 (0.67 g; 0.9 mL; 5.14 mmol) were placed into a Schlenk flask and the reaction mixture was cooled to 0 °C. Me3SiCl (0.56 g; 0.65 mL; 5.14 mmol) was added dropwise and the mixture was stirred for 12 h at 25 °C. Upon filtration and vacuum distillation at 100–105 °C (2 torr), 1.12 g (70%) of a yellow liquid was obtained.
1H–NMR (400 MHz, 25 °C, C6D6): δ = 0.40 (s, 9H, CH3), 1.01 (s, 3H, CH), 1.11–1.15 (m, 18H, CH3) ppm. 13C–NMR APT (100 MHz, 25 °C, C6D6): δ = 4.97, 14.67, 19.09 ppm. 29Si–NMR (99 MHz, 25 °C, C6D6): δ = 10.41, 30.24 ppm. 77Se–NMR (95 MHz, 25 °C, C6D6): δ = −447.10 ppm. EI–MS: m/z = 310 (10, M+), 267 (95), 224 (45), 183 (20), 129 (20), 101 (25), 73 (100).
2.2. DSC and TGA Measurements
Differential scanning calorimetry (DSC) was carried out with a Mettler–Toledo STARe System DSC 2/700 equipped with FRS 6 ceramic sensor and cooling system HUBER TC100–MT RC 23. Thermal behavior of the target compounds was measured in open aluminous crucibles under N2 inert atmosphere. DSC curves were determined with a scanning rate of 3–5 °C/min within the range −70–+400 °C. Thermogravimetric analysis (TGA) was carried out using a Mettler–Toledo STARe System TGA 2 equipped with a horizontal furnace LF (400 W, 1100 °C), balance XP5 (resolution 1 μg) and cooling system HUBER Minichiller 600.
3. Results and Discussion
3.1. Straightforwad Reaction Pathway to Bis(Trialkylstannyl)Selenides
The work of Tanaka et al. [18] reported that Me6Sn2 reacts sluggishly with elemental tellurium in the absence of a phosphine. This observation prompted us to investigate this un-catalyzed reaction in more detail. This goal was indeed achieved by simply tuning the reaction conditions and the compounds of general formula (R3Sn)2Se were prepared in a very straightforward manner without any catalyst (Scheme 2). The reaction carried out at a significantly raised temperature to 180 °C (using a pressure vessel) afforded (Me3Sn)2Se and (Bu3Sn)2Se in almost quantitative yields and no additional purification was required. It should also be highlighted that the starting Me6Sn2 and Bu6Sn2 are readily available and inexpensive compounds. On the contrary, a similar experiment with Me6Si2 with(out) Ph3P did not provide any product.
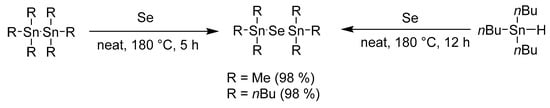
Scheme 2.
Tuned synthesis of (R3Sn)2Se at high temperature.
Triethyltinhydride has been reported as another starting material used for the synthesis of (Et3Sn)2Se [19]. However, its low commercial availability prompted us to attempt a reaction between inexpensive and readily available Bu3SnH and elemental selenium under similar high-temperature conditions (Scheme 2). No matter what amount of tributyltinhydride was used, bis(tributyltin)selenide was always exclusively isolated in almost quantitative yield.
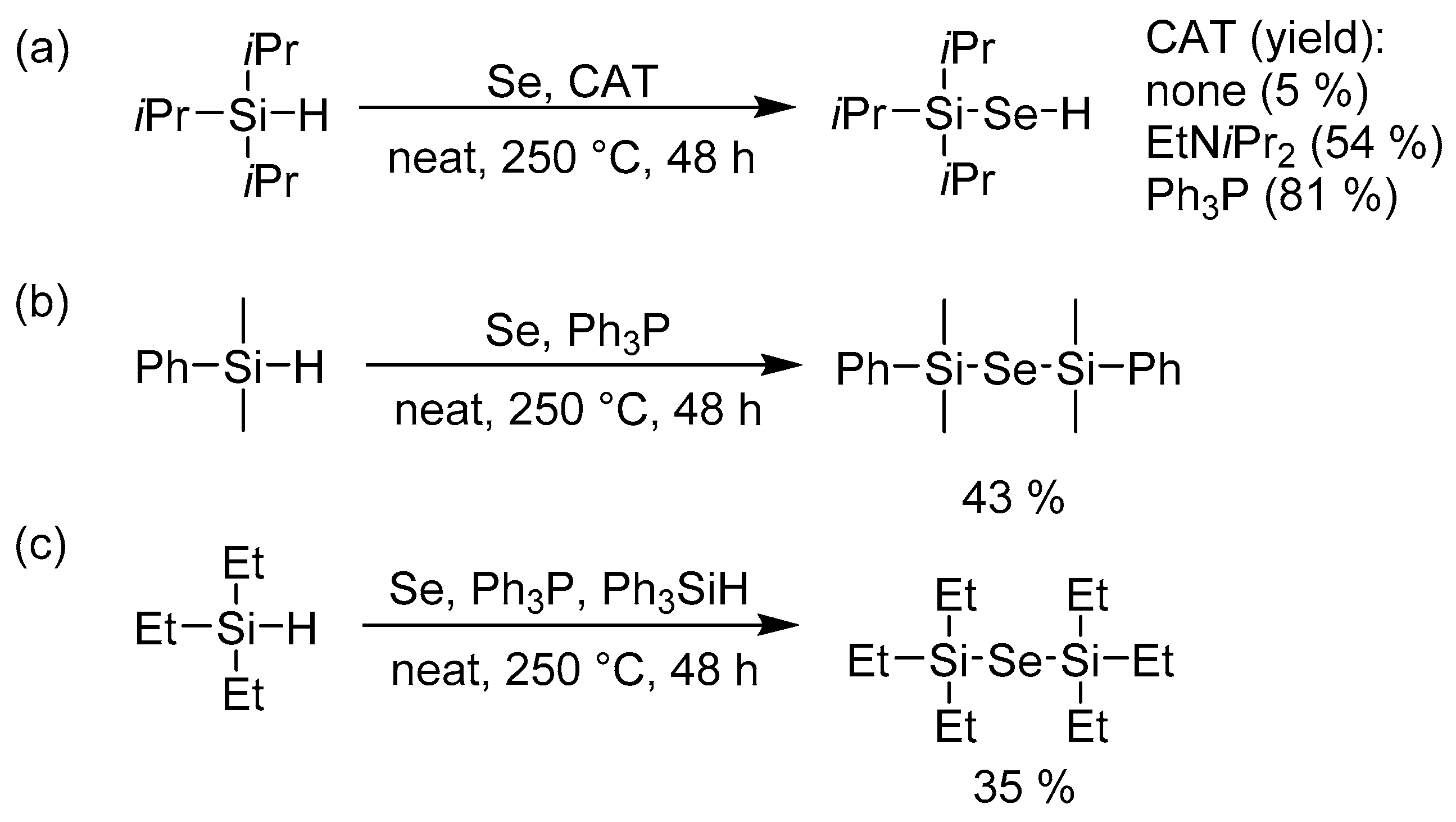
3.2. Elaboration with Silylselenides
Encouraged by the easy and high-yielding reaction with Bu3SnH (Scheme 2), we were also curious about a similar reaction with R3SiH. Such a reaction using one equivalent of trialkylsilane provides organoselenium compounds of general formula R3Si–SeH as reported for Et3Si–SeH [20] and cHex3Si–SeH [21]. Hence, we attempted the synthesis of Et3Si–SeH [20], but even after one week at different temperatures (120, 180, 250 °C) the attained yields did not exceed 1% (as monitored by GC/MS). Using iPr3Si–H as a starting silane afforded 5% of iPr3Si–SeH. Further elaboration revealed that the yield could be significantly improved using a catalytic amount of Lewis bases such as ethyldiisopropylamine or triphenylphosphane (Scheme 3a). Despite working with large excess of iPr3Si–H, we observed exclusive formation of iPr3Si–SeH and only traces of (iPr3Si)2Se were detected on GC/MS. However, when replacing the starting triisopropylsilane by dimethylphenylsilane, PhMe2Si–SeH has been identified as a minor product even if 1 equivalent of PhMe2Si–H was used. PhMe2Si–Se–SiMe2Ph was isolated in 43% yield when using 3 equivalents of PhMe2Si–H (Scheme 3b).
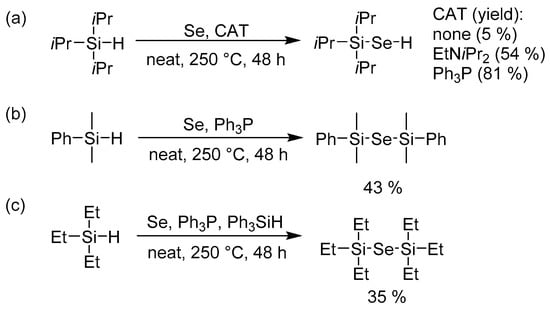
Scheme 3.
Towards trialkylsilylselanole and bis(trialkyl/aryl)silylselenides using triisopropyl- (a), dimethylphenyl- (b), and triethylsilane (c).
The initially unsuccessful preparation of Et3Si–SeH or well-established ALD precursor (Et3Si)2Se using the aforementioned methodology proved to be tricky. However, we further focused our attention to transfer of alkyl/aryl chains, well known for 14 group of elements. Hence, the reaction of selenium with 2 equivalents of Et3Si–H, Ph3P, and catalytic amount of Ph3Si–H provided desired (Et3Si)2Se in 35% yield (Scheme 3c). Only traces of Et3Si–SeH were detected by GC/MS.
In ALD, metal halides (X) are often used as the counter precursors. In this respect, selenoles R3SiSeH seem to be less useful as their joint reaction produces unfavorable and corrosive HX as a by-product. Hence, our further synthetic modifications were focused on overcoming this problem. Scheme 4 shows very simple replacement of the hydrogen (Se–H) by trimethylsilyl group by treating iPr3Si–SeH with Me3SiCl in the presence of a base. This reaction could be performed either with isolated or in situ generated iPr3Si–SeH and allows facile formation of unsymmetrical bis(trialkylsilyl)selenides, which is in contrast to previous reports [8,11].
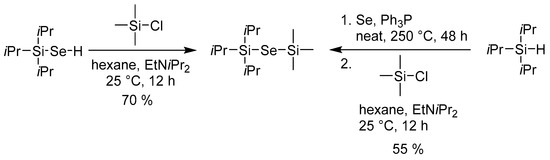
Scheme 4.
Preparation of unsymmetrical bis(trialkylsilyl)selenide.
The structure of the prepared organoselenium materials was determined by heteronuclear NMR spectroscopy and GC/MS analyses; the spectra are found in the Supporting Information files (Figures S1–S15). A very low chemical shift (−2.81 ppm) was observed for Se–H proton of iPr3Si–SeH (Figure S1). 77Se–NMR gated spectrum of this compound also showed typical 77Se–1H coupling with J = 47.17 Hz (Figure S4). The recorded chemical shifts in 77Se–NMR vary from −447.1 ppm (iPr3Si–Se–SiMe3) to −341.15 ppm (PhMe2Si–Se–SiMe2Ph). 29Si–NMR spectra revealed chemical shifts between 5.44 ppm (PhMe2Si–Se–SiMe2Ph) and 32.22 ppm (iPr3Si–SeH). As expected, the GC/MS records always feature M+ as well as R3Y+ ions with a typical isotope layout for selenium accompanied by further fragmentation (Figures S13–S15).
3.3. Thermal Properties
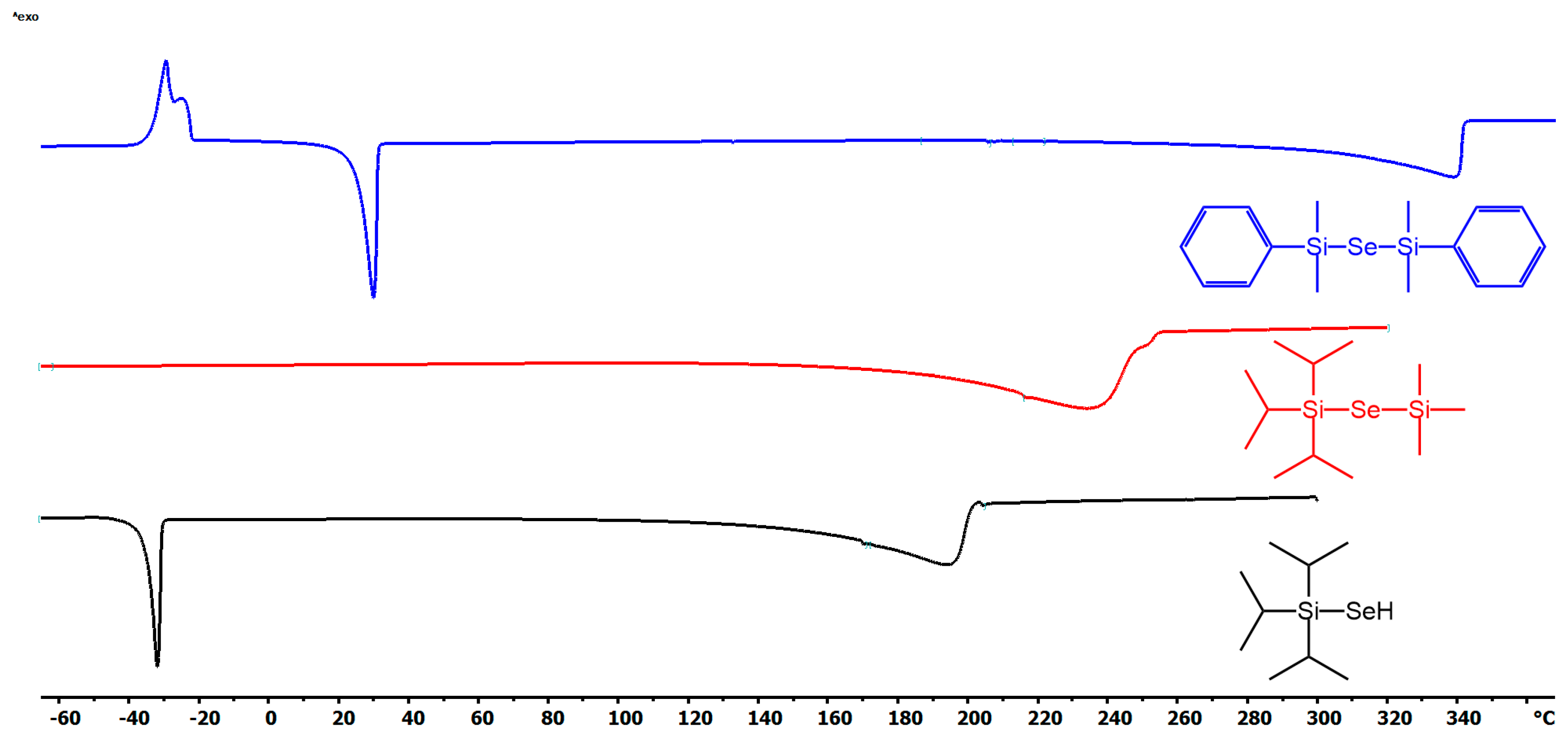
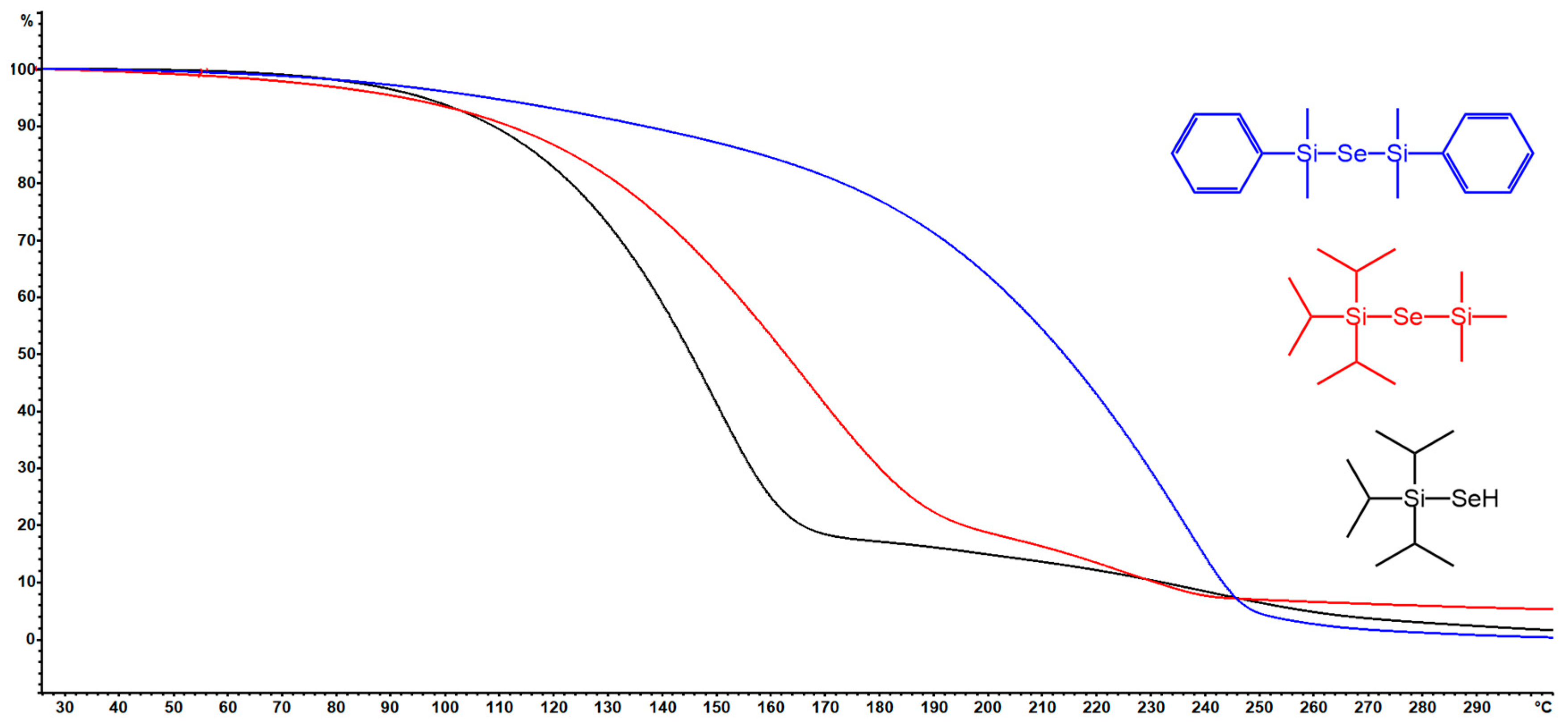
Thermal stability and volatility are crucial properties of ALD precursors. The thermal properties of new organoselanoles and selenides (iPr3Si–SeH, iPr3Si–Se–SiMe3and PhMe2Si–Se–SiMe2Ph) were studied by DSC and TG analyses (see Figures S16–S21 in the Supporting Information for particular thermograms). For thermograms of remaining organoselanes, see our previous report [11]. The DSC curves shown in Figure 1 reveal melting/crystallization at −30/25 °C for iPr3Si–SeH and PhMe2Si–Se–SiMe2Ph, while vigorous evaporation takes place mostly within the range of 120–200 (iPr3Si–SeH), 280–340 (PhMe2Si–Se–SiMe2Ph), and 180–240 °C (iPr3Si–Se–SiMe3). PhMe2Si–Se–SiMe2Ph derivative also showed cold crystallization at around −30 °C. Although the endothermic peaks of evaporation for PhMe2Si–Se–SiMe2Ph is sharp, another (probably exothermic) process accompanies the evaporation of the remaining two precursors. This is corroborated by the performed TG analyses shown in Figure 2. PhMe2Si–Se–SiMe2Ph showed gradual evaporation, while iPr3Si–SeH and PhMe2Si–Se–SiMe3 underwent some additional unidentified thermal processes at around 160 and 190 °C. The mass residues are below 5%. Hence, at ambient pressure the prepared precursors are thermally stable up to 160 (iPr3Si–SeH), 190 (iPr3Si–Se–SiMe3), 200 (Me3Sn)2Se, (Bu3Sn)2Se, and (Et3Si)2Se), and 300 °C (PhMe2Si–Se–SiMe2Ph).
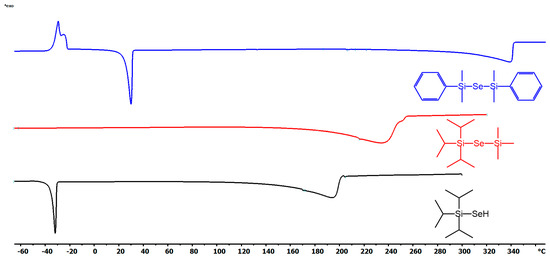
Figure 1.
DSC records for newly prepared organoselenium compounds.
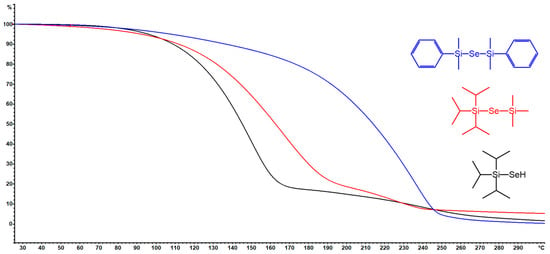
Figure 2.
TGA curves for newly prepared organoselenium compounds.
4. Conclusions
In conclusion, we have successfully optimized the synthesis of bis(trimethyl/butylstannyl)selenides. The synthesis is very straightforward and involves simple heating of the starting (Me3Sn)2Se, (Bu3Sn)2Se or Bu3SnH with selenium powder. Both products were isolated directly without the need for further purification and in almost quantitative yields. Moreover, all starting materials are readily available and inexpensive, which makes the developed synthesis cost-effective and suitable for large production (as generally required by the ALD).
Further attempts were focused on the synthesis of triisopropylselenole, which was prepared in a similar easy way employing triphenylphosphane as a catalyst. Bis(dimethylphenylsilyl)selenide and bis(triethylsilyl)selenide were prepared analogously. We have further demonstrated that the Se–H bond in triisopropylselenole can be modified, affording the first unsymmetrical (triisopropylsilyl)(trimethylsilyl)selenide.
An investigation of the thermal properties with DSC and TGA revealed that thermal stability and volatility of all prepared derivatives are sufficiently high to be applied as Se precursors for the ALD.
Supplementary Materials
The following are available online, Figure S1: 1H NMR (400 MHz, 25 °C, C6D6) spectrum of iPr3Si–SeH, Figure S2: 13C–NMR (125 MHz, 25 °C, C6D6) spectrum of iPr3Si–SeH, Figure S3: 29Si–NMR (99 MHz, 25 °C, C6D6) spectrum of iPr3Si–SeH, Figure S4: 77Se–NMR (95 MHz, 25 °C, C6D6, gated) spectrum of iPr3Si–SeH (detail around 420 ppm as an inset), Figure S5: 1H–NMR (400 MHz, 25 °C, C6D6) spectrum of PhMe2Si–Se–SiMe2Ph, Figure S6: 13C–NMR (125 MHz, 25 °C, C6D6) spectrum of PhMe2Si–Se–SiMe2Ph, Figure S7: 29Si–NMR (99 MHz, 25 °C, C6D6) spectrum of PhMe2Si–Se–SiMe2Ph, Figure S8: 77Se–NMR (95 MHz, 25 °C, C6D6) spectrum of PhMe2Si–Se–SiMe2Ph, Figure S9: 1H NMR (400 MHz, 25 °C, C6D6) spectrum of iPr3Si–Se–SiMe3, Figure S10: 13C–NMR (125 MHz, 25 °C, C6D6) spectrum of iPr3Si–Se–SiMe3, Figure S11: 29Si–NMR (99 MHz, 25 °C, C6D6) spectrum of iPr3Si–Se–SiMe3, Figure S12: 77Se–NMR (95 MHz, 25 °C, C6D6) spectrum of iPr3Si–Se–SiMe3, Figure S13: GC/MS record of iPr3Si–SeH, Figure S14: GC/MS record of PhMe2Si–Se–SiMe2Ph, Figure S15: GC/MS record of iPr3Si–Se–SiMe3, Figure S16: DSC curve of iPr3Si–SeH, Figure S17: DSC curve of PhMe2Si–Se–SiMe2Ph, Figure S18: DSC curve of iPr3Si–Se–SiMe3, Figure S19: TGA curve of iPr3Si–she, Figure S20: TGA curve of PhMe2Si–Se–SiMe2Ph, Figure S21: TGA curve of iPr3Si–Se–SiMe3.
Author Contributions
Conceptualization, F.B., J.C.; methodology, F.B.; software, J.C.; validation, F.B.; formal analysis, F.B.; investigation, J.C., D.P., M.K., V.J.; resources, F.B.; data curation, F.B.; writing—original draft preparation, J.C.; writing—review and editing, F.B.; visualization, J.C.; supervision, F.B.; project administration, F.B.; funding acquisition, F.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Czech Science Foundation, grant number 18-03881S.
Acknowledgments
This article is dedicated to G. Mlostoń on the occasion of his 70th birthday.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, Q.; Lei, Y.; Wang, Y.; Liu, Y.; Song, C.; Zeng, J.; Song, Y.; Duan, X.; Wang, D.; Li, Y. Atomic-scale engineering of chemical vapor deposition-grown 2D transition metal dichalcogenides for electrocatalysis. Energy Environ. Sci. 2020. [Google Scholar] [CrossRef]
- Zhao, M.; Su, J.; Zhao, Y.; Luo, P.; Wang, F.; Han, W.; Li, Y.; Zu, X.; Qiao, L.; Zhai, T. Sodium-Mediated Epitaxial Growth of 2D Ultrathin Sb2Se3 Flakes for Broadband Photodetection. Adv. Funct. Mater. 2020, 30, 1909849. [Google Scholar] [CrossRef]
- Sun, Y.; Dai, T.; He, Z.; Zhou, W.; Hu, P.; Li, S.; Wu, S. Memristive phase switching in two-dimensional 1T’-VSe2 crystals. Appl. Phys. Lett. 2020, 116, 033101. [Google Scholar] [CrossRef]
- Yun, S.J.; Duong, D.L.; Ha, D.M.; Singh, K.; Phan, T.L.; Choi, W.; Kim, Y.M.; Lee, Y.H. Ferromagnetic Order at Room Temperature in Monolayer WSe2 Semiconductor via Vanadium Dopant. Adv. Sci. 2020, 7, 1903076. [Google Scholar] [CrossRef] [PubMed]
- George, S.M.; Yoon, B.; Hall, R.A.; Abdulagatov, A.I.; Hibbs, Z.M.; Lee, Y.; Seghete, D.; Lee, B.H. Atomic Layer Deposition of Nanostructured Materials; Pinna, N., Knez, M., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; ISBN 9780470109526. [Google Scholar]
- Ng, S.; Krbal, M.; Zazpe, R.; Přikryl, J.; Charvot, J.; Dvořák, F.; Strizik, L.; Slang, S.; Sopha, H.; Kosto, Y.; et al. MoSexOy-Nanotube Layers: Efficient Interface for Light-Driven Applications. Adv. Mater. Interfaces 2017, 5, 1701146. [Google Scholar] [CrossRef]
- Balasubramanyam, S.; Merkx, M.J.M.; Verheijen, M.A.; Kessels, W.M.M.; Mackus, A.J.M.; Bol, A.A. Area-selective atomic layer deposition of 2D WS nanolayers Area-selective atomic layer deposition of 2D WS 2 nanolayers Abstract With downscaling of device dimensions, two dimensional (2D) semiconducting transition metal. ACS Mater. Lett. 2020, 2, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Pore, V.; Hatanpää, T.; Ritala, M.; Leskelä, M. Atomic Layer Deposition of Metal Tellurides and Selenides Using Alkylsilyl Compounds of Tellurium and Selenium Atomic Layer Deposition of Metal Tellurides and Selenides Using Alkylsilyl Compounds of Tellurium and Selenium. J. Am. Chem. Soc. 2009, 131, 3478–3480. [Google Scholar] [CrossRef] [PubMed]
- Mahuli, N.; Halder, D.; Paul, A.; Sarkar, S.K. Atomic Layer Deposition of an Sb2Se3 Photoabsorber Layer Using Selenium Dimethyldithiocarbamate as a New Se Precursor. Chem. Mater. 2019, 31, 7434–7442. [Google Scholar] [CrossRef]
- Charvot, J.; Pokorný, D.; Zazpe, R.; Krumpolec, R.; Pavliňák, D.; Hromádko, L.; Přikryl, J.; Rodriguez-Pereira, J.; Klikar, M.; Jelínková, V.; et al. Cyclic Silylselenides: Convenient Selenium Precursors for Atomic Layer Deposition. Chempluschem 2020, cplu.202000108. [Google Scholar] [CrossRef] [PubMed]
- Zazpe, R.; Charvot, J.; Krumpolec, R.; Hromádko, L.; Pavliňák, D.; Dvorak, F.; Knotek, P.; Michalicka, J.; Přikryl, J.; Ng, S.; et al. Atomic Layer Deposition of MoSe2 Using New Selenium Precursors. FlatChem 2020, 21, 100166. [Google Scholar] [CrossRef]
- Detty, M.R.; Seidler, M.D. Bis(trialkylsilyl) Chalcogenides. 1. Preparation and Reduction of Group 6A Oxides. J. Org. Chem. 1982, 47, 1354–1356. [Google Scholar] [CrossRef]
- Syper, L.; Mlochowski, J. Lithium diselenide in aprotic medium—A convenient reagent for synthesis of organic diselenides. Tetrahedron 1988, 44, 6119–6130. [Google Scholar] [CrossRef]
- Hantapää, T.; Pore, V.; Ritala, M.; Leskelä, M. Alkylsilyl Compounds of Selenium and Tellurium: New precursors for ALD. Electrochem. Soc. 2009, 25, 609–616. [Google Scholar]
- Schmidt, M.; Ruf, H. Organosilicium-, -germanium- und -zinnselenide. Angew. Chem. 1961, 73, 64. [Google Scholar] [CrossRef]
- Li, G.M.; Zingaro, R.A.; Segi, M.; Reibenspies, J.H.; Nakajima, T. Synthesis and Structure of Telluroamides and Selenoamides. The First Crystallographic Study of Telluroamides. Organometallics 1997, 16, 756–762. [Google Scholar] [CrossRef]
- Kriegsmann, V.H.; Hoffmann, H.; Geissler, H. Untersuchungen an Zinnverbindungen. IX. Die Infrarot- und RAMAN-Spektren einiger Hexaorganodistannoxane, Hexaorganodistannthiane sowie von Hexamethyldistannselan. Z. Anorg. Allg. Chem. 1965, 341, 24–35. [Google Scholar] [CrossRef]
- Han, L.; Mirzaei, F.; Tanaka, M. The First Phosphine-Catalyzed Insertion of Tellurium into Sn-Sn and Pb-Pb Bonds: A Simple and Efficient Route to R 3 MTeMR 3 (M) Sn, Pb). Organometallics 2000, 19, 722–724. [Google Scholar] [CrossRef]
- Vyzanakin, N.S.; Bocharkev, M.N.; Sanina, L.P. Triethylstannane. J. Gen. Chem. USSR 1966, 36, 175. [Google Scholar]
- Vyzanakin, N.S. Triethylsilane. J. Gen. Chem. USSR 1968, 38, 414. [Google Scholar]
- Grenader, K.; Schüpbach, B.; Peters, A.; Kümmel, O.; Halter, O.; Terfort, A. Catalytic C-Se bond formation under very mild conditions for the two-step, one-pot synthesis of aryl selenoacetates. Adv. Synth. Catal. 2012, 354, 2653–2658. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).