In Vitro Prebiotic Effects of Malto-Oligosaccharides Containing Water-Soluble Dietary Fiber
Abstract
1. Introduction
2. Results
2.1. MOS Content after Differences in the Enzyme and Treatment Process
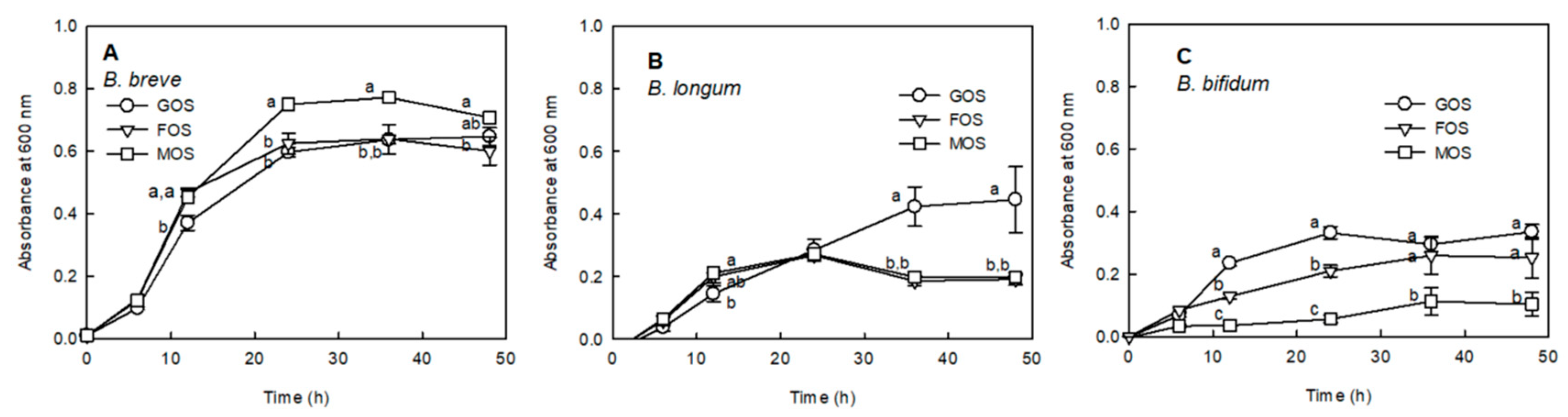
2.2. Proliferation Effect of MOS on Bifidobacterium sp.
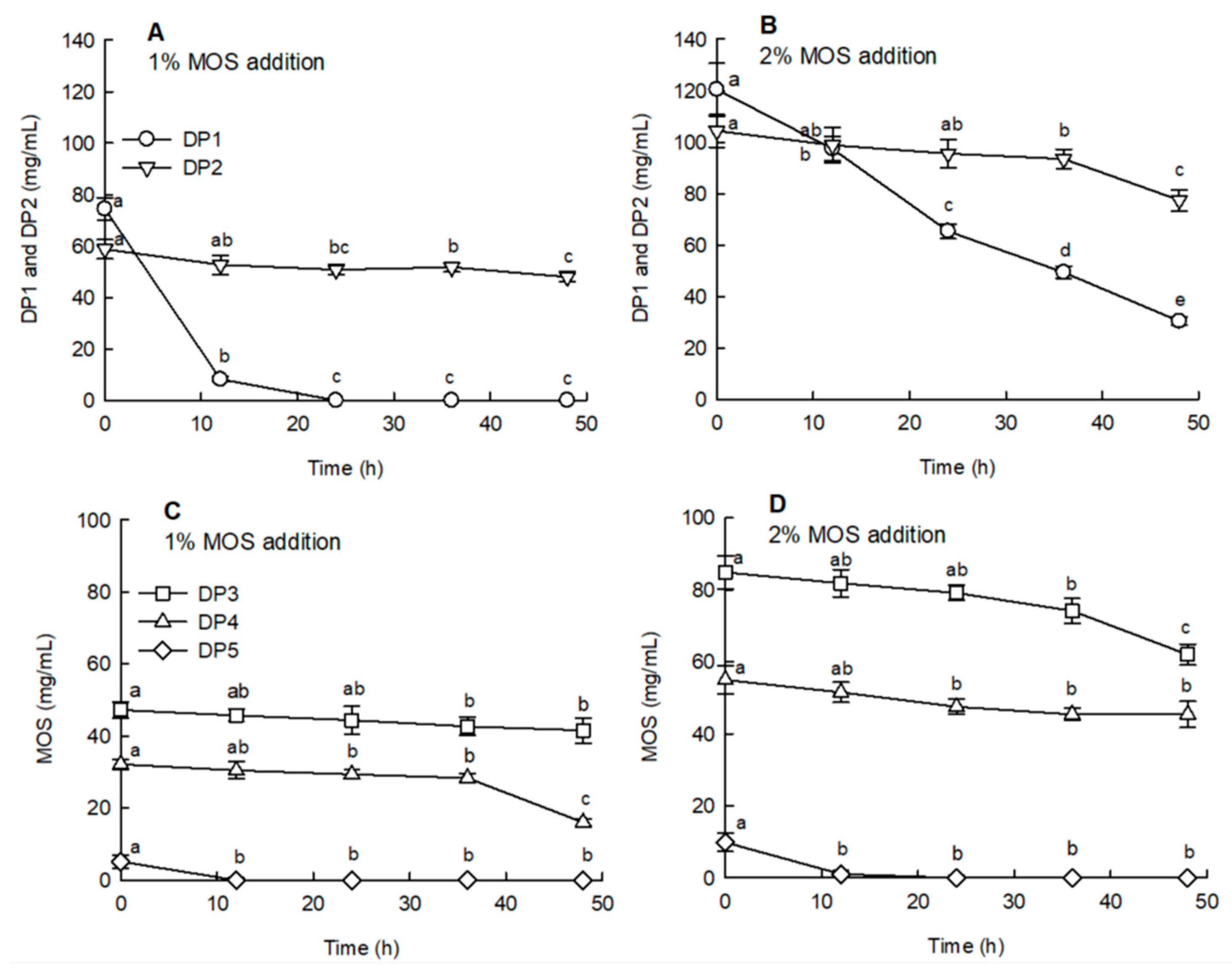
2.3. Effects of the Digestion Process on the Sugar Composition of MOS
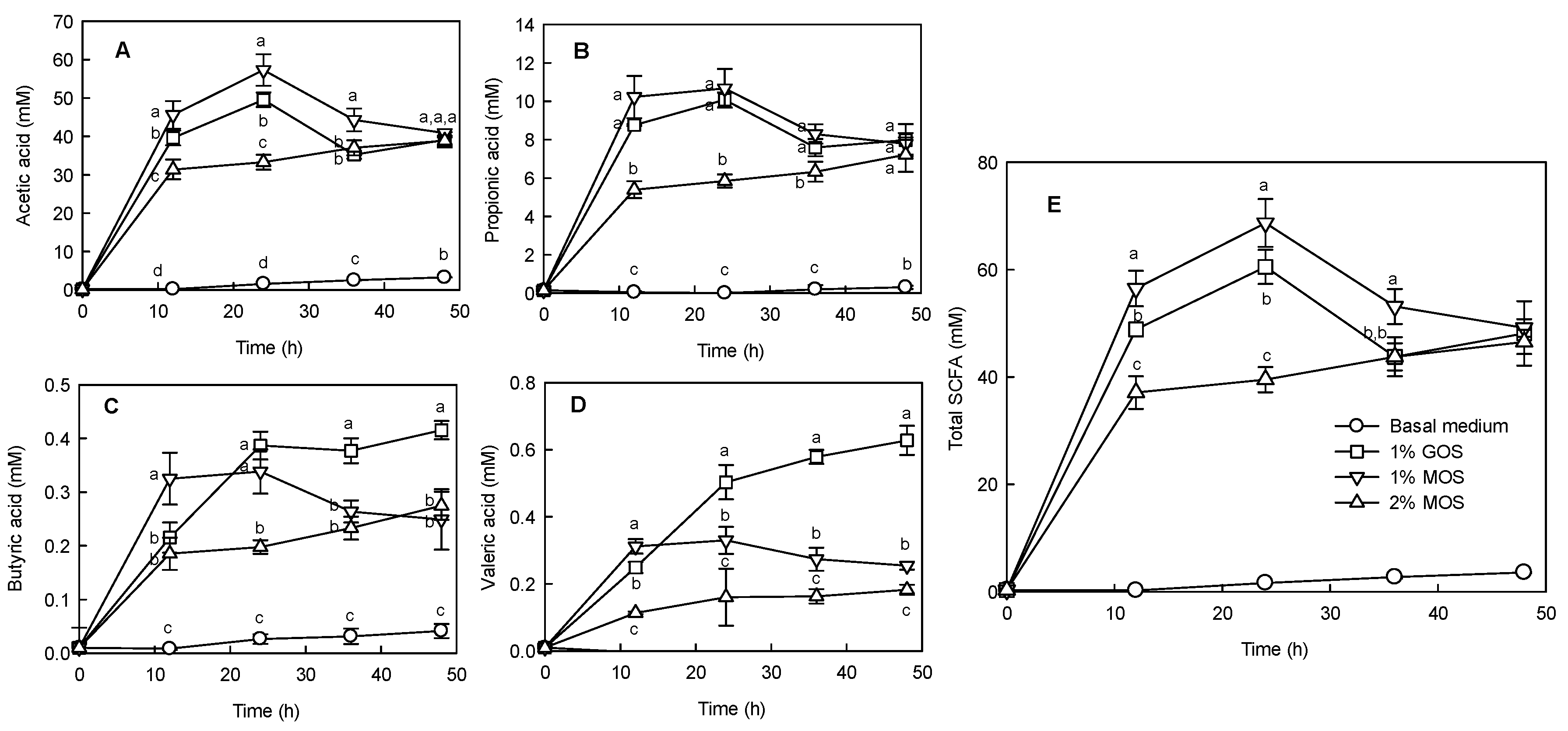
2.4. Effects of MOS on the Sugar Content and SCFA Production during Anaerobic Fermentation
2.5. Species Richness and Diversity Following MOS and GOS Fermentations Using Fecal Microorganisms
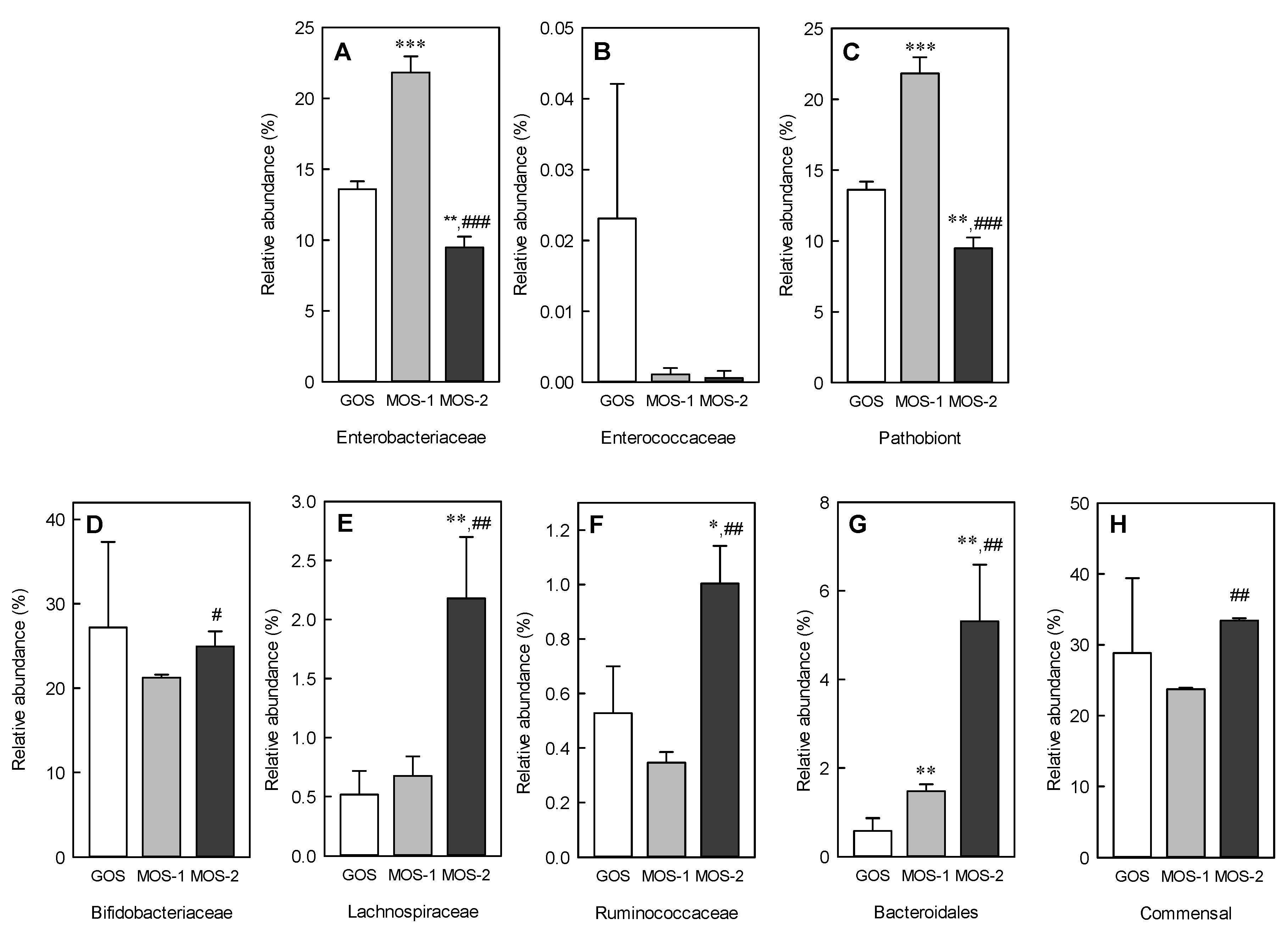
2.6. Changes in Microbiota Following MOS and GOS Fermentation by Using Fecal Microorganisms
3. Discussion
4. Materials and Methods
4.1. MOS Production
4.2. Evaluation of MOS Availability by Bifidobacterium sp.
4.3. In Vitro Digestion of MOS
4.4. In Vitro Fecal Fermentation
4.5. Assay of MOS
4.6. Assay of SCFAs
4.7. Microbial Analysis of MOS Fermentation Broth by Fecal Microorganisms
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lordan, C.; Thapa, D.; Ross, R.P.; Cotter, P.D. Potential for enriching next-generation health-promoting gut bacteria through prebiotics and other dietary components. Gut Microbes 2020, 11, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.; Lane, J.A.; van Sinderen, D.; Hickey, R.M. Human milk oligosaccharides: Shaping the infant gut microbiota and supporting health. J. Funct. Foods. 2020, 72, 104074. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Khangwal, I.; Shukla, P. Prospecting prebiotics, innovative evaluation methods, and their health applications: A review. 3 Biotech. 2019, 9, 187. [Google Scholar] [CrossRef]
- de Paulo Farias, D.; de Araújo, F.F.; Neri-Numa, I.A.; Pastore, G.M. Prebiotics: Trends in food, health and technological applications. Trends.Food. Sci. Technol. 2019, 93, 23–35. [Google Scholar] [CrossRef]
- Pan, S.; Ding, N.; Ren, J.; Gu, Z.; Li, C.; Hong, Y.; Cheng, L.; Holler, T.P.; Li, Z. Maltooligosaccharide-forming amylase: Characteristics, preparation, and application. Biotechnol. Adv. 2017, 35, 619–632. [Google Scholar] [CrossRef]
- Ali, M.B.; Mhiri, S.; Mezghani, M.; Bejar, S. Purification and sequence analysis of the atypical maltohexaose-forming α-amylase of the B. stearothermophilus US100. Enzyme. Microb. Technol. 2001, 28, 537–542. [Google Scholar] [PubMed]
- Park, K. Development of new carbohydrate materials. Food. Sci. Technol. 1992, 25, 73–82. [Google Scholar]
- Xu, Q.; Chao, Y.L.; Wan, Q.B. Health benefit application of functional oligosaccharides. Carbohydr. Polym. 2009, 77, 435–441. [Google Scholar]
- Jang, E.Y.; Ahn, Y.; Suh, H.J.; Hong, K.-B.; Jo, K. Amylase-producing maltooligosaccharide provides potential relief in rats with loperamide-induced constipation. Evid. Based. Complement. Alternat. Med. 2020, 2020, 5470268. [Google Scholar] [CrossRef]
- Ibrahim, O.O. Functional oligosaccharide: Chemicals structure, manufacturing, health benefits, applications and regulations. J. Food. Chem. Nanotechnol. 2018, 4, 65–76. [Google Scholar] [CrossRef]
- Zaman, S.A.; Sarbini, S.R. The potential of resistant starch as a prebiotic. Crit. Rev. Biotechnol. 2016, 36, 578–584. [Google Scholar] [CrossRef]
- Fehlbaum, S.; Prudence, K.; Kieboom, J.; Heerikhuisen, M.; Van den Broek, T.; Schuren, F.H.; Steinert, R.E.; Raederstorff, D. In vitro fermentation of selected prebiotics and their effects on the composition and activity of the adult gut microbiota. Int. J. Mol. Sci. 2018, 19, 3097. [Google Scholar] [CrossRef]
- Carlson, J.L.; Erickson, J.M.; Lloyd, B.B.; Slavin, J.L. Health effects and sources of prebiotic dietary fiber. Curr. Dev. Nutr. 2018, 2, nzy005. [Google Scholar] [CrossRef]
- Rathore, S.; Salmeron, I.; Pandiella, S.S. Production of potentially probiotic beverages using single and mixed cereal substrates fermented with lactic acid bacteria cultures. Food. Microbiol. 2012, 30, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Kedia, G.; Vazquez, J.A.; Pandiella, S.S. Evaluation of the fermentability of oat fractions obtained by debranning using lactic acid bacteria. J. Appl. Microbiol. 2008, 105, 1227–1237. [Google Scholar] [CrossRef][Green Version]
- Gu, F.; Borewicz, K.; Richter, B.; van der Zaal, P.H.; Smidt, H.; Buwalda, P.L.; Schols, H.A. In Vitro Fermentation Behavior of Isomalto/Malto-Polysaccharides Using Human Fecal Inoculum Indicates Prebiotic Potential. Mol. Nutr. Food. Res. 2018, 62, e1800232. [Google Scholar] [CrossRef]
- Guzman-Maldonado, H.; Paredes-Lopez, O. Amylolytic enzymes and products derived from starch: A review. Crit. Rev. Food. Sci. Nutr. 1995, 35, 373–403. [Google Scholar] [CrossRef]
- Sasaki, D.; Sasaki, K.; Ikuta, N.; Yasuda, T.; Fukuda, I.; Kondo, A.; Osawa, R. Low amounts of dietary fibre increase in vitro production of short-chain fatty acids without changing human colonic microbiota structure. Sci. Rep. 2018, 8, 435. [Google Scholar] [CrossRef]
- Min, D.; Chung, Y.; Bai, D.; Yu, J. Production of beta-mannanase by an alkali-tolerant Bacillus sp. YA-14. Foods. Biotechnol. 1995, 4, 285–289. [Google Scholar]
- Roberts, P.J.; Whelan, W.J. The mechanism of carbohydrase action. 5. Action of human salivary alpha-amylase on amylopectin and glycogen. Biochem. J. 1960, 76, 246–253. [Google Scholar] [CrossRef]
- Sako, T.; Matsumoto, K.; Tanaka, R. Recent progress on research and applications of non-digestible galacto-oligosaccharides. Int. Dairy. J. 1999, 9, 69–80. [Google Scholar] [CrossRef]
- Voragen, A.G.J. Technological aspects of functional food-related carbohydrates. Trends. Food. Sci. Technol. 1998, 9, 328–335. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef]
- Patterson, E.; Cryan, J.F.; Fitzgerald, G.F.; Ross, R.P.; Dinan, T.G.; Stanton, C. Gut microbiota, the pharmabiotics they produce and host health. Proc. Nutr. Soc. 2014, 73, 477–489. [Google Scholar] [CrossRef]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vazquez-Baeza, Y.; Van Treuren, W.; Ren, B.Y.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The Treatment-naive microbiome in new-onset Crohn’s disease. Cell. Host. Microbe. 2014, 15, 382–392. [Google Scholar] [CrossRef]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic effects: Metabolic and health benefits. Brit. J. Nutr. 2010, 104, S1–S63. [Google Scholar] [CrossRef]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and their role as members of the human gut microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.Y.; Antonopoulos, D.A.; Kalra, A.; Tonelli, A.; Khalife, W.T.; Schmidt, T.M.; Young, V.B. Decreased diversity of the fecal microbiome in recurrent Clostridium difficile-associated diarrhea. J. Infect. Dis. 2008, 197, 435–438. [Google Scholar] [CrossRef]
- Monteagudo-Mera, A.; Arthur, J.; Jobin, C.; Keku, T.; Bruno-Barcena, J.; Azcarate-Peril, M. High purity galacto-oligosaccharides (GOS) enhance specific Bifidobacterium species and their metabolic activity in the mouse gut microbiome. Benef. Microbes 2016, 7, 247–264. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.J.; Li, Q. Simultaneous determination of maltooligosaccharides in beer using HPLC-ELSD and their influence on beer foam stability. J. Am. Soc. Brew. Chem. 2015, 73, 78–83. [Google Scholar] [CrossRef]
- Kim, O.-S.; Cho, Y.-J.; Lee, K.; Yoon, S.-H.; Kim, M.; Na, H.; Park, S.-C.; Jeon, Y.S.; Lee, J.-H.; Yi, H. Introducing EzTaxon-e: A prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int. J. Syst. Evol. Microbiol. 2012, 62, 716–721. [Google Scholar] [CrossRef]
- Li, W.Z.; Godzik, A. Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar] [CrossRef]
- Chao, A. Nonparametric-estimation of the number of classes in a population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Hill, T.C.J.; Walsh, K.A.; Harris, J.A.; Moffett, B.F. Using ecological diversity measures with bacterial communities. Fems. Microbiol. Ecol. 2003, 43, 1–11. [Google Scholar] [CrossRef]

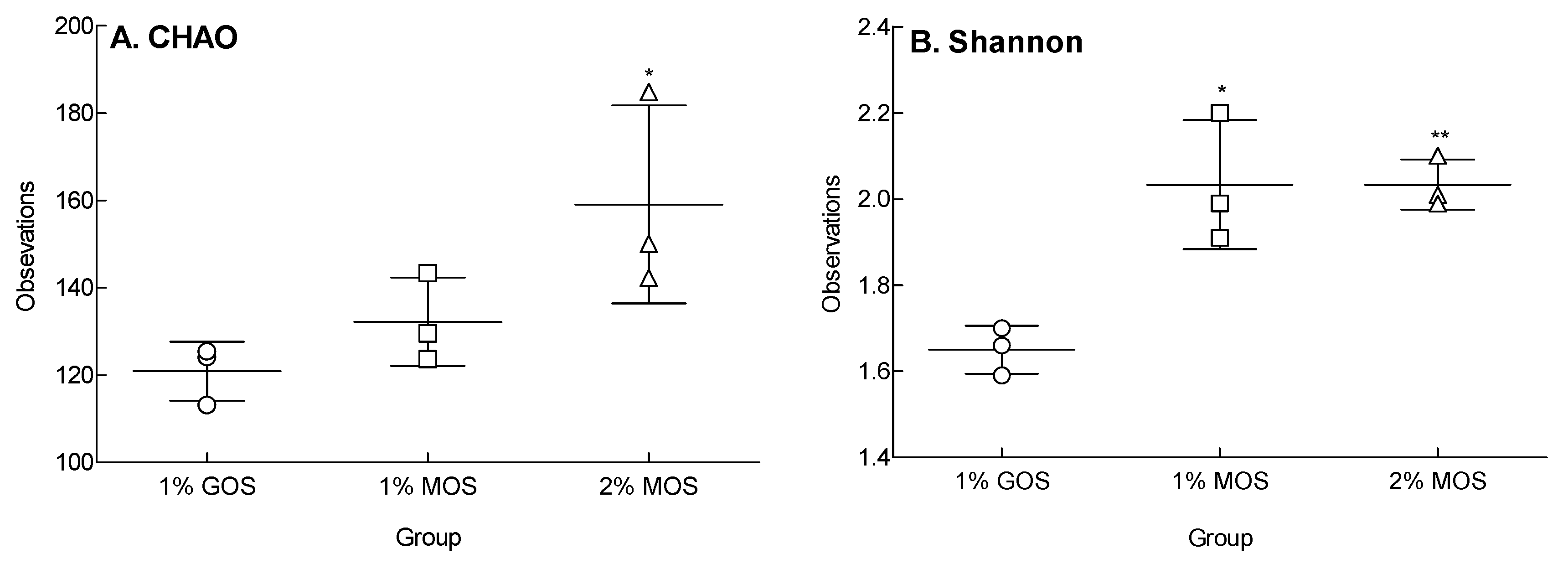

| Process | Time (h) | Maltotriose (%) (DP3) | Maltotetraose (%) (DP4) | Maltopentaose (%) (DP5) | Total MOS (%) |
|---|---|---|---|---|---|
| Liquefaction by MTAA | 4 | 3.32 ± 0.45 | 2.43 ± 0.32 | 2.16 ± 0.26 | 7.91 ± 1.03 |
| Saccharification by Fungamyl 800L | 8 | 5.23 ± 0.32 | 4.64 ± 0.64 | 3.83 ± 0.35 | 13.70 ± 1.31 |
| 13 | 8.27 ± 0.47 | 7.36 ± 0.57 | 6.99 ± 0.75 | 22.62 ± 1.79 | |
| 20 | 14.21 ± 1.24 | 12.11 ± 1.04 | 9.24 ± 1.01 | 35.56 ± 3.29 | |
| 24 | 14.50 ± 0.45 | 12.58 ± 0.85 | 9.63 ± 0.84 | 36.71 ± 2.14 | |
| Simultaneous liquefaction and saccharification by HTAA | 4 | 5.83 ± 0.33 | 5.41 ± 0.38 | 3.53 ± 0.33 | 14.77 ± 1.04 |
| 8 | 9.20 ± 0.45 | 7.95 ± 0.59 | 10.11 ± 0.54 | 27.26 ± 1.58 | |
| 13 | 14.77 ± 0.75 | 13.23 ± 0.65 | 10.93 ± 0.85 | 38.93 ± 2.25 | |
| 20 | 21.74 ± 1.12 | 18.84 ± 0.83 | 11.76 ± 0.93 | 52.34 ± 2.88 | |
| 24 | 21.54 ± 1.01 | 17.32 ± 0.73 | 12.83 ± 0.56 | 51.69 ± 2.30 |
Sample Availability: Samples of the compounds are not available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, E.Y.; Hong, K.-B.; Chang, Y.B.; Shin, J.; Jung, E.Y.; Jo, K.; Suh, H.J. In Vitro Prebiotic Effects of Malto-Oligosaccharides Containing Water-Soluble Dietary Fiber. Molecules 2020, 25, 5201. https://doi.org/10.3390/molecules25215201
Jang EY, Hong K-B, Chang YB, Shin J, Jung EY, Jo K, Suh HJ. In Vitro Prebiotic Effects of Malto-Oligosaccharides Containing Water-Soluble Dietary Fiber. Molecules. 2020; 25(21):5201. https://doi.org/10.3390/molecules25215201
Chicago/Turabian StyleJang, Eun Yeong, Ki-Bae Hong, Yeok Boo Chang, Jungcheul Shin, Eun Young Jung, Kyungae Jo, and Hyung Joo Suh. 2020. "In Vitro Prebiotic Effects of Malto-Oligosaccharides Containing Water-Soluble Dietary Fiber" Molecules 25, no. 21: 5201. https://doi.org/10.3390/molecules25215201
APA StyleJang, E. Y., Hong, K.-B., Chang, Y. B., Shin, J., Jung, E. Y., Jo, K., & Suh, H. J. (2020). In Vitro Prebiotic Effects of Malto-Oligosaccharides Containing Water-Soluble Dietary Fiber. Molecules, 25(21), 5201. https://doi.org/10.3390/molecules25215201





