Evaluation of Antioxidant and Anti-α-glucosidase Activities of Various Solvent Extracts and Major Bioactive Components from the Seeds of Myristica fragrans
Abstract
1. Introduction
2. Results and Discussion
2.1. Determination of Total Phenolic Content (TPC) and Yields in Each Solvent Extract
2.2. 2,2-Diphenyl-1-(2,4,6-trinitrophenyl)hydrazyl (DPPH) Free-Radical Scavenging Activity
2.3. 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) Free-Radical Scavenging Activity
2.4. Superoxide Radical Scavenging Activity
2.5. Hydroxyl Radical Scavenging Activity
2.6. Anti-α-glucosidase Activity Assay
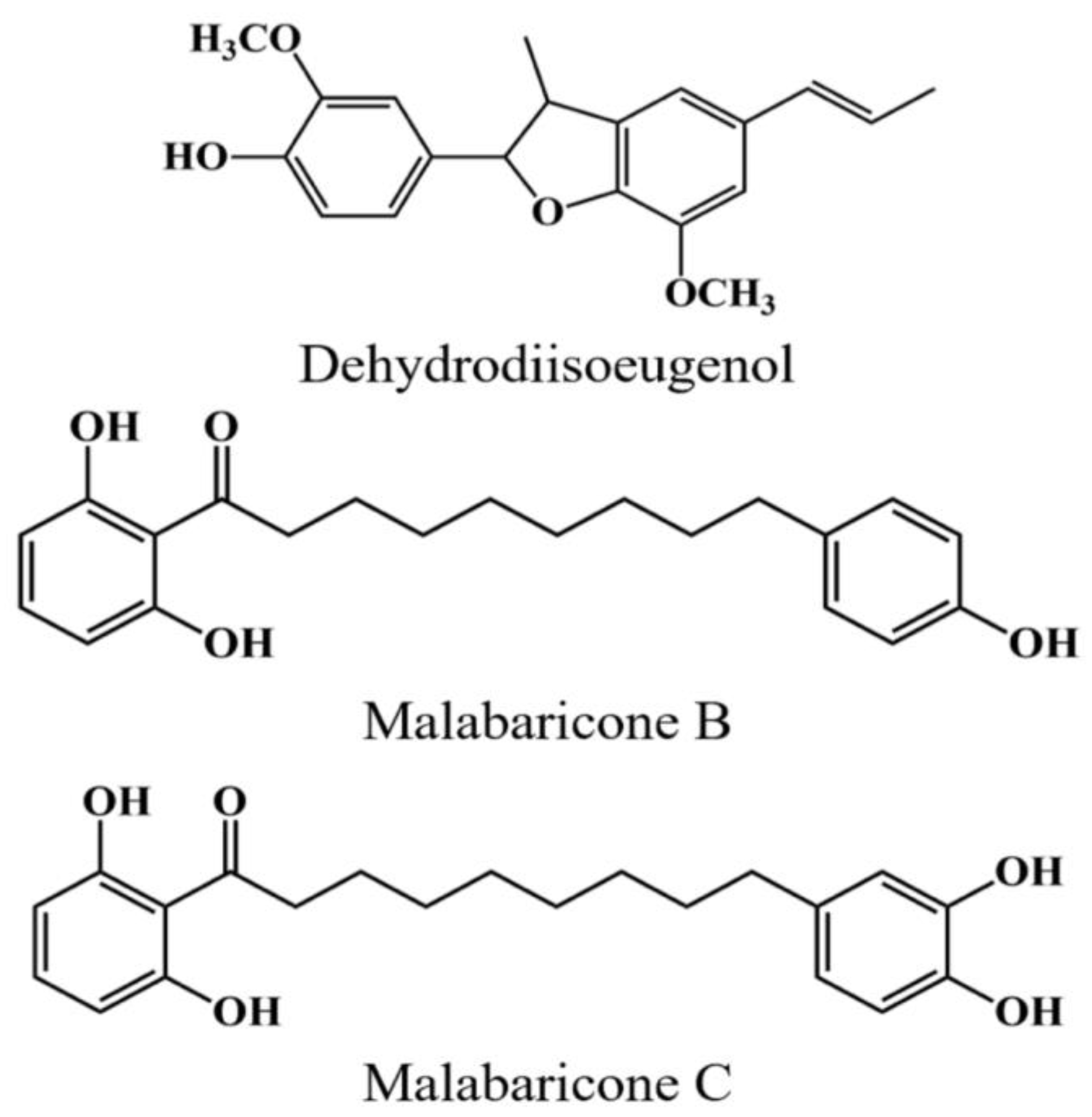
2.7. Quantification of Components
2.8. Quantitation of Active Components in Different Solvent Extracts
2.9. Antioxidant Activities of Isolated Components
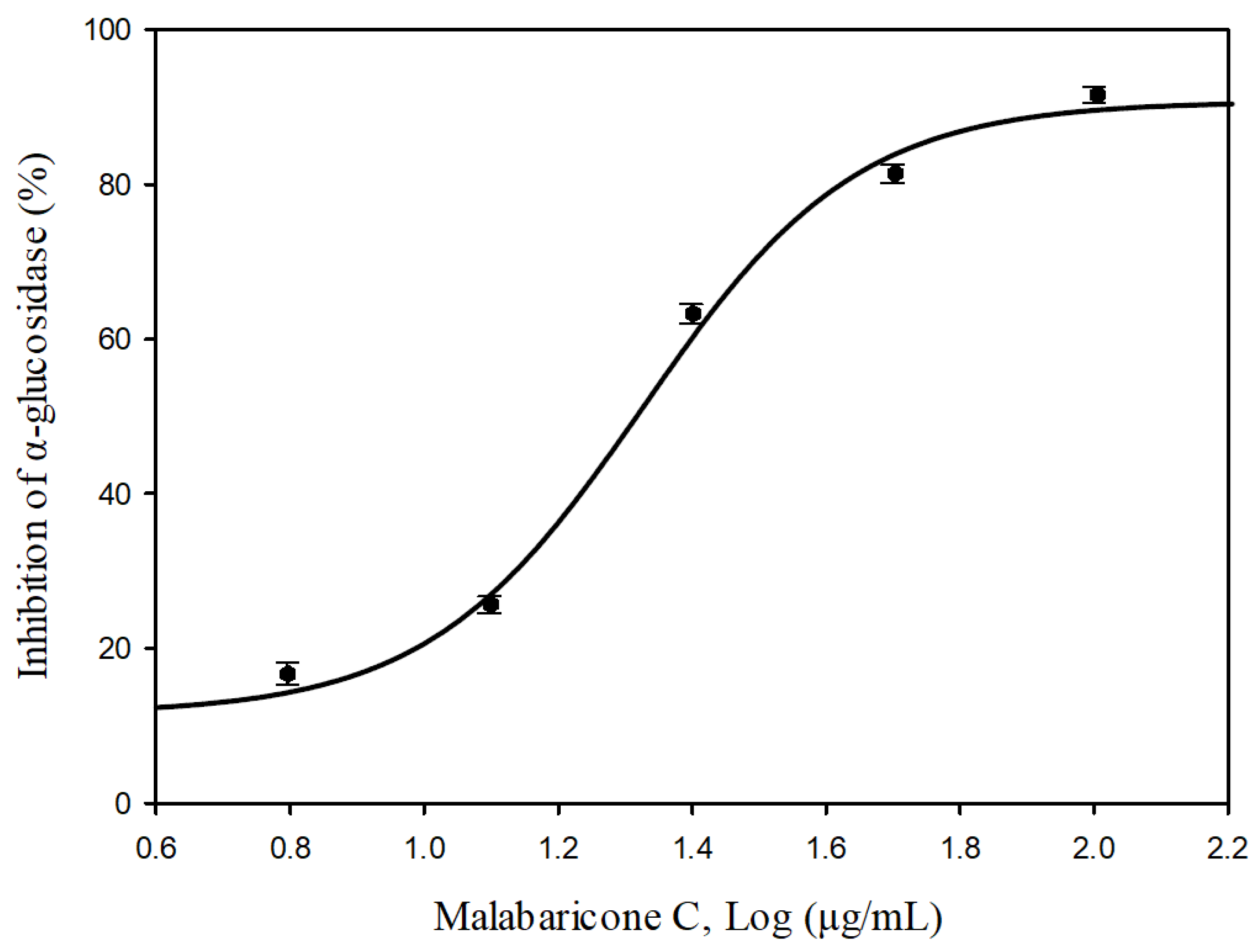
2.10. Anti-α-glucosidase Activities of Isolated Component
3. Materials and Methods
3.1. Chemicals and Antibodies
3.2. Preparation of M. fragrans Extract
3.3. Preparation of Active Components
3.4. Normal-Phase HPLC
3.5. Reverse-Phase HPLC
3.6. Determination of Total Phenolic Content
3.7. DPPH Radical Scavenging Activity
3.8. ABTS Anion Radical Scavenging Activity
3.9. Superoxide Radical Scavenging Activity
3.10. Hydroxyl Radical Scavenging Activity
3.11. α-Glucosidase Inhibitory Activity Assay
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wiseman, H.; Halliwell, B. Damage to DNA by reactive oxygen and nitrogen species: Role in inflammatory disease and progression to cancer. Biochem. J. 1996, 313, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.X.; Kennedy, J.F. Antioxidant activities of different polysaccharide conjugates (CRPs) isolated from the fruiting bodies of Chroogomphis rutilus (Schaeff.: Fr.) O. K. Miller. Carbohydr. Polym. 2010, 82, 510–514. [Google Scholar] [CrossRef]
- Kaur, C.; Kapoor, H.C. Antioxidants in fruits and vegetables-the millennium’s health. Int. J. Food Sci. Technol. 2001, 36, 703–725. [Google Scholar] [CrossRef]
- Gulcin, I.; Oktay, M.; Kirecci, E.; Kufrevioglu, O.I. Screening of antioxidant and antimicrobial activities of anise (Pimpinella anisum L.) seed extracts. Food Chem. 2003, 83, 371–382. [Google Scholar] [CrossRef]
- Kubola, J.; Siriamornpun, S. Phenolic contents and antioxidant activities of bitter gourd (Momordica charantia L.) leaf, stem and fruit fraction extracts in vitro. Food Chem. 2008, 110, 881–890. [Google Scholar] [CrossRef]
- Silva, B.A.; Malva, J.O.; Dias, A.C.P. St. John’s Wort (Hypericum perforatum) extracts and isolated phenolic compounds are effective antioxidants in several in vitro models of oxidative stress. Food Chem. 2008, 110, 611–619. [Google Scholar] [CrossRef]
- Guo, J.M.; Weng, X.C.; Wu, H.; Li, Q.H.; Bi, K.S. Antioxidants from a Chinese medicinal herb—Psoralea corylifolia L. Food Chem. 2005, 91, 287–292. [Google Scholar]
- Han, H.; Weng, X.C.; Bi, K.S. Antioxidants from a Chinese medicinal herb-Lithospermum erythrorhizon. Food Chem. 2008, 106, 2–10. [Google Scholar] [CrossRef]
- Zhu, Y.Z.; Huang, S.H.; Tan, B.K.H.; Sun, J.; Whiteman, M.; Zhu, Y.C. Antioxidants in Chinese herbal medicines: A biochemical Perspective. Nat. Prod. Rep. 2004, 21, 478–489. [Google Scholar] [CrossRef]
- Van Der Zwan, L.P.; Scheffer, P.G.; Dekker, J.M.; Stehouwer, C.D.A.; Heine, R.J.; Teerlink, T. Hyperglycemia and oxidative stress strengthen the association between myeloperoxidase and blood pressure. Hypertension 2010, 55, 1366–1372. [Google Scholar] [CrossRef]
- Ceriello, A.; Davidson, J.; Hanefeld, M.; Leiter, L.; Monnier, L.; Owens, D.; Tajima, N.; Tuomilehto, J. Postprandial hyperglycaemia and cardiovascular complications of diabetes: An update. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 453–456. [Google Scholar] [CrossRef]
- O’Keefe, J.H.; Bell, D.S.H. Postprandial Hyperglycemia/Hyperlipidemia (Postprandial Dysmetabolism) Is a Cardiovascular Risk Factor. Am. J. Cardiol. 2007, 100, 375, 899–904. [Google Scholar] [CrossRef]
- Szablewski, L. Glucose Homeostasis—Mechanism and Defects. In Diabetes—Damages and Treatments; Rigobelo, E., Ed.; InTech Publishing Inc.: Rijeka, Croatia, 2011; Volume 12, pp. 227–256. [Google Scholar]
- Kim, K.Y.; Nguyen, T.H.; Kurihara, H.; Kim, S.M. α-Glucosidase inhibitory activity of bromophenol purified from the red alga Polyopes lancifolia. J. Food Sci. 2010, 75, H145–H150. [Google Scholar] [CrossRef]
- Etxeberria, U.; de la Garza, A.L.; Campión, J.; Martínez, J.A.; Milagro, F.I. Antidiabetic effects of natural plant extracts via inhibition of carbohydrate hydrolysis enzymes with emphasis on pancreatic alpha amylase. Expert Opin. Ther. Targets 2012, 16, 269–297. [Google Scholar] [CrossRef]
- Van De Laar, F.A. Alpha-glucosidase inhibitors in the early treatment of type 2 diabetes. Vasc. Health Risk Manag. 2008, 4, 1189–1195. [Google Scholar] [CrossRef]
- Jaiswal, P.; Kumar, P.; Singh, V.K.; Singh, D.K. Biological Effects of Myristica fragrans. Annu. Rev. Biomed. Sci. 2009, 11, 21–29. [Google Scholar] [CrossRef]
- Grover, J.K.; Khandkar, S.; Vats, V.; Dhunnoo, Y. Pharmacological studies on Myristica fragrans—Antidiarrheal, hypnotic, analgesic and hemodynamic (blood pressure) parameters. Meth. Find. Exp. Clin. Pharmacol. 2003, 24, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Jinno, K.; Kawagishi, H.; Arimoto, Y.; Suganuma, H.; Inakuma, T.; Sugiyama, K. Hepatoprotective effect of myristicin from nutmeg (Myristica fragrans) on lipopolysaccharide/d-galactosamine-induced liver injury. J. Agric. Food Chem. 2003, 51, 1560–1565. [Google Scholar] [CrossRef]
- Sonavane, G.S.; Sarveiya, V.P.; Kasture, V.S.; Kasture, S.B. Anxiogenic activity of Myristica fragrans seeds. Pharmacol. Biochem. Behav. 2002, 71, 239–244. [Google Scholar] [CrossRef]
- Capasso, R.; Pinto, L.; Vuotto, M.L.; Di Carlo, G. Preventive effect of eugenol on PAF and ethanol-induced gastric mucosal damage. Fitoterapia 2000, 71 (Suppl. 1), S131–S137. [Google Scholar] [CrossRef]
- Park, S.; Lee, D.K.; Yang, C.H. Inhibition of fos-jun-DNA complex formation by dihydroguaiaretic acid and in vitro cytotoxic effects on cancer cells. Cancer Lett. 1998, 127, 23–28. [Google Scholar] [CrossRef]
- Ozaki, Y.; Soedigdo, S.; Wattimena, Y.R.; Suganda, A.G. Anti-inflammatory effect of mace, aril of Myristica fragrans Houtt., and its active principles. Jpn. J. Pharmacol. 1989, 49, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Herchi, W.; Kallel, H.; Boukhchina, S. Physicochemical properties and antioxidant activity of Tunisian date palm (Phoenix dactylifera L.) oil as affected by different extraction methods. J. Food Sci. Technol. 2014, 34, 464–470. [Google Scholar] [CrossRef]
- Du, G.R. Study on the Total Antioxidant Capacity and Bioactive Compounds of Kiwi, Persimmon and Apple Fruits; Northwest Agriculture and Forestry University: Xianyang, China, 2009. [Google Scholar]
- Saputri, F.A.; Lestari, K.; Levita, J. Determination of safrole in ethanol extract of Nutmeg (Myristica fragrans Houtt) using reversed-phase high performance liquid chromatography. Int. J. Chem. 2014, 6, 14–20. [Google Scholar] [CrossRef]
- Chiu, S.; Wang, T.; Belski, M.; Abourashed, E.A. HPLC-guided isolation, purification and characterization of phenylpropanoid and phenolic constituents of nutmeg kernel (Myristica fragrans). Nat. Prod. Commun. 2016, 11, 483–488. [Google Scholar] [CrossRef]
- Lim, S.; Choi, A.H.; Kwon, M.; Joung, E.J.; Shin, T.; Lee, S.G.; Kim, N.G.; Kim, H.R. Evaluation of antioxidant activities of various solvent extract from Sargassum serratifolium and its major antioxidant components. Food chem. 2019, 278, 178–184. [Google Scholar] [CrossRef]
- Kim, S.M.; Kang, S.W.; Jeon, J.S.; Jung, Y.J.; Kim, W.R.; Kim, C.Y.; Um, B.H. Determination of major phlorotannins in Eisenia bicyclis using hydrophilic interaction chromatography: Seasonal variation and extraction characteristics. Food Chem. 2013, 138, 2399–2406. [Google Scholar] [CrossRef]
- Koivikko, R.; Loponen, J.; Honkanen, T.; Jormalainen, V. Contents of soluble, cell-wall-bound and exuded phlorotannins in the brown alga Fucus vesiculosus, with implications on their ecological functions. J. Chem. Ecol. 2005, 31, 195–212. [Google Scholar] [CrossRef]
- Takao, T.; Watanabe, N.; Yagi, I.; Sakata, K. A simple screening method for antioxidants and isolation of several antioxidants produced by marine bacteria from fish and shellfish. Biosci. Biotechnol. Biochem. 1994, 58, 1780–1783. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Zhang, Q.-F.; Zhang, Z.-R.; Cheung, H.-Y. Antioxidant activity of Rhizoma Smilacis Glabrae extracts and its key constituent-astilbin. Food Chem. 2009, 115, 297–303. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E. Studies on the antioxidant activities of cinnamon (Cinnamomum verum) bark extracts, through various in vitro models. Food Chem. 2006, 94, 520–528. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, Y.; Cheng, Y.; Wang, Y. Rapid screening and identification of α-glucosidase inhibitors from mulberry leaves using enzyme-immobilized magnetic beads coupled with HPLC/MS and NMR. Biomed. Chromatogr. 2013, 27, 148–155. [Google Scholar] [CrossRef]

| Extracting Solvents | Relative Polarity | TPC (mg/g) a (GAE) | Yields (%) b |
|---|---|---|---|
| n-Hexane | 0.009 | 16.82 ± 0.62 *** | 27.3 ± 1.67 |
| Chloroform | 0.259 | 18.65 ± 0.53 *** | 29.2 ± 0.79 |
| Dichloromethane | 0.269 | 18.97 ± 1.22 ** | 30.7 ± 1.49 |
| Ethyl acetate | 0.288 | 32.93 ± 0.85 *** | 24.5 ± 1.13 |
| Acetone | 0.355 | 70.07 ± 2.28 *** | 21.1 ± 0.23 |
| Methanol | 0.762 | 107.83 ± 0.66 *** | 18.2 ± 0.75 |
| Ethanol | 0.654 | 98.01 ± 2.99 *** | 15.6 ± 1.21 |
| Extracting Solvents | DPPH IC50 (μg/mL) | ABTS IC50 (μg/mL) | Superoxide IC50 (μg/mL) | Hydroxyl IC50 (μg/mL) |
|---|---|---|---|---|
| n-Hexane | 126.57 ± 6.23 * | 103.05 ± 2.41 * | >400 | 51.94 ± 0.79 * |
| Chloroform | 167.17 ± 7.13 | 93.70 ± 5.06 * | >400 | 82.39 ± 2.62 * |
| Dichloromethane | 96.90 ± 7.68 | 82.31 ± 2.15 * | >400 | 88.19 ± 2.09 * |
| Ethyl acetate | 95.12 ± 2.63 * | 91.19 ± 0.88 * | >400 | 55.25 ± 1.25 * |
| Acetone | 65.08 ± 1.44 * | 64.35 ± 1.58 * | >400 | 42.99 ± 0.19 * |
| Methanol | 22.42 ± 0.99 ** | 34.41 ± 0.78 ** | 117.66 ± 2.56 * | 37.81 ± 1.56 * |
| Ethanol | 39.65 ± 0.83 * | 27.68 ± 0.31 ** | >400 | 56.05 ± 2.52 * |
| BHT a | 36.94 ± 0.49 ** | 11.05 ± 0.26 ** | N.A. b | 61.51 ± 2.46 * |
| Extracting Solvents | α-Glucosidase IC50 (μg/mL) |
|---|---|
| n-Hexane | >200 |
| Chloroform | >200 |
| Dichloromethane | >200 |
| Ethyl acetate | 185.36 ± 5.21 |
| Acetone | 29.07 ± 2.30 * |
| Methanol | 4.08 ± 0.12 ** |
| Ethanol | 11.92 ± 0.39 * |
| Quercetin a | 14.99 ± 0.81 ** |
| Extracting Solvents | Malabaricone B (mg/g) | Malabaricone C (mg/g) | Dehydrodiisoeugenol (mg/g) | Total Amount (mg/g) |
|---|---|---|---|---|
| Methanol | 6.17 ± 0.51 | 31.67 ± 1.49 | 13.59 ± 0.50 | 51.43 ± 1.18 |
| Ethanol | 4.65 ± 0.54 | 27.54 ± 1.16 | 10.61 ± 0.59 | 42.80 ± 1.17 |
| Acetone | 2.72 ± 0.13 | 16.41 ± 0.91 | 6.62 ± 0.19 | 25.75 ± 0.67 |
| Ethyl acetate | 2.29 ± 0.28 | 15.12 ± 0.67 | 5.86 ± 0.89 | 23.27 ± 1.72 |
| Chloroform | 2.50 ± 0.05 | 4.48 ± 0.27 | 11.27 ± 0.54 | 18.25 ± 0.65 |
| Dichloromethane | 2.58 ± 0.08 | 3.89 ± 0.59 | 10.18 ± 0.42 | 16.65 ± 0.92 |
| n-Hexane | 1.10 ± 0.13 | N.D. a | 14.40 ± 0.36 | 15.52 ± 0.26 |
| Compounds | DPPH IC50 (μg/mL) | ABTS IC50 (μg/mL) | Superoxide IC50 (μg/mL) | Hydroxyl IC50 (μg/mL) |
|---|---|---|---|---|
| Dehydrodiisoeu-genol | 66.02 ± 2.85 * | 8.43 ± 0.42 *** | >200 | 68.29 ± 0.70 |
| Malabaricone B | >200 | 7.05 ± 0.72 *** | >200 | 95.22 ± 4.20 |
| Malabaricone C | 8.35 ± 2.20 ** | 5.36 ± 0.19 ** | >200 | 72.81 ± 2.58 * |
| BHT a | 34.28 ± 1.40 * | 10.67 ± 0.41 ** | N.A. b | 69.96 ± 4.66 * |
Sample Availability: Samples of the compounds are available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.-W.; Chu, Y.-C.; Huang, C.-Y.; Fu, S.-L.; Chen, J.-J. Evaluation of Antioxidant and Anti-α-glucosidase Activities of Various Solvent Extracts and Major Bioactive Components from the Seeds of Myristica fragrans. Molecules 2020, 25, 5198. https://doi.org/10.3390/molecules25215198
Li C-W, Chu Y-C, Huang C-Y, Fu S-L, Chen J-J. Evaluation of Antioxidant and Anti-α-glucosidase Activities of Various Solvent Extracts and Major Bioactive Components from the Seeds of Myristica fragrans. Molecules. 2020; 25(21):5198. https://doi.org/10.3390/molecules25215198
Chicago/Turabian StyleLi, Cai-Wei, Yi-Cheng Chu, Chun-Yi Huang, Shu-Ling Fu, and Jih-Jung Chen. 2020. "Evaluation of Antioxidant and Anti-α-glucosidase Activities of Various Solvent Extracts and Major Bioactive Components from the Seeds of Myristica fragrans" Molecules 25, no. 21: 5198. https://doi.org/10.3390/molecules25215198
APA StyleLi, C.-W., Chu, Y.-C., Huang, C.-Y., Fu, S.-L., & Chen, J.-J. (2020). Evaluation of Antioxidant and Anti-α-glucosidase Activities of Various Solvent Extracts and Major Bioactive Components from the Seeds of Myristica fragrans. Molecules, 25(21), 5198. https://doi.org/10.3390/molecules25215198









