Saponins as Modulators of the Blood Coagulation System and Perspectives Regarding Their Use in the Prevention of Venous Thromboembolic Incidents
Abstract
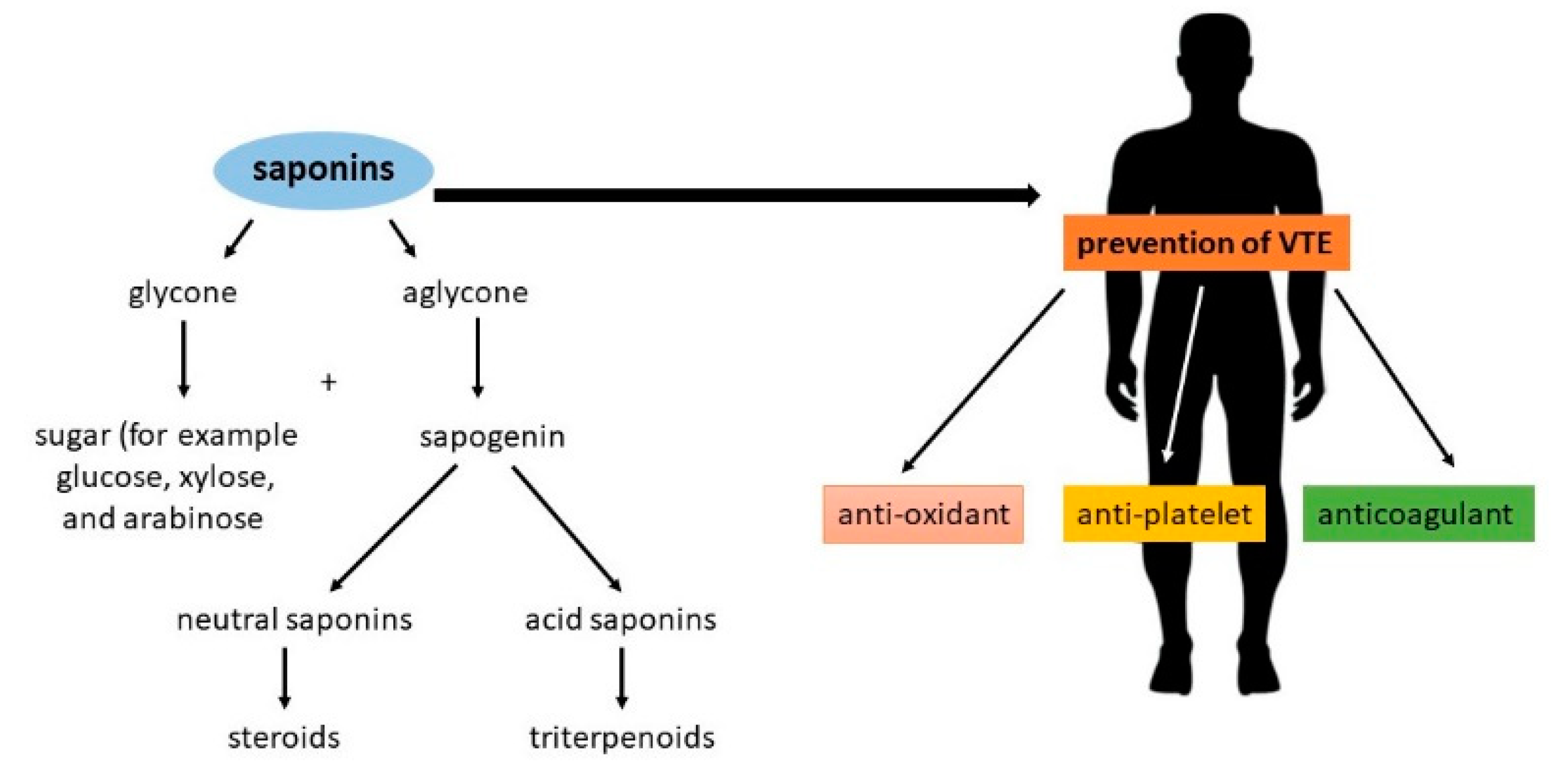
1. Introduction
2. Saponins with Antiplatelet and Anticoagulation Activity
3. The Molecular Mechanism of Saponin Action as Modulators of the Coagulation System
3.1. Arachidonic Acid Pathway
3.2. GPVI Signaling Pathway and Enzymatic Cascades
3.3. Inhibition of Tissue Factor Expression
4. Toxicology and Safety of Saponins
5. Conclusions
Funding
Conflicts of Interest
References
- Chanda, S.; Ramachandra, T.V. A review on some therapeutic aspects of phytochemicals present in medicinal plants. IJPLS 2019, 10, 6064–6067. [Google Scholar]
- El Aziz, M.M.A.; Ashour, A.S.; Melad, A.S.G. A review on saponins from medicinal plants: Chemistry, isolation, and determination. J. Nanomed. Res. 2019, 8, 6–12. [Google Scholar]
- Xu, C.; Wang, W.; Wang, B.; Zhang, T.; Cui, X.; Pu, Y.; Li, N. Analytical methods and biological activities of Panax notoginseng saponins: Recent trends. J. Ethnopharmacol. 2019, 236, 443–465. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.Y.; Zeng, J.Z.; Wong, A.S.T. Chemical structures and pharmacological profiles of ginseng saponins. Molecules 2019, 24, 2443. [Google Scholar] [CrossRef]
- Yang, Y.; Laval, S.; Yu, B. Chemical synthesis of saponins. In Advances in Carbohydrate Chemistry and Biochemistry; Horton, D., Ed.; Academic Press: London, UK, 2014; Volume 77, pp. 137–226. [Google Scholar]
- Cárdenas, P.D.; Almeida, A.; Bak, S. Evolution of structural diversity of triterpenoids. Front. Plant Sci. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hu, Z.; Li, A.; Zhu, Z.; Yang, N.; Ying, Z.; He, J.; Wang, C.; Yin, S.; Cheng, S. Recent advances in biotransformation of saponins. Molecules 2019, 24, 2365. [Google Scholar] [CrossRef] [PubMed]
- Das, T.K.; Banerjee, D.; Chakraborty, D.; Pakhira, M.C.; Shrivastava, B.; Kuhad, R.C. Saponin: Role in animal system. Vet. World 2012, 5, 248–254. [Google Scholar] [CrossRef]
- El Barky, A.R.; Hussein, S.A.; Alm-Eldeen, A.E. Saponins and their potential role in diabetes mellitus. Diabetes Manag. 2017, 7, 148–158. [Google Scholar]
- Liu, Y.; Liu, T.; Zhao, J.; He, T.; Chen, H.; Wang, J.; Zhang, W.; Ma, W.; Fan, Y.; Song, X. Phospholipase Cγ2 signalling contributes to the haemostatic effect of Notoginsenoside Ft1. J. Pharm. Pharmacol. 2019, 71, 878–886. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, L.; Man, J.; Hu, Y.; Cui, X. Chemical and bioactive comparison of Panax notoginseng root and rhizome in raw and steamed forms. J. Ginseng Res. 2019, 43, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhao, X.; Miyamoto, A.; Zhao, S.; Liu, C.; Zheng, W.; Wang, H. Effects of steroidal saponins extract from Ophiopogon japonicus root ameliorates doxorubicin-induced chronic heart failure by inhibiting oxidative stress and inflammatory response. Pharm. Biol. 2019, 57, 176–183. [Google Scholar] [CrossRef]
- Sun, J.; Yu, X.; Huangpu, H.; Yao, F. Ginsenoside Rb3 protects cardiomyocytes against hypoxia/reoxygenation injury via activating the antioxidation signaling pathway of PERK/Nrf2/HMOX1. Biomed. Pharmacother. 2019, 109, 254–261. [Google Scholar] [CrossRef]
- Dong, Y.; Duan, L.; Chen, H.-W.; Liu, Y.-M.; Zhang, Y.; Wang, J. Network Pharmacology-Based Prediction and Verification of the Targets and Mechanism for Panax Notoginseng Saponins against Coronary Heart Disease. Evidence-Based Complement. Altern. Med. 2019, 2019, 6503752. [Google Scholar] [CrossRef]
- Evangelista, M.S.; Slompo, K.; Timi, J.R.R. Venous thromboembolism and route of delivery—Review of the literature. Rev. Bras. Ginecol. Obstet. 2018, 40, 156–162. [Google Scholar] [CrossRef]
- Donnellan, E.; Khorana, A.A. Cancer and venous thromboembolic disease: A review. Oncologist 2017, 22, 199–207. [Google Scholar] [CrossRef]
- Hirsch, E.G.; Viecili, P.R.N.; de Almeida, A.S.; Nascimento, S.; Porto, F.G.; Otero, J.; Schmidt, A.; de Silva, B.; Parisi, M.M.; Klafke, J.Z. Natural products with antiplatelet action. Curr. Pharm. Des. 2017, 23, 1228–1246. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.L.; Mao, W.H.; Wang, C.G.; Pan, Y.M.; Liang, D.; Wang, H.S. Five 11α, 12α-epoxy pentacyclic triterpenoid saponins with antithrombus activities from Glechoma longituba. Fitoterapia 2019, 138, 104345. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Luo, Y.; Chen, Y.; Ma, Y.; Yue, P.; Yang, M. Novel breviscapine nanocrystals modified by Panax notoginseng saponins for enhancing bioavailability and synergistic anti-platelet aggregation effect. Colloids Surf. B Biointerfaces 2019, 175, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Park, K.I. A review of antiplatelet activity of traditional medicinal herbs on integrative medicine studies. Evid. Based Complement. Alternat. Med. 2019, 2019, 7125162. [Google Scholar] [CrossRef]
- Cong, Y.; Wang, L.; Peng, R.; Zhao, Y.; Bai, F.; Yang, C.; Liu, X.; Wang, D.; Ma, B.; Cong, Y. Timosaponin AIII induces antiplatelet and antithrombotic activity via Gq-mediated signaling by the thromboxane A2 receptor. Sci. Rep. 2016, 6, 38757. [Google Scholar] [CrossRef]
- Qi, H.; Huang, Y.; Yang, Y.; Dou, G.; Wan, F.; Zhang, W.; Yang, H.; Wang, L.; Wu, C.; Li, L. Anti-platelet activity of panaxatriol saponins is mediated by suppression of intracellular calcium mobilization and ERK2/p38 activation. BMC Complement. Altern. Med. 2016, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, S.N.; Pei, X.; Hao, K. The multivariate regression statistics strategy to investigate content-effect correlation of multiple components in traditional Chinese medicine based on a partial least squares method. Molecules 2018, 23, 545. [Google Scholar] [CrossRef]
- Kang, L.; Zhang, J.; Cong, Y.; Li, B.; Xiong, C.Q.; Zhao, Y.; Tan, D.W.; Yu, H.S.; Yu, Z.Y.; Cong, W.Y.; et al. Steroidal glycosides from the rhizomes of Anemarrhena asphodeloides and their antiplatelet aggregation activity. Planta Med. 2012, 78, 611–616. [Google Scholar] [CrossRef]
- Zhai, K.F.; Zheng, J.R.; Tang, Y.M.; Li, F.; Lv, Y.N.; Zhang, Y.Y.; Gao, Z.; Qi, J.; Yu, B.Y.; Kou, J.P. The saponin D39 blocks dissociation of non-muscular myosin heavy chain IIA from TNF receptor 2, suppressing tissue factor expression and venous thrombosis. Br. J. Pharmacol. 2017, 174, 2818–2831. [Google Scholar] [CrossRef]
- Li, H.; Huang, W.; Wen, Y.; Gong, G.; Zhao, Q.; Yu, G. Anti-thrombotic activity and chemical characterization of steroidal saponins from Dioscorea zingiberensis CH Wright. Fitoterapia 2010, 81, 1147–1156. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, B.; Du, D.; Guo, X.; Xin, G.; Xing, Z.; Liang, Y.; Chen, Y.; Chen, Q.; He, Y.; et al. Anti-thrombosis effect of diosgenyl saponins in vitro and in vivo. Steroids 2013, 78, 1064–1070. [Google Scholar] [CrossRef]
- Lu, W.Q.; Qiu, Y.; Li, T.J.; Tao, X.; Sun, L.N.; Chen, W.S. Antiplatelet and antithrombotic activities of timosaponin B-II, an extract of Anemarrhena asphodeloides. Clin. Exp. Pharmacol. Physiol. 2011, 38, 430–434. [Google Scholar] [CrossRef]
- Zhou, Z.; Wei, X.; Fu, H.; Luo, Y. Chemical constituents of Callicarpa nudiflora and their anti-platelet aggregation activity. Fitoterapia 2013, 88, 91–95. [Google Scholar] [CrossRef]
- Dahmer, T.; Berger, M.; Barlette, A.G.; Reck, J.; Segalin, J.; Verza, S.; Ortega, G.G.; Gnoatto, S.C.; Guimarães, J.A.; Verli, H.; et al. Antithrombotic effect of chikusetsusaponin IVa isolated from Ilex paraguariensis (Mate). J. Med. Food. 2012, 15, 1073–1080. [Google Scholar] [CrossRef]
- Lee, W.M.; Kim, S.D.; Park, M.H.; Cho, J.Y.; Park, H.J.; Seo, G.S.; Rhee, M.H. Inhibitory mechanisms of dihydroginsenoside Rg3 in platelet aggregation: Critical roles of ERK2 and cAMP. J. Pharm. Pharmacol. 2008, 60, 1531–1536. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, X.J.; Shi, D.Z. Antiplatelet effect of active components derived from Chinese herbal medicine. Chin. J. Integr. Med. 2018, 24, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.G.; Lee, Y.Y.; Kim, S.Y.; Pyo, J.S.; Yun-Choi, H.S.; Park, J.H. Platelet antiaggregating activity of ginsenosides isolated from processed ginseng. Die Pharm. 2009, 64, 602–604. [Google Scholar]
- Li, C.T.; Wang, H.B.; Xu, B.J. A comparative study on anticoagulant activities of three Chinese herbal medicines from the genus Panax and anticoagulant activities of ginsenosides Rg1 and Rg2. Pharm. Biol. 2013, 51, 1077–1080. [Google Scholar] [CrossRef]
- Shin, J.H.; Kwon, H.W.; Cho, H.J.; Rhee, M.H.; Park, H.J. Vasodilator-stimulated phosphoprotein-phosphorylation by ginsenoside Ro inhibits fibrinogen binding to αIIb/β3 in thrombin-induced human platelets. J. Ginseng Res. 2016, 40, 359–365. [Google Scholar] [CrossRef]
- Endale, M.; Lee, W.M.; Kamruzzaman, S.M.; Kim, S.D.; Park, J.Y.; Park, M.H.; Park, H.J.; Cho, J.Y.; Rhee, M.H. Ginsenoside-Rp1 inhibits platelet activation and thrombus formation via impaired glycoprotein VI signalling pathway, tyrosine phosphorylation and MAPK activation. Br. J. Pharmacol. 2012, 167, 109–127. [Google Scholar]
- Irfan, M.; Jeong, D.; Kwon, H.W.; Shin, J.H.; Park, S.J.; Kwak, D.; Kim, T.H.; Lee, D.H.; Park, H.J.; Rhee, M.H. Ginsenoside-Rp3 inhibits platelet activation and thrombus formation by regulating MAPK and cyclic nucleotide signaling. Vascul. Pharmacol. 2018, 109, 45–55. [Google Scholar]
- Son, Y.M.; Jeong, D.H.; Park, H.J.; Rhee, M.H. The inhibitory activity of ginsenoside Rp4 in adenosine diphosphate-induced platelet aggregation. J. Ginseng Res. 2017, 41, 96–102. [Google Scholar]
- Kothiyal, S.K.; Sati, S.C.; Rawat, S.M.M.; Sati, M.D.; Semwal, D.K.; Semwal, R.B.; Sharma, A.; Rawat, B.; Kumar, A. Chemical constituents and biological significance of the genus Ilex (Aquifoliaceae). Nat. Prod. J. 2012, 2, 212–224. [Google Scholar]
- Liu, Y.; Liu, T.; Ding, K.; Liu, Z.; Li, Y.; He, T.; Zhang, W.; Fan, Y.; Ma, W.; Song, X. Phospholipase Cγ2 signaling cascade contribution to the antiplatelet effect of Notoginsenoside Fc. Front. Pharmacol. 2018, 9, 1293. [Google Scholar] [CrossRef]
- Huang, H.C.; Tsai, W.J.; Morris-Natschke, S.L.; Tokuda, H.; Lee, K.H.; Wu, Y.; Kuo, Y.H. Sapinmusaponins F− J, bioactive tirucallane-type saponins from the galls of Sapindus mukorossi. J. Nat. Prod. 2006, 69, 763–767. [Google Scholar] [CrossRef]
- Huang, H.C.; Tsai, W.J.; Liaw, C.C.; Wu, S.H.; Wu, Y.C.; Kuo, Y.H. Anti-platelet aggregation triterpene saponins from the galls of Sapindus mukorossi. Chem. Pharm. Bull. 2007, 55, 1412–1415. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.H.R. Arachidonic acid metabolism, thrombosis, and stroke. J. Cardiol. Cardiovasc. Ther. 2018, 11, 1–3. [Google Scholar] [CrossRef]
- Martin, S.A.; Brash, A.R.; Murphy, R.C. The discovery and early structural studies of arachidonic acid. J. Lipid Res. 2016, 57, 1126–1132. [Google Scholar] [CrossRef]
- Biringer, R.G. The enzymes of the human eicosanoid pathway. Res. Rep. Med. Sci. 2018, 2, 106. [Google Scholar]
- Hao, Y.; Tatonetti, N.P. Predicting G protein-coupled receptor downstream signaling by tissue expression. Bioinformatics 2016, 32, 3435–3443. [Google Scholar] [CrossRef]
- Powell, W.S.; Rokach, J. Biosynthesis, biological effects, and receptors of hydroxyeicosatetraenoic acids (HETEs) and oxoeicosatetraenoic acids (oxo-ETEs) derived from arachidonic acid. Biochim. Biophys. Acta 2015, 1851, 340–355. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.K.; Lee, J.G.; Park, M.K.; Park, S.J.; Lee, C.H.; Park, J.H.; Kwon, S.W. Metabolomic investigation of the anti-platelet aggregation activity of ginsenoside Rk1 reveals attenuated 12-HETE production. J. Proteome Res. 2012, 11, 4939–4946. [Google Scholar] [CrossRef]
- Dütting, S.; Bender, M.; Nieswandt, B. Platelet GPVI: A target for antithrombotic therapy?! Trends Pharmacol. Sci. 2012, 33, 583–590. [Google Scholar] [CrossRef]
- Adam, F.; Kauskot, A.; Rosa, J.P.; Bryckaert, M. Mitogen-activated protein kinases in hemostasis and thrombosis. J. Thromb. Haemost. 2008, 6, 2007–2016. [Google Scholar] [CrossRef]
- Adam, F.; Kauskot, A.; Nurden, P.; Sulpice, E.; Hoylaerts, M.F.; Davis, R.J.; Rosa, J.P.; Bryckaert, M. Platelet JNK1 is involved in secretion and thrombus formation. Blood 2010, 115, 4083–4092. [Google Scholar] [CrossRef]
- Makhoul, S.; Walter, E.; Pagel, O.; Walter, U.; Sickmann, A.; Gambaryan, S.; Smolenski, A.; Zahedi, R.P.; Jurk, K. Effects of the NO/soluble guanylate cyclase/cGMP system on the functions of human platelets. Nitric Oxide 2018, 76, 71–80. [Google Scholar] [CrossRef]
- Shin, J.H.; Kwon, H.W.; Rhee, M.H.; Park, H.J. Inhibitory effects of thromboxane A2 generation by ginsenoside Ro due to attenuation of cytosolic phospholipase A2 phosphorylation and arachidonic acid release. J. Ginseng Res. 2019, 43, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Thibeault, P.E.; Ramachrandran, R. Biased signalling in platelet G-protein-coupled receptors. Can. J. Physiol. Pharmacol. 2020. [Google Scholar] [CrossRef]
- Offermanns, S. Activation of platelet function through G protein–coupled receptors. Circ. Res. 2006, 12, 1293–1304. [Google Scholar] [CrossRef]
- Grover, S.P.; Mackman, N. Tissue factor: An essential mediator of hemostasis and trigger of thrombosis. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Brummel-Ziedins, K.; Mann, K.G. Molecular basis of blood coagulation. In Hematology, 6th ed.; Shaz, B., Hilley, C., Gil, M., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2018; pp. 1885–1905. [Google Scholar]
- Gil, M.R. Overview of the coagulation system. In Transfusion Medicine and Hemostasis; Shaz, B., Hilley, C., Gil, M., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2019; pp. 559–564. [Google Scholar]
- Hoffman, M. The tissue factor pathway and wound healing. Semin. Thromb. Hemost. 2018, 44, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Zhai, K.; Tang, Y.; Zhang, Y.; Li, F.; Wang, Y.; Cao, Z.; Yu, J.; Kou, J.; Yu, B. NMMHC IIA inhibition impedes tissue factor expression and venous thrombosis via Akt/GSK3beta-NF-kappa B signalling pathways in the endothelium. Thromb. Haemost. 2015, 114, 173–185. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Zhao, Y.; Han, H.; Hu, Y.; Liang, D.; Yu, B.; Kou, J. The myosin II inhibitor, blebbistatin, ameliorates FeCl3-induced arterial thrombosis via the GSK3β-NF-κB pathway. Int. J. Biol. Sci. 2017, 13, 630–639. [Google Scholar] [CrossRef][Green Version]
- Singh, D.; Chaudhuri, P.K. Structural characteristics, bioavailability and cardioprotective potential of saponins. Integr. Med. Res. 2018, 7, 33–43. [Google Scholar] [CrossRef]
- DrugBank Database. Available online: https://www.drugbank.ca/ (accessed on 9 January 2020).
- Tian, Z.; Pang, H.; Du, S.; Lu, Y.; Zhang, L.; Wu, H.; Guo, S.; Wang, M.; Zhang, Q. Effect of Panax notoginseng saponins on the pharmacokinetics of aspirin in rats. J. Chromatogr. B 2017, 1040, 136–143. [Google Scholar] [CrossRef]
- Tian, Z.; Pang, H.; Zhang, Q.; Du, S.; Lu, Y.; Zhang, L.; Bai, J.; Li, P.; Zhao, M.; Chen, X. Effect of aspirin on the pharmacokinetics and absorption of Panax notoginseng saponins. J. Chromatogr. B 2018, 1074, 25–33. [Google Scholar] [CrossRef]
- Leon, M.; Johanna, M. The synopsis of biological and pharmacological events in saponins. IJLES 2018, 1, 1–5. [Google Scholar]
- Zhang, X.; Jin, M.; Tadesse, N.; Xian, L.; Zhang, H.; Wang, S.; Dang, J.; Zhang, Y.; Guo, Z.; Ito, Y. Safety investigation on total steroid saponins extracts from Dioscorea zingiberensis CH Wright: Sub-acute and chronic toxicity studies on dogs. Regul. Toxicol. Pharmacol. 2017, 91, 58–67. [Google Scholar] [CrossRef]
- Abou-Hashem, A.A.M. Evaluation of the rodenticidal effects of some plant extracts under laboratory and field conditions. J. Basic Appl. Zool. 2012, 65, 282–288. [Google Scholar] [CrossRef]
- Hansen, S.C.; Stolter, C.; Imholt, C.; Jacob, J. Plant secondary metabolites as rodent repellents: A systematic review. J. Chem. Ecol. 2016, 42, 970–983. [Google Scholar] [CrossRef]
- Abu, O.D.; Adeogun, E.F.; Ebhohon, S.O. Oral LD50 of total saponins and tannins isolated from Dialium guineense. Eur. J. Exp. Biol. 2019, 9, 11–13. [Google Scholar]
- Alias, E.; Zeinelabdin, M.; Bashir, N.; Assad, Y. Acute toxicity of saponins from the fruit of bitter apple Citrullus colocynthis (L.) Schrad, on the Norway rat, Rattus norvegicus (Berkenhout). Gezira J. Agric. Sci. 2015, 13, 1. [Google Scholar]
- Shu, Y.; Cao, M.; Yin, Z.Q.; Li, P.; Li, T.Q.; Long, X.F.; Zhu, L.F.; Jia, R.Y.; Dai, S.J.; Zhao, J. The reproductive toxicity of saponins isolated from Cortex Albiziae in female mice. Chin. J. Nat. Med. 2015, 13, 119–126. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, Y.; Liu, H.; Chang, W.; Xu, Y.; Lin, F. Prediction of hemolytic toxicity for saponins by machine-learning methods. Chem. Res. Toxicol. 2019, 32, 1014–1026. [Google Scholar] [CrossRef]
- Lorent, J.H.; Quetin-Leclercq, J.; Mingeot-Leclercq, M.P. The amphiphilic nature of saponins and their effects on artificial and biological membranes and potential consequences for red blood and cancer cells. Org. Biomol. Chem. 2014, 12, 8803–8822. [Google Scholar] [CrossRef] [PubMed]
- de Groot, C.; Müller-Goymann, C.C. Saponin interactions with model membrane systems—Langmuir monolayer studies, hemolysis and formation of ISCOMs. Planta Med. 2016, 82, 1496–1512. [Google Scholar] [CrossRef]
- Sarikahya, N.B.; Nalbantsoy, A.; Top, H.; Gokturk, R.S.; Sumbul, H.; Kirmizigul, S. Immunomodulatory, hemolytic and cytotoxic activity potentials of triterpenoid saponins from eight Cephalaria species. Phytomedicine 2018, 38, 135–144. [Google Scholar] [CrossRef]
- Vo, N.N.Q.; Fukushima, E.O.; Muranaka, T. Structure and hemolytic activity relationships of triterpenoid saponins and sapogenins. J. Nat. Med. 2017, 71, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Shao, X.; Zhu, D.; Yu, B. Chemical synthesis of marine saponins. Nat. Prod. Rep. 2019, 36, 769–787. [Google Scholar] [CrossRef]
- Zhang, X.J.; Su, H.; Wang, Y.T.; Wan, J.B. Therapeutic potential of ginsenosides in management of atherosclerosis. In Phytotherapies: Efficacy, Safety, and Regulation; Ramzon, I., Ed.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Liu, C.; Feng, R.; Zou, J.; Xia, F.; Wan, J.B. 20(S)-protopanaxadiol saponins mainly contribute to the anti-atherogenic effects of Panax notoginseng in ApoE deficient mice. Molecules 2019, 24, 3723. [Google Scholar] [CrossRef]
- Laine, M.; Paganelli, F.; Bonello, L. P2Y12-ADP receptor antagonists: Days of future and past. World J. Cardiol. 2016, 8, 327–332. [Google Scholar] [CrossRef]
- Ayombil, F.; Camire, R.M. Insights into vitamin K-dependent carboxylation: Home field advantage. Haematologica 2020, 105, 1996–1998. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Ye, C.; Thanki, K.; Leng, D.; van Hasselt, P.M.; Hennink, W.E.; van Nostrum, C.F. Mixed micellar system stabilized with saponins for oral delivery of vitamin K. Colloids Surf. B Biointerfaces 2018, 170, 521–528. [Google Scholar] [CrossRef]
- Tollefsen, S.; Wierød, L.; Skotte, A.; Rob, J.A.; Helgeland, L. Saponin permeabilization of rough microsomes from rat liver reveals a novel prothrombin pool. Biochim. Biophys. Acta 2001, 1526, 249–256. [Google Scholar] [CrossRef]

| No and Compound Name, Concentration Used | Model | Property | Chemical Structure | Reference |
|---|---|---|---|---|
| Steroidal saponins | ||||
| (1) Anemarrhenasaponin A2 (50 and 100 µg/mL) | Male Wistar rats (PRP) | Inhibitory effects on ADP-induced platelet aggregation | 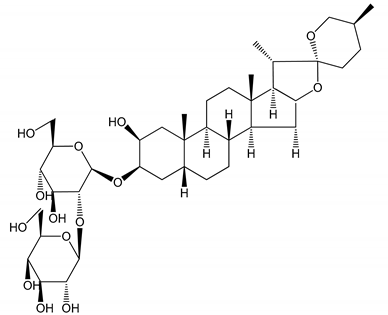 | [24] |
| (2) Anemarsaponin B | Male Wistar rats (PRP and washed blood platelets) | Inhibitory effects on ADP-induced platelet aggregation; delayed thromboplastin time | 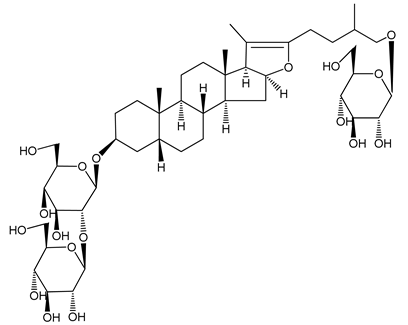 | [20] |
| (3) D39 (0.01–1 µM) | Male C57BL/6J mice and HUVEC cells | Inhibition of thrombus formation | 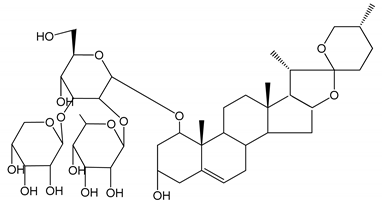 | [25] |
| (4) Dioscin (10 mg/kg/day) | Male Kunming mice and Sprague-Dawley rats (PRP) | Antithrombotic effects by improving anticoagulation activity and inhibiting platelet aggregation | 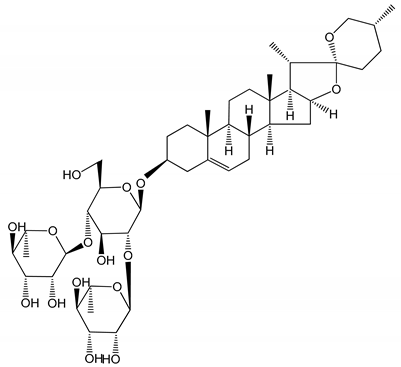 | [26] |
| (5) Diosgenyl β-d-galactopyranosyl-(1→4)-β-d-glucopyranoside (25–100 µM) | Male Wistar rats (PRP) | Inhibition of platelet aggregation, antithrombotic activity (prolongation of APTT, inhibition of factor VIII activities) | 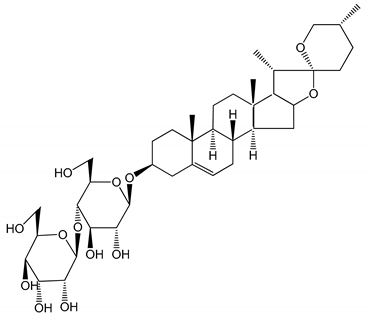 | [27] |
| (6) Timosaponin A-III (50, 60 and 100 µg/mL) | Male Wistar rats; male Balb/c mice (PRP and washed blood platelets) | Inhibitory effects on ADP-induced platelet aggregation; delayed thromboplastin time; antithrombotic activities |  | [20,21,24] |
| (7) Timosaponin B-II (50 and 100 µg/mL) | Male Wistar rats; New Zealand white rabbits (PRP) | Inhibitory effects on ADP-induced platelet aggregation; delayed thromboplastin time; antithrombotic activities | 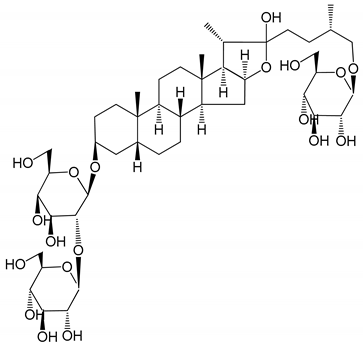 | [20,24,28] |
| Triterpenoid saponins | ||||
| (8) 2α,3α,19α,23-tetrahydroxyurs-12,20(30)-dien-28-oic acid (1–50 µM) | Rats (PRP) | Inhibitory effects on ADP-induced platelet aggregation |  | [29] |
| (9) 2α,3α,19α-trihydroxyurs-12-en-28-oic acid 28-O-β-d-xylopyranosyl (1→2)-β-d-glucopyranoside (1–50 µM) | Rats (PRP) | Inhibitory effects on ADP-induced platelet aggregation | 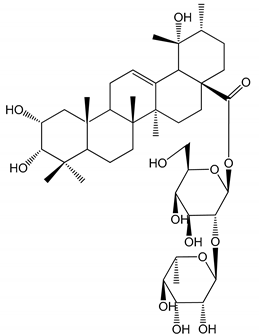 | [29] |
| (10) Chikusetsusaponin IVa (0–2000 µM) | Human in vitro studies (washed platelets) male Wistar rats—in vivo studies | Antithrombotic and antiplatelet activity | 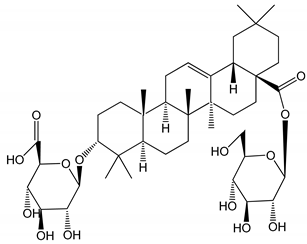 | [30] |
| (11) Dihydroginsenoside Rg3 (5–100 µM) | Male Sprague–Dawley rats (washed platelets) | Inhibition of platelet aggregation induced by collagen and thrombin | 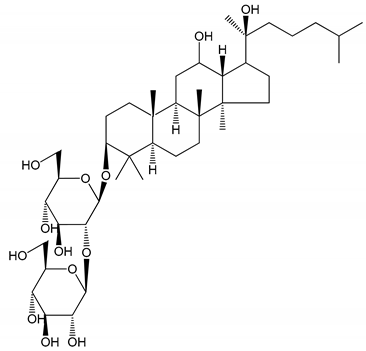 | [31] |
| (12) Ginsengoside-2A | Human in vitro studies (PRP and washed platelets) | Decrease of platelet maximum aggregation rate | 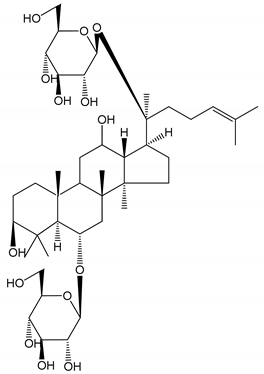 | [32] |
| (13) Ginsenoside 20(R)-Rg3 (2.5–10 µM) | Male ICR mice (PRP) | Inhibition of platelet aggregation induced by ADP, collagen, arachidonic acid and U46619 (mimic agent of TXA2) | 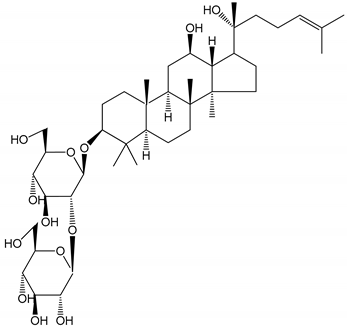 | [33] |
| (14) Ginsenoside 20(S)-Rg3 (2.5–10 µM) | Male ICR mice (PRP) | Inhibition of platelet aggregation induced by ADP, collagen, arachidonic acid and U46619 (mimic agent of TXA2) | 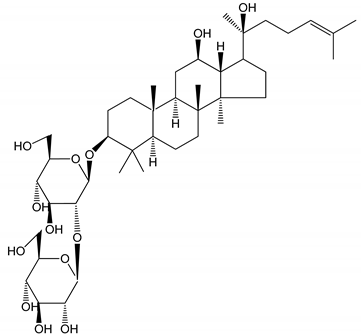 | [33] |
| (15) Ginsenoside R1 (10–100 µM) | New Zealand albino rabbits (PRP) | Inhibition of platelet aggregation induced by ADP, collagen, thrombin | 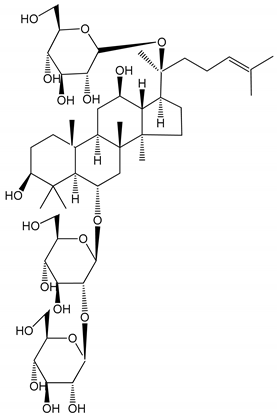 | [22] |
| (16) Ginsenoside Ra3 (100 mg/kg/day) | Male Sprague-Dawley rats (PRP) | Antithrombotic effect | 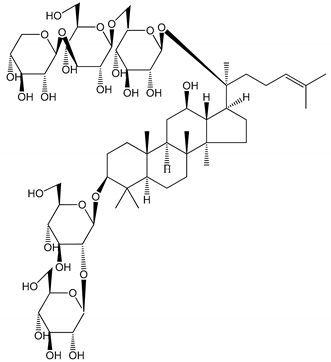 | [23] |
| (17) Ginsenoside Rb1 (100 mg/kg/day) | Male Sprague–Dawley rats (PRP) | Antithrombotic effect |  | [23] |
| (18) Ginsenoside Rb3 | Rabbit | Decrease of platelet maximum aggregation rate | 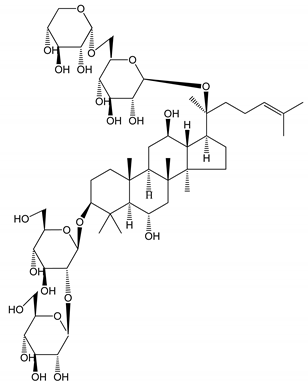 | [32] |
| (19) Ginsenoside Rd (100 mg/kg/day) | Male Sprague–Dawley rats (PRP) | Antithrombotic effect | 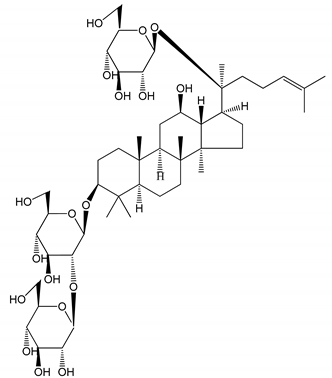 | [23] |
| (20) Ginsenoside Rg1 (10–100 µM) | New Zealand albino rabbits (PRP) Human in vitro plasma coagulation assays (PRP) | Inhibition of platelet aggregation induced by ADP, collagen, thrombin Anticoagulation activity |  | [22] [34] |
| (21) Ginsenoside Rg2 (0.05 mg/mL) | Human in vitro plasma coagulation assays (PRP) | Anticoagulation activity |  | [34] |
| (22) Ginsenoside Rg5 (2.5–10 µM) | Male ICR mice (PRP) | Inhibition of platelet aggregation induced by ADP, collagen, arachidonic acid and U46619 (mimic agent of TXA2) |  | [33] |
| (23) Ginsenoside Re (10–100 µM) | New Zealand albino rabbits (PRP) Male Sprague–Dawley rats (PRP) | Inhibition of platelet aggregation induced by ADP, collagen, thrombin Antithrombotic effect | 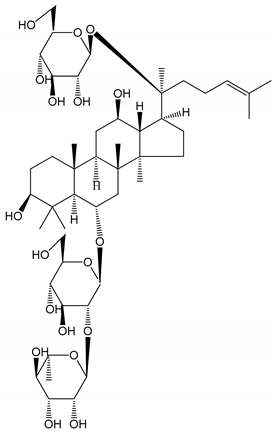 | [22] [23] |
| (24) Ginsenosides Rk1 (2.5–10 µM) | Male Sprague–Dawley rats (PRP) | Inhibition of platelet aggregation induced by ADP, collagen, arachidonic acid and U46619 (mimic agent of TXA2) | 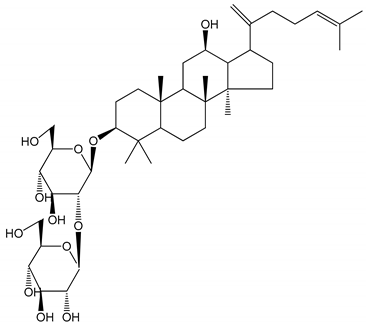 | [3,33] |
| (25) Ginsenoside Ro (50–300 µM) | Human in vitro studies (washed platelets) | Inhibition of platelet activation | 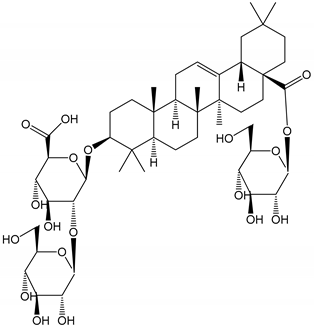 | [35] |
| (26) Ginsenoside Rp1 (2.5–100 µM) | Male Sprague–Dawley rats and male C57BL/6J mice (PRP) | Inhibition of platelet activation and thrombus formation | 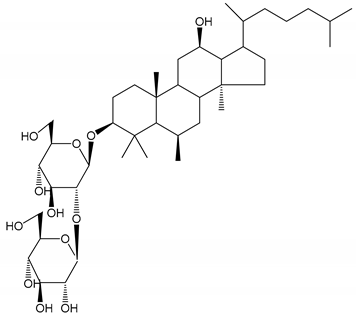 | [36] |
| (27) Ginsenoside Rp3 (1.56–50 µM) | Male Sprague–Dawley rats and C57BL/6J mice (washed platelets) | Inhibition of agonist-platelet aggregation and thrombus formation | 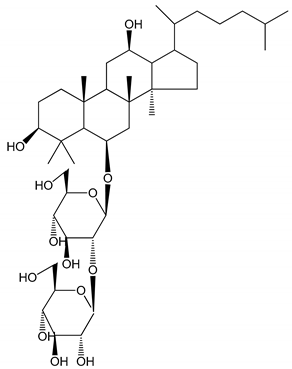 | [37] |
| (28) Ginsenoside Rp4 (6.25–50 µM) | Male Sprague–Dawley rats (PRP) | Inhibition of platelet aggregation induced by ADP | 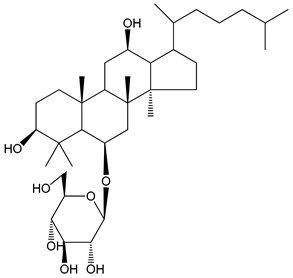 | [38] |
| (29–33) Glechomanosides A–E (20 µM) | Mice and rabbits (PRP) | Glechomanosides A–E antithrombotic activity Glechomanosides C and D anticoagulant effect | 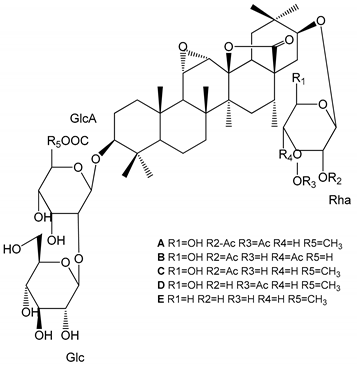 | [18] |
| (34) Ilexoside D (10 µM) | Male Sprague–Dawley rats (PRP) | in vivo and in vitro anticoagulant activity | 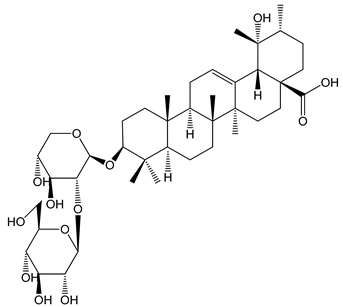 | [39] |
| (34–36) Ilexosides A, D and J (10 µM) | Male Sprague–Dawley rats (PRP) | Strong inhibitory activities on platelet aggregation induced by thrombin | 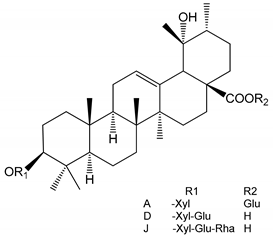 | [40] |
| (37) Notoginsenoside Fc (50–800 µM) | Male Sprague–Dawley rats and Kunming mice (washed platelets) | Inhibition of platelet aggregation induced by ADP, collagen, thrombin | 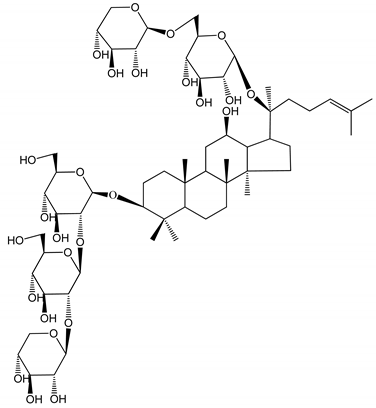 | [40] |
| (38) Notoginsenoside Ft1 (50–800 µM) | Male Sprague–Dawley rats and Kunming mice (washed platelets) | Induction of platelet shape change, but not aggregation; haemostatic activity; potentiation of platelet aggregation induced by thrombin |  | [40] |
| (39) Notoginsenoside R1 (100 mg/kg/day) | Male Sprague–Dawley rats (PRP) | Antithrombotic effect |  | [23] |
| (40–44) Sapinmusaponins F-J (1–100 µM) | Human in vitro studies (washed platelets) | Antiplatelet effect, includding anti-aggregatory properties | 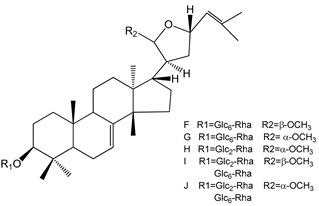 | [41] |
| (45,46) Sapinmusaponins Q and R (1–50 µM) | Human in vitro studies (washed platelets) | Antiplatelet effect (anti-aggregatory effect) potent than aspirin, IC50 ca. 3.4–13.5 mM and 30.5 mM, respectively | 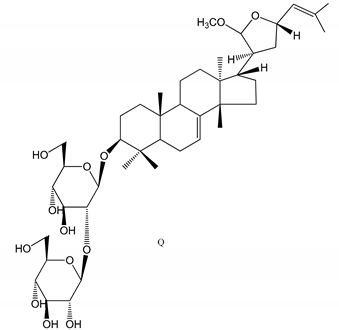 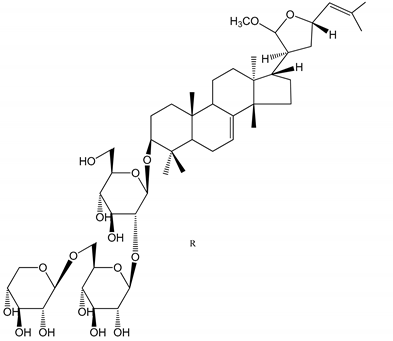 | [42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olas, B.; Urbańska, K.; Bryś, M. Saponins as Modulators of the Blood Coagulation System and Perspectives Regarding Their Use in the Prevention of Venous Thromboembolic Incidents. Molecules 2020, 25, 5171. https://doi.org/10.3390/molecules25215171
Olas B, Urbańska K, Bryś M. Saponins as Modulators of the Blood Coagulation System and Perspectives Regarding Their Use in the Prevention of Venous Thromboembolic Incidents. Molecules. 2020; 25(21):5171. https://doi.org/10.3390/molecules25215171
Chicago/Turabian StyleOlas, Beata, Karina Urbańska, and Magdalena Bryś. 2020. "Saponins as Modulators of the Blood Coagulation System and Perspectives Regarding Their Use in the Prevention of Venous Thromboembolic Incidents" Molecules 25, no. 21: 5171. https://doi.org/10.3390/molecules25215171
APA StyleOlas, B., Urbańska, K., & Bryś, M. (2020). Saponins as Modulators of the Blood Coagulation System and Perspectives Regarding Their Use in the Prevention of Venous Thromboembolic Incidents. Molecules, 25(21), 5171. https://doi.org/10.3390/molecules25215171





