The Flame Retardancy of Polyethylene Composites: From Fundamental Concepts to Nanocomposites
Abstract
1. Introduction
2. PE Grades and Properties
3. FR Approaches and Materials
3.1. Phosphorous and Melamine
3.2. Nitrogen
3.3. Inorganic Hydroxides
3.4. Boron
3.5. Silicon
4. The Role of Nanotechnology in Flame Retardancy of Polymer Nanocomposites
4.1. The Role of Nanomaterials in Improving Flame Retardancy of PE Systems
4.2. Incorporation Methods of Nanomaterials in Polymer Matrices
4.2.1. In Situ Polymerization
4.2.2. Solvent Casting
4.2.3. Melt Mixing
5. Summary and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Khanal, S.; Lu, Y.; Ahmed, S.; Ali, M.; Xu, S. Synergistic effect of zeolite 4A on thermal, mechanical and flame retardant properties of intumescent flame retardant HDPE composites. Polym. Test. 2020, 81, 106177. [Google Scholar] [CrossRef]
- Das, O.; Capezza, J.A.; Mårtensson, J.; Dong, Y.; Neisiany, E.R.; Pelcastre, L.; Jiang, L.; Xu, Q.; Olsson, T.R.; Hedenqvist, S.M. The Effect of Carbon Black on the Properties of Plasticised Wheat Gluten Biopolymer. Molecules 2020, 25, 2279. [Google Scholar] [CrossRef] [PubMed]
- Neisiany, R.E.; Enayati, M.S.; Kazemi-Beydokhti, A.; Das, O.; Ramakrishna, S. Multilayered Bio-Based Electrospun Membranes: A Potential Porous Media for Filtration Applications. Front. Mater. 2020, 7, 67. [Google Scholar] [CrossRef]
- Salasinska, K.; Mizera, K.; Celiński, M.; Kozikowski, P.; Borucka, M.; Gajek, A. Thermal properties and fire behavior of polyethylene with a mixture of copper phosphate and melamine phosphate as a novel flame retardant. Fire Saf. J. 2020, 115, 103137. [Google Scholar] [CrossRef]
- Das, O.; Neisiany, R.E.; Capezza, A.J.; Hedenqvist, M.S.; Försth, M.; Xu, Q.; Jiang, L.; Ji, D.; Ramakrishna, S. The need for fully bio-based facemasks to counter coronavirus outbreaks: A perspective. Sci. Total Environ. 2020, 736, 139611. [Google Scholar] [CrossRef] [PubMed]
- Majka, T.M.; Stachak, P. The studies on the production of polyethylene film with reduced flammability. In Proceedings of the 21st International Electronic Conference on Synthetic Organic Chemistry, Santiago, Spain, 1–30 November 2017. [Google Scholar]
- Babu, K.; Rendén, G.; Afriyie Mensah, R.; Kim, N.K.; Jiang, L.; Xu, Q.; Restás, Á.; Esmaeely Neisiany, R.; Hedenqvist, M.S.; Försth, M. A review on the flammability properties of carbon-based polymeric composites: State-of-the-art and future trends. Polymers 2020, 12, 1518. [Google Scholar] [CrossRef]
- Lai, X.; Tang, S.; Li, H.; Zeng, X. Flame-retardant mechanism of a novel polymeric intumescent flame retardant containing caged bicyclic phosphate for polypropylene. Polym. Degrad. Stab. 2015, 113, 22–31. [Google Scholar] [CrossRef]
- Wang, D.-Y.; Liu, Y.; Wang, Y.-Z.; Artiles, C.P.; Hull, T.R.; Price, D. Fire retardancy of a reactively extruded intumescent flame retardant polyethylene system enhanced by metal chelates. Polym. Degrad. Stab. 2007, 92, 1592–1598. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.-Y.; Wang, J.-S.; Song, Y.-P.; Wang, Y.-Z. A novel intumescent flame-retardant LDPE system and its thermo-oxidative degradation and flame-retardant mechanisms. Polym. Adv. Technol. 2008, 19, 1566–1575. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, B.; Wang, X.; Wang, G.; Ding, D. The flame retardancy and smoke suppression effect of a hybrid containing CuMoO4 modified reduced graphene oxide/layered double hydroxide on epoxy resin. J. Hazard. Mater. 2018, 343, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Majlingova, A.; Kačíková, D.; Xu, Q.; Jin, C. Current Trends in Flame-Retardant Treatment of Selected Polymers—A Review; Technical University in Zvolen: Zvolen, Slovak Republic, 2018. [Google Scholar]
- Lim, K.-S.; Bee, S.-T.; Sin, L.T.; Tee, T.-T.; Ratnam, C.T.; Hui, D.; Rahmat, A.R. A review of application of ammonium polyphosphate as intumescent flame retardant in thermoplastic composites. Compos. Part B Eng. 2016, 84, 155–174. [Google Scholar] [CrossRef]
- Gao, H.; Hu, S.; Han, H.; Zhang, J. Effect of different metallic hydroxides on flame-retardant properties of low density polyethylene/melamine polyphosphate/starch composites. J. Appl. Polym. Sci. 2011, 122, 3263–3269. [Google Scholar] [CrossRef]
- Camino, G.; Grassie, N.; McNeill, I.C. Influence of the fire retardant, ammonium polyphosphate, on the thermal degradation of poly(methyl methacrylate). J. Polym. Sci. Polym. Chem. Ed. 1978, 16, 95–106. [Google Scholar] [CrossRef]
- Dasari, A.; Yu, Z.-Z.; Cai, G.-P.; Mai, Y.-W. Recent developments in the fire retardancy of polymeric materials. Prog. Polym. Sci. 2013, 38, 1357–1387. [Google Scholar] [CrossRef]
- Giri, R.; Nayak, L.; Rahaman, M. Flame and fire retardancy of polymer-based composites. Mater. Res. Innov. 2020, 1–29. [Google Scholar] [CrossRef]
- Luyt, A.S.; Malik, S.S.; Gasmi, S.A.; Porfyris, A.; Andronopoulou, A.; Korres, D.; Vouyiouka, S.; Grosshauser, M.; Pfaendner, R.; Brüll, R. Halogen-free flame-retardant compounds. Thermal decomposition and flammability behavior for alternative polyethylene grades. Polymers 2019, 11, 1479. [Google Scholar] [CrossRef]
- Kurtz, S.M. UHMWPE Biomaterials Handbook: Ultra High Molecular Weight Polyethylene in Total Joint Replacement and Medical Devices; Academic Press: Oxford, UK, 2009. [Google Scholar]
- Hench, L.; Jones, J. Biomaterials, Artificial Organs and Tissue Engineering; Elsevier: Abington, PA, USA, 2005. [Google Scholar]
- Li, D.; Zhou, L.; Wang, X.; He, L.; Yang, X. Effect of Crystallinity of Polyethylene with Different Densities on Breakdown Strength and Conductance Property. Materials 2019, 12, 1746. [Google Scholar] [CrossRef]
- Wilkie, C.A.; Morgan, A.B. Fire Retardancy of Polymeric Materials; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Grand, A.F.; Wilkie, C.A. Fire Retardancy of Polymeric Materials; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Khanal, S.; Zhang, W.; Ahmed, S.; Ali, M.; Xu, S. Effects of intumescent flame retardant system consisting of tris (2-hydroxyethyl) isocyanurate and ammonium polyphosphate on the flame retardant properties of high-density polyethylene composites. Compos. Part A Appl. Sci. Manuf. 2018, 112, 444–451. [Google Scholar] [CrossRef]
- Ma, Z.-L.; Wang, X.-L.; Wei, H.-M.; Song, H.-Z. Flame retardation of dibromoneopentyl glycol on intumescent flame-retardant/low-density polyethylene composites. J. Appl. Polym. Sci. 2015, 132, 41244. [Google Scholar] [CrossRef]
- Yan, J.; Xu, M. Design, synthesis and application of a highly efficient mono-component intumescent flame retardant for non-charring polyethylene composites. Polym. Bull. 2020, 1–20. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.; Xiao, Z.; Yang, Z.; Zhu, H.; Ju, J.W.; Yan, Z. Development of a novel bio-inspired cement-based composite material to improve the fire resistance of engineering structures. Constr. Build. Mater. 2019, 225, 99–111. [Google Scholar] [CrossRef]
- Li, W.; Li, S.; Cheng, Z.; Hu, X.; Yang, W.; Yao, Y. The effect of flame retardant-modified sepiolite nanofibers on thermal degradation and fire retardancy of low-density polyethylene. J. Therm. Anal. Calorim. 2019, 138, 1011–1019. [Google Scholar] [CrossRef]
- Cavdar, A.D.; Torun, S.B.; Ertas, M.; Mengeloglu, F. Ammonium zeolite and ammonium phosphate applied as fire retardants for microcrystalline cellulose filled thermoplastic composites. Fire Saf. J. 2019, 107, 202–209. [Google Scholar] [CrossRef]
- Qin, R.; Zhang, X.; Kong, F.; Yang, J.; Nie, S. Investigation on novel flame retardant low-density polyethylene composites based on THEIC and MCAPP. J. Polym. Res. 2019, 26, 144. [Google Scholar] [CrossRef]
- Hong, H.; Liu, H.; Zhang, H.; He, H.; Liu, T.; Jia, D. Flame retarded polyethylene/wood flour composites with high performances: Satisfying both sides with intumescent flame retardants and synergistic compatibilizers, respectively. Polym. Compos. 2018, 39, 569–579. [Google Scholar] [CrossRef]
- Long, J.; Li, S.; Liang, B. Synthesis and properties of a new halogen-free flame retardant for polyethylene. Pigment Resin Technol. 2018, 47, 208–215. [Google Scholar] [CrossRef]
- Kruger, H.J.; Focke, W.W.; Mhike, W.; Taute, A.; Roberson, A. Thermal properties of polyethylene flame retarded with expandable graphite and intumescent fire retardant additives. Fire Mater. 2017, 41, 573–586. [Google Scholar] [CrossRef]
- Makhlouf, G.; Hassan, M.; Nour, M.; Abdelmonem, Y.; Abdelkhalik, A. A novel intumescent flame retardant: Synthesis and its application for linear low-density polyethylene. Arab. J. Sci. Eng. 2017, 42, 4339–4349. [Google Scholar] [CrossRef]
- Hassan, M.; Nour, M.; Abdelmonem, Y.; Makhlouf, G.; Abdelkhalik, A. Synergistic effect of chitosan-based flame retardant and modified clay on the flammability properties of LLDPE. Polym. Degrad. Stab. 2016, 133, 8–15. [Google Scholar] [CrossRef]
- Matar, M.; Azambre, B.; Cochez, M.; Vahabi, H.; Fradet, F. Influence of modified mesoporous silica SBA-15 on the flammability of intumescent high-density polyethylene. Polym. Adv. Technol. 2016, 27, 1363–1375. [Google Scholar] [CrossRef]
- Altun, Y.; Doğan, M.; Bayramlı, E. The effect of red phosphorus on the fire properties of intumescent pine wood flour—LDPE composites. Fire Mater. 2016, 40, 697–703. [Google Scholar] [CrossRef]
- Xu, C.; Jian, W.; Xing, C.; Zhou, H.; Zhao, Y.; Pan, H.; Xiong, X. Flame retardancy and mechanical properties of thermal plastic composite panels made from Tetra Pak waste and high-density polyethylene. Polym. Compos. 2016, 37, 1797–1804. [Google Scholar] [CrossRef]
- Li, X.; Yang, B. Synergistic effects of pentaerythritol phosphate nickel salt (PPNS) with ammonium polyphosphate in flame retardant of polyethylene. J. Therm. Anal. Calorim. 2015, 122, 359–368. [Google Scholar] [CrossRef]
- Katančić, Z.; Krehula, L.K.; Siročić, A.P.; Grozdanić, V.; Hrnjak-Murgić, Z. Effect of modified nanofillers on fire retarded high-density polyethylene/wood composites. J. Compos. Mater. 2013, 48, 3771–3783. [Google Scholar] [CrossRef]
- Kruger, H.J.; Focke, W.W.; Mhike, W.; Taute, A.; Roberson, A.; Ofosu, O. Cone calorimeter study of polyethylene flame retarded with expandable graphite and intumescent fire-retardant additives. J. Fire Sci. 2014, 32, 498–517. [Google Scholar] [CrossRef]
- Pan, M.; Mei, C.; Song, Y. A novel fire retardant affects fire performance and mechanical properties of wood flour-high density polyethylene composites. BioResources 2012, 7. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Ni, A.; Chen, H.; Shen, P.J.P. Synthesis of a novel phosphorous-nitrogen based charring agent and its application in flame-retardant HDPE/IFR composites. Polymers 2019, 11, 1062. [Google Scholar] [CrossRef]
- Makhlouf, G.; Hassan, M.; Nour, M.; Abdel-Monem, Y.K.; Abdelkhalik, A. Evaluation of fire performance of linear low-density polyethylene containing novel intumescent flame retardant. J. Therm. Anal. Calorim. 2017, 130, 1031–1041. [Google Scholar] [CrossRef]
- Sanchez-Olivares, G.; Sanchez-Solis, A.; Manero, O.; Pérez-Chávez, R.; Jaramillo, M.; Alongi, J.; Carosio, F.J.M. Improving Mechanical Properties and Reaction to Fire of EVA/LLDPE Blends for Cable Applications with Melamine Triazine and Bentonite Clay. Materials 2019, 12, 2393. [Google Scholar] [CrossRef] [PubMed]
- Altun, Y.; Doğan, M.; Bayramlı, E. Flammability and thermal degradation behavior of flame retardant treated wood flour containing intumescent LDPE composites. Eur. J. Wood Wood Prod. 2016, 74, 851–856. [Google Scholar] [CrossRef]
- Kausar, A.; Rafique, I.; Anwar, Z.; Muhammad, B. Recent developments in different types of flame retardants and effect on fire retardancy of epoxy composite. Polym. Technol. Eng. 2016, 55, 1512–1535. [Google Scholar] [CrossRef]
- Yang, Z.-Z.; Li, J.-Q.; Wang, Z.-Z.; Dong, D.-W.; Qi, X.-R.J.B. Tumor-targeting dual peptides-modified cationic liposomes for delivery of siRNA and docetaxel to gliomas. Biomaterials 2014, 35, 5226–5239. [Google Scholar] [CrossRef]
- Russell, L.; Marney, D.; Humphrey, D.; Hunt, A.; Dowling, V.; Cookson, L.J.A.F.; R, W.P.; Corporation, V.D. Combining Fire Retardant and Preservative Systems for Timber Products in Exposed Applications—State of the Art Review; Forest & Wood Products Australia: Melbourne, Australia, 2007. [Google Scholar]
- Levchik, S.; Balabanovich, A.; Levchik, G.; Costa, L.J.F. Effect of melamine and its salts on combustion and thermal decomposition of polyamide 6. Materials 1997, 21, 75–83. [Google Scholar] [CrossRef]
- Ray, S.S.; Kuruma, M. Halogen-Free Flame-Retardant Polymers: Next-generation Fillers for Polymer Nanocomposite Applications; Springer: Cham, Switzerland, 2020; Volume 294. [Google Scholar]
- Morgan, A.B.; Wilkie, C.A. The Non-Halogenated Flame Retardant Handbook; John Wiley & Sons Inc.: Hoboken, NJ, USA; Scrivener Publishing LLC.: Salem, MA, USA, 2014. [Google Scholar]
- Wilén, C.-E.; Pfaendner, R. Design and utilization of nitrogen containing flame retardants based on N-alkoxyamines, azoalkanes and related compounds. In Polymer Green Flame Retardants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 267–288. [Google Scholar]
- Cheng, Y.; Li, J.; He, Y.; Wang, B.; Liu, Y.; Wang, Q.J.P.E. Acidic buffer mechanism of cyclotriphosphazene and melamine cyanurate synergism system flame retardant epoxy resin. Science 2015, 55, 1046–1051. [Google Scholar] [CrossRef]
- Wan, L.; Deng, C.; Zhao, Z.-Y.; Chen, H.; Wang, Y.-Z.J.P. Flame Retardation of Natural Rubber: Strategy and Recent Progress. Polymers 2020, 12, 429. [Google Scholar] [CrossRef]
- Weil, E.D.; Choudhary, V. Flame-retarding plastics and elastomers with melamine. J. Fire Sci. 1995, 13, 104–126. [Google Scholar] [CrossRef]
- Costa, L.; Camino, G.; Luda di Cortemiglia, M.P. Mechanism of Thermal Degradation of Fire-Retardant Melamine Salts; ACS Publications: Washington, DC, USA, 1990. [Google Scholar]
- Liodakis, S.; Tsapara, V.; Agiovlasitis, I.; Vorisis, D. Thermal analysis of Pinus sylvestris L. wood samples treated with a new gel–mineral mixture of short-and long-term fire retardants. Thermochim. Acta 2013, 568, 156–160. [Google Scholar] [CrossRef]
- Lewin, M.; Weil, E.D. Mechanisms and modes of action in flame retardancy of polymers. Fire Retard. Mater. 2001, 1, 31–68. [Google Scholar]
- Beyer, G. Nanocomposites—A new concept for flame retardant polymers. Polym. News (USA) 2001, 26, 370–378. [Google Scholar]
- Weil, E.; Lewin, M.; Rao, D. A search for an interactive flame retardant system for ethylene–vinyl acetate. In Proceedings of the 15th Conference on Recent Advances in Flame Retardancy of Polymeric Materials, Stamford, CT, USA, 20–22 May 1996. [Google Scholar]
- Mouritz, A.P.; Gibson, A.G. Fire Properties of Polymer Composite Materials; Springer Science & Business Media: Dordrecht, The Netherlands, 2007; Volume 143. [Google Scholar]
- Nalawade, P.; Aware, B.; Kadam, V.J.; Hirlekar, R.S. Layered double hydroxides: A review. J. Sci. Ind. Res. 2009, 68, 267–272. [Google Scholar]
- Babu, H.V.; Coluccini, C.; Wang, D.Y. 8—Functional layered double hydroxides and their use in fire-retardant polymeric materials. In Novel Fire Retardant Polymers and Composite Materials; Wang, D.-Y., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 201–238. [Google Scholar] [CrossRef]
- Bravo-Suárez, J.J.; Páez-Mozo, E.A.; Oyama, S.T. Review of the synthesis of layered double hydroxides: A thermodynamic approach. Química Nova 2004, 27, 601–614. [Google Scholar] [CrossRef]
- Arslan, F.; Dilsiz, N. Flame resistant properties of LDPE/PLA blends containing halogen-free flame retardant. J. Appl. Polym. Sci. 2020, 137, 48960. [Google Scholar] [CrossRef]
- Cabrera-Álvarez, E.N.; Ramos-deValle, L.F.; Sánchez-Valdes, S.; Candia-García, A.; Soriano-Corral, F.; Ramírez-Vargas, E.; Ibarra-Alonso, M.C.; Patiño-Soto, P. Study of the silane modification of magnesium hydroxide and their effects on the flame retardant and tensile properties of high density polyethylene nanocomposites. Polym. Compos. 2014, 35, 1060–1069. [Google Scholar] [CrossRef]
- Beltrán-Ramírez, F.I.; Ramos-deValle, L.F.; Ramírez-Vargas, E.; Sánchez-Valdes, S.; Espinoza-Martínez, A.B.; Martínez-Colunga, J.G.; Rodríguez-Fernandez, O.S.; Cabrera-Alvarez, E.N.; López-Quintanilla, M.L. Effect of Nanometric Metallic Hydroxides on the Flame Retardant Properties of HDPE Composites. J. Nanomater. 2014, 2014, 969184. [Google Scholar] [CrossRef]
- Jaerger, S.; Wypych, F. Thermal and flammability properties influenced by Zn/Al, Co/Al, and Ni/Al layered double hydroxide in low-density polyethylene nanocomposites. J. Appl. Polym. Sci. 2020, 137, 48737. [Google Scholar] [CrossRef]
- Sánchez-Valdes, S.; Ramírez-Vargas, E.; Rodriguez-Gonzalez, J.A.; Uribe-Calderón, J.A.; Ramos de-Valle, L.F.; Zuluaga-Parra, J.D.; Martínez-Colunga, J.G.; Solís-Rosales, S.G.; Sánchez-Martínez, A.C.; Flores-Flores, R. Organopalygorskite and Molybdenum Sulfide Combinations to Produce Mechanical and Processing Enhanced Flame-Retardant PE/EVA Blend Composites with Low Magnesium Hydroxide Loading. J. Vinyl Addit. Technol. 2020. [Google Scholar] [CrossRef]
- Zhou, R.; Ming, Z.; He, J.; Ding, Y.; Jiang, J. Effect of Magnesium Hydroxide and Aluminum Hydroxide on the Thermal Stability, Latent Heat and Flammability Properties of Paraffin/HDPE Phase Change Blends. Polymers 2020, 12, 180. [Google Scholar] [CrossRef]
- Wang, C.; Liu, J.; Wang, Y.; Han, Z. Enhanced flame retardance in polyethylene/magnesium hydroxide/polycarbosilane blends. Mater. Chem. Phys. 2020, 253, 123373. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, C.; Liu, X.; Chen, X.; Xiang, Y.; Shao, H. Flammability and mechanical properties of EVA/LDPE blended with MHSH whiskers and ATH. Mater. Res. Express 2019, 6, 095319. [Google Scholar] [CrossRef]
- Albite-Ortega, J.; Sánchez-Valdes, S.; Ramirez-Vargas, E.; Nuñez-Figueredo, Y.; deValle, L.R.; Martínez-Colunga, J.; Graciano-Verdugo, A.; Sanchez-Martínez, Z.; Espinoza-Martínez, A.; Rodriguez-Gonzalez, J. Influence of keratin and DNA coating on fire retardant magnesium hydroxide dispersion and flammability characteristics of PE/EVA blends. Polym. Degrad. Stab. 2019, 165, 1–11. [Google Scholar] [CrossRef]
- Afzal, A.; Usama, M.; Rashid, I.A.; Khalid, Z.; Mohsin, M.; Shakir, M.F.; Tariq, A. Effect of MgOH/TiO2 on flame retardancy and mechanical behavior of composite. Mater. Res. Express 2020, 6, 125352. [Google Scholar] [CrossRef]
- Dorigato, A.; Fredi, G.; Fambri, L.; Lopez-Cuesta, J.-M.; Pegoretti, A. Polyethylene-based single polymer laminates: Synergistic effects of nanosilica and metal hydroxides. J. Reinf. Plast. Compos. 2019, 38, 62–73. [Google Scholar] [CrossRef]
- Savas, L.A.; Arslan, C.; Hacioglu, F.; Dogan, M. Effect of reactive and nonreactive surface modifications and compatibilizer use on mechanical and flame-retardant properties of linear low-density polyethylene filled with huntite and hydromagnesite mineral. J. Therm. Anal. Calorim. 2018, 134, 1657–1666. [Google Scholar] [CrossRef]
- Ameen Khan, M.; Sailaja, R. Nanocomposites of HDPE/LDPE/Nylon 6 reinforced with MWCNT, Kenaf fiber, nano Mg(OH)2, and PEPA with enhanced mechanical, thermal, and flammability characteristics. Polym. Compos. 2018, 39, E1474–E1486. [Google Scholar] [CrossRef]
- Zhang, J.; Mei, C.; Huang, R.; Xu, X.; Lee, S.; Kim, B.J.; Wu, Q. Comparative mechanical, fire-retarding, and morphological properties of high-density polyethylene/(wood flour) composites with different flame retardants. J. Vinyl Addit. Technol. 2018, 24, 3–12. [Google Scholar] [CrossRef]
- Laoutid, F.; Lorgouilloux, M.; Bonnaud, L.; Lesueur, D.; Dubois, P. Fire retardant behaviour of halogen-free calcium-based hydrated minerals. Polym. Degrad. Stab. 2017, 136, 89–97. [Google Scholar] [CrossRef]
- Scarfato, P.; Incarnato, L.; Di Maio, L.; Dittrich, B.; Schartel, B. Influence of a novel organo-silylated clay on the morphology, thermal and burning behavior of low density polyethylene composites. Compos. Part B Eng. 2016, 98, 444–452. [Google Scholar] [CrossRef]
- Pawelec, W.; Tirri, T.; Aubert, M.; Häggblom, E.; Lehikoinen, T.; Skåtar, R.; Pfaendner, R.; Wilén, C.-E. Toward halogen-free flame resistant polyethylene extrusion coated paper facings. Prog. Org. Coat. 2015, 78, 67–72. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhou, Z.; Yu, F.; Ji, J. Synthesis and Flame Retardant Properties of Low Density Polyethylene/Ethylene-Vinyl Acetate/Polyphosphazene Derivative Composites. Asian J. Chem. 2015, 27, 919–924. [Google Scholar] [CrossRef]
- Zhou, S.; Ning, M.; Wang, X.; Yan, Z.; Guo, D.; He, Q.; Zhang, Y.; She, S.; Hu, Y. The influence of γ-irradiation on the mechanical, thermal degradation, and flame retardant properties of EVA/LDPE/ATH blends. J. Therm. Anal. Calorim. 2015, 119, 167–173. [Google Scholar] [CrossRef]
- Liu, S.-P. Flame retardant and mechanical properties of polyethylene/magnesium hydroxide/montmorillonite nanocomposites. J. Ind. Eng. Chem. 2014, 20, 2401–2408. [Google Scholar] [CrossRef]
- Laoutid, F.; Lorgouilloux, M.; Lesueur, D.; Bonnaud, L.; Dubois, P. Calcium-based hydrated minerals: Promising halogen-free flame retardant and fire resistant additives for polyethylene and ethylene vinyl acetate copolymers. Polym. Degrad. Stab. 2013, 98, 1617–1625. [Google Scholar] [CrossRef]
- Sanchez-Olivares, G.; Sanchez-Solis, A.; Calderas, F.; Medina-Torres, L.; Herrera-Valencia, E.E.; Castro-Aranda, J.I.; Manero, O.; Di Blasio, A.; Alongi, J. Flame retardant high density polyethylene optimized by on-line ultrasound extrusion. Polym. Degrad. Stab. 2013, 98, 2153–2160. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y. Effect of ethylene-acrylic acid copolymer on flame retardancy and properties of LLDPE/EAA/MH composites. Polym. Degrad. Stab. 2011, 96, 2215–2220. [Google Scholar] [CrossRef]
- Bellayer, S.P.; Tavard, E.; Duquesne, S.; Piechaczyk, A.; Bourbigot, S. Natural mineral fire retardant fillers for polyethylene. Fire Mater. 2011, 35, 183–192. [Google Scholar] [CrossRef]
- Popescu, C.-M.; Pfriem, A. Treatments and modification to improve the reaction to fire of wood and wood based products—An overview. Fire Mater. 2020, 44, 100–111. [Google Scholar] [CrossRef]
- Levchik, S.V. Introduction to flame retardancy and polymer flammability. In Flame Retardant Polymer Nanocomposites; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 1–29. [Google Scholar] [CrossRef]
- Li, S.; Long, B.; Wang, Z.; Tian, Y.; Zheng, Y.; Zhang, Q. Synthesis of hydrophobic zinc borate nanoflakes and its effect on flame retardant properties of polyethylene. J. Solid State Chem. 2010, 183, 957–962. [Google Scholar] [CrossRef]
- Wu, Z.; Hu, Y.; Shu, W. Effect of ultrafine zinc borate on the smoke suppression and toxicity reduction of a low-density polyethylene/intumescent flame-retardant system. J. Appl. Polym. Sci. 2010, 117, 443–449. [Google Scholar] [CrossRef]
- Schubert, D.M. Hydrated Zinc Borates and Their Industrial Use. Molecules 2019, 24, 2419. [Google Scholar] [CrossRef]
- Yalinkilic, M.K.; Su, W.-Y.; Imamyra, Y.; Takahashi, M.; Demirci, Z.; Yallnkilic, A. Boron effect on decay resistance of some fire-retardant coatings applied on plywood surface. Holz als Roh-und Werkstoff 1998, 56, 347–353. [Google Scholar] [CrossRef]
- Pang, X.-Y.; Ming-Wei, D.; ZHI-XIAO, Z.; Yu, T. An intumescent flame retardant-expandable graphite: Preparation, characteristics and flame retardance for polyethylene. Kuwait J. Sci. 2015, 42, 133–149. [Google Scholar]
- Ciesielski, M.; Burk, B.; Heinzmann, C.; Döring, M. Fire-retardant high-performance epoxy-based materials. In Novel Fire Retardant Polymers and Composite Materials; Woodhead Publishing Elsevier Witney: Oxford, UK, 2017; pp. 3–51. [Google Scholar]
- Xue, E.; Zeng, M. Flame Retardant Science and Application; National Defense Industrial Press: Beijing, China, 1988. [Google Scholar]
- Youming, Y.; Xichang, S.; Ruirong, Z. Flame Retardancy Behavior of Zinc Borate. J. Fire Sci. 1999, 17, 355–361. [Google Scholar] [CrossRef]
- Nyambo, C.; Kandare, E.; Wilkie, C. Thermal stability and flammability characteristics of ethylene vinyl acetate (EVA) composites blended with a phenyl phosphonate-intercalated layered double hydroxide (LDH), melamine polyphosphate and/or boric acid. Polym. Degrad. Stab. 2009, 94, 513–520. [Google Scholar] [CrossRef]
- Demirel, M.; Pamuk, V.; Dilsiz, N. Investigation of flame retardancy and physical–mechanical properties of zinc borate/boric acid polyester composites. J. Appl. Polym. Sci. 2010, 115, 2550–2555. [Google Scholar] [CrossRef]
- Sultygova, Z.K.; Kitieva, L.; Borukaev, T.A. Using Zinc Borate as Effective Flame Retardant. Proc. Key Eng. Mater. 2019, 816, 129–133. [Google Scholar] [CrossRef]
- Abdulrahman, S.T.; Ahmad, Z.; Thomas, S.; Maria, H.J.; Rahman, A. Viscoelastic and thermal properties of natural rubber low-density polyethylene composites with boric acid and borax. J. Appl. Polym. Sci. 2020, 137, 49372. [Google Scholar] [CrossRef]
- Ai, L.; Yang, L.; Hu, J.; Chen, S.; Zeng, J.; Liu, P. Synergistic Flame Retardant Effect of Organic Phosphorus–Nitrogen and Inorganic Boron Flame Retardant on Polyethylene. Polym. Eng. Sci. 2020, 60, 414–422. [Google Scholar] [CrossRef]
- Guo, Z.; Zhao, L.; Fang, Z. The flame retardant and smoke suppression effect of fullerene by trapping radicals in decabromodiphenyl oxide/Sb2O3 flame-retarded high density polyethylene. Fire Mater. 2017, 41, 916–924. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Q.; Li, G.; Li, M.-C.; Sun, X.; Ring, D. Synergistic influence of halogenated flame retardants and nanoclay on flame performance of high density polyethylene and wood flour composites. RSC Adv. 2017, 7, 24895–24902. [Google Scholar] [CrossRef]
- Wu, G.-F.; Xu, M. Effects of Boron Compounds on the Mechanical and Fire Properties of Wood-chitosan and High-density Polyethylene Composites. BioResources 2014, 9, 4173–4193. [Google Scholar] [CrossRef]
- Sun, Z.; Ma, Y.; Xu, Y.; Chen, X.; Chen, M.; Yu, J.; Hu, S.; Zhang, Z. Effect of the particle size of expandable graphite on the thermal stability, flammability, and mechanical properties of high-density polyethylene/ethylene vinyl-acetate/expandable graphite composites. Polym. Eng. Sci. 2014, 54, 1162–1169. [Google Scholar] [CrossRef]
- Alwaan, I.M.; Hassan, A. Effects of zinc borate loading on thermal stability, flammability, crystallization properties of magnesium oxide/(90/10) mLLDPE/(NR/ENR-50) blends. Iran. Polym. J. 2014, 23, 277–287. [Google Scholar] [CrossRef]
- Nour, M.; Elmorsy, S.; Saltout, R.; El-Gendy, A.A. Fire Behavior of HDPE Composite Based on Modified Clay with Phenol Formaldehyde Silane Resin. Arab. J. Sci. Eng. 2017, 42, 153–162. [Google Scholar] [CrossRef]
- Fredi, G.; De Col, A.; Dorigato, A.; Lopez-Cuesta, J.-M.; Fambri, L.; Pegoretti, A. Combined effect of fumed silica and metal hydroxides as fire retardants in PE single-polymer composites. AIP Conf. Proc. 2018, 1981, 020021. [Google Scholar]
- Praveen, T.; Sundara Rajan, J.; Sailaja, R. Evaluation of thermal and flame properties of HDPE-MWCNT-SiO2 nanocomposites. Compos. Interfaces 2017, 24, 215–232. [Google Scholar] [CrossRef]
- Feng, C.; Liang, M.; Jiang, J.; Huang, J.; Liu, H. Flame retardancy and thermal degradation behavior of efficient intumescent flame retardant LDPE composite containing 4A zeotile. J. Anal. Appl. Pyrolysis 2016, 118, 9–19. [Google Scholar] [CrossRef]
- Kratofil Krehula, L.; Katančić, Z.; Marić, G.; Hrnjak-Murgić, Z. Study of Fire Retardancy and Thermal and Mechanical Properties of HDPE-Wood Composites. J. Wood Chem. Technol. 2015, 35, 412–423. [Google Scholar] [CrossRef]
- Kaynak, C.; Ibibikcan, E. Contribution of nanoclays to the flame retardancy of polyethylene-based cable insulation materials with aluminum hydroxide and zinc borate. J. Fire Sci. 2013, 32, 121–144. [Google Scholar] [CrossRef]
- Deng, C.; Zhao, J.; Deng, C.-L.; Lv, Q.; Chen, L.; Wang, Y.-Z. Effect of two types of iron MMTs on the flame retardation of LDPE composite. Polym. Degrad. Stab. 2014, 103, 1–10. [Google Scholar] [CrossRef]
- Pan, M.; Mei, C.; Li, G.; Du, J. Ammonium polyphosphate improving physicochemical properties of rice straw-high density polyethylene composites. Trans. Chin. Soc. Agric. Eng. 2014, 30, 328–333. [Google Scholar]
- Chen, S.; Sun, B.; Huang, G.; Guo, H.; Wang, S. Effects of an (Intumescent flame retardant)-montmorillonite combination on the thermal stability and fire-retardant properties of LDPE/EVA nanocomposites. J. Vinyl Addit. Technol. 2013, 19, 285–292. [Google Scholar] [CrossRef]
- Mingzhu, P.; Hailan, L.; Changtong, M. Flammability of nano silicon dioxide–wood fiber–polyethylene composites. J. Compos. Mater. 2012, 47, 1471–1477. [Google Scholar] [CrossRef]
- Liany, Y.; Tabei, A.; Farsi, M.; Madanipour, M. Effect of nanoclay and magnesium hydroxide on some properties of HDPE/wheat straw composites. Fibers Polym. 2013, 14, 304–310. [Google Scholar] [CrossRef]
- Karlsson, L.; Lundgren, A.; Jungqvist, J.; Hjertberg, T.J.F. Effect of nanofillers on the flame retardant properties of a polyethylene–calcium carbonate–silicone elastomer system. Fire Mater. 2011, 35, 443–452. [Google Scholar] [CrossRef]
- Huang, G.; Zhu, B.; Shi, H. Combination effect of organics-modified montmorillonite with intumescent flame retardants on thermal stability and fire behavior of polyethylene nanocomposites. J. Appl. Polym. Sci. 2011, 121, 1285–1291. [Google Scholar] [CrossRef]
- Sittisart, P.; Farid, M.M. Fire retardants for phase change materials. Appl. Energy 2011, 88, 3140–3145. [Google Scholar] [CrossRef]
- Zielecka, M.; Rabajczyk, A.; Pastuszka, Ł.; Jurecki, L.J.C. Flame Resistant Silicone-Containing Coating Materials. Coatings 2020, 10, 479. [Google Scholar] [CrossRef]
- Alongi, J.; Colleoni, C.; Rosace, G.; Malucelli, G. Phosphorus-and nitrogen-doped silica coatings for enhancing the flame retardancy of cotton: Synergisms or additive effects? Polym. Degrad. Stab. 2013, 98, 579–589. [Google Scholar] [CrossRef]
- Serra, A.; Ramis, X.; Fernández-Francos, X. Epoxy Sol-Gel Hybrid Thermosets. Coatings 2016, 6, 8. [Google Scholar] [CrossRef]
- Jana, R.; Mukunda, P.; Nando, G. Thermogravimetric analysis of compatibilized blends of low density polyethylene and poly (dimethyl siloxane) rubber. Polym. Degrad. Stab. 2003, 80, 75–82. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Vatani, A.; Mohammadi, T. Synergistic interactions between POSS and fumed silica and their effect on the properties of crosslinked PDMS nanocomposite membranes. RSC Adv. 2015, 5, 82460–82470. [Google Scholar] [CrossRef]
- Zhang, W.; Camino, G.; Yang, R. Polymer/polyhedral oligomeric silsesquioxane (POSS) nanocomposites: An overview of fire retardance. Prog. Polym. Sci. 2017, 67, 77–125. [Google Scholar] [CrossRef]
- Waddon, A.; Zheng, L.; Farris, R.; Coughlin, E.B. Nanostructured polyethylene-POSS copolymers: Control of crystallization and aggregation. Nano Lett. 2002, 2, 1149–1155. [Google Scholar] [CrossRef]
- Sun, D.; Yang, C.; Qi, X.; Yang, J.; Wang, Y. Largely enhanced fracture toughness of the PP/EPDM blends induced by adding carbon nanofibers. Compos. Sci. Technol. 2018, 164, 146–152. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Esmaeely Neisiany, R.; Nouri Khorasani, S.; Dinari, M.; Ataei, S.; Koochaki, M.S.; Ramakrishna, S. Development of an epoxy self-healing coating through the incorporation of acrylic acid-co-acrylamide copolymeric gel. Prog. Org. Coat. 2020, 149, 105948. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Du, F.; Douglas, J.F.; Winey, K.I.; Harris, R.H.; Shields, J.R. Nanoparticle networks reduce the flammability of polymer nanocomposites. Nat. Mater. 2005, 4, 928–933. [Google Scholar] [CrossRef]
- Peeterbroeck, S.; Laoutid, F.; Taulemesse, J.M.; Monteverde, F.; Lopez-Cuesta, J.M.; Nagy, J.B.; Alexandre, M.; Dubois, P. Mechanical properties and flame-retardant behavior of ethylene vinyl acetate/high-density polyethylene coated carbon nanotube nanocomposites. Adv. Funct. Mater. 2007, 17, 2787–2791. [Google Scholar] [CrossRef]
- Cai, G.; Dasari, A.; Yu, Z.-Z.; Du, X.; Dai, S.; Mai, Y.-W.; Wang, J. Fire response of polyamide 6 with layered and fibrillar nanofillers. Polym. Degrad. Stab. 2010, 95, 845–851. [Google Scholar] [CrossRef]
- Dasari, A.; Yu, Z.-Z.; Mai, Y.-W.; Cai, G.; Song, H. Roles of graphite oxide, clay and POSS during the combustion of polyamide 6. Polymer 2009, 50, 1577–1587. [Google Scholar] [CrossRef]
- Liu, S.; Fang, Z.; Yan, H.; Chevali, V.S.; Wang, H. Synergistic flame retardancy effect of graphene nanosheets and traditional retardants on epoxy resin. Compos. Part A Appl. Sci. Manuf. 2016, 89, 26–32. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Williams, G.R.; O’Hare, D.; Wang, Q. Layered double hydroxide-oxidized carbon nanotube hybrids as highly efficient flame retardant nanofillers for polypropylene. Sci. Rep. 2016, 6, 35502. [Google Scholar] [CrossRef]
- Huang, G.; Chen, S.; Song, P.; Lu, P.; Wu, C.; Liang, H. Combination effects of graphene and layered double hydroxides on intumescent flame-retardant poly (methyl methacrylate) nanocomposites. Appl. Clay Sci. 2014, 88, 78–85. [Google Scholar] [CrossRef]
- Szustakiewicz, K.; Cichy, B.; Gazińska, M.; Pigłowski, J. Comparative study on flame, thermal, and mechanical properties of HDPE/clay nanocomposites with MPP or APP. J. Reinf. Plast. Compos. 2013, 32, 1005–1017. [Google Scholar] [CrossRef]
- Chuang, T.-H.; Guo, W.; Cheng, K.-C.; Chen, S.-W.; Wang, H.-T.; Yen, Y.-Y. Thermal properties and flammability of ethylene-vinyl acetate copolymer/montmorillonite/polyethylene nanocomposites with flame retardants. J. Polym. Res. 2004, 11, 169–174. [Google Scholar] [CrossRef]
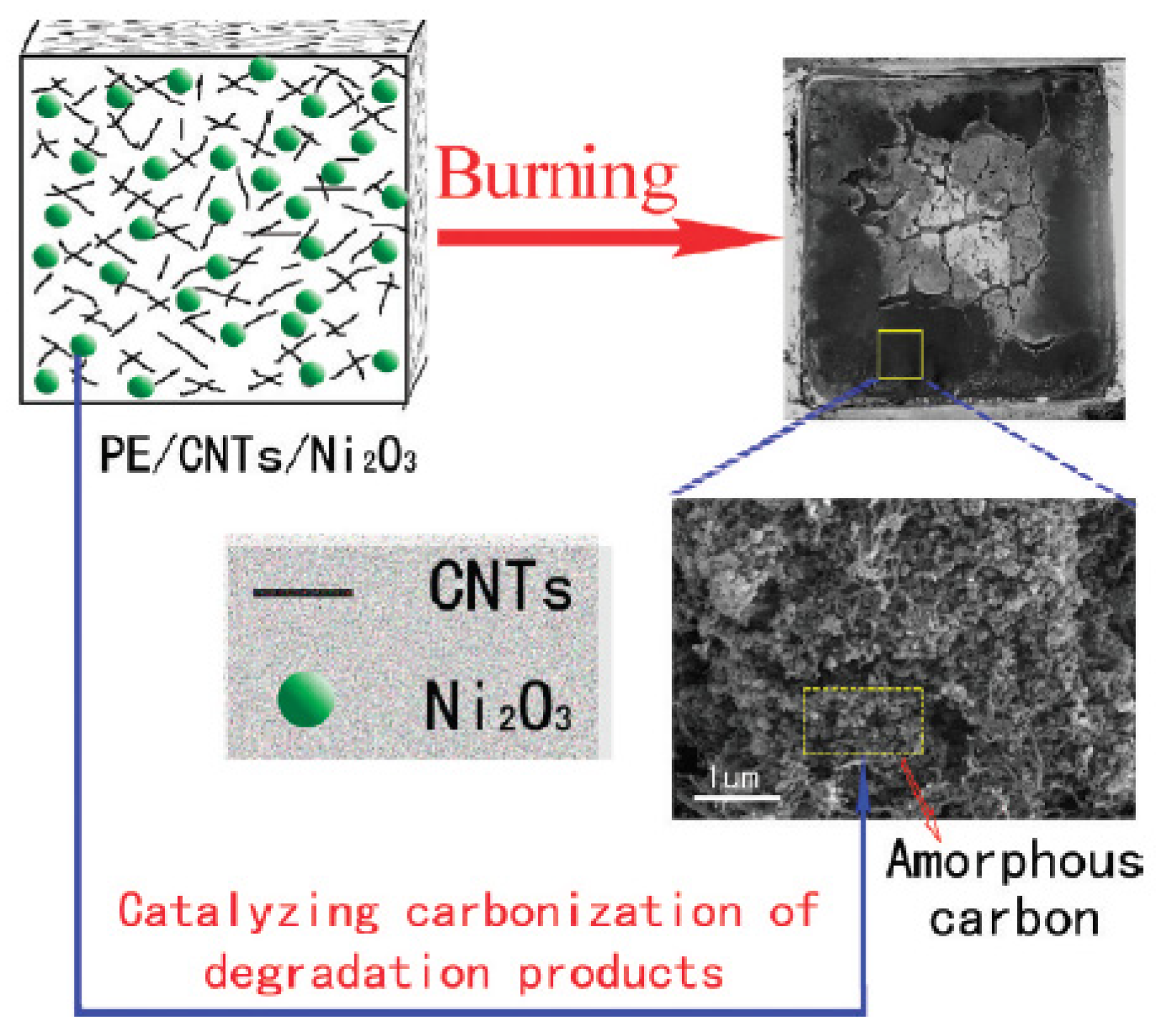
- Yu, H.; Liu, J.; Wang, Z.; Jiang, Z.; Tang, T. Combination of carbon nanotubes with Ni2O3 for simultaneously improving the flame retardancy and mechanical properties of polyethylene. J. Phys. Chem. C 2009, 113, 13092–13097. [Google Scholar] [CrossRef]
- Han, Z.; Wang, Y.; Dong, W.; Wang, P. Enhanced fire retardancy of polyethylene/alumina trihydrate composites by graphene nanoplatelets. Mater. Lett. 2014, 128, 275–278. [Google Scholar] [CrossRef]
- Ran, S.; Guo, Z.; Chen, C.; Zhao, L.; Fang, Z. Carbon nanotube bridged cerium phenylphosphonate hybrids, fabrication and their effects on the thermal stability and flame retardancy of the HDPE/BFR composite. J. Mater. Chem. A 2014, 2, 2999–3007. [Google Scholar] [CrossRef]
- Pan, M.; Mei, C.; Du, J.; Li, G. Synergistic effect of nano silicon dioxide and ammonium polyphosphate on flame retardancy of wood fiber–polyethylene composites. Compos. Part A Appl. Sci. Manuf. 2014, 66, 128–134. [Google Scholar] [CrossRef]
- Lu, H.; Hu, Y.; Xiao, J.; Wang, Z.; Chen, Z.; Fan, W. Magnesium hydroxide sulfate hydrate whisker flame retardant polyethylene/montmorillonite nanocomposites. J. Mater. Sci. 2006, 41, 363–367. [Google Scholar] [CrossRef]
- Zhao, J.; Deng, C.L.; Du, S.L.; Chen, L.; Deng, C.; Wang, Y.Z. Synergistic flame-retardant effect of halloysite nanotubes on intumescent flame retardant in LDPE. J. Appl. Polym. Sci. 2014, 131, 40065. [Google Scholar] [CrossRef]
- Ran, S.; Chen, C.; Guo, Z.; Fang, Z. Char barrier effect of graphene nanoplatelets on the flame retardancy and thermal stability of high-density polyethylene flame-retarded by brominated polystyrene. J. Appl. Polym. Sci. 2014, 131, 40520. [Google Scholar] [CrossRef]
- Visakh, P.; Yoshihiko, A. Flame Retardants: Polymer Blends, Composites and Nanocomposites; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Ajayan, P.M.; Schadler, L.S.; Braun, P.V. Nanocomposite Science and Technology; John Wiley & Sons: Weinheim, Germany, 2006. [Google Scholar]
- Aslzadeh, M.M.; Mir Mohamad Sadeghi, G.; Abdouss, M. Synthesis and characterization of chlorine-containing flame-retardant polyurethane nanocomposites via in situ polymerization. J. Appl. Polym. Sci. 2012, 123, 437–447. [Google Scholar] [CrossRef]
- Chung, D.D. Composite Materials: Science and Applications; Springer: London, UK, 2010. [Google Scholar]
- Kuila, B.K.; Nandi, A.K. Physical, mechanical, and conductivity properties of poly (3-hexylthiophene)–montmorillonite clay nanocomposites produced by the solvent casting method. Macromolecules 2004, 37, 8577–8584. [Google Scholar] [CrossRef]
- Wang, Q.; Dai, J.; Li, W.; Wei, Z.; Jiang, J. The effects of CNT alignment on electrical conductivity and mechanical properties of SWNT/epoxy nanocomposites. Compos. Sci. Technol. 2008, 68, 1644–1648. [Google Scholar] [CrossRef]
- Utracki, L.A.; Wilkie, C.A. Polymer Blends Handbook; Springer: Dordrecht, The Netherlands, 2002; Volume 1. [Google Scholar]
- Tadmor, Z.; Gogos, C.G. Principles of Polymer Processing; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Markarian, J. Flame retardants for polyamides-new developments and processing concerns. Plast. Addit. Compd. 2005, 7, 22–25. [Google Scholar] [CrossRef]
- Ghomi, E.R.; Khorasani, S.N.; Kichi, M.K.; Dinari, M.; Ataei, S.; Enayati, M.H.; Koochaki, M.S.; Neisiany, R.E. Synthesis and characterization of TiO2/acrylic acid-co-2-acrylamido-2-methyl propane sulfonic acid nanogel composite and investigation its self-healing performance in the epoxy coatings. Colloid Polym. Sci. 2020, 298, 213–223. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Terentjev, E.M. Dispersion of carbon nanotubes: Mixing, sonication, stabilization, and composite properties. Polymers 2012, 4, 275–295. [Google Scholar] [CrossRef]
- Kasaliwal, G.; Villmow, T.; Pegel, S.; Pötschke, P. Influence of material and processing parameters on carbon nanotube dispersion in polymer melts. In Polymer–Carbon Nanotube Composites; Elsevier: Amsterdam, The Netherlands, 2011; pp. 92–132. [Google Scholar]


| PE Grades | Structure’s Description | Density (g/cm−3) | Crystallinity | LOI (%) | Thermal Conductivity (W/m·K) | Melt Temperature (°C) | Mw (g/mol) |
|---|---|---|---|---|---|---|---|
| LDPE (Low-density PE) | Branched structure containing long and short chains | 0.915–0.932 | Lower degree of crystallinity | 17–18 | 0.32–0.35 | 105–115 | <50,000 |
| LLDPE (Linear low-density PE) | Branched structure containing short chains | 0.910–0.930 | Slightly higher than LDPE | 17–18 | 0.35–0.45 | 120–130 | <50,000 |
| HDPE (High-density PE) | Linear structure | 0.940–0.970 | Higher degree of crystallinity | 17–18 | 0.45–0.5 | 128–136 | Up to 200,000 |
| Polymer Matrix | Additive(s) | Mechanism(s) | Result(s) | Ref. |
|---|---|---|---|---|
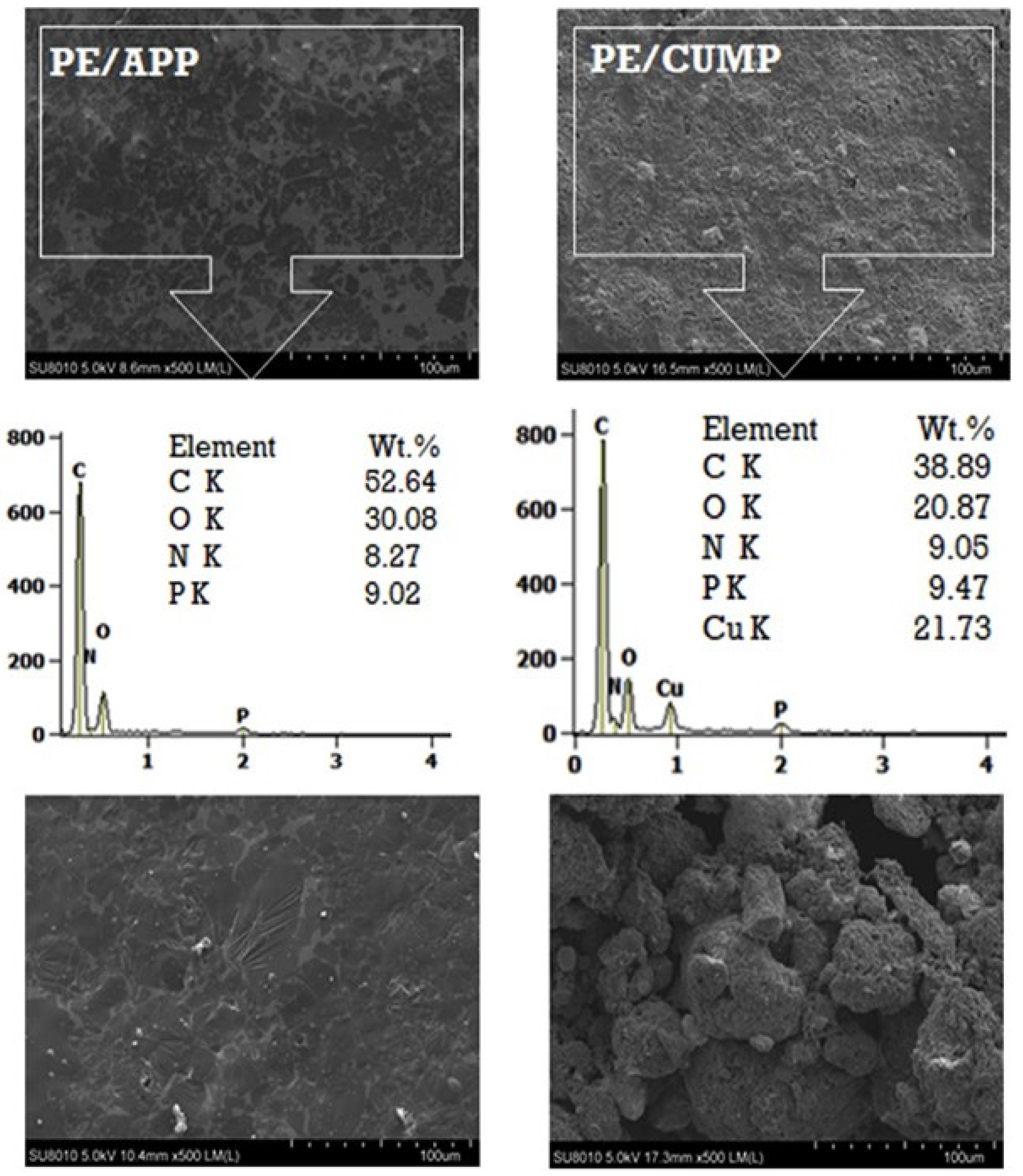
| HDPE | CUMP | Char formation, Emission of non-flammable gases | -Reduction of decomposition rate -Increasing the yield of residue -Reduction of heat release rate (HRR) -Increasing the time of burning | [4] |
| HDPE | APP THEIC | Char forming | -HDPE/APP/THEIC showed the heist LOI value -IFR incorporation significantly declined the PHR rate of HDPE | [24] |
| LDPE | Carbonization agent, APP, MP | Char formation, Physical barrier creation able to deactivate the oxidation-active centers of carbon | -Optimal flame retardancy belongs to carbonization agent/APP/MP with the weight ratio of 7:7:1 -Increasing the maximum temperature of the decomposition peak of LDPE | [10] |
| LDPE | APP, Pentaerythritol (PER), Salt of MP, Dibromoneop-enty Glycol (DBNPG) | Char forming, Thermal barrier | -Improving the char layer -Thermal barrier behavior enhancement -Increase in melt viscosity with proper amount of DBNPG | [25] |
| PE | Poly (piperazine methylphosphonic acid pentaerythritol ester) | Char formation, Exert condensed phase | -Improvement the residual mass and thermal stability -Reduction in HRR, THR -UL-94 V0 rating | [26] |
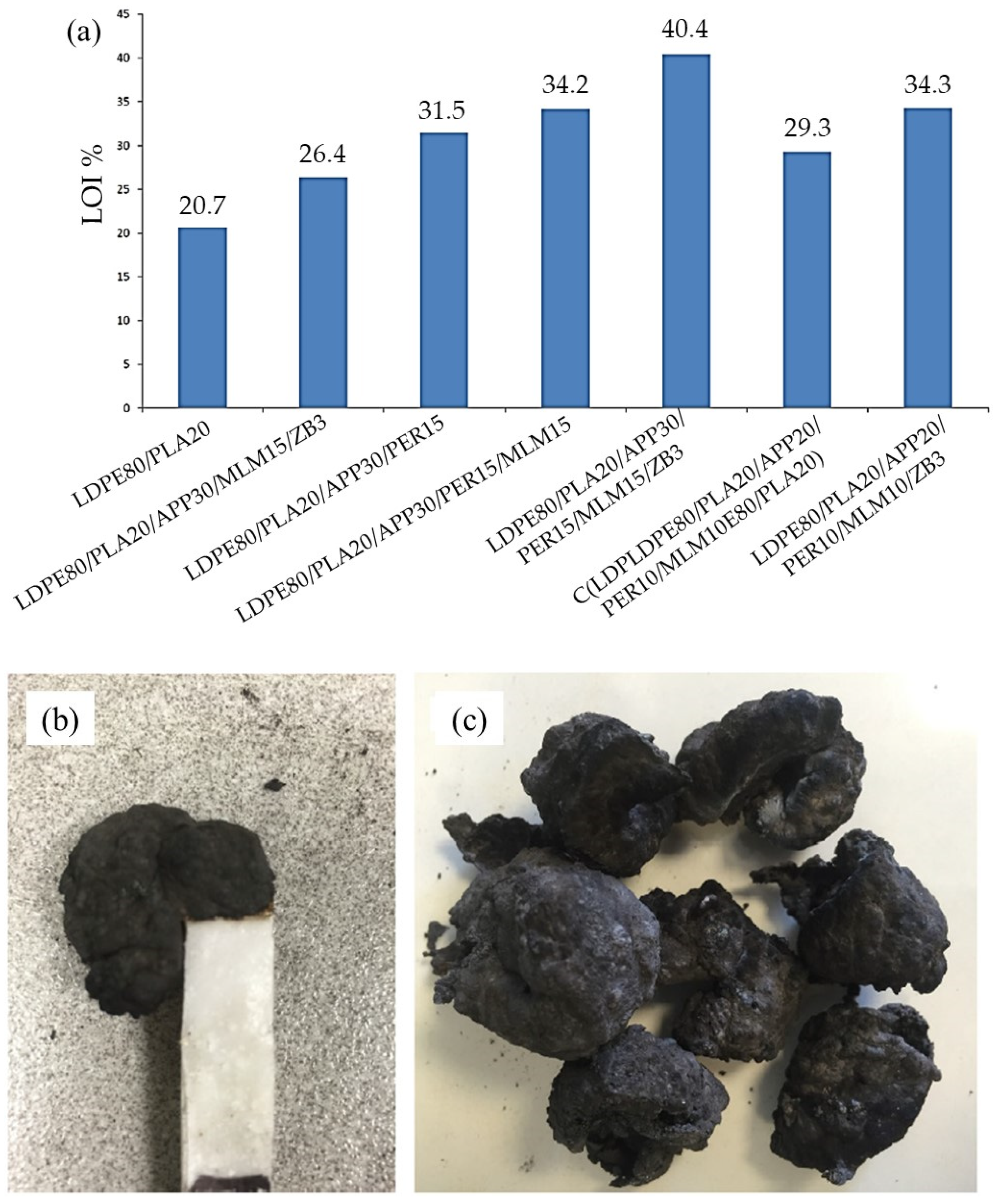
| LDPE | APP, PER, MLM | Char formation | -Increasing in thermal stability | [27] |
| LDPE | PSPHD-SEP | Vapor phase radical-trapping effect | -Reduction in PHR rate, THR -Improving the thermal stability -Increasing the LOI value (21%) compared to neat LDPE -UL-94 V-2 rating | [28] |
| HDPE | 10 wt.% of mono ammonium phosphate (MAP), ammonium zeolite (AZ), and microcrystalline cellulose (MCC) | Char forming | -Slowing down the burning rates of HDPE/MAP10 and HDPE/MCC/MAP5/AZ5 composite by 64% and 62%, respectively -Improving the LOI level and char forming by incorporating FRs | [29] |
| LDPE | THEIC, microencapsulated ammonium polyphosphate (MCAPP) | Formation of a compact char | In the composite with MCAPP/THEIC (2:1): -Achieving V-0 rating of UL-94 -Reduction in PHR rate and THR by 74.8% and 71.9%, respectively compared to pure LDPE -Enhancing thermal stability at high temperature | [30] |
| PE/Wood Flour (WF) | APP | Performance of WF as the charring agent with incorporation IFRs | -Achieving V-0 rating of UL-94 -Positive effect of IFRs and WF to control the fire spreading and the risk of combustion -Reduction in PHR rate of the composites containing IFRs and compatibilizers | [31] |
| PE | Phenyl phosphinic arid di-4-[1-(4-pheny phodphonic acid monophenyl ester-yl)-methyl-ethyl] phenyester dimelaminium (PDEPDM) | Char forming | In composite containing 32 wt.% PDEPDM: -Achieve in V-0 rating of UL-94 -Improvement in LOI, formation of char residue -Increase in char yield from 0.08 wt.% for neat PE to 5.17 wt.% for composite containing 40 wt.% PDEPDM at 800 °C -Reduction in tensile and impact strength | [32] |
| LDPE | Expandable graphite (EG), Ethylenediamine phosphate (EDAP), 3,5-diaminobenzoic acid phosphate (DABAP) | Releasing CO2 gas acting as an effective charring effect | -Substantial reductions in PHR rate for all flame-retarded samples -Decreasing the mass loss rate by adding intumescent additives | [33] |
| LLDPE | MLM salt of pentaerythritol phosphate montmorillonite (MPPM) | thermally stable char forming | -Enhancing the char formation and the thermal stability of LLDPE at high temperatures -Substantial reduction in PHR rate, THR, mean mass loss rate, and fire growth rate index -Achieving V-0 rating in UL-94V test | [34] |
| LLDPE | MLM salt of chitosan phosphate (MCHP), Organically modified montmorillonite (OMMT) | -Char forming | -Increasing the char residue -Improving the thermal stability -Reduction of PHR rate, total heat release (THR), CO, CO2 emissions and fire growth index (FGI) | [35] |
| HDPE | APP, PER, modified porous mesostructured silica (SBA-15) | Intumescent char layer formation | -Better flammability characteristics at low SBA-15 loadings (<2 wt.%) -Enhancement in fire properties affected by formation of crystalline silicone phosphate barrier | [36] |
| LDPE | RP, APP | Intumescent char layer formation | -Increase in LOI value from 17.5 to 24.2 by addition of 30wt% APP -The highest LOI value of 27.2 and UL-94 rating of V0 at ratio of 5:1 (APP:RP) -Increase in the gas phase action by the addition of RP | [37] |
| HDPE | APP, MLM | Intumescent char layer formation | -Improvement in the composite’s tensile strength and combustion process by FR loading’s increase -Improving the thermal stability and char formation’s promotion by FRs | [38] |
| PE | Pentaerythritol phosphate nickel salt (PPNS), APP | Intumescent char layer formation | - LOI value increased from 30% to 34% -Reduction in total HRR by 46.3% and 51.9% -Reduction in average mass loss rate by 40.6 and 87.5% | [39] |
| HDPE/WF | APP, Aluminum trihydroxide (ATH), SiO2, CaCO3 | Char forming | -Increase in both mechanical and fire properties by using nanofiller additive -Combination of APP and SiO2 showed the highest LOI value, and the lowest HRR | [40] |
| PE | DABAP, EDAP, EG | Char forming | -Higher decomposition temperature was attributed to DABAP -The best char yields was belonged to PE/DABAP -PE/EDAP/EG showed the best flame retardancy behavior | [41] |
| HDPE/WF | APP | Char forming | -APP decreased HRR and total smoke values of system -The heat of ignition remained constant -Maximum reduction of HRR obtained by increasing the amount of APP to 4 wt.% | [42] |
| HDPE | Phosphorous–nitrogen-based charring agent (PDTBP), APP | Intumescent char layer formation | -UL-94 V-0 rating -Low migration percentage (2.2%) -Decrease in PHR rate, THR, and fire hazard value -High tensile and flexural strength | [43] |
| LLDPE | MLM salt of montmorillonite phosphate (MMP), zinc borate (ZB) | Char layer formation | -Increasing in thermal stability and char formation -Reduction in PHR rate, mean HRR, THR, and mean mass loss rate -Reduction in the fire risk -UL-94 V-0 rating for the composite with 30 wt.% MMP and 5 wt.% ZB -Highest char residue formation for the composite with 32 wt.% MMP and 3 wt.% ZB -Max. fire performance index (142%) for the system with only MMP (40 wt.%) | [44] |
| Ethylene-vinyl acetate (EVA)/LLDPE | MLM, TRZ, and Bentonite Clay | Strengthening the protective char barrier produced by ATH | E-PE/120ATH in comparison with the conventional E-PE/185ATH achieved: -Self-extinguishing behavior (UL-94 V-0 rating) -Reduction in the stiffness and improvement in elongation at break, Composites with TRZ and clay showed 23% reduction in PHR rate and 11% in smoke production | [45] |
| LDPE/WF | APP, WF | Char forming | Increasing the LOI value from 17.5 to 24.2 with addition of 30 wt.% m-APP 25% reduction in THE in the LDPE/WF/APP. | [46] |
| Polymer Matrix | Additive(s) | Mechanism(s) | Result(s) | Ref. |
|---|---|---|---|---|
| HDPE | MH, Modified MH | Char forming with both MH and modified MH | -The flame sustainability of HDPE/modified MH was higher than HDPE/MH -The flame retardancy behavior of HDPE/modified MH did not enhance compared to HDPE/MH | [67] |
| HDPE | ATH, MH | Endothermic decomposition reaction and heat absorption | -The HDPE/ATH/MH system demonstrated the lowest value of PHR rate -The lowest amount of THR belonged to HDPE/ATH/MH system -Combination of ATH and MH indicated the significant non-flammability behavior | [68] |
| LDPE | Zn/Al, Ni/Al, Co/Al | Char forming | -The incorporation of Zn/Al-LDH and Ni/Al-LDH with LDPE showed more decrease of flammability compared to Co/Al-LDH incorporation -The composite containing LDH alternated with organic onions indicated more flammability reduction in comparison with composite containing inorganic anions | [69] |
| LDPE | ATH, MH, Ferric oxyhydroxide (FH) | Char forming, Restriction of oxygen diffusion | -The higher LOI value of composites containing ATH compared to composites containing MH and FH -LDPE/MPP/Starch (ST)/ATH system indicated the more protective charred layer with smaller pores on it compared to other systems | [14] |
| LDPE/EVA | Organopalygorskite (OPGS), Molybdenum sulfide (MoS2), MH | Char formation | -Increasing the LOI value (26%) -Reduction in the burning rate (66%) and PHR rate (83%) compared neat polymer blend -Indicative the UL-94 V-0 rating | [70] |
| Paraffin/HDPE | MH, ATH, EG | Char formation, physical barrier | -Increasing in thermal stability and carbonization ability -Increasing the amount of char residue -Reduction in the THR and PHR rate | [71] |
| PE/PCS | MH | Exert condensed phase, barrier effects of char formation | -Improving thermal stability -Forming the multi-layered char structure | [72] |
| EVA/LDPE | ATH, Magnesium hydroxide sulfate hydrate (MHSH) | Char forming | -Improving thermal stability -Reduction in thermal degradation rate in the temperature ranges of 410 °C∼510 °C -Indicative V-0 in UL-94 test | [73] |
| LDPE/EVA | MH, Keratin fibers (KF), deoxyribose nucleic acid (DNA) | Char forming | -Increasing the LOI up to 24.5% -Reducing the HRR by 82% compared to PE/EVA sample with 55 wt.% MH | [74] |
| LDPE/EVA | MH, TiO2 | Char forming | -Reaching to V-0 with LOI value of 24.9% -Increasing mass residue from 5 wt.% for blend to 25 wt.% for the composite containing both FRs -Increasing tensile strength and modulus of LDPE/EVA blend from 6.4 MPa to 7.1 MPa and 127 MPa to 133 MPa respectively by incorporation of both FRs -Improving impact strength from 27.8 to 35.2 KJ·mm−2 | [75] |
| LLDPE | MH, SiO2 | Char forming | -Improving thermal degradation resistance and the LOI value -Reduction in PHR rate and THR | [76] |
| LLDPE | Huntite and hydromagnesite (HH) | Char forming | -Increase in value of LOI and elastic modulus -Reduction in the horizontal burning rate, tensile strength, and elongation at break | [77] |
| HDPE/LDPE/Nylon 6 | MH, MWCNT, Kenaf fiber | Char forming | -Increasing the tensile strength value by 50% at 0.5/0.5 wt.% loading of Mg(OH)2/MWCNT compared to composite without filler -Reduction in PHR rate with addition of Mg(OH)2/MWCNT | [78] |
| HDPE/WF | MH, 1,2-bis(pentabromophenyl) ethane, Aluminum hydroxide | Char forming | -Significant decrease in the HRR and THR -Best fire resistance for composite containing 1,2-bis(pentabromophenyl) ethane | [79] |
| MDPE | MH, Calcium-based hydrated minerals | Formation of cohesive CaCO3 combustion residue | -Reduction in PHR rate for Ca-based composites -Generation of an intumescent mineral residue during the combustion by calcium hydroxide | [80] |
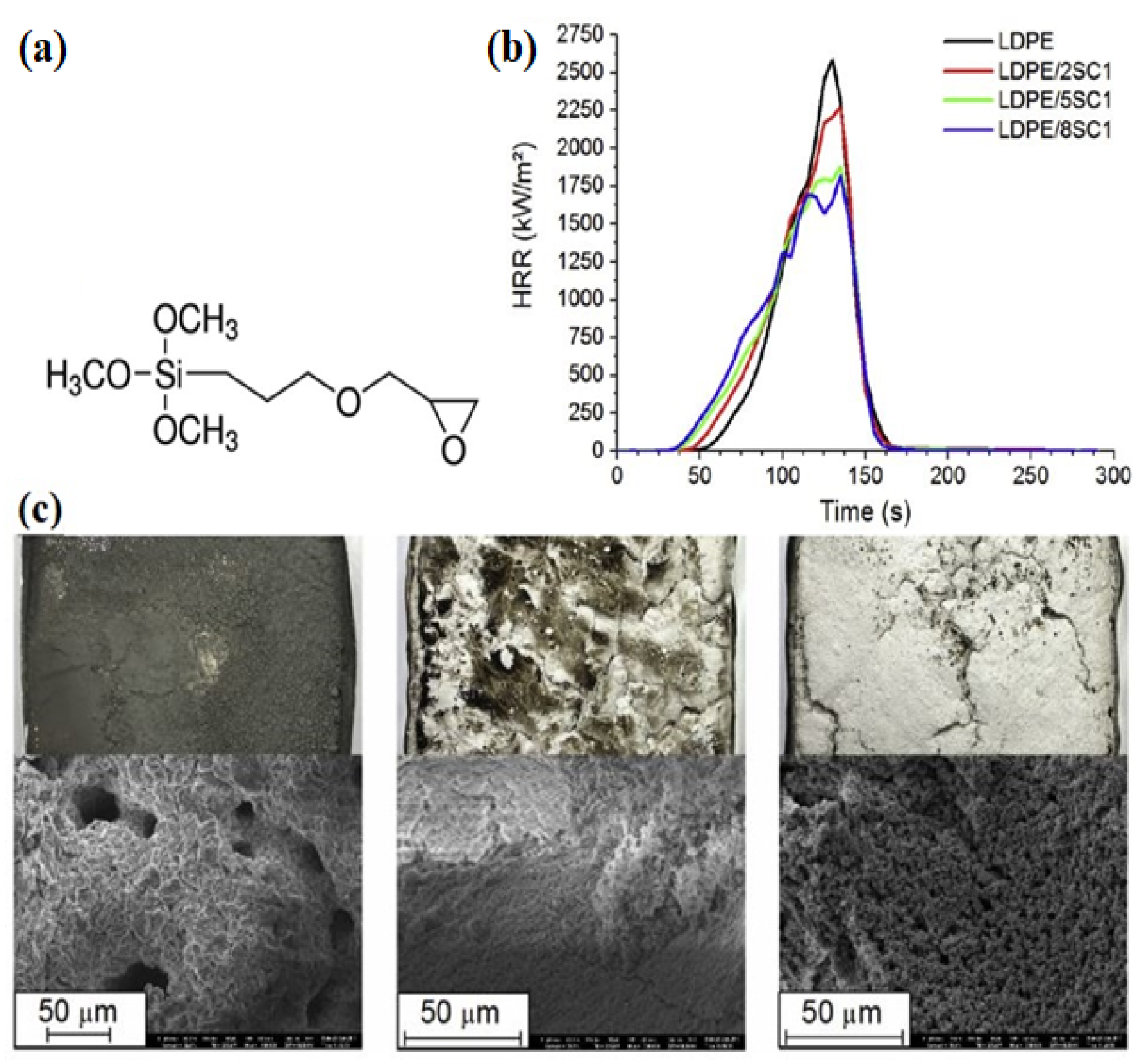
| LDPE | MH, Montmorillonite (MMT) | Char forming | -Higher interlayer spacing is observed for organosilylated clay (SC1) compared to original MMT -Improving thermal stability compared to commercial organoclays | [81] |
| LDPE | Azocyclohexane (AZO), Bis(cyclohexylazocyclohexylmethane) (BISAZO), FlameStab® NOR116, Magnesium dihydroxide (MDH), Luvogard MB81/PE | Intumescent char layer formation | -Better performance in flame retardancy when using AZO and BISAZO compared to the other additives | [82] |
| LDPE/EVA | Hexaphenoxylcyclotriphosphazene, Mg(OH)2, Al(OH)3 | Char forming | -Blends showed better flame retardancy when composited with Mg(OH)2 and Al(OH)3 -The maximum specific optical density is reduced from 370.65 to 91.72 -An increase in the residual volume and compactness of solid residue surface layer based on SEM morphology is observed | [83] |
| LDPE | ATH, EVA | Char forming | -Flame resistance of EVA/LDPE/ATH blends is slightly enhanced after γ-irradiation -Increase in the cross-linking density caused an enhancement in electrical and thermal properties -γ-irradiation delayed the thermal degradation process of EVA/LDPE/ATH blends | [84] |
| LDPE/Cross-linked polyethylene (XLPE) | MMT, MH, LDPE-g-MA | Char forming | -The increase in the tensile and impacts strengths induced by the addition of clay and LDPE-g-MA -Thermal stability at high temperatures is enhanced due to the increase in char residual of nanocomposites -XLPE nanocomposites showed efficient level of flame retardancy | [85] |
| MDPE/EVA | MDH, Hydrated lime, Hydrated dolomitic limes | Intumescent char layer formation | -Ca-based MDPE composites depicted similar rates of PHR with MDH composite -Lower PHR rate observed for Ca-based fillers in EVA compositions -The formation of an intumescent cohesive residue in the combustion process is induced by an effective role of calcium di-hydroxide | [86] |
| HDPE | ATH, ZB | Char forming | -2 phr organo-clay additive is used to achieve V0 rating -FR materials with high processability and mechanical properties is obtained when using HDPE rendering | [87] |
| LLDPE/Ethylene-acrylic acid (EAA) | MH | Char forming | -Addition of EAA improved LOI value of LLDPE/EAA/MH from 28% to 30% -Reduction of HRR and SPR values was occurred because of the acceptable dispersion of MH -Improvement of thermal oxidative stability of LLDPE/EAA/MH due to the EAA presence | [88] |
| LLDPE | CaCO3, MgCO3, Talc | Intumescent char layer formation | -HRR peaks were considerably reduced with incorporation of all mineral fillers -Improvement of nanoparticle dispersion in LLDPE by stearic acid | [89] |
| Polymer Matrix | Additive(s) | Mechanism(s) | Result(s) | Ref. |
|---|---|---|---|---|
| PE | ZB, Phosphorus–Nitrogen (DOPO-N) | Exert condensed phase and gas phase | For the PE/20%ZB/10%DOPO-N composite: -Increasing in thermal stability -Reduction in PHR, THR, average heat combustion, and FGI | [104] |
| HDPE | Fullerene (C60), Decabromodiphenyl oxide/Sb2O3 (brominated FRs) | trapping radical ability in condensed phase and gaseous phase by C60 and BFR, respectively | -Improving the thermal and thermo-oxidative stability of HDPE/BFR blends by adding C60 -A remarkable reduction in PHR rate especially at higher concentration of C60 | [105] |
| HDPE/WF | 1,2-bis(pentabromophenyl) and ethylene bis(tetrabromophthalimide), and nanoclay, MAPE as compatibilizer | Trapping the free radical produced from WF by Bromine radicals Char forming by WF and nanoclay | -Decreasing the composite strength by adding FRs -Synergistic effect in 1,2-bis(pentabromophenyl)-clay-MAPE system by reducing PHR rate and increasing thermal stability | [106] |
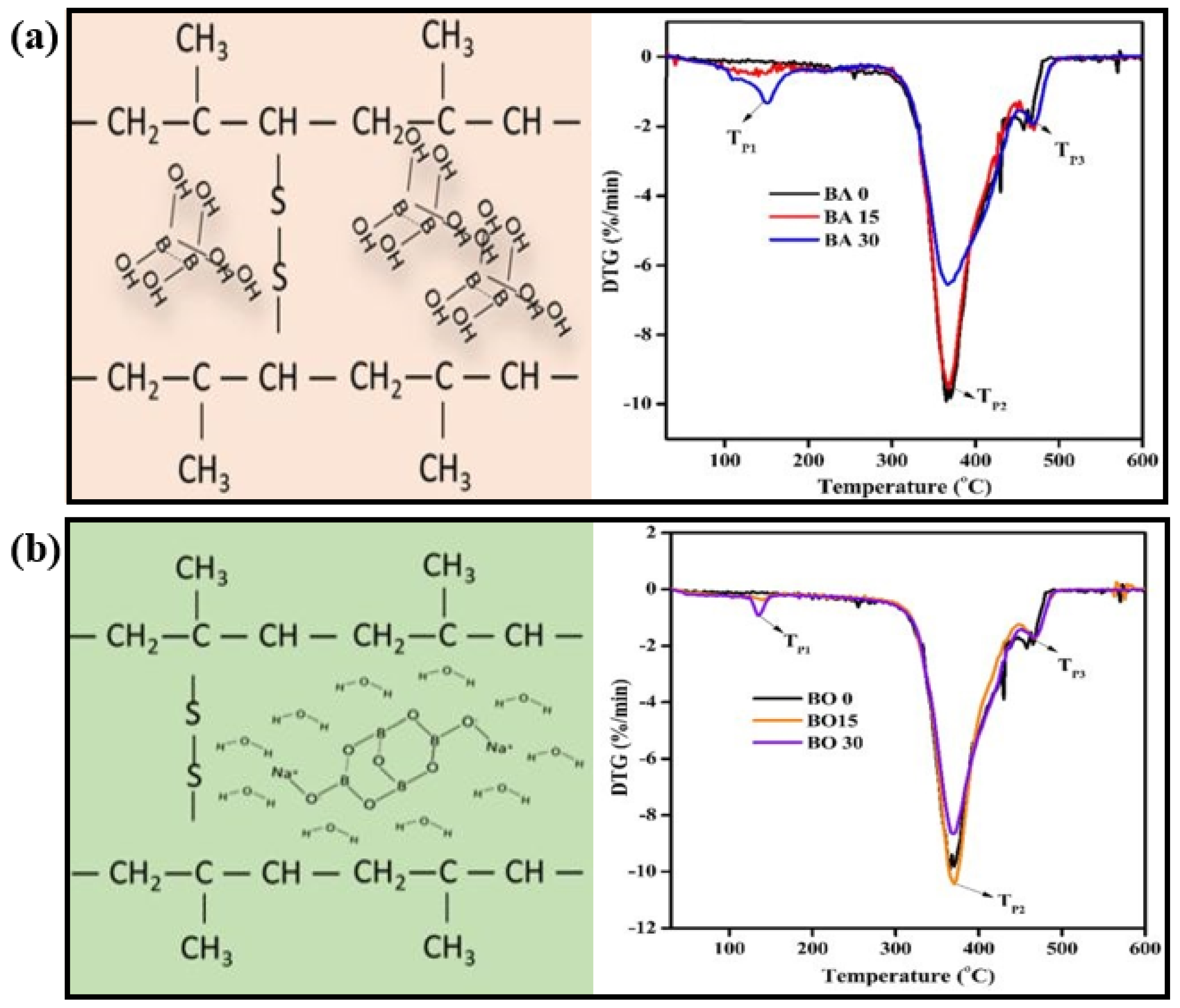
| HDPE | WF, BA, borax (BX) | Char forming | -CCT showed that the addition of BA/BX improved the fire performance of the samples -Increasing the ratio of BA/BX has a negative effect on ignition time, HRR, smoke production rate, and specific extinction area | [107] |
| HDPE/EVA | Two different particle sizes of EG | Char forming | -According to TGA and CCT tests, thermal stability and fire resistance of HDPE/EVA blend considerably increased due to the existence of EG -EG incorporation decreased the mechanical properties | [108] |
| mLLDPE/(NR/ENR-50) | ZB | Char formation | -Improvement in crystallinity of all the blends due to ZB presence and the best crystallinity was obtained at 6 phr ZB blend -Increasing the thermal stability of NR because of ZB incorporation -The best thermal stability was achieved at 8 phr ZB blend -Incorporation of ZB enhanced the LOI value of mLLDPE | [109] |
| HDPE | Modified Clay | Decomposition of fillers and char layer formation | -The decrease in PHR from 13 to 62% by adding 3, 5 and 7 wt.% of each PFS1 or PFS2 and their OMMTPFS1 and O-MMTPFS2 -62.41% reduction in PHR rate for the composite containing 7 wt.% of O-MMTPFS2 -TTI was higher or similar to initial HDPE for all samples -Decrease in the fire growth rate for all composites by increasing the filler loading | [110] |
| LLDPE | Aerosil® r974 organically treated fumed silica (Ar974) in combination with Al hydroxide Alufy® 2 (AF) or Mg hydroxide Hydrofy® G1.5 (HF) | Char Formation | -Both PE/HF/Ar974 composites with 20 wt.% HF and (2 or 5 wt.% Ar974) self-extinguished (LOI values were 31.9% and 35.2%, respectively) -Effect of nanosilica on decreasing the PHR rate is significant in synergistic systems -Composite containing 20 wt.% HF and 5 wt.% Ar974 showed best fire performance based on LOI and CCTs | [111] |
| HDPE | Aminosilane modified silica in combination with MWCNT | Char layer formation that can be promoted by MWCNT | -Composite with 5% MWCNT and no nanosilica represented the max. value of LOI: 26.0 (36.8% higher than that of neat HDPE) and the min. value of the PHR rate (54% reduction) -Increase in MWCNT loading decreased PHR rate -Lowest smoke production for the composite with only nanosilica and highest with the ones with only MWCNT -Higher MWCNT loading, thicker and more homogeneous char layer -Slight synergism between fillers | [112] |
| LDPE | 4A zeolite | Intumescent char layer formation | -Enhancement in the LDPE/IFR’s LOI value -Successful passing in the UL-94 V-0 rating test for all composites -Improvement in the strength and compactness of the char surface | [113] |
| HDPE | SiO2 or CaCO3, APP, PER | Intumescent char layer formation | -Sample composition has significant role in WPCs’ properties -Best properties obtained when using SiO2 as the filler | [114] |
| LDPE/EVA | Nanoclay, ATH, ZB | Char formation | -Using nanoclays improved many parameters of flammability including ignition time, FGI, and PHR -Nanoclays effects are intensified when combined with traditional aluminum hydroxide or aluminum hydroxide | [115] |
| LDPE | Fe-MMT, Fe-OMMT | Intumescent char layer formation | -Lower HRR and lower THR observed for LDPE/IFR/Fe-MMT compared to LDPE/IFR/Fe-OMMT for the same loading percentage | [116] |
| HDPE | APP, SiO2 | Char formation | -Lower initial temperature and peak temperature of thermal degradation is achieved for RPC compared to wood-HDPE composites (WPC) -Introducing APP to RPCN expedites the thermal degradation of RPC -Better flame retardancy is observed for RPC | [117] |
| LDPE/EVA | OMMT, Piperazine spirocyclic pentaerythritol bisphosphonate) (PPSPB) | Intumescent char layer formation | -Thermal stability increased while flammability considerably decreased -PHR rate, THR, and average mass loss rate reduced significantly -The PHR rate of LDPE/EVA/PPSPB/OMMT showed 50% reduction compared to the LDPE/EVA blend. | [118] |
| Wood fiber-HDPE | Nano-SiO2 | Char formation | -Reduced the HRR, THR, and total smoke release of wood fiber-HDPE composites -Tensile and flexural strength improved | [119] |
| HDPE/Wheat straw | Mg(OH)2, Nanoclay | Char formation | -Increasing the nanoclay and Mg(OH)2 content reduced the burning rate, tensile and impact strength of the samples -Increasing the nanoclay weight percentage increased the tensile modulus and impact strength | [120] |
| PE | MMT, Sepiolite, POSS | Char formation | -HRR of CaSiEBA significantly increased after MMT nanofibers addition -Flammability retardancy of CaSiEBA and CaSiEMAA remained unchanged after sepiolite incorporation -Reduction of dripping was occurred due to the addition of only small amount of POSS -POSS enhanced HRR value of CaSiEMAA | [121] |
| PE | OMMT, Diphenylmethanamine spirocyclic pentaerythritol bisphosphonate (PSPD) | Intumescent char layer formation | -Combination of PSPD and montmorillonite (MT) improved the thermal stability of LDPE -The flammability of LDPE Extremely reduced due to the addition of PSPD/MT -51% decrease in the PHR rate of LDPE/PSPD/OMMT in comparison with LDPE | [122] |
| HDPE | MH, Aluminium hydroxide, EG, APP, PER, MMT | Char formation | -Improved flame retardancy behavior obtained by using APP/PER/MMT and APP/EG -Increasing the thermal stability of HDPE due to the FRs incorporation | [123] |
| Nanomaterial and Its Loading Amount | Types of FR and Its Loading | Result(s) | Ref. |
|---|---|---|---|
| Ce-MWCNTs, 3 wt.% | Brominated FR, 10 wt.% | 25% reduction in PHR rate observed from CCT, improved the UL-94 from V-2 to V-0 | [144] |
| Nano-SiO2, 6 wt.% | APP, 8 wt.% | 42% and 44% reduction in average HRR and PHR rate, respectively, 78% increase in TTI | [145] |
| Organic-modified montmorillonite, 10 wt.% | MHSH, 30 wt.% | 84% reduction in PHR rate and increase in tign observed from CCT. | [146] |
| Organic-modified montmorillonite, 5 wt.% | IFRs, 15 wt.% | 51% reduction in PHR rate observed from CCT | [122] |
| Halloysite nanotubes, 2 wt.% | IFRs, 28 wt.% | 92% and 75% decrease in PHR rate and THR, respectively. | [147] |
| Graphene, 1 wt.% | Brominated polystyrene/antimony trioxide, 6.2 wt.% | Increase LOI value from 23.4% to 24.1%, change UL-94 grades from NG to V-2. | [148] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezvani Ghomi, E.; Khosravi, F.; Mossayebi, Z.; Saedi Ardahaei, A.; Morshedi Dehaghi, F.; Khorasani, M.; Neisiany, R.E.; Das, O.; Marani, A.; Mensah, R.A.; et al. The Flame Retardancy of Polyethylene Composites: From Fundamental Concepts to Nanocomposites. Molecules 2020, 25, 5157. https://doi.org/10.3390/molecules25215157
Rezvani Ghomi E, Khosravi F, Mossayebi Z, Saedi Ardahaei A, Morshedi Dehaghi F, Khorasani M, Neisiany RE, Das O, Marani A, Mensah RA, et al. The Flame Retardancy of Polyethylene Composites: From Fundamental Concepts to Nanocomposites. Molecules. 2020; 25(21):5157. https://doi.org/10.3390/molecules25215157
Chicago/Turabian StyleRezvani Ghomi, Erfan, Fatemeh Khosravi, Zahra Mossayebi, Ali Saedi Ardahaei, Fatemeh Morshedi Dehaghi, Masoud Khorasani, Rasoul Esmaeely Neisiany, Oisik Das, Atiye Marani, Rhoda Afriyie Mensah, and et al. 2020. "The Flame Retardancy of Polyethylene Composites: From Fundamental Concepts to Nanocomposites" Molecules 25, no. 21: 5157. https://doi.org/10.3390/molecules25215157
APA StyleRezvani Ghomi, E., Khosravi, F., Mossayebi, Z., Saedi Ardahaei, A., Morshedi Dehaghi, F., Khorasani, M., Neisiany, R. E., Das, O., Marani, A., Mensah, R. A., Jiang, L., Xu, Q., Försth, M., Berto, F., & Ramakrishna, S. (2020). The Flame Retardancy of Polyethylene Composites: From Fundamental Concepts to Nanocomposites. Molecules, 25(21), 5157. https://doi.org/10.3390/molecules25215157












