3. Experimental
3.1. Chemistry
The chemicals and amino acids reported here were obtained from Sigma-Aldrich (Munich, Germany) or Alfa Aesar (Tewksbury, MA, USA). The solvents were obtained from Himmed (Moscow, Russia). The solvents were purified and dried by standard methods. Thin-layer chromatography (TLC) using silica gel on aluminum sheets (Merck, Darmstadt, Germany) was used to observe the chemical reactions; chloroform and methanol (9:1) were used as the eluent. The TLC was visualized in ultraviolet light or using the tolidine test. The melting points were measured using Optimelt MPA 100 (Stanford Research Systems, Sunnyvale, CA, USA). 1H-NMR spectra were recorded using Bruker Fourier 300 (Bruker Corporation, Leipzig, Germany) with DMSO-d6 (solvent). Chemical shifts (δ) are indicated in ppm (part per million), with tetramethylsilane (TMS) as a reference; coupling constants (J) were recorded (Hz). The NMR shifts were documented as follows: s, singlet; d, doublet; and m, multiplet. The optical rotation values were measured using an ADP 410 automatic digital polarimeter (Bellingham Stanley, Tunbridge Wells, Kent, Great Britain) at the wavelength of the D-line of the sodium spectrum (589.3 nm). The values of specific optical rotations were calculated by the formula: [α]D = (α × V) ÷ (l × m), where—α is observed optical rotation in degrees; V is the volume of solution in mL; l is the length of the measuring cell in dm; m is the weight of substance in grams.
General procedure for the synthesis of N-hydroxysuccinimide esters of carboxylic and amino acids. The mixture of 30.00 mmol acid in 200–300 mL of ethylacetate and 34.50 mmol (15% excess) of N-hydroxysuccinimide was cooled to +5 °C using ice-water bath. Then 35.10 mmol (17% excess) of dicylohexylcarbodiimide was added. The mixture was stirred for 30 min at +5 °C and then for 5 h at room temperature. The reaction was monitored by TLC. The dicyclohexylurea precipitate was filtered off and the solvent was removed in vacuo. The resulting viscous oil was dissolved in 100 mL of ethylacetate. The solution was kept for 24 h at +8 °C; the re-formed dicyclohexylurea precipitate was filtered off again; the filtrate was washed with 5% NaHCO3 (2 × 100 mL) and 100 mL of distilled water. The organics were dried over anhydrous Na2SO4 and then filtered; the solvent was removed in vacuo.
N-Boc–l-Leu–OSu. Glassy crystals; yield: 73%; m.p. 110–112 °C;
= −40° (c = 2, dioxane). (Lit. data: m.p. 116 °C (diisopropyl esther),
= −41.8° (c = 2, dioxane) [
23]);
1H-NMR (DMSO-
d6) δ, ppm: 0.82–0.88 (m, 6H, 2C
δH
3 Leu), 1.39 (s, 9H, -OC(CH
3)
3), 1.45 (m, 2H, C
βH Leu), 1.56–1.73 (m, 1H, C
γH
2 Leu), 2.82 (m, 4H, OSu), 3.86–3.88 (m, 1H, C
αH Leu), 7.93 (d,
J = 8.38 Hz, 1H, NH Leu).
Boc–d–Ile–OSu. Oil (yield: 91%); Rf 0.90. 1H-NMR (DMSO-d6, δ, ppm): 0.86 (m, CδH3 Ile, 3H), 0.97 (d, J = 6.7 Hz, CγºH3 Ile, 3H), 1.27 and 1.52 (two m, CγH2 Ile, 2H), 1.40 (s, -OC(CH3)3, 9H), 1,87 (1H, m, CβH Ile), 2,80 (4 H, m, OSu), 4.24 (m, CαH Ile, 1H), 7.57 (1 H, d, J = 8.40 Hz, NH Ile).
N-Z-l-Trp–OSu. Cream foam (yield: 92%), m.p. 137–140 °C = −60.0° (c = 1, DMF); Rf 88. 1H-NMR (DMSO-d6) δ, ppm: 2.78 (m, 4H, OSu), 3.01–3.25 (m, 2H, CβH Trp), 3.98 (m, 1H, CαH Trp), 4.97 (s, 2H, -OCH2C6H5), 6.73–7.62 (m, 10H, Ar), 8.56 (d, J = 8.8 Hz 1H, NH Trp), 10.78 (1H, c, NHindole).
N-Z-d-Trp–OSu. Cream foam (yield: 96%); = +44.3 ° (c 1, DMF); Rf 87; 1H-NMR (DMSO-d6, δ, ppm): 2.80 (m, OSu, 4H), 3.07–3.25 (m, CβH Trp, 2H), 4.00 (m, CαH Trp, 1H), 4.95 (s, -OCH2C6H5, 2H), 6.94–7.77 (m, -OCH2C6H5, indole, 10H), 8.56 (d, J = 8.8 Hz, NH Trp, 1H), 10.80 (1H, s, NH indole).
Boc–Gly–OSu. White powder (yield 78%), m.p. 165–167 °C, Rf 0,66 (CHCl3/MeOH 9/1). 1H-NMR (DMSO-d6) δ, ppm: 1.39 (s, -OC(CH3)3, 9H), 2.81 (m, OSu, 4H), 4.08 (d, J = 6.1 Hz, CH2 Gly, 2H), 7.48 (t, J = 5.9 Hz, NH Gly, 1H).
Ph(CH2)2C(O)–Gly–OSu. Viscous white oil (yield 75%). 1H-NMR (DMSO-d6) δ, ppm: 2,37 (t, 2H, CH2CO), 2,69 (m, 2H, CH2C6H5), 2.80 (m, 4H, OSu), 3.56 (m, 2H, CH2 Gly), 7.27–7.43 (m, 5H, C6H5,), 7.47 (t, J = 5.8 Hz, NH Gly, 1H).
Ph(CH2)2C(O)Su. White crystals (yield: 45%) m.p. 83–84 °C. 1H-NMR (DMSO-d6) δ, ppm: 2.81 (m, 4H, OSu), 2.97–3.01 (m, 2H, CH2C6H5), 3.34 (s, 2H, CH2CO), 7.16–7.31 (m, 5H, Ar).
PhCH2C(O)–OSu. White powder (yield 96%); m.p. 111–112 °C. 1H-NMR (DMSO-d6) δ, ppm: 2.81 (m, OSu, 4H), 4.10 (s, CH2C6H5,2H), 7.20–7.42 (m, C6H5, 5H).
CH3(CH2)4C(O)–OSu. White glassy crystalls (yield 76%); m.p. 53–55 °C; Rf 0.85; 1H-NMR (DMSO-d6) δ, ppm: 0.87 (m, 3H, CH3), 1.32 (m, 2H, CδH2,), 1.62 (m, 4H, CγH2CβH2), 2.63–2.68 (m, 2H, CαH2), 2,80 (m, OSu, 4H).
3.2. Preparation of Starting Compounds
Boc–Gly–NH2. To a solution of 6.00 g (27 mmol) Boc–Gly–OSu in 20 mL of DMF, 40 mL of aqueous ammonia was added. The resulting suspension was sustained at room temperature for 24 h without stirring. The resulting solution was concentrated by rotary evaporation. Yellow oil was obtained in the yield of 99%. (lit data m.p. 91–92 °C [
30])
1H-NMR (DMSO-
d6) δ, ppm: 1.38 (s, -OC(CH
3)
3, 9H), 4.10 (d,
J = 6.1 Hz, CH
2 Gly, 2H), 7.49 (t,
J = 5.9 Hz, NH Gly, 1H), 7.41 and 7.10 (two s, NH
2 amide, 2H).
N-Boc–l-Leu–NH2. To a solution of 8.00 g (24.36 mmol) of Boc–
l-Leu–OSu in 15 mL of DMF, 100 mL of an aqueous solution of ammonia was added. The resulting suspension was stirred for 30 min. The precipitate was kept for 3 h at +8°. The product was filtered, washed with distilled water until neutral and dried at 100 °C. The yield was 3.37 g (60%) of Boc–
l-Leu–NH
2 as white powder with m.p. 164–165 °C,
= −11.0° (c = 1, DMF). (Lit. data: m.p. 150–152 C,
= −11.9° (c = 1, methanol) [
31])
1H-NMR (DMSO-
d6) δ, ppm: 0.82–0.85 (m, 6H, 2C
δH
3 Leu), 1.08–1.22 (m, 1H, C
γH
2 Leu), 1.36 (s, 9H, -OC(CH
3)
3), 1.45 (m, 2H, C
βH Leu), 3.86–3.88 (m, 1H C
αH Leu), 6.72 (d,
J = 8.2 Hz, 1H, NH Leu), 6.89 and 7.19 (2s, 2H, NH
2amide).
N-Boc–d-Leu–NH2. Obtained similarly to Boc–l-Ile–NH2 using Boc–d-Leu–OSu. White powder; yield 75%; m.p. 148–149 °C; = +88.4° (c = 1, DMF); Rf 0.47 (CHCl3:MeOH 9:1). 1H-NMR (DMSO-d6) δ, ppm: 0.82–0.87 (m, 6H, 2CδH3 Leu), 1.08–1.22 (m, 1H, CγH Leu), 1.37 (s, 9H, -OC(CH3)3), 1.45 (m, 2H, CβH2 Leu), 3.86–3.89 (m, 1H CαH Leu), 6.72 (d, J = 8.38 Hz, 1H, NH Leu), 6.88 and 7.03 (2s, 2H, NH2amide).
TFA*[l-Leu–NH2]. The suspension of 12.00 g (52.10 mmol) of Boc–l-Leu–NH2 in 30 mL of dichloromethane was treated with 60 mL of TFA (150% excess) and stirred for 2 h at room temperature. The solution was re-evaporated with diethyl ether (3 × 50 mL). The obtained white oil was triturated with diethyl ether, a white powder was obtained and the ether was decanted. The product was dried on air at room temperature. The yield was 11.88 g (99%) of TFA*[H-Leu–NH2], white powder with m.p. 118–119 °C, = −18° (c = 1, DMF). 1H-NMR (DMSO-d6) δ, ppm: 0.83–0.89 (m, 6H, 2CδH3 Leu), 1.08–1.22 (m, 1H, CγH Leu), 1.45 (m, 2H, CβH2 Leu), 3.69 (m, 1H, CαH Leu), 7.52 and 7.93 (2s, 2H, NH2 amide), 8.12 (broad s, 3H, N+H3 Leu).
TFA*[d-Leu–NH2]. Obtained similarly to TFA*[l-Leu–NH2] Boc–d-Leu–NH2. Yield 99%; white powder; m.p. 134–135; = +34.4°, (c = 1, DMF); 1H-NMR (DMSO-d6) δ, ppm: 0.88–0.90(6 H, m, 2CδH3 Leu), 1.08–1.27 (1 H, m, CγH Leu), 1.55 (2 H, m, CβH2 Leu), 3.63 (1H, m, CαH Leu), 7.53 and 7.90 (2H, 2s, NH2 amide), 8.07 (3H, broad s, N+H3 Leu).
Z-l-Trp-l-Leu–NH2. 3.30 g (13.52 mmol) of TFA*[l-LeuNH2] was dissolved in 30 mL of DMF with the addition of 2.35 mL (13.52 mmol) of DIPEA. This mixture was stirred for half an hour. Then 7.06 g (16.22 mmol, 20% excess) of Z-l-TrpOSu in 30 mL of DMF was added. The reaction mixture was stirred for 12 h at room temperature. The solvent was evaporated. The resulting fluent orange oil was dissolved in 200 mL of ethylacetate; washed with 3% H2SO4 (2 × 100 mL), 5% NaHCO3 solution (2 × 100 mL) and distilled water (1 × 100 mL); the organics were dried over Na2SO4; the solvent was evaporated. The resulting oil was dissolved in a minimum amount of DMF and diluted with 200 mL of distilled water; the white precipitate was obtained. The precipitate was maintained for 12 h at +5 °C, filtered, and washed with distilled water and hexane. The product was dried in vacuo over CaCl2 and paraffin. The yield was 5.78 g (94%), white powder with m.p. 166–169 °C; = −31° (c = 1, DMF). 1H-NMR (DMSO-d6) δ, ppm: 0.82–0.88 (m, 6H, 2CδH3 Leu), 1.08–1.22 (m, 1H, CγH2 Leu), 1.45 (m, 2H, CβH2 Leu), 2.90–3.09 (m, 2H, CβH Trp), 4.28–4.31 (m, 2H, CαH Leu and CαH Trp), 4.93 (m, 2H, CH2CO), 6.99–7.30 (m, 10H, Aryl), 7.25 and 7.33 (2s, 2H, NH2 amide), 7.62 (d, J = 7.53 Hz, 1H, NH Leu), 7.97 (d, J = 7.83 Hz, 1H, NH Trp), 10.81 (s, 1H, NH indole).
Z-d-Trp–l-Leu–NH2 Obtained similarly to Z-l-Trp-l-Leu–NH2 using Z-d-Trp–OSu. White powder (yield 73%); m.p. 180–122 °C, [α]D23 = +28.0° (c 1, DMF). 1H-NMR (DMSO-d6) δ, ppm: 0.71–0.80 (6H, m, 2CδH3 Leu), 1.22–1.27 (1H, m, CγH Leu), 1.40–1.45 (2H, m, CβH2 Leu), 2.93–3.04 (2H, 2m, CβH Trp), 4.16 (1H, m, CαH Leu), 4.31 (1H, m, CαH Trp), 4.96 (2H, m, -OCH2C6H5), 6.97–7.32 (10H, m, Aryl), 7.15 and 7.32 (2H, 2s, NH2 amide), 7.62 (1 H, d, J = 8.75 Hz, NH Trp), 7.97 (1H, d, J = 7.73 Hz, NH Leu), 10.81 (1H, s, NH indole).
Z-l-Trp–d-Leu–NH2 was obtained similarly to Z-l-Trp-l-Leu–NH2 using TFA*[d-Leu–NH2]. White powder (yield 76%); m.p. 188–189 °C. 1H-NMR (DMSO-d6) δ, ppm: 0.82–0.88 (6 H, m, 2CδH3 Leu), 1.45 (2H, t, CβH2 Leu), 1.56–1.73 (1H, m, CγH Leu), 2.90–3.09 (2H, m, CβH Trp), 4.22 (1H, m, CαH Leu), 4.54 (1H, m, CαH Trp), 6.99–7.20 (10H, m, Aryl), 7.33 and 7.60 (2H, 2 s, NH2 amide), 7.93 (1H, d, J = 8.47 Hz, NH Leu), 8.08 (1H, d, J = 7.73 Hz NH Trp), 10.78 (1H, s, NH indole).
H-l-Trp–l-Leu–NH2. Through the suspension of 5.78 g (12.8 mmol) of Z-l-Trp-l-Leu–NH2 and 0.500 g of 10% Pd/C in 50 mL of methanol a stream of hydrogen was passed from the gas-holder for 2 h at vigorous stirring. After reaction was complete, the catalyst was filtered and washed with methanol. The methanol was evaporated in vacuo. The product was obtained in the amount of 4.71 g (99%) as a gray foam without a clear melting point, = −25° (c = 1, DMF). 1H-NMR (DMSO-d6) δ, ppm: 0.82–0.88 (2dd, J = 12.95 Hz and J = 12.85 Hz, 6H, 2CδH3 Leu), 1.09–1.26 (m, 1H, CγH2 Leu), 1.45 (m, 2H, CβH2 Leu), 2.90 and 3.08 (2dd, 2H, CβH Trp), 3.50 (m, 2H, CαH Trp), 4.14 (m, 1H, CαH Leu), 6.94–7.55 (m, 5H, Ar), 7.05 and 7.41 (2s, 2H, NH2 amide), 7.60 (d, J = 7.83 Hz 1H, NH Leu), 8.05 (d, 1H, J = 8.29 Hz NH Trp), 10.85 (broad s, 1H, NH indole).
H-l-Trp–d-Leu–NH2. Obtained similarly to H-l-Trp-l-Leu–NH2 using Z-l-Trp-d-Leu–NH2. Grey powder (yield 96%); m.p. 110 °C. = −5° (c = 1, DMF)1H-NMR (DMSO-d6) δ, ppm: 0.78–0.83 (m, 6H, 2CδH3 Leu), 0.88 (m, 2H, CγH2 Leu), 1.40 (m, 1H, CβH Leu), 2.71−3.03 (m, 2H, CβH2 Trp), 3.50 (m, 2H, CαH Trp), 4.21 (dd, J = 14.99 Hz and J = 7.26 Hz, 1H, CαH Leu), 6.96–7.33 (m, 5H, Ar), 7.05 and 7.41 (2s, 2H, NH2 amide), 7.55 (d, J = 7.92 Hz, 1H, NH Leu), 7.94 (d, J = 7.93 Hz, 1H, NH Trp), 10.82 (broad s, 1H, NH indole).
H-d-Trp–l-Leu–NH2. Obtained similarly to H-l-Trp-l-Leu–NH2 using Z-d-Trp-l-Leu–NH2. Cream foam without a clear melting point (yield 99%), = −17° (c = 1, DMF). 1H-NMR (DMSO-d6) δ, ppm: 0.81–0.88 (2dd, J = 12.95 Hz and J = 12.85 Hz, 6H, 2CδH3 Leu), 0.88 (m, 2H, CγH2 Leu), 1.40 (m, 1H, CβH Leu), 2.71–3.03 (m, 2H, CβH2 Trp), 3.50 (m, 2H, CαH Trp), 4.21 (m, 1H, CαH Leu), 6.96–7.33 (m, 5H, Ar), 7.05 and 7.41 (2s, 2H, NH2 amide), 7.55 (d, J = 7.54 Hz, 1H, NH Leu), 7.94 (d, J = 7.64 Hz, 1H, NH Trp), 10.82 (broad s, 1H, NH indole).
Z-l-Trp–l-Leu–OMe. In total, 2.43 g (13.36 mmol) of HCl*[
l-Leu–OMe] was dissolved in 30 mL of DMF with 2.54 mL (14.57 mmol) of DIPEA; this mixture was stirred for half an hour. Then 6.98 g (16.03 mmol) of Z-
l-Trp–OSu was added. The mixture was stirred for 24 h at room temperature. The solvent was evaporated; the resulting fluid oil was diluted with 300 mL of ethylacetate. The solution was washed with 3% H
2SO
4 (2 × 300 mL), and then with 5% NaHCO
3 (2 × 300 mL) and distilled water (1 × 300 mL). The organic fraction was dried over anhydrous MgSO
4 for half an hour, and then filtered and evaporated to a state of fluid oil. This oil was diluted with 200 mL of distilled water. The white precipitate was obtained, the water was decanted and the product was dried in vacuo over CaCl
2 and paraffin. The yield was 5.95 g (95.6%) of the thick white oil without a clear melting point,
Rf=0.79,
= −21.8° (c = 1, DMF). (Lit data: m.p. 55–57 °C [
32]).
1H-NMR (DMSO-
d6) δ, ppm: 0.83–0.88 (m, 6H, 2C
δH
3 Leu), 1.17 (m, 2H, C
γH Leu), 1.25 (m, 1H, C
βH
2 Leu), 2.96–3.10 (m, 2H, C
βH Trp), 3.62 (s, 3H, -OCH
3), 4.29 (m, 1H, C
αH Leu), 4.41 (m, 1H, C
αH Trp), 4.96 (m, 2H, -OC
H2C
6H
5), 6.98–7.51 (m, 10H, Aryl), 7.66 (d,
J = 7.64 Hz, 1H, NH Trp), 8.27 (d,
J = 7.54 Hz, 1H, NH Leu), 10.83 (s, 1H, NH indole).
H-l-Trp–l-Leu–OMe. Through the suspension of 5.79 g (12.44 mmol) of Z-l-Trp-l-Leu–OMe and 1.00 g of 10% Pd/C in 100 mL of methanol the stream of hydrogen was bubbled for 2 h with vigorous stirring. After the reaction was complete the catalyst was filtered and washed with methanol. Methanol was removed in vacuo; 4.15 g of product was obtained as grey thick oil with = −28.6° (c = 1, DMF). 1H-NMR (DMSO-d6) δ, ppm: 0.83–0.88 (m, 6H, 2CδH3 Leu), 1.06–1.35 (m, 2H, CβH2 Leu), 1.58 (m,1H, CγH Leu), 2.90–3.06 (m, 2H, CβH2 Trp), 3.63 (s, 3H, -OCH3), 4.29 (dd, 1H, CαH Leu), 4,41 (m, 1H, CαH Trp), 6.98–7.51 (m, 1H, Aryl), 7.67 (1d, J = 7.92 Hz, 1H, NH Trp), 8.27 (d, J = 7.92 Hz, 1H, NH Leu), 10.83 (s, 1H, NH indole).
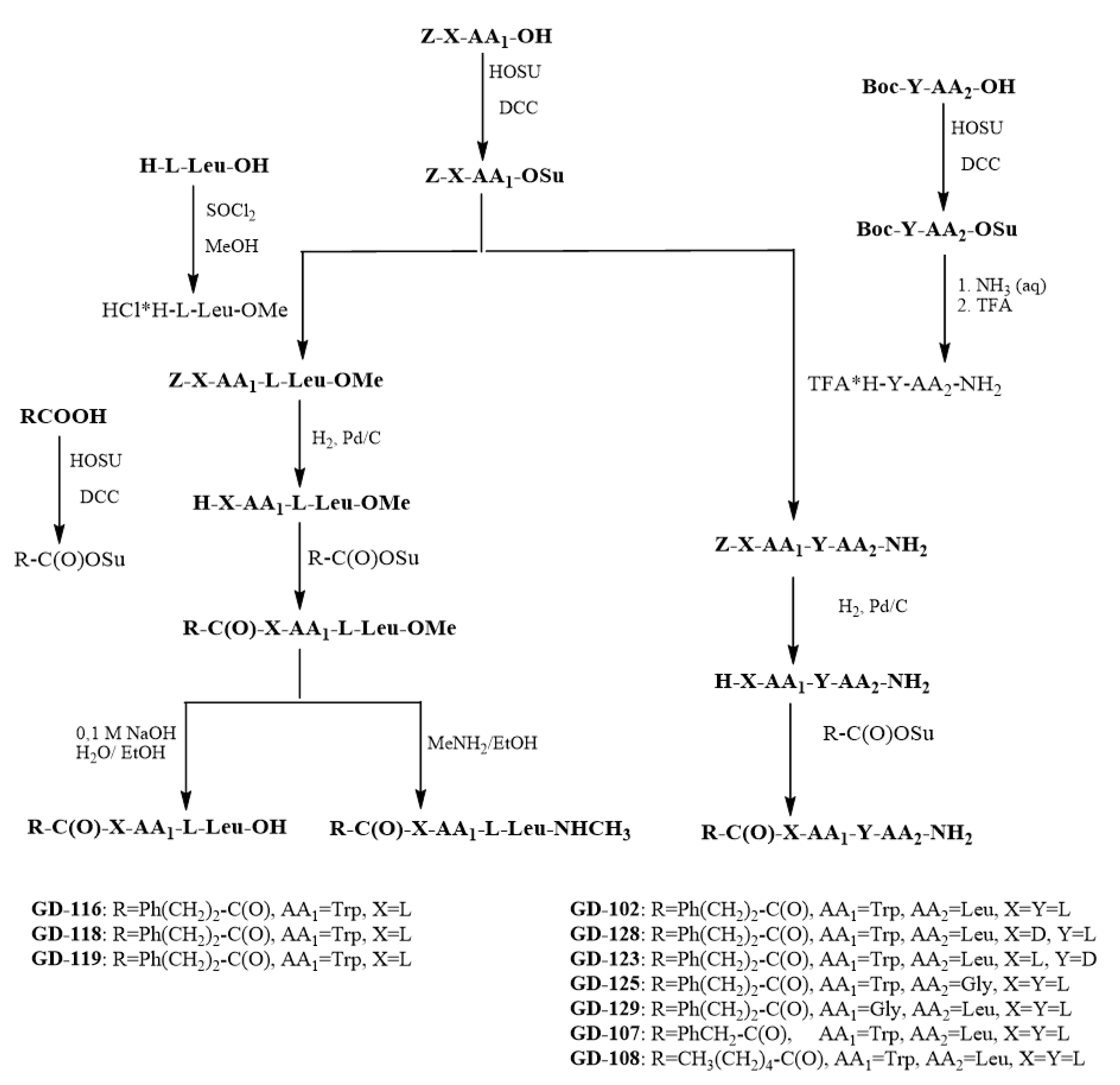
3.3. Preparation of Target Dipeptides
N-Ph(CH2)2C(O)–l-Trp–l-Leu–OMe (GD-116). 3.67 g (14.85 mmol, 15% excess) phenylpropionic acid succinimide ester was added to the solution of 4.10 g (12.37 mmol) of H-l-Trp–l-Leu–OMe in 70 mL DMF. The solution was stirred for 12 h. Excess of the succinimide ester was quenched by adding 0.2 mL of DMAPA. The solvent was evaporated; diluted with 300 mL of ethylacetate; and washed with 3% H2SO4 (1 × 300 mL), 5% NaHCO3 (2 × 300 mL), and a saturated solution of NaCl (1 × 300 mL), and then with distilled water (1 × 300 mL). The organic fraction was dried over anhydrous MgSO4, evaporated to dryness. The product in a form of white oil was twice re-evaporated with diethyl ether to obtain stable white foam. The foam was crushed into powder and washed with hexane on the glass filter. The product was dried in vacuo over Na2SO4 and paraffin. The yield was 4.25 g (74%) of white powder with m.p. 134–135 °C, = −41.4° (c = 1, DMF). 1H-NMR (DMSO-d6) δ, ppm: 0.84–0.91 (6 H, m, 2CδH3 Leu), 1.06–1.27 (2H, m, CβH Leu), 1.48–1.58 (1H, m, CγH2 Leu), 2.32 (2H, m, CH2 chain), 2.50 (H, m, CH2 chain), 2.92–3.09 (2H, m, CβH Trp), 3.61 (3H, s, -OCH3), 4.32 (1H, m, CαH Leu), 4,63 (1H, m, CαH Trp), 6.93–7.61 (10H, m, Aryl), 8.02 (1H, d, J = 7.73 Hz, NH Trp), 8.35 (1 H, d, J = 7.29 Hz, NH Leu), 10.91 (1 H, s, NH indole).
N-Ph(CH2)2C(O)-l-Trp-l-Leu–OH (GD-118). The solution of 0.19 g (4.64 mmol, 200% excess) NaOH in 10 mL of water was added dropwise to the solution of 1.10 g (2.32 mmol) of N-Ph(CH2)2C(O)-l-Trp-l-Leu–OMe in 20 mL of methanol. The mixture was stirred for 3 h, and then diluted with 100 mL of cold water (+5–8 °C); finally, it was acidified with 5% H2SO4 to pH 4–5. The product was obtained by extraction with ethyl acetate (3 × 30 mL). Organics were dried over Na2SO4, the solvent was removed in vacuo, and then re-evaporated 3 times with diethyl ether to obtain the white foam. The foam was crushed, put to a glass filter and washed with hexane. The yield was 0.95 g (91%) with m.p. 91–92 °C, = −19.8° (c = 1, DMF). 1H-NMR (DMSO-d6) δ, ppm: 0.83–0.88 (6H, m, 2CδH3 Leu), 1.07–1.20 (2H, m, CβH Leu), 1.58 (1H, m, CγH2 Leu), 2.37 (2H, m, CH2chain), 2.68 (H, m, CH2chain), 2.79–2.88 (2H, m, CβH Trp), 3.61 (3 H, s, -OCH3), 4.23–4.30 (1H, m, CαH Leu), 4.59–4.65 (1H, m, CαH Trp), 6.95–7.31 (10H, m, Aryl), 7.96 (1H, d, J = 8.29 Hz, NH Trp), 8.35 (1H, d, J = 8.01 Hz, NH Leu), 10.91 (1 H, s, NH indole) 12.63 (1H, broad s, OH).
N-Ph(CH2)2C(O)-l-Trp-Leu–NHCH3 (GD-119). In total, 1.10 g (2.37 mmol) of N-Ph(CH2)2C(O)–l-Trp–l-Leu–OMe was added to the fresh-made solution of methylamine in ethyl alcohol (23 g of solution containing 5.7 g of pure methylamine). The mixture was left in the closed flask for 6 days at room temperature without stirring. The solution was evaporated, and re-evaporated twice with diethyl ether to obtain white powder. The yield was 1.05 g (96%) of product with m.p. 201 °C (with decomposition), [α] = −16.6° (c = 1, DMF). 1H-NMR (DMSO-d6) δ, ppm: 0.81–0.88 (m 2CδH3 Leu, 6H), 1.06–1.27 (m, CβH Leu, 2H), 1.58 (m CγH2 Leu, 1H), 2.15 (s, -NHCH3, 3H), 2.37 (m, 2H CH2 chain), 2.69 (m, 2H chain), 2.87–3.95 (m, CβH Trp, 2H), 4.12 (m, CαH Leu, 1H), 4.35 (m, CαH Trp, 1H), 6.91–7.28 (m, 10H, Aryl), 7.60 (d, J = 8.20 Hz, NH Trp, 1H), 7.85 (d, J = 7.82 Hz, NH Leu, 1H), 10.83 (s, NH indole, 1H).
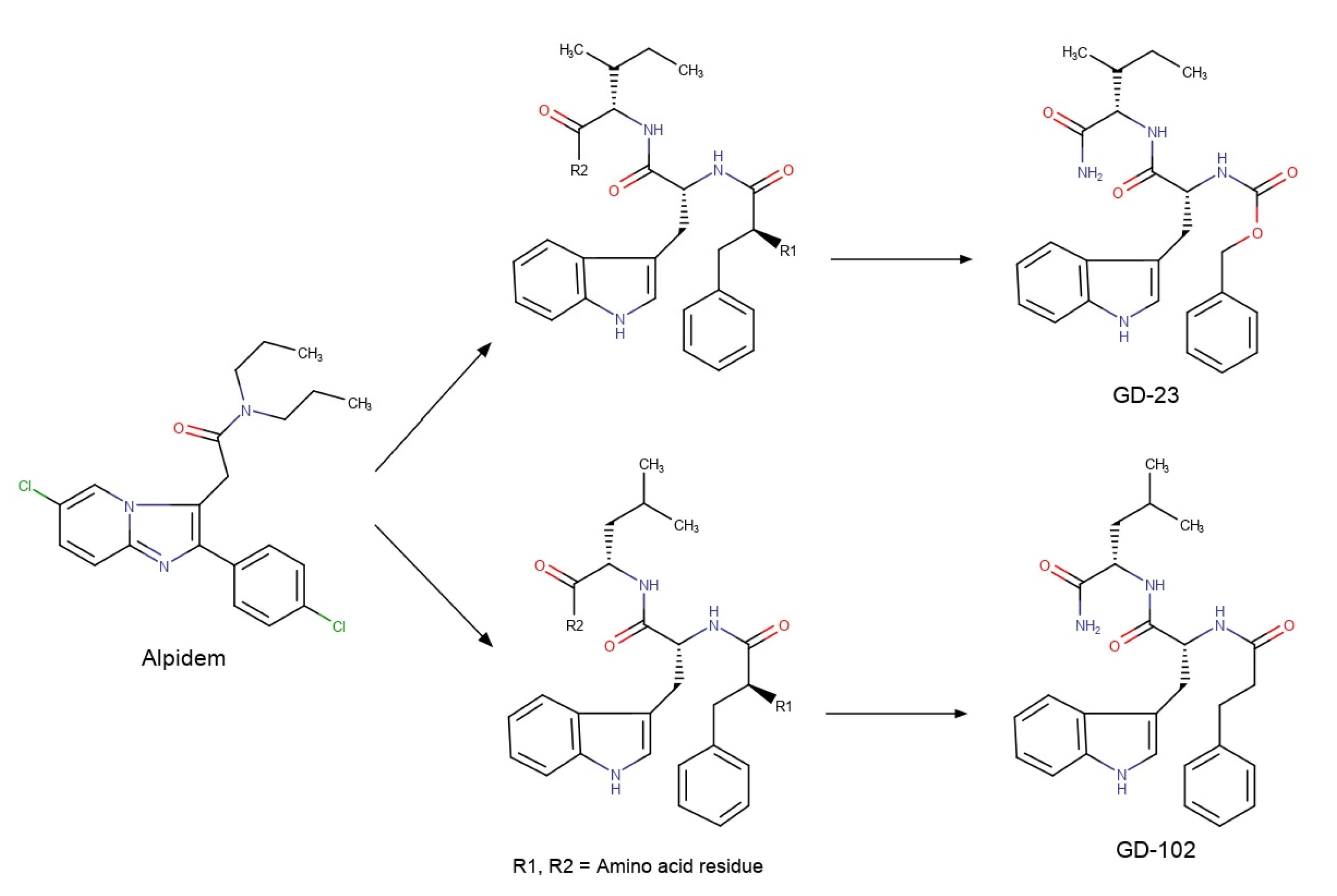
N-Ph(CH2)2C(O)-l-Trp-l-Leu–NH2 (GD-102). A total of 4.50 g (14.3 mmol) of H-l-Trp-l-Leu–NH2 was dissolved in 50 mL of DMF; then 3.86 g (15.6 mmol, 10% excess) of Ph(CH2)2C(O)Su was added and stirred for 12 h at room temperature. The solvent was evaporated to half the volume. The residue was diluted with 100 mL of water to obtain an insoluble product. The resultant crystals were washed with the glass filter with 100 mL of warm (43–45 °C) water, then with 100 mL of hexane and then with 50 mL of diethyl ether. The yield was 4.49 g (70%) of the product in the form of a white powder, with a m.p. 197–198 °C, = −27° (c = 1, DMF). 1H-NMR (DMSO-d6, δ, ppm): 0.82–0,88 (6H, 2 dd, J = 12.95 Hz and J = 12.85 Hz, 2CδH3 Leu), 1.45 (2H, t, CβH Leu), 1.56–1.73 (1 H, m, CγH2 Leu), 2.38 (2H, m, CH2C6H5 chain), 2.69 (2H, m, CH2CO chain), 2.90–3.09 (2 H, 2dd, J = 9.66 Hz and J = 4.66 Hz, CβH Trp), 4.22 (1H, dd, J = 15.95 Hz and J = 7.64 Hz, CαH Leu), 4.54 (1H, m, CαH Trp), 6.99–7.20 (10H, m, Aryl), 7.33 and 7.60 (2H, 2 s, NH2 amide), 7.93 (1H, d, J = 8.29 Hz, NH Leu), 8.08 (1H, d, J = 8.01 Hz NH Trp), 10.80 (1 H, s, NH indole).
N-PhCH2C(O)-l-Trp-l–Leu–NH2 (GD-108). The 0.355g (1.76 mmol) of H-l-Trp-l-Ile-NH2 was dissolved in 20 mL of DMF; then 0.46g (2.11 mmol, 20% excess) of PhCH2C(O)Su was added and stirred for 12 h at room temperature. The solvent was evaporated to half the volume. The residue was diluted with 100 mL of water to obtain an insoluble product. The resulting precipitate was maintained for 12 h at room temperature, filtered over glassy filter and washed with 50 mL of water and 50 mL of hexane. The yield was 80% of product in the form of white powder with m.p. 226–230 °C; = −16° (c = 1, DMF). 1H-NMR (DMSO-d6) δ, ppm: 0.82–0.88 (6H, m, 2CδH3 Leu), 1.45 (2H, t, J = 14.25 Hz and J = 7.26 Hz, CβH Leu), 1.56–1.73 (1 H, m, CγH2 Leu), 2.95 and 3.09 (2 H, 2dd, CβH Trp), 3.39 (2H, s, CH2C6H5), 4.15 (1H, dd, J = 15.27 Hz and J = 7.73 Hz CαH Leu), 4.62 (1H, m, CαH Trp), 6.97–7.38 (10H, m, Aryl), 7.07 and 7.58 (2 H, 2s, NH2 amide), 7.77 (1H, d, J = 8.38 Hz, NH Leu), 8.28 (1H, d, J = 7.92 Hz, NH Trp), 10.81 (1H, s, NH indole).
CH3(CH2)4C(O)-l-Trp-l-Leu–NH2 (GD-107). The 0.65 g (1.92 mmol) of H–l-Trp–l-Leu–NH2 was dissolved in 50 mL of DMF, then 0.45 g (2.11 mmol, 10% excess) of CH3(CH2)4C(O)Su was added and stirred for 12 h at room temperature. The solvent was evaporated to half the volume. The residue was diluted with 70 mL of ethylacetate and washed twice with 70 mL 5% NaHCO3, then with 70 mL 3% H2SO4 and then with 70 mL of distilled water. The organics were dried over anhydrous MgSO4 for half an hour; the solvent was filtered and evaporated. The yield was 0.74 g (92%), white powder with a m.p. 166 °C, = −20° (c = 1, DMF). 1H-NMR (DMSO-d6) δ, ppm: 0.82–0,88 (9H, m, 2CδH3 Leu and CH3 chain), 1.32 (2H, m, CδH2), 1,45 (2H, m, CβH Leu), 1,56–1,73 (1H, m, CγH2 Leu), 1.62 (4H, m, CγH2CβH2), 2.63–2.68 (2H, m, CαH2), 2.95 and 3.09 (2 H, m, CβH Trp), 3.39 (2H, s, CH2C6H5), 4.15 (1H, m, CαH Leu), 4,62 (1 H, m, CαH Trp), 6.97–7.38 (10H, m, Aryl), 7.07 and 7.58 (2H, 2d, NH2 amide), 7.77 (1H, d, J = 7.73 Hz, NH Leu), 8.28 (1H, d, J = 8.Hz, NH Trp), 10.81 (1H, s, NH indole).
N-Ph(CH2)2C(O)-d-Trp-l-Leu–NH2 (GD-128). Obtained similarly to N-Ph(CH2)2C (O)-l-Trp-l-Leu–NH2 from 1.00 g (3.16 mmol) of H-d-Trp-l-Leu–NH2 and 1.17 g (4.74 mmol, 15% excess) of Ph(CH2)2C(O)Su. The yield was 1.04 g (74%) of the product in the form of a cream powder, with a m.p. 122 °C, = −5° (c = 1, DMF). 1H-NMR (DMSO-d6, δ, ppm): 0.82–0,88 (6H, m, 2CδH3 Leu), 1.45 (2H, t, CβH Leu), 1.56–1.73 (1H, m, CγH2 Leu), 2.38 (2H, m, CH2C6H5), 2.69 (2H, m, CH2CO), 2.90–3.09 (2 H, m, CβH Trp), 4.22 (1H, dd, CαH Leu), 4.54 (1H, m, CαH Trp), 6.99–7.20 (10H, m, Aryl), 7.33 and 7.60 (2H, 2 s, NH2 amide), 7.93 (1H, d, J = 7.92 Hz, NH Leu), 8.08 (1H, d, J = 8.56 Hz, NH Trp), 10.80 (1H, s, NH indole).
N-Ph(CH2)2C(O)-l-Trp-d-Leu–NH2 (GD-123). Obtained similarly to N-Ph(CH2)2C (O)-l-Trp-l-Leu–NH2 from 1.00 g (3.16 mmol) of H-l-Trp-d-Leu–NH2 and 1.17 g (4.74 mmol, 15% excess) of Ph(CH2)2C(O)Su. The yield was 1.12 g (79%) of the product in the form of a cream powder, with a m.p. 210 °C (with decomposition), = +17.7° (c = 1, DMF). 1H-NMR (DMSO-d6, δ, ppm): 0.78–0,81 (6H, m, 2CδH3 Leu), 1.35 (2 H, m, CβH Leu), 1.60–1.74 (1H, m, CγH2 Leu), 2.35–2.41 (2H, m, CH2C6H5), 2.69–2.74 (2H, t, J = 7.82 Hz, CH2CO), 2.91–3.03 (2H, m, CβH Trp), 4.22 (1H, dd, J = 8.29 Hz and J = 4.47 Hz, CαH Leu), 4.54 (1H, m, CαH Trp), 6.99–7.20 (10 H, m, Aryl), 7.33 and 7.60 (2H, 2 s, NH2 amide), 7.93 (1H, d, J = 7.73 Hz, NH Leu), 8.08 (1H, d, J = 8.47 Hz, NH Trp), 10.80 (1H, s, NH indole).
N-Ph(CH2)2C(O)-Gly-l-Leu–NH2 (GD-129). The mixture of 2.51 g (10.29 mmol) TFA*[l-LeuNH2], 2.00 mL (11.31 mmol) DIPEA and 30 mL DMF was stirred for 0.5 h. Then the solution of 3.39 g (11.31 mmol 10% excess) of Ph(CH2)2C(O)-Gly–OSu in 30 mL of DMF was added. This mixture was stirred for 12 h at room temperature. The solvent was evaporated to half the volume. The residue was diluted with 100 mL of water to obtain an insoluble product. The resulting precipitate was maintained for 24 h at +8 °C, washed with 100 mL of water and 100 mL of hexane. The yield was 1.50 g (46%) of product in the form of white powder with m.p. 162 °C, = −5.2° (c = 1, DMF). 1H-NMR (DMSO-d6, δ, ppm): 0.85 (6H, m, 2CδH3 Leu), 1.45 (2H, m, CβH Leu), 1.56–1.73 (1H, m, CγH2 Leu), 2.37 (2H, t, CH2CO), 2.69 (2H, t, CH2C6H5), 3.56 (2H, m, CH2 Gly), 4.47 (1 H, dd, J = 8.29 Hz and J = 4.47 Hz, CαH Leu), 6.99–7.20 (10H, m, Aryl), 7.33 and 7.60 (2H, 2s, NH2 amide), 7.93 (1H, d, J = 7.36 Hz, NH Leu), 8.22 (1H, t, J = 5.59 Hz, NH Gly), 10.79 (1H, s, NH indole).
N-Ph(CH2)2C(O)-l-Trp-Gly-NH2 (GD-125). A total of 4.50g (14.3 mmol) of H-l-Trp-l-Gly-NH2 was dissolved in 50 mL of DMF, then 3.86 g (15.6 mmol, 10% excess) of Ph(CH2)2C(O)Su was added and stirred for 12 h at room temperature. The solvent was evaporated to half the volume. The residue was diluted with 100 mL of water to obtain an insoluble product. The resulting precipitate was maintained for 24 h at +8˚C, filtered, washed with 100 mL of water and with 50 mL of hexane. The yield was 4.49 g (70%) of product in the form of white powder with m.p. 118 °C, = −33° (c = 1, DMF). 1H-NMR (DMSO-d6, δ, ppm): 2.92–3.11 (2H, m, CβH Trp), 2.37 (2H, m, CH2CO), 2.69 (2H, t, CH2C6H5), 3.40 (2H, m, CH2 Gly), 4.34 (1 H, m, CαH Trp), 6.52–7.77 (10H, m, Aryl), 7.57 (1H, d, J = 7.73 Hz, NH Trp), 7.41 and 7.10 (2H, 2s, NH2 amide), 8.14 (1H, d, J = 7.36, NH Gly), 10.12 (1H, s, NH indole).
3.4. Pharmacology
3.4.1. Animals
Adult male Balb/c mice and ICR mice weighing 19–25 g were used in this study. The animals were obtained from the nursery of laboratory animals of Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry of the Russian Academy of Sciences and adapted for 2 weeks prior to testing in the Zakusov Institute of Pharmacology vivarium. The mice were maintained in an environmentally controlled facility with a 12 h light-dark cycle (lights on at 08:00 o’clock), the temperature of 22 °C ± 2 °C and the humidity of 60 ± 10%. All the mice were put on a standard diet with food and water available ad libitum. The animals were split into groups of 8 according to body weight with a deviation from the mean value of no more than ±10%. All animals were kept for 24 h in the experimental room at “home” cages before experiments.
3.4.2. Ethical Approval
The study complied with the requirements of Order of the Ministry of Health of the Russian Federation Number 199 “On Approval of the Rules of Good Laboratory Practice” and with accordance with GOST 33215-2014 “Guidelines for accommodation and care of animals. Environment, housing and management” (
http://protect.gost.ru/document.aspx?control=7&id=202494) and Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 “On the Protection of Animals Used for Scientific Purposes.” The animals were kept in accordance with the sanitary and epidemiological rules 2.2.1.3218-14 “On Sanitary and Epidemiological Requirements for the Design, Equipment, and Maintenance of Experimental Biological Clinics (Vivariums),” approved by the Resolution of the Chief State Sanitary Doctor of the Russian Federation Number 51 of 29 August 2014. All manipulations with the animals were approved by the Bioethical Commission. All the experiments were approved by the Institutional Animal Care and Use Committee of V.V. Zakusov Research Institute of Pharmacology, Moscow (order number 68 of 17 October 2016). The work was performed within the framework of the government contracts of the Ministry of Science and Higher Education of the Russian Federation (Project 0521-2019-0002) in accordance with the protocol number 28 from 5 December 2018 and protocol number 2 from 9 February 2019. The article doesn’t include human studies and studies using human biomaterials, and therefore doesn’t contradict the rules of the WMA Declaration of Helsinki.
3.4.3. Open Field Test
The open field apparatus was constructed as a white plywood arena with a diameter of 75 cm with white boards of 30 cm high. The arena was illuminated with 1500 lx by shadowless lamps fixed at the height of 60 cm above the floor. The arena of the open field was divided by 4 concentric circles, which in turn were divided into sectors by radii in a way that the peripheral circle consisted of 16 identical curvilinear squares. All surfaces of the OP were smooth, matte, without protrusions. The markings on the floor of the OP were applied with black matte paint, which did not protrude above the floor’s surface.
The experiment was carried out in accordance with the procedure described in [
25]. Adult male Balb/c mice were used in this experiment. After 30 min of intraperitoneal administration of vehicle and compounds in the form of suspension in 1% Tween 80 water solution, the animals were placed in a dark chamber for 1 min, and then on the one of the peripheral squares of the arena. The number of squares crossed in the periphery (SCP) and in the central regions (SCC), the number of entries into the center (C) and number of rearing (R) were traced during 3 min. After each individual test session, the equipment was washed with soap and water, cleaned with 70% ethanol and dried. Then, the subsequent mouse was tested.
3.4.4. Elevated Plus-Maze Test (EPM)
The EPM apparatus consisted of four arms elevated by 40 cm above the floor, with each arm positioned at 90° relative to the adjacent arms. Two of the arms were enclosed with high walls (65 × 5 × 15 cm), and the other arms were connected via a central area (5 × 5 cm) to form a plus sign. The maze floor and the walls of enclosed arms were painted in black. The room was illuminated with 50 lx.
The experiment was carried out in accordance with the procedure described in [
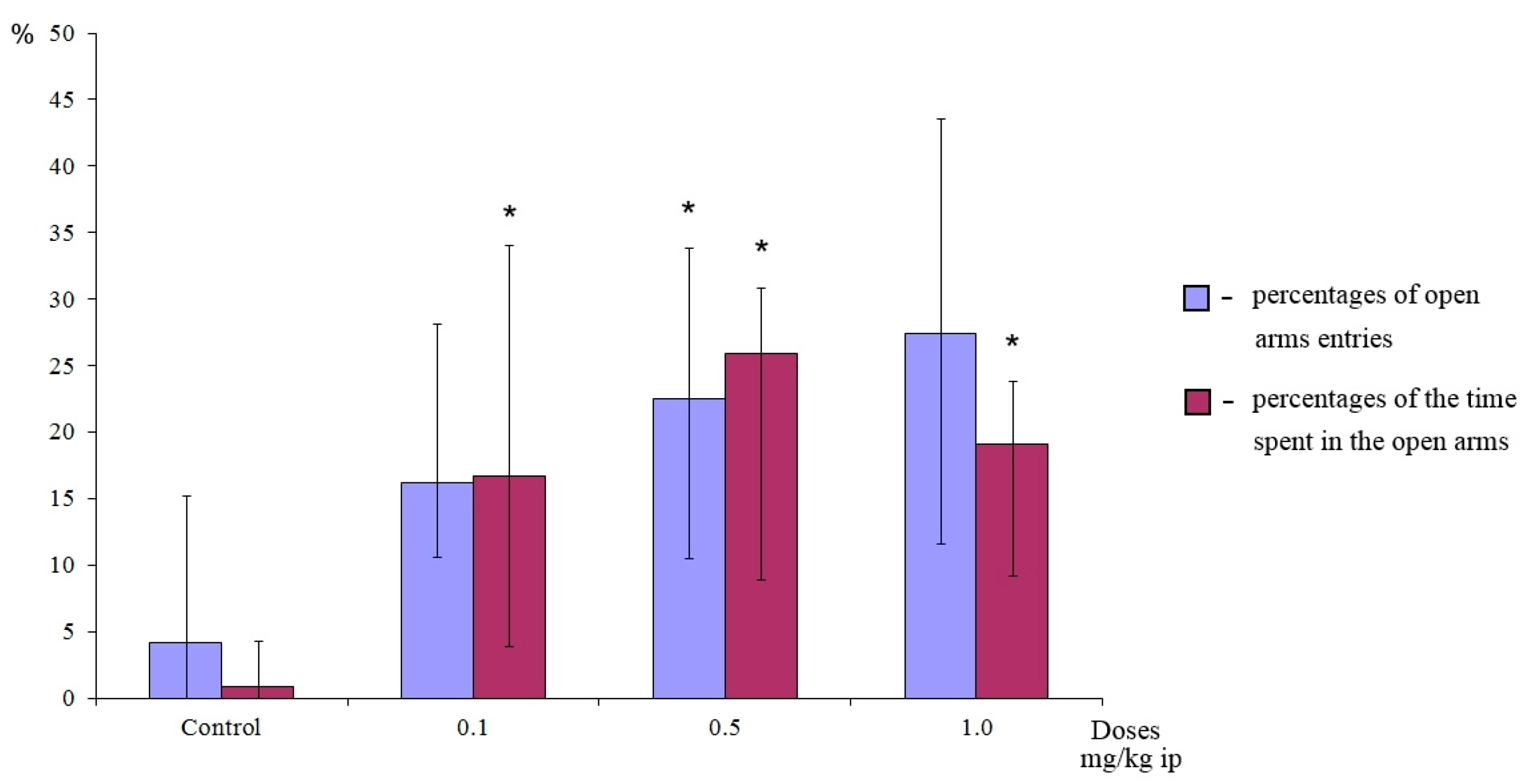
28]. Adult male ICR mice were used in this experiment. The animals were treated with distilled water and compounds as a suspension in 1% Tween 80 water intraperitoneally, 30 min prior to the test. The experiment was performed between 09:00 and 14:00. Each mouse was individually placed on the central platform facing toward an open arm. The frequency and durations of entries into the open and closed arms were observed for 5 min. An entry was counted when all four paws of the mouse entered an open or closed arm. The percentage of time spent (duration) in the open arms (100 × open/(open + enclosed)) and the percentage of the number of open arm entries (frequency, 100 × open/total entries) were calculated for each animal. The apparatus was thoroughly cleaned after each run.
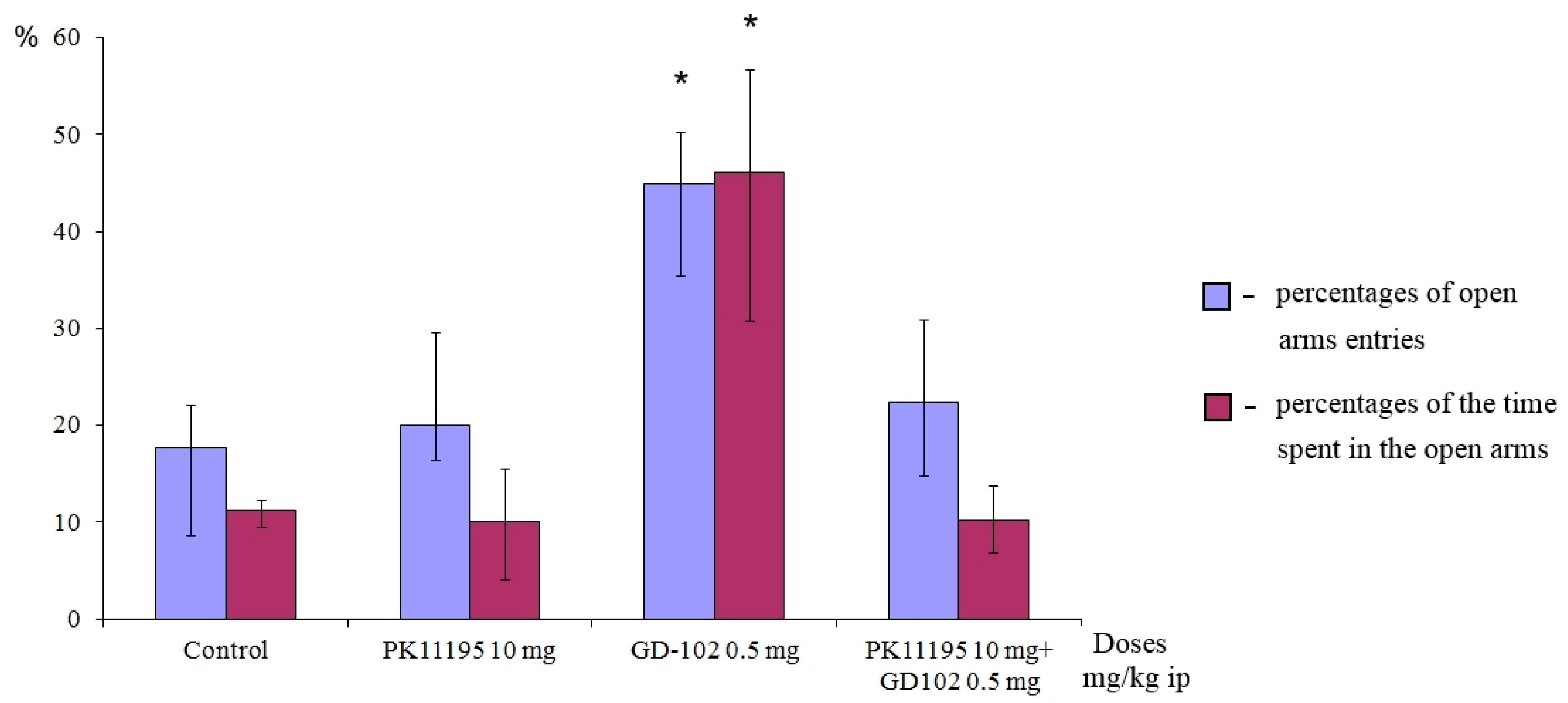
3.4.5. Influence of the PK11195 on Anxiolytic Activity of GD-102 in EPM Test
The experiment was carried out in accordance with the procedure described in [
28]. Adult male ICR mice were used in this experiment. The animals were divided into four groups, 8 mice in each group. Group 1 consisted of control animals. Control group received distilled water. Distilled water was administered twice: 60 min and 30 min before testing. Group 2: PK11195 (10.0 mg/kg) was administered 60 min before and distilled water 30 min before testing. Group 3: distilled water was administered 60 min before and compound GD-102 (0.5 mg/kg) 30 min before testing. Group 4: PK11195 (10.0 mg/kg) was administered 60 min before and compound GD-102 (0.5 mg/kg) 30 min before testing. Animal behavior was evaluated in EPM test. The animals were treated with vehicle and compounds as suspensions in 1% Tween 80 water solution intraperitoneally.
3.4.6. Registration of Spontaneous Motor Activity
The infrared actimeter Panlab (Spain) was used to register spontaneous locomotor activity [
33]. The system consists of 2 dimensional (x and y coordinates) square frames, a supporting frame and a control device. Each frame includes 16 × 16 infrared beams. The frames were controlled by a control unit and registered with the ActiTrack software, which exports the data via RS 232 serial port to the Microsoft Excel compatible format. Measured parameters: general motor activity (total indicator of horizontal activity and stereotypy), stereotyping, i.e., movement in the absence of movement, horizontal activity, maximum travel speed (cm/s), average travel speed (cm/s), distance travelled (cm). The animals were preliminarily adapted in the experimental chamber for 20 min to exclude the orientation component of motor activity. The measurements for spontaneous locomotor were carried out within 10 min.
3.4.7. Statistical Analysis
The statistical analysis was carried out using a nonparametric analogue of analysis of variance according to Kruskal–Wallis with further processing according to the Mann–Whitney test with Bonferoni’s correction.
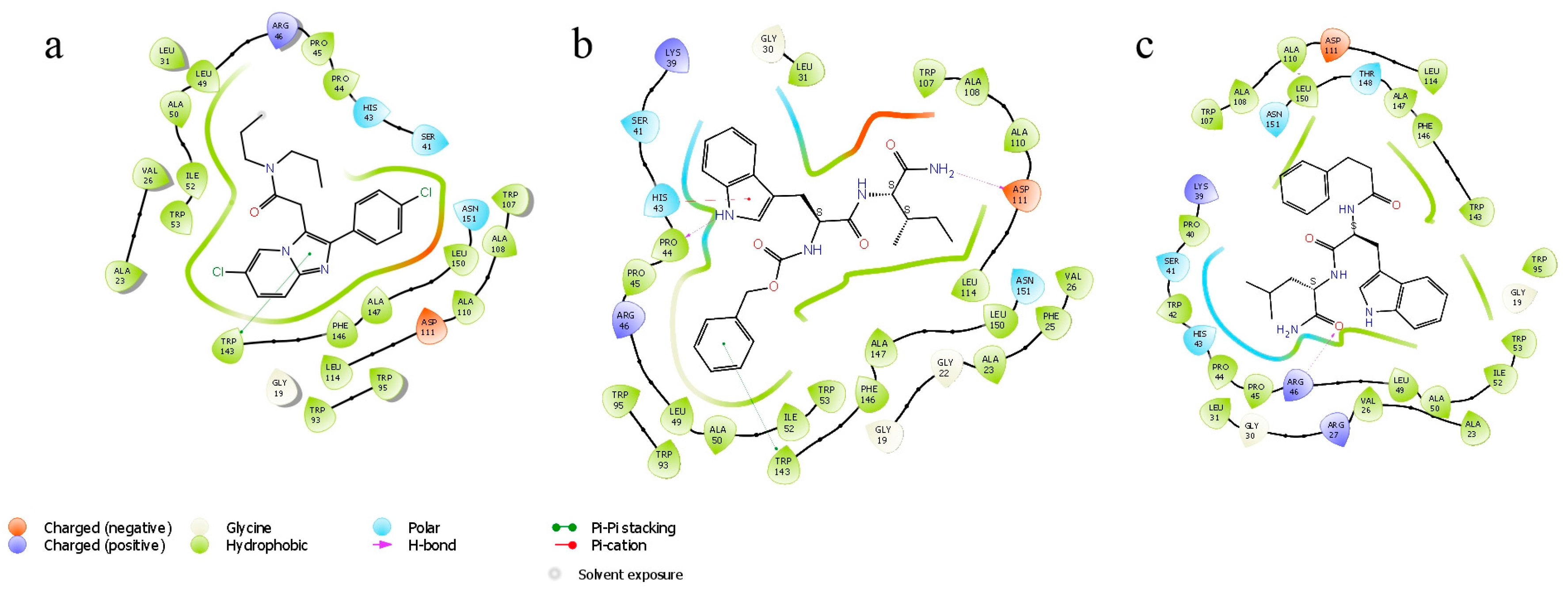
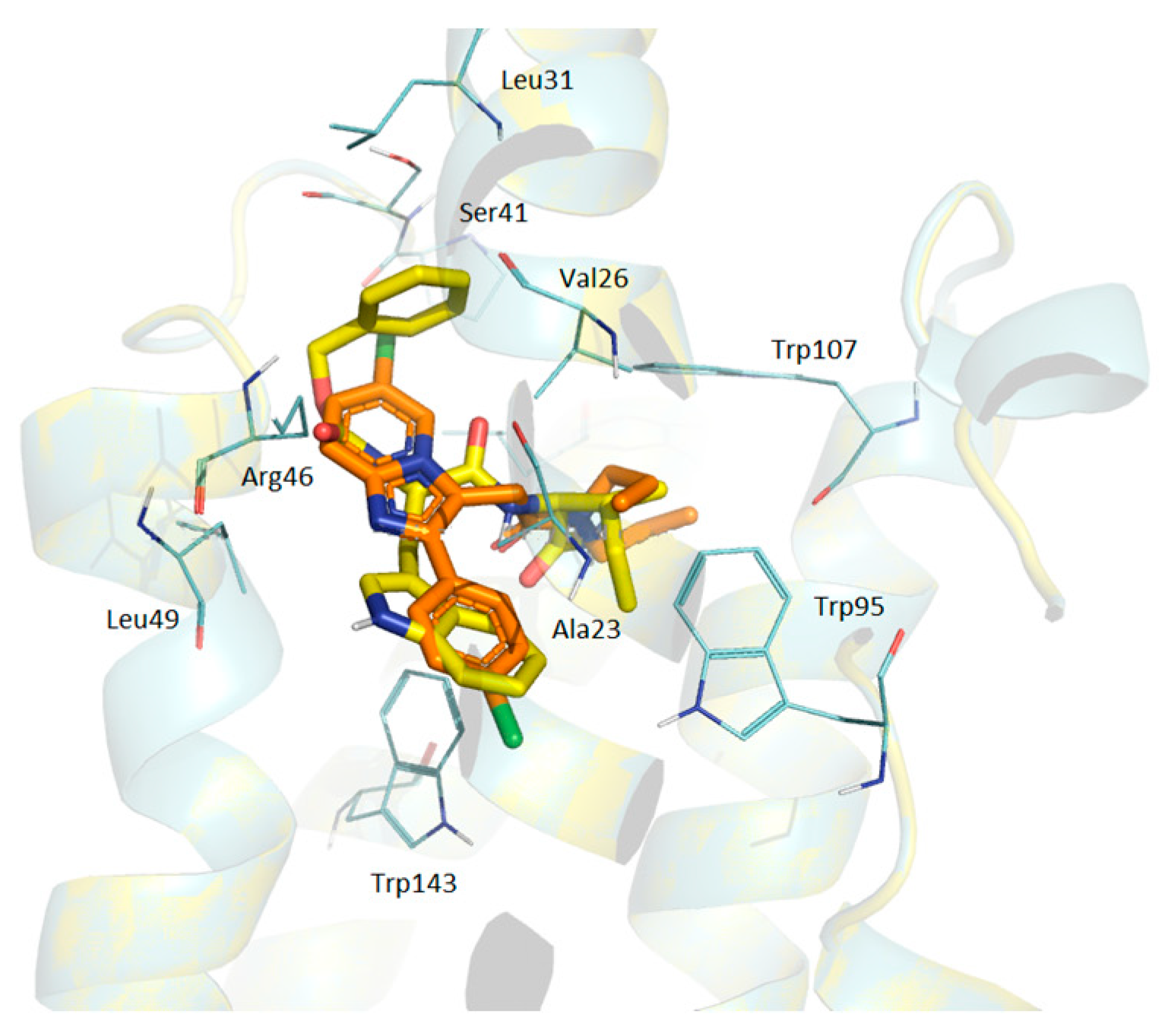
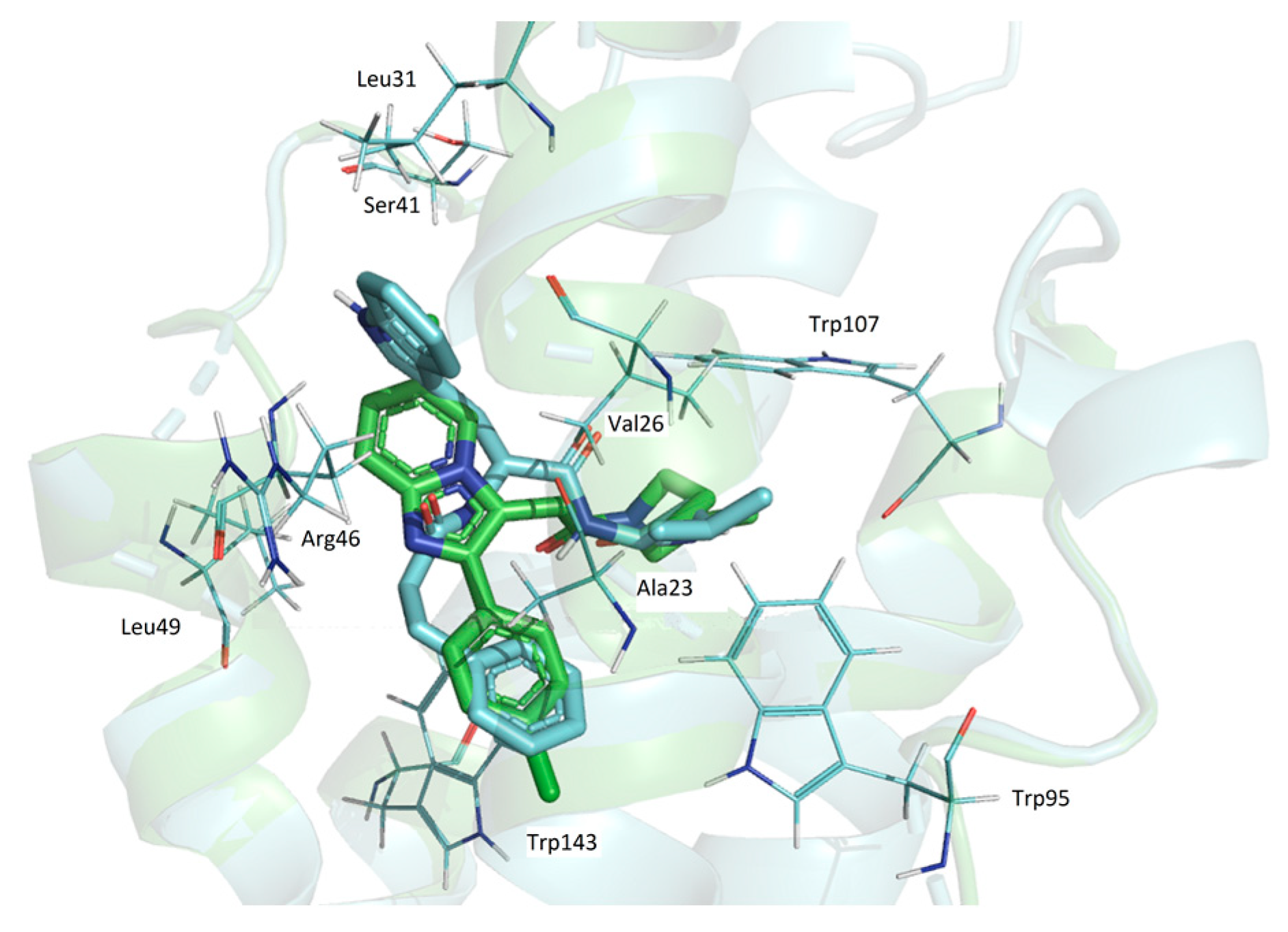
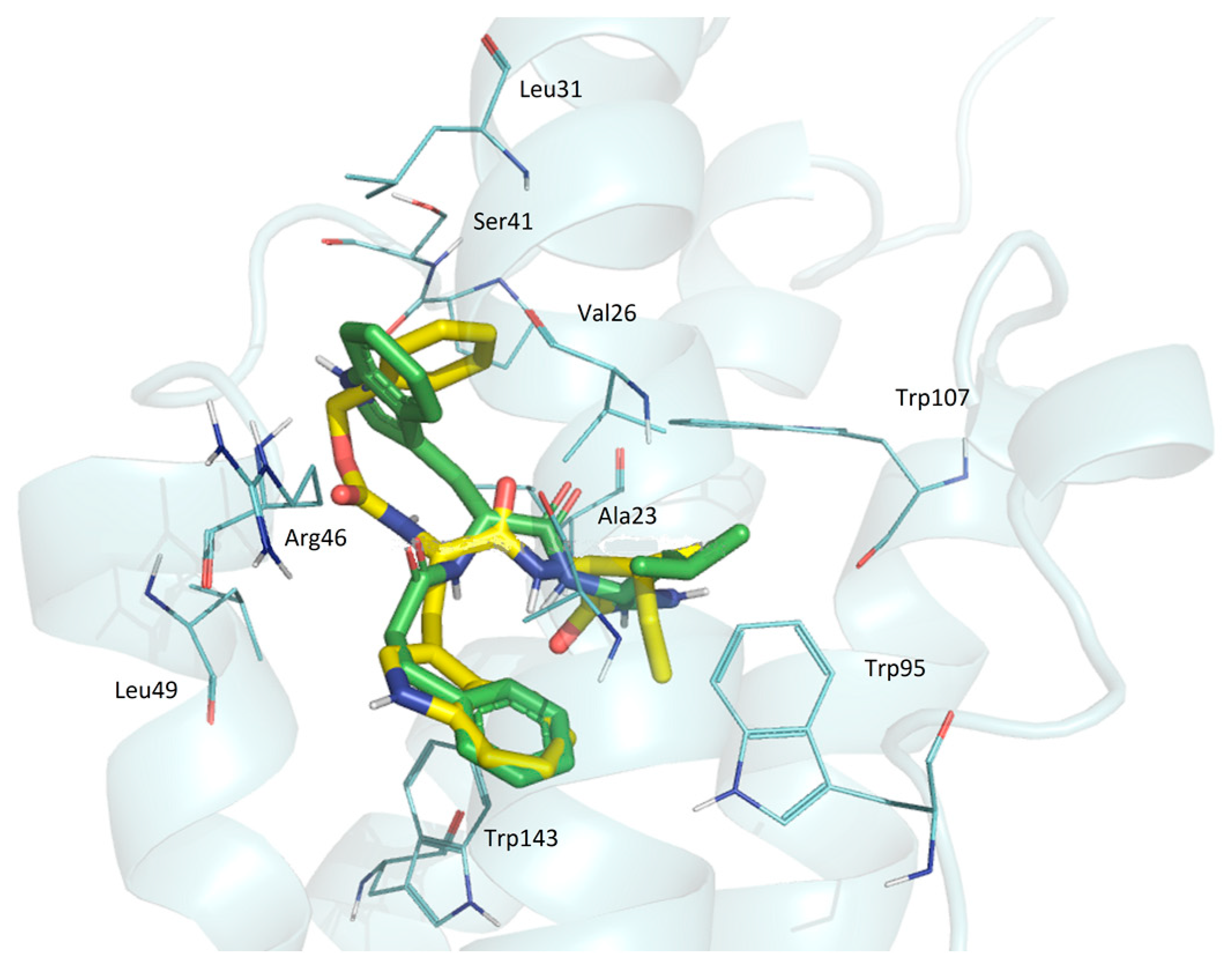
3.4.8. Docking Simulations Details
Molecular docking for compounds was performed with the active center 2MGY in complex with PK1195 taken from the (RCSB Protein Data Bank) PDB database. Protein and peptides were prepared using the Schrodinger Protein Preparation Wizard using the protocol [
34,
35]. Water molecules were removed because they do not participate in the ligand–receptor interaction. Ligands were prepared by the Ligprep module using Epik in the Schrödinger package to enhance protonation and tautomeric states at 7.0 ± 2.0 pH units. Confgen with OPLS 2005 force field was used to generate conformers. Docking settings: flexible ligands, solid receptor. The binding site was defined as a box surrounding PK 11195 at a distance of 20 A. Docking was performed in Glide v8.1. using SP peptide settings, poses were rendered in Maestro.
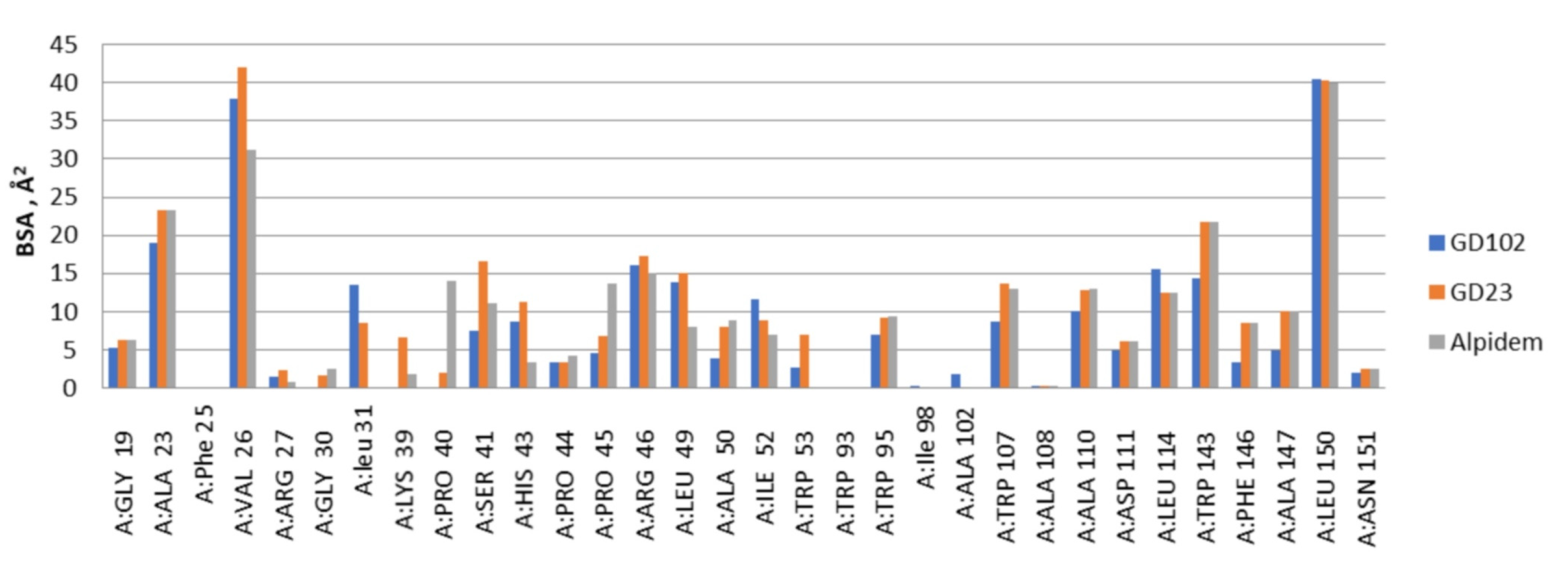
Pisa program (“Protein interfaces, surfaces and assemblies” PISA service at the European Bioinformatics Institute web server (
http://www.ebi.ac.uk/pdbe/prot_int/pistart.html)) was used for calculations of the ligand–receptor interfaces and buried surface areas [
22]. The superpositions of the TSPO and ligands complexes were performed by Cα atoms of all the residues (the Lsqkab program from the CCP4 library was used). Results of modeling were visualized with PyMol software (The PyMOL Molecular Graphics System, Version 0.99 Schrödinger, LLC., New York, NY, USA).