Abstract
Bacteria are social organisms able to build complex structures, such as biofilms, that are highly organized surface-associated communities of microorganisms, encased within a self- produced extracellular matrix. Biofilm is commonly associated with many health problems since its formation increases resistance to antibiotics and antimicrobial agents, as in the case of Pseudomonas aeruginosa and Staphylococcus aureus, two human pathogens causing major concern. P. aeruginosa is responsible for severe nosocomial infections, the most frequent of which is ventilator-associated pneumonia, while S. aureus causes several problems, like skin infections, septic arthritis, and endocarditis, to name just a few. Literature data suggest that natural products from plants, bacteria, fungi, and marine organisms have proven to be effective as anti-biofilm agents, inhibiting the formation of the polymer matrix, suppressing cell adhesion and attachment, and decreasing the virulence factors’ production, thereby blocking the quorum sensing network. Here, we focus on plant derived chemicals, and provide an updated literature review on the anti-biofilm properties of terpenes, flavonoids, alkaloids, and phenolic compounds. Moreover, whenever information is available, we also report the mechanisms of action.
1. Introduction
The classical concept of microorganisms as solitary entities was revised when it appeared clear that bacteria, as well as fungi, are social organisms able to build complex communities, like biofilms. This condition facilitates survival in adverse conditions, allowing microorganisms to grow and colonize host tissues or inert surfaces, including implants and urinary catheters [1], with adverse effects on human health. Therefore, a great effort is needed to find new drugs able to counteract this phenomenon, with natural products in the hotspot as possible promising candidates.
Biofilm has a very complex architecture. Microorganisms embedded in the biofilm are not simply attached to a surface; complex molecular signals, inducing the spatial and temporal reorganization of planktonic cells, are activated in response to environmental stimuli. As a consequence, bacteria in the biofilm show altered expression of surface molecules, nutrient utilization, and virulence factors, along with increased stress resistance. These factors allow their survival in unfavorable environments, but also resistance to antimicrobial compounds and evasion of host immunity [2,3]. Therefore, it is important for the host to eliminate bacteria before biofilm is organized.
Even more complex is the situation in which biofilms are polymicrobial [1]. Co-infecting species in the polymicrobial biofilm can aggravate the severity of the disease, complicating the choice of antibiotic therapy, with a consequent delay in host recovery. One example of a polymicrobial infection is given by Pseudomonas aeruginosa and Staphylococcus aureus. They separately colonize different niches (soil and water in the former, and the respiratory tract and skin in the latter), but can cause chronic wound infections that are resistant to conventional antimicrobial therapy [4].
P. aeruginosa is one of the most common pathogens in nosocomial and ventilator-associated pneumonia, cystic fibrosis (CF), meningitis, abscess, soft tissue and urinary tract infections, infection of the cornea, and erythema of conjunctiva. In addition, it can cause catheter-associated and chronic lung infections in immunocompromised patients [5]. Considering its ability to form biofilm on medical devices and to take advantage on the host with an altered normal flora, due to administration of broad-spectrum antibiotics, P. aeruginosa has induced researchers to study and develop new therapeutic strategies.
On the other hand, S. aureus is a leading cause of both community- and hospital-acquired infections associated with high morbidity and mortality, due also to the emergence of multi-drug resistant strains such as MRSA (Methicillin-Resistant S. aureus) [6]. Although it is found in the environment, and can be part of normal human flora (colonizing the skin and mucous membranes of healthiest individuals), if it finds the conditions to enter the bloodstream or internal tissues it may be responsible for different, potentially serious, infections.
Cystic fibrosis, otitis media, periodontitis, urinary tract infections, and osteomyelitis, are all polymicrobial [7]. For example, P. aeruginosa and S. aureus are often found to coinfect the lungs of patients with CF, and alginate overproduction may be an important factor driving P. aeruginosa coinfection with S. aureus [8,9].
Multiple and complex are the mechanisms that lead to acquired resistance to antibiotics in microorganisms. Among these, reduced permeability through the cell membrane, modification of the molecular target, increased efflux pump expression, and degradation of the antibiotics are all important factors that reduce drug efficacy on free planktonic cells. In addition, in bacterial biofilm, other modifications are induced, like decreased growth rates and metabolism, and induction of cell biofilm-specific phenotypes, known as persister cells [10]. As a consequence, antibiotics active against planktonic cells can result in being ineffective on sessile/dormant cells. Many studies have attempted to understand the molecular mechanism underlying antibiotic inefficacy in sessile bacteria. The reckless use of antibiotics has led to the development of multidrug resistant microorganisms, due to the selective pressure exerted on their survivability; therefore, gene expression modulation of microorganism virulence factors, rather than killing them, has been widely explored. Natural products are mainly directed at inhibiting bacterial growth or at reducing their pathogenicity, acting on specific genes that control important virulence factors [11]. In addition, several natural compounds have also been explored for their properties as quorum sensing (QS) inhibitors [12].
Plants, microorganisms, as well as marine organisms, represent an inestimable source of anti-biofilm agents. Examples of these compounds are styrylpyrones and quinic acid derivatives from the polar extract of Helichrysum italicum (active against P. aeruginosa [13]), pholretin, (specifically reducing enterohemorrhagic Escherichia coli O157:H7 (EHEC) biofilm formation [14]), or alkaloids from marine sponges (active against gram-positive and gram-negative bacteria [15]).
Many studies have been conducted with the aim of discovering novel antimicrobial and anti-biofilm agents [16]. Anti-biofilm intervention can be aimed at mechanical eradication/destruction (modification of surface properties of the biofilm carrier and mechanical stability of the biofilm, application of hydrolytic enzymes disrupting its structure and composition, and others) as well as acting on the regulatory system of its formation (QS and virulence factors) [17].
The aim of this review is to examine the most recent literature in the field of plant-derived natural products as potential novel anti-biofilm agents against P. aeruginosa and S. aureus. We briefly summarize the mechanisms of biofilm formation and QS for both bacteria objects of the present review. The anti-biofilm effects of natural products, mainly relying on the inhibition of formation of the polymer matrix, suppression of cell adhesion and attachment, and decrease of virulence factor production, thereby blocking QS network, are summarized. Furthermore, as mentioned above, biofilm formation is driven by sophisticated regulatory mechanisms, involving events both at single-cell level and at cell population level. Thus, in this paper we will discuss the molecular mechanisms associated with the anti-biofilm effects of terpene, flavonoids, alkaloids, and phenolic compounds.
2. Quorum Sensing Mechanism
Among the regulatory mechanisms that ensure timely adaptation of microorganisms to the environment, QS is the most studied since it plays a critical role in the formation of biofilm and its surrounding extracellular polymeric substance (EPS). The latter is important to keeping the basic architecture of a biofilm matrix, and forms the defense shield for bacteria inside the biofilm [18]. EPS quantification can directly correlate with the extent of biofilm formation. The EPS protects bacteria from the antimicrobial activity of antibiotics. It comprises 50–90% of the total organic mass of the biofilm and contains exopolysaccharides, extracellular DNA (eDNA), proteins, lipids, and humic substances [19]. Biofilm is not just a barrier to avoid the deleterious effect of antibiotics but also increases bacteria pathogenicity, through the activation of genes that control their virulence. QS is a cell-to-cell communication, depending on density population, and it is differently gene-controlled in gram-negative and gram-positive bacteria, as well as in fungi. It controls the expression of important bacterial genes that encode for virulence factors [20,21]. The QS system is mediated by autoinducers (AIs), identified as oligopeptides and acylated homoserine lactones (AHLs) in gram-positive and gram-negative bacteria, respectively.
2.1. QS Molecular Signaling Network of Gram-Negative Bacteria
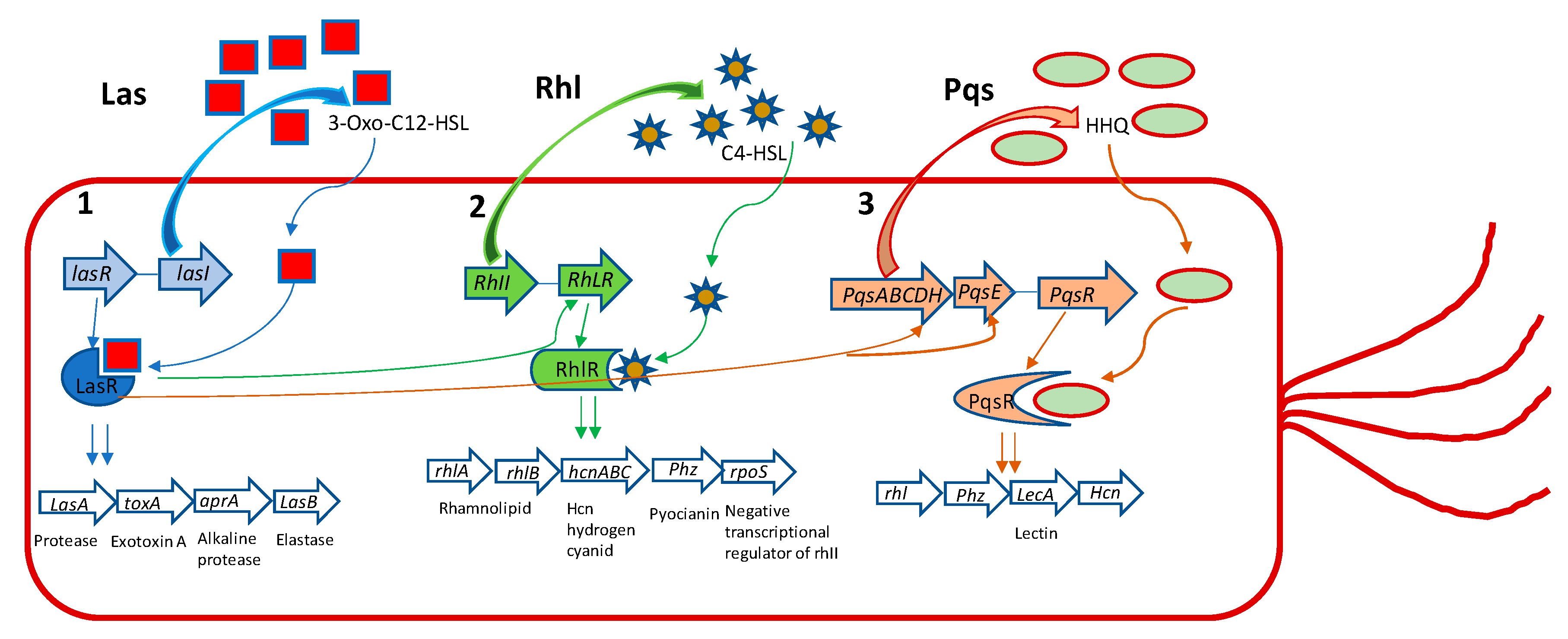
P. aeruginosa has three main QS systems, named las, rhl, and pqs. Las and rhl modulate the synthesis of AI’s N-(3-oxododecanoyl)-l-homoserine lactone (3-oxo-C12-HSL) and N-butanoyl-l-homoserine lactone (C4-HSL) as their autoinducers, respectively (Figure 1).
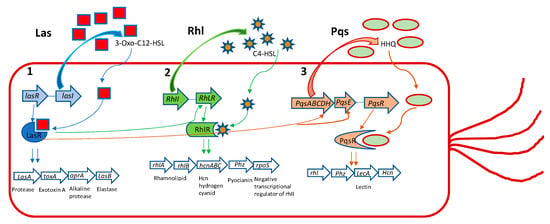
Figure 1.
The three major Pseudomonas aeruginosa QS systems with their main effects. (1) LasI produces 3-oxo-C12-HSL, which acts on LasR. This leads to induction of aprA, lasA, las B, and toxA genes and other virulence genes that are under its regulation. (2) RhlI produces C4-HSL, that acts on RhlR, which induces phz, lasA, rpoS, lasB, rhlAB, and hcnABC gene expression. (3) PqsABCDH produces HHQ that acts on PqsR, regulating the gene expression of LecA, Phz, Hcn, and rhl. Additionally, LasR positively regulates 2-heptyl-1H-quinolin-4-one (HHQ) through the complex LasR-3-Oxo-C12-HSL on PqsH. LasR positively regulates rhlR, again through the complex LasR-3-oxo-C12-HSL and rhII. Finally, LasR positively regulates HHQ through PqsE. Elastase and protease exert their effect on disruption of the epithelial barrier and matrix protein (collagen, elastin, etc.). ToxinA induces cell death favoring the establishment of infection and colonization. The alkaline protease is involved in degradation of the host complement system and cytokines, playing a role in immune evasion and persistent colonization. Rhamnolipids favor immune evasion and biofilm formation. Hydrogen cyanide reduces lung function. Pyocyanin, among various effects, causes oxidative stress and, like lectinA, induces paralysis of airway cilia. RpoS is a negative transcriptional regulator of rhlI.
The third QS system in P. aeruginosa is Pseudomonas quinolone signal (pqs), that is a non-AHL-mediated QS signaling pathway, using alkyl-4-quinolones (AQs), among which 2-heptyl-3-hydroxy-1H-quinolin-4-one (PQS) and 2-heptyl-1H-quinolin-4-one (HHQ) are signal molecules [22,23]. Even though different kinds of AIs are used by these QS systems, they are interconnected and modulate the activities of each other. As signal molecules bind to receptor LasR, RhlR, or PqsR they subsequently activate the expression of the QS-related genes of P. aeruginosa, regulating the production of virulence factors, such as exoenzymes, proteases, elastases, pyocyanine, rhamnolipds, alginate, EPS etc., and other important cellular processes that allow the bacteria to establish an infection in the host tissue [23].
2.2. QS Molecular Signaling Network of Gram-Positive Bacteria
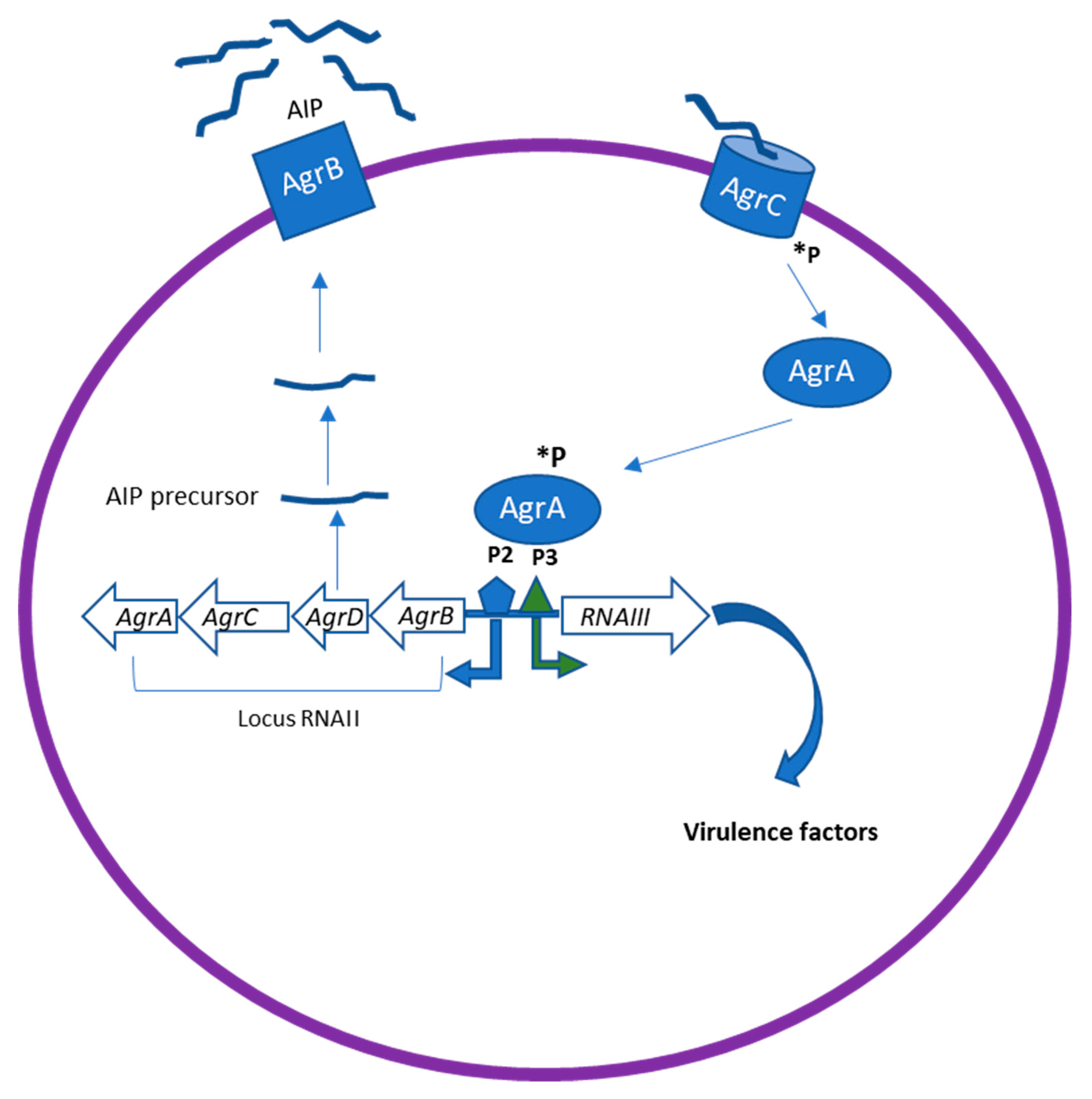
The Agr system has been identified as the most classical QS system in gram-positive bacteria. It therefore plays a major role in staphylococcal pathogenesis [24]. The Agr locus comprises two divergent transcriptional units, RNAII and RNAIII, containing genes responsible for the production of many virulence factors in Staphylococcus spp (Figure 2).
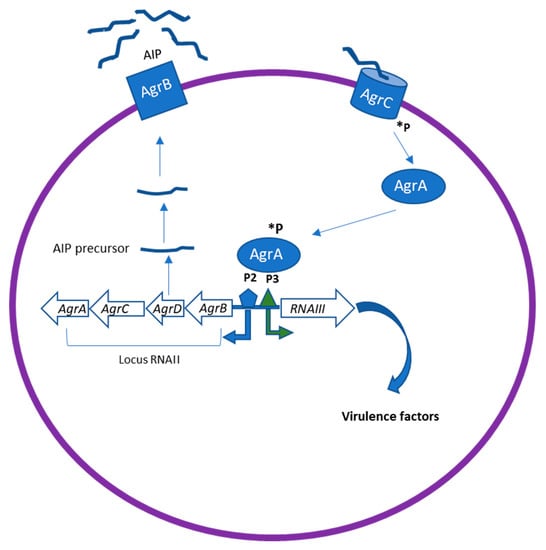
Figure 2.
The Agr quorum sensing (QS) system in Staphylococcus spp. The Agr locus comprises two divergent transcriptional units, RNAII and RNAIII, containing genes responsible for the production of many virulence factors in S.aureus. AgrD encodes the precursor of AIP, which is then processed and transported through AgrB. The processed AIP interacts with a histidine sensor kinase receptor AgrC, which in turn leads to the phosphorylation (*P) of AgrA. This leads to the activation of the regulator AgrA, which binds to the chromosomal P2 and P3 promoter regions to upregulate transcription of RNAII and RNAIII. RNAIII can induce upregulation of virulence factor expression as proteases, toxins, and degradative enzymes.
RNAII encodes the core QS circuit protein AgrABCD, whereas RNAIII regulates the expression of multiple virulence genes. AgrB and D are involved in the production of the auto-inducing octa-peptides (AIPs) [24]. AgrD encodes the precursor of AIP, which is then processed and transported to the extracellular environment by the integral membrane protease AgrB. When it is released in the environment at high concentration, AIP binds to the kinase receptors (AgrC) on the bacteria membrane, which in turn leads to the phosphorylation of AgrA. This leads to the activation of the regulator AgrA, which binds to the chromosomal P2 and P3 promoter regions to upregulate transcription of RNAII and RNAIII [24]. RNAIII is thus the intracellular effector of the Agr system. Agr can downregulate the expression of cell surface-associated proteins (microbial surface components recognizing adhesive matrix molecules, MSCRAMMs) and upregulate the expression of virulence factors, including toxins (phenol-soluble modulins PSMs, alpha-toxin, delta-toxin (hld), etc.) and degradative exoenzymes (proteases SspA, SspB, Spl, etc.). In addition, Agr induces an increased expression of methicillin resistance genes [25]. Inhibition of AgrA and RNAIII transcription represent an effective strategy for suppressing the virulence of S. aureus.
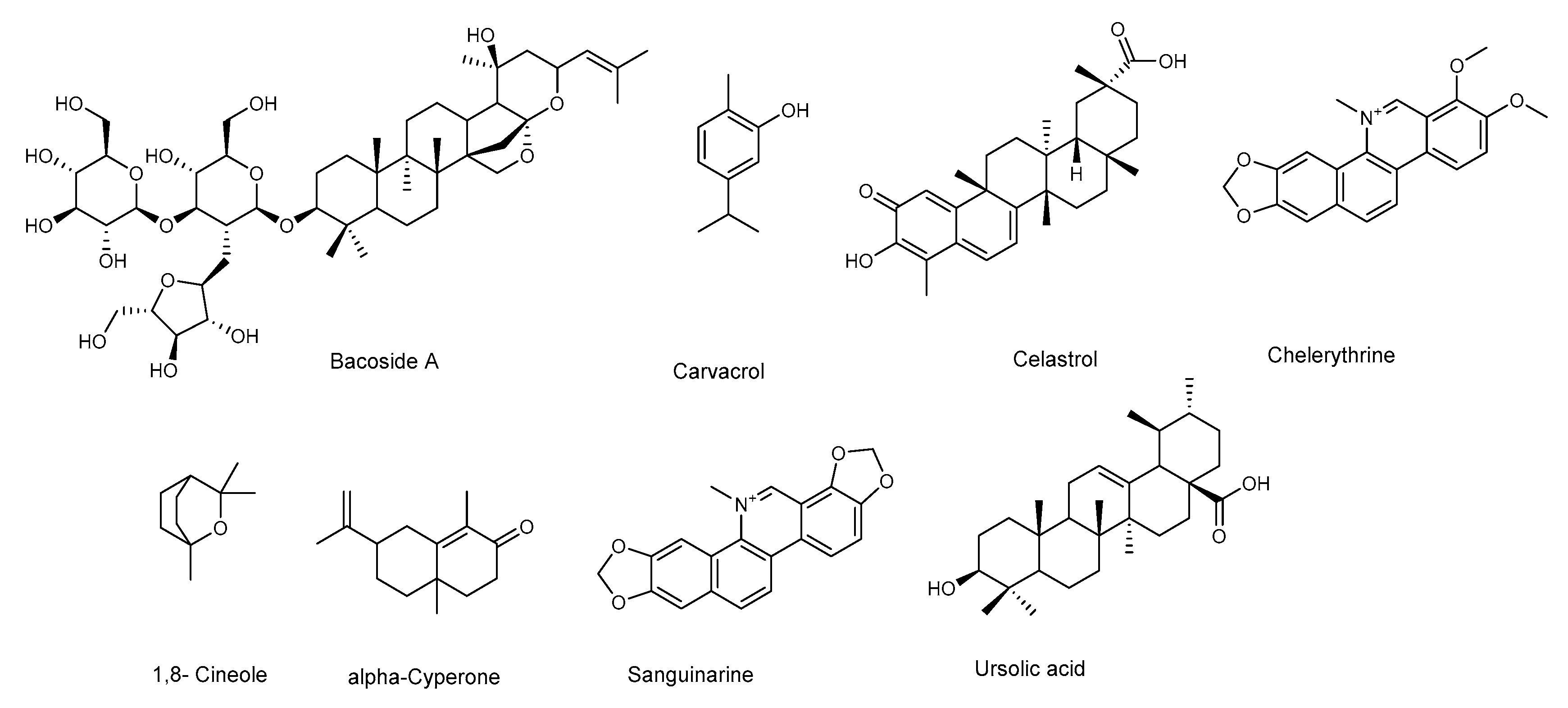
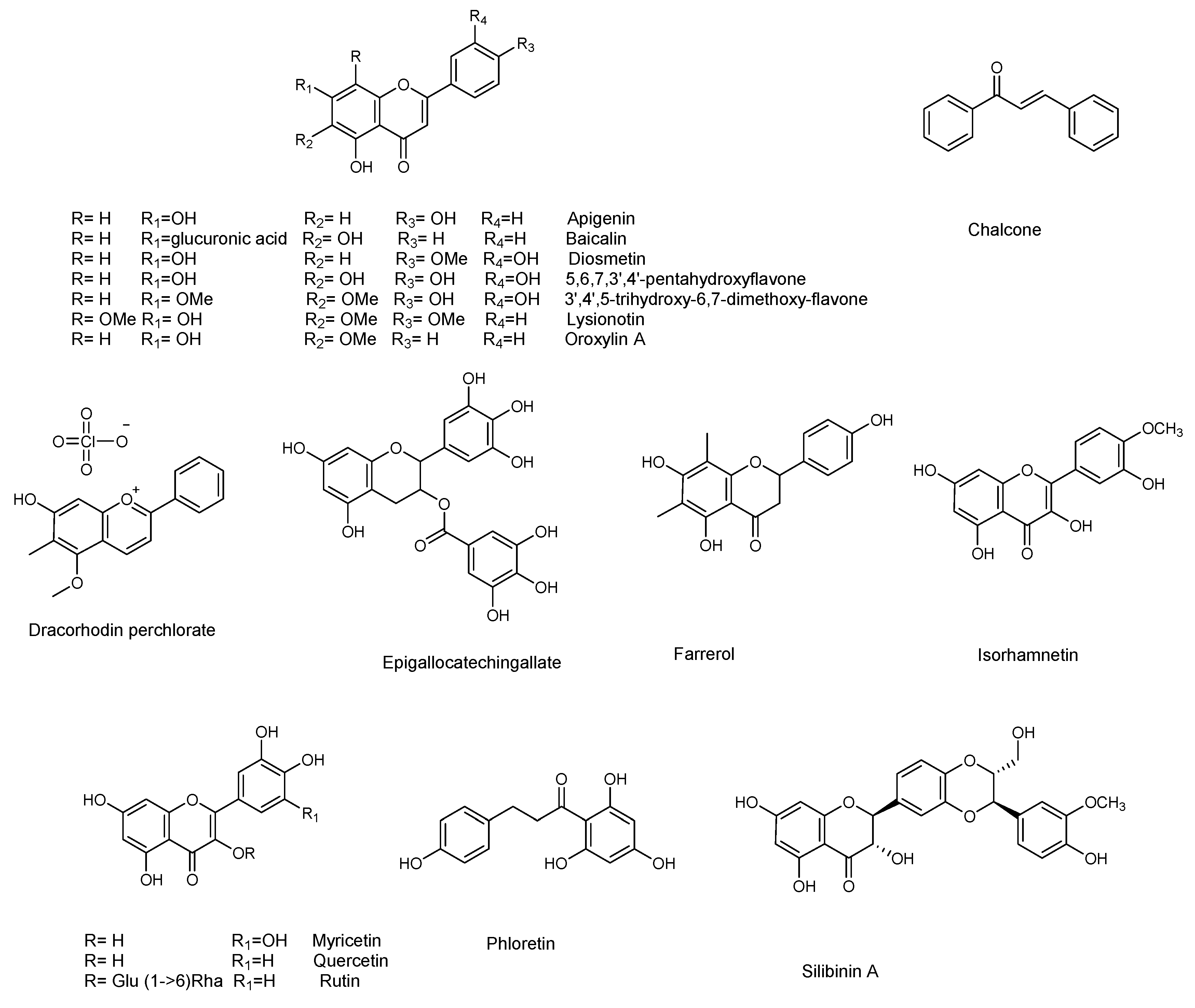
3. Anti-Biofilm Activity of Natural Compounds Against Pseudomonas aeruginosa
P. aeruginosa is responsible for a wide range of opportunistic infections, but more complicated to manage are the biofilm-related nosocomial infections, including cystic fibrosis, urinary tract, and eye and burn wounds in immunocompromised patients. Increasing incidents of resistant biofilm infection have resulted in high mortality rates worldwide. Plant-derived anti-biofilm products identified against P. aeruginosa include alkaloids, organosulfur compounds, flavonoids, phenolic compounds, and terpenoids (Figure 3 and Figure 4). A number of natural products have been tested for their anti-biofilm potential using mainly crystal violet, or safranin staining method, the evaluation of QS-related antivirulent activity, as well as the capacity to eradicate preformed-biofilm. The section below describes the molecular mechanisms associated with the anti-biofilm effects of the above-mentioned classes of natural products.
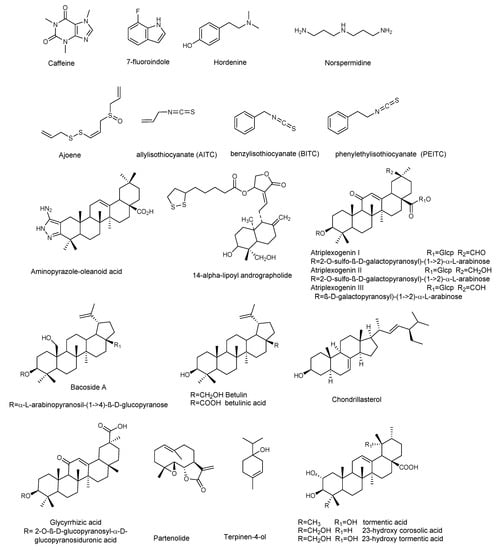
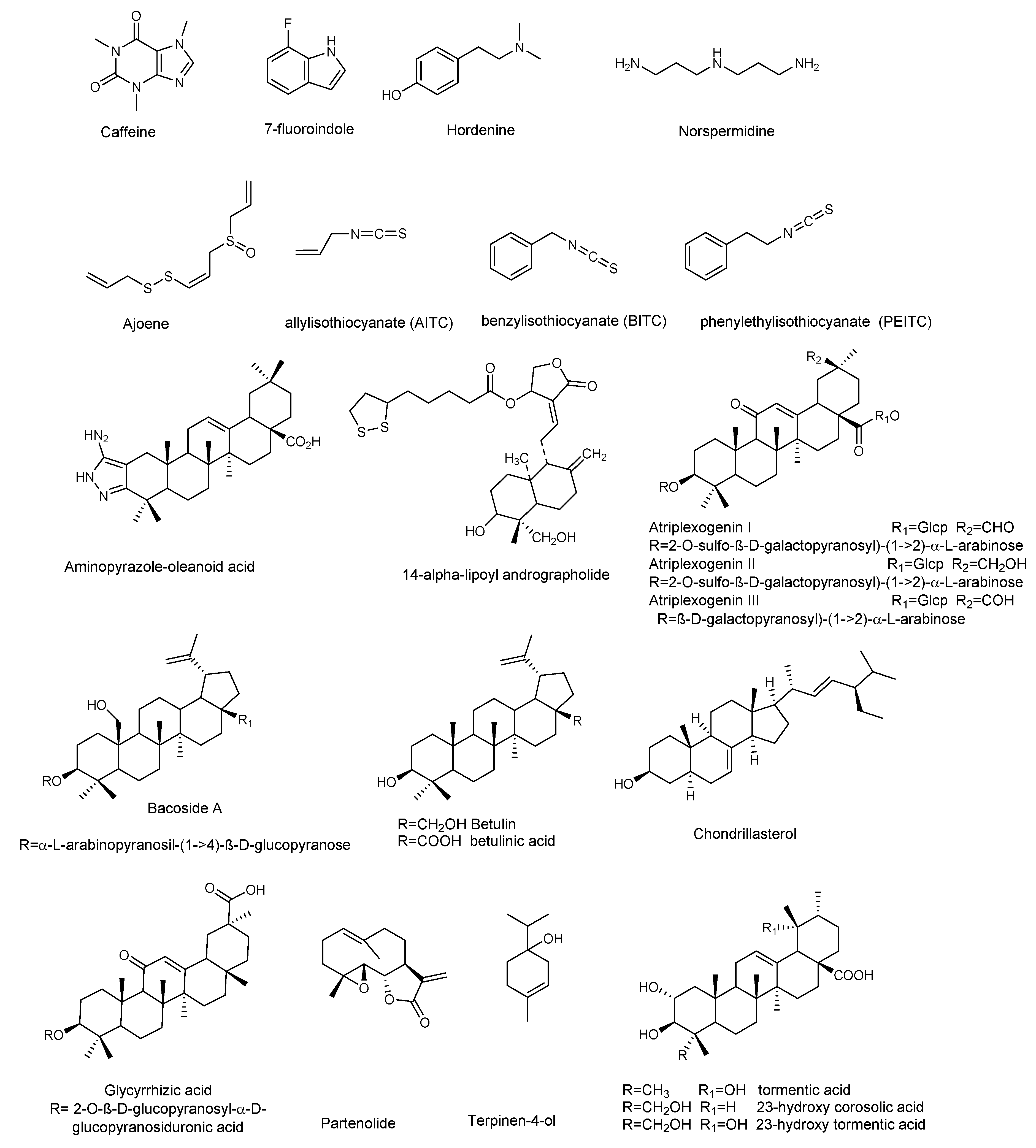
Figure 3.
Chemical structures of compounds containing nitrogen, organosulfur compounds, and terpenoids active against P. aeruginosa.
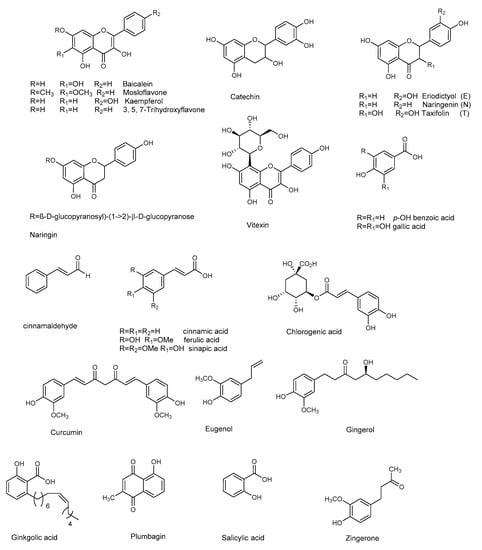
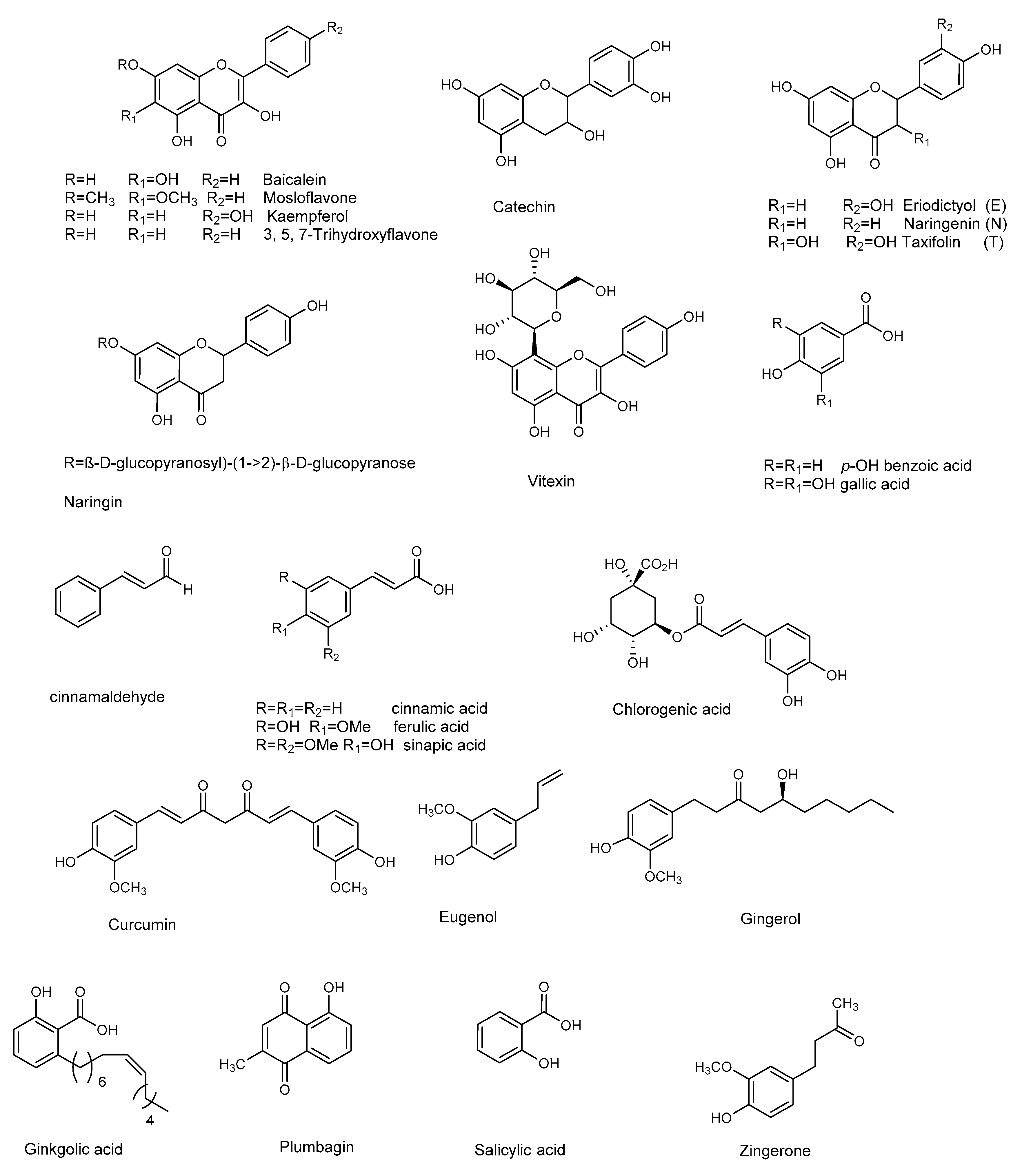
Figure 4.
Chemical structures of flavonoids and other phenolic compounds active against P. aeruginosa.
3.1. Alkaloids and Nitrogen-Containing Compounds
Alkaloids, a large group of basic (mostly) heterocyclic nitrogen containing natural products, are promising candidates for drug discovery. Hordenin is a dietary phytochemical from sprouting barley, traditionally known for its properties as an antimicrobial compound, inhibitor of monoamine oxidase B, stimulator of gastrin release, and as a vasoconstrictive [26]. Recently, Zhou et al. investigated the properties of hordenine as a QS-inhibitor anti-biofilm agent and as an aminoglycoside antibiotic-accelerant against P. aeruginosa PAO1 [27]. Hordenine reduced AHLs production and, subsequently, biofilm formation, motility, and virulence factors as protease, elastase, rhamnolipid, pyocyanin, and pyoverdine (Table 1 and Table 2), all important indicators of QS operon in P. aeruginosa. The authors analyzed the effect of hordenine on the expression of four QS-related genes, that is lasI, lasR, rhlI, and rhlR, in P. aeruginosa PAO1. They observed a significant down-regulation of all genes after exposure to 1.0 mg/mL of hordenine. The interest of these results lies in the ability of hordenine to work as a competitive inhibitor of QS (Table 2), exerting a fine gene regulation of major virulence factors in P. aeruginosa, thus contrasting the infection. Rhamnolipids are a class of glycolipids regulated by the rhl system (Figure 1), and play a vital role in surface motility and biofilm initiation. They are important bacterial surfactants and a key virulence determinant in P. aeruginosa [28]. Rhamnolipids facilitate the degradation of the biofilm matrix and activate the motility to favor the metastatic colonization of new sites. Moreover, production of rhamnolipids by P. aeruginosa that colonizes intubated patients was associated with the development of ventilator-associated pneumonia [29]. Other alkaloids were reported for their antibiofilm effects: caffeine [30] and 7-fluoro indole, a synthetic indole-derivative [31]. Both compounds significantly inhibited the biofilm development of P. aeruginosa and interfered with the QS by targeting swarming, motility, and several virulence factors.

Table 1.
Compounds containing nitrogen and sulfur inhibiting biofilm formation and motility in P. aeruginosa 1.

Table 2.
Compounds containing nitrogen and sulfur inhibiting P. aeruginosa virulence factors regulated by QS 1.
Polyamines are small organic nitrogen-containing compounds, positively charged at the physiological pH required for normal cell growth in both eukaryotes and prokaryotes [32]. Among them, norspermidine displayed remarkable properties, since it can inhibit the formation of P. aeruginosa biofilm and eradicate established biofilms [33]. Norspermidine significantly inhibited the transcription level of lasR/I, rhlR/I, and mvfR, and modulated the QS-related virulence factors (pyocyanin, elastase activity, and protease) [33].
3.2. Terpenoids
Terpenes are a wide group of natural compounds characterized by enormous structural diversity, and originating from the coupling of isoprene units. Monoterpenes present in essential oils as well as di- and triperpenoids have long been used as natural medicaments, because of their antimicrobial and anti-biofilm properties. Terpinen-4-ol, the main bioactive constituent of tea tree oil, exhibited QS inhibition at sub-MIC (sub minimum inhibitory concentration) values [36], and reduced the expression of QS genes (lasI, lasR, rhlI, rhlR, rhlAB, lasB, aprA, toxA, and plcH). Further analyses showed the decrease of virulence factors in treated P. aeruginosa PAO1, thus confirming the results of QS gene expression analyses. Moreover, Terpinen-4-ol acted synergistically when used in combination with ciprofloxacin, enhancing the effectiveness of the antibiotic against P. aeruginosa. This feature makes this natural product very remarkable, because the combined use of old drugs in association with a new antimicrobial, able to potentiate or restore their efficacy, appears as a good strategy to safeguard the future effectiveness of critically important antibiotics.
Parthenolide (Figure 3) is a sesquiterpene lactone obtained from Tanacetum parthenium, a plant with well-known medicinal properties, attributable to the active components, sesquiterpenes and sesquiterpene lactones [37]. A study by Kalia et al. [38] demonstrated the ability of parthenolide to contrast P. aeruginosa PAO1 biofilm formation, reducing the production of 3-oxo-C12 HSL. Significant decrease in virulence factors and biofilm formation was observed when P. aeruginosa was treated with a sub-MIC concentration (Table 4) of parthenolide. At this concentration bacterial growth was not affected. Real time PCR demonstrated the down-regulation of autoinducer synthases (lasI, rhlI), as well as their receptors (LasR and RhlR), correlated with the down-regulation of various virulence factors like pyocyanin, protease, and swarming (Table 3 and Table 4). All the analyzed virulence factors were reduced to a level equivalent to that of the double negative mutant ΔlasIΔrhlI. The addition of autoinducers restored the virulence phenotypes, thus suggesting that parthenolide might interfere with either the synthesis or the reception of AHL. Finally, molecular docking studies evidenced the binding of parthenolide to the active site of the LasR, which may be responsible for the repression of its expression.

Table 3.
Terpenoids inhibiting biofilm formation and motility in P. aeruginosa 1.

Table 4.
Terpenoids inhibiting P. aeruginosa virulence factors regulated by QS 1.
Plant derived triterpenes also represent a good scaffold for the synthesis of analogues with improved activities. Indeed, a series of analogs of oleanolic acid showed efficacy in inhibiting biofilm formation and swarming against clinical isolates of P. aeruginosa [39]. In particular, the aminopyrazole analog (Table 3) demonstrated potent anti-swarming activity against different strains of gram-negative clinical and agricultural isolates. Notably, the authors analyzed the levels of 24 motility genes in P. aeruginosa HONKR grown on swarm plates, with or without this aminopyrazole analog, demonstrating a dose- and time-dependent reduction of gene expression of algR, whose product regulates P. aeruginosa virulence factors, including type IV pili. The results suggest that the aminopyrazole analog of oleanolic acid acts by interfering with regulation of genes for Type IV pili, bacterial appendages required for motility, thus representing a good candidate for the treatment of persistent P. aeruginosa lung infections in cystic fibrosis patients.
Ghosh et al. [40] investigated the anti-biofilm properties against P. aeruginosa of tormentic acid and 23-hydroxycorosolic acid, two ursane triterpenes isolated from Sarcochlamys pulcherrima (Roxb.) Gaud, an ethnomedicinal plant traditionally used for its anti-microbial and anti-inflammatory properties [48]. Ghosh et al. [40] observed that tormentic and 23-hydroxycorosolic acids (Figure 3) inhibited the growth of planktonic P. aeruginosa MTCC 2488 bacteria at MIC of 55 and 40 μg/mL, respectively, in comparison to untreated control. At sub-inhibitory doses, they did not inhibit bacterial growth, while being effective at reducing biofilm formation. Both compounds significantly increased the membrane potential of P. aeruginosa at the MIC values, enhancing cell membrane damage and, consequently, cell death. Notably, tormentic and 23-hydroxycorosolic acids reduced the swarming motility and the secretion of proteases and pyoverdine (Table 3 and Table 4), and in vitro and in vivo toxicity studies suggested that they were non-toxic. It was also observed that the treatment with these two triterpenes significantly reduced the bacterial load on a catheter, as well as in liver and spleen. The authors demonstrated that both triterpenoids reduced lasR, lasI, lasB, rhlI, and rhlR gene expression with respect to the untreated control. These genes are all interconnected and represent a valid tool to verify the QS modulation by natural compounds. The lasB gene encodes the metalloproteinase elastase, an important virulence factor in P. aeruginosa, since a lasB mutation decreases the virulence of the bacterium [49]. It is under the transcriptional control of lasI, which encodes a synthase that leads to formation of 3O–C12-HSL. The latter diffuses toward the surrounding cells initiating QS, interacts lasR with the transcription factor, and activates multiple virulence genes, including lasB. In silico docking studies with proteins, like the las family (lasA, lasI, and lasR), luxR, and pil family (pilB, pilT, and pilY1), showed that tormentic and 23-hydroxycorosolic acids [40], as well as a third ursane triperpene, 23-hydroxytormentic acid from Mussaenda roxburghii [41], have good binding affinity with all the selected proteins.
Other pentacyclic triterpenes [42], such as aglycones or saponins [43,44,45], significantly inhibited the formation of P. aeruginosa biofilm. Atriplexogenin I-III, oleanane-type saponins from Atriplex tatarica [44] in combination with ampicillin and streptomycin acted synergistically, enhancing the effectiveness of antibiotics against P. aeruginosa. The same effect was shown by glycyrrhizic acid in combination with ciprofloxacin [45].
3.3. Organosulfur Compounds
Jakobsen et al. [35] reported the anti-biofilm properties of ajone, an organosulfide which represents a natural remedy for some human diseases. To determine the QSI (QS Inhibitor) activity of ajoene the authors performed fine experiments by using three reporter systems, which contain fusions of the QS-controlled lasB promoter and rhlA promoter to gfp (ASV), encoding an unstable GFP variant in a P. aeruginosa background. The third was a QS reporter system harbored in an E. coli background, where the luxR gene and the promoter region of the luxI were fused to gfp (ASV). Microarray analysis showed that ajoene induced a concentration-dependent down-regulation of a few, but central, QS-controlled virulence genes of P. aeruginosa (lasA, chiC, lecA, rhlA, rhlB, prpL, cbpD), with the best activity at 80 μg/mL. Attempts to repress more genes were successful only with higher concentrations, also affecting cell growth. DNA microarray studies represent an important tool in the investigation of a plethora of QS-regulating genes. Microarray data were confirmed by RT-PCR analysis of two QS-regulated genes lasB and rhlA. Due to rhlA gene down-regulation, the rhamnolipid content was drastically reduced when the cells were treated with 80 μg/mL ajoene (Table 2). Ajoene demonstrated a clear synergistic effect, with tobramycin killing bacteria embedded in biofilm, and inhibited the lytic necrosis of polymorphonuclear leukocytes. Furthermore, during in vivo studies on a mouse model of pulmonary infection, a significant clearing of infecting P. aeruginosa was detected in ajoene-treated mice compared to a nontreated control group.
Isothiocyanates, another class of compounds containing sulfur, known for their antimicrobial activity, also showed significant activity in the treatment of biofilm-related infections caused by P. aeruginosa. In particular allylisothiocyanate (AITC), benzylisothiocyanate (BITC), and phenylethylisothiocyanate (PEITC), found in plants such as nasturtium (Tropaeolum majus) and horseradish (Armoracia rusticana), were analyzed on mature and developing biofilms of clinical P. aeruginosa (blood culture isolates, multidrug-resistant (MDR) and extensively drug-resistant (XD) Pa strains from invasive and non-invasive clinical samples) isolated either from clinical patients with signs and symptoms of infection, or from the hospital environment [34]. PEITC was the most effective on the development of P. aeruginosa biofilms (500 µg/mL) while AITC preparations showed effectiveness on established P. aeruginosa biofilms, reducing their metabolic activity (between 200 and 800 μg/mL) to a level comparable to the mixture of all three compounds (ITCM, 500–1000 μg/mL). The combination of isothiocyanates with the antibiotic meropenem showed a synergistic effect, with better results when compared to either preparation alone [34].
3.4. Flavonoids
Flavonoids are natural products ubiquitously present in the plant kingdom. They are classified based on the chemical functionalization of the C ring in: flavones (α-β unsaturated ketone), flavanones (ketone at C-4), flavonols (the 3-hydroxy derivative of flavones), and flavan-3-ol (hydroxyl at C-3). These compounds are often also present in glycoside form. Several flavonoids have been evaluated for their anti-biofilm activities, mainly QS-activities. Baicalein (Figure 4) is the most abundant flavone monomer extracted from the roots of Scutellaria baicalensis, and used as a medicine in the Chinese Pharmacopoeia for the treatment of fever, sore throat, and upper respiratory tract infection [50,51]. Baicalein is commercially produced as oral tablets for the treatment of bacteria-induced diarrhea. In addition to its antimicrobial properties baicalein has demonstrated important anti-inflammatory properties [52]. The latter is an important result since a hallmark of P. aeruginosa pulmonary infection is the secretion of various proinflammatory cytokines and a massive recruitment of neutrophils to the infection site. Such excessive inflammatory responses are harmful to the host, contributing to severe tissue damage and organ dysfunction. Therefore, the contemporary administration of an anti-inflammatory drugs is necessary, to slow the progression of chronic infectious diseases by interrupting the infection and inflammation.
Along with anti-QS activity (attenuation of P. aeruginosa virulence factors, including swarming and twitching, and down-regulation of QS-regulated genes transcription) baicalein (128 μg/mL) significantly attenuated IL-1β, IL-6, IL-8, and TNFα secretion at sub-MIC level compared with the PAO1-infected group in the absence of baicalein treatment. At the same concentration, baicalein significantly prevented P. aeruginosa-induced IκBα phosphorylation and the subsequent nuclear translocation and DNA-binding activity of NFκB (p65), compared to the untreated P. aeruginosa PAO1. In summary, the results showed that baicalein represents a promising candidate for combating P. aeruginosa infection, since it can attenuate bacterial pathogenesis by interfering with the QS system, and for its notable anti-inflammatory effect. The flavanones naringenin and taxifolin [53], as well as the flavan-3-ol catechin [54], also showed promising anti-biofilm properties, due to the ability to reduce the production of QS-controlled virulence factors in P. aeruginosa PAO1 (e.g., pyocyanin and elastase) and to modulate the expression of several QS-controlled genes (Table 5 and Table 6). Naringin, a glycoside of naringenin, was screened for its capacity to inhibit the QS-controlled factors, and its antibiofilm efficacy by Vandeputte [53], and recently by Dey et al. [55]. Although naringin showed antibiofilm activities, in addition to its combinatorial performances with antibiotics ciprofloxacin and tetracycline [55], RT-PCR showed that this compound did not reduce the expression of any of the selected QS genes (lasI, lasR, lasB, rhlI, rhlR, rhlA, and aceA) [53].

Table 5.
Flavonoids inhibiting biofilm formation and motility in P. aeruginosa 1.

Table 6.
Flavonoids inhibiting P. aeruginosa virulence factors regulated by QS 1.
Thanks to the low risk and contextual multitargeted actions, the combination of nanoparticles (NPs) and natural compounds has gained a lot of attention in biomedical applications [58]. Zinc and copper play vital roles in several biological processes. Due to their biomedical applications and selective binding to phytochemicals, zinc oxide nanoparticles or zinc and copper thin film techniques are becoming attractive in biomedical applications. Recently, flavonoid-loaded nanoparticles were assessed for their anti-biofilm properties in order to evaluate the potential antibacterial effects, in comparison to the parent flavonoid. In particular, the use of dual drug-like molecules (rutin-benzamide) loaded in a poly vinyl alcohol (PVA) surface modified single nanocarrier (PEG−PLGA) represents a potential anti-biofilm therapy, based on interesting results in term of EPS reduction as well as the extent (%) of biofilm inhibition compared to the control [59]. In addition to pure flavonoids, crude extracts containing flavonoid derivatives as principal constituents also attenuated QS-mediated virulence and biofilm formation. In particular, the binding affinity of mosloflavone for RhlR, detected in the methanolic extract of Plectranthus tenuiflorus [60], was observed to be comparatively higher than its natural ligand, while kaempferol constituted the major constituent of Centella asiatica, a herb with proven anti-QS properties [61].
3.5. Other Phenolic Compounds
Curcumin, present in the rhizome of turmeric (Curcuma longa L.), has many properties and a long-term use in traditional Indian medicine as an antimicrobial agent [62]. Anti-biofilm properties (see Table 7) at sub-MIC concentration are ascribed to curcumin, that down-regulate the P. aeruginosa PAO1 QS system and related virulence factor (pyocyanin, protease and elastase, Table 8) [63]. To overcome its poor water solubility, and enhance its antimicrobial properties, curcumin has been loaded onto zinc oxide nanoparticles (ZnO-NCs), excellent drug carriers due to their low toxicity and biodegradable nature [64]. This considerably improved the anti-QS effect of curcumin against P. aeruginosa PAO1. ZnC-NCs suppressed the LasR-RhlR transcriptional activators and was capable of triggering ROS generation. The ZnC-NC-induced O2− generation was responsible for its anti-biofilm effect against P. aeruginosa PAO1. Molecular docking analysis confirmed the molecular mechanism, showing how curcumin better fits inside the binding site of LasR protein (−5.9730) and RhlR protein (−6.5435).

Table 7.
Phenolic compounds inhibiting biofilm formation and motility in P. aeruginosa 1.

Table 8.
Phenolic compounds inhibiting P. aeruginosa virulence factors regulated by QS 1.
Another natural product showing good antibacterial and anti-biofilm properties against P. aeruginosa (MTCC 424, MTCC 2488) is the naphthoquinone plumbagin [65]. This compound has been used as a traditional medicine in India for its antiparasitic, antioxidant, anticancer, and antimicrobial properties, and can be isolated from the roots of Plumbaginaceae plants [66]. It was demonstrated that plumbagin alone, and in combination with gentamicin, significantly reduced the secretion of virulent enzymes and virulence factors against both strains of P. aeruginosa. The expression of lasB, lasI, and lasR genes was also significantly reduced following plumbagin treatment of P. aeruginosa MTCC 424 and MTCC 2488, at 250 and 150 μg/mL, respectively. In addition, plumbagin showed a synergistic interaction with gentamicin. This combinatorial approach, which represents a novel strategy for the reduction of biofilm formation by P. aeruginosa, also encourages the use of existing antibiotics at lower doses. Plumbagin’s mechanism of action was assessed by protein-ligand docking analysis. The compound showed good affinity for the ligand binding site of Las family and Pil family proteins: the former is related to QS, while the latter to pilus assembly. This result led the authors to hypothesize that plumbagin may affect pilus assembly, inhibiting the QS and swarming motility.
Many other phenol derivatives (Figure 4) exhibited remarkable anti-biofilm properties (Table 7 and Table 8) against P. aeruginosa: cinnammic acid [67], ginkgolic acid [68], gallic, chlorogenic, sinapic, and ferulic acids, as well as eugenol [69,70,71]. Moreover, synergistic effects due to the combination of two or more phenolic compounds have been detected, as in the case of salicylic acid and trans-cinnamaldehyde [72].
4. Anti-Biofilm Properties of Natural Compounds against Staphylococcus aureus
Staphylococcus aureus is a gram-positive pathogen, frequently the cause of biofilm-associated infections on indwelling medical devices [77]. Like for P. aeruginosa, staphylococcal biofilms show enhanced resistance toward antibiotics and the immune response, thus representing an important therapeutic challenge in clinics worldwide. A recent study has already provided an accurate overview of natural products isolated from plants and microorganisms with activity against the major virulence factors of S. aureus [78]. In this section, we report the latest updates and, when the information is available, the molecular mechanisms associated with the anti-biofilm effects of terpenes, flavonoids, and phenolic compounds (Figure 5 and Figure 6, Table 9, Table 10 and Table 11).
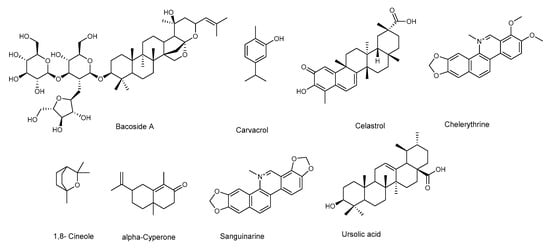
Figure 5.
Chemical structures of terpenoids active against Staphylococcus aureus.
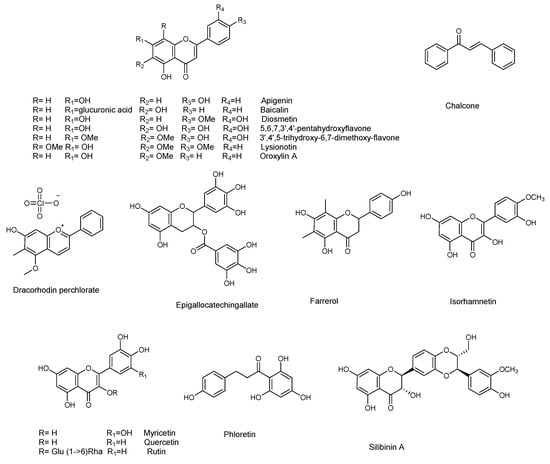
Figure 6.
Chemical structures of flavonoids active against S. aureus.

Table 9.
Terpenoids inhibiting S. aureus biofilm formation and production of virulence factors regulated by QS 1.

Table 10.
Flavonoids inhibiting S. aureus. biofilm formation and production of virulence factors regulated by QS 1.

Table 11.
Phenolic compounds inhibiting S. aureus biofilm formation and production of virulence Figure 1.
4.1. Terpenes
Among monoterpenes, 1,8-cineole (Figure 5) and carvacrol [79,80] were shown to act against biofilm formation, while eugenyl acetate was active against alfa-hemolysin [81].
The sesquiterpene (+)-nootkatone is present in essential oils from Alaska yellow cedar trees, some herbs, and grapefruit. It has been approved by the Food and Drug Administration (FDA) as a flavoring agent in citrus-flavored foods and beverages. Farha et al. [82] demonstrated that (+)-nootkatone at 200 μg/mL significantly disrupted S. aureus preformed biofilm, and reduced the viability of cells within matured biofilm, suggesting that the compound penetrates through the biofilm. Additionally, the molecular analysis showed that (+)-nootkatone suppressed the expression levels of sarA, icaA, agrA, RNAIII, and spa; major genes involved in biofilm formation. The compound was also able to inhibit the sliding motility of S.aureus, thus contrasting the initial phase of bacterial surface colonization and biofilm formation. Moreover, up to 50 μg/mL, sub-MIC concentration, at which the inhibition of biofilm formation was observed, (+)-nootkatone was non-toxic to normal fibroblast cells.
The diterpenes, salvipisone and aethiopinone, isolated from hairy roots of Salvia sclarea, showed activity against methicillin-resistant S. aureus. They reduced the resistance to the antibiotic oxacillin, and caused a reduction of the biofilm biomass, as well as the disruption of the biofilm structure [83].
The triterpene celestrol (Table 9) was shown to inhibit biofilm formation, and to possess antimicrobial activity against S. aureus ATCC 29,213 (a reference strain of methicillin-sensitive S. aureus (MSSA)) and a clinical methicillin-resistant S. aureus (MRSA) isolate [84]. The compound was not only active on planktonic cells (with a MIC of 2 µM and a MBC of 32 µM), but it was also effective in dispersing preformed biofilms of the clinical MRSA isolates, as evaluated by confocal laser scanning microscopy. Furthermore, it inhibited the secretion of EPS, which are crucial for the formation of the matrix that facilitates the adherence of these microorganisms on the target surfaces. Therefore, the compound has a great potential, since it is not only inhibiting to the formation of biofilms, but it can further act by eradicating preformed biofilms, while also being active on the planktonic cells. However, this compound also showed a certain cytotoxicity against hFOB 1.19 cells (osteoblast).
Ursolic acid (Table 9) was active against the formation of biofilm by S. aureus subsp. aureus COL, a MRSA strain, resistant to several antibiotics, including penicillin and tetracycline. The RNA-Seq-based transcriptome analysis showed that ursolic acid reduces the metabolism of some amino acids and the expression of adhesins [85].
A mixture of triterpenoid saponins, known as Bacoside A (Figure 5), was reported for its antimicrobial and anti-biofilm activity against S. aureus MTCC 96. It is very likely that these saponins alter the structure and permeability of the bacterial cell membrane. Furthermore, Bacoside A, also dispersed preformed biofilm. The treated biofilm showed altered cell structure and a loss of EPS that caused biofilm dispersion [43].
4.2. Flavonoids
Among the flavonoids, baicalein (which is also active against P. aeruginosa) was active against the QS system, by inhibiting the transcription of AgrA and RNAIII, and inhibits biofilm formation [88]. The biofilm formation was also inhibited by myricetin [89]. Myricetin, quercetin, farrerol, isorhamnetin, dracorhodin, lysionotin, diosmetin, silibinin, apigenin, epicallocatechin gallate, oroxylin A, and baicalin (Figure 6) were active against alfa-haemolysin [89,90,91,92,93,94,95,96,97,98,99,100]. The flavonoid rutin showed a concentration dependent reduction of biofilm formation (Table 10). However, it did not significantly decrease the biomass, while it reduced the secretion of EPS. Therefore, it probably acts by interfering with the adhesion, and with all the other functions, of EPS [101]. Pro-antocyanidin A2 inhibited de-novo biofilm formation, without showing bactericidal activity, nor inhibiting activity on planktonic growth. Furthermore, it also appeared to have no activity on mature biofilm [86]. Two flavonoids isolated from Teucrium polium, namely 3′,4′,5-trihydroxy-6,7-dimethoxyflavone and 5,6,7,3′,4′-pentahydroxyflavone, inhibited biofilm growth of Staphylococcus aureus (Table 10) AH133 strain [102]
A recent study explored the capacity of kaempferol to inhibit S. aureus biofilm formation, and the associated potential molecular mechanisms [103]. Kaempferol inhibited the attachment phase of biofilm formation, by reducing S. aureus adhesion, since its action was evident only if added immediately after the inoculation of bacteria to plates. This was mediated by blocking the activity of Sortase A (SrtA), an enzyme essential in the anchoring of surface proteins to the cell wall of gram-positive bacteria. This has important consequences for the onset of acute infection by S. aureus, since the bacteria cannot display functional surface adhesins in the cell wall envelope. In addition, the authors analyzed the expression of adhesion-related genes. They demonstrated that the compound reduced the expression of clfA and clfB, which encode clumping factor A (ClfA), and ClfB, fnbA, and fnbB which encode fibronectin-binding proteins (FnbpA and FnbpB), and sarA, a global regulator gene that is closely related to biofilm formation, and positively regulates fnbA and fnbB. The results reported suggest that kaempferol represents a potential compound with a novel mechanism of biofilm inhibition.
4.3. Other Phenolic Compounds
Several other plant-derived phenolic compounds have already been discussed in the work by Wu et al., including chalcone [106], resveratrol ([107], phloretin [104], alfa-cyperone [87], curcumin [108], osthole [105], and brazilin [109]. Among these, resveratrol (Table 11) was active against α-hemolysin [107] and, used in combination with vancomycin, inhibited biofilm formation. It was suggested that resveratrol would disturb the expression of genes related to QS, surface and secreted proteins, and capsular polysaccharides [85].
Besides these, gallic and ferulic acid were also tested for their activity against S. aureus, although they were active only at relatively high concentrations. Only ferulic acid completely inhibited colony spreading. Furthermore, it was hypothesized that changes in motility could affect the ability of the bacteria to form a biofilm [71]
The tannin, hamamelitannin, was shown to inhibit the quorum sensing regulator RNAIII [110,111], while punicalagin was active against α-hemolysin [112], and exerted a remarkable inhibitory effect on biofilm formation [113]. The activity of punicalagin against S. aureus was further investigated, with the aim of understanding the possible mode of action. Punicalagin exhibited a MIC of 0.25 mg/mL and induced morphological damage to the cell membrane, also inducing an efflux of potassium.
Tannic acid showed antibacterial and anti-biofilm formation activity, although further studies are needed to understand the mechanism of action [114].
1,2,3,4,6-Penta-O-galloyl-d-glucopyranose (PGG) prevented biofilm formation at 6.25 µM of several strains of S. aureus, while showing no bactericidal activity at this concentration [115]. Arylbenzylfuran was active against clinical strains of methicillin-resistant S. aureus (MRSA), and was able to induce a significant reduction in S. aureus ATCC 12600S biofilm viability [116].
Several aromatic polyketides isolated from plants have been reported, in particular aloe-emodin [117], acting against the Agr quorum-sensing system. The same compound and the structurally related rhein [117] were able to inhibit biofilm formation.
Finally, noteworthy is the activity of capsaicin, which acts against α-hemolysin by suppressing the expression of Hla and AgrA [95].
5. Conclusions
Biofilms represent one of the most successful strategies used by bacteria to increase their survival in terms of resistance to antibiotics and antimicrobial agents. If biofilm forming microorganisms are a big challenge, even more complex is the fight against polymicrobial biofilms, like the ones formed by S. aureus and P. aeruginosa. Therefore, finding new anti-biofilm chemicals is crucial. In this context, plants are an extraordinarily rich source of compounds endowed with several different biological activities, including antimicrobial and antibiofilm properties. These compounds often act via modes of action that are different than the ones of currently used antibiotics, thus also offering a tool for combating antibiotic resistance.
Many studies have been published on the topic in recent years, and the latest advances in the discovery of plant-derived natural products with anti-biofilm properties against P. aeruginosa and S. aureus have been herewith reviewed. Knowing the molecular mechanisms underlying the biological activity is very important, especially if these compounds are to be further studied for possible applications. Therefore, when known, the molecular mechanisms were also herewith reported and discussed, with the aim of providing a clear overview of the state of the art.
Funding
This research received no external funding.
Acknowledgments
M.S. acknowledges Valere Program of University of Campania Luigi Vanvitelli for funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stacy, A.; McNally, L.; Darch, S.E.; Brown, S.P.; Whiteley, M. The biogeography of polymicrobial infection. Nat. Rev. Microbiol. 2016, 14, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Kostakioti, M.; Hadjifrangiskou, M.; Hultgren, S.J. Bacterial biofilms: Development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb. Perspect. Med. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Roilides, E.; Simitsopoulou, M.; Katragkou, A.; Walsh, T.J. How biofilms evade host defenses. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef]
- Trivedi, U.; Parameswaran, S.; Armstrong, A.; Burgueno-Vega, D.; Griswold, J.; Dissanaike, S.; Rumbaugh, K.P. Prevalence of multiple antibiotic resistant infections in diabetic versus nondiabetic Wounds. J. Pathog. 2014, 173053. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, M.; Anju, C.P.; Biswas, L.; Kumar, V.A.; Mohan, C.G.; Biswas, R. Antibiotic resistance in Pseudomonas aeruginosa and alternative therapeutic options. Int. J. Med. Microbiol. 2016, 306, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, D.; Peters, B.M.; Li, L.; Li, B.; Xu, Z.; Shirliff, M.E. Staphylococcal chromosomal cassettes mec (SCCmec): A mobile genetic element in methicillin-resistant Staphylococcus aureus. Microb. Pathog. 2016, 101, 56–67. [Google Scholar] [CrossRef]
- Ibberson, C.B.; Whiteley, M. The social life of microbes in chronic infection. Curr. Opin. Microbiol. 2020, 53, 44–50. [Google Scholar] [CrossRef]
- Limoli, D.H.; Whitfield, G.B.; Kitao, T.; Ivey, M.L.; Davis, M.R., Jr.; Grahl, N.; Hogan, D.A.; Rahme, L.G.; Howell, P.L.; O’Toole, G.A.; et al. Pseudomonas aeruginosa alginate overproduction promotes coexistence with Staphylococcus aureus in a model of cystic fibrosis respiratory infection. MBio 2017, 8, e00186-17. [Google Scholar] [CrossRef]
- Limoli, D.H.; Hoffman, L.R. Help, hinder, hide and harm: What can we learn from the interactions between Pseudomonas aeruginosa and Staphylococcus aureus during respiratory infections? Thorax 2019, 74, 684–692. [Google Scholar] [CrossRef]
- Borges, A.; Abreu, A.C.; Dias, C.; Saavedra, M.J.; Borges, F.; Simões, M. New perspectives on the use of phytochemicals as an emergent strategy to Control Bacterial Infections Including Biofilms. Molecules 2016, 21, 877. [Google Scholar] [CrossRef]
- Shaw, E.; Wuest, W.M. Virulence attenuating combination therapy: A potential multi-target synergy approach to treat Pseudomonas aeruginosa infections in cystic fibrosis patients. RSC Med. Chem. 2020, 11, 358–369. [Google Scholar] [CrossRef]
- Asfour, H.Z. Anti-quorum sensing natural compounds. J. Microsc. Ultrastruct. 2018, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- D’Abrosca, B.; Buommino, E.; D’Angelo, G.; Coretti, L.; Scognamiglio, M.; Severino, V.; Pacifico, S.; Donnarumma, G.; Fiorentino, A. Spectroscopic identification and anti-biofilm properties of polar metabolites from the medicinal plant Helichrysum italicum against Pseudomonas aeruginosa. Bioorg. Med. Chem. 2013, 21, 7038–7046. [Google Scholar] [CrossRef]
- Lee, J.H.; Regmi, S.C.; Kim, J.A.; Cho, M.H.; Yun, H.; Lee, C.S.; Lee, J. Apple flavonoid phloretin inhibits Escherichia coli O157:H7 biofilm formation and ameliorates colon inflammation in rats. Infect. Immun. 2011, 79, 4819–4827. [Google Scholar] [CrossRef]
- Stowe, S.D.; Richards, J.J.; Tucker, A.T.; Thompson, R.; Melander, C.; Cavanagh, J. Anti-Biofilm Compounds Derived from Marine Sponges. Mar. Drugs 2011, 9, 2010–2035. [Google Scholar] [CrossRef]
- Masák, J.; Čejková, A.; Schreiberová, O.; Rezanka, T. Pseudomonas biofilms: Possibilities of their control. FEMS Microbiol. Ecol. 2014, 89, 1–14. [Google Scholar] [CrossRef]
- Song, X.; Xia, Y.X.; He, Z.D.; Zhang, H.J. A Review of Natural Products with Anti-biofilm Activity. Curr. Org. Chem. 2018, 22, 789–817. [Google Scholar] [CrossRef]
- Waters, C.M.; Bassler, B. Quorum sensing: Cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 2005, 21, 319–346. [Google Scholar] [CrossRef]
- Flemming, H.; Wingende, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Lu, L.; Hu, W.; Tian, Z.; Yuan, D.; Yi, G.; Zhou, Y.; Cheng, Q.; Zhu, J.; Li, M. Developing natural products as potential anti-biofilm agents. Chin. Med. 2019, 14, 11. [Google Scholar] [CrossRef]
- Wu, H.; Moser, C.; Wang, H.Z.; Høiby, N.; Song, Z.J. Strategies for combating bacterial biofilm infections. Int. J. Oral Sci. 2015, 7, 1–7. [Google Scholar] [CrossRef]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal-response systems in gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef]
- García-Reyes, S.; Soberón-Chávez, G.; Cocotl-Yanez, M. The third quorum-sensing system of Pseudomonas aeruginosa: Pseudomonas quinolone signal and the enigmatic PqsE protein. J. Med. Microbiol. 2020, 69, 25–34. [Google Scholar] [CrossRef]
- Le, K.Y.; Otto, M. Quorum-sensing regulation in staphylococci-an overview. Front. Microbiol. 2015, 6, 1174. [Google Scholar] [CrossRef]
- Cheung, G.Y.; Wang, R.; Khan, B.A.; Sturdevant, D.E.; Otto, M. Role of the accessory gene regulator agr in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. Infect. Immun. 2011, 79, 1927–1935. [Google Scholar] [CrossRef]
- Hapke, H.; Strathmann, W. Pharmacological effects of hordenine. Dtsch. Tierarztl. Wochenschr. 1995, 102, 228–232. [Google Scholar]
- Zhou, J.W.; Luo, H.Z.; Jiang, H.; Jian, T.K.; Chen, Z.Q.; Jia, A.Q. Hordenine: A Novel Quorum Sensing Inhibitor and Anti-biofilm Agent against Pseudomonas aeruginosa. J. Agric. Food Chem. 2018, 66, 1620–1628. [Google Scholar] [CrossRef]
- O’May, C.; Tufenkji, N. The swarming motility of Pseudomonas aeruginosa is blocked by cranberry proanthocyanidins and other tannin-containing materials. Appl. Environ. Microbiol. 2011, 77, 3061–3067. [Google Scholar] [CrossRef]
- Köhler, T.; Guanella, R.; Carlet, J.; van Delden, C. Quorum sensing-dependent virulence during Pseudomonas aeruginosa colonisation and pneumonia in mechanically ventilated patients. Thorax 2010, 65, 703–710. [Google Scholar] [CrossRef][Green Version]
- Chakraborty, P.; Dastidar, D.G.; Paul, P.; Dutta, S.; Basu, D.; Sharma, S.R.; Basu, S.; Sarker, R.K.; Sen, A.; Sarkar, A.; et al. Inhibition of biofilm formation of Pseudomonas aeruginosa by caffeine: A potential approach for sustainable management of biofilm. Arch. Microbiol. 2020, 202, 623–635. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Cho, M.H.; Kim, J.A.; Lee, J. 7-fluoroindole as an antivirulence compound against Pseudomonas aeruginosa. FEMS Microbiol. Lett. 2012, 329, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Tabor, C.W.; Tabor, H. Polyamines. Ann. Rev. Biochem. 1984, 53, 749–790. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; She, P.; Wang, Y.; Liu, F.; Zhang, D.; Chen, L.; Luo, Z.; Xu, H.; Qi, Y.; Wu, Y. Effects of norspermidine on Pseudomonas aeruginosa biofilm formation and eradication. Microbiologyopen 2016, 5, 402–412. [Google Scholar] [CrossRef]
- Kaiser, S.J.; Mutters, N.T.; Blessing, B.; Günther, F. Natural isothiocyanates express antimicrobial activity against developing and mature biofilms of Pseudomonas aeruginosa. Fitoterapia 2017, 119, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, T.H.; van Gennip, M.; Phipps, R.K.; Shanmugham, M.S.; Christensen, L.D.; Alhede, M.; Skindersoe, M.E.; Rasmussen, T.B.; Friedrich, K.; Uthe, F.; et al. Ajoene, a Sulfur-Rich Molecule from Garlic, Inhibits Genes Controlled by Quorum Sensing. Antimicrob. Agents Chemother. 2012, 56, 2314. [Google Scholar] [CrossRef]
- Bose, S.K.; Chauhan, M.; Dhingra, N.; Chhibber, S.; Harjai, K. Terpinen-4-ol attenuates quorum sensing regulated virulence factors and biofilm formation in Pseudomonas aeruginosa. Future Microbiol. 2020, 15, 127–142. [Google Scholar] [CrossRef]
- Pareek, A.; Suthar, M.; Rathore, G.S.; Bansal, V. Feverfew (Tanacetum parthenium L.): A systematic review. Pharmacogn. Rev. 2011, 5, 103–110. [Google Scholar] [CrossRef]
- Kalia, M.; Yadav, V.K.; Singh, P.K.; Sharma, D.; Narvi, S.S.; Agarwal, V. Exploring the impact of parthenolide as anti-quorum sensing and anti-biofilm agent against Pseudomonas aeruginosa. Life Sci. 2018, 199, 96–103. [Google Scholar] [CrossRef]
- Lawrence, J.A.; Huang, Z.; Rathinavelu, S.; Hua, J.F.; Garoa, E.; Ellis, M.; Normana, V.L.; Buckle, R.; Williams, R.B.; Starks, C.M.; et al. Optimized plant compound with potent anti-biofilm activity across gram-negative. Bioorg. Med. Chem. 2020, 28, 115–229. [Google Scholar] [CrossRef]
- Ghosh, C.; Bhowmik, J.; Ghosh, R.; Das, M.C.; Sandhu, P.; Kumari, M.; Acharjee, S.; Daware, A.V.; Akhter, Y.; Banerjee, B.; et al. The anti-biofilm potential of triterpenoids isolated from Sarcochlamys pulcherrima (Roxb.) Gaud. Microb. Pathog. 2020, 139, 103901. [Google Scholar] [CrossRef]
- Ghosh, R.; Das, M.C.; Sarkar, A.; Das, A.; Sandhu, P.; Dinda, B.; Akhter, Y.; Bhattacharjee, S.; De, U.C. Exploration of Phytoconstituents from Mussaenda roxburghii and Studies of their Antibiofilm Effect. Chem. Biodivers. 2017, 14, e1700165. [Google Scholar] [CrossRef] [PubMed]
- Rajkumari, J.; Borkotoky, S.; Murali, A.; Suchiang, K.; Mohanty, S.K.; Busi, S. Attenuation of quorum sensing controlled virulence factors and biofilm formation in Pseudomonas aeruginosa by pentacyclic triterpenes, betulin and betulinic acid. Microb. Pathog. 2018, 118, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Parai, D.; Islam, E.; Mitra, J.; Mukherjee, S.K. Effect of Bacoside A on growth and biofilm formation by Staphylococcus aureus and Pseudomonas aeruginosa. Can. J. Microbiol. 2017, 63, 169–178. [Google Scholar] [CrossRef]
- Stanković, J.; Gođevac, D.; Tešević, V.; Dajić-Stevanović, Z.; Ćirić, A.; Soković, M.; Novaković, M. Antibacterial and Antibiofilm Activity of Flavonoid and Saponin Derivatives from Atriplex tatarica against Pseudomonas aeruginosa. J. Nat. Prod. 2019, 82, 1487–1495. [Google Scholar] [CrossRef]
- Chakotiya, A.S.; Tanwar, A.; Narula, A.; Sharma, R.K. Alternative to antibiotics against Pseudomonas aeruginosa: Effects of Glycyrrhiza glabra on membrane permeability and inhibition of efflux activity and biofilm formation in Pseudomonas aeruginosa and its in vitro time-kill activity. Microb. Pathog. 2016, 98, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Mozirandi, W.; Tagwireyi, D.; Mukanganyama, S. Evaluation of antimicrobial activity of chondrillasterol isolated from Vernonia adoensis (Asteraceae). BMC Complement. Altern. Med. 2019, 249. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Liu, X.; Bian, J.; Pei, G.; Dai, H.; Polyak, S.W.; Song, F.; Ma, L.; Wang, Y.; Zhang, L. Synergistic effect of 14-alpha-lipoyl andrographolide and various antibiotics on the formation of biofilms and production of exopolysaccharide and pyocyanin by Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2011, 55, 3015–3017. [Google Scholar] [CrossRef]
- Dietz, B.M.; Hajirahimkhan, A.; Dunlap, T.L.; Bolton, J.L. Botanicals and their bioactive phytochemicals for women’s health. Pharmacol. Rev. 2016, 68, 1026–1073. [Google Scholar] [CrossRef]
- Kuang, Z.; Hao, Y.; Walling, B.E.; Jeffries, J.L.; Ohman, D.E.; Gee, W.L. Pseudomonas aeruginosa elastase provides an escape from phagocytosis by degrading the pulmonary surfactant protein-A. PLoS ONE 2011, 6, e7091. [Google Scholar] [CrossRef]
- Chaudhary, A.; Harminder, A.K.; Singh, V. A review on the taxonomy, ethnobotany, chemistry and pharmacology of Oroxylum indicum Vent. Indian J. Pharm Sci. 2011, 73, 483–490. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Q.; Zhou, W.; Gao, S.; Lin, H.; Ye, S.; Xu, S.; Cai, J. Chinese medicine injection Shuanghuanglian for treatment of acute upper respiratory tract infection: A systematic review of randomized controlled trials. J. Evid. Based Complement. Altern. Med. 2013, 2013, 987326. [Google Scholar] [CrossRef]
- Luo, J.; Kong, J.L.; Dong, B.Y.; Huang, H.; Wang, K.; Wu, L.H.; Hou, C.C.; Liang, Y.; Li, B.; Chen, Y.Q. Baicalein attenuates the quorum sensing-controlled virulence factors of Pseudomonas aeruginosa and relieves the inflammatory response in P. aeruginosa-infected macrophages by downregulating the MAPK and NFκB signal-transduction pathways. Drug Des. Dev. Ther. 2016, 10, 183–203. [Google Scholar] [CrossRef]
- Vandeputte, O.; Kiendrebeogo, M.; Rasamiravaka, T.; Stévigny, C.; Duez, P.; Rajaonson, S.; Diallo, B.; Mol, A.; Baucher, M.; El Jaziri, M. The flavanone naringenin reduces the production of quorum sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Microbiology 2011, 157, 2120–2132. [Google Scholar] [CrossRef]
- Vandeputte, O.M.; Kiendrebeogo, M.; Rajaonson, S.; Diallo, B.; Mol, A.; Jaziri, M.E.; Bacher, M. Identification of catechin as one of the flavonoids from Combretum albiflorum bark extract that reduces the production of quorum-sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Appl. Environ. Microbiol. 2010, 76, 243–253. [Google Scholar] [CrossRef]
- Dey, P.; Parai, D.; Banerjee, M.; Hossain, S.T.; Mukherjee, S.K. Naringin sensitizes the antibiofilm effect of ciprofloxacin and tetracycline against Pseudomonas aeruginosa biofilm. Int. J. Med. Microbiol. 2020, 310, 151410. [Google Scholar] [CrossRef]
- Abinaya, M.; Gayathri, M. Inhibition of biofilm formation, quorum sensing activity and molecular docking study of isolated 3, 5, 7-Trihydroxyflavone from Alstonia scholaris leaf against P. aeruginosa. Bioorg. Chem. 2019, 87, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Das, M.C.; Sandhu, P.; Gupta, P.; Rudrapaul, P.; De, U.C.; Tribedi, P.; Akhter, Y.; Bhattacharjee, S. Attenuation of Pseudomonas aeruginosa biofilm formation by Vitexin: A combinatorial study with azithromycin and gentamicin. Sci. Rep. 2016, 6, 23347. [Google Scholar] [CrossRef] [PubMed]
- Lalitha, K.; Sandeep, M.; Prasad, Y.S.; Sridharan, V.; Maheswari, C.U.; Srinandan, C.S.; Nagarajan, S. Intrinsic Hydrophobic Antibacterial Thin Film from renewable Resources: Application in the Development of Anti-Biofilm Urinary Catheters. Acs Sustain. Chem. Eng. 2017, 5, 436–449. [Google Scholar] [CrossRef]
- Deepika, M.S.; Thangam, R.; Sundarraj, S.; Sheena, T.S.; Sivasubramanian, S.; Kulandaivel, J.; Thirumurugan, R. Co-delivery of Diverse Therapeutic Compounds Using PEG-PLGA Nanoparticle Cargo against Drug-Resistant Bacteria: An Improved Anti-biofilm Strategy. ACS Appl. Bio Mater. 2020, 3, 385–399. [Google Scholar] [CrossRef]
- Hnamte, S.; Subhaswaraj, P.; Ranganathan, S.K.; Ampasala, D.R.; Muralitharan, G.; Siddhardha, B. Methanolic Extract of Plectranthus tenuiflorus Attenuates Quorum Sensing Mediated Virulence and Biofilm Formation in Pseudomonas aeruginosa PAO1. J. Pure Appl. Microbiol. 2018, 12, 1985–1996. [Google Scholar] [CrossRef]
- Vasavi, H.S.; Arun, A.B.; Rekha, P.D. Anti-quorum sensing activity of flavonoid-rich fraction from Centella asiatica L. against Pseudomonas aeruginosa PAO1. J. Microbiol. Immunol. Infect. 2016, 49, 8–15. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Kadir, H.A.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Res. Int. 2014, 2014, 186864. [Google Scholar] [CrossRef]
- Rudrappa, T.; Bais, H.P. Curcumin, a known phenolic from Curcuma longa, attenuates the virulence of Pseudomonas aeruginosa PAO1 in whole plant and animal pathogenicity models. J. Agric Food Chem. 2008, 56, 1955–1962. [Google Scholar] [CrossRef]
- Prateeksha Rao, C.V.; Das, A.K.; Barik, S.K.; Singh, B.N. ZnO/Curcumin Nanocomposites for Enhanced Inhibition of Pseudomonas aeruginosa Virulence via LasR-RhlR Quorum Sensing Systems. Mol. Pharm. 2019, 16, 3399–3413. [Google Scholar] [CrossRef]
- Gupta, P.; Sarkar, A.; Sandhu, P.; Daware, A.; Das, M.C.; Akhter, Y.; Bhattacharjee, S. Potentiation of antibiotic against Pseudomonas aeruginosa biofilm: A study with plumbagin and gentamicin. J. Appl. Microbiol. 2017, 123, 246–261. [Google Scholar] [CrossRef]
- Acharya, B.R.; Bhattacharyya, B.; Chakrabarti, G. The natural naphthoquinone plumbagin exhibits antiproliferative activity and disrupts the microtubule network through tubulin binding. Biochemistry 2008, 47, 7838–7845. [Google Scholar] [CrossRef]
- Rajkumari, J.; Borkotoky, S.; Murali, A.; Suchiang, K.; Mohanty, S.K.; Busi, S. Cinnamic acid attenuates quorum sensing associated virulence factors and biofilm formation in Pseudomonas aeruginosa PAO1. Biotechnol. Lett. 2018, 40, 1087–1100. [Google Scholar] [CrossRef]
- Tahrioui, A.; Ortiz, S.; Azuama, O.C.; Bouffartigues, E.; Benalia, N.; Tortuel, D.; Maillot, O.; Chemat, S.; Kritsanida, M.; Feuilloley, M.; et al. Membrane-interactive compounds from Pistacia lentiscus L. thwart Pseudomonas aeruginosa virulence. Front. Microbiol. 2020, 11, 1068. [Google Scholar] [CrossRef]
- Zhou, L.; Zheng, H.; Tang, Y.; Yu, W.; Gong, Q. Eugenol inhibits quorum sensing at sub-inhibitory concentrations. Biotechnol. Lett. 2013, 35, 631–637. [Google Scholar] [CrossRef]
- Plyuta, V.; Zaitseva, J.; Lobakova, E.; Zagoskina, N.; Kuznetsov, A.; Khmel, I. Effect of plant phenolic compounds on biofilm formation by Pseudomonas aeruginosa. APMIS 2013, 121, 1073–1081. [Google Scholar] [CrossRef]
- Borges, A.; Saavedra, M.J.; Simões, M. The activity of ferulic and gallic acids in biofilm prevention and control of pathogenic bacteria. Biofouling 2012, 28, 755–767. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Rudden, M.; Smyth, T.J.; Dooley, J.S.; Marchant, R.; Banat, I.M. Natural quorum sensing inhibitors effectively down-regulate gene expression of Pseudomonas aeruginosa virulence factors. Appl. Microbiol. Biotechnol. 2019, 103, 3521–3535. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S.; Byun, Y.; Park, H.D. 6-Gingerol reduces Pseudomonas aeruginosa biofilm formation and virulence via quorum sensing inhibition. Sci. Rep. 2015, 5, 8656. [Google Scholar] [CrossRef]
- Kumar, L.; Chhibber, S.; Harjai, K. Zingerone inhibit biofilm formation and improve antibiofilm efficacy of ciprofloxacin against Pseudomonas aeruginosa PAO1. Fitoterapia 2013, 90, 73–78. [Google Scholar] [CrossRef]
- Kumar, L.; Chhibber, S.; Kumar, R.; Kumar, M.; Harjai, K. Zingerone silences quorum sensing and attenuates virulence of Pseudomonas aeruginosa. Fitoterapia 2015, 102, 84–95. [Google Scholar] [CrossRef]
- Ulrey, R.K.; Barksdale, S.M.; Zhou, W.; van Hoek, M.L. Cranberry proanthocyanidins have anti-biofilm properties against Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2014, 14, 499. [Google Scholar] [CrossRef]
- Schilcher, K.; Horswill, A.R. Staphylococcal Biofilm Development: Structure, Regulation, and Treatment Strategies. Microbiol. Mol. Biol. 2020, 84, e00026-19. [Google Scholar] [CrossRef]
- Wu, S.C.; Liu, F.; Zhu, K.; Shen, J.Z. Natural Products That Target Virulence factors in Antibiotic-Resistant Staphylococcus aureus. J. Agric. Food Chem. 2019, 67, 13195–13211. [Google Scholar] [CrossRef]
- Merghni, A.; Noumi, E.; Hadded, O.; Dridi, N.; Panwar, H.; Ceylan, O.; Mastouri, M.; Snoussi, M. Assessment of the antibiofilm and antiquorum sensing activities of Eucalyptus globulus essential oil and its main component 1,8-cineole against methicillin-resistant Staphylococcus aureus strains. Microb. Pathog. 2018, 118, 74–80. [Google Scholar] [CrossRef]
- Burt, S.A.; Ojo-Fakunle, V.T.; Woertman, J.; Veldhuizen, E.J. The Natural Antimicrobial Carvacrol Inhibits Quorum Sensing in Chromobacterium violaceum and Reduces Bacterial Biofilm Formation at Sub-Lethal Concentrations. PLoS ONE 2014, 9, e93414. [Google Scholar] [CrossRef]
- Musthafa, K.S.; Voravuthikunchai, S.P. Anti-virulence potential of eugenyl acetate against pathogenic bacteria of medical importance. Antonie Leeuwenhoek. 2015, 107, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Farha, A.K.; Yang, Q.Q.; Kim, G.; Zhang, D.; Mavumengwana, V.; Habimana, O.; Li, H.B.; Corke, H.; Gana, R.Y. Inhibition of multidrug-resistant foodborne Staphylococcus aureus biofilms by a natural terpenoid (+)-nootkatone and related molecular mechanism. Food Control. 2020, 107154. [Google Scholar] [CrossRef]
- Walencka, E.; Rozalska, S.; Wysokinska, H.; Rozalski, M.; Kuzma, L.; Rozalska, B. Salvipisone and aethiopinone from Salvia sclarea hairy roots modulate staphylococcal antibiotic resistance and express anti-biofilm activity. Planta Med. 2007, 73, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.G.; Lee, S.Y.; Lee, S.M.; Lim, K.H.; Ha, E.J.; Eom, Y.B. Activity of novel inhibitors of Staphylococcus aureus biofilms. Folia Microbiol. 2017, 62, 157–167. [Google Scholar] [CrossRef]
- Qin, N.; Tan, X.; Jiao, Y.; Liu, L.; Zhao, W.; Yang, S.; Jia, A. RNA-Seq-based transcriptome analysis of methicillin-resistant Staphylococcus aureus biofilm inhibition by ursolic acid and resveratrol. Sci. Rep. 2014, 4, 1–9. [Google Scholar] [CrossRef]
- Artini, M.; Papa, R.; Barbato, G.; Scoarughi, G.L.; Cellini, A.; Morazzoni, P.; Bombardelli, E.; Selan, L. Bacterial biofilm formation inhibitory activity revealed for plant derived natural compounds. Bioorg. Med. Chem. 2012, 20, 920–926. [Google Scholar] [CrossRef]
- Luo, M.; Qiu, J.; Zhang, Y.; Wang, J.; Dong, J.; Li, H.; Leng, B.; Zhang, Q.; Dai, X.; Niu, X.; et al. alpha-Cyperone Alleviates Lung Cell Injury Caused by Staphylococcus aureus via Attenuation of alpha-Hemolysin Expression. J. Microbiol. Biotechnol. 2012, 22, 1170–1176. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, T.; Wang, K.; Hou, C.; Cai, S.; Huang, Y.; Du, Z.; Huang, H.; Kong, J.; Chen, Y. Baicalein Inhibits Staphylococcus aureus Biofilm Formation and the Quorum Sensing System In Vitro. PLoS ONE 2016, 11, e0153468. [Google Scholar] [CrossRef]
- Silva, L.N.; Da Hora, G.C.; Soares, T.A.; Bojer, M.S.; Ingmer, H.; Macedo, A.J.; Trentin, D.S. Myricetin protects Galleria mellonella against Staphylococcus aureus infection and inhibits multiple virulence factors. Sci. Rep. 2017, 7, 2823. [Google Scholar] [CrossRef]
- Lopez, G.C.; Sanchez, C.A. Quercetin attenuates Staphylococcus aureus virulence by reducing alpha-toxin secretion. Rev. Argent. Microbiol. 2018, 50, 131–135. [Google Scholar] [CrossRef]
- Qiu, J.Z.; Xiang, H.; Hu, C.; Wang, Q.A.; Dong, J.; Li, H.E.; Luo, M.J.; Wang, J.F.; Deng, X.M. Subinhibitory concentrations of farrerol reduce alpha-toxin expression in Staphylococcus aureus. FEMS Microbiol. Lett. 2011, 315, 129–133. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jiang, L.; Li, H.; Wang, L.; Song, Z.; Shi, L.; Li, W.; Deng, X.; Wang, J. Isorhamnetin Attenuates Staphylococcus aureus-Induced Lung Cell Injury by Inhibiting Alpha-Hemolysin Expression. J. Microbiol. Biotechnol. 2016, 26, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.H.; Shi, D.X.; Liu, H.Y.; Shen, Z.Y.; Zha, Y.H.; Li, W.H.; Deng, X.M.; Wang, J.F. Lysionotin attenuates Staphylococcus aureus pathogenicity by inhibiting alpha-toxin expression. Appl. Microbiol. Biotechnol. 2017, 101, 6697–6703. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhou, X.; Li, W.H.; Zhang, H.; Zhang, B.; Li, G.; Liu, B.W.; Deng, X.M.; Peng, L.P. Diosmetin inhibits the expression of alpha-hemolysin in Staphylococcus aureus. Antonie Leeuwenhoek. 2015, 108, 383–389. [Google Scholar] [CrossRef]
- Dong, J.; Qiu, J.Z.; Wang, J.F.; Li, H.E.; Dai, X.H.; Zhang, Y.; Wang, X.; Tan, W.; Niu, X.D.; Deng, X.M.; et al. Apigenin alleviates the symptoms of Staphylococcus aureus pneumonia by inhibiting the production of alpha-hemolysin. FEMS Microbiol. Lett. 2013, 338, 124–131. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Chen, C.Z.; Pan, J.; Deng, X.M.; Wang, J.F. Epigallocatechin gallate can attenuate human alveolar epithelial cell injury induced by alpha-haemolysin. Microb. Pathog. 2018, 115, 222–226. [Google Scholar] [CrossRef]
- Qiu, J.Z.; Niu, X.D.; Dong, J.; Wang, D.C.; Wang, J.F.; Li, H.G.; Luo, M.J.; Li, S.T.; Feng, H.H.; Deng, X.M. Baicalin Protects Mice From Staphylococcus aureus Pneumonia Via Inhibition of the Cytolytic Activity of alpha-Hemolysin. J. Infect. Dis. 2012, 206, 292–301. [Google Scholar] [CrossRef]
- Dong, J.; Qiu, J.Z.; Zhang, Y.; Lu, C.J.; Dai, X.H.; Wang, J.F.; Li, H.G.; Wang, X.; Tan, W.; Luo, M.J.; et al. Oroxylin A Inhibits Hemolysis via Hindering the Self-Assembly of alpha-Hemolysin Heptameric Transmembrane Pore. PLoS Comput. Biol. 2013, 9, e1002869. [Google Scholar] [CrossRef]
- Liu, Y.M.; Shi, D.X.; Guo, Y.; Li, M.; Zha, Y.H.; Wang, Q.K.; Wang, J.F. Dracorhodin Perochlorate attenuates Staphylococcus aureus USA300 virulence by decreasing alpha-toxin expression. World J. Microbiol. Biotechnol. 2017, 33, 17. [Google Scholar] [CrossRef]
- Wang, X.; Dong, J.; Dai, X.H.; Zhang, Y.; Wang, J.F.; Li, H.G.; Lu, C.J.; Tan, W.; Gao, X.H.; Deng, X.M.; et al. Silibinin In Vitro Protects A549 Cells from Staphylococcus aureus-Mediated Injury and In Vivo Alleviates the Lung Injury of Staphylococcal Pneumonia. Planta Med. 2013, 79, 110–115. [Google Scholar] [CrossRef]
- Al-Shabib, N.A.; Husain, F.M.; Ahmad, I.; Khan, M.S.; Khan, R.A.; Khan, J.M. Rutin inhibits mono and multi-species biofilm formation by foodborne drug resistant Escherichia coli and Staphylococcus aureus. Food Control. 2017, 79, 325–332. [Google Scholar] [CrossRef]
- Elmasri, W.A.; Yang, T.; Tran, P.; Hegazy, M.E.; Hamood, A.N.; Mechref, Y.; Paré, P.W. Teucrium polium phenylethanol and iridoid glycoside characterization and flavonoid inhibition of biofilm-forming Staphylococcus aureus. J. Nat. Prod. 2015, 78, 2–9. [Google Scholar] [CrossRef]
- Ming, D.; Wang, D.; Cao, F.; Xiang, H.; Mu, D.; Cao, J.; Li, B.; Zhong, L.; Dong, X.; Zhong, X.; et al. Kaempferol Inhibits the Primary Attachment Phase of Biofilm Formation in Staphylococcus aureus. Front. Microbiol. 2017, 8, 2263. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, S.; Li, W.H.; Zhang, B.; Liu, B.W.; Liu, Y.; Deng, X.M.; Peng, L.P. Phloretin derived from apple can reduce alpha-hemolysin expression in methicillin-resistant Staphylococcus aureus USA300. World J. Microbiol. Biotechnol. 2015, 31, 1259–1265. [Google Scholar] [CrossRef]
- Liu, S.; Liu, B.W.; Luo, Z.Q.; Qiu, J.M.; Zhou, X.A.; Li, G.; Zhang, B.; Deng, X.M.; Yang, Z.G.; Wang, J.F. The combination of osthole with baicalin protects mice from Staphylococcus aureus pneumonia. World J. Microbiol. Biotechnol. 2017. [Google Scholar] [CrossRef]
- Zhang, B.; Teng, Z.H.; Li, X.H.; Lu, G.J.; Deng, X.M.; Niu, X.D.; Wang, J.F. Chalcone Attenuates Staphylococcus aureus Virulence by Targeting Sortase A and Alpha-Hemolysin. Front. Microbiol. 2017, 8, 1. [Google Scholar] [CrossRef]
- Duan, J.; Li, M.; Hao, Z.; Shen, X.; Liu, L.; Jin, Y.; Wang, S.; Guo, Y.; Yang, L.; Wang, L.; et al. Subinhibitory concentrations of resveratrol reduce alpha-hemolysin production in Staphylococcus aureus isolates by down-regulating saeRS. Emerg. Microbes Infect. 2018, 7, 1. [Google Scholar] [CrossRef]
- Wang, J.F.; Zhou, X.; Li, W.H.; Deng, X.M.; Deng, Y.H.; Niu, X.D. Curcumin protects mice from Staphylococcus aureus pneumonia by interfering with the self-assembly process of alpha-hemolysin. Sci. Rep. 2016, 6, 28254. [Google Scholar] [CrossRef]
- Peng, D.; Chen, A.L.; Shi, B.; Min, X.; Zhang, T.; Dong, Z.L.; Yang, H.; Chen, X.L.; Tian, Y.B.; Chen, Z.H. Preliminary study on the effect of brazilin on biofilms of Staphylococcus aureus. Exp. Ther. Med. 2018, 16, 2108–2118. [Google Scholar] [CrossRef]
- Vermote, A.; Brackman, G.; Risseeuw MD, P.; Vanhoutte, B.; Cos, P.; Van Hecke, K.; Breyne, K.; Meyer, E.; Coenye, T.; Van Calenbergh, S. Hamamelitannin Analogues that Modulate Quorum Sensing as Potentiators of Antibiotics against Staphylococcus aureus. Angew. Chem. Int. Ed. 2016, 55, 6551–6555. [Google Scholar] [CrossRef]
- Kiran, M.D.; Adikesavan, N.V.; Cirioni, O.; Giacometti, A.; Silvestri, C.; Scalise, G.; Ghiselli, R.; Saba, V.; Orlando, F.; Shoham, M.; et al. Discovery of a quorum-sensing inhibitor of drug resistant staphylococcal infections by structure-based virtual screening. Mol. Pharmacol. 2008, 73, 1578–1586. [Google Scholar] [CrossRef]
- Mun, S.H.; Kong, R.; Seo, Y.S.; Zhou, T.; Kang, O.H.; Shin, D.W.; Kwon, D.Y. Subinhibitory concentrations of punicalagin reduces expression of virulence-related exoproteins by Staphylococcus aureus. FEMS Microbiol. Lett. 2016, 363, fnw253. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, C.; Wu, Q.; Zheng, Z.; Liu, P.; Li, G.; Peng, X.; Xia, X. Antimicrobial Activity of Punicalagin Against Staphylococcus aureus and Its Effect on Biofilm Formation. Foodborne Pathog. Dis. 2017, 14, 282–287. [Google Scholar] [CrossRef]
- Dong, G.; Liu, H.; Yu, X.; Zhang, X.; Lu, H.; Zhou, T.; Cao, J. Antimicrobial and anti-biofilm activity of tannic acid against Staphylococcus aureus. Nat. Prod. Res. 2018, 32, 2225–2228. [Google Scholar] [CrossRef]
- Lin, M.H.; Chang, F.R.; Hua, M.Y.; Wu, Y.C.; Liu, S.T. Inhibitory effects of 1, 2, 3, 4, 6-penta-O-galloyl-β-D-glucopyranose on biofilm formation by Staphylococcus aureus. Antimicrob. Agents Chemother. 2011, 55, 1021–1027. [Google Scholar] [CrossRef]
- Vollaro, A.; Catania, M.R.; Iesce, M.R.; Sferruzza, R.; D’Abrosca, B.; Donnarumma, G.; De Filippis, A.; Cermola, F.; DellaGreca, M.; Buommino, E. Antimicrobial and anti-biofilm properties of novel synthetic lignan-like compounds. New Microbiol. 2019, 42, 21–28. [Google Scholar]
- Zhang, L.; Quan, C.; Zhang, X.; Xiong, W.; Fan, S. Proteoliposome-based model for screening inhibitors targeting histidine kinase AgrC. Chem. Biol. Drug Des. 2019, 93, 712. [Google Scholar] [CrossRef]
- Saising, J.; Ongsakul, M.; Voravuthikunchai, S.P. Rhodomyrtus tomentosa (Aiton) Hassk. ethanol extract and rhodomyrtone: A potential strategy for the treatment of biofilm-forming staphylococci. J. Med. Microbiol. 2011, 60, 1793–1800. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).