Deposition of Tetracoordinate Co(II) Complex with Chalcone Ligands on Graphene
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Crystal Structure of 1
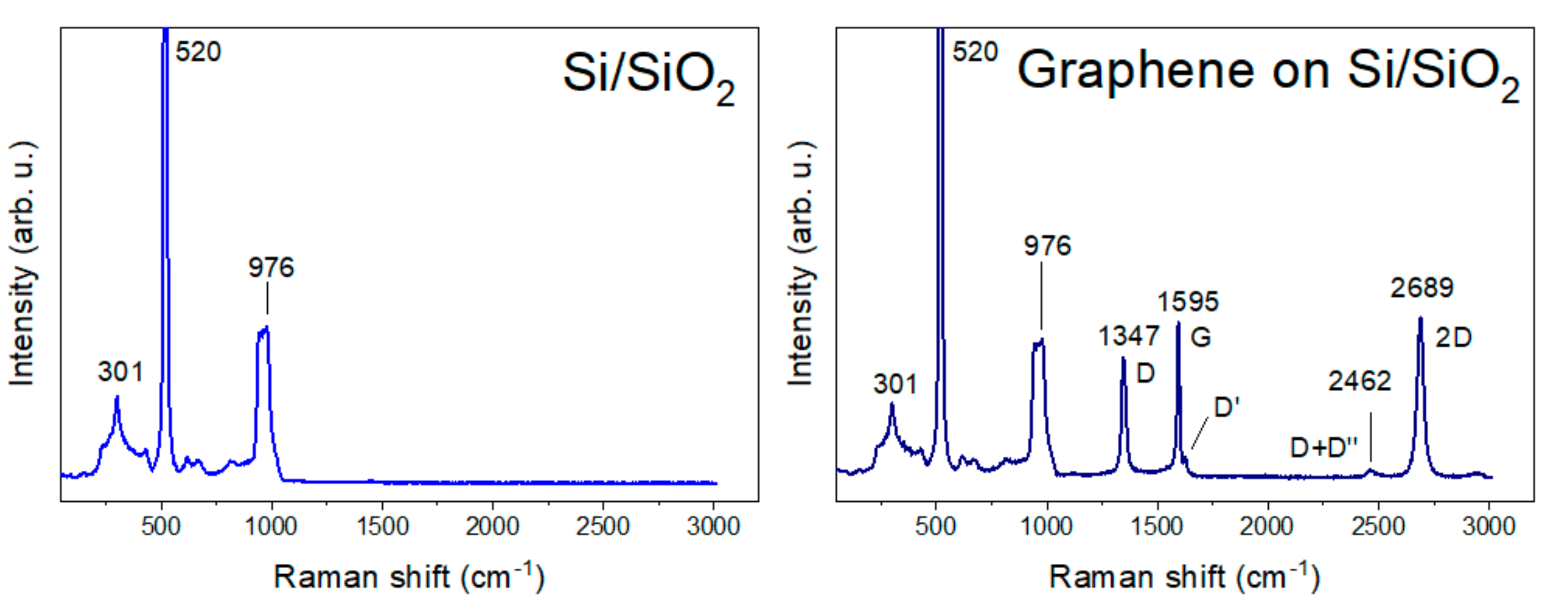
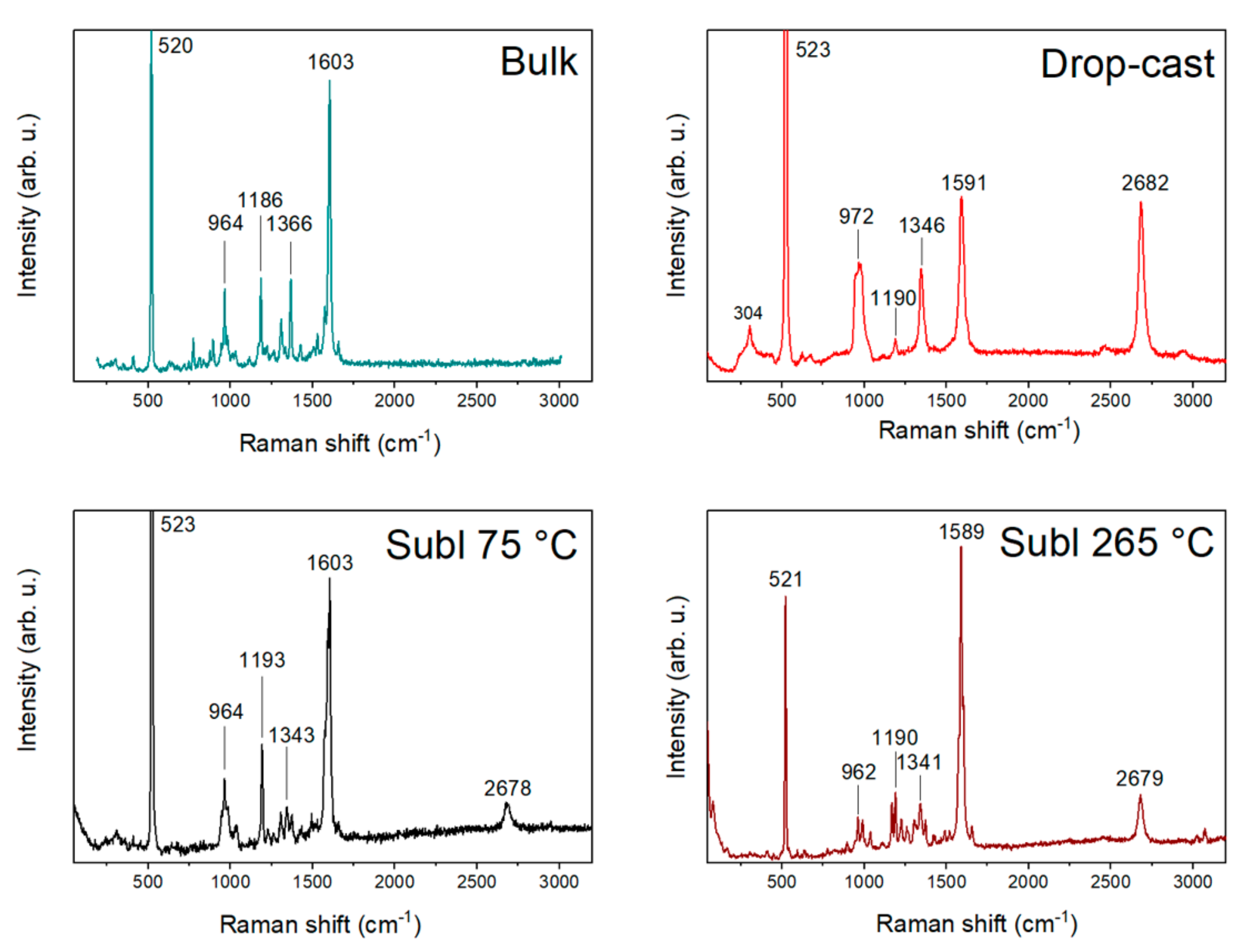
2.2. Raman Vibrations
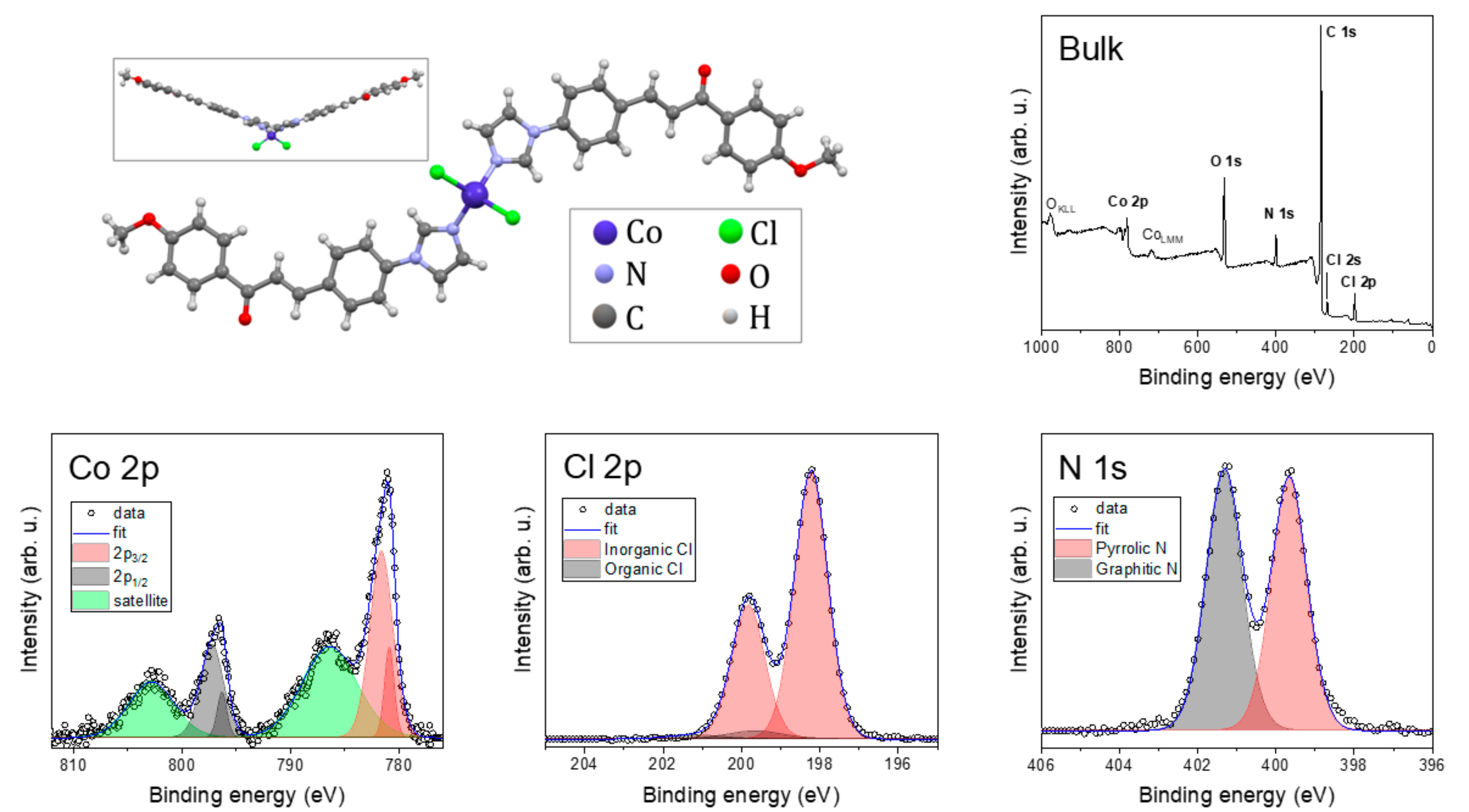
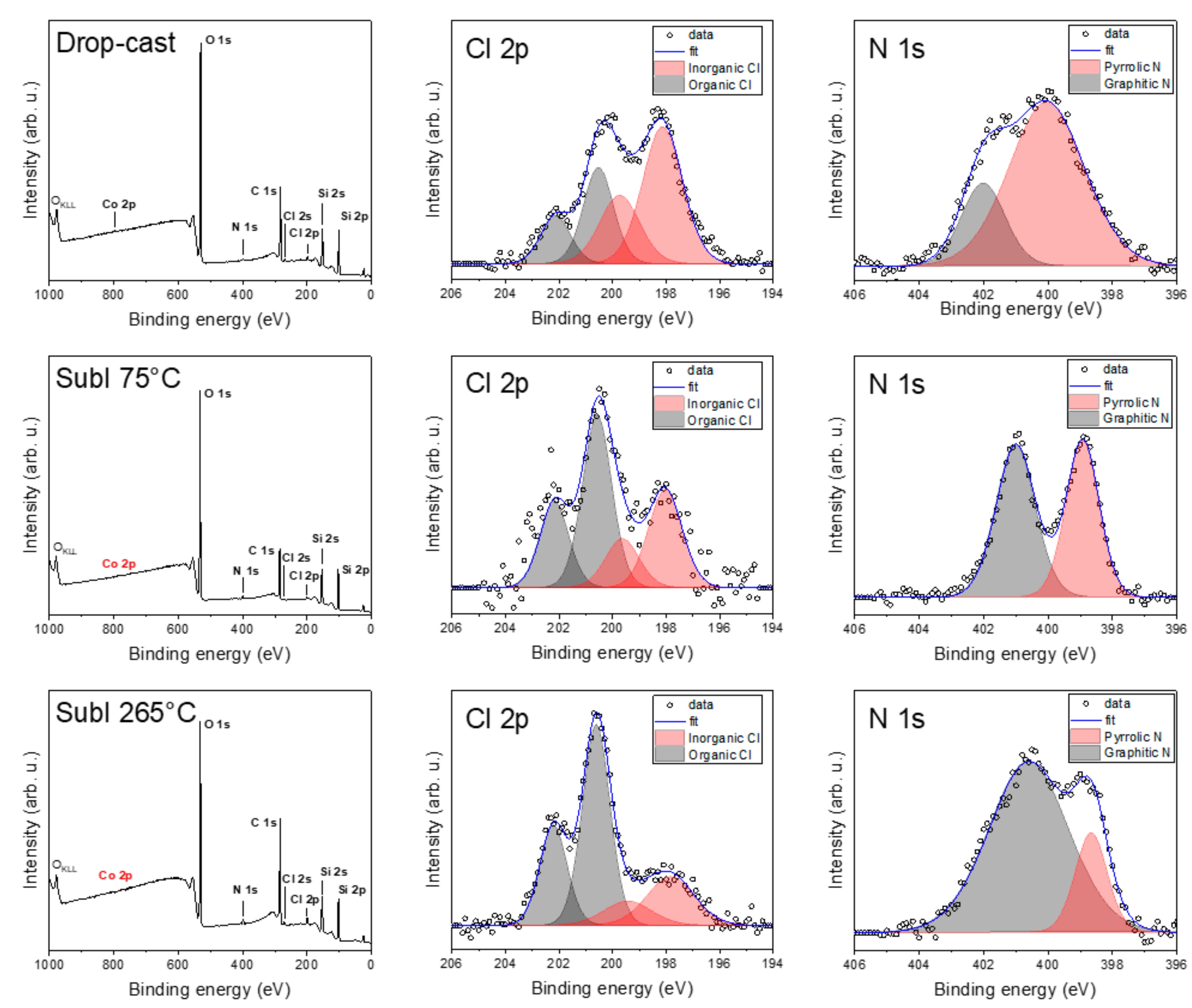
2.3. Chemical Composition and Bonds
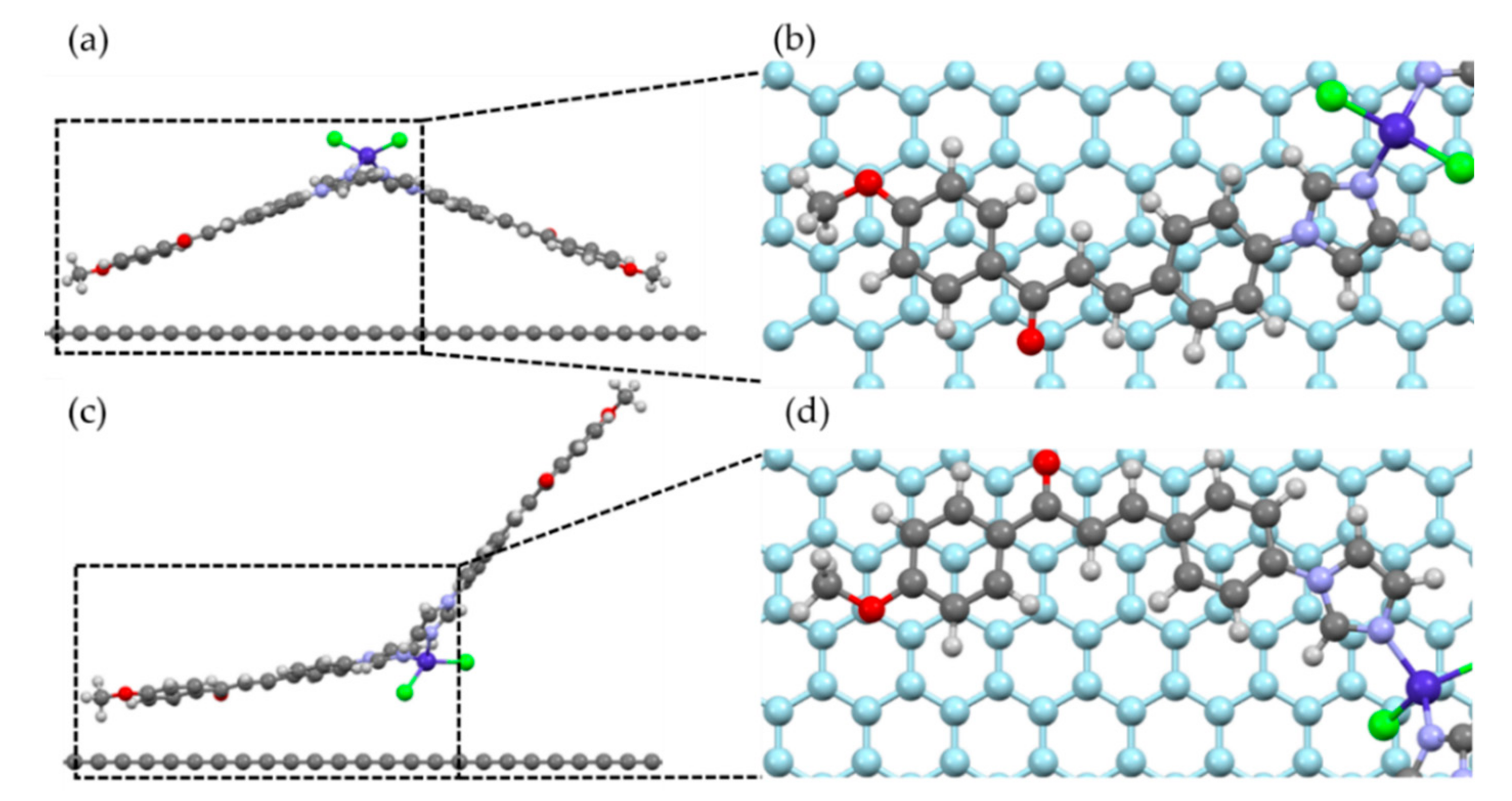
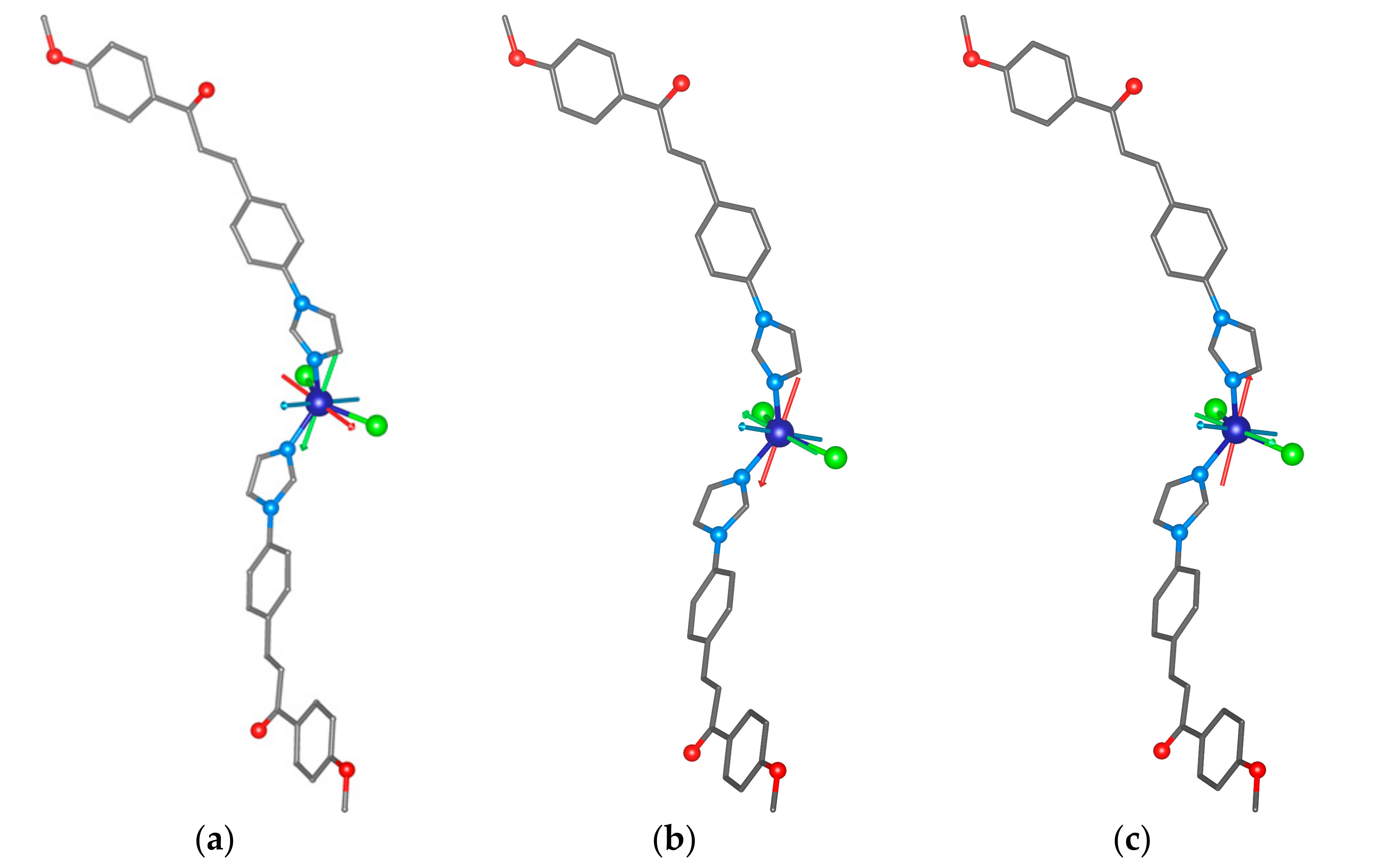
2.4. Molecular Adsorption by DFT
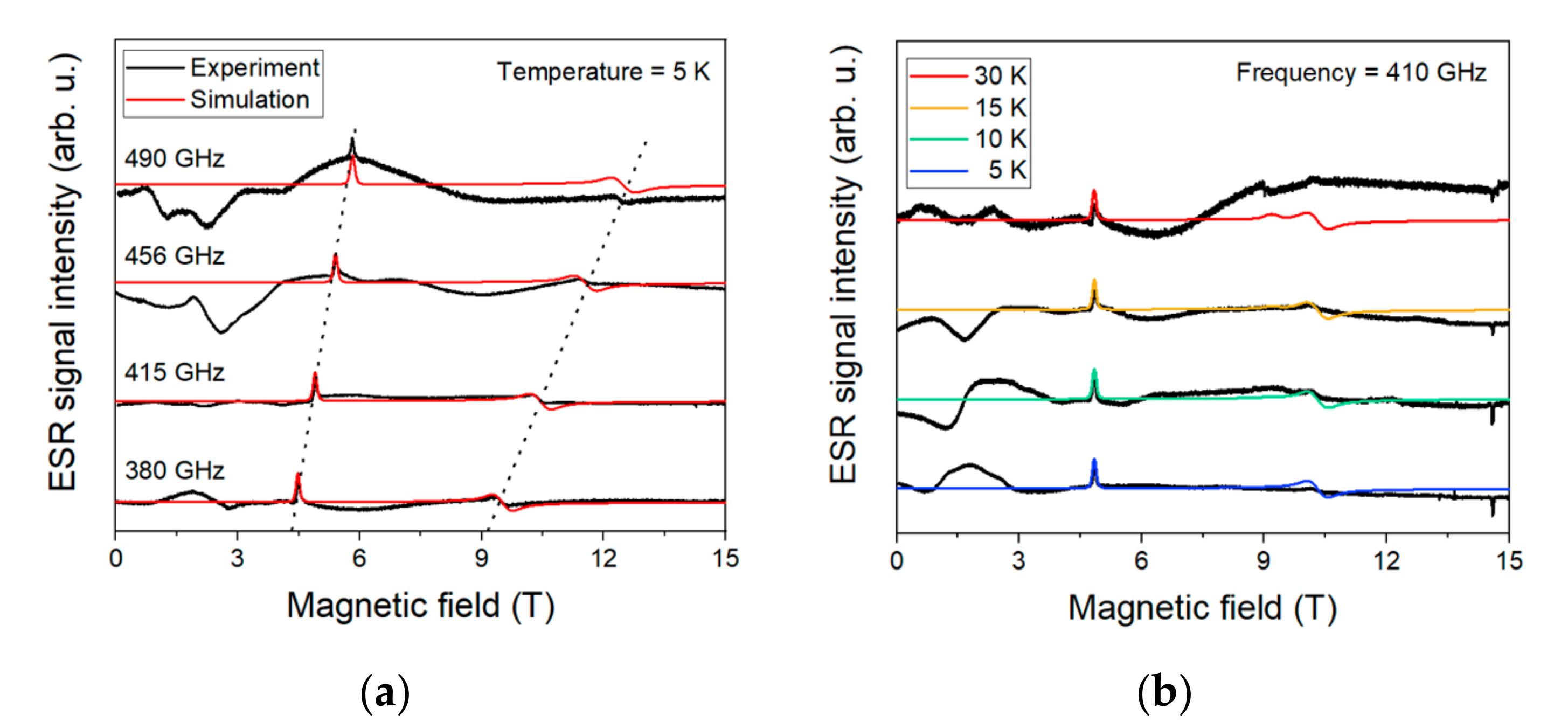
2.5. HF-ESR Spectroscopy
2.6. CASSCF Calculations
3. Materials and Methods
3.1. Materials
3.2. Synthesis
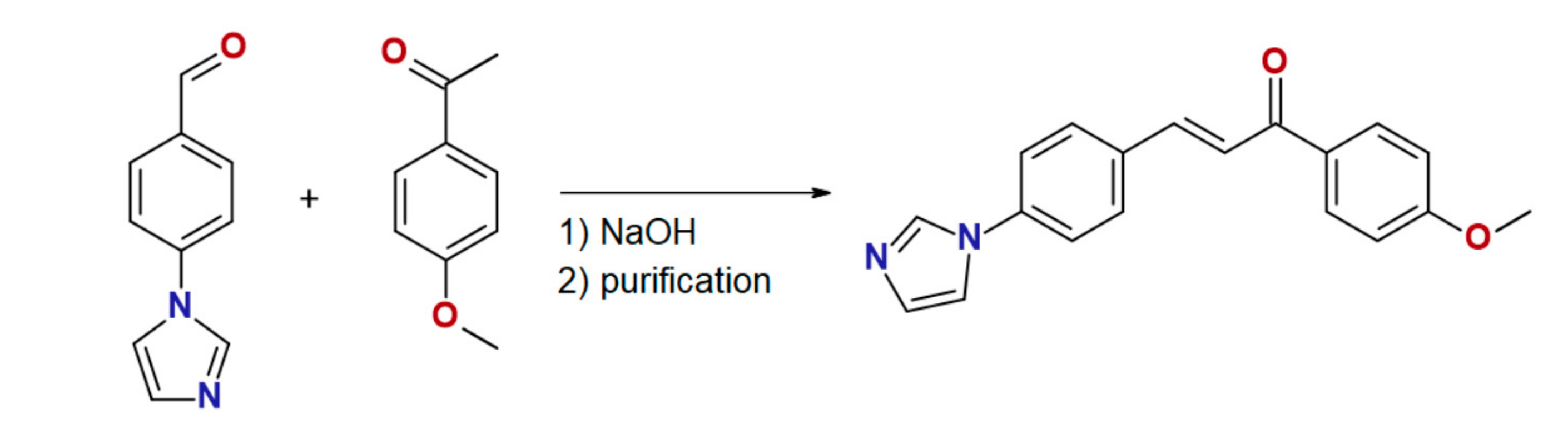
3.2.1. (2E)-3-[4-(1H-imidazol-1-yl)phenyl]-1-(4-methoxyphenyl)prop-2-en-1-one (4MeO-L)
3.2.2. Complex [Co(4MeO-L)2(Cl)2] (1)
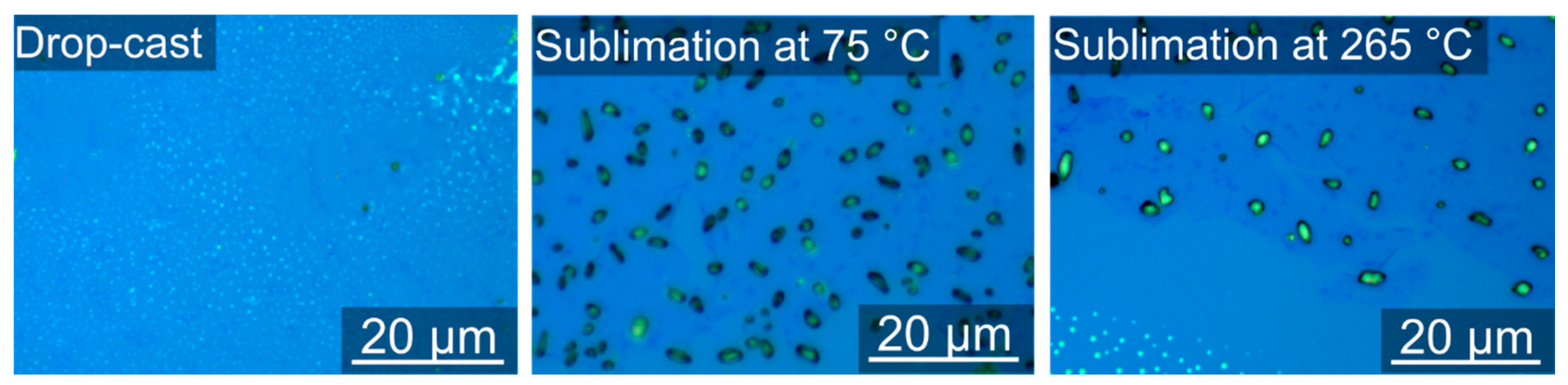
3.3. Deposited Samples
3.4. Raman Spectroscopy (RS)
3.5. Atomic Force Microscopy (AFM)
3.6. X-ray Photoelectron Spectroscopy (XPS)
3.7. High-Frequency Electron Spin Resonance (HF-ESR)
3.8. Density Functional Theory (DFT)
3.9. Theoretical Methods (CASSCF/NEVPT2)
3.10. Elemental Analyses (EA)
3.11. Mass Spectrometry (MS)
3.12. NMR Spectroscopy
3.13. Infrared Spectroscopy
3.14. Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Caneschi, A.; Gatteschi, D.; Sessoli, R.; Barra, A.L.; Brunei, L.C.; Guillot, M. Alternating Current Susceptibility, High Field Magnetization, and Millimeter Band EPR Evidence for a Ground S=10 State in [Mn12O12(CH3COO)16(H2O)4].2CH3COOH.4H2O. J. Am. Chem. Soc. 1991, 113, 5873–5874. [Google Scholar] [CrossRef]
- Sessoli, R.; Gatteschi, D.; Tsai, H.L.; Hendrickson, D.N.; Schake, A.R.; Wang, S.; Vincent, J.B.; Christou, G.; Folting, K. High-Spin Molecules: [Mn12O12(O2CR)16(H2O)4]. J. Am. Chem. Soc. 1993, 115, 1804–1816. [Google Scholar] [CrossRef]
- Sessoli, R.; Gatteschi, D.; Caneschi, A.; Novak, M.A. Magnetic bistability in a metal-ion cluster. Nature 1993, 365, 141–143. [Google Scholar] [CrossRef]
- Gatteschi, D.; Sessoli, R.; Villain, J. Molecular Nanomagnets, 1st ed.; Oxford University Press: Oxford, UK, 2007; ISBN 9780198567530. [Google Scholar]
- Neese, F.; Pantazis, D.A. What is not required to make a single molecule magnet. Faraday Discuss. 2011, 148, 229–238. [Google Scholar] [CrossRef]
- Ishikawa, N.; Sugita, M.; Ishikawa, T.; Koshihara, S.Y.; Kaizu, Y. Lanthanide double-decker complexes functioning as magnets at the single-molecular level. J. Am. Chem. Soc. 2003, 125, 8694–8695. [Google Scholar] [CrossRef]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. Magnetic hysteresis up to 80 kelvin in a dysprosium metallocene single-molecule magnet. Science 2018, 362, 1400–1403. [Google Scholar] [CrossRef]
- Wolf, S.A. Spintronics: A Spin-Based Electronics Vision for the Future. Science 2001, 294, 1488–1495. [Google Scholar] [CrossRef] [PubMed]
- Leuenberger, M.N.; Loss, D. Quantum computing in molecular magnets. Nature 2001, 410, 789–793. [Google Scholar] [CrossRef]
- Bogani, L.; Wernsdorfer, W. Molecular spintronics using single-molecule magnets. Nat. Mater. 2008, 7, 179–186. [Google Scholar] [CrossRef]
- Barra, A.L.; Gatteschi, D.; Sessoli, R. High-frequency EPR spectra of a molecular nanomagnet: Understanding quantum tunneling of the magnetization. Phys. Rev. B Condens. Matter Mater. Phys. 1997, 56, 8192–8198. [Google Scholar] [CrossRef]
- Barra, A.L.; Gatteschi, D.; Sessoli, R. High-frequency EPR spectra of [Fe8O2(OH)12(taCn)6]Br8: A critical appraisal of the barrier for the reorientation of the magnetization in single-molecule magnets. Chem. Eur. J. 2000, 6, 1608–1614. [Google Scholar] [CrossRef]
- Barra, A.L. High-frequency EPR spectroscopy of single-molecule magnets: A case study. Appl. Magn. Reson. 2001, 21, 619–628. [Google Scholar] [CrossRef]
- Krzystek, J.; Ozarowski, A.; Zvyagin, S.A.; Telser, J. High spin Co(I): High-frequency and -field EPR spectroscopy of CoX(PPh 3) 3 (X = Cl, Br). Inorg. Chem. 2012, 51, 4954–4964. [Google Scholar] [CrossRef] [PubMed]
- Rechkemmer, Y.; Fischer, J.E.; Marx, R.; Dörfel, M.; Neugebauer, P.; Horvath, S.; Gysler, M.; Brock-Nannestad, T.; Frey, W.; Reid, M.F.; et al. Comprehensive Spectroscopic Determination of the Crystal Field Splitting in an Erbium Single-Ion Magnet. J. Am. Chem. Soc. 2015, 137, 13114–13120. [Google Scholar] [CrossRef]
- Realista, S.; Fitzpatrick, A.J.; Santos, G.; Ferreira, L.P.; Barroso, S.; Pereira, L.C.J.; Bandeira, N.A.G.; Neugebauer, P.; Hrubý, J.; Morgan, G.G.; et al. A Mn(III) single ion magnet with tridentate Schiff-base ligands. Dalton Trans. 2016, 45, 12301–12307. [Google Scholar] [CrossRef]
- Kultaeva, A.; Biktagirov, T.; Neugebauer, P.; Bamberger, H.; Bergmann, J.; Van Slageren, J.; Krautscheid, H.; Pöppl, A. Multifrequency EPR, SQUID, and DFT Study of Cupric Ions and Their Magnetic Coupling in the Metal-Organic Framework Compound ∞3[Cu(prz-trz-ia)]. J. Phys. Chem. C 2018, 122, 26642–26651. [Google Scholar] [CrossRef]
- Neugebauer, P.; Bloos, D.; Marx, R.; Lutz, P.; Kern, M.; Aguilà, D.; Vaverka, J.; Laguta, O.; Dietrich, C.; Clérac, R.; et al. Ultra-broadband EPR spectroscopy in field and frequency domains. Phys. Chem. Chem. Phys. 2018, 20, 15528–15534. [Google Scholar] [CrossRef]
- Bucinsky, L.; Breza, M.; Malček, M.; Powers, D.C.; Hwang, S.J.; Krzystek, J.; Nocera, D.G.; Telser, J. High-Frequency and -Field EPR (HFEPR) Investigation of a Pseudotetrahedral Cr IV Siloxide Complex and Computational Studies of Related Cr IV L 4 Systems. Inorg. Chem. 2019, 58, 4907–4920. [Google Scholar] [CrossRef]
- Hrubý, J.; Dvořák, D.; Squillantini, L.; Mannini, M.; van Slageren, J.; Herchel, R.; Nemec, I.; Neugebauer, P. Co(ii)-Based single-ion magnets with 1,1’-ferrocenediyl-bis(diphenylphosphine) metalloligands. Dalton Trans. 2020, 2, 11697–11707. [Google Scholar] [CrossRef]
- Hrubý, J.; Santana, V.T.; Kostiuk, D.; Bouček, M.; Lenz, S.; Kern, M.; Šiffalovič, P.; van Slageren, J.; Neugebauer, P. A graphene-based hybrid material with quantum bits prepared by the double Langmuir–Schaefer method. RSC Adv. 2019, 9, 24066–24073. [Google Scholar] [CrossRef]
- Marie, L.S.; El Fatimy, A.; Hrubý, J.; Nemec, I.; Hunt, J.; Myers-Ward, R.; Gaskill, D.K.; Kruskopf, M.; Yang, Y.; Elmquist, R.; et al. Nanostructured graphene for nanoscale electron paramagnetic resonance spectroscopy. J. Phys. Mater. 2020, 3, 014013. [Google Scholar] [CrossRef]
- Ciccullo, F.; Glaser, M.; Sättele, M.S.; Lenz, S.; Neugebauer, P.; Rechkemmer, Y.; Van Slageren, J.; Casu, M.B. Thin film properties and stability of a potential molecular quantum bit based on copper(ii). J. Mater. Chem. C 2018, 6, 8028–8034. [Google Scholar] [CrossRef]
- Holmberg, R.J.; Murugesu, M. Adhering magnetic molecules to surfaces. J. Mater. Chem. C 2015, 3, 11986–11998. [Google Scholar] [CrossRef]
- Caneschi, A.; Gatteschi, D.; Totti, F. Molecular magnets and surfaces: A promising marriage. A DFT insight. Coord. Chem. Rev. 2015, 289–290, 357–378. [Google Scholar] [CrossRef]
- Mannini, M.; Pineider, F.; Sainctavit, P.; Danieli, C.; Otero, E.; Sciancalepore, C.; Talarico, A.M.; Arrio, M.A.; Cornia, A.; Gatteschi, D.; et al. Magnetic memory of a single-molecule quantum magnet wired to a gold surface. Nat. Mater. 2009, 8, 194–197. [Google Scholar] [CrossRef]
- Saywell, A.; Magnano, G.; Satterley, C.J.; Perdigão, L.M.A.; Britton, A.J.; Taleb, N.; Del Carmen Giménez-López, M.; Champness, N.R.; O’Shea, J.N.; Beton, P.H. Self-assembled aggregates formed by single-molecule magnets on a gold surface. Nat. Commun. 2010, 1, 75. [Google Scholar] [CrossRef]
- Rozbořil, J.; Rechkemmer, Y.; Bloos, D.; Münz, F.; Wang, C.N.; Neugebauer, P.; Čechal, J.; Novák, J.; van Slageren, J. Magneto-optical investigations of molecular nanomagnet monolayers. Dalton Trans. 2016, 45, 7555–7558. [Google Scholar] [CrossRef]
- Malavolti, L.; Lanzilotto, V.; Ninova, S.; Poggini, L.; Cimatti, I.; Cortigiani, B.; Margheriti, L.; Chiappe, D.; Otero, E.; Sainctavit, P.; et al. Magnetic bistability in a submonolayer of sublimated Fe4 single-molecule magnets. Nano Lett. 2015, 15, 535–541. [Google Scholar] [CrossRef]
- Kiefl, E.; Mannini, M.; Bernot, K.; Yi, X.; Amato, A.; Leviant, T.; Magnani, A.; Prokscha, T.; Suter, A.; Sessoli, R.; et al. Robust Magnetic Properties of a Sublimable Single-Molecule Magnet. ACS Nano 2016, 10, 5663–5669. [Google Scholar] [CrossRef]
- Lanzilotto, V.; Malavolti, L.; Ninova, S.; Cimatti, I.; Poggini, L.; Cortigiani, B.; Mannini, M.; Totti, F.; Cornia, A.; Sessoli, R. The Challenge of Thermal Deposition of Coordination Compounds: Insight into the Case of an Fe4 Single Molecule Magnet. Chem. Mater. 2016, 28, 7693–7702. [Google Scholar] [CrossRef]
- Cañon-Mancisidor, W.; Miralles, S.G.; Baldoví, J.J.; Espallargas, G.M.; Gaita-Ariño, A.; Coronado, E. Sublimable Single Ion Magnets Based on Lanthanoid Quinolinate Complexes: The Role of Intermolecular Interactions on Their Thermal Stability. Inorg. Chem. 2018, 57, 14170–14177. [Google Scholar] [CrossRef]
- Miralles, S.G.; Bedoya-Pinto, A.; Baldoví, J.J.; Cañon-Mancisidor, W.; Prado, Y.; Prima-Garcia, H.; Gaita-Ariño, A.; Mínguez Espallargas, G.; Hueso, L.E.; Coronado, E. Sublimable chloroquinolinate lanthanoid single-ion magnets deposited on ferromagnetic electrodes. Chem. Sci. 2018, 9, 199–208. [Google Scholar] [CrossRef]
- Margheriti, L.; Mannini, M.; Sorace, L.; Gorini, L.; Gatteschi, D.; Caneschi, A.; Chiappe, D.; Moroni, R.; de Mongeot, F.B.; Cornia, A.; et al. Thermal Deposition of Intact Tetrairon(III) Single-Molecule Magnets in High-Vacuum Conditions. Small 2009, 5, 1460–1466. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Du, X.; Skachko, I.; Andrei, E.Y.; Barker, A. Approaching ballistic transport in suspended graphene. Nat. Nanotechnol. 2008, 3, 491–495. [Google Scholar] [CrossRef]
- Neugebauer, P.; Orlita, M.; Faugeras, C.; Barra, A.L.; Potemski, M. How perfect can graphene be? Phys. Rev. Lett. 2009, 103, 136403. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic of Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Seol, J.H.; Jo, I.; Moore, A.L.; Lindsay, L.; Aitken, Z.H.; Pettes, M.T.; Li, X.; Yao, Z.; Huang, R.; Broido, D.; et al. Two-dimensional phonon transport in supported graphene. Science 2010, 328, 213–216. [Google Scholar] [CrossRef]
- Novoselov, K.S.S.; Geim, A.K.K.; Morozov, S.V.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.V.; Grigorieva, I.V.V.; Firsov, A.A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Warner, J.H.; Mukai, M.; Kirkland, A.I. Atomic structure of ABC rhombohedral stacked trilayer graphene. ACS Nano 2012, 6, 5680–5686. [Google Scholar] [CrossRef]
- VanMil, B.L.; Myers-Ward, R.L.; Tedesco, J.L.; Eddy, C.R., Jr.; Jernigan, G.G.; Culbertson, J.C.; Campbell, P.M.; McCrate, J.M.; Kitt, S.A.; Gaskill, D.K. Graphene Formation on SiC Substrates. Mater. Sci. Forum 2009, 615–617, 211–214. [Google Scholar] [CrossRef]
- Coleman, J.N.; Lotya, M.; O’Neill, A.; Bergin, S.D.; King, P.J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.J.; et al. Two-dimensional nanosheets produced by liquid exfoliation of layered materials. Science 2011, 331, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Ri Kim, H.; Song, Y.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, M.; Aravena, D.; Suturina, E.; Bill, E.; Maganas, D.; Neese, F. First principles approach to the electronic structure, magnetic anisotropy and spin relaxation in mononuclear 3d-transition metal single molecule magnets. Coord. Chem. Rev. 2015, 289–290, 177–214. [Google Scholar] [CrossRef]
- SHAPE, Version 2.1; University of Barcelona: Barcelona, Spain, 2013.
- Alvarez, S. Polyhedra in (inorganic) chemistry. Dalton Trans. 2005, 13, 2209–2233. [Google Scholar] [CrossRef]
- Mankad, V.; Gupta, S.K.; Jha, P.K.; Ovsyuk, N.N.; Kachurin, G.A. Low-frequency Raman scattering from Si/Ge nanocrystals in different matrixes caused by acoustic phonon quantization. J. Appl. Phys. 2012, 112, 54318. [Google Scholar] [CrossRef]
- Spizzirri, P.G.; Fang, J.H.; Rubanov, S.; Gauja, E.; Prawer, S. Nano-Raman spectroscopy of silicon surfaces. arXiv 2010, arXiv:1002.2692. [Google Scholar]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron-phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Akula, S.; Parthiban, V.; Peera, S.G.; Singh, B.P.; Dhakate, S.R.; Sahu, A.K. Simultaneous Co-Doping of Nitrogen and Fluorine into MWCNTs: An In-Situ Conversion to Graphene Like Sheets and Its Electro-Catalytic Activity toward Oxygen Reduction Reaction. J. Electrochem. Soc. 2017, 164, F568–F576. [Google Scholar] [CrossRef]
- Fiedler, R.; Herzschuh, R. An XPS investigation of the effects of heat treatment on the chlorine surface chemistry of some lignites. Fuel 1993, 72, 1501–1505. [Google Scholar] [CrossRef]
- Poneti, G.; Mannini, M.; Cortigiani, B.; Poggini, L.; Sorace, L.; Otero, E.; Sainctavit, P.; Sessoli, R.; Dei, A. Magnetic and spectroscopic investigation of thermally and optically driven valence tautomerism in thioether-bridged dinuclear cobalt-dioxolene complexes. Inorg. Chem. 2013, 52, 11798–11805. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B Condens. Matter Mater. Phys. 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple [Phys. Rev. Lett. 77, 3865 (1996)]. Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Hussain, T.; Siddiqui, H.L.; Zia-ur-Rehman, M.; Masoom Yasinzai, M.; Parvez, M. Anti-oxidant, anti-fungal and anti-leishmanial activities of novel 3-[4-(1H-imidazol-1-yl) phenyl]prop-2-en-1-ones. Eur. J. Med. Chem. 2009, 44, 4654–4660. [Google Scholar] [CrossRef]
- Masaryk, L.; Moncol, J.; Herchel, R.; Nemec, I. Halogen Bonding in New Dichloride-Cobalt(II) Complex with Iodo Substituted Chalcone Ligands. Crystals 2020, 10, 354. [Google Scholar] [CrossRef]
- Shirley, D.A. High-resolution x-ray photoemission spectrum of the valence bands of gold. Phys. Rev. B 1972, 5, 4709–4714. [Google Scholar] [CrossRef]
- Yeh, J.J.; Lindau, I. Atomic subshell photoionization cross sections and asymmetry parameters: 1 ≤ Z ≤ 103. At. Data Nucl. Data Tables 1985, 32, 1–155. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Hellweg, A.; Hättig, C.; Höfener, S.; Klopper, W. Optimized accurate auxiliary basis sets for RI-MP2 and RI-CC2 calculations for the atoms Rb to Rn. Theor. Chem. Acc. 2007, 117, 587–597. [Google Scholar] [CrossRef]
- Malmqvist, P.Å.; Roos, B.O. The CASSCF state interaction method. Chem. Phys. Lett. 1989, 155, 189–194. [Google Scholar] [CrossRef]
- Angeli, C.; Cimiraglia, R.; Evangelisti, S.; Leininger, T.; Malrieu, J.P. Introduction of n-electron valence states for multireference perturbation theory. J. Chem. Phys. 2001, 114, 10252. [Google Scholar] [CrossRef]
- Ganyushin, D.; Neese, F. First-principles calculations of zero-field splitting parameters. J. Chem. Phys. 2006, 125, 024103. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. Efficient and accurate approximations to the molecular spin-orbit coupling operator and their use in molecular g-tensor calculations. J. Chem. Phys. 2005, 122, 034107. [Google Scholar] [CrossRef] [PubMed]
- Maurice, R.; Bastardis, R.; de Graaf, C.; Suaud, N.; Mallah, T.; Guihéry, N. Universal theoretical approach to extract anisotropic spin hamiltonians. J. Chem. Theory Comput. 2009, 5, 2977–2984. [Google Scholar] [CrossRef]
- Palatinus, L.; Chapuis, G. SUPERFLIP—A computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Crystallogr. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- MacRae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]

| D/cm−1 | E/D | gx | gy | gz | gav | |
|---|---|---|---|---|---|---|
| 1 | +14.5 | 0.150 | 2.325 | 2.378 | 2.163 | 2.289 |
| Geometry 1 | +16.4 | 0.090 | 2.346 | 2.364 | 2.150 | 2.287 |
| Geometry 2 | +17.5 | 0.132 | 2.345 | 2.381 | 2.143 | 2.290 |
| HF-ESR | +14.6 | 0.235 | 2.320 | 2.380 | 2.160 | 2.287 |
Sample Availability: Samples are available from the authors upon request in limited amount. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hrubý, J.; Vavrečková, Š.; Masaryk, L.; Sojka, A.; Navarro-Giraldo, J.; Bartoš, M.; Herchel, R.; Moncol, J.; Nemec, I.; Neugebauer, P. Deposition of Tetracoordinate Co(II) Complex with Chalcone Ligands on Graphene. Molecules 2020, 25, 5021. https://doi.org/10.3390/molecules25215021
Hrubý J, Vavrečková Š, Masaryk L, Sojka A, Navarro-Giraldo J, Bartoš M, Herchel R, Moncol J, Nemec I, Neugebauer P. Deposition of Tetracoordinate Co(II) Complex with Chalcone Ligands on Graphene. Molecules. 2020; 25(21):5021. https://doi.org/10.3390/molecules25215021
Chicago/Turabian StyleHrubý, Jakub, Šárka Vavrečková, Lukáš Masaryk, Antonín Sojka, Jorge Navarro-Giraldo, Miroslav Bartoš, Radovan Herchel, Ján Moncol, Ivan Nemec, and Petr Neugebauer. 2020. "Deposition of Tetracoordinate Co(II) Complex with Chalcone Ligands on Graphene" Molecules 25, no. 21: 5021. https://doi.org/10.3390/molecules25215021
APA StyleHrubý, J., Vavrečková, Š., Masaryk, L., Sojka, A., Navarro-Giraldo, J., Bartoš, M., Herchel, R., Moncol, J., Nemec, I., & Neugebauer, P. (2020). Deposition of Tetracoordinate Co(II) Complex with Chalcone Ligands on Graphene. Molecules, 25(21), 5021. https://doi.org/10.3390/molecules25215021






